Abstract
Objective
The prevalence of osteoarthritis (OA) varies between joints. Cartilage in eight different joints was evaluated to elucidate the disparate susceptibilities between joints to post-traumatic OA (PTOA) and provide evidence for joint specific clinical treatments. The hypothesis was that cartilage in different joints would have varying cell death and anabolic gene expression profiles after injury.
Methods
Adult equine cartilage explants were harvested from shoulder (SH), elbow (EL), carpal (CA), metacarpophalangeal (MC), patellofemoral (FP), tarsal (TA), metatarsophalangeal (MT), and proximal interphalangeal (PP) joints, and were injured by loading with 30 MPa within 1 second. Fractional dissipated energy, cell density, cell death, and gene expression were quantified.
Results
PP had the highest fractional dissipated energy (94%, 95% confidence interval [CI] 88–101%). Cell density was most dense in superficial zone in all samples, with MC and MT having the highest peak density. Injured samples had significantly higher cell death (13.5%, 95% CI 9.1–17.9%) than non-injured samples (6.8%, 95% CI 2.5–11.1%, p=0.016); however, cell death after injury was not significantly different between joints. Gene expression was significantly different between joints. CD-RAP expression in normal cartilage was lowest in FP (Cp=21, 95% CI −80–122). After injury, the change in CD-RAP expression increased and was highest in FP (147% relative increase after injury, 95% CI 64–213).
Conclusion
Different joints have different baseline characteristics, including cell density and gene expression, and responses to injury, including energy dissipation and gene expression. These unique characteristics may explain differences in OA prevalence and suggest differences in susceptibility to PTOA.
Clinical Relevance
Understanding differences in the response to injury and potential susceptibility of OA can lead to the development of preventative or treatment strategies.
Key terms: Gene expression, cartilage injury, chondrocyte, multiphoton microscopy, cartilage biomechanical properties
INTRODUCTION
Osteoarthritis (OA) affects the worldwide population,1 with an estimated 5.6 million Americans suffering from post-traumatic osteoarthritis (PTOA) of lower-extremities, creating a $3 billion annual cost to society.2 Research in OA is often performed by examining a single joint such as the knee, and the findings might be extrapolated to other unrelated joints. With this approach, there is an underlying assumption that different joints respond similarly to varying stimuli and share similar susceptibility to OA. Yet, the prevalence and type of OA is not the same between joints. In 500 patients with combinations of unilateral, bilateral, and multiple-joint OA, the distribution of 847 OA-affected joints has been reported as 41.2% knees, 30.0% hands, 19.0% hips, 4.4% ankles, 3.2% shoulders, 1.6% elbows, and 0.6% wrists.3 Different types of OA have different prevalences in each joint. PTOA which is associated with prior injury, accounts for 12.5%4 of OA in the knee but 54%4–78%5 in the ankle. Conversely, spontaneously-occurring OA not associated with any pathologies, accounts for 82%4 of OA in the knee but only 9%5–14.6%4 in the ankle. The hand can be subcategorized to further demonstrate variability of OA between joints with similar motion. The prevalence of OA in the proximal interphalangeal and metacarpophalangeal joints in 1300 women is 16.5% and 6.8%, respectively.6 Within a single joint, such as the knee, there is varying prevalence of OA between regions. In a three year study, 24% of patients had radiographic signs of OA in the patellofemoral joint, 4% in the tibiofemoral joint, and 41% in both regions of the knee.7 With these differences in prevalence of OA, understanding the differences of healthy cartilage and the resilience to injury between different joints can help facilitate the development of OA therapeutics, particularly joint-specific treatments.
Differences in susceptibility to OA between joints may be influenced by variance in response to stimuli or in biochemical or biomechanical properties of cartilage between joints. These differences have been shown in a small subset of joints. Knee and ankle cartilage respond differently to stimuli. Cytokine stimulation with interleukin-1β decreases the synthesis of glycosaminoglycans (GAGs) in the knee to a greater extent than in the ankle, where 3.5 pg/ml and 35 pg/ml, respectively, are needed to elicit the same 50% reduction in GAG synthesis.8 Mechanical stimulation by cyclic hydrostatic pressure (0.33 Hz, 16 kPa max gauge pressure) increases aggrecan expression in knee chondrocytes but does change the expression in ankle chondrocytes.9 Knee and ankle cartilages have different biochemical and mechanical properties. Dynamic stiffness and GAG content are higher in the ankle than the knee.10 The reported differences between this small subset of joints suggest that differences might occur more widely among joints than previously thought.
Cellular distribution and cartilage thickness vary between joints and correlate with biomechanical properties. These characteristics may elucidate differences in susceptibility to OA. Chondrocyte density inversely correlates with thickness of cartilage: thinner cartilage has a higher cell density (as many as 330,000 cells mm−3), and thicker cartilage has a lower cell density (up to 14,000 cells mm−3).11 Thicker tissue has both a smaller proportion of superficial zone cartilage12 and a lower superficial zone cell density.11 The superficial layer is important to compressive and shear biomechanical properties,12, 13 with superficial zone thickness having a positive correlation with isotropic indentation modulus.12 While cartilage thickness has been shown to be unrelated to normal standing stress,14 it can be altered by injury.15 This suggests that cartilage thickness and density may contribute to but not define susceptibility to OA. Cellular distribution as a function of depth may help further characterize differences between joints and be a factor in resilience to cartilage damage and development of OA.
Current literature suggests variability between a small subset of major joints exists, both with respect to baseline cellular density, synthesis activities, and response to injury. While injury has been established to cause cell death in cartilage,16, 17 the difference between multiple joints in response to injury has not been studied. Understanding these differences will lead to a better understanding the unique progression of PTOA between joints and the potential for future development of joint-specific OA treatments.
In the present study, the aim was to answer the following questions by evaluating cartilage in eight major equine joints:
Are there variations between joints in normal cartilage characteristics, as defined by cell viability, distribution, and density, and gene expression?
