Abstract
Better understanding of biases related to selective prescribing of, and adherence to, preventive treatments has led to improvements in the design and analysis of pharmacoepidemiologic studies. One influential development has been the “active comparator, new user” study design, which seeks to emulate the design of a head-to-head randomized controlled trial. In this review, we first discuss biases that may affect pharmacoepidemiologic studies and describe their direction and magnitude in a variety of settings. We then present the historical foundations of the active comparator, new user study design and explain how this design conceptually mitigates biases leading to a paradigm shift in pharmacoepidemiology. We offer practical guidance on the implementation of the study design using administrative databases. Finally, we provide an empirical example in which the active comparator, new user study design addresses biases that have previously impeded pharmacoepidemiologic studies.
Keywords: pharmacoepidemiology, confounding, selection bias, study design
Introduction
Over the past 40 years pharmacoepidemiologists have made substantial contributions to public health by generating evidence regarding the use, benefit and harm of drugs, biologics, vaccines and medical devices in the population. During this time there have been rapid methodological advances within the field. In this paper, we review biases that can affect pharmacoepidemiologic research and one of the most influential methodological developments used to address these biases: the active comparator, new user (ACNU) study design. We review the study design's historical foundations, provide practical guidance for its implementation using administrative databases and examine the study design's benefits and limitations in practice using a contemporary example. For brevity throughout the remainder of the review, we will refer to any drug, biologic, vaccine, medical device, or any other medical intervention simply as the “treatment.”
Potential for bias in pharmacoepidemiology
Confounding
In routine clinical practice, treatments are administered to patients for a specific reason (i.e., the medical indication): to prevent an adverse health outcome from occurring or to treat an adverse health outcome that has already occurred (and to prevent future occurrences). The indication for treatment leads to selective prescribing of treatments, and in pharmacoepidemiologic studies, confounding by indication may arise if the indication for the treatment is also related to prognosis (the risk of the outcome).(1, 2) Confounding by indication can be illustrated using a hypothetical study seeking to estimate the effect of beta-agonists on asthma-related mortality among asthma patients. Asthma patients with severe disease are more likely than patients with less severe disease to receive beta-agonists and to die from their asthma. Such confounding would tend to make beta-agonists appear as though they were associated with asthma mortality.
In general, confounding by indication is likely to be of greatest concern in studies that compare initiators of treatments to non-initiators. Schneeweiss and colleagues provide support for this concern(3) in a study evaluating the association between statin use and 1-year mortality among adults age 65+ years. Through incremental restriction of the study cohort, the authors demonstrate a large shift in the overall estimate when new statin users were compared with new users of an unrelated preventive treatment (anti-glaucoma medication) instead of non-users, indicating a large reduction in bias.
Another type of confounding bias common in pharmacoepidemiologic studies is the healthy initiator bias.(4-6) This bias can arise through two distinct pathways.(7, 8) The first involves the selective initiation of preventive treatments among healthy and health-conscious patients, who through the effects of their healthy lifestyle, are also at decreased risk of a number of adverse health outcomes. The second involves the selective channeling of treatments away from frail individuals, who are at an increased risk of adverse outcomes. Under both scenarios, the healthy initiator bias will lead to spurious associations, where the beneficial effect of a given drug will be exaggerated.
The healthy initiator bias has been documented in studies of influenza vaccine effectiveness in older adults and other populations in poor health.(9, 10) Older adults and particularly those with severe comorbid illness and decreased physiologic reserves are unlikely to receive influenza vaccination, but are at an increased risk for death. This type of channeling has led a number of non-experimental studies to report implausibly strong preventive effects of influenza vaccination on all-cause mortality in the range of a 50% relative risk reduction.(11-13)
Selection bias
One common form of selection bias that occurs in pharmacoepidemiologic studies is the healthy adherer bias,(6, 14-16) which extends the healthy initiator and frailty bias to patients who adhere to treatment. Individuals who adhere to preventive treatments over prolonged periods are: 1) more likely to adhere to other healthy behaviors and preventative care and 2) less likely to have experienced changes in frailty (or underlying health status) compared with their non-adherent counterparts. One of the most striking examples of the healthy adherer bias was demonstrated in a meta-analysis evaluating the effect of adherence to placebo in randomized controlled trials (RCTs).(17) This study reported that patients who adhered to placebo had substantially reduced mortality compared with patients who were non-adherent to placebo. As placebo could not plausibly have any effect on mortality, the observed reduction in mortality can be solely attributable to selection bias.
The impact of: 1) confounding by indication and 2) the healthy user bias (the combination of the healthy initiator and healthy adherer biases) work in opposite directions. Confounding by indication distorts drug-outcome associations so that treatment looks “bad” or “harmful”, while the healthy user bias distorts drug-outcome associations so that treatment looks “good” or “beneficial.” The relative importance of these biases depends upon the specific drug-outcome association and population of interest.
