Introduction:
Overfill of tissue expanders is a commonly used modality to achieve customized dimensions in breast reconstruction. Little formal study of the dynamics of hyperexpansion of these devices has been performed to date, however.
Methods:
Overfill trials were performed using both Natrelle 133 MV and Mentor 8200 tissue expanders of indicated capacities ranging from 250 to 800 mL. Each expander was initially filled to its indicated capacity with normal water and then injected in regular increments to 400% overfill. Measurements of each expander’s width, height, and projection were made at indicated capacity and with each successive incremental overfill injection, and these results were then recorded, collated, and analyzed.
Results:
Over the first 50% overfill, all expanders demonstrated a logarithmic increase in projection (mean increase, 143 ± 9%) while maintaining essentially stable base dimensions. Overfill levels in excess of 50% were accompanied by linear increases in height, width, and projection, during which projection approached, but never equaled, base dimensions. Stress versus strain analyses demonstrated nonlinear biomechanical dynamics during the first 50% overfill, followed by standard elastic dynamics up to 400% overfill. At no point during the study, did expander tensions outstrip elastic properties, thereby explaining the lack of device rupture.
Conclusions:
Through overfilling, tunable geometries of tissue expanders can be accessed that may provide for increasing customization of reconstructions, particularly at overfill volumes up to 50% over indicated capacity. This study should serve to guide tissue expander selection and fill volumes that surgeons may implement in obtaining ideal reconstructed breast shapes.
Breast cancer remains the most common malignancy in woman with almost 300,000 new cases diagnosed in 2013.1 Of these cases, it is estimated that 20–40% of women who undergo mastectomy will elect to have breast reconstruction through either implant-based breast reconstruction or autologous tissue–based reconstructive method.2–5 Recent data have demonstrated that although the rate of autologous tissue–based breast reconstruction has remained relatively constant, implant-based reconstruction rates have risen by approximately 203%.5 In fact, in 2013, plastic surgeons in the United States performed a total of 95,589 breast reconstruction procedures, of which 68,607 (72%) involved tissue expansion for the reconstructive process.6 Therefore, tissue expander–based reconstruction represents the dominant modality of breast reconstruction in the United States.
Breast reconstruction seeks to recreate the natural aesthetic of the breast after mastectomy. Success depends on a final outcome that produces pleasing breast contour and projection within the confines of the patient’s natural anatomy.7,8 Reconstructive surgeons routinely utilize a 2-staged approach characterized by initial tissue expander placement to create and maintain tissue pockets that will be eventually utilized for implant-based or autologous tissue–based reconstructions. Although many methods can be used to tailor the pocket at the second stage of reconstruction, these often cannot fully correct an initial pocket that is less than ideal.9
Surgeons must choose tissue expanders from a set catalogue of available devices with restricted dimensions. These expanders have set base widths and projections for each volume within a given style. Therefore, expander reconstructions must balance base diameter, volume, and projection—often sacrificing shape in 1 dimension for the betterment of others. Furthermore, although a tissue expander is placed with a certain result in mind, during expansion, volumes are often adjusted to meet changes in patient preference or simply to obtain the initially desired outcome.
As replacement of one expander style with another requires another operative procedure, patient’s tissue pockets are often confined to the expansion parameters dictated by the expander’s inherent dimensions. To ameliorate this limitation, reconstructive surgeons often overfill tissue expanders to increase breast volume or redefine the mastectomy pocket without exchange of the initial expander. Although no data on the prevalence of overfilling breast tissue expanders are published, this technique is commonly used.9 Overfilling of the expander leads to increased breast volume; however, its effects on the overall shape of the expander have not been examined.
Toward this end, this study sought to address how tissue expander overfilling affects expander dimensions with increasing volume. It is our belief that determination of expander hyperinflation dynamics may better inform efforts to create truly customized dimensions for ideal mastectomy pocket creation, thereby improving the end results that may be achieved for expander-related breast reconstruction.
