Abstract
Background
In vitro diagnostic (IVD) investigations are indispensable for routine patient management. Appropriate testing allows early-stage interventions, reducing late-stage healthcare expenditure (HCE).
Aim
To investigate HCE on IVDs in two developed markets and to assess the perceived value of IVDs on clinical decision-making. Physician-perceived HCE on IVD was evaluated, as well as desired features of new diagnostic markers.
Methods
Past and current HCE on IVD was calculated for the US and Germany. A total of 79 US/German oncologists and cardiologists were interviewed to assess the number of cases where: physicians ask for IVDs; IVDs are used for initial diagnosis, treatment monitoring, or post-treatment; and decision-making is based on an IVD test result. A sample of 201 US and German oncologists and cardiologists was questioned regarding the proportion of HCE they believed to be attributable to IVD testing. After disclosing the actual IVD HCE, the physician’s perception of the appropriateness of the amount was captured. Finally, the association between physician-rated impact of IVD on decision-making and perceived contribution of IVD expenditure on overall HCE was assessed.
Results
IVD costs account for 2.3% and 1.4% of total HCE in the US and Germany. Most physicians (81%) believed that the actual HCE on IVDs was >5%; 19% rated the spending correctly (0–4%, p<0.001). When informed of the actual amount, 64% of physicians rated this as appropriate (p<0.0001); 66% of decision-making was based on IVD. Significantly, more physicians asked for either additional clinical or combined clinical/health economic data than for the product (test/platform) alone (p<0.0001).
Conclusions
Our results indicate a poor awareness of actual HCE on IVD, but a high attributable value of diagnostic procedures for patient management. New markers should deliver actionable and medically relevant information, to guide decision-making and foster improved patient outcomes.
Introduction
In vitro diagnostic (IVD) testing has become an indispensable tool in clinical practice for diagnosing and monitoring of diseases, as well as providing prognosis and predicting treatment response [1, 2]. In addition, IVD is used to assess the potential risk of developing a disease or disorder and to guide patient management [1]. IVD of analytes originating from body specimens, including blood and tissue biopsies, is used alone or in combination with clinical investigations [2] and is perceived as an important tool for high-quality medical outcomes [3]. There are over 40,000 different IVD products available that provide information to doctors and patients on a huge range of conditions. These comprise markers for inorganic chemistry (electrolytes, toxins, and heavy metals), markers for organic chemistry/biochemistry (proteins, lipids, and carbohydrates), as well as molecular biologic procedures (sequencing and polymerase chain reaction). One German study revealed that up to 187 of 584 diagnoses can be confirmed exclusively by an IVD testing [4]. Routine diagnostics and population screening programs, such as the Pap smear for cervical carcinoma, have the potential to identify high-risk individuals and to prevent disease onset or progression [5, 6]. The introduction of cervical cancer screening programs in Europe has led to a substantial decrease in mortality [7, 5]. Furthermore, timely IVD testing allows more early-stage and cost-effective interventions, instead of advanced-stage therapy, which is generally associated with worse prognosis and a higher use of healthcare resources [8, 9].
A New Trend towards Companion Diagnostics
The contemporary concept of companion diagnostics is based on identifying patients with a high likelihood of response to a specific drug, hence curbing total costs of healthcare due to targeted patient management. A well-known example is Herceptest®–the companion diagnostic for HER2-positive breast cancer and gastric cancer–which identifies patients eligible for trastuzumab treatment [10]. Other examples of Food and Drug Administration (FDA)-approved drugs with companion diagnostics include cetuximab, imatinib, and vemurafenib, which are used to treat metastatic colorectal cancer, gastrointestinal stroma tumor, and late-stage melanoma, respectively [11, 12] With the emergence of new molecular technologies identifying tumor aberrations that can be treated with targeted agents, the number of companion diagnostic tests used in oncology will significantly increase in the future.
Companion diagnostics has the potential to enable the selection of the correct drug dose at the appropriate time of a patient`s treatment course, thereby reducing overall therapy cost. The investment of developing companion diagnostic drugs is substantial, however [13]. This is particularly true for immunotherapy treatments, such as those targeting cytotoxic T-lymphocyte antigen 4 (CTLA-4), programmed cell death-1 (PD-1), or programmed cell death 1 ligand 1 (PD-L1). For example, the cost of using the PD-1 inhibitor pembrolizumab has already exceeded an annual cost per patient of US$1 million [13]. In an initiative to help manage costs while maintaining high quality care, the American Society of Clinical Oncology (ASCO) has recently proposed a framework to assess the value of cancer treatment options [14].
Value in Healthcare
The question of how to measure value in healthcare has been discussed controversially. Nonetheless, there is agreement on the overarching concept of assessing health outcomes achieved per dollar spent [15]. In economic terms, the value component would equal clinical utility and cost-effectiveness [16]. The value term simultaneously involves patients (utility and efficacy) and payers (efficiency), describing a framework for performance improvement in healthcare [17]. The Joint Commission of Healthcare Organizations has defined the value term as “the degree to which patient care services increase the probability of desired patient outcomes and reduce the probability of undesired outcomes, given the current state of knowledge” [18].
The Value of IVD
IVD tests have been under increasing cost pressure over the last decade as a result of their increasing use and concerns about uncontrollable healthcare expenditures [19]. Furthermore, the diagnostic industry is now facing stricter regulatory hurdles for product approval [20]. For many years, registration of diagnostic tests in the European Union only required the CE label; however, in the light of financial shortfalls, health authorities are increasingly requesting proof that diagnostic tests not only have reasonable pricing, but also add considerable value to society [21].
Many articles introduce frameworks of how best to assess the value of laboratory diagnostics [22, 23]. Basically, IVD value may be defined as:
Performance is mandated to give the highest accuracy, referring to outcome reliability and reproducibility, with the lowest turnaround time. Efficiency is derived from the percentage of confident clinical decisions made (clinical utility) over costs. While costs refer to resource usage for a given process [24], utility speaks to driving the most accurate conclusion given available evidence for a diagnostic test [25].
While the performance of IVD testing devices is fairly comparable across the diagnostic industry, the efficiency component is the main differentiator and determines the medical value component. Although the utility–cost relationship is difficult to assess, it is important to quantify this amount and to estimate the current value of IVD testing in proportion to its cost relative to overall healthcare expenditure (HCE).
