Abstract
HIV testing and counselling is the first crucial step for linkage to HIV treatment and prevention. However, despite high HIV burden in sub-Saharan Africa, testing coverage is low, particularly among young adults and men. Community-based HIV testing and counselling (testing outside of health facilities) has the potential to reduce coverage gaps, but the relative impact of different modalities is not well assessed. We conducted a systematic review of HIV testing and counselling modalities, characterizing facility and community (home, mobile, index, key populations, campaign, workplace and self-testing) approaches by population reached, HIV-positivity, CD4 count at diagnosis and linkage. Of 2,520 abstracts screened, 126 met eligibility criteria. Community HIV testing had high coverage and uptake and identified HIV-positive individuals at higher CD4 counts than facility testing. Mobile HIV testing reached the highest proportion of men of all modalities examined (50%, 95% CI = 47–54%) and home with self-testing reached the highest proportion of young adults (66%, 95% CI = 65–67%). Few studies evaluated HIV testing and counselling for key populations (commercial sex workers and men who have sex with men), but these interventions yielded high HIV positivity (38%, 95% CI = 19–62%) combined with the highest proportion of first-time testers (78%, 95% CI = 63–88%), indicating service gaps. Facilitated linkage (for example, counsellor follow-up to support linkage) achieved high linkage to care (95%, 95% CI = 87–98%) and ART initiation (75%, 95% CI = 68–82%). Expanding mobile HIV testing, self-testing and outreach to key populations with facilitated linkage can increase the proportion of men, young adults and high-risk individuals linked to HIV treatment and prevention.
Globally, there are around 2.3-million new HIV infections annually, 80% of which occur in sub-Saharan Africa1. Despite the high burden, only one-third of adults in sub-Saharan Africa have been tested for HIV in the past year and less than 50% of HIV-positive individuals know their status2,3. Knowledge of one’s serostatus is vital for accessing lifesaving anti-retroviral therapy (ART) and linking to HIV prevention. Conventional facility-based HIV testing and counselling (HTC) has not achieved high testing coverage in sub-Saharan Africa and will probably be insufficient to meet UNAIDS ambitious 90–90–90 targets — 90% of HIV-positive individuals knowing their status, 90% of HIV-positive individuals who are aware of their status on ART, and 90% of individuals on ART virally suppressed4,5. Barriers to facility testing include distance from clinic, long wait times, costs (transportation, lost wages and childcare), confidentiality concerns, low perceived risk and infrequent contact with the health-care system6. In addition, patients often present at facilities late in the course of their illness, increasing HIV morbidity, mortality and transmission7. Community-based HTC (conducted outside of a health facility) has the potential to overcome these barriers, achieve high coverage, and identify asymptomatic HIV-positive individuals at high CD4 counts8,9. In addition, community HTC may reach more men, young adults, and key populations than facility HTC. Community-based strategies also require minimal infrastructure allowing for easier scale up10–12.
Community HTC modalities include: home, mobile, workplace, index partner/family members (sexual partners or family members of HIV-positive persons) and as part of a campaign. Uptake and demographics of population reached can vary widely by modality9. A large number of studies on HTC have been conducted in sub-Saharan Africa and a previous systematic review was completed in 2012, but facility testing was not included and uptake in men and young adults was not assessed. In addition, several large-scale interventions have been published since 201211,13–15. Recently, the World Health Organization’s released guidelines that strongly recommend implementing community HTC16. As most countries have multiple and varying epidemics, UNAIDS recommends creating regional policies tailored to the macro-epidemic rather than nation-wide approaches.17 Local policymakers will need to determine the optimal combination of community HTC interventions to increase testing in the context of their country’s HIV epidemic.
To provide evidence for decision makers, we summarize the literature on community and facility-based HTC. We characterize each modality by population coverage, since high coverage is beneficial to both HIV-positive and -negative people. HTC can reduce risk behaviour in HIV-negative individuals, while providing a means to link them to primary prevention (including circumcision and pre-exposure prophylaxis (PrEP))18–21. We evaluate effectiveness in reaching men and young adults (both groups have a disproportionately high risk of HIV acquisition and poorer clinical outcomes once infected22–24) and targeted HTC for key populations (men who have sex with men (MSM), commercial sex workers (CSWs) and people who inject drugs (PWID)) — groups that generally have very high HIV prevalence and low access to health care25. We assess HIV positivity to characterize yield and examine CD4 count at diagnosis to identify modalities that have the potential to link infected persons to care earlier in their disease course. Estimates from our analysis can also be used as parameters in mathematical models to project the long-term impact of HTC interventions.
Methods
Inclusion criteria
We conducted a systematic literature review following Cochrane and PRISMA (preferred reporting items for systematic reviews and meta-analyses) guidelines26. Studies were eligible for inclusion if they reported data on at least one of the following outcomes: coverage (individuals who accepted HTC/eligible target population); uptake (individuals who accepted HTC/individuals offered HTC); proportion of young adults (age <25 or <30 years; proportion of men; proportion of first-time testers; HIV positivity (number positive/total tested); proportion with a CD4 count of 350 cells μL−1 or less; proportion linked to care (those who had visited a clinic, obtained a CD4 count or initiated ART); proportion retained in care (individuals retained/individuals who initiated ART); or cost per person tested. The target population was defined as eligible population in the catchment area, either enumerated by the study (often the case for home HTC) or estimated (often the case for mobile and campaign HTC). For facility HTC, the target population was defined as people visiting the clinic, and for index partner or family members it was defined as all sexual partners or cohabitating family members listed by index patient. With the exception of HTC targeted to key populations, we excluded HTC studies not related to general population screening, including case reports and studies limited to antenatal or paediatric settings, or to patients with specific diseases (for example, tuberculosis). Observational (cross-sectional and cohort) studies and randomized trials were eligible for inclusion. Studies were included in the analyses more than once if they had different arms or multiple study sites (for example, urban and rural settings or different countries). If more than one wave of a survey or intervention was completed, only the most recent was used.
Search strategy
Literature searches were conducted with the help of a librarian on July 22, 2014 and updated on June 10, 2015. Briefly, we searched PubMed, EMBASE, Cochrane Library, Global Health Database, African Index Medicus, and conference abstracts (CROI, R4P, IAS) using MeSH terms for PubMed and comparable terms for other databases. Search terms included “HIV Infections/diagnosis” AND “Africa South of the Sahara” AND (“mass screening” OR test OR tests OR testing OR screen* OR diagnosis OR “counseling”). Bibliographies of relevant papers were screened and authors were contacted for missing outcomes. Searches were limited to human studies published between 2000 and 2015. Full strategy is described in the Supplementary Information.
Definitions of HTC modalities
Community-based HTC was defined as that conducted outside of health facilities. Facility-based HTC modalities were conducted in health-care facilities (for example, clinics, hospitals, fixed stand-alone voluntary counselling and testing sites). Facility HTC was divided into two categories: voluntary counselling and testing (VCT), which is patient-initiated testing; and provider-initiated testing counselling (PITC), which is routine or opt-out HTC that is initiated by a provider. Community HTC modalities included home (offering HTC door-to-door to a catchment area), mobile (setting up a mobile van or container to provide HTC in a central area of a community), index partner or family member (offering HTC to individuals who may have been exposed to HIV by a sexual partner or who have an HIV-positive household member), campaign (short — generally one to two weeks — intensive community mobilization followed by mobile testing, often partnered with other health interventions), key populations (targeted to MSM, CSWs and PWID) and workplace (offered at a place of employment). We examined a subset of home and workplace HTC that used self-testing.
Data screening and extraction
M.S., R.Y. and R.V.B. screened abstracts for initial inclusion. Disagreements were adjudicated by reviewing the full text. M.S., R.V.B., R.Y. and G.T. reviewed papers for eligibility and used a standardized extraction form to characterize eligible studies (Supplemental Information 2). Study quality was rated low; moderate; or high; based on representativeness of underlying population, follow-up (present or absent), assessment of outcomes, and number of outcomes presented. Costs were inflated to 2012 US dollars by converting to local currency units, multiplying by the ratio of each country’s gross domestic product deflator (2012 deflator or base year deflator) and converting back to US dollars27.
Statistical analysis
Random effects meta-analysis of single proportions with binomial exact confidence intervals (CI) was used to summarize results. Proportions were stabilized using the Freeman-Tukey double arcsine transformation unless the number of events was less than ten, in which case a logit transformation was used because of convergence issues. Heterogeneity was quantified using the I2 statistic. For modalities with enough data (ten studies or more), trends were examined by year before 2005 (when the HIV rapid diagnostic test was introduced), country and facilitated linkage. Analyses were conducted in R software using the metaprop function in the meta package28.
Results
We identified 126 eligible studies out of 2,520 abstracts (Supplementary Figure S0.a). Overall, 64% of studies were rated moderate or high quality (Supplementary Information 2). Most studies included in our analysis evaluated facility and home HTC. We identified far fewer studies on other types of community HTC: home with self-testing (n = 2), workplace with self-testing (n = 2), index partner/family member (n = 5), key populations (n = 5), campaign (n = 5) and workplace (n = 4). Forest plots of each outcome by modality are available in the Supplementary Information with pooled estimates presented here. I2 values of pooled estimates varied from 90% to 100%, reflecting high heterogeneity in study designs and countries included (Supplementary Information). The countries represented varied by outcome with the greatest number reporting data for home and facility HTC coverage, uptake and tester demographics. Far fewer studies reported CD4 count at diagnosis and linkage to care outcomes; studies containing these data were mainly conducted in South Africa, Kenya and Uganda. All home self-testing studies were conducted in Malawi and the most key population studies were conducted in Nigeria. Overall, the largest number of studies were conducted in South Africa.
