Abstract
Bees provide critical pollination services to 87% of angiosperm plants; however, the reliability of these services may become threatened as bee populations decline. Agricultural intensification, resulting in the simplification of environments at the landscape scale, greatly changes the quality and quantity of resources available for female bees to provision their offspring. These changes may alter or constrain the tradeoffs in maternal investment allocation between offspring size, number and sex required to maximize fitness. Here we investigate the relationship between landscape scale agricultural intensification and the size and number of individuals within a wild ground nesting bee species, Andrena nasonii. We show that agricultural intensification at the landscape scale was associated with a reduction in the average size of field collected A. nasonii adults in highly agricultural landscapes but not with the number of individuals collected. Small females carried significantly smaller (40%) pollen loads than large females, which is likely to have consequences for subsequent offspring production and fitness. Thus, landscape simplification is likely to constrain allocation of resources to offspring through a reduction in the overall quantity, quality and distribution of resources.
Introduction
Pollinators, in particular bees, are critical ecosystem service providers, responsible for the pollination of 87% of angiosperm plants [1] including more than 70% of crop species [2]. Although declines in bee populations have been documented [3,4], the causes of these declines are poorly understood and likely multifaceted [5].
Agricultural intensification resulting in the simplification of environments at the landscape scale greatly alters the resources available for bees and has been demonstrated to alter the abundance and distribution of a number of taxa [6,7,8]. Landscape simplification is associated with a transition of perennial natural habitats to arable fields, destruction of edge habitats and simplification of overall land-use types resulting in a reduction in habitat connectivity and greater fragmentation and isolation of remaining natural habitat patches [9].
As central place foragers, landscape composition is likely to have a strong influence on a female bee’s ability to locate and obtain quality nesting and provisioning resources. Agricultural intensification resulting in the reduction and fragmentation of the natural and semi-natural habitat patches that bees rely on for floral and nesting resources may increase foraging times [10,11], decrease the quality of resources [12] and increase pesticide exposure [13]. As floral and nesting resources become scarce, bees are more likely to use resources farther from the nesting site [14]. Longer foraging distances over a fixed nesting period equate to fewer overall foraging trips and therefore lower resource acquisition [15]. Potentially, longer foraging trips may also lead to a greater risk of adult predation as well as increasing the nest’s vulnerability to cleptoparasitism [16,17, 18] and adult exposure to pesticides. The likelihood of females collecting pesticide-laden pollen to provision brood cells may also be higher in simplified landscapes where pesticide use increases ([13], but see [19]).
Parental investment in offspring is optimized to maximize fitness through tradeoffs between the size, number and sex ratios of offspring [20, 21, 22]. Ecological and environmental conditions such as resource distribution [14, 14], abundance [23, 24], quality [25] and competition [16] can alter or constrain the tradeoffs in parental offspring investment. The cost of acquiring resources varies in changing environments and influences how parents allocate resources to offspring [26, 27]. Roulston and Cane [25] reported that offspring of the ground nesting bee Lasioglossum zephyrum grew larger on a high protein pollen diet, and although females responded to changes in floral resource abundance by altering provision mass size they did not compensate in response to lower pollen quality, suggesting that lower quality resources in agriculturally intensified landscapes could play a role in reducing body size.
Hymenopterans in particular have been a fertile ground for developing and testing theories on maternal offspring investment, as they tend to be strongly sexually dimorphic in size and females have control through haplodiploidy over the primary sex ratio of offspring. Bees provide an excellent system to study maternal resource allocation tradeoffs since all food consumed by the offspring prior to adulthood is provided by the mother and the amount of food provisioned to individual offspring is correlated to the subsequent size of the progeny at maturity [28, 29, 30, 23, 31]. Additionally, the heritability of body size appears low [32] and may be adaptively adjusted by females in response to seasonally changing resource levels [27].
When resources vary in space and time, females should alter the optimal amount of resources provisioned per offspring in order to maximize fitness [26, 27]. Seasonal changes in weather conditions and in resource availability have been shown to influence adult bee size in the following year in both solitary [33, 34] and social bee species [35]. Previous studies have relied on managed bee populations to isolate individual factors affecting maternal resource allocation such as foraging distance [15] and specific resource levels [23, 24] but in reality these factors are likely to be correlated and also linked to additional factors such as resource quality, competition and parasite pressure. Measurements of bee size and number in natural populations allows for the assessment of the cumulative effects of these factors simultaneously.