Are some joints more resilient to compressive loading and the resulting cell death and catabolic gene expression than other joints?
By answering these questions, this paper presents evidence supporting the hypothesis that cartilage in different joints is inherently different. Consequently, susceptibility to PTOA is different. Our findings of in vitro cell death and gene expression reveal that the pathogenesis of PTOA is likely joint-specific.
METHODS
Tissue collection and injury model
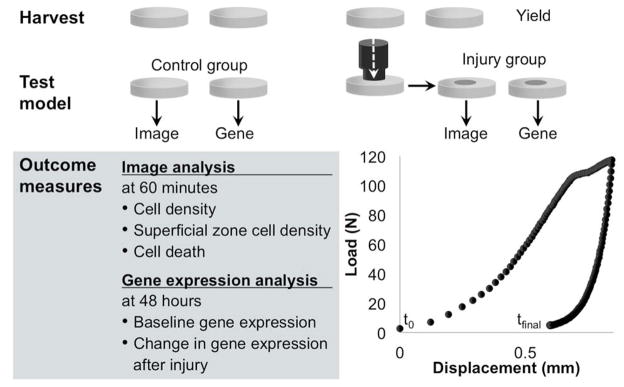
Six millimeter diameter cartilage biopsy punches were harvested aseptically from young adult horses (ages 2.5–4 years, n=4) immediately after euthanasia with approval from the Institutional Animal Care and Use Committee. Articular cartilage was harvested from the high impact region of caput humeri of the shoulder joint (SH), condylus lateralis radii of the elbow joint (EL), proximal surface of os carpale III of the carpal joint (CA),18 condylus lateralis metacarpi III of the metacarpophalangeal joint (MC),19, 20 condylus lateralis phalanx proximalis III of the proximal interphalangeal joint (PP), condylus lateralis femoris/trochlea ossis femoris of the patellofemoral joint (FP),21 the distal surface of os tarsi centrale of the tarsal joint (TA),21 and condylus lateralis metatarsi III of the metatarsophalangeal joint (MT).19–21 Cartilage was grossly evaluated and scored using the International Cartilage Repair Society Clinical Cartilage Injury Evaluation System - 2000.22, 23 Explants were equally divided into control and injury groups and then equally distributed for either imaging or gene expression analysis (Figure 1). Cartilage explants used for imaging were marked with a standard laboratory permanent marker at harvest to ensure that all images were acquired in the same anterior-posterior orientation. Explants were placed in phenol red-free MEM with 25 mM Hepes, 100 IU/ml penicillin, and 100 μg/ml streptomycin.
Figure 1.
Schematic of methods. Biopsy punches (diameter=6 mm) were harvested (n=4 animals), divided equally into control and injured groups, and distributed to image or gene expression analysis.
For compressive injury, explants were briefly removed from media and placed in a custom chamber containing the described media. The chamber was then placed under 2.25 mm-diameter indenter on an EnduraTEC ELF3200 mechanical test frame (EnduraTec, Minnetonka, MN). The articular surface was injured with a single compression of 117.4 N (95% confidence interval [CI] 117.6–117.2 N), to achieve a stress of 30 MPa24, 25 under load control within 1 second. Samples were subjected to a mean peak stress rate of 130 MPa sec−1 (95% CI 125–136), with no significant differences of peak stress rates found between samples (p=0.731). Temporal load and displacement data were recorded, and force-displacement curves (Figure 1) were generated for each sample by plotting the raw data. The load at which the sample yielded was found by generating the second derivative of the force-displacement curve, with respect to displacement by numerical differentiation using Matlab (Mathworks, Natick, MA). The yield point was defined as the minima on the second derivative curve. Fractional dissipated energy was calculated from the force-displacement curve using the following equation
where E represents the fractional dissipated energy, F represents the force, x represents displacement, and t represents time where tm is the time at maximum displacement and tf is the end of the impact. This quantity measures the energy dissipated within the system normalized to the maximum energy applied to the system. It represents the fraction of the energy applied during loading that is dissipated.
After injury, explants for imaging were immediately replaced into media and incubated for 60 minutes at 37°C at 5% CO2 to allow any immediate biological changes and cell death to occur. Gene expression explants were transferred to Ham’s F-12 medium, containing 25 mM Hepes, 2 mM L-glutamine, 50 μg/ml ascorbic acid, 30 μg/ml α-ketoglutaric acid, and 10% FBS and incubated for 48 hours at 37°C at 5% CO2 to capture changes in expression of a diverse profile of genes.
Multiphoton data acquisition and analysis
After the 60 minute incubation, explants were cut in cross-section and placed in 1 μM sodium fluorescein (AK-FLUOR 25%, Akorn, Inc., Lake Forest, IL) in PBS for multiphoton microscopy (MPM) imaging.25 Images were collected using a Tsunami titanium:sapphire laser (Newport Corp., Irvine, CA) with 780 nm wavelength at 100 fs pulses and 80 MHz. Emission spectra were collected through a 670 nm long-pass dichroic and photomultiplier tubes using filters of 380–490 nm to collect second harmonic generation, and 510–650 nm to collect fluorescein emission.
To quantify cell density, images from normal controls were converted to binary images using Fiji with Sauvola local threshold.26 Binary images were processed with custom code in MATLAB (MathWorks, Natick, MA) to identify and quantify all chondrocytes as a function of depth, using 50 μm binning. The relationship of cell density and depth for each sample was evaluated by fitting an exponential decay curve to the data points to characterize how the density of cells changed from the articular surface to the deep zone. The exponential decay curve’s decay length (“b”, where b=x in y=Ae−x/b) was quantified for each individual sample to utilize a numerical characteristic to describe the change in cell density with depth. A smaller value of decay rate length indicates a relatively dense region of cells at the surface and a significantly less dense region of cells in the middle and deep zones. A larger value of decay rate length indicates a more uniform distribution of cells among the surface, middle, and deep zones. This decay rate length was compared between joints.