METHODS
Overfill trials were performed using both Natrelle 133 MV (Actavis + Allergan, Parsippany, N.J.) and Mentor Medium Height Style 8200 (Mentor Worldwide, Santa Barbara, Calif.) tissue expanders of indicated capacities ranging from 250 to 800 mL (Refs. 133 MV 11–16 and 354-82 11–16, respectively). Each device was injected by accessing its port with a 21-gauge winged Luer lock needle set (BD, Franklin Lakes, N.J., catalogue no. 367281) and a 60-mL syringe (BD, catalogue no. 309653). Each expander was initially filled to its indicated capacity with normal water and then overfilled in 50-mL increments to 5 times its indicated capacity (ie, 400% overfill). Measurements of each expander’s base diameter (width), height, and projection were made at indicated capacity and with each successive incremental overfill injection. Dimension measurements were obtained using 3-inch standardized calipers (H&H Industrial Products, Chino CA, N.J., UNSPC Code 23241601) with each expander on a flat, level surface. The results of these changes in dimensions were then recorded, collated, and analyzed.
RESULTS
Six distinct volume expanders were overfilled for each manufacturer, with indicated capacities of 250, 300, 400, 500, 600, and 700 mL for the Natrelle expanders and indicated capacities of 275, 350, 450, 550, 650, and 800 mL for the Mentor expanders. With each size expander for both manufacturers, initial overfill up to 50% the indicated capacity resulted in logarithmic increases in projection with comparatively little change in expander base dimensions (Fig. 1); the mean projection change observed during 50% overfill was 148 ± 9% relative to projection at indicated capacity, compared with mean height and width changes of 98 ± 4% and 96 ± 2%, respectively (Table 1). With overfill volumes more than 50%, increasing overfill demonstrated linear increases in all 3 expander dimensions up to 400% overfill of indicated capacity (Fig. 2). With overfill volumes more than 50%, increases in projection continued to outpace those of both height and width, whereas height and width tended to increase in a nearly 1:1 fashion (Fig. 3); however, projection dimensions remained consistently below those of height and width during this linear growth phase. These behaviors were characteristic of all studied expanders, regardless of indicated capacity; trendline analyses of aggregated data demonstrated R-squared values for height, width, and projection changes with increasing overfill of 0.7600, 0.9212, and 0.8482, respectively (Fig. 4).
Fig. 1.
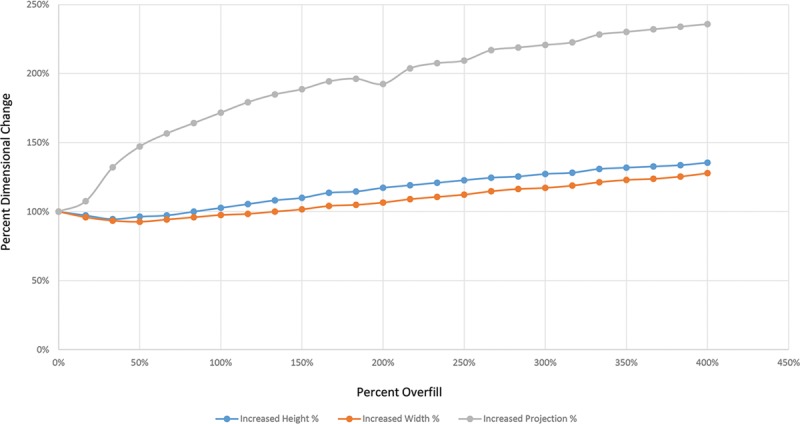
Representative overfill dynamics for Natrelle 133MV 400-mL tissue expander. Progressive overfill of a 400-mL tissue expander demonstrates a logarithmic increase in projection during the first 50% overfill period, during which height, and width remain essentially unchanged. Beyond 50% overfill, linear increases in all expander dimensions are observed.
Table 1.