Aims
In 2005, a report by the Lewin Group revealed that diagnostics comprise less than 5% of hospital costs and approximately 1.6% of all Medicare costs, while accounting for 60–70% of clinical decisions [26]. However, the authors do not provide citable references for their claims. The main objective of this study was to find statistical backing for the surprising utility–cost ratio.
Methods
This study was conducted in three stages: literature review, interviews with medical oncologists and cardiologists, and a confirmatory internet-based multiple-choice survey (SERMO). Given that the study was based on interview responses and did not involve active treatment of human participants, it was not necessary to include an institutional review board (ethics committee).
Table 1 provides an overview of all stages of the study.
Table 1. Study Design.
| Study Stage | Quality | Objectives/Aims | Methods | Countries | Physicians |
|---|---|---|---|---|---|
| Stage 1 | Quantitative | • % of healthcare expenditures used for IVD | Systematic literature research | Germany and US | None |
| • % of healthcare expenditures used for IVD in hospital and private practice | |||||
| Stage 2 | Qualitative & Quantitative | • Patients seen per week | Interviews | Germany | Onc (N = 20) Card (N = 20 |
| • Distribution | US | Onc (N = 20) | |||
| ○ New patients | Card (N = 19) | ||||
| ○ Patients undergoing treatment | |||||
| ○ Patients in post-treatment phase | |||||
| • Overall and specific amount of IVD testing | |||||
| ○ In initial diagnostic phase | |||||
| • IVD subtype use | |||||
| • Rated importance of IVD subtype | |||||
| ○ In treatment phase | |||||
| ○ In post-treatment follow-up | |||||
| • Treatment decision based on IVD-testing | |||||
| Stage 3 | Quantitative | • % of healthcare expenditures used for IVD | Questionnaire | Germany | Onc (N = 30) Card (N = 51) |
| • Perceived HCE on IVD testing | US | Onc (N = 70) Card (N = 50) | |||
| • Perception of spending appropriateness | |||||
| • Design of optimal biomarker | Germany, US, UK, Canada, Norway, Switzerland | Onc (N = 102) Card (N = 102) GP (N = 38) Int. M (N = 38) Path (N = 68) |
Display of study design, objectives and methods used in the three different parts of the analysis. Number of sources, included physicians, their specializations and country of origin.
IVD, in-vitro diagnostic; Onc, oncologist; Card, cardiologist; GP, general practitioner; Int. M, internal medicine; Path, pathologist; HCE, healthcare expenditure.
Stage 1
A literature review was conducted to assess the total percentage of HCE on IVDs in two countries–the US and Germany. Data for healthcare and diagnostic expenditures were derived from government and private industry sources. Secondary sources were assessed for information and data on IVD HCE including government websites, healthcare agencies, industry, and market reports. Various search terms were used to ascertain IVD spending data, including “clinical laboratory industry revenues”, “in-vitro diagnostic spending”, “clinical laboratory market”, and “reimbursement for clinical laboratories”.
Data for total US HCE for the period 1993–2011 were derived from the Centers for Medicare and Medicaid Services [27]. At the time of this analysis, 2009–2013 data for IVD expenditure in the US had not yet been published, therefore an estimate for spending during that period was made based on an average annual growth rate of 4% [28]. For the years 1994–1997, an average growth rate of 5.3% was applied. The 1993 IVD spending was an estimate based on the ratio of IVD manufacturers’ revenues over total IVD spending in other years.
For Germany, both total government healthcare spending and IVD spending were captured from the Federal Statistical Office [29]. All calculations were based on German government HCE, which accounts for about 77% of overall healthcare costs [30].
The percentage of total healthcare spending on IVDs was calculated by dividing the total IVD spending by the total HCE.
Selection of Countries
The US and Germany were selected as they represented approximately the estimated global HCE in 2009 [31] and therefore provide satisfactory proxies for other countries in developed markets. Fig 1 displays the percentage of GDP allocated to HCE and the total HCE split according to payers for both countries in 2013.
Fig 1. Percentage of public and private HCE of GDP (pie) in the US and Germany in 2013.
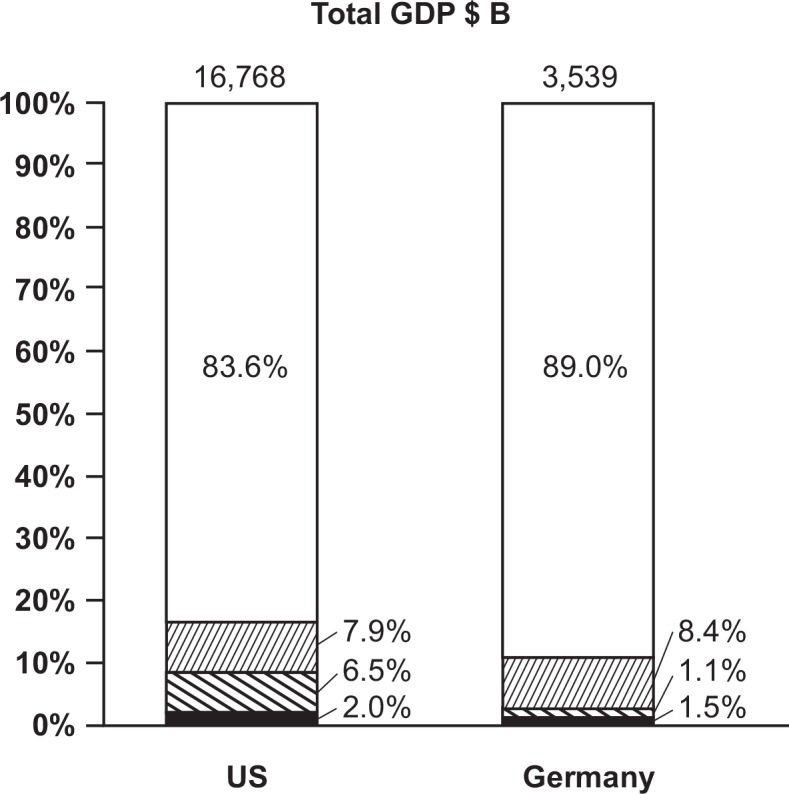
Thin lines, public HCE; bold lines, private HCE without out-of-pocket; black fill, out-of-pocket HCE; white fill, rest of GDP (non-HCE) HCE, healthcare expenditure; GDP, gross domestic product; $B, US$ billion.