To quantify the density of cells in the superficial zone,, cells from the superficial zone were identified in the above described images by selecting those cellular profiles demonstrating an orientation within [−16°, 16°] compared to the articular surface. A histogram distribution of cellular profiles with 50 μm binning was calculated to determine the maximum density and depth at which maximal density occurred. Cell death was manually quantified in normal controls and injured samples using 50 μm binning.
Gene expression analysis
After 48 hours, explants for gene expression were rinsed in PBS, transferred to RLT lysis buffer (Qiagen, Germantown, MD), snap frozen in liquid nitrogen, and stored at −80°C until processing. The explant and buffer were pulverized in liquid nitrogen using a mortar and pestle. Then total RNA was isolated using the RNeasy Fibrous Tissues mini kit (Qiagen). Species-specific intron-spanning equine primers were used to amplify cartilage genes collagen type 2α1 (COL2A1), aggrecan (AGG), cartilage-derived retinoic acid-sensitive protein (CD-RAP), heat shock protein 90 (HSP90) and inflammatory genes serum amyloid A (SAA), matrix metalloproteinase 1 (MMP-1), and MMP-13. Primers are listed in Table 1. Quantitative real-time RT-PCR (qPCR) was performed using the LightCycler® Fast Start DNA Master SYBR Green I and LightCycler ® Real-Time PCR System (Roche Diagnostics, Indianapolis, IN). Results were calculated using the efficiency corrected calculation method also known as the Roche Applied Sciences E(efficiency)-method: Normalized relative ratio = EtCT (target calibrator) – CT (target sample) / Er CT (reference calibrator) – CT (reference sample).27 All genes were normalized to 18S. Expression is reported as normalized Cp ratios with higher normalized ratio values indicating higher gene expression.
Table 1.
Primers used for real time quantitative RT-PCR. Primers were designed by the authors unless otherwise indicated.34
| Primer name | Primer sequence (5′ → 3′) | Primer source |
|---|---|---|
| 18S F | GAT ACC GCA GCT AGG AAT | DQ222453.1 |
| 18S R | ATC TGT CAA TCC TGT CCG | |
| Aggrecan F | CTT AGA GGA CAG AAA GCG AC | Trumble et al34 |
| Aggrecan R | ACT TTG GGC GGA AGA AGG | |
| CD-RAP F | ATG CCC AAG CTG GCT GA | EF679787 |
| CD-RAP R | CTT CGA TTT TGC CAG GTT TC | |
| Collagen type 2α1 F | TCT GCA GAA TGG GCA GAG GTA TA | NM_031163 |
| Collagen type 2α1 R | GAT AAT GTC ATC GCA GAG GAC ATT C | |
| HSP 90 F | GGA TCT GGT CAT CCT GCT CTA C | NM_001163955.1 |
| HSP 90 R | ACG TGT CGT CAT CTC CTT CA | |
| MMP-1 F | CAG TGC CTT CAG AAA CAC GA | AF148882.1 |
| MMP-1 R | GCT TCC CAG TCA CTT TCA GC | |
| MMP-13 F | GCT GCC TAT GAG CAT CCT TC | NM_001081804.1 |
| MMP-13 R | ACC TCC AGA CCT GGT TTC CT | |
| Serum amyloid A F | CCT GGG CTG CTA AAG TCA TC | AF240364.1 |
| Serum amyloid A R | AGG CCA TGA GGT CTG AAG TG |
Statistical analysis
To determine the differences between joints in cartilage compressive loading at yield point, fractional dissipated energy, chondrocyte density decay rate length, superficial zone chondrocyte peak density and density z-depth location, cell death in control and after injury, a mixed model ANOVA while treating joint as fixed factor and the horse ID as random factor with an LSD post-hoc analysis was used. Assumptions of normality of residuals were verified. All statistical analyses were performed with SPSS version 22 (IBM Corporation, Armonk, NY), and significance was evaluated at p<0.05.
RESULTS
Cartilage injury yield and fractional dissipative energy
To compare the mechanical response to compressive loading, the peak load at the yield point and the fractional dissipative energy was determined. The peak load at which tissue yielded was not significantly different between joints (p=0.185). PP yielded at the highest load of 93 N (95% CI 78–108), followed by MT (91 MPa, 95% CI 77–106), MC (90, 75–104), TA (89, 74–104), SH (87, 70–104), FP (78, 61–95), CA (77, 62–92), and EL (67, 53–82). The fractional dissipated energy was significantly different between joints (p=0.003, Table 2 with maximum displacement), with PP (94%, 95% CI 88–101%) having the highest fractional dissipated energy, followed by SH, EL, FP, TA, CA, MT, and the lowest MC (78%, 95% CI 72–85%).
Table 2.
The fractional dissipated energy was significantly different between shoulder (SH), elbow (EL), carpal (CA), metacarpal (MC), proximal interphalangeal (PP), patellofemoral (FP), tarsal (TA), and metatarsal (MT) joints (p=0.003). The maximum displacement as distance (mm) and fractional dissipated energy as percentage is reported with 95% confidence interval (CI95). An LSD post-hoc test was used to determine differences between joints and is reported with non-shaded boxes having p≥0.05, light gray boxes having 0.001≤p<0.05, and dark gray boxes having p<0.001.
