Background:
Recent papers and guidelines agree that patients with locally advanced breast cancer (LABC) should be offered breast reconstruction. Yet, the type of reconstruction in this group of patients is still a point of controversy.
Methods:
One hundred fourteen patients, treated for LABC from 2007 to 2013, were divided into 3 groups based on the reconstructive option: no reconstruction (NR), implant-based/expander-based reconstruction (IBR), and autologous tissue reconstruction (ATR). We analyzed demographics and compared delay in adjuvant therapy, length of hospitalization, surgical complications, failure of reconstruction, local recurrence, and disease-free survival.
Results:
Twenty-six patients had NR, 38 had IBR, and 50 had ATR. No significant difference was found in the percentage of patients who had their adjuvant treatment delayed [16% (NR) vs 22% (IBR) vs 14% (ATR)]. Mean length of hospitalization for the NR, IBR, and ATR groups was 2.7, 6, and 7.5 days, respectively. Complication rates requiring readmission were 36% (NR), 42% (IBR), and 32% (ATR). In the IBR group, 37% of implants were removed because of complications. Failure of reconstruction was 37% and 0% for the IBR and ATR groups, respectively. Local recurrence rates in the NR and Reconstruction (groups IBR and ATR combined) groups were 7% and 2%, respectively. Mean survival times in patients were 18 (NR), 10.3 (IBR), and 12.2 (ATR) months.
Conclusions:
No significant difference was found in the hospital stay length, adjuvant treatment delay, and complication rates between IBR and ATR. High rates of failed reconstruction suggest that the use of implants should be considered very carefully in patients with LABC.
Locally advanced breast cancers (LABCs) are characterized by tumors larger than 5 cm; the presence of fixed axillary lymph nodes; positive ipsilateral supraclavicular, infraclavicular, or internal mammary nodes; skin ulceration; dermal infiltration; erythema or “peau d’orange”; tumor fixation to the ribs or muscles; and inflammatory carcinomas.1
The oncological treatment of LABC is well established; however, the safety and the efficacy of immediate reconstruction in this group of patients remain unknown.2–4 Radiotherapy is typically indicated for these patients, and the effects of such treatment on breast reconstruction are the subject of ongoing debate.5–13 Moreover, the type and the timing of reconstruction for LABC patients continue to be points of major controversy.2–4,14 These patients are considered difficult candidates for reconstruction because of the significantly greater risk of local recurrence, more aggressive adjuvant and neoadjuvant treatments, and reduced survival.
Currently, the incidence of locally advanced cancer is lower than it has been because of improved diagnostic techniques and greater public awareness of breast cancer.15 However, 3% to 5% of all of the breast cancer cases and 3000 to 5000 cases per annum in the United Kingdom present as locally advanced disease. Among the locally advanced population, we have observed that patients request reconstruction despite knowing that their mortality prognosis might not be favorable. Oncologically safe reconstruction with low complication rates is a high priority for this group of patients.
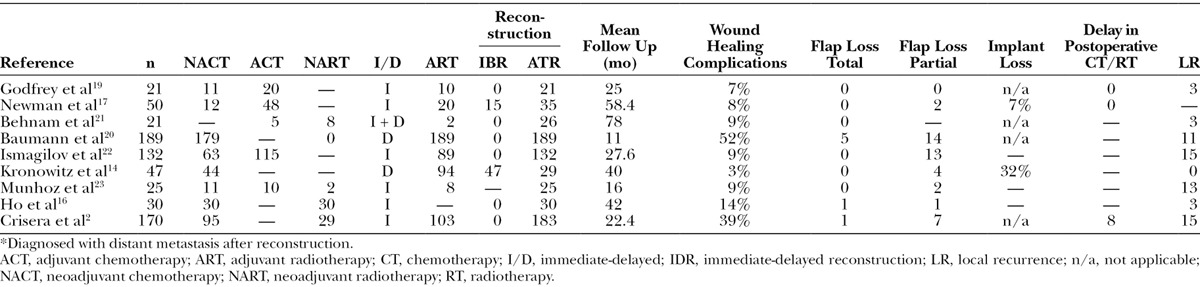
The number of currently available comparative studies of patients with LABC is limited, and the outcomes are controversial2,14,16–23 (Table 1). The purposes of this study were to provide an objective analysis of the outcomes of different types of reconstruction based on a large cohort of patients with LABC who were treated in a single centre and to compare implant-/expander-based reconstruction (IBR), autologous tissue reconstruction (ATR), and control no reconstruction (NR) groups.