Stage 2
Interviews were conducted with medical oncologists and cardiologists in the US and Germany. The interview was designed to assess (i) the number of patients seen per week and (ii) the distribution of patients according to treatment stages. Furthermore we assessed (iii) in how many cases physicians were asking for IVD and (iv) in how many cases IVD was used for either initial diagnosis, treatment monitoring or post-treatment follow up. With this in mind we also analyzed which IVD subtypes were used frequently during initial patient work up and how important these subtypes were rated by physicians. We then investigated (v) in how many cases a treatment decision (defined as stopping, initiating or continuing treatment) is based on IVD-test results.
Interviews were conducted by three neutral researchers (all male; one MD, one PhD, and one BS, MBA) employed by the Enterprise Analysis Corporation (EAC; Stamford, CT, US). Interviewers had a strong knowledge of diagnostics testing and were experienced in conducting interviews with physicians, laboratory workers and other healthcare professionals.
Participants were contacted by telephone and asked to participate in the study; the nature of the study was briefly described and an honorarium was offered for participation. Interviewers had no prior relationship with physicians. Physicians were required to see ≥20 patients per week in their practice. These interviews took place by phone and were scheduled to take 1 hour; no non-participants were involved in the interviews. A pilot-tested, structured interview was conducted. Interview questions were not shared with the physician in advance of the interview. There were no repeat interviews. In general, interviews were not recorded although some may have been if the physician consented to recording. Notes were taken during the interview; additional comments and notes were added to the interview protocol directly after the interview whilst fresh in the mind of the interviewer. Transcripts were not returned as there were few open-ended questions and transcripts were not lengthy.
Data from the interviews were entered into a database (Microsoft Access) by one person; this was reviewed for accuracy by a project manager at EAC.
Stage 3
This stage involved a confirmatory internet-based multiple-choice survey of physician, the purpose of which was (vi) to assess how much physicians believed to be spent on IVD testing and to compare their assumption with the actual HCE spent on IVD calculated in Stage 1 and (vii) to assess, after disclosing the actual HCE on IVDs, if physicians felt this amount was appropriate. Finally, the perceived value of IVDs was correlated with the physician’s estimated cost.
Stage 3 was executed using SERMO, an anonymized internet-based multiple-choice survey, executed by Genentech (South San Francisco, CA, US), hosted by WorldOne (Boston, MA, US). SERMO is a shared service, with a facility that allows multiple companies to gain quick and comprehensive insights on conceptual questions via physician surveys. Interested physicians apply to take part in surveys and receive questions from several companies. Invitations to participate in this cross-sectional survey were sent to physicians from the US, UK, Germany, Canada, Norway, and Switzerland; 348 physicians responded to the invitation to participate. Participating physicians received financial compensation.
Statistical Analysis
Student’s t test was applied to compare mean values of patient numbers seen by physicians, by country and specialty (Germany vs US; cardiologists vs oncologists). A comparison of patients undergoing IVD testing between countries and specialties was performed with a χ2 test (with 3 degrees of freedom) after recalculating the number of patients by weighted average. The correlation between the replies regarding value of IVD in clinical practice and perceived costs was estimated with the Spearman’s rank correlation coefficient. A Likert Scale (1 = lowest importance, 5 = highest importance) was used for the assessment of the relative importance of IVD subtypes during the initial patient work up phase. All statistical analyses were performed using SAS version 9.4 (SAS Institute Inc, Cary, NC, US).
Results
Stage 1
IVD Spending as a Percentage of Total HCE
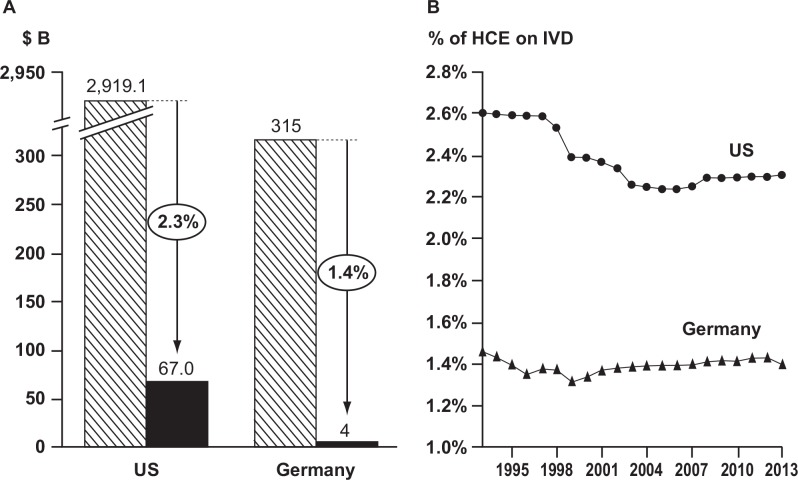
The literature research revealed that healthcare spending on IVDs (defined as payments to clinical laboratories for testing services) represents approximately 2.3% of all healthcare spending in the US (S1 Table). In Germany, 1.4% of public healthcare expenditure is used for IVD (S1 Table; Fig 2A). Although government spending on IVD testing is well documented, private sector spending is not systematically tracked on an annual basis, thus fewer data resources are available.
Fig 2.
(A) Percentage of HCE on IVD in 2013 and (B) evolution of HCE on IVD 1993–2013. HCE, healthcare expenditure; IVD, in vitro diagnostics; $ B, US$ billion.
A retrospective analysis from 1993 through 2013 revealed that IVD spending in the US has grown at an annual rate of 5.3% from US$30 billion in 1998 to an estimated US$67 billion in 2013. In Germany, spending on IVD has grown at a more modest annual rate of 3.1% since 1993, reaching US$ 4.5 billion in 2013. Overall, this has resulted in a relatively consistent HCE on IVD testing in Germany, whereas a slight decline of 0.2% was observed in the US (Fig 2B).
Stage 2
IVD Use for Patients and Clinical Decision-Making
A total of 40 oncologists and 39 cardiologists participated in physician interviews. On average, 93 patients were seen per week by oncologists and cardiologists in the US and Germany. Significantly more patients were treated by physicians in the US compared with Germany (p = 0.005). A comparable number of patients underwent IVD testing in the US (74%) and Germany (76%) (p = 0.119; average 75%). Overall, IVD testing was used in 88%, 77%, and 72% of patients for initial diagnosis, treatment monitoring, and follow-up, respectively.