| PP | MT | TA | FP | MC | CA | EL | SH | |
|---|---|---|---|---|---|---|---|---|
| Max disp (CI95) | 0.84 (0.76–0.91) | 0.44 (0.33–0.56) | 0.34 (0.29–0.38) | 1.12 (0.77–1.46) | 0.44 (0.37–0.51) | 0.29 (0.27–0.30) | 0.57 (0.54–0.59) | 0.88 (0.73–1.04) |
| % (CI95) | 94 (88,101) | 80 (74,87) | 83 (77,89) | 87 (80,94) | 78 (72,85) | 82 (76,89) | 87 (81,94) | 93 (86,101) |
| PP | 0.001 | 0.007 | 0.090 | <0.001 | 0.004 | 0.069 | 0.811 | |
| MT | 0.457 | 0.102 | 0.572 | 0.604 | 0.076 | 0.004 | ||
| TA | 0.322 | 0.198 | 0.819 | 0.278 | 0.019 | |||
| FP | 0.037 | 0.233 | 0.993 | 0.169 | ||||
| MC | 0.284 | 0.024 | 0.001 | |||||
| CA | 0.193 | 0.012 | ||||||
| EL | 0.142 |
Chondrocyte distribution
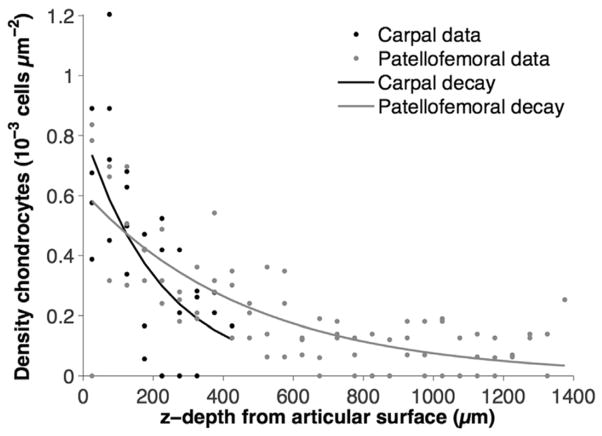
The distribution of cells was fit to an exponential decay curve to determine if cell density varied in cartilage between different joints (Table 3, Figure 2). The exponential decay rate length of cell density was not statistically different between joints (p=0.254). Maximal decay rate length was found in the FP joint (560 μm, 95% CI −169–1290) indicating a more diffuse distribution of cells from surface to deep zone. Minimal decay rate length was found in the CA joint (273 μm, 95% CI 48–497) indicating a high density of the cells at the surface and a rapid decrease in the density of cells with depth. The goodness of fit for the exponential model was evaluated by the correlation R2. The best fit was found in the MT joint (R2=0.78). The worst fit was found in the SH joint (R2=0.57).
Table 3.
Chondrocyte density decay rate length with goodness of fit as R2. An exponential decay curve was fit to the data points of cell density as a function of depth. A smaller value has a shorter exponential decay of cell density than a larger value. Cartilage in the shoulder (SH), elbow (EL), carpal (CA), metacarpal (MC), proximal interphalangeal (PP), patellofemoral (FP), tarsal (TA), and metatarsal (MT) joints were evaluated. Decay rate lengths were not statistically different between joints (p=0.289, n=4 horses). CI=confidence interval.
| SH | EL | CA | MC | PP | FP | TA | MT | |
|---|---|---|---|---|---|---|---|---|
| Decay length (μm) | 573 | 281 | 273 | 345 | 420 | 560 | 236 | 266 |
| 95% CI: lower | −242 | 122 | 48 | −520 | −285 | −169 | 134 | 151 |
|
| ||||||||
| 95% CI: upper | 1390 | 440 | 497 | 1210 | 1124 | 1290 | 338 | 381 |
|
| ||||||||
| R2 | 0.56 | 0.75 | 0.60 | 0.71 | 0.67 | 0.61 | 0.77 | 0.78 |
| 95% CI: lower | −0.15 | 0.57 | 0.29 | 0.03 | −0.03 | −0.14 | 0.08 | 0.60 |
| 95% CI: upper | 1.28 | 0.92 | 0.91 | 1.39 | 1.36 | 1.37 | 1.46 | 0.96 |
Figure 2.
Exponential decay rate length of cell density in cartilage, by y=Ae−x/b. The thickest cartilage with the longest decay rate length was found in the patellofemoral joint (FP, mean=573 μm, 95% CI −169–1290 μm) and is shown with the thinnest cartilage with the shortest decay rate length that was found in the carpal joint (CA, mean=273 μm, 95% CI 48–497 μm). Lengths of exponential decay rate between joints were not statistically different from each other (p=0.289, n=4).
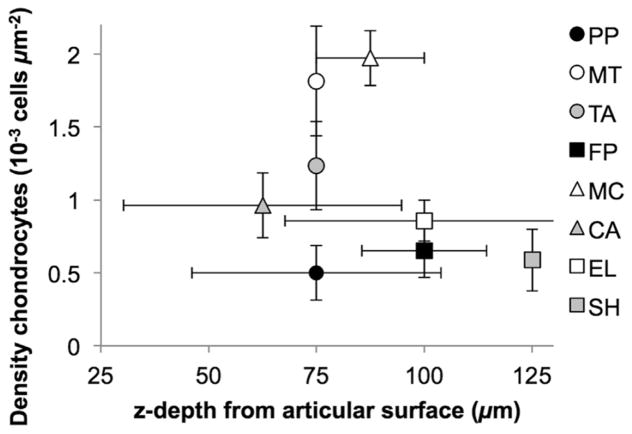
Density of chondrocytes within the superficial zone was different between joints, range: 0.20–2.54x10−3 cells/μm2 (Figure 3, Table 4). MC had the highest mean density and was significantly higher than FP with the lowest density (p<0.001), PP (p=0.001), SH (p=0.002), EL (p=0.002), CA (p=0.005), and TA (p=0.032). MT had the second highest density and was significantly higher than FP (p=0.002), PP (p=0.003), SH (p=0.007), EL (p=0.007), and CA (p=0.015). The depth at which the maximal cell density occurred was not statistically different between joints (p=0.306, Figure 3). Peak density occurred at 0–50 μm in 10% of samples, 50–100 μm in 60%, 100–150 μm in 27%, and 150–200 μm in 3% of samples. The overall mean depth of peak density was 87 μm (95% CI 75–99 μm).
Figure 3.