Average Percentage Dimensional Changes with Increasing Overfill
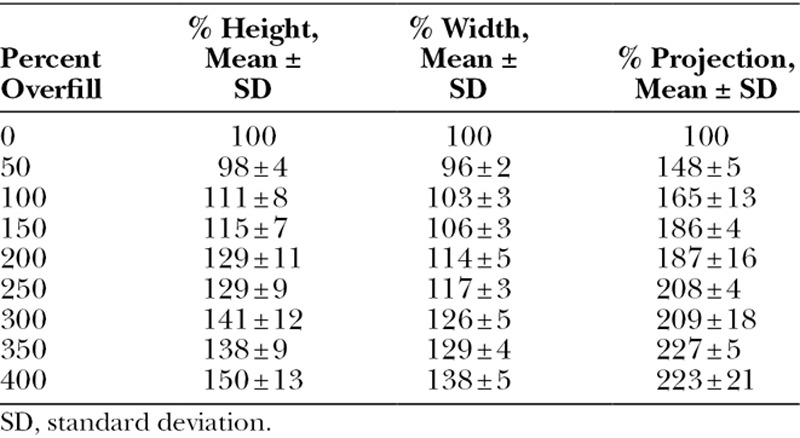
Fig. 2.
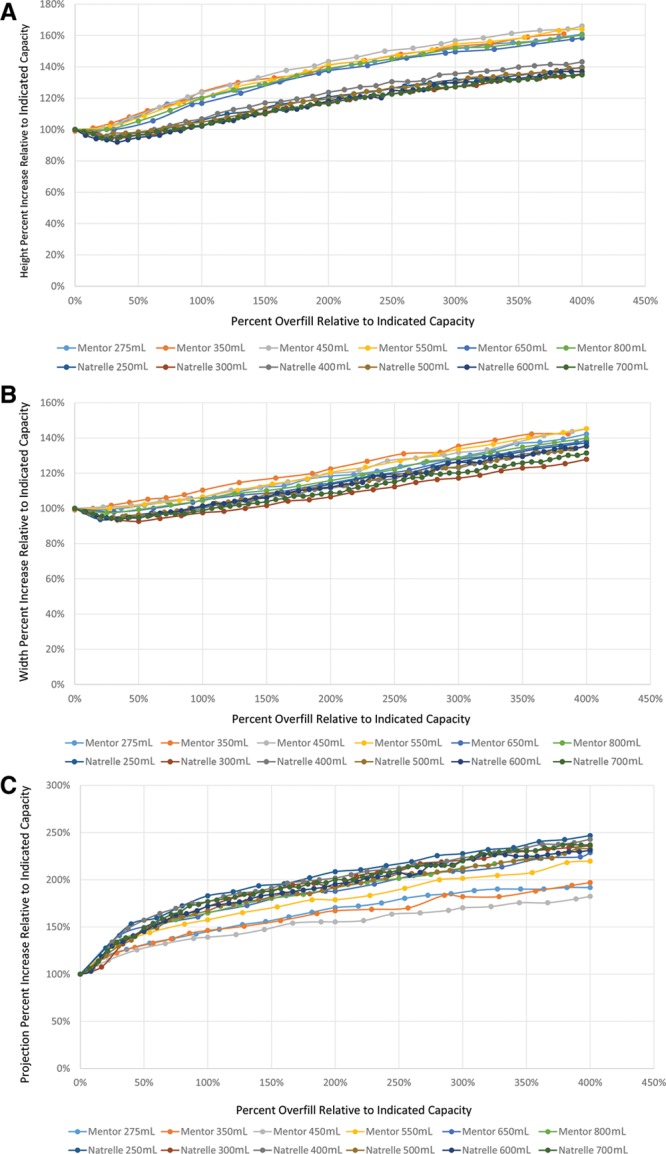
Dimensional percentage changes as a function of overfill percentage. Plots of percentage changes in height (A), width (B), and projection (C) as a function of overfill percentage demonstrate highly conserved dynamics across all expanders studied, regardless of indicated capacity and manufacturer.
Fig. 3.