A new standard: The active comparator, new user (ACNU) design
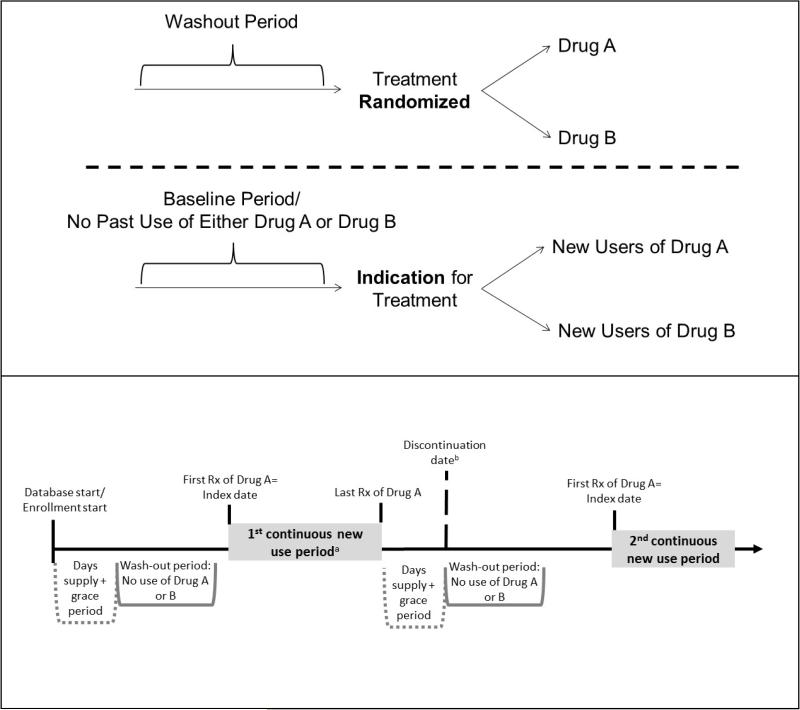
The ACNU design has been one of the most influential methodological advances in pharmacoepidemiology. This study design was developed to avoid many of the biases mentioned above and is regarded as the standard for pharmacoepidemiology, to be implemented whenever possible.(18) In brief, the ACNU design emulates the intervention part of a RCT. Cohorts of new drug users (i.e., individuals newly prescribed an index drug A and individuals newly prescribed a therapeutic alternative or comparator drug B) are assembled and followed over time for the health outcome(s) of interest (Figure 1, top panel). Notably, this design does not compare treatment users or initiators to non-users.
Figure 1. Active comparator, new user (ACNU) study design schematics.
The top panel illustrates how the ACNU study is designed to emulate a head-to-head randomized controlled trial. The bottom panel provides a detailed picture of how to identify periods of new use of drug A (the same process would apply to drug B) in a claims or other healthcare database. a One individual can have multiple new use periods. The individual can also be a new user of drug A and later a new user of drug B (or vice versa). Often, analyses will be restricted to the first period of new use. b The date of discontinuation (or switching or augmenting) may be used as a censoring date in as-treated analyses.
The active comparator (AC) component of the design helps to mitigate bias by restricting the study to individuals with an indication for treatment and without contraindications, including frailty, while the new user (NU) component mitigates bias by aligning individuals at a uniform point in time to start follow-up (i.e., treatment initiation) and ensuring the correct temporality between covariate and exposure assessment. The foundations of this study design have evolved over time and we first review a number of the contributions from the literature.
Historical foundations of the active comparator, new user (ACNU) study design
Contributions from occupational epidemiology
In occupational epidemiology, the healthy worker bias(19-21) arises through similar mechanisms as the healthy user bias, whereby healthy individuals are more likely to become employed (healthy hire bias)(22, 23) and remain in the workforce (healthy worker survivor bias)(19, 20) compared with less healthy individuals. Healthy hire bias is typically controlled by restricting the study to individuals who are employed, comparing disease occurrence between subgroups of workers within the study,(20, 24) lending early support to the use of active comparators in epidemiologic studies.
The healthy worker survivor bias is a form of selection bias when changes in underlying health status cause people to leave the workforce.(20, 25) Those who remain employed over prolonged periods of time are therefore an increasingly selected group of people who stayed healthy rather than a random sample of all those who entered the workforce. Similarly, patients persistent on preventive treatments for chronic conditions over prolonged periods of time will often be a healthy selection of all patients who initiated the treatment. This selection bias can be minimized by starting follow-up at hire (referred to as an inception cohort) or initiation of treatment (as in a new user design) and not conditioning on prolonged persistence (e.g., employment or treatment) or addressed analytically, assuming information on time-varying predictors of persistence is available.
Contributions from clinical epidemiology and pharmacoepidemiology
Active comparator
In a paper published in 1987, Mark Kramer and colleagues(26) highlighted a number of methodological issues affecting non-experimental studies of adverse drug effects. The authors succinctly recommended the use of an active comparator to avoid confounding in pharmacoepidemiologic studies, stating that “Even if it is clear to whom a particular drug poses a risk, and over what period of time, it is important to compare that risk with that of some other real therapeutic option for patients with the same clinical indication. Just as in a clinical trial investigating treatment efficacy, any epidemiologic study of treatment risks should compare two or more viable treatment alternatives” and “... measuring risks conditionally on clinical indication is ... essential to reduce confounding.”