Table 1.
Studies of Reconstruction for Locally Advanced Breast Cancer Patients
METHODS
Patient Groups
A retrospective evaluation was performed for all patients with LABC who underwent surgery with or without immediate reconstruction at St. Thomas’s Hospital, London, United Kingdom from February 2007 to April 2013. We compared 3 groups: NR group, IBR, to reflect a relatively quick and simple reconstructive technique with no donor site that involves the use of implant materials, and a group with ATR, reflecting a more complex reconstructive technique with a donor tissue site that brings in nonirradiated tissue and does not involve the use of implants. The data were collected from the case notes and included patient demographics, length of hospital stay, median follow-up time, delays in adjuvant therapy, surgical complications, failure of reconstruction, local recurrence, and survival.
Statistical Methods
The statistical analyses were performed using SPSS Statistics version 20. Pearson’s χ2 tests were used for analyses of survival, delays in adjuvant treatment, and complications. Mann-Whitney and Kruskal Wallis tests were used to evaluate the length of hospital stay, and Student’s t test was used to examine age and tumor size. Statistical significance was taken at P < 0.05.
RESULTS
Patient Demographics
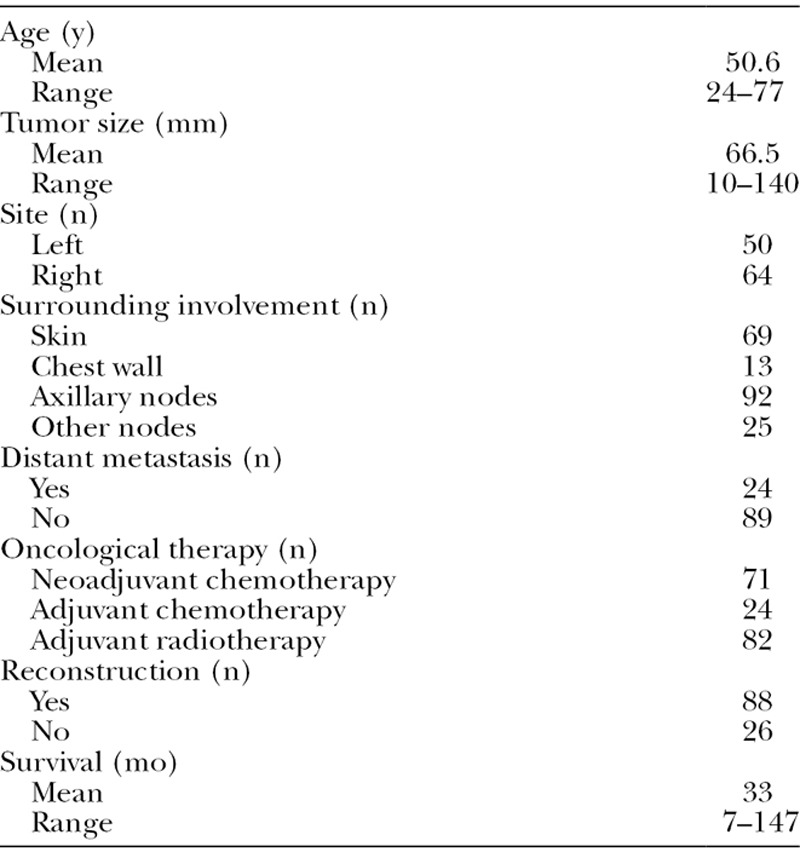
Of the 114 identified patients, 50 patients underwent ATR with a deep inferior epigastric perforator, transverse rectus abdominis myocutaneous, or latissimus dorsi flaps; 38 patients underwent reconstruction with implants or expanders; and 26 patients underwent NR. The patients’ demographic data are presented in Table 2.
Table 2.