Significantly more oncology patients underwent IVD testing than cardiology patients (92% vs 60%, respectively; p<0.0001) in both, the US (p<0.0001) and Germany (p<0.0001) (Table 2). Overall, 75% of patients underwent IVD testing across both disciplines, testing that led to a substantial clinical decision in 66% of these patients (Table 2).
Table 2. Number of Patients Receiving IVD Testing per Week and General Use of IVD Testing During Different Phases of Care.
| Patients treated with IVD | General use of IVD (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Country | Specialty | Setting | Mean No. of patients/week | Patients with IVD testing (%) | Clinical decisions affected by IVD (%) | Initial diagnosis | Treatment monitoring | Post-treatment monitoring |
| US | ||||||||
| Oncologist | Total | 62 | 91 | 58 | 95 | 95 | 79 | |
| Private | 87 | 94 | 62 | 97 | 97 | 81 | ||
| Hospital | 45 | 88 | 54 | 92 | 92 | 76 | ||
| Cardiologist | Total | 86 | 62 | 68 | 86 | 52a | 52a | |
| Private | 99 | 42 | 59 | 79 | 44a | 44a | ||
| Hospital | 74 | 80 | 76 | 93 | 61a | 61a | ||
| Average US | 74 | 74 | 64 | 90 | 71 | 64 | ||
| Germany | ||||||||
| Oncologist | Total | 114 | 92 | 63 | 100 | 94 | 87 | |
| Private | 95 | 88 | 58 | 99 | 92 | 77 | ||
| Hospital | 133 | 96 | 67 | 100 | 96 | 95 | ||
| Cardiologist | Total | 112 | 59 | 71 | 75 | 68a | 68a | |
| Private | 175 | 35 | 64 | 57 | 51a | 51a | ||
| Hospital | 95 | 92 | 80 | 99 | 90a | 90a | ||
| Average Germany | 113 | 76 | 67 | 87 | 81 | 78 | ||
| Overall | ||||||||
| Average oncologists | 88 | 92 | 62 | 97 | 94 | 84 | ||
| Average cardiologists | 99 | 60 | 70 | 82 | 61a | 61a | ||
| Average Overall (%) | 75 | 66 | 88 | 77 | 72 | |||
| Average Overall (in No. of patients/week) | 93 | 71 | 61 | |||||
IVD, in vitro diagnostics. aFor cardiologists, only one question was asked on treatment monitoring and post-treatment monitoring.
IVD Subtype Use and Rated Subtype Importance
During the initial work-up phase, on average physicians used clinical chemistry and hematology assessments in nearly 100% of patients, followed by immunology (86%). Basic and Advanced Tissue Staining as well as molecular diagnostics was used in approximately half of patients during the initial work-up phase. The average rating of IVD subtype importance corresponded with its usage (S1 Fig). Detailed information about country- and specialty-related IVD usage and rated importance is displayed in S2 Table.
Stage 3
Actual and Perceived Spending of IVD on HCE
Our analysis yielded a discrepancy between the actual amount of IVD on HCE (2.3% in the US; 1.4% in Germany) and the perceived amount of HCE by physicians. Throughout all specializations and irrespective of country, physicians tended to overestimate IVD-related monetary expenditure; this was most pronounced among US cardiologists. In total, 81% of physicians estimated IVD expenditure to be >5% of the total HCE.19% rated spending to be 0–4% (p<0.001) (Table 3).
Table 3. Physicians’ estimation of IVD expenditure as a proportion of total HCE.
| IVD expenditure, % of total HCE | ||||
|---|---|---|---|---|
| Country | 0–4% | 5–10% | 11–20% | >20% |
| Germany, % physicians | ||||
| Oncologist | 20.0 | 43.3 | 20.0 | 16.7 |
| Cardiologist | 17.6 | 49.0 | 25.5 | 7.8 |
| Total | 18.5 | 46.9 | 23.5 | 11.1 |
| US, % physicians | ||||
| Oncologist | 22.5 | 31.0 | 32.4 | 14.1 |
| Cardiologist | 14.0 | 38.0 | 26.0 | 22.0 |
| Total | 19.0 | 33.9 | 29.8 | 17.4 |
| Overall | 18.8 | 39.1 | 27.2 | 14.9 |
HCE, healthcare expenditure; IVD, in vitro diagnostic.
Assessment of Appropriateness of IVD Costs on Overall HCE
After disclosure of the actual proportion of HCE incurred by IVD testing (2.3% and 1.4% for the US and Germany, respectively), 92% of all physicians rated the current IVD expenditure as either appropriate or too low and 7% as too high (p<0.0001). No major differences were observed within countries (Spearman’s correlation coefficient 0.291 for Germany, 0.003 for US) or specialization (Spearman’s correlation coefficient 0.118 for cardiology, 0.031 for oncology).
On a more granular level, 64% of physicians rated current IVD spending as appropriate; 28% of physicians assessed this spending as too low. 8% of the latter believed that innovative assays deserve to command a higher price, even if total healthcare spend were to increase, while 20% of the latter that innovative assays deserve a higher price but cost cuts would need to be made in other segments of the healthcare value chain. Among the remaining 8% who rated the current spending as too high, 5% believed that IVD savings should be added to other segments of the healthcare value chain.
Association Between Impact of IVD on Clinical Decisions and Perceived Cost Contribution
Physicians who rated the impact of IVD testing as rather low also believed that the associated cost of such procedures was low. Conversely, physicians who rated the value of IVD testing as high considered it to be more expensive (Spearman’s rank correlation coefficient 0.28617; p≤0.0001). For example, 26.7% of physicians who based >85% of their clinical decisions on IVDs estimated the cost impact of IVDs at over 20% of the overall HCE (Table 4).
Table 4. Relationship between percentage of clinical decisions based on IVD testing and perceived HCE on IVDs by physicians.
| Clinical decisions based on IVD | Perceived HCE on IVD | |||
|---|---|---|---|---|
| 0–4% | 5–10% | 11–20% | >20% | |
| <20% | 34.7%*** | 40.8%*** | 16.3%** | 8.2%* |
| 20–44% | 19.7%** | 44.3%*** | 19.7%** | 16.4%** |
| 45–64% | 7.7%* | 36.5%*** | 40.4%*** | 15.4%** |
| 65–85% | 8.3%* | 41.7%*** | 33.3%*** | 16.7%** |
| >85% | 13.3%* | 20.0%** | 40.0%*** | 26.7%** |
*0–14.9%
**15–29.9%
***30–44.9%
HCE, healthcare expenditure; IVD, in vitro diagnostic.