Coverage and uptake
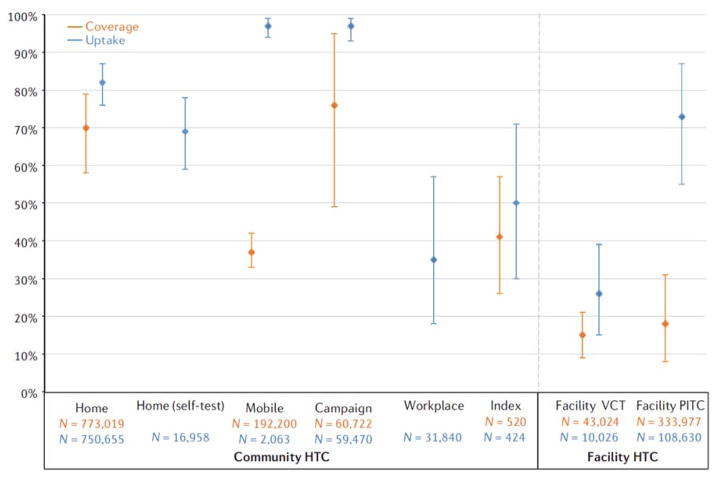
Coverage was reported in 19 home HTC studies15,18,29–45, one mobile13, two campaign46,47, three index partner/family member48–50, five facility VCT51–55, and five facility PITC studies56–61. Overall, community HTC modalities achieved higher coverage than facility, with home (70%, 95% CI = 58–79) and campaign (76%, 95% CI = 49–95%) having the highest population coverage (Fig. 1). Home HTC consistently achieved high coverage across 19 studies, whereas campaign coverage was also high, but based on only two studies. Pooled coverage was 37% (95%, CI = 33–42%) for mobile HTC, from one study conducted in three countries (South Africa, Tanzania and Zimbabwe). Coverage of index HTC was heterogeneous depending on target group (family members compared with sexual partners) and type of contact tracing (active or passive referral). Figure 1 shows results for sexual partner tracing only (41%); full results are shown in Supplementary Figure S18. Facility VCT (15%, 95% CI = 9–21%) and PITC (18%, 95% CI = 18–31%) attained the lowest coverage.
Figure 1.
Pooled coverage and uptake of HIV testing and counselling modalities. Coverage is defined as total number of people tested/total number of people in the target population. Uptake is defined as total number of people tested/total number of people offered testing. Bars indicate 95% confidence intervals of random effects meta-analyses. n, sample size.
Uptake was reported in 31 home HTC studies5,14,15,18,27,29–38,40–45,53,62–74, two home with self-testing11,75, two mobile10,68, three index partner or family member48–50, four campaign46,47,76,77, three workplace78–80, three facility VCT54,56,81, and 11 facility PITC studies56,57,59,60,81–87. Overall, community modalities had high uptake (Fig. 1). Home HTC had a pooled uptake of 82% (95% CI = 76–87%) and home with self-testing had slightly lower uptake (69%, 95% CI = 59–78). Mobile and campaign had the highest uptake (both at97%). Index uptake was 89% (95% CI = 88–90%) for home testing of family members (Supplementary Figure S10) and 52% for sexual partners (95% CI = 30–71%; Fig. 1). Uptake for facility VCT was defined as number tested divided by number referred for VCT by provider, for facility PITC it was defined as number tested divided by number offered PITC. We found higher uptake for people given routine PITC (73%, 95% CI = 55–87%) compared with those referred to on site VCT (26%, 95% CI = 15–39%).
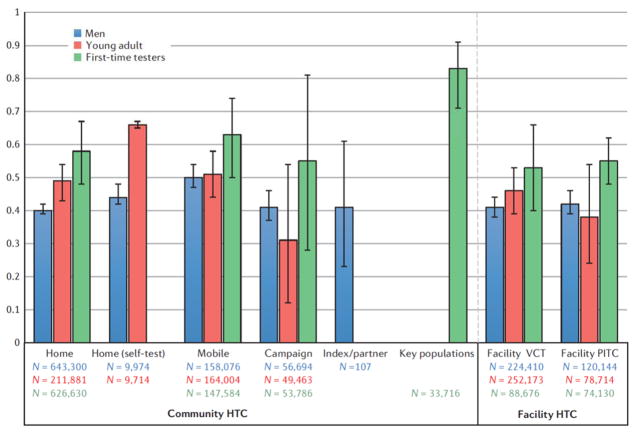
Demographics of testers
The percentage of men out of total persons tested was reported in 25 home HTC studies5,14,18,29,31,32,37,38,41–45,63,64,66,68–72,88–90, two home with self-testing11,75, 10 mobile10,13,68,72,91–99, 3 index partner47,49,88, 3 campaign46,47,76, 2 workplace100,101, 20 facility VCT52,54,61,64,81,88,89,92,93,95,96,98,102–107, and 13 facility PITC58,60,82–84,86,99,108–113 (Fig. 2). Mobile had the highest percentage of men (50%, 95% CI = 47–54%), whereas home had the lowest for general population HTC (40%, 95% CI = 39–41%). Index partner testing had 41% men (95% CI = 23–61%), but varied greatly by tracing strategy; active tracing had 50% men whereas passive clinic referral had only 15% (Supplementary Figure S18). Facility VCT and PITC both had 42% men.
Figure 2.
Pooled percentage of men, young adults and first-time testers by HIV testing and counselling modality. Bars indicate 95% confidence intervals of random effects meta-analyses. n, sample size.
Percentage of participants reporting testing for the first time was included in 20 home HTC studies5,14,18,29,31,32,38,41–44,63,65,66,68–72,88, 11 mobile10,12,68,93–95,97,103,114, 3 campaign46,47,77, 3 key populations25,115,116, and 7 facility VCT12,54,91,93,95,106, and 5 facility PITC58,86,88,111,112. Pooled percentages of first-time testers were higher for community than facility modalities (Fig. 2). Percentages varied by country, with South Africa consistently having the lowest percentage of first-time testers across modalities (Supplementary Figures S23–S27). Key population interventions had the highest proportion of first-time testers (83%, 95% CI = 71–91%), and mobile had the highest percentage among the general population (63%, 95% CI = 50–74%). Home HTC had 58% first-time testers (95% CI = 48–67%), and campaign had 55% (95% CI = 20–91%), but was highly variable depending on the setting (Supplementary Figure S25). Facility VCT had 53% (95% CI = 40–66%) and PITC had 55% (95% CI = 48–62%) first-time testers.
The percentage of testers who were young adults testers <age 25 or <30 years) was reported in 17 home HTC studies5,18,29–31,35,37,38,45,63,64,68–70,73,74,90,117, one home with self-testing11, 13 mobile10,12,13,68,91,93,95–97,103,107,114, two index partner48,88, two campaign47,77, 20 facility VCT12,51,52,54,64,88,89,91–93,95,104–107,114,118–120, and six facility PITC58,82,86,88,110,113. Results varied considerably by study (Supplementary Figures S29–S35). Community HTC generally tested a higher proportion of young adults than facility modalities; home with self-testing had the largest percentage (66%, 95% CI = 65–67%), followed by mobile, and then home (Fig. 2). Campaign reported 31% young adults, but varied from 20–50% depending on study (Supplementary Figure S32). Facility VCT had 46% (95% CI = 39–53%) and PITC had 38% (95% CI = 39–53%).
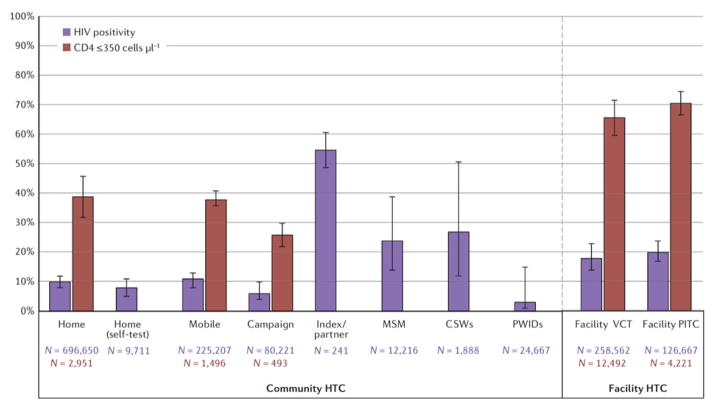
HIV positivity and CD4 count of less than 350 cells μL−1 at diagnosis
Yield of HIV positive individuals (HIV positivity) was reported in 29 home studies14,15,18,27,29–32,34,36,38,41–45,63,65,66,68,70–73,88,89, one home with self-testing11, 12 mobile10,13,68,72,92–95,97,98,103,107,114, five campaign46,47,76,77,120, three workplace79,80,121, four key population12,115,116,122, four index partner48–50,88, 27 facility VCT54–56,64,81,84,88,91–93,95,98,102,104–107,114,118–120,123–127, and 17 facility PITC56,57,59,60,81,83–88,99,110–113,126 studies. Community-based strategies for the general population had lower HIV positivity (6–11%) than facility HTC (18–20%), whereas targeted community HTC for key populations and sexual partners of index patients had the highest HIV yield (Fig. 3). HTC interventions targeting sexual partners of index cases had 28% positivity (95% CI = 13–50%), those for MSM had 24% (95% CI = 14–39%), for CSWs had 27% (95% CI = 12–51%), and interventions targeting PWIDs had the lowest positivity (3%, 95% CI = 1–15%). Index HTC for family members had similar HIV yield to home and mobile HTC (9%, 95% CI = 5–14%) (Supplementary Figure S42). Forest plots of HIV positivity for each modality stratified by country are shown in Supplementary Figures S36–S44). HIV positivity for community HTC in the general population largely mirrored prevalence of the country where the study was conducted, with the exception of four countries with the highest prevalence: Mozambique, Swaziland, Botswana and Lesotho. These countries have adult HIV prevalence ranging from 22–27%128, but HIV yield from home, mobile and campaign HTC was 5–12%. HIV positivity for facility VCT and PITC was generally higher than prevalence in the general population.