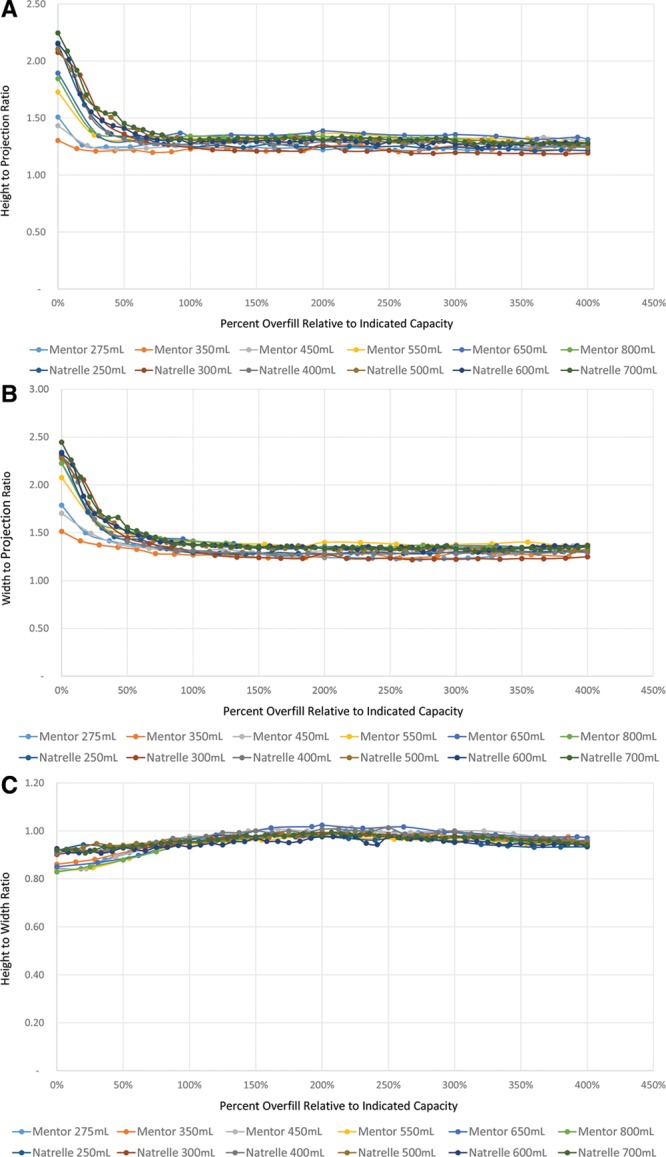
Dimensional ratio changes as a functional of overfill percentage. Analyses of ratios of height to projection (A), width to projection (B), and height to width (C) demonstrate increasing parity of projection to base dimensions and a largely stable 1:1 relationship of base dimensions with increasing overfill. Projection never demonstrates an equivalency to base dimensions, however.
Fig. 4.
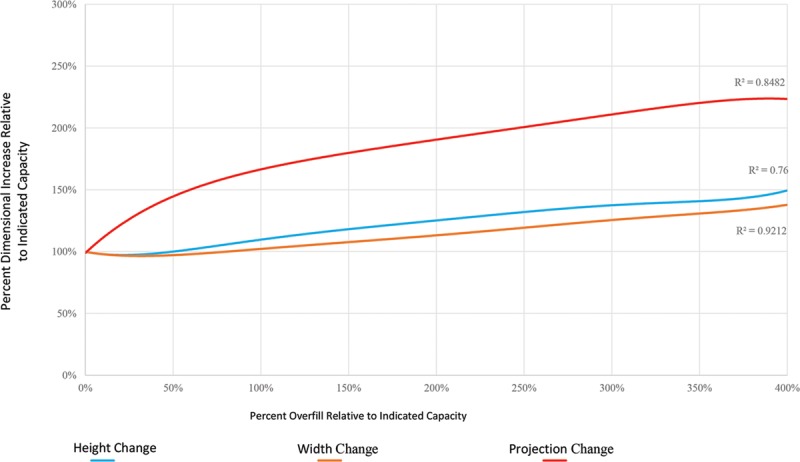
Composite dimensional changes with increasing overfill. Trendline analyses based on aggregate data from all studied expanders illustrate the general dynamics of changes in device height, width, and projection with increasing overfill. A high degree of uniformity is noted, as reflected in R2 values greater than 0.75 for all analyses.