Physicians’ Expectations of IVD Markers. Regarding the prospective development of new IVD markers, 53% of physicians believed that IVD tests would need to demonstrate additional clinical evidence of improved patient outcomes (p<0.0001 vs other criteria), 29% stated that IVDs must provide health economic benefits plus evidence for improved patient outcome, whereas only 8% of physicians selected health economic benefits to be the exclusive purchasing factor. Overall, a significant proportion of physicians (83%) asked either for additional clinical data or combined clinical and health economic data. Thus, these combined health economic and outcomes benefits were more frequently requested than the sole provision of a diagnosis (p<0.0001), indicating that the latter will be insufficient to cater for the future demand of physicians.
Discussion
To our knowledge this is the first comprehensive analysis to investigate the relationship between the value of IVDs and their associated cost in two major developed markets. Such an analysis is particularly important as recognition and reimbursement levels for IVDs have decreased significantly within the last 15 years [32]. Assessing the IVD utility–cost ratio is therefore important in raising awareness of IVDs as a cost-efficient tool for patient management. The present study has revealed at least four important findings:
The actual IVD spend as a part of overall HCE is low compared with other segments of the health value chain, accounting for 2.3% and 1.4% in the US and Germany, respectively.
IVD testing guides approximately 66% of clinical decisions.
Physicians overrate the costs of IVD as a proportion of HCE.
Physicians demand diagnostic tests that show both clinical utility and cost-effectiveness.
1. Our investigations revealed that the HCE on IVD in the US and Germany is 2.3% and 1.4%. This is in line with the statement from the Lewin Group report, concluding that diagnostics account for <5% of hospital costs and about 1.6% of all Medicare costs [26]. This cost is rather low when compared with other segments of the medical value chain, such as pharmaceuticals and medical aids, which in Germany accounted for 15% and 5%, respectively, of public HCE in 2013 [29]. Pharmaceutical spending on prescription medicines and over-the-counter products as a proportion of the overall HCE in 2013 was estimated to be 11.9% in the US and 17.5% in Canada [33].
As the percentage spent on IVDs relative to total HCE has remained fairly stable over the last 20 years, the results of the present study indicate that IVDs have contributed to the growth of the HCE at a constant low level (Fig 2). However, despite continuous discussion about cost containment, it must not be forgotten that newer predictive companion diagnostics are economically favorable. They allow patients who will benefit from a specific treatment to be identified and treated, while those who will not respond do not incur the cost for ineffective treatment and management of possible side effects. Indeed, evidence exists for a high cost–benefit ratio for identifying patients with KRAS and BRAF wild-type metastatic colorectal cancer suitable for treatment with cetuximab and those with HER2-positive breast carcinomas who will respond to trastuzumab [34, 35]. Furthermore, IVD-based screening programs may allow a reduction in the number of expensive late-stage treatments through earlier interventions [8].
2. The present study confirms the widespread belief that IVDs play an important role in clinical practice, as they influence 66% of clinical decision-making. This verifies the statement from the Lewin Group, which reported this number to be between 60–70% [26], which was a central aim of our study. Our investigation shows that clinical chemistry and hematology assessments play a pivotal role for clinical decision making in the initial patient work-up phase. This holds true for both the cardiology and oncology disease areas. Major differences in the use of molecular testing between the oncology and cardiology settings illustrates the excellent progress made in the field of personalized healthcare in cancer management but concerns remain over the low use of molecular testing in the cardiology field. Not surprisingly caution regarding the future of hypertension pharmacogenetics is warranted in various studies [36].
The strong influence of IVD on clinical decisions also underlines the responsibility of diagnostic laboratories and companies to physicians and patients. The manufacturers of IVD products play an important role in the reduction of laboratory errors by ensuring the highest possible safety and efficacy of their products [37]. Despite that fact that pre-analytical and post-analytical steps are more error prone than the analytical phase and errors due to analytical problems have been significantly reduced over the last two decades, laboratory errors are known to have a serious impact on patients and their safety [38,39].
While there has been substantial progress in reducing errors associated with IVD testing, additional challenges in the reduction of diagnostic errors and hence patient safety remain to be addressed [40,41]. In fact, the frequency of diagnostic errors related to IVD may still be as high as one out of 330 tests [38]. A recent publication from the US Institute of Medicine addresses this challenge with eight goals for improving diagnosis [40].
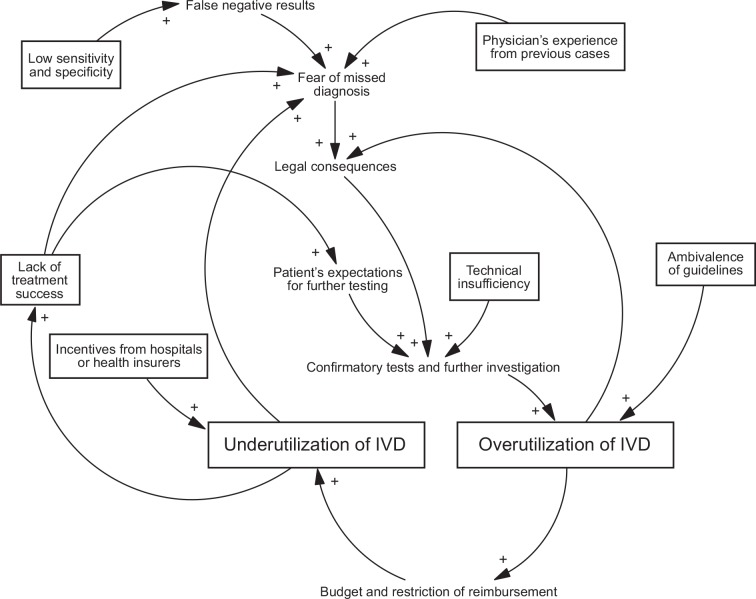
3. In the present study, IVD costs were generally overestimated by physicians. This might be triggered by the general belief of IVD overutilization, which has reported to be between 10–50% [42]. However, a recent meta-analysis suggests that IVD underutilization is more prevalent than overutilization (44.8% vs. 20.6%) [43]. Interestingly is also the fact that there were four times as many studies found on over- compared to underutilization during the assessed 15-year period and, despite in-depth literature research, only 42 studies finally matched the criteria for review, indicating a poor level of evidence on inappropriate use of IVD testing [43]. Root causes for IVD over- and underutilization are summarized in the causal-loop diagram shown in Fig 3.
