Abstract
TIPE2, also known as TNFAIP8L2, a member of the tumor necrosis factor-alpha-induced protein-8 (TNFAIP8) family, is known as an inhibitor in inflammation and cancer, and its overexpression induces cell death. We examined the role of TIPE2 with respect to adjuvant arthritis (AA)-associated pathogenesis by analyzing the TIPE2 regulation of death receptor (DR5)-mediated apoptosis in vitro. The results showed that TIPE2 was detected in normal fibroblast-like synoviocytes (FLSs), but scarcely observed in AA-FLSs. Therefore, recombinant MIGR1/TIPE2+/+ and control MIGR1 lentivirus vectors were transfected to AA-FLSs, which were denoted as TIPE2+/+-FLSs and MIGR1-FLSs, respectively. Our results showed that TIPE2+/+-FLSs were highly susceptible to ZF1-mediated apoptosis, and ZF1 was our own purification of an anti-DR5 single chain variable fragment antibody. Under the presence of TIPE2, the expression of DR5 was significantly increased compared with that of the MIGR1-FLS group. In contrast, the level of phosphorylated nuclear factor-kappa B (pNF-κB) was lower in the TIPE2+/+-FLS group treated with ZF1, whereas the activity of caspase was higher. Moreover, the rate of apoptosis in the TIPE2+/+-FLS group, which was pretreated with caspase inhibitor Z-VAD-FMK, was significantly decreased. In contrast, the apoptosis occurrence in the MIGR1-FLS group increased significantly with the pretreatment of the NF-κB inhibitor Bay. These results indicated that TIPE2 increased the apoptosis of AA-FLSs by enhancing DR5 expression levels, thereby promoting the activation of caspase and inhibiting the activation of NF-κB in AA-FLSs. TIPE2 might potentially act as a therapeutic target for rheumatoid arthritis.
Keywords: rheumatoid arthritis, adjuvant arthritis, fibroblast-like synoviocytes, DR5, TIPE2
Introduction
Rheumatoid arthritis (RA) synovial tissue contains large numbers of infiltrated inflammatory cells and excessively proliferated synovial lining cells.1 Fibroblast-like synoviocytes (FLSs), which fail to precede apoptosis, play an important role in RA pathology.2–4 Adjuvant arthritis (AA) is a model of experimental arthritis that is induced by the injection of complete Freund’s adjuvant.5 The symptoms of AA in joints, along with the cellular and humoral immunities, show a similar pathology to those observed in RA.
Tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) induces apoptosis by binding to TRAIL receptor 1 (death receptor 4, DR4) and TRAIL receptor 2 (DR5), which antagonize the death signal.6 Previous reports have indicated that RA synovial tissue and fibroblasts express high levels of DR5 and are highly susceptible to DR5-mediated apoptosis.7 A novel agonistic antihuman DR5 single chain variable fragment antibody (Ab), ZF1, is known to induce strong apoptosis response without hepatocellular toxicity.8 Although the high expression levels of DR5 in RA synovial fibroblasts are thought to sensitize these cells for apoptosis, cell hyperplasia and lymphocyte activation are severe.
A new member of the TNF-alpha-induced protein-8 (TNFAIP8) family, which is commonly referred to as the TIPE family, was recently identified from an inflamed spinal cord, this is known as TNFAIP8-like 2 (TIPE2). TIPE2 has been defined as a negative regulator of innate and adaptive immunity, and maintains immune homeostasis.9 TIPE2 is downregulated in patients with chronic inflammatory diseases, such as systemic lupus erythematosus, and its expression is inversely correlated with disease progression.10 The forced expression of TIPE2 in hepatocellular carcinoma-derived cell lines markedly inhibits tumor cell growth11 and the proliferation of vascular smooth muscle.12 In contrast, downregulated TIPE2 is associated with a poor prognosis and promotes cell proliferation in non-small-cell lung cancer, and TIPE2-deficient T cells are significantly less sensitive to apoptosis.13,14 Therefore, we hypothesized that TIPE2 would have a similar effect on RA FLSs in orchestrating cell proliferation and death. More specifically, we postulated that the expression of TIPE2 would influence ZF1-induced apoptosis in AA-FLS cells.