Here we focus on the relationship between landscape scale agricultural intensification and the size and number of individuals of the wild ground nesting bee species, Andrena nasonii. We hypothesize that changes in resource quantity and quality associated with gradients in agricultural land-use intensity constrain maternal investment tradeoffs such that 1) smaller individuals and 2) fewer individuals are produced in highly agricultural landscapes. Further, we explore the ecological consequences of a smaller bee size and test the prediction that smaller female bees carry smaller pollen loads than larger females.
Methods
Study System
Though bees in the family Andrenidae are among the most common and speciose of the vernal community, detailed life history information is sparse for the majority of species. A. nasonii Robertson 1895, is a polylectic bee with a short flight period in early spring [36]. It is an abundant floral visitor to many early spring fruit crops including apple [37], blueberry [38] and strawberry [39]. Although little is known about A. nasonii nesting biology specifically, bees in the genus Andrena are known to excavate nesting tunnels in the ground either singly or in aggregations but always with each female constructing and occupying a single nest. Nests are comprised of a simple tunnel up to 1 m in length ending in a small number of brood cells, which are sequentially provisioned with a mass of pollen mixed with nectar [40]. Following completion of the provision, a single egg is laid on the mass and the entrance to the brood cell is filled with soil [40].
Landscape Parameters
We identified 16 farms in the Finger Lakes Region of New York USA along a gradient in landscape complexity and established standardized 100m2 strawberry (Fragaria x ananassa) plots of the variety “Jewel”. Plots were located on Cornell University research farms or on private farms (landowners gave permission to conduct the study at these sites) and varied in the cover of agricultural land use in the surrounding landscape. Radii of 750 m and 1 km were selected as strawberry pollinator populations, which are dominated by A. nasonii, have been shown to respond to landscape gradients at these scales [39]. Agricultural lands were defined as pastoral and cultivated crops and estimated from the 2013 & 2014 NASS Cropland Data Layers [41]. Natural and semi-natural habitats included forests, wetlands, scrublands, fallows, open and low intensity developed land (roadsides etc). Because many early season flowering crops provide key floral resources for A. nasonii and A. nasonii may use these habitats as well as pastures and grasslands as nesting habitats, we included in our analysis the cover of early season blooming crops as well as pastures and cultivated crops separately.
Bee Size and Number
Bee specimens were collected using sweep nets along 50m transects once a week over four weeks in May of 2014 and 2015. Individuals were quickly killed in ethyl acetate killing jars and frozen (-20°C) until further processed. Species identification of each bee was verified using reference materials and DiscoverLife.org keys. Only five male A. nasonii were collected but were excluded from analyses given the small sample size. Two morphological measures of size were taken for each female bee: inter-tegular distance (ITD) and head capsule width using an ocular micrometer. These measures provide accurate estimates of bee size and have been used in a number of other studies [42, 43]
Pollen Load
To estimate the effect of bee size on its ability to collect pollen resources, we randomly selected 12 individuals from the upper and 12 individuals from the lower quartiles of bees collected based on inter-tegular distances. These 24 specimens were placed into pre-weighed 2 ml eppendorf tubes with 1 ml of 65°C 70% ethanol and vortexed for 15 minutes. The bees were then washed with 0.5 ml of 25°C 70% ethanol to remove any remaining pollen from the body and the tubes were centrifuged for 5 minutes at 13,000 RPM. The ethanol was decanted from each tube and the tubes were placed on a 65°C heating block to dry until they had reached a stable weight (approx. 3 hours). The final weight of the tube was recorded to the nearest 0.0001g from which the initial weight of the tube was subtracted to estimate the total weight of the pollen.
Statistical Analyses
Separate linear mixed effects models were fit using the nlme package [44] in R (v 3.1.0) [45] to determine the effects of landscape simplification on our measures of bee size (ITD and head capsule width) with each land cover variable as a fixed effect and sampling date within farm within year as hierarchical random effects. Land cover variables were correlated among themselves (S1 Table); thus, separate models were fit for each cover type and scale, and comparisons of model fit were made using AICc values. Models including with all agricultural lands at 750m (ITD AICc = 13.72) and 1km (ITD AICc = 11.83) were equivalent based on AICc. Therefore, the data for all other landscape variables are presented at the 1km scale. The effect of landscape simplification on abundance was tested with a linear mixed effects model with total number of individuals collected per site per year as the response variable and the most predictive land cover variable from the previous analysis as a fixed effect. Farm within year was included in the model as a hierarchical random effect. A t-test was used to determine whether pollen loads were different between the two bee size categories.