Fig 3. Causal loop diagram displaying the root causes for over- and under-utilization of IVD testing. Key drivers displayed in boxes; antecedents and secondary drivers displayed as plain text.
IVD, in vitro diagnostic; →(+), positive causal links amplifying the behavior of target variable.
Recently, the American Board of Internal Medicine has launched the “Choosing Wisely” initiative [44], which aims to identify tests of little clinical value with potential for over-diagnosis [24]. The Swiss Society of Internal Medicine launched a similar campaign in 2014 called “Smarter Medicine” [45].
Despite initial overestimation of IVD expenditure in the present analysis, most physicians rated the actual IVD cost as appropriate or too low, clearly demonstrating a low awareness of price structures among healthcare professionals. In a French study investigating cost awareness of overall hospital expenditure among physicians, only 29% of their overall ratings were within 50% of the true costs [46]. In another survey, only 19% of general practitioners estimated the true costs of laboratory and radiology tests in hospitals to be within 25% of the actual range [47]. This is in line with the present study results, which indicate that only 19% of physicians surveyed correctly estimated actual IVD costs.
4. The last finding of this analysis is that the mere supply of diagnostic tests will not be sufficient for physicians in the future, because evidence of accuracy does not automatically transfer to evidence of efficiency [48]. The present study has shown that >50% of all physicians demand proven clinical utility. These results reflect those of a study in which service provision (defined as the provision of validated treatment algorithms) was rated as a significantly stronger purchasing factor than technical preciseness [49]. There is evidence to suggest that physicians are reluctant to use diagnostic tools when a test result cannot be sufficiently translated into clinical actions [50]. Medical value in terms of clinical utility studies is able to close this gap by demonstrating improved patient outcomes by either decreasing triage time [51] or appropriate choice of treatment (companion diagnostics) [52].
The results of the present study furthermore demonstrate that 29% of all physicians demand new IVD markers with health economic benefits. Although pharmaceutical companies incorporated the concept of economic value decades ago, vendors of IVDs still see themselves primarily as providers of accurate technical equipment. This results in limited awareness of the economic value of IVDs, neglecting the fact that regular testing can fundamentally reduce healthcare costs, especially over the long term. In the US alone, US$1.3 billion could have been saved in 2004 if half of the patients with atrial fibrillation in routine medical care were optimally treated with oral anticoagulation [53].
Limitations
This analysis has several limitations. The literature review was based on available public sources. As a result of a lack of some reference points, calculations and assumptions were necessary to fill gaps, which can lead to deviations from the actual spending. In Germany, the IVD cost as a percentage of HCE was calculated based on public HCE only, whereas total HCE was assessed in the US. An additional problem was the absence of a clear definition of “healthcare spending”. Consequently, associated costs may differ between the US and Germany. The survey included a relatively small sample size of interviewed physicians and there is a need for validation of the result using a larger sample base. In addition, the study was conducted for two developed markets only and thus validity of the results for the rest of the world remains to be proven.
Conclusions
IVDs are an indispensable tool in clinical practice as they govern approximately 66% of clinical decision-making while accounting for approximately 2.3% and 1.4% of healthcare spending in the US and Germany, respectively. Although the presumed HCE on IVDs is generally overestimated by the majority of physicians, actual costs were considered as appropriate. IVDs can be regarded a cost-effective measure to maximize treatment outcomes. When used with established diagnostic algorithms, IVD testing can reduce direct and indirect healthcare costs [24], generate better clinical outcomes [54], and thus create Medical Value [49].
Supporting Information
(DOCX)
(DOCX)
(DOCX)
(XLSX)
(DOCX)
(XLSX)
(DOCX)
A) Percentage of IVD subtype use during initial patient workup according to specialty and country; B) Rated importance of IVD subtypes for clinical practice and decision making during initial patient workup according to specialty and country (rating based on Likert scale, 1 = very low, 5 very high).
(DOCX)
Acknowledgments
Editing assistance for this manuscript was provided by Miller Medical Communications Ltd (UK), with funding provided by Roche Diagnostics. The physician interviews were conducted by the Enterprise Analysis Corporation (Stamford, US).
Data Availability
The authors have included all relevant material important to display. Because the interviews have been performed by an independent third party and because this topic is a general topic and not related to any Roche products, the authors are confident to share all documents that have been generated during this investigation. This investigation was initiated by Roche Medical and Scientific Affairs, which partly covers educational and research activities independently of the business. The questions and raw data for stages 2 and 3 of the study have been uploaded as supplemental files to accompany the manuscript.
Funding Statement
The study has been funded by Roche Divisional Diagnostics, Medical and Scientific Affairs. Roche was involved in the study design, data collection and analysis, decision to publish and preparation of the manuscript.