In a 1989 paper,(27) Ray and Griffin suggest an alternative control selection strategy to mitigate confounding by indication when population controls “... may be unsatisfactory if the outcome is plausibly associated with factors related to receiving the drug that are difficult to measure.” The authors suggest that to “...compensate for such differences, one can select controls from persons receiving similar drugs. In a case-control study, this can be achieved by comparing the odds ratio for these other drugs to that of the drugs under consideration.”
Burt Gerstman and colleagues also supported the use of active comparators to control for confounding by indication in a 1990 paper(28) stating, “One means of approximating a match based on indication is to restrict the study base to individuals using drugs within a single therapeutic category. Rates of suspected adverse reactions may then be compared among alternative therapies presumably used to treat similar underlying conditions.” The authors continue that “Studies restricted to patients using drugs within a particular class may also aid in controlling for selection and detection biases.”
New user
One of the central tenants of a new user design is that the start of follow-up should be anchored to the initiation of a treatment. This concept of a “time zero”, the date of treatment initiation, was first raised by Alvin Feinstein in 1971(29) in writings on “chronology bias”, a general discussion of biases impacting studies of disease prognosis and medical interventions. Feinstein advocates for the use of “inception cohorts” because the use of survivor cohorts “create major distortions in cohort statistics unless the investigator ascertains that the [actual] inception cohorts would have been similar in their short-term (and intermediate) survival rates.”
Kramer and colleagues further the rationale for a new user approach,(26) arguing that, “The risk posed by a drug for a particular event is not generally the same in the sixth month of chronic therapy as in the first or second week. Using the total time exposed as the denominator in estimating risk is therefore incorrect, particularly if many patients are on chronic therapy... The two approaches are equivalent only if the risk remains constant as a function of time, i.e. the timing distribution is uniform and the expected time per course of therapy is known. The first of these criteria is almost never true, and the second is clearly untrue for the drugs under study ...”
In 1989, Harry Guess expanded on the issue of time varying hazards for drug side effects after drug initiation.(30) Guess states that “The possibility of temporally non-constant hazard functions should be considered in the study design. This requires that drug exposure time be measured not only in relation to onset of the study disease but also in relation to start of therapy with the study drug [italics by author].” Guess continues that “If the duration-specific incidence ratios differ widely, the odds ratio will depend not only on the drug but also on its usage pattern in the study population.”
In 1994, Moride and Abenhaim(31) highlight the concept of depletion of susceptibles (i.e., selection bias, to explain time-varying hazards, also mentioned by Guess(30)). The authors concluded that their counterintuitive results, where the risk of upper gastrointestinal bleeding using a non-user comparator was increased for individuals who were new users of non-steroidal anti-inflammatory (NSAIDs), but decreased for long-term past users of NSAIDs, indicted “...either a lack of cumulative effect of the drugs, or more likely, a selection process by which patients who have used the drugs in the past and tolerated them well remain on the drugs while patients who are susceptible to gastropathy select themselves out of the population at risk. This process is analogous to the well-known “healthy worker effect”.... If not taken into account in comparative studies, it could introduce a selection bias.”
Throughout the 1990's, additional research underscored concerns regarding potential for bias due to time-varying hazards and the mixing of new and prevalent users with contributions by Gerstman et al,(28) Hartzema(32) and McMahon and colleagues.(33, 34) In the most cited paper (479 citations, according to Web of Science (August 19, 2015) on the topic published in 2003 in the American Journal of Epidemiology, Wayne Ray coined the phrase “new user design”.(35) In addition to reiterating the above pitfalls of time-varying hazards, Ray mentions for the first time a concern regarding the timing of covariate assessment where “...covariates for [prevalent] drug users at study entry often are plausibly affected by the [earlier] drug itself.” He then argues that the “new-user design eliminates these biases by restricting the analysis to persons under observation at the start of the current course of treatment.” Ray's paper focused on the application of the new user design in the cohort setting, and as a result has become the landmark study on the topic, leading to wide dissemination of the underlying message and substantial changes in best practices in pharmacoepidemiology.(18)
Practical guidance on implementation of the active comparator, new user (ACNU) design
When planning a study using the ACNU design, it is necessary to carefully consider: 1) the selection of an active comparator, 2) the definitions used to identify “new use” and 3) treatment changes over time. We review each of these considerations below and provide practical advice and an illustration to aid implementation for pharmacoepidemiologic research.
Selection of an active comparator
The purpose of selecting an active comparator is to mitigate confounding by indication and other unmeasured patient characteristics (e.g., healthy initiator, frailty). Therefore, the more “substitutable” one treatment is for another, the less potential unmeasured confounding. Clinical treatment guidelines are an obvious resource to consult when selecting an active comparator for an index treatment of interest, as they are used to guide practicing clinicians in treatment decision-making. Another option, particularly in the setting where multiple active comparators are available, is to evaluate the similarity of measured characteristics of patients initiating the treatment of interest and each of the potential active comparator treatments. For example, it would be possible to assess which comparator population most closely matches the distribution of the index treatment population with respect to age, sex, comorbidity and concomitant medication use. If none of the measured factors is a strong predictor of initiating the treatment of interest rather than the comparator, it is more likely that unmeasured factors would not affect treatment choice, and thus the assumption of no unmeasured confounding may be more plausible.