No expanders ruptured within the measured overfill volumes. Estimated load versus strain diagrams (Fig. 5) of the overfilled expanders demonstrated that as the load on any given device was increased from 10 to 30 N, the expander underwent nonlinear deformation within a strain regime of 0–80%. Once 80% strain and 30 N was reached, the expander underwent linear stress–strain relationships consistent with elastic behavior. Within the ranges tested, 400% overfill resulted in 150% strain in the projection dimension, during which the expander remained elastic, having not reached its yield strength.
Fig. 5.
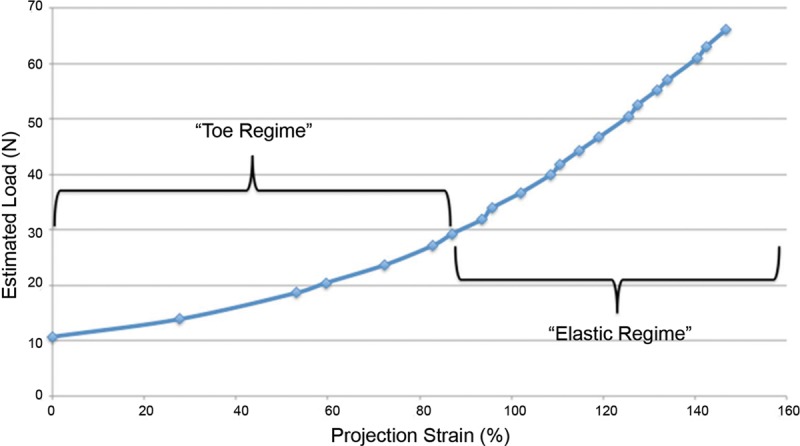
Estimated stress versus strain for representative Natrelle 133MV 250-mL tissue expander with increasing overfill. Stress versus strain analyses demonstrate a classic nonlinear initial toe regime. At approximately 80% strain, the curve becomes linear, consistent with elastic material behavior. Of note, the yield strength was not approached in the experimental space.
DISCUSSION
Currently, tissue expander–based methodologies remain the dominant technique to achieve breast reconstruction in the United States.5 These techniques are often preferred because they provide multiple stages for tailoring of the reconstructed breast to match the aesthetic goals of both the patient and the surgeon. Successful breast reconstruction demands the creation of an aesthetic unit of size and contour appropriate to a patient’s baseline anatomy. One of the greatest challenges to achieve this aim stems from difficulty in matching the volume of the reconstructed breast conus within the anatomically defined breast footprint.10 Tissue expanders are often placed with their base dimensions matched to the breast footprint and then overfilled in an attempt to achieve the desired projection within this footprint.10–13 Previous studies have demonstrated that overfilling a tissue expander up to 15 times the manufacturer’s stated fill is safe14; however, the effect of overfilling on the dimensions of the expander has not been reported.
This study demonstrates that overfilling Natrelle and Mentor tissue expanders results in predictable and consistent changes in the overall shape of the tissue expanders. Expander overfilling up to 50% of the indicated expander capacity results in a preferential increase in projection with minimal impact on expander width and height. Overfilling beyond 50% of the indicated expander capacity results in increases in device projection, width, and height at linear rates, producing increasing spherical expander shapes with ongoing overfill (Fig. 6). These uniform increases in all dimensions persist at overfill volumes up to 400% beyond indicated expander capacity.
Fig. 6.

Morphological changes in expander shape with increasing overfill. Representative photographs of a Mentor 8200 350-mL capacity expander at increasing fill volumes demonstrate gradual evolution of a nearly spherical shape with ongoing expansion.
These results are notable in that they suggest the possibility of accessing previously unobtainable expander dimensions (namely projection) through overfilling, particularly at overfill volumes up to 50% of the indicated expander capacity. For example, a 400-mL Natrelle 133 MV expander overfilled to 600 mL demonstrates dimensions of 12.4 (width) × 11.6 (height) × 8.8 (projection) cm, whereas a 600-mL Natrelle 133MX high-profile expander has catalog dimensions of 14 (width) × 13 (height) × 7.1 (projection) cm.15 This represents a highly tunable platform by which ideal breast shape can be possibly achieved for customized breast reconstruction. A surgeon may, therefore, opt to overfill an expander to provide a highly projecting breast that does not reach beyond the natural footprint of the breast. Thus, these findings support a new paradigm for initial tissue expander selection.