Materials and methods
Cell lines and plasmids
AA inducement, FLS preparation, and identification techniques were conducted according to a previous publication.15 RAW 264.7 cells, normal FLSs, and AA-FLSs were grown in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% fetal bovine serum, penicillin, and streptomycin. To generate stable cell lines, AA-FLSs were infected with MIGR1/TIPE2+/+ recombinant lentiviral vectors, and MIGR1 lentiviral vector was used as the negative control (gift from Dr Chen Youhai, University of Pennsylvania School of Medicine). The culture medium was replaced 24 hours after infection. After an additional 48 hours, the infected cells were observed under fluorescence microscopy and infected cells constituted more than 90% of the total cell count.
Antihuman DR5 single chain variable fragment Ab, ZF1
The anti-DR5 single chain variable fragment Ab, ZF1, reported previously in the invention patent CN 101717775A, was used in this study at >98% purity, through a nickel ion column (Merck KGaA, Kenilworth, NJ, USA). Protein purification was performed as previously described.8
Quantitative polymerase chain reaction (qPCR) and reverse transcription (RT)-PCR analysis
Total RNA was isolated using TRIzol reagent (Invitrogen, Waltham, MA, USA) and purified with RNeasy Mini kits (Qiagen, Hilden, Germany) according to the manufacturers’ instruction.
After processing with RNase-free DNase I (Invitrogen), RNA samples were reversely transcribed with oligo (dT) and SuperScript II transcriptase (Invitrogen). qPCR was performed using specific Quantitect Primers for mouse glyceraldehyde-phosphate dehydrogenase (GAPDH), TIPE1, TIPE2, and TIPE3 (Qiagen) in an Applied Biosystems 7500 system using Power SYBR Green PCR Master Mix (Applied Biosystems, Thermo Fisher Scientific, Waltham, MA, USA). Relative gene expression levels were determined using GAPDH as the control.
Western blot analysis
Aliquots of synovial cell lysates were separated with 12% sodium dodecyl sulfate–polyacrylamide gel electrophoresis, blotted onto a polyvinylidene difluoride membrane, and probed with primary antibodies (Abcam, Cambridge, UK) for 75 minutes at room temperature. The membranes were incubated with rabbit anti-actin Ab (Huaan Biotechnology, Hangzhou, People’s Republic of China), anti-TIPE2 (Abcam), anti-caspase-8 (p18) (Abcam), anti-DR5 antibodies (Abcam), antinuclear factor-kappa Bp (anti-NF-κBp) 105/p50 (phospho) (Abcam), anti-caspase-3 (pro) (Epitomics, Burlingame, CA, USA), anti-caspase-3 (active) (Epitomics), anti-caspase-9 (Epitomics), anti-Bid (Epitomics), and anti-Bax (Epitomics). The protein bands were visualized by electrochemiluminescence reactions. Immunoblot analysis was performed as previously described.16
AA-FLS proliferation assay
FLSs (1×104 cells/well) were added in quintuplicate to a 96-well plate (Corning Inc., Corning, NY, USA) in the presence of various concentration of ZF1 (0.0063, 0.0125, 0.025, 0.050, 0.1, and 0.2 mg/mL); the cells were then cultured in DMEM with 10% fetal bovine serum at 37°C in a humidified 5% CO2 atmosphere for 12 hours. Then, 20 μL of a 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide (MTT) solution (5 mg/mL) was added to each well, and the plates were incubated for an additional 4 hours. All of the supernatant was removed before 150 μL of dimethyl sulfoxide was added to each well to dissolve the formazan crystals produced by MTT. Finally, the optical density (OD) value was measured by a multiscanner autoreader (MR 5000; Dynatech, Chantilly, VA, USA) at 490 nm. Inhibition ratio (%) was calculated using the following equation:
Detection of DR5 expression and cell apoptosis with flow cytometry