Distribution of superficial zone chondrocyte profiles. Peak superficial zone cell density was significantly higher in the metacarpus (MC) than the patellofemoral (FP, p=0.001), proximal interphalangeal (PP, p=0.001), shoulder (SH, p=0.003), elbow (EL, p=0.002), carpal (CA, p=0.005), and tarsal (TA, p=0.033) joints. Density in the metatarsus (MT) was significantly higher than FP (p=0.002), PP (p=0.004), SH (p=0.008), EL (p=0.007), and CA (p=0.016).
Table 4.
Distribution of superficial zone chondrocytes. Peak superficial zone cell density was significantly higher in the metacarpus (MC) than the patellofemoral (FP, p<0.001), proximal interphalangeal (PP, p=0.001), shoulder (SH, p=0.002), elbow (EL, p=0.002), carpal (CA, p=0.005), and tarsal (TA, p=0.032) joints. Density in the metatarsus (MT) was significantly higher than FP (p=0.002), PP (p=0.003), SH (p=0.007), EL (p=0.007), and CA (p=0.015).
| SH | EL | CA | MC | PP | FP | TA | MT | |
|---|---|---|---|---|---|---|---|---|
| Peak density | 0.78*° | 0.86*° | 0.96*° | 1.97 | 0.67*° | 0.67*° | 1.23* | 1.80 |
| 95% CI: lower | 0.30 | 0.41 | 0.26 | 1.37 | 0.15 | 0.06 | 0.27 | 0.62 |
| 95% CI: upper | 1.27 | 1.31 | 1.67 | 2.57 | 1.19 | 1.24 | 2.20 | 3.01 |
indicates p<0.05 difference from MC;
°indicates p<0.05 difference from MT. CI=confidence interval; n=4 horses. Quantities reported as 10−3 cells μm−2.
Cell death after injury
Cell death was compared between control and injured samples to determine if there was a difference in susceptibility to death after injury between joints. Overall injured samples had significantly higher cell death (13.5%, 95% CI 9.1–17.9%) than non-injured samples (6.8%, 95% CI 2.5–11.1%, p=0.016). However, cell death was not significantly different between joints within controls ((p=0.567) or within injured joints (p=0.995). MC had the highest relative increase in cell death after injury (16.7% difference, 95% CI 1.5–32.6% difference), followed by PP (16.7, −2.4–35.8), TA (14.8, 1.3–28.3), MT (14.7, −0.9–30.3), FP (13.3, −2.3–28.8), EL (12.3, −1.2–25.8), TA (12.1, −1.4–25.6), and SH (8.9, −6.6–24.5).
Gene expression
Gene expression in control, uninjured samples was evaluated to determine if there were differences in chondrocyte expression between joints. Expression levels of AGG p=0.039, CD-RAP (p<0.001), COL2A1 (p=0.001), HSP90 (p=0.024), SAA (p=0.039), and MMP-1 (p=0.001) were significantly different between joints (CD-RAP Table 5; all others Supplemental Table). No significant differences in baseline gene expression were found in MMP-13 (p=0.138).
Table 5.
Baseline mRNA levels and change in mRNA levels after injury was significantly different between joints (p<0.001 and p=0.028, respectively). Increased Cp ratio values represent an increase in gene expression levels, and decreased Cp ratio values represent a decrease in gene expression; mRNA expression after injury is shown as percent difference between control and injured. A mixed model ANOVA with joint as a fixed factor and the horse as a random factor was used to compare gene expression between shoulder (SH), elbow (EL), carpal (CA), metacarpal (MC), proximal interphalangeal (PP), patellofemoral (FP), tarsal (TA), and metatarsal (MT) joints. The mean Cp value with 95% confidence interval (95CI) is reported. An LSD post-hoc test was used to determine differences between joints and is reported with non-shaded boxes having p≥0.05, light gray boxes having≤0.001 p<0.05, and dark gray boxes having p<0.001
| CD-RAP: contr | PP | MT | TA | FP | MC | CA | EL | SH |
|---|---|---|---|---|---|---|---|---|
| Cp | 344 (243,444) | 171 (70,272) | 309 (209,410) | 21 (−80,122) | 243 (143,344) | 53 (−48,153) | 81 (−20,182) | 58 (−43,159) |
| PP | 0.016 | 0.605 | <0.001 | 0.143 | <0.001 | 0.001 | <0.001 | |
| MT | 0.05 | 0.035 | 0.289 | 0.089 | 0.189 | 0.103 | ||
| TA | <0.001 | 0.331 | 0.001 | 0.002 | 0.001 | |||
| FP | 0.003 | 0.641 | 0.378 | 0.585 | ||||
| MC | 0.009 | 0.023 | 0.011 | |||||
| CA | 0.673 | 0.936 | ||||||
| EL | 0.733 |
| CD-RAP: injury | PP | MT | TA | FP | MC | CA | EL | SH |
|---|---|---|---|---|---|---|---|---|
| Cp | 72 (44,100) | 39 (22,55) | 64 (43,85) | 140 (91,190) | 3 (2,4) | 277 (44,510) | 21 (14,27) | 21 (21,22) |
| % difference | −34 (−117,49) | 18 (−65,102) | 30 (−54,113) | 147 (64,231) | −57 (−140,26) | 101 (5,197) | 25 (−58,108) | −41 (−125,42) |
| PP | 0.368 | 0.275 | 0.004 | 0.689 | 0.039 | 0.312 | 0.898 | |
| MT | 0.844 | 0.033 | 0.198 | 0.194 | 0.910 | 0.305 | ||
| TA | 0.050 | 0.141 | 0.260 | 0.933 | 0.225 | |||
| FP | 0.002 | 0.457 | 0.042 | 0.003 | ||||
| MC | 0.017 | 0.164 | 0.785 | |||||
| CA | 0.673 | 0.936 | ||||||
| EL | 0.733 |
AGG expression in PP was 7.0x higher than in FP (p=0.005), 8.3x higher than CA (p=0.004), 4.9x higher than EL (p=0.008), and 2.4x higher than SH (p=0.045). AGG expression was also 5.3x and 6.3x higher in PP than FP (p=0.033) and CA (p=0.028), respectively.