Identifying new users
New users of a treatment have initiated therapy after some defined period of non-use and are at the beginning of their treatment course. Prevalent users include both new users and current users of a treatment and are observed at a number of different time points in their treatment course (i.e., there is no uniform “time zero”). By including only new users in pharmacoepidemiologic studies and starting follow-up at treatment initiation, time-varying hazards can be described and evaluated and the temporality of covariate assessment is preserved.
Ideally, new users would be first-time users of a treatment. To assess this type of use, lifetime treatment use data would be necessary (e.g., Danish prescription databases). In most settings, however, defining new use requires consideration of a “washout period” (Figure 1, top panel). This period represents a fixed window prior to the first prescription (i.e., treatment initiation) where the individual has drug coverage, but does not have any use of either the index treatment or the active comparator treatment, a requirement for entry into the new user cohort. Washout windows of 6-12 months are common, although evaluation of longer periods has revealed misclassification of “true” new use with relatively short wash-out periods.(36)
Lack of lifetime treatment data when implementing an ACNU design results in the possibility for individuals to have multiple new use periods. This would occur when an individual initiates treatment, but subsequently discontinues (i.e., stops refilling the prescription) and re-initiates after the washout period. Because the exact date of treatment discontinuation is generally unknown, the discontinuation date is usually defined as the last prescription dispensing date + the days supply + a grace period. A person refilling a prescription of the same drug before the discontinuation date is classified as a continuous user. The grace period is necessary to allow for some leeway when refilling prescriptions, for patients forgetting to take some pills, or even splitting pills. It is frequently based on a proportion (e.g., 50%) of the typical days supply of the drug but can also be based on the empirical distribution of days between refills. Researchers interested in allowing for multiple new use periods should add the length of the days supply plus the grace period to the initial wash-out window in order to provide uniform washout windows for all new use periods (Figure 1, lower panel). They should also index the new use periods per patient so that the first one can easily be identified if an analysis restricted to the first new use period is desired (first new use period could be the first ever use period for some individuals in contrast to subsequent ones). Once new use is established, the index date is defined as the first prescription dispensing date after being free of both the index and comparator treatments during the washout period. In some settings, a second prescription within a given amount of time from the first prescription is required for cohort entry, in which case the index date would be the second prescription dispensing date.
By requiring the start of follow-up to occur at the point of treatment initiation, analysis of the changing hazards of events of interest is straightforward. For example, the rate of an adverse event can be evaluated among the index and active comparator cohorts in the first 30, 60 or 90 days following therapy initiation. If the hazard for these events is highest in the early periods and subsequently decreases in later periods, this may be an indication of the depletion of susceptibles, the phenomena described above where individuals who develop events are removed from the cohort early on, leaving a cohort of individuals who tolerate the treatment without any side effects.
Analysis of treatment changes over time
An additional advantage of the ACNU design is that researchers can avoid the potential for the healthy adherer bias by ignoring stopping of the initially prescribed treatment (non-adherence). If stopping is ignored in those who initiated drug A as well as drug B, then there is no selection bias induced. This “first treatment carried forward” approach is the equivalent of the “intent to treat” analysis in RCTs. While this approach avoids selection bias, another bias, exposure misclassification, is introduced and would tend to increase over time since treatment initiation. This analysis is usually seen as introducing bias towards the null and is often acceptable in conventional RCTs because it is conservative when assessing efficacy. When assessing adverse outcomes, however, as in many pharmacoepidemiologic studies, bias towards the null may mask potential harm, is therefore not conservative, and should be avoided. It is also worth mentioning that in RCTs where major crossover between treatments is not assumed, bias is very likely to be towards the null. With major crossover between treatments, it would be possible for bias to cause the treatment effect estimate to cross the null.
An alternative to the first treatment carried forward design is the “as-treated” analysis, the parallel to the “per-protocol” analysis in RCTs. This analytic approach in pharmacoepidemiologic studies can account for not only stopping treatments (described above in Figure 1, lower panel), but also switching of treatments (switching from drug A to drug B) and augmenting treatments (adding drug B to drug A, and vice versa). The distinction between switching and augmenting can only be made after a period of time, because it will depend on whether or not the individual refills the initial treatment after start of the alternative treatment. The period of time that should be used to assess a refill of the initial treatment should be based on e.g., the days supply of the previous prescription and the grace period.
Once these events (starting, stopping, switching, augmenting) have been identified for each patient, researchers must then hypothesize about three distinct periods to define time at risk periods: the induction period (i.e., time required for the treatment to biologically effect the outcome), latent period (i.e., time required between disease onset and clinical diagnosis) and potential carryover effects (i.e., time that the treatment remains active in the body) based on available information on the treatment and outcome of interest. In practice, carryover effects tend to be short, only accounting for a few days and therefore are generally ignored or assumed to be part of the latent period following discontinuation.