Patient Demographics
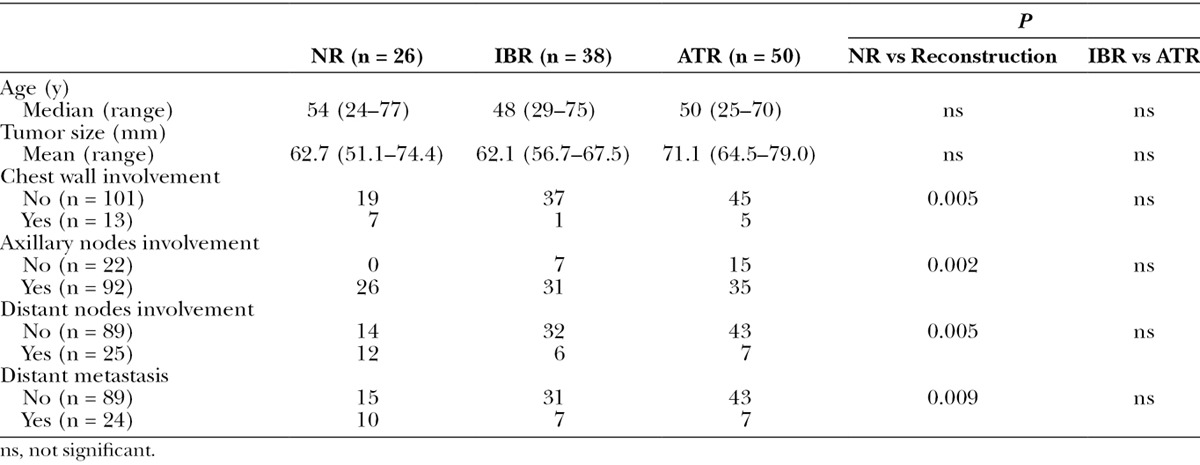
The median ages were 48 (29–75) years in the IBR group, 50 (25–70) years in the ATR group, and 54 (24–77) years in the NR group. The patients who underwent reconstruction were slightly younger than the nonreconstructed patients (Table 2). The ATR group tended to have larger tumors than the IBR group (71.7 vs 62.1 mm in mean size). The NR group had an average tumor size of 62.7 mm, although this was not significantly different from the mean tumor size of the patients who underwent reconstruction (ie, the R group; Table 3). The patients with chest wall involvement were less likely to undergo reconstruction. Only 6 of 13 patients with chest wall involvement underwent reconstruction compared with 82 of 101 patients with no chest wall involvement. All 22 patients with no axillary node involvement underwent reconstruction, whereas 66 of 92 patients with nodal involvement did so (P = 0.002). Among the 89 patients with no evidence of metastasis, 74 patients underwent reconstruction. Of the 24 patients who had distant metastases, 14 patients underwent reconstruction based on the multidisciplinary treatment recommendation and patients’ preference.
Table 3.
Comparisons of Age, Tumor Size, Distant Metastasis, and Chest Wall, Axillary, and Distant Nodal Involvement Among the 3 Groups (NR, IBR, and ATR Groups)
Delay in Adjuvant Therapy
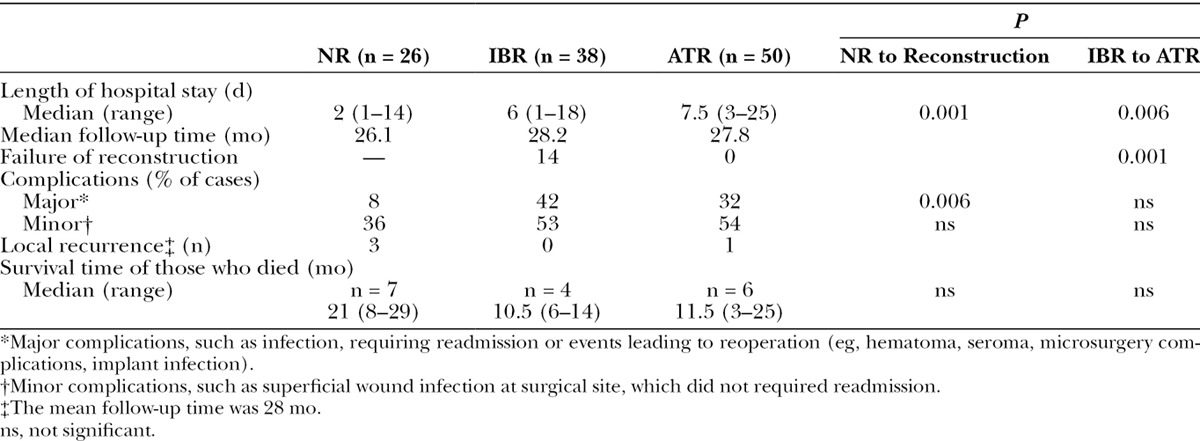
Seventy-one patients received neoadjuvant chemotherapy, and 24 patients received adjuvant chemotherapy. Eighty-six of 114 patients had adjuvant radiotherapy with no significant difference between the reconstruction groups and NR group (69% in the IBR group and 68% in the ATR group vs 71% in the NR group). A delay in adjuvant therapy was defined as a delay of 4 or more weeks. No significant differences were found in the percentages of patients who had their adjuvant treatment delayed (chemotherapy or radiotherapy) between the different groups (16% in the NR vs 18% in the Reconstruction (groups IBR and ATR combined) group (R group); 22% in the IBR vs 14% in the ATR groups).