FLSs were trypsinized gently, washed with phosphate-buffered saline (PBS, with 3% bovine serum albumin), incubated with 1 μg anti-DR5 Ab for 1 hour at 4°C washed again with PBS (with 3% bovine serum albumin), and incubated with 1 μg of an anti-mouse IgG-allophycocyanin Ab for 30 minutes at room temperature. Finally, the cells were analyzed using a FACS scan flow cytometer (BD Biosciences, San Jose, CA, USA). For apoptosis analyses, FLSs were plated at a concentration of 5×105 cells/mL in a six-well plate, incubated for 24 hours, then treated with ZF1 (0, 0.0125, 0.025, 0.050, and 0.1 mg/mL) for 12 hours. After treatment, the cells were harvested by 0.25% trypsin (without EDTA), washed with PBS, and suspended in binding buffer (1×). A 100 μL aliquot of the cell suspension was incubated with 5 μL of annexin V–APC and 5 μL of propidium iodide for 15 minutes in the dark, then 1 mL of binding buffer (1×) was added to each sample. Cells were analyzed directly by a flow cytometer as previously described.17
Caspase inhibition and NF-κB inhibition assay
Cells were plated at a concentration of 5×105 cells/mL in a six-well plate, incubated for 24 hours. Before stimulation with 0.04 mg/mL ZF1, the TIPE2+/+-FLS group was pretreated with caspase inhibitor Z-VAD-FMK (Sigma-Aldrich Co., St Louis, MO, USA) and the MIGR1-FLS group was pretreated with the NF-κB inhibitor Bay (Sigma, USA). After treatment, the cells were harvested by 0.25% trypsin (without EDTA), washed with PBS, and suspended in binding buffer (1×) and detected cell apoptosis by a flow cytometer as previously described.
Statistical analysis
The statistical analysis was performed using SPSS 11.5 software for Windows. The data were expressed as mean ± standard deviation, with the number of respective experiments run in triplicate. The significance of the differences in the mean values between/within multiple groups was examined by an independent-samples t-test and two-way analysis of variance, respectively. A value of P<0.05 was considered statistically significant.
Results
TIPE2 is not expressed in AA-FLSs
We observed an amplicon of TIPE2 in normal FLSs, but not in AA-FLSs (Figure 1A). The qPCR results were consistent with this observation (Figure 1B). To further confirm TIPE2 expression, we used Western blotting techniques in conjunction with a specific Ab against TIPE2. TIPE2 was observed in the normal FLSs in a clear band at ~21 kDa, while it was absent in the AA-FLSs samples (Figure 1C and D). These results indicated that TIPE2 may play an important role in the pathology of AA.
Figure 1.
Overexpression of TIPE2 in AA-FLSs.
Notes: PCR and Western blot analyses of TIPE2 expression in freshly harvested RAW 264.7 cells, normal FLSs, AA-FLSs, and AA-FLSs stably transfected with the lentivirus vector MIGR1/TIPE2+/+ plasmid and the control vector MIGR1 plasmid. TIPE2 expression in RAW 264.7 cells was used as a positive control. (A) RT-PCR analysis of TIPE2 mRNA. GAPDH was used as a loading control, whereas H2O was used as the background control. (B) Quantification of mRNA expression by quantitative real-time PCR. Data are mean ± SD (n=3), ***P<0.001 vs the normal FLS group. (C) Whole cell lysates were prepared. An aliquot (30 μg) of each whole cell lysate was fractionated on a 12% SDS gel, and TIPE2 was analyzed by Western blotting. β-Actin was used as a loading control. (D) Grayscale analysis of Figure 1C, ***P<0.001 vs the normal FLS group. (E) mRNA levels of TNFAIP8 family members were determined by RT-PCR using total RNA isolated from cell preparations. GAPDH was used as a loading control, whereas H2O was used as the background control. (F) Quantification of mRNA expression by quantitative real-time PCR. Data are mean ± SD (n=3), ***P<0.001 vs the MIGR1-FLS group. (G) Whole cell lysates were prepared. An aliquot (30 μg) of each whole cell lysate was fractionated on a 12% SDS gel, and TIPE2 and β-actin were analyzed by Western blotting. (H) Grayscale analysis of Figure 1G, ***P<0.001 vs the MIGR1-FLS group.
Abbreviations: AA, adjuvant arthritis; FLS, fibroblast-like synoviocyte; GAPDH, glyceraldehyde-phosphate dehydrogenase; mRNA, messenger RNA; RT-PCR, reverse transcription polymerase chain reaction; SD, standard deviation; SDS, sodium dodecyl sulfate.