Results
A total of 112 female A. nasonii were collected over the course of strawberry bloom in May 2014 and 2015. An average of 5 individuals were collected per site with a minimum of 1 and a maximum of 10. The inter-tegular distance of female A. nasonii ranged from 1.4615 to 1.9615 mm and the head capsule width ranged from 2.1538 to 2.7692 mm.
Bee Size and Number
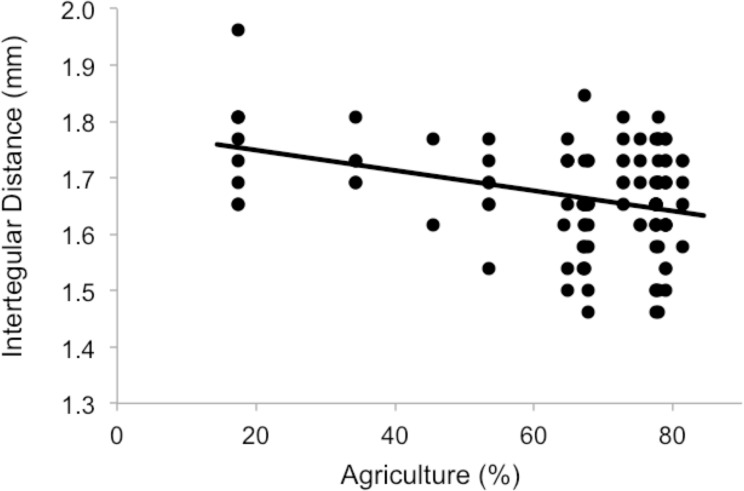
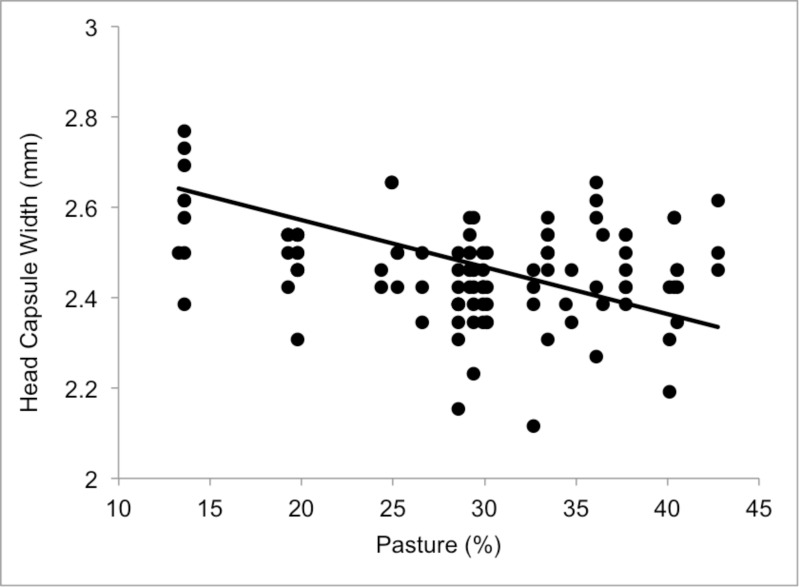
A significant positive correlation was found between inter-tegular distance and head capsule width (Pearson’s r = 0.67, p<0.001, N = 109). The top models describing the effects on ITD included all agricultural land use types at the 1 km scale and at the 750 m scale (Table 1). Increasing cover of agriculture at both scales had a negative effect on bee size, measured by ITD (1 km: F(1,21) = 11.99 P = 0.002, Fig 1, 750 m: F(1,21) = 9.94 P = 0.004). For every 10% increase in the percent agriculture at 1 km, ITD decreases by 0.047 mm. Although a similar effect of agriculture was found on head capsule width (F(1,21) = 4.96 P = 0.036); the best model included only the percentage of pasture cover (F(1,21) = 4.99 P = 0.036, Table 1, Fig 2).
Table 1. Model selection statistics for models describing inter-tegular distance and head capsule width as a function of percent agricultural land cover (cropland including pastures), cropland only, pastures only, natural and semi-natural cover (forest, fallows and open land), and percent cover of insect pollinated blooming crops at 750m and 1000m radii from the collection sites.