These concepts can be illustrated using two hypothetical examples. First, consider a study of the effect of an antibiotic on the adverse outcome of anaphylaxis. In this case, we assume that treatment has an immediate effect on the incidence of anaphylaxis (i.e., an induction period=0 days) and is instantly diagnosed by a healthcare provider (i.e., latent period=0 days), and as such, we would start evaluating time-at-risk at drug initiation. Furthermore, if we assume that this effect would immediately cease upon stopping the treatment (i.e., carryover period=0 days), then time-at-risk would stop at drug discontinuation. Note that we would likely miss any effect if we did not implement a new user design and did not enroll a considerable proportion of first-time users.
Second, consider a study of the effect of antidepressant medications on risk of cancer. We may hypothesize that antidepressants have a late-acting effect in the carcinogenesis process (e.g., relatively short induction period, say of 3 to 6 months) and would need to add a latent period for it to be clinically detected (e.g., an additional 3 to 6 months). It is likely that the carryover effect of antidepressants is relative short (only a few days). As such we may consider varying the combined induction and latent period between 6 months to one year. We would then start follow-up 6 months after drug initiation (and vary this up to 12 months, the combined induction and latent periods) and stop follow-up 3 months after drug use has ended (and vary this up to 6 months, the latent period only) (i.e., assigning person-time and events until 3 months after the actual stopping date). Sensitivity analysis varying the length of the assumed induction, latent and carry-over periods, ideally in both directions (longer and shorter), can help assess robustness of estimated effects and are recommended.
Finally, the ACNU design may aid in the evaluation of the cumulative effects of drug exposures on health outcomes. Because all individuals in the cohort are followed from drug initiation, the event rate in those who continue on therapy on drug A (e.g., for 2-years) can be compared to individuals who continue therapy on drug B (for 2-years). Consideration of the mechanisms and patterns of treatment stopping, switching and augmenting is important for the validity of this approach, as informative censoring, where individuals who are censored from the analysis are at increased (or decreased) risk of the outcome of interest, may still induce selection bias. Analytic approaches that account for informative censoring may be applied, if detailed information is available on the predictors of non-adherence. Many of these methods have been developed and gained popularity in both the occupational epidemiology literature(25) and the HIV literature,(37, 38) perhaps based on the ability to predict reasons for employment cessation and changes in antiretroviral treatments, respectively.
Benefits of the active comparator, new user (ACNU) design: A contemporary example
To illustrate the benefits of the ACNU design, we will briefly review a study by Stürmer and colleagues evaluating the long-term effects of insulin glargine on cancer risk.(39) One of the major challenges in evaluating the effects of insulins on cancer risk in patients with type 2 diabetes is likely strong confounding by indication (e.g., diabetes not controlled by oral antidiabetics due to severity and diabetes duration) and obesity, which are impossible or difficult to measure in administrative data. An additional challenge is the need for a clear timeline in order to evaluate whether the duration of treatment may impact cancer risk.
Using clinical guidelines, the authors selected the second most prescribed long acting insulin, human NPH insulin, as the active comparator. The researchers defined a wash-out period of 19 months to identify new users of the study drugs, to represent a typical 30-day supply of insulin + a grace period of 6 months + a wash-out period of 12 months, using an approach similar to Figure 1. The relatively long wash-out period was derived from the empirical distribution of days between refills, indicating that any shorter period would have led to misclassify many apparently continuous users as having stopped treatment.
Potential confounders were assessed in the 12-months prior to the first prescription for insulin, which ensures the correct temporality of covariate assessment in relation to the drug initiation (i.e., prior to initiation). The primary analysis used an as-treated approach where follow-up began at the date of the second prescription and stopped after treatment discontinuation, date of treatment switch (i.e., filling a prescription for another long-acting insulin), death, end of enrollment, end of the study period or the date of a cancer event. Sensitivity analysis varied induction and latent periods (i.e., excluding incident cancer cases for up to 12 months after insulin initiation and allowing for diagnoses to be made up to 24 months or indefinitely after stopping treatment [first treatment carried forward]). Furthermore, the cumulative effects of insulin on cancer risk were assessed by comparing imitators who remained on treatment for 0-less than 6 months, 6 months to less than 12 months, 12 to less than 24 months and greater than 24 months. The authors found no indication for an increased risk for any of the specific cancers.
Overall, the measured baseline patient characteristics of initiators of insulin glargine vs. human NPH insulin were similar, with slight differences noted for age, sex, year of treatment initiation and concomitant medication use. After further adjustment approaches using propensity score weighting, these differences were reduced to a clinically insignificant level or eliminated entirely. To address concern about residual confounding by BMI, the authors conducted two validation studies in external populations where BMI information was available for initiators of insulin glargine and human NPH insulin. In both studies, the distribution of BMI was virtually identical in initiators of insulin glargine and in initiators of human NPH insulin.