Overexpression of TIPE2 enhanced susceptibility to DR5-mediated apoptosis
AA-FLSs were stably transfected with the recombinant TIPE2 and control MIGR1 lentivirus vectors; TIPE2 mRNA was readily detected in the TIPE2+/+-FLS group, but not in the MIGR1-FLS group. In contrast, similar levels of TIPE1 and TIPE3 were detected in the normal FLS, TIPE2+/+, and MIGR1-FLS groups (Figure 1E). The results demonstrated that TIPE2 overexpression did not affect the expression of other member genes within the family. This observation was also consistent with qPCR measurements (Figure 1F). Protein expression was measured using Western blotting, where TIPE2 appeared as a 21 kDa fusion protein (Figure 1G and H). The expression levels of the TIPE2 protein were significantly higher in the TIPE2+/+-FLS group compared with the MIGR1-FLS group.
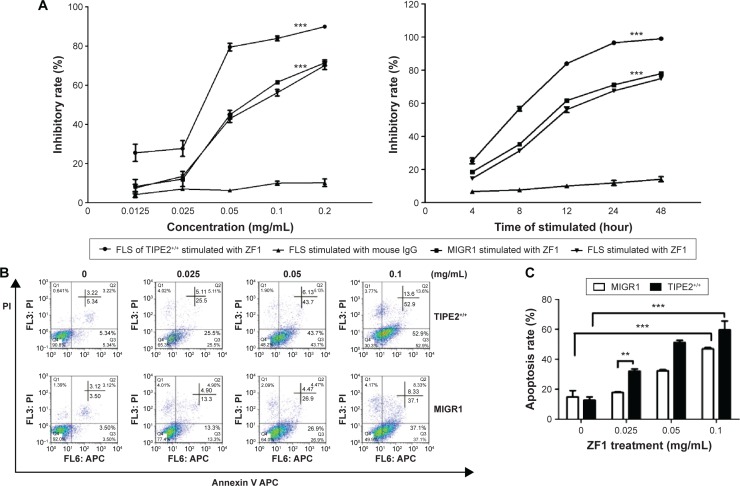
We next explored whether AA-FLSs with different TIPE2 expression levels exhibited different sensitivities to ZF1 in vitro using proliferation assays. The results showed that the cell growth inhibition rate was increased in the TIPE2+/+-FLS group in a dose- and time-dependent manner (Figure 2A). We next set out to determine whether the inhibitory effect was caused by apoptosis or necrosis using flow cytometry. The rate of apoptosis for the control group was 6.62%–45.43%, whereas the apoptosis rate for the TIPE2+/+-FLS group was 8.56%–66.5% (Figure 2B). In addition, the results showed that ZF1 induced AA-FLSs’ apoptosis in a dose-dependent manner, and increased TIPE2 expression enhanced the sensitivity of ZF1-mediated apoptosis in the TIPE2+/+-FLS group in vitro.
Figure 2.
Overexpression of TIPE2 enhanced susceptibility to DR5-mediated apoptosis.
Notes: (A) Growth inhibition of AA-FLSs. The cells were stimulated with the indicated amounts of plate-bound ZF1 for 12 hours or 0.1 mg/mL of matrine for different time periods (0, 4, 8, 12, 24, and 48 hours) prior to the MTT assay. Mouse IgG (concentration 0.0125–0.2 mg/mL) on FLSs was used as the homotype control. The data shown are the mean from three parallel experiments (mean ± SD), ***P<0.001 vs the IgG group. (B) Flow cytometry detection of apoptosis with annexin V/PI in AA-FLSs stimulated with ZF1 at concentrations of 0, 0.025, 0.05, and 0.1 mg/mL. (C) The percentages of apoptotic cells (Q2 + Q3) (shown in Figure 2B) are presented as the mean ± SD of three independent experiments, **P<0.01; ***P<0.001.
Abbreviations: AA, adjuvant arthritis; DR, death receptor; FLS, fibroblast-like synoviocyte; MTT, 20 μL of a 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide; PI, propidium iodide; SD, standard deviation; DR, death receptor; APC, allophycocyanin; IgG, intravenous gamma globulin.