CD-RAP expression in PP was 2.0x higher than in MT (p=0.016), 16.3x higher than in FP (p<0.001), 6.6x higher than in CA (p<0.001), 4.3x higher than in EL (p=0.001), and 5.9x higher than in SH (p<0.001). CD-RAP expression in TA was 1.8x higher than in MT (p=0.050), 14.6x higher than in FP (p<0.001), 5.9x higher than in CA (p=0.001), 3.8x higher than in EL (p=0.002), and 5.3x higher than in SH (p=0.001). CD-RAP expression in CA was 11.5x higher than FP (p=0.003), 4.6x higher than TA (p=0.009), 3.0x higher than EL (p=0.023), and 4.2x higher than SH (p=0.003).
COL2A1 expression in PP was 3.5x higher than in MT (p<0.001), 2.5x higher than in TA (p=0.002), 13.9x higher than in FP (p<0.001), 2.8x higher than in MC (p=0.001), 5.8x higher than in CA (p<0.001), 5.8x higher than in EL (p<0.001), and 9.6x higher than in SH (p<0.001).
HSP90 expression in PP was 2.2x higher than in MT (p=0.030), 5.5x higher than in FP (p=0.002), 3.2x higher than in MC (p=0.008), 3.8x higher than in CA (p=0.005), 6.0x higher than in EL (p=0.002), and 6.1x higher than in SH (p=0.002).
MMP-1 expression in TA was 4.4x higher than in PP, 4.4x higher than in MT, 4.7x higher than in FP, 2.8x higher than in MC, 2.7x higher than in CA, 4.5x higher than in EL, and 4.1x higher than in SH (all p<0.001).
SAA expression in TA was 13.4x higher than PP (p=0.003), 2.6x higher than MC (p=0.034), 4.5x higher than CA (p=0.009), 3.3x higher than EL (p=0.018), and 4.4x higher than SH (p=0.018). SAA expression in FP was 10.3x higher than in PP (p=0.018).
Changes in gene expression after injury were analyzed to evaluate differences in the gene response of chondrocytes between joints. After injury, there was a significant difference between joints in gene expression of CD-RAP (p=0.028, Table 5). No other significant differences were found (18S p=0.582, AGG p=0.589, COL2A1 p=0.063, HSP90=0.328, MMP-1 p=0.761, MMP-13 p=0.191, SAA p=0.248).
DISCUSSION
Comparison of baseline characteristics in normal cartilage and response to injury in eight different joints was performed to better understand the response of cartilage to traumatic compressive injury. Between joints, differences were found in fractional dissipated energy, superficial zone cell density, and gene expression. These results suggest joints may have varying susceptibilities to the development of PTOA.
Explants were loaded with a peak stress previously shown to cause cell death,24 with a mean peak stress rate shown to cause surface fissures and cell death.28 This loading condition was used to create an injury model that results in increased but not 100% cell death. Similar loading conditions have been used as injury models to understand PTOA. While different joints will experience different loading in vivo, a similar loading condition that represents about 2–3-fold the normal physiologic loading in the equine forelimb29 was used in the present study for all joints to minimize variability within the model. Using the same loading condition for all joints may not be reflective of joint-specific in vivo conditions and would have also led to the resulting strains of explants being different between joint. However, without established joint-specific injury models, this load-control model helped to minimize other varying factors. The peak stress at which samples yielded was not significantly different. The relative quantity of energy that was dissipated from loading was significantly different between joints. This fractional dissipated energy was highest in PP followed by SH, EL, and FP. Joints with lower fractional dissipated energy were TA, CA, MT, and the lowest MC. A negative correlation between shoulder and knee cartilage thickness and stiffness determined by equilibrium modulus has been previously reported in beagles,12 suggesting that joints with different thickness cartilage may dissipate energy differently during compressive loading. Similarly, FP was found to have higher dissipated energy and has been shown to have thicker cartilage than CA or MC.30 However, thickness of cartilage is unrelated to normal standing stress.14 This suggests that although a single joint in a limb will see similar forces in vivo, cartilages will have different thickness between joints and different abilities to absorb compressive loading. While this information may not directly help minimize the risk for developing OA in thicker cartilages, it conveys a need to treat and study OA differently between these very different types of cartilage.
Cell density was quantified to determine which joints had differences in exponential decay rate length of cell density from superficial to deep zones. The cell density decay rate was not statistically different between joints. This suggests that distribution of cells within cartilage from articular surface to the deep zone was similar between joints, with most cells found in the superficial zone. Full-depth cellular distribution may not be a key factor in susceptibility to PTOA development. However, cell density within the superficial zone was significantly different between joints. Cell density in the superficial zone was higher in both the MC and MT than in other joints. The importance of the superficial zone in absorbing energy from trauma has become more apparent in recent research.13 For example, thickness of the superficial zone has been associated with mechanical properties of cartilage, including having a positive correlation with the isotropic indentation modulus.12 Furthermore, differences in the response to cytokine and mechanical stimuli8, 9 and equilibrium modulus and stiffness of cartilage10, 21 may result from differences in concentration of superficial zone cells. This suggests that if the superficial zone is different between joints, one joint may be able to absorb energy without mechanical damage while another joint under the same conditions may experience matrix damage.
Cell death was increased with the chosen loading condition, which is similar to previous studies,24, 25 allowing for comparison of death and gene expression after injury. Yet, no significant differences in cell death between joints were found, suggesting that although cell death could be associated with development of disease, it might not be correlated with varying prevalence of PTOA between joints.