The example provides evidence that the ACNU study design can enhance the validity of non-experimental studies through: 1) aligning individuals at a uniform point in time to start follow-up (i.e., initiation of a drug), 2) ensuring the correct temporality between covariate and exposure assessment and 3) mitigating measured and unmeasured confounding by design through the selection of an appropriate active comparator that led to the inclusion of patients that had the indication for treatment augmentation.
There are important limitations of this approach worthy of note. One of the primary limitations is that as-treated approach highlighted in this study limits the evaluation of the cumulative effect of treatment use beyond 2 years. However, this limitation is mainly a function of “real world” treatment dynamics, where patients do not use insulin glargine or human NPH insulin over extended periods of time due to stopping or switching of treatments. This may be important for interpretation of the study findings and whether concerns over the “longer-term” effects (e.g., 2+ years) of treatment exposure on cancer risk are warranted from a public health perspective. Furthermore, data on long-term use available when studying prevalent rather than incident users, would not answer a clinically relevant question (we cannot “assign” anyone to long-term use) and would suffer from the selection introduced by focusing on a very small fraction of all those that have ever been exposed to the drug. Another potential limitation of the as-treated approach is the concern about informative censoring. The first treatment carried forward analyses that eliminates selection bias, support the primary as treated analysis, however. Further work on evaluating the impacts of informative censoring in an ACNU design are indicated. Finally, we need to assume that the BMI balance observed in the external validation studies would be transportable to the main study. Given the arguments about synchronizing cohorts on the indication for treatment augmentation and the moderate differences in covariates observed between treatment cohorts, this seems a quite plausible assumption.
Conclusions
Advances in our understanding of how non-experimental study design and treatment dynamics impact the validity of the pharmacoepidemiologic studies have laid the foundation for the modern implementation of the ACNU study design. This design offers the theoretical advantage of mitigating confounding bias by indication, healthy user, and frailty at the design stage, prior to the application of analytic methods and as a result is considered the current standard in pharmacoepidemiologic research. The ACNU design can be easily implemented within typical databases used for pharmacoepidemiologic research (e.g., administrative claims databases). As evidence accrues with respect to the scenarios under which the theoretical benefits are most likely to be actualized, the ACNU design is expected to gain further popularity.
References
- 1.Miettinen OS. The need for randomization in the study of intended effects. Statistics in medicine. 1983;2(2):267–71. doi: 10.1002/sim.4780020222. Epub 1983/04/01. [DOI] [PubMed] [Google Scholar]
- 2.Csizmadi I, Collet J-P. Bias and Confounding in Pharmacoepidemiology. In: Strom BL, Kimmel SE, editors. Textbook of Pharmacoepidemiology. John Wiley & Sons, Ltd; West Sussex, England: 2006. doi: 10.1002/9781118707999.ch16. [Google Scholar]
- 3.Schneeweiss S, Patrick AR, Sturmer T, Brookhart MA, Avorn J, Maclure M, et al. Increasing levels of restriction in pharmacoepidemiologic database studies of elderly and comparison with randomized trial results. Medical care. 2007;45(10 Supl 2):S131–42. doi: 10.1097/MLR.0b013e318070c08e. Epub 2007/10/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Humphrey LL, Chan BK, Sox HC. Postmenopausal hormone replacement therapy and the primary prevention of cardiovascular disease. Annals of internal medicine. 2002;137(4):273–84. doi: 10.7326/0003-4819-137-4-200208200-00012. Epub 2002/08/21. [DOI] [PubMed] [Google Scholar]