The role of TIPE2 in DR5-induced cell death partly occurs through upregulating caspase activation while downregulating NF-κB activation in TIPE2+/+-FLSs
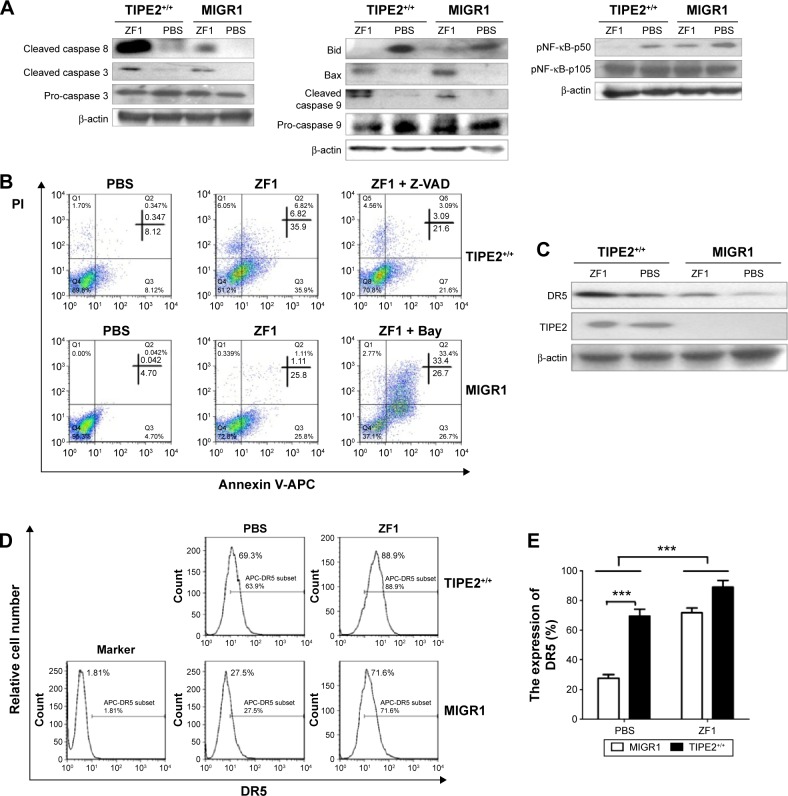
Induction of ZF1 in the TIPE2+/+-FLS and MIGR1-FLS groups showed increased activation of caspases 8, 9, and 3 (Figure 3A). These results indicated that ZF1 is an agonistic anti-DR5 Ab, and that it induces apoptosis in a caspase-dependent fashion. Thus, we showed that increased TIPE2 was able to increase the levels of apoptosis-related proteins; in this case, cleaved caspase 3 and caspase 8 were increased, while the levels of pro-caspase 3 were decreased (Figure 3A). Furthermore, to examine the mitochondrial apoptotic events associated with DR5-induced apoptosis, we analyzed the expression of its associated proteins via Western blot. The results showed that the expression of Bid was decreased, whereas Bax was increased after treatment with ZF1 for 6 hours. Meanwhile, caspase 9 cleavage was significantly activated after ZF1 treatment. Such results were in line with further observation, where the mitochondrial pathway was involved in DR5-induced apoptosis (Figure 3A).
Figure 3.
TIPE2 overexpression enhanced DR5 and caspase expression and downregulated the activation of NF-κB.
Notes: (A) The FLSs were stimulated with 0.1 mg/mL ZF1 in ZF1 groups or PBS in no ZF1 groups for 6 hours. The total protein was extracted, and the expressions of apoptosis-related and intrinsic mitochondrial pathway-related proteins and pNF-κB-p105/p50 proteins were analyzed by Western blot assay. (B) Flow cytometry detection of apoptosis with annexin V/PI in AA-FLSs stimulated with ZF1. Before stimulation with 0.04 mg/mL ZF1, the TIPE2+/+-FLS group was pretreated with caspase inhibitor Z-VAD-FMK and the MIGR1-FLS group was pretreated with the NF-κB inhibitor Bay. (C) The FLSs were stimulated with 0.1 mg/mL ZF1 in ZF1 groups or without ZF1 in PBS groups for 6 hours. The total protein was extracted and the expressions of TIPE2 and DR5 proteins were analyzed using Western blot assay. (D) TIPE2 upregulated the expression of DR5 on FLSs. Flow cytometry detection of DR5 expression with anti-DR5 and mouse IgG-APC in AA-FLSs stimulated with 0.1 mg/mL ZF1 or without ZF1 for 12 hours was carried out. (E) TIPE2 up-regulated the expression of DR5 on FLSs. The data are presented as the mean ± SD of three independent experiments, ***P<0.001. Data are representative of three independent experiments, which showed similar results.