References
- 1.Raman G, Avendano E, Chen M. Update on Emerging Genetic Tests Currently Available for Clinical Use in Common Cancers, in Technology Assessment Report Rockville, MD: Agency for Healthcare Research and Quality; 2013. [PubMed] [Google Scholar]
- 2.Billings PR. Three barriers to innovative diagnostics. Nat Biotechnol. 2006;24:917–918. [DOI] [PubMed] [Google Scholar]
- 3.Blendon RJ, Schoen C, DesRoches CM, Osborn R, Zapert K, Raleigh E. Confronting competing demands to improve quality: a five-country hospital survey. Health Aff (Millwood). 2004;23:119–135. [DOI] [PubMed] [Google Scholar]
- 4.Wilke MH, Schenker M, Hoffmann G. Detection and documentation of DRG-relevant comorbidities using laboratory tests. Aust Health Rev. 2002;25:152–160. [DOI] [PubMed] [Google Scholar]
- 5.Peto J, Gilham C, Fletcher O, Matthews FE. The cervical cancer epidemic that screening has prevented in the UK. Lancet. 2004;364:249–256. [DOI] [PubMed] [Google Scholar]
- 6.Liu S, Semenciw R, Probert A, Mao Y. Cervical cancer in Canada: changing patterns in incidence and mortality. Int J Gynecol Cancer. 2001;11:24–31. [DOI] [PubMed] [Google Scholar]
- 7.Levi F, Lucchini F, Negri E, Franceschi S, la Vecchia C. Cervical cancer mortality in young women in Europe: patterns and trends. Eur J Cancer. 2000;36:2266–2271. [DOI] [PubMed] [Google Scholar]
- 8.Mignogna MD, Fedele S, Lo Russo L, Ruoppo E, Lo Muzio L. Costs and effectiveness in the care of patients with oral and pharyngeal cancer: analysis of a paradox. Eur J Cancer Prev. 2002;11:205–208. [DOI] [PubMed] [Google Scholar]
- 9.Cressman S, Lam S, Tammemagi MC, Evans WK, Leighl NB, Regier DA, et al. Resource utilization and costs during the initial years of lung cancer screening with computed tomography in Canada. J Thorac Oncol. 2014;9:1449–1458. 10.1097/JTO.0000000000000283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rüschoff J, Hanna W, Bilous M, Hofmann M, Osamura RY, Penault-Llorca F, et al. HER2 testing in gastric cancer: a practical approach. Mod Pathol. 2012;25: 637–650. 10.1038/modpathol.2011.198 [DOI] [PubMed] [Google Scholar]
- 11.US Food and Drug Administration. List of cleared or approved companion diagnostic devices (in vitro and imaging tools); 2015 [cited October 1, 2015]. Available: http://www.fda.gov/medicaldevices/productsandmedicalprocedures/invitrodiagnostics/ucm301431.htm.
- 12.US Food and Drug Administration. Paving the way for personalized medicine: FDA’s role in a new era of medical product development; 2013 [cited October 1, 2015]. Available: http://www.fda.gov/downloads/ScienceResearch/SpecialTopics/PersonalizedMedicine/UCM372421.pdf. Accessed 1 October 2015.
- 13.Saltz L. 2015 ASCO Annual Meeting: Perspectives on Value–Plenary Session Including the Science of Oncology Award and Lecture. 2015: Chicago.
- 14.Schnipper LE, Davidson NE, Wollins DS, Tyne C, Blayney DW, Blum D, et al. American Society of Clinical Oncology Statement: A conceptual framework to assess the value of cancer treatment options. J Clin Oncol. 2015;33:2563–2677. 10.1200/JCO.2015.61.6706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Porter ME. What is value in health care? N Engl J Med. 2010;363:2477–2481. 10.1056/NEJMp1011024 [DOI] [PubMed] [Google Scholar]
- 16.Drummond MF, O’Brien B, Stoddart GL, Torrance GW. Methods for the economic evaluation of health care programmes, 2nd ed. Oxford: Oxford University Press; 1997. [Google Scholar]
- 17.Porter ME, Teisberg EO. Redefining competition in health care. Harv Bus Rev. 2004;82:64–76, 136. [PubMed] [Google Scholar]
- 18.Bissell MG. Laboratory-related measures of patient outcomes: An introduction Washington, DC, US: AACC Press; 2000. [Google Scholar]
- 19.Sarata AK, Johnson JA. Regulation of clinical tests: In vitro diagnostic (IVD) devices, laboratory developed tests (LDTs), and genetic tests Washington, DC, US: Congressional Research Service; 2014. Available: https://www.fas.org/sgp/crs/misc/R43438.pdf. [Google Scholar]
- 20.Smith KM, Kates JA. Regulatory hurdles in bringing an in vitro diagnostic device to market. Clin Chem. 1996;42:1556–1557. [PubMed] [Google Scholar]
- 21.Miller I, Ashton-Chess J, Spolders H, Fert V, Ferrara J, Kroll W, et al. Market access challenges in the EU for high medical value diagnostic tests. Personalized Medicine. 2011;8:137–148. [DOI] [PubMed] [Google Scholar]
- 22.Anonychuk A, Beastall G, Shorter S, Kloss-Wolf R, Neumann P. A framework for assessing the value of laboratory diagnostics. Healthc Manage Forum. 2012;25(3 Suppl):S4–S11. [Google Scholar]
- 23.Price CP. Evidence-based laboratory medicine: supporting decision-making. Clin Chem. 2000;46:1041–1050. [PubMed] [Google Scholar]
- 24.Lazar EJ, Fleischut P, Regan BK. Quality measurement in healthcare. Annu Rev Med. 2013;64:485–496. 10.1146/annurev-med-061511-135544 [DOI] [PubMed] [Google Scholar]
- 25.Durtschi A, Julicher P. Assessing the value of cardiac biomarkers: going beyond diagnostic accuracy? Future Cardiol. 2014;10:367–380. 10.2217/fca.14.26 [DOI] [PubMed] [Google Scholar]
- 26.The Lewin Group, Inc. The value of diagnostics innovation, adoption and diffusion into health care; 2005. Available: http://www.lewin.com/~/media/Lewin/Site_Sections/Publications/ValueofDiagnostics.pdf.
- 27.Centers for Medicare & Medicaid Services. National health expenditure data (research, statistics, data and systems). Available: https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/NationalHealthAccountsHistorical.html.
- 28.Quest Diagnostics 31st Annual J. P. Morgan Healthcare Conference January 9, 2013. Available: http://ir.questdiagnostics.com/phoenix.zhtml?c=82068&p=irol-presentations.
- 29.Health expenditures in Germany as share of GDP and in millions of Euro (absolute and per inhabitant). Health expenditure, time series. Available: http://www.gbe-bund.de/gbe10/trecherche.prc_them_rech?tk=19200&tk2=19300&p_uid=gast&p_aid=71688746&p_sprache=E&cnt_ut=10&ut=19310.
- 30.Organisation for Economic Co-operation and Development . Country note: How does health spending in Germany compare? OECD Health Statistics; 2015. Available: http://www.oecd.org/els/health-systems/Country-Note-GERMANY-OECD-Health-Statistics-2015.pdf. [Google Scholar]
- 31.World Health Organization. WHO global health expenduture atlas. Available: http://apps.who.int/nha/atlasfinal.pdf.