Gene expression was quantified to investigate differences in cellular activity between joints. AGG, CD-RAP, COL2A1, MMP-1, and SAA had significantly different mRNA expression between joints in control cartilage. Expression of CD-RAP is associated with increased cell proliferation and is diminished in severe OA.31 CD-RAP was examined because its expression can change during very early response to stimuli (<48 hours) and is down-regulated by IL-1β and TGF-β.32 Synthesis of collagen type II is important for maintaining the cartilage matrix.33 Aggrecan is a key component of the matrix. SAA and MMP-1 are indicators of cellular catabolism. Overall, FP tended to have lower anabolic gene expression, including AGG, CD-RAP, and COL2A1, and higher expression of SAA. High SAA and MMP-1 expression was similarly found in TA. PP tended to have the opposite trend. After injury, CD-RAP expression was significantly increased in FP, indicating that it is a faster responder to injury than other joints. However, this immediate response cannot be extrapolated to long term response or long term healing. Interestingly, superficial zone cell density was found to be less in FP and TA than MC. This could indicate that joints such as MCs with high superficial zone cellular density might respond differently than cartilage of low superficial zone cellular density joints. However, additional factors not examined in this paper, such as in vivo loading conditions during every day activities, may contribute to these gene expression differences.
The random effect of individual animal was used in the statistical model. The animals were not bred for research and did not have controlled activity, as would be similarly found with people. While there was individual variability present, one individual was not consistently more resilient to injury than another. Images from injured samples were manually counted due to the high irregularity of collagen and fluorescence signals. Decreased signal emission in the impacted site made identification of cells, dead or alive, difficult and may have lead to a diminished area of damaged tissue being counted, creating a potential lower reported cell death. Yet, significance was found between control and injured groups despite this potential limitation.
Overall, differences were found between joints in fractional dissipated energy, superficial zone cell density, and gene expression. Superficial zone chondrocytes were most dense in MT and MC, suggesting cells in this zone may be different between joints. Baseline CD-RAP was lower in FP, yet after injury, CD-RAP expression increased in FP. These differences between joints suggest findings from a single joint cannot be extrapolated to other joints. Baseline characteristics of chondrocytes in different joints may have an effect on how cartilage responds differently to injury between joints. This may result in different pathologies of PTOA between joints and subsequently in joint-specific future targets for PTOA treatment.
Supplementary Material
What is known about the subject
The prevalence of OA is variable among joints; however, most laboratory studies are performed on a single joint – most commonly the knee and extrapolated to other joints such as the ankle or shoulder. A small number of studies have compared knee and ankle cartilage and reported differences in mechanical properties and gene expression.
What this study adds to existing knowledge
There are differences in baseline cell density and gene expression and differences in response to injury, including gene expression and cell death. This suggests that there are inherent differences leading to varying susceptibilities in OA prevalence among joints. Joint-specific treatments may improve OA therapies.
Acknowledgments
The authors would like to Jesse Silverberg for assistance with image analysis and Hussni Mohammed and Laura Goodman for assistance with statistics.
FUNDING SOURCES
This investigation was supported by the Harry M. Zweig Foundation for Equine Research (LAF) and grant TL1RR000459 of the Clinical and Translational Science Center at Weill Cornell Medical College (KDN).
Footnotes
AUTHOR CONTRIBUTIONS
Corresponding author Lisa Fortier, Lise Berg, Kira Novakofski, and Ilaria Bronzini designed the project. Kira Novakofski, Lise Berg, Ilaria Bronzini, and Sarah Poland collected data. Kira Novakofski, Lise Berg, Edward Bonnevie, and Lawrence Bonassar performed data analysis. Kira Novakofski drafted the paper; all other authors reviewed the paper for important intellectual content. All authors approved the final submitted version.
CONFLICT OF INTEREST
The authors have no competing financial interests to disclose.
References
- 1.Woolf AD, Pfleger B. Burden of major musculoskeletal conditions. Bull World Health Organ. 2003;81:646–56. [PMC free article] [PubMed] [Google Scholar]
- 2.Brown TD, Johnston RC, Saltzman CL, Marsh JL, Buckwalter JA. Posttraumatic osteoarthritis: a first estimate of incidence, prevalence, and burden of disease. J Orthop Trauma. 2006;20:739–44. doi: 10.1097/01.bot.0000246468.80635.ef. [DOI] [PubMed] [Google Scholar]
- 3.Cushnaghan J, Dieppe P. Study of 500 patients with limb joint osteoarthritis. I. Analysis by age, sex, and distribution of symptomatic joint sites. Ann Rheum Dis. 1991;50:8–13. doi: 10.1136/ard.50.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saltzman CL, Salamon ML, Blanchard GM, Huff T, Hayes A, Buckwalter JA, et al. Epidemiology of ankle arthritis: report of a consecutive series of 639 patients from a tertiary orthopaedic center. Iowa Orthop J. 2005;25:44. [PMC free article] [PubMed] [Google Scholar]