- 5.Majumdar SR, McAlister FA, Eurich DT, Padwal RS, Marrie TJ. Statins and outcomes in patients admitted to hospital with community acquired pneumonia: population based prospective cohort study. BMJ. 2006;333(7576):999. doi: 10.1136/bmj.38992.565972.7C. Epub 2006/10/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ray WA, Daugherty JR, Griffin MR. Lipid-lowering agents and the risk of hip fracture in a Medicaid population. Injury prevention : journal of the International Society for Child and Adolescent Injury Prevention. 2002;8(4):276–9. doi: 10.1136/ip.8.4.276. Epub 2002/12/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brookhart MA, Sturmer T, Glynn RJ, Rassen J, Schneeweiss S. Confounding control in healthcare database research: challenges and potential approaches. Medical care. 2010;48(6 Suppl):S114–20. doi: 10.1097/MLR.0b013e3181dbebe3. Epub 2010/05/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sturmer T, Jonsson Funk M, Poole C, Brookhart MA. Nonexperimental comparative effectiveness research using linked healthcare databases. Epidemiology. 2011;22(3):298–301. doi: 10.1097/EDE.0b013e318212640c. Epub 2011/04/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jackson LA, Jackson ML, Nelson JC, Neuzil KM, Weiss NS. Evidence of bias in estimates of influenza vaccine effectiveness in seniors. International journal of epidemiology. 2006;35(2):337–44. doi: 10.1093/ije/dyi274. Epub 2005/12/22. [DOI] [PubMed] [Google Scholar]
- 10.Jackson LA, Nelson JC, Benson P, Neuzil KM, Reid RJ, Psaty BM, et al. Functional status is a confounder of the association of influenza vaccine and risk of all cause mortality in seniors. International journal of epidemiology. 2006;35(2):345–52. doi: 10.1093/ije/dyi275. Epub 2005/12/22. [DOI] [PubMed] [Google Scholar]
- 11.Hak E, Buskens E, van Essen GA, de Bakker DH, Grobbee DE, Tacken MA, et al. Clinical effectiveness of influenza vaccination in persons younger than 65 years with high-risk medical conditions: the PRISMA study. Archives of internal medicine. 2005;165(3):274–80. doi: 10.1001/archinte.165.3.274. Epub 2005/02/16. [DOI] [PubMed] [Google Scholar]
- 12.Nichol KL, Nordin JD, Nelson DB, Mullooly JP, Hak E. Effectiveness of influenza vaccine in the community-dwelling elderly. The New England journal of medicine. 2007;357(14):1373–81. doi: 10.1056/NEJMoa070844. Epub 2007/10/05. [DOI] [PubMed] [Google Scholar]
- 13.Nordin J, Mullooly J, Poblete S, Strikas R, Petrucci R, Wei F, et al. Influenza vaccine effectiveness in preventing hospitalizations and deaths in persons 65 years or older in Minnesota, New York, and Oregon: data from 3 health plans. The Journal of infectious diseases. 2001;184(6):665–70. doi: 10.1086/323085. Epub 2001/08/23. [DOI] [PubMed] [Google Scholar]
- 14.Brookhart MA, Patrick AR, Dormuth C, Avorn J, Shrank W, Cadarette SM, et al. Adherence to lipid-lowering therapy and the use of preventive health services: an investigation of the healthy user effect. American journal of epidemiology. 2007;166(3):348–54. doi: 10.1093/aje/kwm070. Epub 2007/05/17. [DOI] [PubMed] [Google Scholar]
- 15.Petitti DB. Coronary heart disease and estrogen replacement therapy. Can compliance bias explain the results of observational studies? Annals of epidemiology. 1994;4(2):115–8. doi: 10.1016/1047-2797(94)90056-6. Epub 1994/03/01. [DOI] [PubMed] [Google Scholar]
- 16.Rossouw JE. Debate: The potential role of estrogen in the prevention of heart disease in women after menopause. Current controlled trials in cardiovascular medicine. 2000;1(3):135–8. doi: 10.1186/cvm-1-3-135. Epub 2001/11/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simpson SH, Eurich DT, Majumdar SR, Padwal RS, Tsuyuki RT, Varney J, et al. A meta-analysis of the association between adherence to drug therapy and mortality. BMJ. 2006;333(7557):15. doi: 10.1136/bmj.38875.675486.55. Epub 2006/06/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson ES, Bartman BA, Briesacher BA, Fleming NS, Gerhard T, Kornegay CJ, Nourjah P, Sauer B, Schumock GT, Sedrakyan A, Stürmer T, West SL, Schneeweiss S, The Incident User Design in Comparative Effectiveness Research . Effective Health Care Program Research Report No. 32. (Prepared under Contract No. HHSA290200500161). AHRQ Publication No. 11(12)- EHC054-EF. Agency for Healthcare Research and Quality; Rockville, MD: May, 2012. http://effectivehealthcare.ahrq.gov/reports/final.cfm. [Google Scholar]
- 19.Checkoway H, Pearce N, Kriebel D. Monographs in Epidemiology and Biostatistics. Vol. 34. Oxford University Press; New York, NY: 1989. Research Methods in Occupational Epidemiology. [Google Scholar]
- 20.Arrighi HM, Hertz-Picciotto I. The evolving concept of the healthy worker survivor effect. Epidemiology. 1994;5(2):189–96. doi: 10.1097/00001648-199403000-00009. Epub 1994/03/01. [DOI] [PubMed] [Google Scholar]
- 21.McMichael AJ. Standardized mortality ratios and the “healthy worker effect”: Scratching beneath the surface. J Occup Med. 1976;18(3):165–8. doi: 10.1097/00043764-197603000-00009. [DOI] [PubMed] [Google Scholar]
- 22.Wilcosky T, Wing S. The healthy worker effect. Selection of workers and work forces. Scandinavian journal of work, environment & health. 1987;13(1):70–2. doi: 10.5271/sjweh.2078. Epub 1987/02/01. [DOI] [PubMed] [Google Scholar]