- 32.Busse R, Riesberg A. Health care systems in transition Copenhagen: WHO Regional Office for Europe on behalf of the European Observatory on Health Systems and Policies; 2014. [Google Scholar]
- 33.Organisation for Economic Co-operation and Development. Pharmaceutical spending (indicator). Available: https://data.oecd.org/healthres/pharmaceutical-spending.htm.
- 34.Blank PR, et al. KRAS and BRAF mutation analysis in metastatic colorectal cancer: a cost-effectiveness analysis from a Swiss perspective. Clin Cancer Res. 2011;17:6338–6346. 10.1158/1078-0432.CCR-10-2267 [DOI] [PubMed] [Google Scholar]
- 35.Blank PR, Schwenkglenks M, Moch H, Szucs TD. Human epidermal growth factor receptor 2 expression in early breast cancer patients: a Swiss cost-effectiveness analysis of different predictive assay strategies. Breast Cancer Res Treat. 2010;124:497–507. 10.1007/s10549-010-0862-7 [DOI] [PubMed] [Google Scholar]
- 36.Arnett DK, Claas SA, Glaser SP. Pharmacogenetics of antihypertensive treatment. Vascular Pharmacology 2006;44:107–118. [DOI] [PubMed] [Google Scholar]
- 37.Stankovic A. The role of in vitro diagnostic companies in reducing laboratory error. Clin Chem Lab Med 2007;45:781–788. [DOI] [PubMed] [Google Scholar]
- 38.Plebani M. Errors in clinical laboratories or errors in laboratory medicine? Clinical Chemical Laboratory Medicine. Band 44, Heft 6, Seiten 750–759, ISSN (Online) 1437–4331, ISSN (Print) 1434–6621, 10.1515/CCLM.2006.123, May 2006. [DOI] [PubMed] [Google Scholar]
- 39.Plebani M. The detection and prevention of errors in laboratory medicine. Ann Clin Biochem 2010;47:101–10. 10.1258/acb.2009.009222 [DOI] [PubMed] [Google Scholar]
- 40.Lippi G, Plebani M, Graber ML. Building a bridge to safe diagnosis in healthcare. The role of the clinical laboratory. Clin Chem Lab Med. 2015. December 2. pii:/j/cclm.ahead-of-print/cclm-2015-1135/cclm-2015-1135.xml. [Epub ahead of print]). [DOI] [PubMed] [Google Scholar]
- 41.Epner PL, Gans JE, Graber ML. When diagnostic testing leads to harm: a new outcomes-based approach for laboratory medicine. BMJ Qual Saf 2013;22(Suppl 2):ii6–ii10. 10.1136/bmjqs-2012-001621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lewandrowski K. Managing utilization of new diagnostic tests. Clin Leadersh Manag Rev. 2003;17:318–324. [PubMed] [Google Scholar]
- 43.Zhi M, Ding EL, Theisen-Toupal J, Whelan J, Arnaout R. The landscape of inappropriate laboratory testing: a 15-year meta-analysis. PLoS One 2013;8(11):e78962 10.1371/journal.pone.0078962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.American Board of Internal Medicine. Choosing Wisely; [cited August 19, 2015]. Available: http://www.choosingwisely.org/.
- 45.Swiss Society of General Internal Medicine. Smarter Medicine; [cited August 19, 2015]. Available:www.smartermedicine.ch.
- 46.Sinaiko AD, Rosenthal MB. Increased price transparency in health care–challenges and potential effects. N Engl J Med. 2011;364:891–894. 10.1056/NEJMp1100041 [DOI] [PubMed] [Google Scholar]
- 47.Carlson J, Dachs RJ. Family medicine residents remain unaware of hospital charges for diagnostic testing. Fam Med. 2015;47:466–469. [PubMed] [Google Scholar]
- 48.Ferrante di Ruffano L, Hyde CJ, McCaffery KJ, Bossuyt PM, Deeks JJ. Assessing the value of diagnostic tests: a framework for designing and evaluating trials. BMJ. 2012;344:e686 10.1136/bmj.e686 [DOI] [PubMed] [Google Scholar]
- 49.Schäfer HH, Filser L, Rohr UP, Laubender RP, Dieterle T, Maitland R, et al. Medical value as a new strategy to increase corporate viability: Market chances and limitations in the diagnostic industry. J Entrepren Organiz Manag. 2015;4:131. [Google Scholar]
- 50.Jeremias A, Gibson CM. Narrative review: alternative causes for elevated cardiac troponin levels when acute coronary syndromes are excluded. Ann Intern Med. 2005;142:786–791. [DOI] [PubMed] [Google Scholar]
- 51.Mair J, Smidt J, Lechleitner P, Dienstl F, Puschendorf B. Rapid accurate diagnosis of acute myocardial infarction in patients with non-traumatic chest pain within 1 h of admission. Coron Artery Dis. 1995;6:539–545. [PubMed] [Google Scholar]
- 52.Timp JF, Lijfering WM, Flinterman LE, van Hylckama Vlieg A, le Cessie S, Rosendaal FR, et al. Predictive value of factor VIII levels for recurrent venous thrombosis: results from the MEGA follow-up study. J Thromb Haemost. 2015;13:1823–1832. 10.1111/jth.13113 [DOI] [PubMed] [Google Scholar]
- 53.Caro JJ. An economic model of stroke in atrial fibrillation: the cost of suboptimal oral anticoagulation. Am J Manag Care. 2004;10(14 Suppl):S451–S458; discussion S458-S461. [PubMed] [Google Scholar]
- 54.Knottnerus JA, Buntinx F. The evidence base of clinical diagnosis: Theory and methods of diagnostic research Oxford, UK: BMJ Books; 2011. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
(XLSX)
(DOCX)
(XLSX)
(DOCX)
A) Percentage of IVD subtype use during initial patient workup according to specialty and country; B) Rated importance of IVD subtypes for clinical practice and decision making during initial patient workup according to specialty and country (rating based on Likert scale, 1 = very low, 5 very high).
(DOCX)
Data Availability Statement
The authors have included all relevant material important to display. Because the interviews have been performed by an independent third party and because this topic is a general topic and not related to any Roche products, the authors are confident to share all documents that have been generated during this investigation. This investigation was initiated by Roche Medical and Scientific Affairs, which partly covers educational and research activities independently of the business. The questions and raw data for stages 2 and 3 of the study have been uploaded as supplemental files to accompany the manuscript.