Length of Hospital Stay
The median length of hospitalization for the NR, IBR, and ATR groups was 2, 6, and 7.5 days, respectively (Table 4). As expected, there were significant differences between the length of the hospital stay of the R and NR groups (P < 0.001) and between those of the IBR and ATR groups (P = 0.006).
Table 4.
Comparisons of the Length of Hospital Stay, Median Follow-up Time, Failure of Reconstruction, Major and Minor Complications, Local Recurrence, and Survival Time Among the 3 Groups (NR, IBR, and ATR Groups)
Medial Follow-up Time
The median follow-up time for the IBR, ATR, and NR groups was 28.2, 27.8, and 26.1 months, respectively (Table 4).
Complications
The rates of complications that required readmission were 42% and 32% in the IBR and ATR groups, respectively (Table 4). As expected, the frequency of major complications was higher among the reconstructed patients than the nonreconstructed patients (36% vs 8% P = 0.006). In the IBR group, 37% of the implants were removed because of acute or late complications.
Failure of Reconstruction
In the IBR group, 37% of the implants had to be removed because of early or late complications, whereas in the ATR group, there was no complication of flap failure (Table 4). There was a significant difference in the rate of failure of the reconstruction, 37% versus 0% (P = 0.001).
Survival and Local Recurrence
At a mean follow-up time of 28 months, the local recurrence rates in the NR and R groups were 7% and 2%, respectively (Table 4). Among the reconstructed patients, the local recurrence rates were 4% in the ATR group and 0% in the IBR group. At the time of the completion of the study, 27% of the NR patients and 12.5% of the R patients had died. The median survival times of the deceased patients were 21, 10.5, and 11.5 months in the NR (n = 7), IBR (n = 4), and ATR (n = 6) groups, respectively.
DISCUSSION
Reconstruction in LABC requires a very cautious and individual approach. Guidelines advise that reconstruction should be offered to all breast cancer patients. Opinions regarding the timing and types of reconstruction can vary substantially among surgeons, particularly in cases of advanced disease.24 Important factors, such as disease progression and response to neoadjuvant treatment, need to be taken into consideration, especially the effect of radiotherapy. Authors like Cordeiro and Nava have supported the use of implants for breast reconstruction; however, they acknowledge the higher failure rates and capsular contracture in patients who have had radiotherapy.9,10 Our study supports the benefits of autologous breast reconstruction over IBR in LABC.
Skin-sparing mastectomy followed by immediate reconstruction provides the best aesthetic outcome; studies supporting the use of skin-sparing mastectomy for LABC patients are uncommon, and the majority of such reports are limited to relatively small groups of patients.16,25–27 In our series, skin-sparing mastectomy was offered to all patients with (noninflammatory) locally advanced cancer with no skin involvement regardless of cancer stage. We ensured that the skin incisions were well clear of any disease and that there was a clear plane between the most superficial tumor and overlying skin. Adjuvant postmastectomy radiotherapy is indicated for the following conditions: tumors larger than 4 cm, tumors with close or positive margins at mastectomy, the presence of more than 3 positive axillary lymph nodes, and cases of local recurrence.28 Factors such as tumor grade are also taken into consideration. According to our protocol, all patients with LABC received postoperative radiotherapy; however, some of the patients in our group had previously received radiotherapy after previous wide local excisions. The effects of reconstruction on the delivery of radiotherapy remain a worrisome issue; however, no delays in delivery of adjuvant radiotherapy were found. We studied the outcomes of breast reconstruction offered to the patients dividing them into a group of patients who had IBR and a group of patients who had ATR. We acknowledge that our ATR group is relatively heterogeneous, including DIEPs, superior gluteal artery perforators, transverse rectus abdominis myocutaneous, and latissimus dorsi reconstructions; however, all of these reconstructions have in common that they bring in fresh nonirradiated tissue, are relatively long operations (usually >4 hours), and have donor sites, with the potential for additional morbidity while they do not need the use of implant materials, with their associated complications.