The overall best model and competing models (AICc ≤ 2) are bolded. Asterisks (*) indicate models with significant (p <0.05) terms.
| Response | AICc | ΔAICc | Scale | Metric | Coeff | Weight |
|---|---|---|---|---|---|---|
| Inter-tegular Distance | 11.83 | 0.00 | 1000m | All Agriculture | -0.0047* | 0.464 |
| 13.72 | 1.89 | 750m | All Agriculture | -0.0040* | 0.181 | |
| 14.28 | 2.45 | 1000m | Natural | 0.0061* | 0.136 | |
| 14.37 | 2.54 | 1000m | Cropland only | -0.0051* | 0.180 | |
| 15.58 | 3.75 | 1000m | Pastures | -0.0074* | 0.071 | |
| 18.00 | 6.17 | 1000m | Blooming Crops | -0.0039 | 0.010 | |
| Head Capsule Width | 26.09 | 0.00 | 1000m | Pastures | -0.0091* | 0.505 |
| 28.40 | 2.31 | 1000m | All Agriculture | -0.0039* | 0.159 | |
| 29.02 | 2.93 | 750m | All Agriculture | 0.0034(*) | 0.117 | |
| 29.59 | 3.50 | 1000m | Natural | -0.0041 | 0.088 | |
| 30.07 | 3.98 | 1000m | Cropland only | -0.0033 | 0.069 | |
| 30.30 | 4.21 | 1000m | Blooming Crops | -0.0030 | 0.062 |
Fig 1. Inter-tegular distances of adult female A. nasonii in relation to the percentage of agricultural land uses within a 1 km radius from the collection sites.
Fig 2. Head capsule widths of adult female A. nasonii in relation to the percentage of pasture land within a 1 km radius from the collection sites.
We found no effect of either percent agriculture or pastures at the 1-km scale on the number of A. nasonii individuals collected per site within a given year (AG: F(1,20) = 0.281 P = 0.60; PAST: F(1,20) = 1.26 P = 0.27).
Pollen load
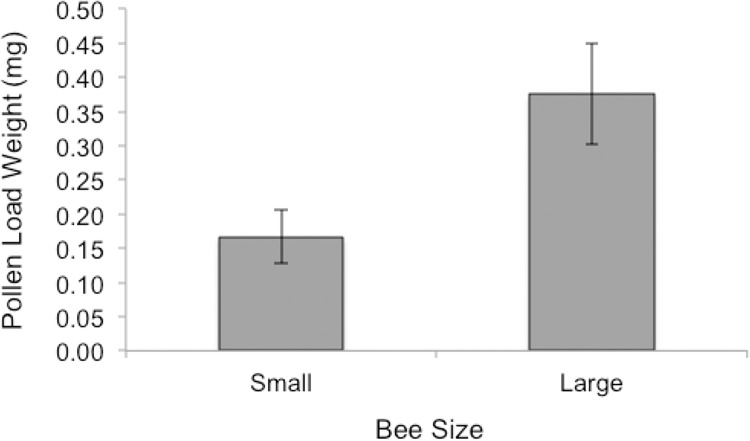
Bee size was found to significantly impact the weight of pollen loads with large bees carrying heavier pollen loads than smaller bees (t = 2.4942, df = 17.193, p-value = 0.02309). Bees in the large category were 20% larger (mean = 1.788 +/- 0.0075 SE), on average, than bees in the small category (mean = 1.538 +/- 0.031 SE), and they carried 40% more pollen on average (Fig 3).
Fig 3. Average weight of pollen load (+/- SE) of small and large female A. nasonii collected while foraging on standardized strawberry plots.
Discussion
Understanding how current land use practices affect pollinator fitness is a crucial step towards proposing sustainable management practices that take into account pollinator health. Using a natural population of the wild bee A. nasonii, we show here that landscape scale variation in agricultural intensification was inversely related to the average size but not the number of field collected A. nasonii adults.
One explanation for the observed reduction in size could be an increased exposure to pesticide residues in highly agricultural landscapes. However, research in this area has been scarce. Morandin and Winston [46] reported no effect of exposure to multiple pesticides at field realistic doses on worker weight in two Bombus species. In this study and others however, insecticide exposure did result in a reduction in foraging efficiency. Bombus terrestris workers exposed to field realistic doses of imidicloprid collected 30% less pollen on average than control workers [12]. This reduction in foraging efficiency could lead to a reduction in the size of individuals in subsequent generations similar to the results observed in our study.
Resource quality is also likely to be impacted by landscape simplification, as agricultural intensification is associated with a change in plant communities and a shift toward less preferred plant species [14, 11, 47]. Both pollen nutrient content [25, 48] and chemical defenses [49, 50] are known to impact bee larval growth.