Figure 3.
Pooled HIV positivity and proportion of newly diagnosed HIV positivity with CD4 count of 350 cells μL−1 or less by HIV testing and counselling modality. Bars indicate 95% confidence intervals of random effects meta-analyses. n, sample size.
The proportion of individuals with CD4 count of less than 350 cells μL−1 at HIV diagnosis was reported in 7 home14,38,42,43,65,72,73, 3 mobile91,94,114, 3 campaign46,47,76, 8 facility VCT60,81,107,126,127,129–131 and 5 facility PITC studies61,81,99,126,130. Community-based strategies identified HIV-positive individuals at higher CD4 counts than facility HTC, with campaign having the lowest proportion with CD4 count less than 350 cells μL−1 (26%, 95% CI = 22–30%) (Fig. 3). Home (39%, 95% CI = 32–46%) and mobile (38%, 95% CI = 36–41%) had similar proportions of HIV-positive individuals with a CD4 count less than 350 cells μL−1, whereas facility VCT (66%, 95% CI = 60–72%) and PITC (71%, 95% CI = 67–75%) had the highest proportion with low CD4 count.
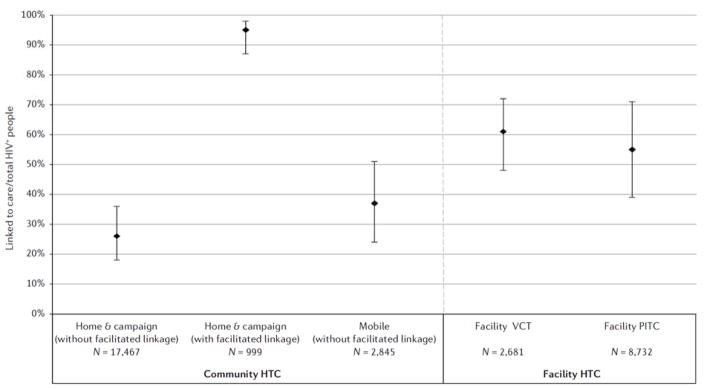
Linkage and retention in care for HIV-positive individuals
Linkage to care was defined as visiting a clinic for community HTC and returning to the clinic to obtain CD4 count results (or enrolling in pre-ART care) for facility HTC. Linkage was reported for ten home14,15,29,34,41–43,65,72,132, six mobile72,91,92,94,133–135, two campaign76,77, eight facility VCT56,81,84,91,92,123,126,136 and five facility PITC studies60,84,87,111,126. Home and campaign interventions achieved a high proportion of individuals linked (95%, 95% CI = 87–98%) when paired with facilitated linkage to care strategies (for example lay-counsellor follow up to encourage clinic visit); interventions without facilitated linkage achieved lower proportions of HIV positive individuals visiting a clinic (26%, 95% CI = 18–36%) (Fig. 4). Mobile HTC achieved 37% (95% CI = 24–51%) linkage; rates were highest in two intervention conducted in South Africa, one which used incentivized monetary recruitment and another which used a call centre to encourage linkage after HTC94,134. Linkage to care from facility VCT was 61% (95% CI = 48–72%) and from PITC was 55% (95% CI = 39–71%) (Fig. 4). Time from HTC to linkage to care ascertainment varied by study (ranging from 1 to 12 months); the method of ascertainment (participant self-report or clinic record) also varied.
Figure 4.
Linkage to care after community and facility HIV testing and counselling
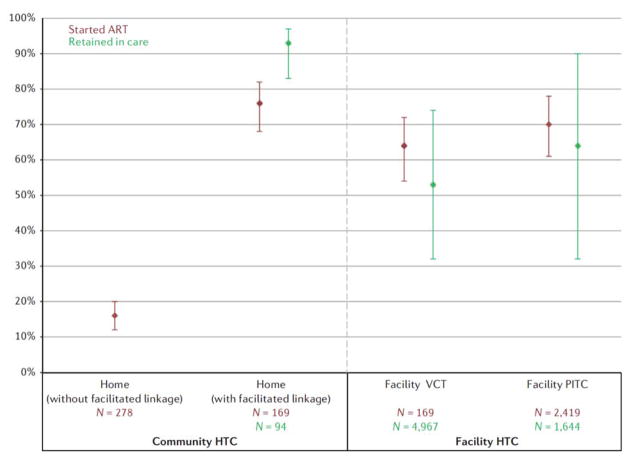
Four home HTC studies reported ART initiation among those eligible14,41,43,65. Similar to linkage to care, ART initiation was higher in home interventions with facilitated linkage (76%, 95% CI = 26–82%) compared with those without facilitated linkage (16%, 95% CI = 12–20%) (Fig. 5). ART initiation rates after home HTC with facilitated linkage were similar to those achieved through facility HTC. Initiation among those eligible was 64% (95% CI = 54–72%) in facility VCT and 70% (95% CI = 61–78%) in facility PITC with three studies reporting initiation rates for VCT61,126,130 and four for facility PITC60,81,84,87,111. Self-testing showed an ART initiation rate of 29% (95% CI = 17–45%), although this number is among all HIV-positive individuals and is not restricted to those who are ART eligible since POC CD4 counts were not conducted11 (Supplementary Figure S55).
Figure 5.
Pooled percentage initiated ART between those eligible and retained in care among those who initiated anti-retroviral therapy. Bars indicate 95% confidence intervals of random effects meta-analyses. n, sample size.
One study reported retention in care at12 months after ART initiation for home HTC14 and two studies for both facility VCT and PITC reported retention — one at 6 months60 and one at 12 months130. Not surprisingly, linkage rates were higher in the 6 month compared with the 12 month retention study (Supplementary Figure S59). Retention was highest for home HTC, although the sample size was small (93%, 95% CI = 83–97%) (Fig. 5). Facility VCT achieved 53% (95% CI = 32–71%) retention, and PITC retention achieved 64% (95% CI = 32–90%).
Cost per person tested
The average cost per person tested (2012 US dollars) for community HTC was $27.38 for mobile, $16.60 for index, $11.17 for campaign and $8.58 for home HTC88,93,103,137–141 (Supplementary Table S2 and Figure S61). The cost per person tested was highest for stand-alone VCT ($36.78)88,93,142 whereas hospital and clinic HTC had similar costs ($12.56 and $12.32, respectively)81,88,93,140,142–147 (Supplementary Table S3 and Figure S62). Costs were dependent on the country where the study was conducted, which costs were included (start-up or ongoing only) and the intervention scale.
Discussion
Across modalities, community HTC successfully reached target groups (men, young adults and first-time testers) with higher coverage than facility HTC. High uptake of community HTC reflects population acceptability of testing outside of health-care facilities. Community HTC identified HIV-positive individuals with higher CD4 counts who were likely to be earlier in their disease course. Combined with the potential of community HTC with facilitated linkage to achieve high linkage to treatment with similar retention rates as facility HTC, this suggests that scaling up community interventions could reduce the morbidity, mortality and transmission associated with late or non-initiation of ART. Although community interventions test a large number of HIV-negative individuals, HTC can reduce risky sexual behaviour74 and provide a means to link uninfected persons to primary prevention. This is particularly crucial for young women, who have high HIV incidence and can benefit from PrEP21. Preventing HIV infections averts future treatment costs as well as morbidity. A recent modelling study found that ART scale-up should be combined with primary prevention such as PrEP to achieve maximum HIV reduction148. High coverage of HTC can also reduce stigma around testing.
Each HTC modality reaches distinct sub-populations and a combination of strategies will probably be necessary to achieve high ART coverage. Mobile and campaign HTC had high uptake (97%), as individuals who present at a mobile van or during a campaign are probably seeking out testing, but home HTC also achieved high uptake among people at home who were offered testing (82%). Home HTC also attained high population coverage, probably because offering testing door-to-door removes substantial barriers, including eliminating the need to actively seek out HIV testing149. However, home HTC is less likely to reach men and young adults. A recent home HTC intervention in Botswana reached 85% of women in the target population compared with just 50% of men150. This may be because women are more likely to be home at the times when the intervention is conducted.
Campaign HTC has the potential to attain high coverage in large catchment areas and identify HIV-positive individuals at high CD4 counts (one-third of newly diagnosed HIV-positive individuals had a CD4 count of less than 350 cells μL−1 compared with two-thirds or more for facility HTC). The multidisease focus of campaigns may reduce stigma of HIV testing interventions. Our results suggest that campaign HTC can be a successful strategy for countries seeking to increase overall testing coverage in a short time frame.
Home HTC with self-testing reached the greatest proportion of young adults of all modalities examined11 and is a promising strategy to achieve high uptake151. Young adults (age 15 to 24 years) represent 39% of new infections in those over 15-years old23, but have lower access to HTC and HIV care and poorer clinical outcomes than other age groups24. Home HTC with self-testing had slightly lower coverage and reached fewer first-time testers than home HTC administered by counsellors. The World Health Organization recommends HIV self-testing as an option for individuals who are unable or unwilling to receive counsellor-administered HTC. However, supervision improves interpretation of results151 and a reactive self-test should not be considered a definitive diagnosis, as standard testing is needed to confirm results. More studies evaluating linkage to care following a positive self-test are needed16.