Abbreviations: AA, adjuvant arthritis; DR, death receptor; FLS, fibroblast-like synoviocyte; NF-κB, nuclear factor-kappa B; PBS, phosphate-buffered saline; PI, propidium iodide; SD, standard deviation; APC, Allophycocyanin.
The activation of NF-κB in TIPE2+/+-FLSs was further analyzed using Western blot (Figure 3A). Compared with the control PBS group, the expression level of phosphorylated NF (pNF)-κB was reduced in the presence of ZF1. The level of pNF-κB was even lower in the TIPE2+/+-FLS group treated with ZF1 than in the MIGR1 group, suggesting that TIPE2 might act as a negative regulator in NF-κB pathways.
To further examine the molecular mechanisms involved in TIPE2-increased ZF1-mediated apoptosis, we used the caspase inhibitor Z-VAD-FMK and the NF-κB inhibitor Bay. The TIPE2+/+-FLS group pretreated with caspase inhibitor Z-VAD-FMK showed a significantly decreased apoptosis incident. In parallel, the pretreated MIGR1-FLS group with the NF-κB inhibitor Bay showed an increased apoptosis rate (Figure 3B).
The role of TIPE2 in DR5-induced cell death partly occurs through increasing DR5 expression in TIPE2+/+-FLSs
To determine the induction status of DR5 expression in TIPE2+/+-FLS cells, we compared the DR5 expression in TIPE2+/+-FLSs before and after ZF1 treatment. The protein level of TIPE2 did not change with the addition of ZF1, but the protein level of DR5 significantly increased (Figure 3C). DR5 expression in the TIPE2+/+-FLS group was increased from 69.3% to 88.9% after ZF1 stimulation, but a much more dramatic increase was seen in the MIGR1 group, from 27.5% to 71.6% (Figure 3D). These results further demonstrated that TIPE2 overexpression could enhance DR5 expression.
Discussion
RA is a chronic systemic autoimmune disorder that mainly affects joints. The pathogenesis of RA is not clear, however, it is believed to include a combination of genetic and environmental factors that cause an attack on joints from immune system. All the mechanisms result in inflammation and thickening of the joint capsule cartilage and bones. Hyperproliferation of synovial fibroblast cells is a characteristic feature of RA. Such cells have been described as “transformed-appearing synoviocytes”.18
TIPE2 contains a putative death effector domain-like domain that shows significant nucleotide identity and amino acid similarity to other known DED sequences.9 Some DED-containing proteins are capable of regulating apoptosis. T cells with a TIPE2 knockdown showed resistance to Fas ligand-induced apoptosis.19 In parallel to the current study, the TIPE2 protein was not detected in AA-FLSs; however, it was expressed in the normal FLSs. The results reported here indicate that TIPE2 is a critical regulator of DR5-mediated apoptosis in AA-FLSs.
Our findings suggest that TIPE2 overexpression enhanced both the intrinsic and extrinsic apoptosis in AA-FLS lines, which had been induced by ZF1, and this effect was dose- and time-dependent. Ichikawa et al20 reported that RA synovial cells express high levels of DR5 and are highly susceptible to DR5-mediated apoptosis.21 Both, Li et al’s15 findings and our results, suggested that AA-FLSs cells express DR5 and are sensitive to DR5-mediated apoptosis. In addition, DR5 expression was enhanced after TIPE2 overexpression in AA-FLSs, even without ZF1 treatment. Thus, we postulated that TIPE2 may regulate DR5-mediated apoptosis by altering the levels of death factors that control the DR5 apoptotic pathway. Our results suggest that caspases 8 and 3 have been significantly activated in TIPE2+/+-FLS cells; using the caspase inhibitor Z-VAD-FMK significantly decreased apoptosis mediated by ZF1.