- 23.Flanders WD, Cardenas VM, Austin H. Confounding by time since hire in internal comparisons of cumulative exposure in occupational cohort studies. Epidemiology. 1993;4(4):336–41. doi: 10.1097/00001648-199307000-00009. Epub 1993/07/01. [DOI] [PubMed] [Google Scholar]
- 24.Choi BC. Definition, sources, magnitude, effect modifiers, and strategies of reduction of the healthy worker effect. Journal of occupational medicine : official publication of the Industrial Medical Association. 1992;34(10):979–88. Epub 1992/10/01. [PubMed] [Google Scholar]
- 25.Buckley JP, Keil AP, McGrath LJ, Edwards JK. Evolving methods for inference in the presence of healthy worker survivor bias. Epidemiology. 2015;26(2):204–12. doi: 10.1097/EDE.0000000000000217. Epub 2014/12/24. [DOI] [PubMed] [Google Scholar]
- 26.Kramer MS, Lane DA, Hutchinson TA. Analgesic use, blood dyscrasias, and case-control pharmacoepidemiology. A critique of the International Agranulocytosis and Aplastic Anemia Study. Journal of chronic diseases. 1987;40(12):1073–85. doi: 10.1016/0021-9681(87)90073-7. Epub 1987/01/01. [DOI] [PubMed] [Google Scholar]
- 27.Ray WA, Griffin MR. Use of Medicaid data for pharmacoepidemiology. American journal of epidemiology. 1989;129(4):837–49. doi: 10.1093/oxfordjournals.aje.a115198. Epub 1989/04/01. [DOI] [PubMed] [Google Scholar]
- 28.Gerstman BB, Lundin FE, Stadel BV, Faich GA. A method of pharmacoepidemiologic analysis that uses computerized Medicaid. Journal of clinical epidemiology. 1990;43(12):1387–93. doi: 10.1016/0895-4356(90)90106-y. Epub 1990/01/01. [DOI] [PubMed] [Google Scholar]
- 29.Feinstein AR. Clinical biostatistics. XI. Sources of ‘chronology bias’ in cohort statistics. Clinical pharmacology and therapeutics. 1971;12(5):864–79. doi: 10.1002/cpt1971125864. Epub 1971/09/01. [DOI] [PubMed] [Google Scholar]
- 30.Guess HA. Behavior of the exposure odds ratio in a case-control study when the hazard function is not constant over time. Journal of clinical epidemiology. 1989;42(12):1179–84. doi: 10.1016/0895-4356(89)90116-9. Epub 1989/01/01. [DOI] [PubMed] [Google Scholar]
- 31.Moride Y, Abenhaim L. Evidence of the depletion of susceptibles effect in non-experimental pharmacoepidemiologic research. Journal of clinical epidemiology. 1994;47(7):731–7. doi: 10.1016/0895-4356(94)90170-8. Epub 1994/07/01. [DOI] [PubMed] [Google Scholar]
- 32.Hartzema AG. Guide to interpreting and evaluating the pharmacoepidemiologic literature. The Annals of pharmacotherapy. 1992;26(1):96–8. doi: 10.1177/106002809202600117. Epub 1992/01/01. [DOI] [PubMed] [Google Scholar]
- 33.McMahon AD, Evans JM, McGilchrist MM, McDevitt DG, MacDonald TM. Drug exposure risk windows and unexposed comparator groups for cohort studies in pharmacoepidemiology. Pharmacoepidemiology and drug safety. 1998;7(4):275–80. doi: 10.1002/(SICI)1099-1557(199807/08)7:4<275::AID-PDS363>3.0.CO;2-N. Epub 2004/04/10. [DOI] [PubMed] [Google Scholar]
- 34.McMahon AD, MacDonald TM. Design issues for drug epidemiology. British journal of clinical pharmacology. 2000;50(5):419–25. doi: 10.1046/j.1365-2125.2000.00289.x. Epub 2000/11/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ray WA. Evaluating medication effects outside of clinical trials: new-user designs. American journal of epidemiology. 2003;158(9):915–20. doi: 10.1093/aje/kwg231. Epub 2003/10/31. [DOI] [PubMed] [Google Scholar]
- 36.Riis AH, Johansen MB, Jacobsen JB, Brookhart MA, Sturmer T, Stovring H. Short look-back periods in pharmacoepidemiologic studies of new users of antibiotics and asthma medications introduce severe misclassification. Pharmacoepidemiology and drug safety. 2015;24(5):478–85. doi: 10.1002/pds.3738. Epub 2015/01/21. [DOI] [PubMed] [Google Scholar]
- 37.Cole SR, Hernan MA. Constructing inverse probability weights for marginal structural models. American journal of epidemiology. 2008;168(6):656–64. doi: 10.1093/aje/kwn164. Epub 2008/08/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hernan MA, Brumback B, Robins JM. Marginal structural models to estimate the causal effect of zidovudine on the survival of HIV-positive men. Epidemiology. 2000;11(5):561–70. doi: 10.1097/00001648-200009000-00012. Epub 2000/08/24. [DOI] [PubMed] [Google Scholar]
- 39.Sturmer T, Marquis MA, Zhou H, Meigs JB, Lim S, Blonde L, et al. Cancer incidence among those initiating insulin therapy with glargine versus human NPH insulin. Diabetes care. 2013;36(11):3517–25. doi: 10.2337/dc13-0263. Epub 2013/07/24. [DOI] [PMC free article] [PubMed] [Google Scholar]