In addition, our study demonstrates that these overfill dynamics are remarkably consistent across all volumes of studies expanders, regardless of indicated capacity or manufacturer. Our experimental overfilling of expanders of varying indicated capacities yielded nearly identical dimension percentage change and ratio changes as a function of percent overfill across all devices. These data demonstrate an engineering similitude with geometric similarity among those expanders studied. The practical implication of this finding to the reconstructive surgeon is that experiences and behaviors evident in overfilling a smaller expander can be safely extrapolated to all volumes of expanders within the same class (eg, Natrelle 133 MV or Mentor 8200).
Although previous studies have demonstrated that tissue expanders can be safely filled to up to 15 times their recommended maximum fill, no mechanical explanation for this observation has been provided.14 In this study, the stress–strain curves of overfilled tissue expanders were graphed demonstrating strains of 140% at 400% over indicated capacity. Initially, stresses from 2000 to 4000 mN and strains form 0% to 80% result in the expanders undergoing a “toe regime” of deformation consistent with behavior seen in biologic tissues and polymeric materials.16–18 These regions are consistent with high deformation with low stresses. This material behavior explains the rapid increase in projection in the initial stages of overfill. At 80% projection strain, the material behavior becomes elastic with stresses resulting in linear increases in strain. At 400% overfill, the material remains in the elastic regime and does not reach its yield strength and, therefore, does not become ductile. This indicates that the material has not undergone plastic deformation and, therefore, should resist rupture.
The implications of our study must be considered with its limitations in mind. First, our study only examined the overfill dynamics of 2 brands and classes of tissue expanders and may, therefore, not be generalizable to all types of breast tissue expander devices. We selected the devices for this study based on the fact that the Natrelle 133 MV and Mentor 8200 each represent the most popular models of expander produced by each manufacturer; as such, we expect the findings of this study to be relevant to a large segment of reconstructive plastic surgeons. That being said, variances in the geometry and shape of other devices may result in overfill behaviors distinct from those evidenced in this study; the overfill dynamics of other expanders are, therefore, worthy of study in further investigations. In addition, the dimensional changes witnessed in our investigation were measured ex vivo and, therefore, do not account for external strains and deformational forces to which breast tissue expanders would be exposed in a human subject (eg, the dynamic interplay of overlying contractile skeletal musculature and underlying chest wall). Although it is uncertain to what extent these biological forces might alter the device dynamics described earlier, we believe that the inherent rigidity of expander form may resist major deviations from our study results in vivo. Confirmation of this supposition should be the subject of further investigations.
CONCLUSIONS
Overfilling of tissue expanders is a commonly utilized technique in expander-based breast reconstructions. This study is the first of its kind to analyze the effect overfilling of expanders has on the shape and behavior of tissue expanders. Through overfilling, tunable geometries of tissue expanders can be accessed that may provide for increasing customization of reconstructions, particularly at overfill volumes up to 50% over indicated capacity. This study should serve to guide tissue expander selection and fills that surgeons may implement in obtaining ideal reconstructed breast shapes.
Footnotes
Disclosure: The authors have no financial interest to declare in relation to the content of this article. The Article Processing Charge was paid for by the authors.