Most studies of the delay in adjuvant treatment after immediate reconstruction have described short-time delays or no delays in the start of chemotherapy after reconstruction. There is no evidence related to the oncologic repercussions of the 1- or 2-week delays that can be caused by surgical complications.29 In our study, the NR group exhibited a rate of delay in adjuvant treatment (chemotherapy or radiotherapy) that was nearly identical to that of the R group.
Immediate, delayed-immediate, and delayed reconstructions have each been considered to be the preferred option for patients with LABC by different authors. It has been shown that immediate reconstruction does not compromise survival or recurrence rates at any stage of breast cancer.2,4,17,24,30 Neoadjuvant chemotherapy and adjuvant radiotherapy are indicated for most LABC patients. These procedures might increase the risk of surgical complications. Baumann et al20 provided evidence that the optimal timing for delayed ATR should be 12 months after the completion of radiotherapy. However, in situations of high-risk cancers and potentially short life expectancies, many women do not wish to wait for a delayed reconstruction.
As expected, the rate of major complications was higher in our R patients than in our NR patients. The difference in major complications between the ATR and the IBR groups was not statistically significant, but this rate was higher in the implant group. The 6-day median hospital stay of the IBR groups was unexpectedly high. This reflects the practice at the time of keeping patients in hospital until drain removal. Usual practice is now to discharge patients home with drains in situ, and so hospital stay for IBR is usually 24 to 48 hours. The incidence of loss of implants and expanders we observed in patients with LABC was 37%, which is similar to the findings of other studies.14,31 This rate is significantly higher than the implant loss rates of non-LABC patients9,10; the failure of reconstruction was significantly higher in the IBR compared with the ATR group, as no flaps failed opposed to the 37% of implants that had to be removed. Therefore, the use of IBRs for LABC patients should be considered very carefully.
Why is expander loss so detrimental to further ATR? The majority of the losses of expanders and implants that we observed were caused by recurrent infections.31 Infection causes inflammation and scarring, and it delays any further reconstruction. Considering the poorer survival prognoses of the patients with LABC, many of these women might ultimately not only lose their expander but also be without any reconstruction at all. The inflammation and capsule formation caused by expanders in cases of delayed-immediate reconstruction make the preparation of the recipient sites for flaps more technically difficult.2 The methods that have been proposed to reduce implant loss after radiotherapy include partial deflation of the expander during the period of radiotherapy, extension of the pocket, and meticulous drain management; thus, this issue remains an important area for future research.14
The high rate of expander loss observed in the LABC patients, as experienced in our unit, supports the use of ATR for patients who are undergoing chemotherapy and adjuvant radiotherapy because this type of reconstruction is potentially less risky and more cost-effective than IBR, leading to significantly higher rate of successful reconstruction. In addition, the patient satisfaction rates with reconstruction in settings of radiotherapy have been reported to be significantly higher for ATR than for IBR.11,12 However, with careful patient selection, other authors have reported a relatively lower rate of failure of IBR.9,10 Identifying the optimal reconstructive method for each individual patient is an important skill and priority, particularly for patients with LABC.
CONCLUSIONS
Postmastectomy reconstruction is important and should be considered for all patients with LABC. Patients must be adequately informed of the risks, complications, and potential long-term cosmetic outcomes. The potential superiority of ATR over IBR observed in our series should be noted.
Footnotes
Disclosure: The authors have no financial interest to declare in relation to the content of this article. The Article Processing Charge was paid for by the authors.