Landscape simplification may also limit the allocation of resources to offspring through a reduction in the overall quantity and distribution of available resources. The solitary bee, Megachile rotundata was found to reduce the size of female offspring and the total number of offspring produced when presented with experimentally reduced floral resources [24]. This effect may not simply be a consequence of reduced resources but may be an adaptive response to resource variability. Smaller offspring require fewer resources to produce, thus in a landscape with few floral resources small body size may lead to higher fitness. Indeed, multivoltine Megachile apicalis adjust female offspring body size in order to maximize performance under seasonally variable resource conditions [27].
Variation in the distribution of resources and habitat isolation associated with landscape simplification may increase foraging distances, which directly impacts the allocation of resources to offspring by reducing total quantity provisioned per unit time. Osmia cornifrons nesting in agricultural landscapes in the Central Valley of California preferred to collect pollen from native plant sources and were forced to forage further from nesting sites in areas isolated from natural habitats resulting in a decrease in offspring production and survival [14]. Increased foraging distance also indirectly increases the risk of predation [51] and nest parasitism [16, 17, 18]. Together these factors are likely to constrain the quantity or quality of provisions allocated by female A. nasonii and explain the observed reduction in size in simplified landscapes. While average bee size was reduced in highly agricultural landscapes, the total number of individuals collected remained constant, suggesting that females may be constrained in adjusting allocation of resources between offspring size and offspring number. It is possible however; that with decreasing cover of semi-natural habitat at the landscape scale, flowers in the local patch become more attractive to pollinators [52] thus leading to an overestimation of A. nasonii numbers in simplified landscapes. Recently, floral resource plantings incorporated in agricultural landscapes have been shown to increase the abundance of bees in adjacent habitats [53, 54]. The results of these studies highlight the importance of floral resources and suggest that increasing abundance and diversity of floral resources are likely to ease the constraints on maternal resource allocation to offspring; although, this prediction has not yet been explicitly tested.
In our study, large A. nasonii females carried significantly larger (40%) pollen loads than small females. These large females should therefore be capable of provisioning larger or a greater number of brood cells per unit time in landscapes with abundant resources [55, 56]. Because smaller bees require fewer resources to produce, small females may still provision equivalent number of smaller offspring in landscapes with limited floral resources. Large females should complete individual brood cells with fewer foraging trips and therefore should experience a reduced probability of nest parasitism [16, 17, 18] or desiccation of the incomplete provision mass, both of which are known to increase the overwintering mortality of brood [33]. However, producing smaller provision masses may also decrease time to completion and reduce risks associated with parasitism and desiccation. Similarly, though large females may be more likely to usurp the nests of smaller females [57, 55] when nest sites are limited, small females may have broader range of nest availability than larger females [27], especially in cavity nesting species.
The advantage of lower resource requirements may outweigh the costs associated with smaller body size in landscapes with limited resource availability. Although large females are likely to forage farther [58] and earlier in the day [59] than small females, large bodied bee species are declining at a greater rate that smaller species [60, 47], possibly due to greater pollen requirements [61]. Although the approach take in this study allows for the examination of multiple factors that vary across broad spatial scales, it is unclear whether the observed response of bee size to land use intensification represents a negative impact of reduced resources and/or increased pesticide exposure or an adaptive response. Future research efforts should focus on evaluating the performance of large and small A. nasonii females under varying resource conditions and on examining number of brood cells and quality of pollen masses in the nests of A. nasonii or similar wild bee species across a landscape gradient.
Although the number of individuals collected was similar across landscapes in our study, the reduction in average bee size may have cascading consequences for subsequent generations. At the population and community level, this trend may explain the pattern of reduced abundance and species richness of bees in highly agricultural landscapes [7, 60, 39]. These results contribute to our understanding of the causes of bee decline and provide insights into management strategies to improve pollinator health, such as increasing floral resources in simplified, highly agricultural landscapes.
Supporting Information
Upper value is the correlation coefficient. Lower value is p-value.
(DOCX)
Acknowledgments
We would like to thank Sasha Hernandez for her invaluable assistance in the field and lab. We would also like to thank the farmers who allowed us to work in their fields.
Data Availability
Data are available on Dryad using the following DOI: 10.5061/dryad.kr577.