REFERENCES
- 1.American Cancer Society. Breast Cancer Facts and Figures 2013–2014. Atlanta, GA:: American Cancer Society, Inc.; 2013. [Google Scholar]
- 2.Alderman AK, Wei Y, Birkmeyer JD. Use of breast reconstruction after mastectomy following the Women’s Health and Cancer Rights Act. JAMA. 2006;295:387–388. doi: 10.1001/jama.295.4.387. [DOI] [PubMed] [Google Scholar]
- 3.Kruper L, Holt A, Xu XX, et al. Disparities in reconstruction rates after mastectomy: patterns of care and factors associated with the use of breast reconstruction in Southern California. Ann Surg Oncol. 2011;18:2158–2165. doi: 10.1245/s10434-011-1580-z. [DOI] [PubMed] [Google Scholar]
- 4.Reuben BC, Manwaring J, Neumayer LA. Recent trends and predictors in immediate breast reconstruction after mastectomy in the United States. Am J Surg. 2009;198:237–243. doi: 10.1016/j.amjsurg.2008.11.034. [DOI] [PubMed] [Google Scholar]
- 5.Albornoz CR, Bach PB, Mehrara BJ, et al. A paradigm shift in U.S. Breast reconstruction: increasing implant rates. Plast Reconstr Surg. 2013;131:15–23. doi: 10.1097/PRS.0b013e3182729cde. [DOI] [PubMed] [Google Scholar]
- 6.Surgeons ASoP. 2013 Plastic Surgery Statistics Report. Arlington Heights, IL: ASPS National Clearinghouse of Plastic Surgery Procedural Statisics; 2013. [Google Scholar]
- 7.Lee CN, Hultman CS, Sepucha K. Do patients and providers agree about the most important facts and goals for breast reconstruction decisions? Ann Plast Surg. 2010;64:563–566. doi: 10.1097/SAP.0b013e3181c01279. [DOI] [PubMed] [Google Scholar]
- 8.Lee CN, Hultman CS, Sepucha K. What are patients’ goals and concerns about breast reconstruction after mastectomy? Ann Plast Surg. 2010;64:567–569. doi: 10.1097/SAP.0b013e3181bffc9b. [DOI] [PubMed] [Google Scholar]
- 9.Kim JY, Connor CM. Focus on technique: two-stage implant-based breast reconstruction. Plast Reconstr Surg. 2012;130(5 suppl 2):104S–115S. doi: 10.1097/PRS.0b013e31825f2538. [DOI] [PubMed] [Google Scholar]
- 10.Blondeel PN, Hijjawi J, Depypere H, et al. Shaping the breast in aesthetic and reconstructive breast surgery: an easy three-step principle. Plast Reconstr Surg. 2009;123:455–462. doi: 10.1097/PRS.0b013e3181954cc1. [DOI] [PubMed] [Google Scholar]
- 11.Pietilä JP, Nordström RE, Virkkunen PJ, et al. Accelerated tissue expansion with the “overfilling” technique. Plast Reconstr Surg. 1988;81:204–207. doi: 10.1097/00006534-198802000-00011. [DOI] [PubMed] [Google Scholar]
- 12.Pusic AL, Cordeiro PG. An accelerated approach to tissue expansion for breast reconstruction: experience with intraoperative and rapid postoperative expansion in 370 reconstructions. Plast Reconstr Surg. 2003;111:1871–1875. doi: 10.1097/01.PRS.0000056871.83116.19. [DOI] [PubMed] [Google Scholar]
- 13.Woods JE, Mangan MA. Breast reconstruction with tissue expanders: obtaining an optimal result. Ann Plast Surg. 1992;28:390–396. doi: 10.1097/00000637-199204000-00019. [DOI] [PubMed] [Google Scholar]
- 14.Hallock GG. Safety of clinical overinflation of tissue expanders. Plast Reconstr Surg. 1995;96:153–157. doi: 10.1097/00006534-199507000-00023. [DOI] [PubMed] [Google Scholar]
- 15.Allergan. Allergan Natrelle Product Catalog. 2014. [Google Scholar]
- 16.Hooley CJ, McCrum NG, Cohen RE. The viscoelastic deformation of tendon. J Biomech. 1980;13:521–528. doi: 10.1016/0021-9290(80)90345-0. [DOI] [PubMed] [Google Scholar]
- 17.Gupta HS, Seto J, Krauss S, et al. In situ multi-level analysis of viscoelastic deformation mechanisms in tendon collagen. J Struct Biol. 2010;169:183–191. doi: 10.1016/j.jsb.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 18.Wang Z, Volinsky AA, Gallant ND. Crosslinking effect on polydimethylsiloxane elastic modulus measured by custom-built compression instrument. J Appl Polym Sci. 2014;131:41050–41054. [Google Scholar]