REFERENCES
- 1.Rodger A, Leonard RC, Dixon JM. ABC of breast disease. Locally advanced breast cancer. BMJ. 1994;309:1431–1433. doi: 10.1136/bmj.309.6966.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crisera CA, Chang EI, Da Lio AL, et al. Immediate free flap reconstruction for advanced-stage breast cancer: is it safe? Plast Reconstr Surg. 2011;128:32–41. doi: 10.1097/PRS.0b013e3182174119. [DOI] [PubMed] [Google Scholar]
- 3.Nahabedian MY. Discussion: immediate free flap reconstruction of advanced-stage breast cancer: is it safe? Plast Reconstr Surg. 2011;128:42–43. doi: 10.1097/PRS.0b013e3182173dad. [DOI] [PubMed] [Google Scholar]
- 4.Nedumpara T, Jonker L, Williams MR. Impact of immediate breast reconstruction on breast cancer recurrence and survival. Breast. 2011;20:437–443. doi: 10.1016/j.breast.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 5.Kronowitz SJ. Current status of implant-based breast reconstruction in patients receiving postmastectomy radiation therapy. Plast Reconstr Surg. 2012;130:513e–523e. doi: 10.1097/PRS.0b013e318262f059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jhaveri JD, Rush SC, Kostroff K, et al. Clinical outcomes of postmastectomy radiation therapy after immediate breast reconstruction. Int J Radiat Oncol Biol Phys. 2008;72:859–865. doi: 10.1016/j.ijrobp.2008.01.055. [DOI] [PubMed] [Google Scholar]
- 7.Albino FP, Koltz PF, Ling MN, et al. Irradiated autologous breast reconstructions: effects of patient factors and treatment variables. Plast Reconstr Surg. 2010;126:12–16. doi: 10.1097/PRS.0b013e3181da878f. [DOI] [PubMed] [Google Scholar]
- 8.Wong JS, Ho AY, Kaelin CM, et al. Incidence of major corrective surgery after post-mastectomy breast reconstruction and radiation therapy. Breast J. 2008;14:49–54. doi: 10.1111/j.1524-4741.2007.00522.x. [DOI] [PubMed] [Google Scholar]
- 9.Cordeiro PG, Albornoz CR, McCormick B, et al. The impact of postmastectomy radiotherapy on two-stage implant breast reconstruction: an analysis of long-term surgical outcomes, aesthetic results, and satisfaction over 13 years. Plast Reconstr Surg. 2014;134:588–595. doi: 10.1097/PRS.0000000000000523. [DOI] [PubMed] [Google Scholar]
- 10.Nava MB, Pennati AE, Lozza L, et al. Outcome of different timings of radiotherapy in implant-based breast reconstructions. Plast Reconstr Surg. 2011;128:353–359. doi: 10.1097/PRS.0b013e31821e6c10. [DOI] [PubMed] [Google Scholar]
- 11.Chawla AK, Kachnic LA, Taghian AG, et al. Radiotherapy and breast reconstruction: complications and cosmesis with TRAM versus tissue expander/implant. Int J Radiat Oncol Biol Phys. 2002;54:520–526. doi: 10.1016/s0360-3016(02)02951-6. [DOI] [PubMed] [Google Scholar]
- 12.Lee BT, A Adesiyun T, Colakoglu S, et al. Postmastectomy radiation therapy and breast reconstruction: an analysis of complications and patient satisfaction. Ann Plast Surg. 2010;64:679–683. doi: 10.1097/SAP.0b013e3181db7585. [DOI] [PubMed] [Google Scholar]
- 13.Evans GR, Schusterman MA, Kroll SS, et al. Reconstruction and the radiated breast: is there a role for implants? Plast Reconstr Surg. 1995;96:1111–1115. discussion, 1116. [PubMed] [Google Scholar]
- 14.Kronowitz SJ, Lam C, Terefe W, et al. A multidisciplinary protocol for planned skin-preserving delayed breast reconstruction for patients with locally advanced breast cancer requiring postmastectomy radiation therapy: 3-year follow-up. Plast Reconstr Surg. 2011;127:2154–2166. doi: 10.1097/PRS.0b013e3182131b8e. [DOI] [PubMed] [Google Scholar]
- 15.Newman LA. Epidemiology of locally advanced breast cancer. Semin Radiat Oncol. 2009;19:195–203. doi: 10.1016/j.semradonc.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 16.Ho AL, Tyldesley S, Macadam SA, et al. Skin-sparing mastectomy and immediate autologous breast reconstruction in locally advanced breast cancer patients: a UBC perspective. Ann Surg Oncol. 2012;19:892–900. doi: 10.1245/s10434-011-1989-4. [DOI] [PubMed] [Google Scholar]