DR5 can activate the NF-κB pathway in addition to transducing apoptosis signals.22 Downstream, NF-κB activation transduces an anti-apoptosis signal.23,24 Correspondingly, genetic deficiencies of the NF-κB transcription factor increase spontaneous and TNF-α-induced lymphocyte apop-tosis.25 NF-κB activation is significantly enhanced in TIPE2 knockdown RAW 264.7 cells.9
The apoptosis rate of the MIGR1-FLS group was increased significantly when pretreated with the NF-κB inhibitor Bay. These results suggest a role of TIPE2 in AA-FLS apoptosis, which might be related to caspase enhancement and the inhibition of NF-κB activation.
Overall, we solidly demonstrated that TIPE2 protein could be detected in normal FLSs, but not in AA-FLSs. Through regulating the DR5-caspase-NF-κB pathway, TIPE2 overexpression enhanced AA-FLS apoptosis, which was induced by the antihuman DR5 single chain variable fragment Ab.
Acknowledgments
This work was supported by the Scientific Research Foundation for the National Nature Science Foundation of China (Grants 81072472, 81272720) and the Fujian Province Natural Science Fund (Grant 2012J01416).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003;423:356–361. doi: 10.1038/nature01661. [DOI] [PubMed] [Google Scholar]
- 2.Bottini N, Firestein GS. Duality of fibroblast-like synoviocytes in RA: passive responders and imprinted aggressors. Nat Rev Rheumatol. 2013;9:24–33. doi: 10.1038/nrrheum.2012.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nakano K, Boyle D, Firestein GS. Regulation of DNA methylation in rheumatoid arthritis synoviocytes. J Immunol. 2013;190:1297–1303. doi: 10.4049/jimmunol.1202572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bartok B, Firestein GS. Fibroblast-like synoviocytes: key effector cells in rheumatoid arthritis. Immunol Rev. 2010;233:233–255. doi: 10.1111/j.0105-2896.2009.00859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bendele A, McComb J, Gould T, et al. Animal models of arthritis: relevance to human disease. Toxicol Pathol. 1999;27:134–142. doi: 10.1177/019262339902700125. [DOI] [PubMed] [Google Scholar]
- 6.Wiley S, Schooley K, Smolak P, et al. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity. 1995;3:673–682. doi: 10.1016/1074-7613(95)90057-8. [DOI] [PubMed] [Google Scholar]
- 7.Secchiero P, Gonelli A, Carnevale E, et al. Trail promotes the survival and proliferation of primary human vascular endothelial cells by activating the Akt and ERK pathways. Circulation. 2003;107:2250–2256. doi: 10.1161/01.CIR.0000062702.60708.C4. [DOI] [PubMed] [Google Scholar]
- 8.Yang J, Huang X, Luo F, et al. Preparation and functional studies of hydroxyethyl chitosan nanoparticles loaded with anti-human death receptor 5 single-chain antibody. Onco Targets Ther. 2014;7:779–787. doi: 10.2147/OTT.S59872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun H, Gong S, Carmody R, et al. TIPE2, a negative regulator of innate and adaptive immunity that maintains immune homeostasis. Cell. 2008;133:415–426. doi: 10.1016/j.cell.2008.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li D, Song L, Fan YJ, et al. Down-regulation of TIPE2 mRNA expression in peripheral blood mononuclear cells from patients with systemic lupus erythematosus. Clin Immunol. 2009;133:422–427. doi: 10.1016/j.clim.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 11.Cao X, Zhang L, Shi Y, et al. Human tumor necrosis factor (TNF)-alpha-induced protein 8-like 2 suppresses hepatocellular carcinoma metastasis through inhibiting Rac1. Mol Cancer. 