Mobile HTC is the most effective strategy for reaching men, a target group in sub-Saharan Africa. Men are more likely to be lost at each step of the HIV treatment cascade; they are less likely to undergo testing, more likely to start ART at an advanced disease stage and more likely to interrupt treatment — all of which leads to increased morbidity and mortality22. Qualitative studies highlight men’s preference to test outside of facilities152, so scale up of community interventions can meet this need. Future studies could investigate HTC at predominantly male workplaces, nightclubs or bars.
HIV testing of sexual partners through active contact tracing is an efficient high yield method that should be scaled up. HIV positivity was high (55%) and the intervention attained a high coverage (41%). The HIV prevalence we report is similar to that found in the literature — 45–50% in cohabitating partners of HIV-positive adults, most of whom are unaware of their status48. Interestingly, high coverage of males was achieved only through active contact tracing, whereas passive tracing identified more women (Supplementary Figure S18).
Facilitated linkage strategies are a key component of successful community-based HTC. Persons testing at an HIV facility generally have higher rates of linking to care and initiating ART than those who test outside the health-care system. However, we report that high linkage rates (comparable with, or higher than, facility HTC) can be achieved with community HTC when individuals are followed-up to encourage linkage.
Although scaling up community HTC with facilitated linkage is important, the benefits of improving facility HTC coverage should not be overlooked. Consistent with previous studies, our analysis finds opt-out facility PITC had much greater uptake than referring patients to VCT56. However, coverage of PITC in health facilities is low, demonstrating missed opportunities to identify HIV-positive individuals and to link them to care. For example, a Ugandan hospital reported only 50% of inpatients with HIV-related diagnoses were tested for HIV before leaving the hospital86. PITC is an underused strategy in sub-Saharan Africa and scaling up testing would provide a safety net for those who do not independently seek HTC61,112. Because PITC identifies mainly symptomatic HIV-positive persons at low CD4 counts as well as those with health-care access, it should be coupled with other modalities to maximize population coverage.
Our review identified gaps where additional evidence is needed. A large proportion of CD4 count and linkage data came from South Africa, with Uganda and Kenya also well represented. South Africa has the lowest percentage of first-time testers, reflecting the successful scale-up of HTC. There are fewer studies from other parts of sub-Saharan Africa, which may limit how much the pooled estimates can be generalized. Also, few studies followed patients longitudinally and measured linkage to care, ART initiation, retention and viral suppression. In addition, although many studies evaluated home HTC, more data are needed for other community modalities, including campaign and workplace.
Data were also limited for key populations. Despite having an HIV prevalence up to eight times higher than the general population, interventions for key populations are scarce and scale up is urgently needed115,153. Key population interventions can reduce the spread of HIV in the general population154. Currently, numerous policy barriers exist that restrict the availability and access of HIV-related services for MSM and CSWs, including police harassment and criminal laws155. Only three HTC interventions were targeted to MSM and only one was targeted to CSWs and PWIDs. Most key population HTC studies were from Nigeria, so data are needed from other parts of sub-Saharan Africa. We report a high HIV positivity combined with a high proportion of first-time testers in MSM and CSW groups, highlighting the need for service expansion. We found a lower HIV prevalence in PWIDs compared with MSM and CSW groups, reflecting sexual transmission as the main mode of HIV spread in sub-Saharan Africa. Successful HTC programmes for key populations are community-based (particularly mobile), as many high-risk groups are marginalized and do not have access to conventional health systems122. Community-based HTC for MSM and PWIDs have been shown to have higher acceptance and greater HIV yield than clinic referral for HTC115. In addition, self-testing is a potential strategy to reach key populations, as it demonstrates high acceptability and is considered convenient and private156.
Costs of community-based and facility-based HTC vary by modality, country, scale of intervention, linkage strategy and costs included. Generally, community-based HTC and integrated facility HTC costs were comparable. However, stand-alone HTC had the highest cost per person tested indicating integrated HTC may be more cost-efficient than stand-alone services (Supplementary Table S3).
The limitations of our analysis included the heterogeneity across studies, which may not be accurately reflected in the pooled estimates. Differences in study design, geographical location (country, urban or rural area) and intervention year added to the heterogeneity. To address this, we used random effects meta-analysis and stratified on key variables (year <2005, country and facilitated linkage). In addition, large numbers of HIV-positive individuals were lost to follow up in studies that reported linkage so we considered these persons unlinked in our analyses. If individuals linked at another clinic, our estimates may be conservative157. Furthermore, assessment of linkage to care differed by study (self-report or clinic records review), as did time to linkage assessment, which varied from 1 to 12 months after HTC. In addition, CD4 count at diagnosis and ART uptake among those with eligible CD4 counts could only be assessed in community HTC interventions employing point-of-care CD4, as studies that report CD4 only for those visiting a clinic would not provide accurate denominators. Only studies reporting linkage to care among those eligible for ART were included in our main analysis. Also, estimates of coverage vary in their precision because some studies conducted population enumeration and others used census estimates of the catchment area. Finally, proportion of first-time testers, men and young adults tested are crude measures of relative uptake. For example, for home HTC, it is not possible to discern whether 40% of those tested being men reflects a lower coverage of men, or a greater coverage of women, or a combination of the two. Future studies reporting the number of men, first-time testers and young adults offered testing compared with those accepting testing would increase the accuracy of these measures. Our findings on uptake, HIV positivity and CD4 count at diagnosis are similar to a previously published meta-analysis9.
This analysis characterizes linkage and populations reached by HTC modalities to inform policymakers who are charged with addressing gaps in testing. Facility HTC, although important, is unlikely to be sufficient to curb the HIV epidemic since many people in sub-Saharan Africa do not have regular access to health care. Scaling a combination of community HTC, mobile testing to reach men, self-testing to reach young adults and outreach to high-risk populations, as appropriate to the local epidemic setting, is crucial to achieve high knowledge of serostatus and linkage to HIV treatment and prevention in sub-Saharan Africa.
Supplementary Material
Table 1.
Summary of HIV testing and counselling coverage and tester demographics*
| Home | Mobile | Self-testing (home) | Campaign | Index | Key populations | Facility VCT | Facility PITC | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | |
| Coverage (accepted/target population) | 70 | 58–79 | 37 | 33–42 | 76 | 49–95 | 41 | 26–57 | 15 | 9–21 | 18 | 8–31 | ||||
| Uptake (accepted/offered) | 82 | 76–87 | 97 | 94–99 | 69 | 59–78 | 97 | 93–99 | 50 | 31–71 | 26 | 15–39 | 73 | 55–87 | ||
| Young adult (age <25 or <30) | 49 | 43–54 | 51 | 44–58 | 66 | 65–67 | 31 | 12–54 | 46 | 39–53 | 38 | 24–54 | ||||
| Men | 40 | 39–41 | 50 | 47–54 | 44 | 42–48 | 41 | 37–46 | 41 | 23–61 | 41 | 38–44 | 42 | 39–46 | ||
| First-time testers | 58 | 48–67 | 63 | 50–74 | 55 | 28–81 | 83 | 71–91 | 53 | 40–66 | 55 | 48–62 | ||||
| CD4 ≤350 cells μL−1 | 42 | 34–51 | 38 | 36–41 | 26 | 22–30 | 61 | 51–71 | ||||||||
| HIV positivity | 10 | 8–12 | 11 | 8–13 | 8 | 5–11 | 6 | 4–10 | 55 | 49–61 | 16 | 9–26 | 18 | 13–23 | 20 | 17–24 |
PITC, provider-initiated testing counselling; VCT, voluntary counselling and testing
References
- 1. [Accessed on 22/5/15];UNAIDS 2013 Report on the Global AIDS Epidemic. Available from: http://www.unaids.org/en/resources/campaigns/globalreport2013/globalreport.
- 2.Kranzer K, Govindasamy D, Ford N, Johnston V, Lawn SD. Quantifying and addressing losses along the continuum of care for people living with HIV infection in sub-Saharan Africa: a systematic review. Journal of the International AIDS Society. 2012;15:17383. doi: 10.7448/IAS.15.2.17383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. [Accessed on 25/5/15];UNAIDS World AIDS Day Report. 2012 Available from: http://www.unaids.org/sites/default/files/media_asset/JC2434_WorldAIDSday_results_en_1.pdf.
- 4.UNAIDS. Fast-Track: Ending the AIDS Epidemic by 2030. 2014 < http://www.unaids.org/sites/default/files/media_asset/JC2686_WAD2014report_en.pdf>.