Funding Statement
Northeast Sustainable Agriculture Research and Extension Graduate Student Grant to HC, GNE12-03 nesare.org. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Ollerton J, Winfree R, Tarrant S. How many flowering plants are pollinated by animals? Oikos. 2011; 120(3), 321–326. [Google Scholar]
- 2.Klein AM, Vaissiere BE, Cane JH, Steffan-Dewenter I, Cunningham SA, Kremen C, et al. Importance of pollinators in changing landscapes for world crops. Proc R Soc Lond [Biol]. 2007; 274(1608), 303–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cameron SA, Lozier JD, Strange JP, Koch JB, Cordes N, Solter LF, et al. Patterns of widespread decline in North American bumble bees. PNAS. 2011; 108(2), 662–667. 10.1073/pnas.1014743108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burkle LA, Marlin JC, Knight TM. Plant-pollinator interactions over 120 years: loss of species, co-occurrence, and function. Science. 2013; 339(6127), 1611–1615. 10.1126/science.1232728 [DOI] [PubMed] [Google Scholar]
- 5.Goulson D, Nicholls E, Botías C, Rotheray EL. Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science. 2015; 347(6229), 1255957 10.1126/science.1255957 [DOI] [PubMed] [Google Scholar]
- 6.Smith HG, Dänhardt J, Lindström Å, Rundlöf M. Consequences of organic farming and landscape heterogeneity for species richness and abundance of farmland birds. Oecol. 2010; 162(4), 1071–1079. [DOI] [PubMed] [Google Scholar]
- 7.Batáry P, Báldi A, Kleijn D, Tscharntke T. Landscape-moderated biodiversity effects of agri-environmental management: a meta-analysis. Proc R Soc Lond [Biol]. 2011; 278(1713), 1894–1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flohre A, Fischer C, Aavik T, Bengtsson J, Berendse F, Bommarco R, et al. Agricultural intensification and biodiversity partitioning in European landscapes comparing plants, carabids, and birds. Ecol Appl. 2011; 21(5), 1772–1781. [DOI] [PubMed] [Google Scholar]
- 9.Tscharntke T, Klein AM, Kruess A, Steffan‐Dewenter I, Thies C. Landscape perspectives on agricultural intensification and biodiversity–ecosystem service management. Ecol Lett. 2005; 8(8), 857–874. [Google Scholar]
- 10.Jha S, Kremen C. Resource diversity and landscape-level homogeneity drive native bee foraging. PNAS. 2013; 110(2), 555–558. 10.1073/pnas.1208682110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Palladini JD, Maron JL. Reproduction and survival of a solitary bee along native and exotic floral resource gradients. Oecol. 2014; 176(3), 789–798. [DOI] [PubMed] [Google Scholar]
- 12.Feltham H, Park K, Goulson D. Field realistic doses of pesticide imidacloprid reduce bumblebee pollen foraging efficiency. Ecotoxicology. 2014; 23(3), 317–323. 10.1007/s10646-014-1189-7 [DOI] [PubMed] [Google Scholar]
- 13.Meehan TD, Werling BP, Landis DA, Gratton C. Agricultural landscape simplification and insecticide use in the Midwestern United States. PNAS. 2011; 108(28), 11500–11505. 10.1073/pnas.1100751108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Williams NM, Kremen C. Resource distributions among habitats determine solitary bee offspring production in a mosaic landscape. Ecol Appl. 2007; 17(3), 910–921. [DOI] [PubMed] [Google Scholar]
- 15.Peterson JH, Roitberg BD. Impacts of flight distance on sex ratio and resource allocation to offspring in the leafcutter bee, Megachile rotundata. Behav Ecol Sociobiol. 2006; 59(5), 589–596. [Google Scholar]
- 16.Visscher PK, Danforth BN. Biology of Calliopsis pugionis (Hymenoptera: Andrenidae): nesting, foraging, and investment sex ratio. Ann Entomol Soc Am. 1993; 86(6), 822–832. [Google Scholar]
- 17.Goodell K. Food availability affects Osmia pumila (Hymenoptera: Megachilidae) foraging, reproduction, and brood parasitism. Oecol. 2003; 134(4), 518–527. [DOI] [PubMed] [Google Scholar]
- 18.Seidelmann K. Open-cell parasitism shapes maternal investment patterns in the Red Mason bee Osmia rufa. Behav Ecol. 2006; 17(5), 839–848. [Google Scholar]
- 19.Larsen AE. Agricultural landscape simplification does not consistently drive insecticide use. PNAS, 2013; 110(38), 15330–15335. 10.1073/pnas.1301900110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fisher RA. The genetical theory of natural selection: a complete variorum edition Oxford University Press, 1930. [Google Scholar]