- 17.Newman LA, Kuerer HM, Hunt KK, et al. Feasibility of immediate breast reconstruction for locally advanced breast cancer. Ann Surg Oncol. 1999;6:671–675. doi: 10.1007/s10434-999-0671-6. [DOI] [PubMed] [Google Scholar]
- 18.Gieni M, Avram R, Dickson L, et al. Local breast cancer recurrence after mastectomy and immediate breast reconstruction for invasive cancer: a meta-analysis. Breast. 2012;21:230–236. doi: 10.1016/j.breast.2011.12.013. [DOI] [PubMed] [Google Scholar]
- 19.Godfrey PM, Godfrey NV, Romita MC. Immediate autogenous breast reconstruction in clinically advanced disease. Plast Reconstr Surg. 1995;95:1039–1044. doi: 10.1097/00006534-199505000-00013. [DOI] [PubMed] [Google Scholar]
- 20.Baumann DP, Crosby MA, Selber JC, et al. Optimal timing of delayed free lower abdominal flap breast reconstruction after postmastectomy radiation therapy. Plast Reconstr Surg. 2011;127:1100–1106. doi: 10.1097/PRS.0b013e3182043652. [DOI] [PubMed] [Google Scholar]
- 21.Behnam AB, Nguyen D, Moran SL, et al. TRAM flap breast reconstruction for patients with advanced breast disease. Ann Plast Surg. 2003;50:567–571. doi: 10.1097/01.SAP.0000069075.27321.BC. [DOI] [PubMed] [Google Scholar]
- 22.Ismagilov AK, Khasanov RS, Navrusov SN, et al. Study on possibilities of reconstructive–plastic surgery in patients with stage III breast cancer. Bratisl Lek Listy. 2011;112:686–690. [PubMed] [Google Scholar]
- 23.Munhoz AM, Montag E, Arruda E, et al. Immediate locally advanced breast cancer and chest wall reconstruction: surgical planning and reconstruction strategies with extended V-Y latissimus dorsi myocutaneous flap. Plast Reconstr Surg. 2011;127:2186–2197. doi: 10.1097/PRS.0b013e318213a038. [DOI] [PubMed] [Google Scholar]
- 24.Durrant CA, Khatib M, Macneill F, et al. Mastectomy and reconstruction in stage IV breast cancer: a survey of UK breast and plastic surgeons. Breast. 2011;20:373–379. doi: 10.1016/j.breast.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 25.Lim W, Ko BS, Kim HJ, et al. Oncological safety of skin sparing mastectomy followed by immediate reconstruction for locally advanced breast cancer. J Surg Oncol. 2010;11:39–42. doi: 10.1002/jso.21573. [DOI] [PubMed] [Google Scholar]
- 26.Liang TJ, Wang BW, Liu SI, et al. Recurrence after skin-sparing mastectomy and immediate transverse rectus abdominis musculocutaneous flap reconstruction for invasive breast cancer. World J Surg Oncol. 2013;11:194. doi: 10.1186/1477-7819-11-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prabhu R, Godette K, Carlson G, et al. The impact of skin-sparing mastectomy with immediate reconstruction in patients with stage III breast cancer treated with neoadjuvant chemotherapy and postmastectomy radiation. Int J Radiat Oncol Biol Phys. 2012;82:e587–e593. doi: 10.1016/j.ijrobp.2011.09.024. [DOI] [PubMed] [Google Scholar]
- 28.National Comprehensive Cancer Network. NCCN Breast Cancer Clinical Practice Guidelines. 2013. Available at: www.ncn.org. [Google Scholar]
- 29.Alderman AK, Collins ED, Schott A, et al. The impact of breast reconstruction on the delivery of chemotherapy. Cancer. 2010;116:1791–1800. doi: 10.1002/cncr.24891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Noone RB, Frazier TG, Noone GC, et al. Recurrence of breast carcinoma following immediate reconstruction: a 13-year review. Plast Reconstr Surg. 1994;93:96–106. discussion 107. [PubMed] [Google Scholar]
- 31.Peled AW, Stover AC, Foster RD, et al. Long-term reconstructive outcomes after expander-implant breast reconstruction with serious infectious or wound-healing complications. Ann Plast Surg. 2012;68:369–373. doi: 10.1097/SAP.0b013e31823aee67. [DOI] [PubMed] [Google Scholar]