- 5.Fylkesnes K, et al. Strong effects of home-based voluntary HIV counselling and testing on acceptance and equity: a cluster randomised trial in Zambia. Social science & medicine (1982) 2013;86:9–16. doi: 10.1016/j.socscimed.2013.02.036. [DOI] [PubMed] [Google Scholar]
- 6.Musheke M, et al. A systematic review of qualitative findings on factors enabling and deterring uptake of HIV testing in Sub-Saharan Africa. BMC public health. 2013;13:220. doi: 10.1186/1471-2458-13-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siedner MJ, et al. Trends in CD4 count at presentation to care and treatment initiation in sub-Saharan Africa, 2002–2013: a meta-analysis. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2015;60:1120–1127. doi: 10.1093/cid/ciu1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paulin HN, et al. HIV testing service awareness and service uptake among female heads of household in rural Mozambique: results from a province-wide survey. BMC public health. 2015;15:1388. doi: 10.1186/s12889-015-1388-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suthar AB, et al. Towards universal voluntary HIV testing and counselling: a systematic review and meta-analysis of community-based approaches. PLoS medicine. 2013;10:e1001496. doi: 10.1371/journal.pmed.1001496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Rooyen H, et al. Mobile VCT: reaching men and young people in urban and rural South African pilot studies (NIMH Project Accept, HPTN 043) AIDS and behavior. 2013;17:2946–2953. doi: 10.1007/s10461-012-0368-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.MacPherson P, et al. Effect of optional home initiation of HIV care following HIV self-testing on antiretroviral therapy initiation among adults in Malawi: A randomized clinical trial. JAMA - Journal of the American Medical Association. 2014;312:372–379. doi: 10.1001/jama.2014.6493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ahmed S, et al. HIV counseling and testing and access-to-care needs of populations most-at-risk for HIV in Nigeria. AIDS care. 2013;25:85–94. doi: 10.1080/09540121.2012.686597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coates TJ, et al. Effect of community-based voluntary counselling and testing on HIV incidence and social and behavioural outcomes (NIMH Project Accept; HPTN 043): a cluster-randomised trial. The Lancet. Global health. 2014;2:e267–277. doi: 10.1016/s2214-109x(14)70032-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barnabas RV, et al. Initiation of antiretroviral therapy and viral suppression after home HIV testing and counselling in KwaZulu-Natal, South Africa, and Mbarara district, Uganda: a prospective, observational intervention study. The lancet. HIV. 2014;1:e68–e76. doi: 10.1016/S2352-3018(14)70024-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Genberg BL, et al. Linkage to and engagement in HIV care in western Kenya: an observational study using population-based estimates from home-based counselling and testing. The Lancet HIV. 2015;2:e20–e26. doi: 10.1016/s2352-3018(14)00034-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.WHO. [Accessed on 27 July 2015];Consolidated guidelines on HIV testing services. from: http://www.who.int/hiv/pub/guidelines/hiv-testing-services/en/
- 17.UNAIDS. [Accessed on 10/3/2015];The Gap Report. from: http://www.unaids.org/sites/default/files/media_asset/UNAIDS_Gap_report_en.pdf.
- 18.Sekandi JN, et al. High acceptance of home-based HIV counseling and testing in an urban community setting in Uganda. BMC public health. 2011;11:730. doi: 10.1186/1471-2458-11-730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosenberg NE, et al. Assessing the effect of HIV counselling and testing on HIV acquisition among South African youth. AIDS (London, England) 2013;27:2765–2773. doi: 10.1097/01.aids.0000432454.68357.6a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barnabas RV, van Rooyen H, Tumwesigye E, Brantley J, Krows M, van Heerden A, Turyamureeba B, Hughes J, Baeten J, Celum C. Community-based HIV testing and linkage effectively delivers combination HIV prevention: Results from a multisite randomized trial 8th IAS Conference on HIV Pathogenesis, Treatment and Prevention (IAS 2015); Vancouver, BC, Canada. 2015. [Google Scholar]
- 21.Baeten J, Heffron R, Kidoguchi L, et al. Near Elimination of HIV Transmission in a Demonstration Project of PrEP and ART. 2015 Conference on Retroviruses and Opportunistic Infections. Seattle; February 23–24, 2015; p. Abstract 24. [Google Scholar]
- 22.Cornell M, McIntyre J, Myer L. Men and antiretroviral therapy in Africa: our blind spot. Tropical medicine & international health : TM & IH. 2011;16:828–829. doi: 10.1111/j.1365-3156.2011.02767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kurth AE, Lally MA, Choko AT, Inwani IW, Fortenberry JD. HIV testing and linkage to services for youth. Journal of the International AIDS Society. 2015;18:19433. doi: 10.7448/ias.18.2.19433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.MacPherson P, et al. Service delivery interventions to improve adolescents’ linkage, retention and adherence to antiretroviral therapy and HIV care. Tropical medicine & international health : TM & IH. 2015 doi: 10.1111/tmi.12517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mine M, et al. Performance of rapid HIV testing by lay counselors in the field during the behavioral and biological surveillance survey among female sex workers and men who have sex with men in Botswana. Journal of acquired immune deficiency syndromes (1999) 2015;68:365–368. doi: 10.1097/qai.0000000000000434. [DOI] [PubMed] [Google Scholar]
- 26.Liberati A, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ (Clinical research ed) 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Uwimana J, Zarowsky C, Hausler H, Jackson D. Training community care workers to provide comprehensive TB/HIV/PMTCT integrated care in KwaZulu-Natal: lessons learnt. Tropical medicine & international health : TM & IH. 2012;17:488–496. doi: 10.1111/j.1365-3156.2011.02951.x. [DOI] [PubMed] [Google Scholar]
- 28.RStudio. RStudio: Integrated development environment for R (Version 0.98.1103) [Computer software] Boston, MA: 2015. Retrieved May 20, 2014. Available from http://www.rstudio.org/ [Google Scholar]
- 29.Bigogo G, et al. The impact of home-based HIV counseling and testing on care-seeking and incidence of common infectious disease syndromes in rural western Kenya. BMC infectious diseases. 2014;14:376. doi: 10.1186/1471-2334-14-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gonzalez R, et al. High HIV prevalence in a southern semi-rural area of Mozambique: a community-based survey. HIV medicine. 2012;13:581–588. doi: 10.1111/j.1468-1293.2012.01018.x. [DOI] [PubMed] [Google Scholar]
- 31.Helleringer S, Kohler HP, Frimpong JA, Mkandawire J. Increasing uptake of HIV testing and counseling among the poorest in sub-Saharan countries through home-based service provision. Journal of acquired immune deficiency syndromes (1999) 2009;51:185–193. doi: 10.1097/QAI.0b013e31819c1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kimaiyo S, et al. Home-based HIV counselling and testing in western Kenya. East African medical journal. 2010;87:100–108. doi: 10.4314/eamj.v87i3.62195. [DOI] [PubMed] [Google Scholar]
- 33.Kranzer K, et al. Individual, household and community factors associated with HIV test refusal in rural Malawi. Tropical medicine & international health : TM & IH. 2008;13:1341–1350. doi: 10.1111/j.1365-3156.2008.02148.x. [DOI] [PubMed] [Google Scholar]
- 34.Medley A, et al. Early uptake of HIV clinical care after testing HIV-positive during home-based testing and counseling in western Kenya. AIDS and behavior. 2013;17:224–234. doi: 10.1007/s10461-012-0344-5. [DOI] [PubMed] [Google Scholar]
- 35.Michelo C, Sandoy IF, Dzekedzeke K, Siziya S, Fylkesnes K. Steep HIV prevalence declines among young people in selected Zambian communities: population-based observations (1995–2003) BMC public health. 2006;6:279. doi: 10.1186/1471-2458-6-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Molesworth AM, et al. High accuracy of home-based community rapid HIV testing in rural Malawi. Journal of acquired immune deficiency syndromes (1999) 2010;55:625–630. doi: 10.1097/QAI.0b013e3181f98628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Negin J, Wariero J, Mutuo P, Jan S, Pronyk P. Feasibility, acceptability and cost of home-based HIV testing in rural Kenya. Tropical medicine & international health : TM & IH. 2009;14:849–855. doi: 10.1111/j.1365-3156.2009.02304.x. [DOI] [PubMed] [Google Scholar]
- 38.Ng’ang’a A, et al. The status of HIV testing and counseling in Kenya: results from a nationally representative population-based survey. Journal of acquired immune deficiency syndromes (1999) 2014;66(Suppl 1):S27–36. doi: 10.1097/qai.0000000000000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bailey RC, et al. Male circumcision for HIV prevention in young men in Kisumu, Kenya: a randomised controlled trial. Lancet. 2007;369:643–656. doi: 10.1016/s0140-6736(07)60312-2. [DOI] [PubMed] [Google Scholar]
- 40.Shisana O, et al. South African national household survey of HIV/AIDS prevalence, behavioural risks and mass media impact —detailed methodology and response rate results. South African medical journal = Suid-Afrikaanse tydskrif vir geneeskunde. 94:283–288. [PubMed] [Google Scholar]
- 41.Tumwebaze H, et al. Household-based HIV counseling and testing as a platform for referral to HIV care and medical male circumcision in Uganda: a pilot evaluation. PloS one. 2012;7:e51620. doi: 10.1371/journal.pone.0051620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tumwesigye E, Wana G, Kasasa S, Muganzi E, Nuwaha F. High uptake of home-based, district-wide, HIV counseling and testing in Uganda. AIDS patient care and STDs. 2010;24:735–741. doi: 10.1089/apc.2010.0096. [DOI] [PubMed] [Google Scholar]
- 43.van Rooyen H, et al. High HIV testing uptake and linkage to care in a novel program of home-based HIV counseling and testing with facilitated referral in KwaZulu-Natal, South Africa. Journal of acquired immune deficiency syndromes (1999) 2013;64:e1–8. doi: 10.1097/QAI.0b013e31829b567d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wachira J, et al. HIV testing uptake and prevalence among adolescents and adults in a large home-based HIV testing program in Western Kenya. Journal of acquired immune deficiency syndromes (1999) 2014;65:e58–66. doi: 10.1097/QAI.0b013e3182a14f9e. [DOI] [PubMed] [Google Scholar]
- 45.Welz. Continued very high prevalence of HIV infection in rural KwaZulu-Natal, South Africa: a population- based longitudinal study. 2007. [DOI] [PubMed] [Google Scholar]
- 46.Chamie G, et al. Uptake of community-based HIV testing during a multi-disease health campaign in rural Uganda. PloS one. 2014;9:e84317. doi: 10.1371/journal.pone.0084317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lugada E, et al. Rapid implementation of an integrated large-scale HIV counseling and testing, malaria, and diarrhea prevention campaign in rural Kenya. PloS one. 2010;5:e12435. doi: 10.1371/journal.pone.0012435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lugada E, et al. Comparison of home and clinic-based HIV testing among household members of persons taking antiretroviral therapy in Uganda: results from a randomized trial. Journal of acquired immune deficiency syndromes (1999) 2010;55:245–252. doi: 10.1097/QAI.0b013e3181e9e069. [DOI] [PubMed] [Google Scholar]