- 21.Trivers RL, Willard DE. Natural selection of parental ability to vary the sex ratio of offspring. Science. 1973; 179(4068), 90–92. [DOI] [PubMed] [Google Scholar]
- 22.Smith CC, Fretwell SD. The optimal balance between size and number of offspring. Amer Nat. 1974; 499–506. [Google Scholar]
- 23.Kim JY. Influence of resource level on maternal investment in a leaf-cutter bee (Hymenoptera: Megachilidae). Behav Ecol. 1999; 10(5), 552–556. [Google Scholar]
- 24.Peterson JH, Roitberg BD. Impact of resource levels on sex ratio and resource allocation in the solitary bee, Megachile rotundata. Environ Entomol. 2006b; 35(5), 1404–1410. [Google Scholar]
- 25.Roulston TH, Cane JH. The effect of pollen protein concentration on body size in the sweat bee Lasioglossum zephyrum (Hymenoptera: Apiformes). Evol Ecol. 2002; 16(1), 49–65. [Google Scholar]
- 26.Rosenheim JA, Nonacs P, Mangel M. Sex ratios and multifaceted parental investment. Amer Nat. 1996; 501–535. [Google Scholar]
- 27.Kim JY, Thorp RW. Maternal investment and size-number trade-off in a bee, Megachile apicalis, in seasonal environments. Oecol. 2001; 126(3), 451–456. [DOI] [PubMed] [Google Scholar]
- 28.Klostermeyer EC, Mech SJ Jr, Rasmussen WB. Sex and weight of Megachile rotundata (Hymenoptera: Megachilidae) progeny associated with provision weights. J Kans Entomol Soc. 1973; 536–548. [Google Scholar]
- 29.Freeman BE. Parental investment and its ecological consequences in the solitary wasp Sceliphron assimile (Dahlbom)(Sphecidae). Behav Ecol Sociobiol. 1981; 9(4), 261–268. [Google Scholar]
- 30.Johnson MD. The relationship of provision weight to adult weight and sex ratio in the solitary bee, Ceratina calcarata. Ecol Entomol. 1988; 13(2), 165–170. [Google Scholar]
- 31.Bosch J, Vicens N. Body size as an estimator of production costs in a solitary bee. Ecol Entomol. 2002; 27(2), 129–137. [Google Scholar]
- 32.Tepedino VJ, Thompson R, Torchio PF. Heritability for size in the megachilid bee Osmia lignaria propinqua Cresson. Apidologie. 1984; 15(1), 83–87. [Google Scholar]
- 33.Bosch J. Production of undersized offspring in a solitary bee. Anim Behav. 2008; 75(3), 809–816. [Google Scholar]
- 34.Richards MH, Packer L. The socioecology of body size variation in the primitively eusocial sweat bee, Halictus ligatus (Hymenoptera: Halictidae). Oikos. 1996; 68–76. [Google Scholar]
- 35.Radmacher S, Strohm E. Factors affecting offspring body size in the solitary bee Osmia bicornis (Hymenoptera, Megachilidae). Apidologie. 2010; 41(2), 169–177. [Google Scholar]
- 36.Larkin LL, Neff JL, Simpson BB. The evolution of a pollen diet: host choice and diet breadth of Andrena bees (Hymenoptera: Andrenidae). Apidologie. 2008; 39(1), 133–145. [Google Scholar]
- 37.Park MG, Orr MC, Danforth BN, Hall C. The role of native bees in apple pollination. NY Fruit Quart. 2010; 18, 21–25. [Google Scholar]
- 38.Tuell JK, Ascher JS, Isaacs R. Wild bees (Hymenoptera: Apoidea: Anthophila) of the Michigan highbush blueberry agroecosystem. Ann Entomol Soc Am. 2009; 102(2), 275–287. [Google Scholar]
- 39.Connelly H, Poveda K, Loeb G. Landscape simplification decreases wild bee pollination services to strawberry. Agric Ecosyst Environ. 2015; 211, 51–56. [Google Scholar]
- 40.Neff JL, Simpson BB. Nesting and foraging behavior of Andrena (Callandrena) rudbeckiae Robertson (Hymenoptera: Apoidea: Andrenidae) in Texas. J Kans Entomol Soc. 1997; 100–113. [Google Scholar]
- 41.USDA, National Agricultural Statistics Service. 2014 New York cropland data layer (2014 ed.). USDA, NASS Marketing and Information Services Office, Washington, D.C. 2014. [Google Scholar]
- 42.Williams NM, Crone EE, Roulston TH, Minckley RL, Packer L, Potts SG. Ecological and life-history traits predict bee species responses to environmental disturbances. Biol Conserv. 2010; 143(10), 2280–2291. [Google Scholar]
- 43.Guedot C, Bosch J, Kemp WP. Relationship between body size and homing ability in the genus Osmia (Hymenoptera; Megachilidae). Ecol Entomol. 2009; 34(1), 158–161. [Google Scholar]
- 44.Pinheiro J, Bates D, Debroy S, Sarkar D. R Core Team. Nlme: Linear and nonlinear mixed effects models. (R package version 3. 1–117 ed.), 2014.