2013;12:149. doi: 10.1186/1476-4598-12-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang G, Zhang W, Lou Y, et al. TIPE2 deficiency accelerates neointima formation by downregulating smooth muscle cell differentiation. Cell Cycle. 2013;12:501–510. doi: 10.4161/cc.23325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ruan Q, Wang P, Wang T, et al. MicroRNA-21 regulates T-cell apoptosis by directly targeting the tumor suppressor gene Tipe2. Cell Death Dis. 2014;5:e1095. doi: 10.1038/cddis.2014.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Y, Li X, Liu G, et al. Downregulated TIPE2 is associated with poor prognosis and promotes cell proliferation in non-small cell lung cancer. Biochem Biophys Res Commun. 2015;457(1):43–49. doi: 10.1016/j.bbrc.2014.12.080. [DOI] [PubMed] [Google Scholar]
- 15.Li W, Liu Z, Zhuang G, et al. Anti-DR5 mAb ameliorate adjuvant arthritis rats through inducing synovial cells apoptosis. Exp Biol Med. 2009;234:1468–1476. doi: 10.3181/0811-RM-342. [DOI] [PubMed] [Google Scholar]
- 16.Zhang L, Fu X, Yu Y, et al. Treatment with CA-074Me, a cathepsin B inhibitor, reduces lung interstitial inflammation and fibrosis in a rat model of polymyositis. Lab Invest. 2015;95:65–77. doi: 10.1038/labinvest.2014.135. [DOI] [PubMed] [Google Scholar]
- 17.Lin Y, Chen W, Li J, et al. Overexpression of Jagged-1 combined with blockade of CD40 pathway prolongs allograft survival. Immunol Cell Biol. 2015;93(2):213–217. doi: 10.1038/icb.2014.84. [DOI] [PubMed] [Google Scholar]
- 18.Trabandt A, Gay R, Gay S. Oncogene activation in rheumatoid synovium. APMIS. 1992;100:861–875. doi: 10.1111/j.1699-0463.1992.tb04012.x. [DOI] [PubMed] [Google Scholar]
- 19.Gus-Brautbar Y, Johnson D, Zhang L, et al. The anti-inflammatory TIPE2 is an inhibitor of the oncogenic Ras. Mol Cell. 2012;45:610–618. doi: 10.1016/j.molcel.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ichikawa K, Liu W, Fleck M, et al. TRAIL-R2 (DR5) mediates apoptosis of synovial fibroblasts in rheumatoid arthritis. J Immunol. 2003;171:1061–1069. doi: 10.4049/jimmunol.171.2.1061. [DOI] [PubMed] [Google Scholar]
- 21.Seol D, Li J, Seol MH, Park SY, Talanian RV, Billiar TR. Signaling events triggered by tumor necrosis factor-related apoptosis-inducing ligand (trail): caspase-8 is required for trail-induced apoptosis. Cancer Res. 2001;61:1138–1143. [PubMed] [Google Scholar]
- 22.Chaudhary PM, Eby M, Jasmin A, Bookwalter A, Murray J, Hood L. Death receptor 5, a new member of the TNFR family, and DR4 induce FADD-dependent apoptosis and activate the NF-κB pathway. Immunity. 1997;7:821–830. doi: 10.1016/s1074-7613(00)80400-8. [DOI] [PubMed] [Google Scholar]
- 23.Oya M, Ohtsubo M, Takayanagi A, Tachibana M, Shimizu N, Murai M. Constitutive activation of nuclear factor-kappa b prevents trail-induced apoptosis in renal cancer cells. Oncogene. 2001;20:3888–3896. doi: 10.1038/sj.onc.1204525. [DOI] [PubMed] [Google Scholar]
- 24.Bernard D, Quatannens B, Vandenbunder B, Abbadie C. Rel/NF-κB transcription factors protect against tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL)-induced apoptosis by up-regulating the TRAIL decoy receptor DcR1. J Biol Chem. 2001;276:27322–27328. doi: 10.1074/jbc.M011183200. [DOI] [PubMed] [Google Scholar]
- 25.Zheng Y, Vig M, Lyons J, Van Parijs L, Beg AA. Combined deficiency of p50 and cRel in CD4(+) T cells reveals an essential requirement for nuclear factor kappa B in regulating mature T cell survival and in vivo function. J Exp Med. 2003;197:861–874. doi: 10.1084/jem.20021610. [DOI] [PMC free article] [PubMed] [Google Scholar]