- 49.Brown LB, et al. HIV partner notification is effective and feasible in sub-Saharan Africa: opportunities for HIV treatment and prevention. Journal of acquired immune deficiency syndromes (1999) 2011;56:437–442. doi: 10.1097/qai.0b013e318202bf7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Armbruster B, Helleringer S, Kalilani-Phiri L, Mkandawire J, Kohler HP. Exploring the relative costs of contact tracing for increasing HIV case finding in sub-Saharan countries. Journal of acquired immune deficiency syndromes (1999) 2011;58:e29–36. doi: 10.1097/QAI.0b013e31822a9fa8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bwambale FM, Ssali SN, Byaruhanga S, Kalyango JN, Karamagi CA. Voluntary HIV counselling and testing among men in rural western Uganda: implications for HIV prevention. BMC public health. 2008;8:263. doi: 10.1186/1471-2458-8-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cawley C, et al. Low rates of repeat HIV testing despite increased availability of antiretroviral therapy in rural Tanzania: findings from 2003–2010. PloS one. 2013;8:e62212. doi: 10.1371/journal.pone.0062212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fylkesnes K, Siziya S. A randomized trial on acceptability of voluntary HIV counselling and testing. Tropical medicine & international health : TM & IH. 2004;9:566–572. doi: 10.1111/j.1365-3156.2004.01231.x. [DOI] [PubMed] [Google Scholar]
- 54.Isingo R, et al. Trends in the uptake of voluntary counselling and testing for HIV in rural Tanzania in the context of the scale up of antiretroviral therapy. Tropical medicine & international health : TM & IH. 2012;17:e15–25. doi: 10.1111/j.1365-3156.2011.02877.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.MacPherson P, et al. Suboptimal patterns of provider initiated HIV testing and counselling, antiretroviral therapy eligibility assessment and referral in primary health clinic attendees in Blantyre, Malawi. Tropical medicine & international health : TM & IH. 2012;17:507–517. doi: 10.1111/j.1365-3156.2011.02946.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dalal S, et al. Provider-initiated HIV testing and counseling: increased uptake in two public community health centers in South Africa and implications for scale-up. PloS one. 2011;6:e27293. doi: 10.1371/journal.pone.0027293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fetene NW, Feleke AD. Missed opportunities for earlier HIV testing and diagnosis at the health facilities of Dessie town, North East Ethiopia. BMC public health. 2010;10:362. doi: 10.1186/1471-2458-10-362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kayigamba FR, et al. Provider-initiated HIV testing and counselling in Rwanda: acceptability among clinic attendees and workers, reasons for testing and predictors of testing. PloS one. 2014;9:e95459. doi: 10.1371/journal.pone.0095459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kharsany AB, Karim QA, Karim SS. Uptake of provider-initiated HIV testing and counseling among women attending an urban sexually transmitted disease clinic in South Africa - missed opportunities for early diagnosis of HIV infection. AIDS care. 2010;22:533–537. doi: 10.1080/09540120903254005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Topp SM, et al. Opt-out provider-initiated HIV testing and counselling in primary care outpatient clinics in Zambia. Bulletin of the World Health Organization. 2011;89:328–335a. doi: 10.2471/blt.10.084442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Topp SM, et al. Does provider-initiated counselling and testing (PITC) strengthen early diagnosis and treatment initiation? Results from an analysis of an urban cohort of HIV-positive patients in Lusaka, Zambia. Journal of the International AIDS Society. 2012;15:17352. doi: 10.7448/ias.15.2.17352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Angotti N, et al. Increasing the acceptability of HIV counseling and testing with three C’s: convenience, confidentiality and credibility. Social science & medicine (1982) 2009;68:2263–2270. doi: 10.1016/j.socscimed.2009.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cherutich P, et al. Lack of knowledge of HIV status a major barrier to HIV prevention, care and treatment efforts in Kenya: results from a nationally representative study. PloS one. 2012;7:e36797. doi: 10.1371/journal.pone.0036797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chirawu P, et al. Acceptability and challenges of implementing voluntary counselling and testing (VCT) in rural Zimbabwe: evidence from the Regai Dzive Shiri Project. AIDS care. 2010;22:81–88. doi: 10.1080/09540120903012577. [DOI] [PubMed] [Google Scholar]
- 65.Dalal W. Home-Based HIV Testing and Counseling in Rural and Urban Kenyan Communities. 2013. [DOI] [PubMed] [Google Scholar]
- 66.Doherty T, et al. Effect of home based HIV counselling and testing intervention in rural South Africa: cluster randomised trial. BMJ (Clinical research ed) 2013;346:f3481. doi: 10.1136/bmj.f3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hensen B, et al. Factors associated with HIV-testing and acceptance of an offer of home-based testing by men in rural Zambia. AIDS Behav. 2015;19:492–504. doi: 10.1007/s10461-014-0866-0. [DOI] [PubMed] [Google Scholar]
- 68.Maheswaran H, Thulare H, Stanistreet D, Tanser F, Newell ML. Starting a home and mobile HIV testing service in a rural area of South Africa. Journal of acquired immune deficiency syndromes (1999) 2012;59:e43–46. doi: 10.1097/QAI.0b013e3182414ed7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mutale W, Michelo C, Jurgensen M, Fylkesnes K. Home-based voluntary HIV counselling and testing found highly acceptable and to reduce inequalities. BMC public health. 2010;10:347. doi: 10.1186/1471-2458-10-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Naik R, Tabana H, Doherty T, Zembe W, Jackson D. Client characteristics and acceptability of a home-based HIV counselling and testing intervention in rural South Africa. BMC public health. 2012;12:824. doi: 10.1186/1471-2458-12-824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nyigo V, et al. Magnitude of HIV infection among older people in Mufindi and Babati districts of the Tanzania mainland. HIV/AIDS - Research and Palliative Care. 2014;6:75–79. doi: 10.2147/HIV.S54610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Parker LA, et al. Feasibility and effectiveness of two community-based HIV testing models in rural Swaziland. Tropical medicine & international health : TM & IH. 2015;20:893–902. doi: 10.1111/tmi.12501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shapiro AE, et al. Community-based targeted case finding for tuberculosis and HIV in household contacts of patients with tuberculosis in South Africa. American journal of respiratory and critical care medicine. 2012;185:1110–1116. doi: 10.1164/rccm.201111-1941OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wolff B, et al. Evaluation of a home-based voluntary counselling and testing intervention in rural Uganda. Health policy and planning. 2005;20:109–116. doi: 10.1093/heapol/czi013. [DOI] [PubMed] [Google Scholar]
- 75.Choko AT, et al. The uptake and accuracy of oral kits for HIV self-testing in high HIV prevalence setting: a cross-sectional feasibility study in Blantyre, Malawi. PLoS medicine. 2011;8:e1001102. doi: 10.1371/journal.pmed.1001102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Granich R, Muraguri N, Doyen A, Garg N, Williams BG. Achieving universal access for human immunodeficiency virus and tuberculosis: potential prevention impact of an integrated multi-disease prevention campaign in kenya. AIDS research and treatment. 2012;2012:412643. doi: 10.1155/2012/412643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Labhardt ND, et al. Home-based versus mobile clinic HIV testing and counseling in rural Lesotho: a cluster-randomized trial. PLoS medicine. 2014;11:e1001768. doi: 10.1371/journal.pmed.1001768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Corbett EL, et al. Uptake of workplace HIV counselling and testing: a cluster-randomised trial in Zimbabwe. PLoS medicine. 2006;3:e238. doi: 10.1371/journal.pmed.0030238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Van der Borght SF, et al. Long-term voluntary counseling and testing (VCT) uptake dynamics in a multicountry HIV workplace program in sub-Saharan Africa. AIDS care. 2010;22:195–205. doi: 10.1080/09540120903111486. [DOI] [PubMed] [Google Scholar]
- 80.Bemelmans M, et al. Keeping health staff healthy: evaluation of a workplace initiative to reduce morbidity and mortality from HIV/AIDS in Malawi. Journal of the International AIDS Society. 2011;14:1. doi: 10.1186/1758-2652-14-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bassett IV, et al. Routine voluntary HIV testing in Durban, South Africa: the experience from an outpatient department. Journal of acquired immune deficiency syndromes (1999) 2007;46:181–186. doi: 10.1097/QAI.0b013e31814277c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Abdurahman S, Seyoum B, Oljira L. Factors affecting acceptance of provider-initiated hiv testing and counseling services among outpatient clients in selected health facilities in harar town, Eastern Ethiopia. HIV/AIDS - Research and Palliative Care. 2015;7:157–165. doi: 10.2147/HIV.S81649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.LaCourse SM, et al. Implementation of Routine Counselor-Initiated Opt-Out HIV Testing on the Adult Medical Ward at Kamuzu Central Hospital, Lilongwe, Malawi. Journal of acquired immune deficiency syndromes (1999) 2015;69:e31–35. doi: 10.1097/qai.0000000000000542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Leon N, et al. A comparison of linkage to HIV care after provider-initiated HIV testing and counselling (PITC) versus voluntary HIV counselling and testing (VCT) for patients with sexually transmitted infections in Cape Town, South Africa. BMC health services research. 2014;14:350. doi: 10.1186/1472-6963-14-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Moodley J, Bryan M, Tunkyi K, Khedun SM. A clinical audit of provider-initiated hiv counselling and testing in a gynaecological ward of a district hospital in kwazulu-natal, South Africa. South African Journal of Obstetrics and Gynaecology. 2014;20:8–11. [Google Scholar]
- 86.Wanyenze RK, et al. Acceptability of routine HIV counselling and testing, and HIV seroprevalence in Ugandan hospitals. Bulletin of the World Health Organization. 2008;86:302–309. doi: 10.2471/BLT.07.042580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Waxman MJ, et al. Initial outcomes of an emergency department rapid HIV testing program in western Kenya. AIDS patient care and STDs. 2007;21:981–986. doi: 10.1089/apc.2007.0075. [DOI] [PubMed] [Google Scholar]