- 45.R Core Team. R: A language and environment for statistical computing (3.1.0 ed.). Vienna, Austria: R Foundation for Statistical Computing, 2014. [Google Scholar]
- 46.Morandin LA, Winston ML. Effects of novel pesticides on bumble bee (Hymenoptera: Apidae) colony health and foraging ability. Environ Entomol. 2003; 32(3), 555–563. [Google Scholar]
- 47.Scheper J, Reemer M, van Kats R, Ozinga WA, van der Linden GT, Schaminée JH, et al. Museum specimens reveal loss of pollen host plants as key factor driving wild bee decline in The Netherlands. PNAS. 2014; 111(49), 17552–17557. 10.1073/pnas.1412973111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vanderplanck M, Moerman R, Rasmont P, Lognay G, Wathelet B, Wattiez R, et al. How does pollen chemistry impact development and feeding behaviour of polylectic bees. PLOS ONE. 2014; 9(1), e86209 10.1371/journal.pone.0086209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sedivy C, Müller A, Dorn S. Closely related pollen generalist bees differ in their ability to develop on the same pollen diet: evidence for physiological adaptations to digest pollen. Funct Ecol. 2011; 25(3), 718–725. [Google Scholar]
- 50.Arnold SE, Idrovo MEP, Arias LJL, Belmain SR, Stevenson PC. Herbivore defence compounds occur in pollen and reduce bumblebee colony fitness. J Chem Ecol. 2014; 40(8), 878–881. 10.1007/s10886-014-0467-4 [DOI] [PubMed] [Google Scholar]
- 51.da Silva-Matos EV, Garófalo CA. Worker life tables, survivorship, and longevity in colonies of Bombus (Fervidobombus) atratus (Hymenoptera: Apidae). Rev Biol Trop. 2000; 48(2–3), 657–664. [PubMed] [Google Scholar]
- 52.Kleijn D, Van Langevelde F. Interacting effects of landscape context and habitat quality on flower visiting insects in agricultural landscapes. Basic Appl Ecol, 2006; 7(3), 201–214. [Google Scholar]
- 53.Morandin LA, Kremen C. Hedgerow restoration promotes pollinator populations and exports native bees to adjacent fields. Ecol Appl. 2013; 23(4), 829–839. [DOI] [PubMed] [Google Scholar]
- 54.Blaauw BR, Isaacs R. Flower plantings increase wild bee abundance and the pollination services provided to a pollination‐dependent crop. J Appl Eco. 2014; 51(4), 890–898. [Google Scholar]
- 55.Bosch J, Vicens N. Relationship between body size, provisioning rate, longevity and reproductive success in females of the solitary bee Osmia cornuta. Behav Ecol Sociobiol. 2006; 60(1), 26–33. [Google Scholar]
- 56.Neff JL. Components of nest provisioning behavior in solitary bees (Hymenoptera: Apoidea). Apidologie. 2008; 39(1), 30–45. [Google Scholar]
- 57.Kim JY. Female size and fitness in the leaf‐cutter bee Megachile apicalis. Ecol Entomol. 1997; 22(3), 275–282. [Google Scholar]
- 58.Kuhn-Neto B, Contrera FA, Castro MS, Nieh JC. Long distance foraging and recruitment by a stingless bee, Melipona mandacaia. Apidologie. 2009; 40(4), 472–480. [Google Scholar]
- 59.Stone GN. Endothermy in the solitary bee Anthophora plumipes: independent measures of thermoregulatory ability, costs of warm-up and the role of body size. J Exp Biol. 1993; 174(1), 299–320. [Google Scholar]
- 60.Bartomeus I, Ascher JS, Gibbs J, Danforth BN, Wagner DL, Hedtke SM, Winfree R. Historical changes in northeastern US bee pollinators related to shared ecological traits. PNAS, 2013; 110(12), 4656–4660. 10.1073/pnas.1218503110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Müller A, Diener S, Schnyder S, Stutz K, Sedivy C, Dorn S. Quantitative pollen requirements of solitary bees: Implications for bee conservation and the evolution of bee–flower relationships. Biol Conserv. 2006; 130(4), 604–615. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Upper value is the correlation coefficient. Lower value is p-value.
(DOCX)
Data Availability Statement
Data are available on Dryad using the following DOI: 10.5061/dryad.kr577.