- 88.Menzies N, et al. The costs and effectiveness of four HIV counseling and testing strategies in Uganda. AIDS (London, England) 2009;23:395–401. doi: 10.1097/QAD.0b013e328321e40b. [DOI] [PubMed] [Google Scholar]
- 89.Mulogo EM, Abdulaziz AS, Guerra R, Baine SO. Facility and home based HIV Counseling and Testing: a comparative analysis of uptake of services by rural communities in southwestern Uganda. BMC health services research. 2011;11:54. doi: 10.1186/1472-6963-11-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wachira J, Kimaiyo S, Ndege S, Mamlin J, Braitstein P. What is the impact of home-based HIV counseling and testing on the clinical status of newly enrolled adults in a large HIV care program in Western Kenya? Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2012;54:275–281. doi: 10.1093/cid/cir789. [DOI] [PubMed] [Google Scholar]
- 91.Bassett IV, et al. Linkage to care following community-based mobile HIV testing compared with clinic-based testing in Umlazi Township, Durban, South Africa. HIV medicine. 2014;15:367–372. doi: 10.1111/hiv.12115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bassett IV, et al. Finding HIV in Hard to Reach Populations: Mobile HIV Testing and Geospatial Mapping in Umlazi Township, Durban, South Africa. AIDS and behavior. 2015 doi: 10.1007/s10461-015-1012-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Grabbe KL, et al. Increasing access to HIV counseling and testing through mobile services in Kenya: strategies, utilization, and cost-effectiveness. Journal of acquired immune deficiency syndromes (1999) 2010;54:317–323. doi: 10.1097/QAI.0b013e3181ced126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kranzer K, et al. Incentivized recruitment of a population sample to a mobile HIV testing service increases the yield of newly diagnosed cases, including those in need of antiretroviral therapy. HIV medicine. 2012;13:132–137. doi: 10.1111/j.1468-1293.2011.00947.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mabuto T, et al. Four models of HIV counseling and testing: utilization and test results in South Africa. PloS one. 2014;9:e102267. doi: 10.1371/journal.pone.0102267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Meehan SA, Naidoo P, Claassens MM, Lombard C, Beyers N. Characteristics of clients who access mobile compared to clinic HIV counselling and testing services: a matched study from Cape Town, South Africa. BMC health services research. 2014;14:658. doi: 10.1186/s12913-014-0658-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Morin SF, et al. Removing barriers to knowing HIV status: same-day mobile HIV testing in Zimbabwe. Journal of acquired immune deficiency syndromes (1999) 2006;41:218–224. doi: 10.1097/01.qai.0000179455.01068.ab. [DOI] [PubMed] [Google Scholar]
- 98.Sweat M, et al. Community-based intervention to increase HIV testing and case detection in people aged 16–32 years in Tanzania, Zimbabwe, and Thailand (NIMH Project Accept, HPTN 043): a randomised study. The Lancet infectious diseases. 2011;11:525–532. doi: 10.1016/s1473-3099(11)70060-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Van Rie A, et al. High uptake of systematic HIV counseling and testing and TB symptom screening at a primary care clinic in South Africa. PloS one. 2014;9:e105428. doi: 10.1371/journal.pone.0105428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kalibala S, et al. Factors associated with acceptability of HIV self-testing among health care workers in Kenya. AIDS and behavior. 2014;18(Suppl 4):S405–414. doi: 10.1007/s10461-014-0830-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pant Pai N, et al. Will an unsupervised self-testing strategy for HIV work in health care workers of South Africa? A cross sectional pilot feasibility study. PloS one. 2013;8:e79772. doi: 10.1371/journal.pone.0079772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Arendt V, et al. Clinical screening for HIV in a health centre setting in urban Kenya: an entry point for voluntary counselling, HIV testing and early diagnosis of HIV infection? Tropical doctor. 2007;37:45–47. doi: 10.1258/004947507779951899. [DOI] [PubMed] [Google Scholar]
- 103.Bassett IV, et al. Mobile HIV screening in Cape Town, South Africa: clinical impact, cost and cost-effectiveness. PloS one. 2014;9:e85197. doi: 10.1371/journal.pone.0085197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Creek TL, et al. Botswana’s Tebelopele voluntary HIV counseling and testing network: use and client risk factors for HIV infection, 2000–2004. Journal of acquired immune deficiency syndromes (1999) 2006;43:210–218. doi: 10.1097/01.qai.0000230525.71717.5d. [DOI] [PubMed] [Google Scholar]
- 105.Fiscus SA, et al. Rapid, real-time detection of acute HIV infection in patients in Africa. The Journal of infectious diseases. 2007;195:416–424. doi: 10.1086/510755. [DOI] [PubMed] [Google Scholar]
- 106.Mwangi M, et al. Factors Associated with Uptake of HIV Test Results in a Nationally Representative Population-Based AIDS Indicator Survey. The open AIDS journal. 2014;8:7–16. doi: 10.2174/1874613601408010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.van Schaik N, Kranzer K, Wood R, Bekker LG. Earlier HIV diagnosis--are mobile services the answer? South African medical journal = Suid-Afrikaanse tydskrif vir geneeskunde. 2010;100:671–674. doi: 10.7196/samj.4162. [DOI] [PubMed] [Google Scholar]
- 108.Abdallah TM, Ali AA, Adam I. Provider-initiated HIV testing and counseling among tuberculosis patients in Kassala, Eastern Sudan. Journal of infection and public health. 2012;5:63–66. doi: 10.1016/j.jiph.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 109.Ansa GA, Walley JD, Siddiqi K, Wei X. Delivering TB/HIV services in Ghana: a comparative study of service delivery models. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2014;108:560–567. doi: 10.1093/trstmh/tru110. [DOI] [PubMed] [Google Scholar]
- 110.Bondo M, Modiba MC, Becker P. HIV infection in general surgical patients at the Ga-Rankuwa/MEDUNSA complex South Africa. East African medical journal. 2001;78:395–397. doi: 10.4314/eamj.v78i8.8987. [DOI] [PubMed] [Google Scholar]
- 111.Kiene SM, et al. Initial outcomes of provider-initiated routine HIV testing and counseling during outpatient care at a rural Ugandan hospital: risky sexual behavior, partner HIV testing, disclosure, and HIV care seeking. AIDS patient care and STDs. 2010;24:117–126. doi: 10.1089/apc.2009.0269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Silvestri DM, et al. A comparison of HIV detection rates using routine opt-out provider-initiated HIV testing and counseling versus a standard of care approach in a rural African setting. Journal of acquired immune deficiency syndromes (1999) 2011;56:e9–32. doi: 10.1097/qai.0b013e3181fdb629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.O’Laughlin KN, et al. Clinic-based routine voluntary HIV testing in a refugee settlement in Uganda. Journal of acquired immune deficiency syndromes (1999) 2014;67:409–413. doi: 10.1097/QAI.0000000000000317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nglazi MD, et al. An incentivized HIV counseling and testing program targeting hard-to-reach unemployed men in Cape Town, South Africa. Journal of acquired immune deficiency syndromes (1999) 2012;59:e28–34. doi: 10.1097/QAI.0b013e31824445f0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Adebajo S, et al. Evaluating the effect of HIV prevention strategies on uptake of HIV counselling and testing among male most-at-risk-populations in Nigeria; a cross-sectional analysis. Sex Transm Infect. 2015 doi: 10.1136/sextrans-2014-051659. [DOI] [PubMed] [Google Scholar]
- 116.Charurat ME, et al. Uptake of treatment as prevention for HIV and continuum of care among HIV-positive men who have sex with men in Nigeria. Journal of acquired immune deficiency syndromes (1999) 2015;68(Suppl 2):S114–123. doi: 10.1097/QAI.0000000000000439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Nyblade LC, et al. Population-based HIV testing and counseling in rural Uganda: participation and risk characteristics. Journal of acquired immune deficiency syndromes (1999) 2001;28:463–470. doi: 10.1097/00042560-200112150-00010. [DOI] [PubMed] [Google Scholar]
- 118.Arthur GR, et al. The role for government health centers in provision of same-day voluntary HIV counseling and testing in Kenya. Journal of acquired immune deficiency syndromes (1999) 2005;40:329–335. doi: 10.1097/01.qai.0000166376.23846.38. [DOI] [PubMed] [Google Scholar]
- 119.Gresenguet G, et al. Voluntary HIV counseling and testing: experience among the sexually active population in Bangui, Central African Republic. Journal of acquired immune deficiency syndromes (1999) 2002;31:106–114. doi: 10.1097/00126334-200209010-00014. [DOI] [PubMed] [Google Scholar]
- 120.Hood JE, et al. Client characteristics and gender-specific correlates of testing HIV positive: a comparison of standalone center versus mobile outreach HIV testing and counseling in Botswana. AIDS and behavior. 2012;16:1902–1916. doi: 10.1007/s10461-012-0253-7. [DOI] [PubMed] [Google Scholar]
- 121.Corbett EL, et al. HIV incidence during a cluster-randomized trial of two strategies providing voluntary counselling and testing at the workplace, Zimbabwe. AIDS (London, England) 2007;21:483–489. doi: 10.1097/QAD.0b013e3280115402. [DOI] [PubMed] [Google Scholar]
- 122.Mulongo S, et al. Applying innovative approaches for reaching men who have sex with men and female sex workers in the Democratic Republic of Congo. Journal of acquired immune deficiency syndromes (1999) 2015;68(Suppl 2):S248–251. doi: 10.1097/QAI.0000000000000449. [DOI] [PubMed] [Google Scholar]
- 123.Appiah LT, et al. Efficacy and acceptability of rapid, point-of-care HIV testing in two clinical settings in Ghana. AIDS patient care and STDs. 2009;23:365–369. doi: 10.1089/apc.2008.0224. [DOI] [PubMed] [Google Scholar]
- 124.Kouassi-M’Bengue A, et al. Co-infection of HIV and HBV in voluntary counseling and testing center in Abidjan. Asian Pacific Journal of Tropical Disease. 2011;1:275–278. [Google Scholar]
- 125.Akhigbe RE, Bamidele JO. Prevalence and pattern of utilization of voluntary counseling and testing services and HIV infection in Ogbomoso, southwestern Nigeria. Journal of natural science, biology, and medicine. 2013;4:163–166. doi: 10.4103/0976-9668.107283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kikaya V, et al. Voluntary medical male circumcision programs can address low HIV testing and counseling usage and ART enrollment among young men: lessons from Lesotho. PloS one. 2014;9:e83614. doi: 10.1371/journal.pone.0083614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Govender S, et al. CD4 counts and viral loads of newly diagnosed HIV-infected individuals: implications for treatment as prevention. PloS one. 2014;9:e90754. doi: 10.1371/journal.pone.0090754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.World Bank Development Indicators. [Accessed on 07/07/2015];Prevalence of HIV, (% of total population ages 15–49) from http://data.worldbank.org/indicator/SH.DYN.AIDS.ZS/countries?display=default.
- 129.Agaba PA, et al. Patients who present late to HIV care and associated risk factors in Nigeria. HIV medicine. 2014;15:396–405. doi: 10.1111/hiv.12125. [DOI] [PubMed] [Google Scholar]
- 130.Clouse K, et al. Impact of systematic HIV testing on case finding and retention in care at a primary care clinic in South Africa. Tropical medicine & international health : TM & IH. 2014;19:1411–1419. doi: 10.1111/tmi.12387. [DOI] [PubMed] [Google Scholar]
- 131.Haskew J, et al. Stage of HIV presentation at initial clinic visit following a community-based HIV testing campaign in rural Kenya. BMC public health. 2015;15:16. doi: 10.1186/s12889-015-1367-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Naik R, et al. Linkage to care following a home-based HIV counselling and testing intervention in rural South Africa. Journal of the International AIDS Society. 2015;18:19843. doi: 10.7448/ias.18.1.19843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Govindasamy D, et al. Linkage to HIV care from a mobile testing unit in South Africa by different CD4 count strata. Journal of acquired immune deficiency syndromes (1999) 2011;58:344–352. doi: 10.1097/QAI.0b013e31822e0c4c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.van Zyl MA, Brown LL, Pahl K. Using a call center to encourage linkage to care following mobile HIV counseling and testing. AIDS care. 2015;27:921–925. doi: 10.1080/09540121.2015.1015483. [DOI] [PubMed] [Google Scholar]
- 135.Larson BA, et al. Rapid point-of-care CD4 testing at mobile HIV testing sites to increase linkage to care: an evaluation of a pilot program in South Africa. Journal of acquired immune deficiency syndromes (1999) 2012;61:e13–17. doi: 10.1097/QAI.0b013e31825eec60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Larson BA, et al. Lost opportunities to complete CD4+ lymphocyte testing among patients who tested positive for HIV in South Africa. Bulletin of the World Health Organization. 2010;88:675–680. doi: 10.2471/blt.09.068981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Terris-Prestholt F, et al. The role of community acceptance over time for costs of HIV and STI prevention interventions: analysis of the Masaka Intervention Trial, Uganda, 1996–1999. Sexually transmitted diseases. 2006;33:S111–116. doi: 10.1097/01.olq.0000175389.10289.ba. [DOI] [PubMed] [Google Scholar]
- 138.Kahn JG, et al. Cost of community integrated prevention campaign for malaria, HIV, and diarrhea in rural Kenya. BMC health services research. 2011;11:346. doi: 10.1186/1472-6963-11-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Sharma M, Van Rooyen H, Celum C, Baeten J, Levin C, Barnabas R. The Cost of Community Based HIV Counseling and Testing and Linkage to Care in Rural South Africa: Estimates from the Linkages Randomized Control Trial. Research for Prevention (R4P); Capetown, SA. November, 2014. [Google Scholar]
- 140.Mulogo EM, Batwala V, Nuwaha F, Aden AS, Baine OS. Cost effectiveness of facility and home based HIV voluntary counseling and testing strategies in rural Uganda. African health sciences. 2013;13:423–429. doi: 10.4314/ahs.v13i2.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.McConnel CE, et al. The cost of a rapid-test VCT clinic in South Africa. South African medical journal = Suid-Afrikaanse tydskrif vir geneeskunde. 2005;95:968–971. [PubMed] [Google Scholar]
- 142.Sweat M, et al. Cost-effectiveness of voluntary HIV-1 counselling and testing in reducing sexual transmission of HIV-1 in Kenya and Tanzania. Lancet. 2000;356:113–121. doi: 10.1016/S0140-6736(00)02447-8. [DOI] [PubMed] [Google Scholar]
- 143.Aliyu HB, et al. What is the cost of providing outpatient HIV counseling and testing and antiretroviral therapy services in selected public health facilities in Nigeria? Journal of acquired immune deficiency syndromes (1999) 2012;61:221–225. doi: 10.1097/QAI.0b013e3182683b04. [DOI] [PubMed] [Google Scholar]
- 144.Obure CD, et al. Optimising the cost and delivery of HIV counselling and testing services in Kenya and Swaziland. Sex Transm Infect. 2012;88:498–503. doi: 10.1136/sextrans-2012-050544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Forsythe S, et al. Assessing the cost and willingness to pay for voluntary HIV counselling and testing in Kenya. Health policy and planning. 2002;17:187–195. doi: 10.1093/heapol/17.2.187. [DOI] [PubMed] [Google Scholar]
- 146.Hausler HP, et al. Costs of measures to control tuberculosis/HIV in public primary care facilities in Cape Town, South Africa. Bulletin of the World Health Organization. 2006;84:528–536. doi: 10.2471/blt.04.018606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Thielman NM, et al. Cost-effectiveness of free HIV voluntary counseling and testing through a community-based AIDS service organization in Northern Tanzania. Am J Public Health. 2006;96:114–119. doi: 10.2105/AJPH.2004.056796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Stover J, et al. How can we get close to zero? The potential contribution of biomedical prevention and the investment framework towards an effective response to HIV. PloS one. 2014;9:e111956. doi: 10.1371/journal.pone.0111956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Sabapathy K, Van den Bergh R, Fidler S, Hayes R, Ford N. Uptake of home-based voluntary HIV testing in sub-Saharan Africa: a systematic review and meta-analysis. PLoS medicine. 2012;9:e1001351. doi: 10.1371/journal.pmed.1001351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Novitsky V, et al. Estimated age and gender profile of individuals missed by a home-based HIV testing and counselling campaign in a Botswana community. J Int AIDS Soc. 2015;18:19918. doi: 10.7448/ias.18.1.19918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Choko AT, et al. Uptake, Accuracy, Safety, and Linkage into Care over Two Years of Promoting Annual Self-Testing for HIV in Blantyre, Malawi: A Community-Based Prospective Study. PLoS medicine. 2015;12:e1001873. doi: 10.1371/journal.pmed.1001873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Leblanc NM, Andes KL. An exploration of men’s knowledge, attitudes, and perceptions of HIV, HIV risk, and willingness to test for HIV in Yendi District, Northern Ghana. J Assoc Nurses AIDS Care. 2015;26:281–295. doi: 10.1016/j.jana.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 153.Wheeler T, Wolf RC, Kapesa L, Cheng Surdo A, Dallabetta G. Scaling-up HIV responses with key populations in West Africa. Journal of acquired immune deficiency syndromes (1999) 2015;68(Suppl 2):S69–73. doi: 10.1097/QAI.0000000000000534. [DOI] [PubMed] [Google Scholar]
- 154.Bekker LG, et al. Combination HIV prevention for female sex workers: what is the evidence? Lancet. 2015;385:72–87. doi: 10.1016/s0140-6736(14)60974-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Duvall S, et al. Assessment of policy and access to HIV prevention, care, and treatment services for men who have sex with men and for sex workers in Burkina Faso and Togo. Journal of acquired immune deficiency syndromes (1999) 2015;68(Suppl 2):S189–197. doi: 10.1097/QAI.0000000000000450. [DOI] [PubMed] [Google Scholar]
- 156.Figueroa C, Johnson C, Verster A, Baggaley R. Attitudes and Acceptability on HIV Self-testing Among Key Populations: A Literature Review. AIDS and behavior. 2015;19:1949–1965. doi: 10.1007/s10461-015-1097-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Nsanzimana S, et al. HIV care continuum in Rwanda: a cross-sectional analysis of the national programme. The lancet. HIV. 2015;2:e208–215. doi: 10.1016/S2352-3018(15)00024-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.