- 5.Valderrabano V, Horisberger M, Russell I, Dougall H, Hintermann B. Etiology of ankle osteoarthritis. Clin Orthop Relat Res. 2009;467:1800–6. doi: 10.1007/s11999-008-0543-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haugen IK, Englund M, Aliabadi P, Niu J, Clancy M, Kvien TK, et al. Prevalence, incidence and progression of hand osteoarthritis in the general population: the Framingham Osteoarthritis Study. Ann Rheum Dis. 2011;70:1581–6. doi: 10.1136/ard.2011.150078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duncan R, Peat G, Thomas E, Hay E, Croft P. Incidence, progression and sequence of development of radiographic knee osteoarthritis in a symptomatic population. Ann Rheum Dis. 2011;70:1944–8. doi: 10.1136/ard.2011.151050. [DOI] [PubMed] [Google Scholar]
- 8.Eger W, Schumacher BL, Mollenhauer J, Kuettner KE, Cole AA. Human knee and ankle cartilage explants: catabolic differences. J Orthop Res. 2002;20:526–34. doi: 10.1016/S0736-0266(01)00125-5. [DOI] [PubMed] [Google Scholar]
- 9.Orazizadeh M, Cartlidge C, Wright M, Millward-Sadler S, Nieman J, Halliday B, et al. Mechanical responses and integrin associated protein expression by human ankle chondrocytes. Biorheology. 2006;43:249–58. [PubMed] [Google Scholar]
- 10.Treppo S, Koepp H, Quan EC, Cole AA, Kuettner KE, Grodzinsky AJ. Comparison of biomechanical and biochemical properties of cartilage from human knee and ankle pairs. J Orthop Res. 2000;18:739–48. doi: 10.1002/jor.1100180510. [DOI] [PubMed] [Google Scholar]
- 11.Stockwell R. The interrelationship of cell density and cartilage thickness in mammalian articular cartilage. J Anat. 1971;109:411. [PMC free article] [PubMed] [Google Scholar]
- 12.Korhonen R, Wong M, Arokoski J, Lindgren R, Helminen H, Hunziker E, et al. Importance of the superficial tissue layer for the indentation stiffness of articular cartilage. Med Eng Phys. 2002;24:99–108. doi: 10.1016/s1350-4533(01)00123-0. [DOI] [PubMed] [Google Scholar]
- 13.Buckley MR, Gleghorn JP, Bonassar LJ, Cohen I. Mapping the depth dependence of shear properties in articular cartilage. J Biomech. 2008;41:2430–7. doi: 10.1016/j.jbiomech.2008.05.021. [DOI] [PubMed] [Google Scholar]
- 14.Simon WH. Scale effects in animal joints. I. Articular cartilage thickness and compressive stress. Arthritis Rheum. 1970;13:244–55. doi: 10.1002/art.1780130305. [DOI] [PubMed] [Google Scholar]
- 15.Waters NP, Stoker AM, Carson WL, Pfeiffer FM, Cook JL. Biomarkers affected by impact velocity and maximum strain of cartilage during injury. J Biomech. 2014;47:3185–95. doi: 10.1016/j.jbiomech.2014.06.015. [DOI] [PubMed] [Google Scholar]
- 16.Natoli RM, Scott CC, Athanasiou KA. Temporal effects of impact on articular cartilage cell death, gene expression, matrix biochemistry, and biomechanics. Ann Biomed Eng. 2008;36:780–92. doi: 10.1007/s10439-008-9472-5. [DOI] [PubMed] [Google Scholar]
- 17.Sauter E, Buckwalter JA, McKinley TO, Martin JA. Cytoskeletal dissolution blocks oxidant release and cell death in injured cartilage. J Orthop Res. 2012;30:593–8. doi: 10.1002/jor.21552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Palmer JL, Bertone AL, Litsky A. Contact area and pressure distribution changes of the equine third carpal bone during loading. Equine Vet J. 1994;26:197–202. doi: 10.1111/j.2042-3306.1994.tb04369.x. [DOI] [PubMed] [Google Scholar]
- 19.Brommer H, Brama P, Barneveld A, Weeren Pv. Differences in the topographical distribution of articular cartilage degeneration between equine metacarpo-and metatarsophalangeal joints. Equine Vet J. 2004;36:506–10. doi: 10.2746/0425164044877369. [DOI] [PubMed] [Google Scholar]
- 20.Brama PAJ, Tekoppele JM, Bank RA, Karssenberg D, Barneveld A, Weeren PR. Topographical mapping of biochemical properties of articular cartilage in the equine fetlock joint. Equine Vet J. 2000;32:19–26. doi: 10.2746/042516400777612062. [DOI] [PubMed] [Google Scholar]
- 21.Garcia-Seco E, Wilson DA, Cook JL, Kuroki K, Kreeger JM, Keegan KG. Measurement of articular cartilage stiffness of the femoropatellar, tarsocrural, and metatarsophalangeal joints in horses and comparison with biochemical data. Vet Surg. 2005;34:571–8. doi: 10.1111/j.1532-950X.2005.00090.x. [DOI] [PubMed] [Google Scholar]
- 22.Brittberg M, Aglietti P, Gambardella R, Hangody L, Hauselmann H, Jakob R. ICRS clinical cartilage injury evaluation system-2000. 2000 [Google Scholar]
- 23.Brittberg M, Winalski CS. Evaluation of cartilage injuries and repair. J Bone Joint Surg Am. 2003;85:58. doi: 10.2106/00004623-200300002-00008. [DOI] [PubMed] [Google Scholar]
- 24.Milentijevic D, Rubel IF, Liew ASL, Helfet DL, Torzilli PA. An in vivo rabbit model for cartilage trauma: a preliminary study of the influence of impact stress magnitude on chondrocyte death and matrix damage. J Orthop Trauma. 2005;19:466. doi: 10.1097/01.bot.0000162768.83772.18. [DOI] [PubMed] [Google Scholar]
- 25.Novakofski KD, Williams RM, Fortier LA, Mohammed HO, Zipfel WR, Bonassar LJ. Identification of cartilage injury using quantitative multiphoton microscopy. Osteoarthr Cartilage. 2014;22:355–62. doi: 10.1016/j.joca.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sauvola J, Pietikäinen M. Adaptive document image binarization. Pattern Recognit. 2000;33:225–36. [Google Scholar]
- 27.Tellmann G, Geulen O. LightCycler® 480 Real-Time PCR system: Innovative solutions for relative quantification. 2006;4:16. [Google Scholar]
- 28.Ewers B, Dvoracek-Driksna D, Orth M, Haut R. The extent of matrix damage and chondrocyte death in mechanically traumatized articular cartilage explants depends on rate of loading. J Orthop Res. 2001;19:779–84. doi: 10.1016/S0736-0266(01)00006-7. [DOI] [PubMed] [Google Scholar]
- 29.Witte T, Knill K, Wilson A. Determination of peak vertical ground reaction force from duty factor in the horse (Equus caballus) J Exp Biol. 2004;207:3639–48. doi: 10.1242/jeb.01182. [DOI] [PubMed] [Google Scholar]
- 30.Lee H, Kirkland WG, Whitmore RN, Theis KM, Young HE, Richardson AJ, et al. Comparison of equine articular cartilage thickness in various joints. Connect Tissue Res. 2014:1–9. doi: 10.3109/03008207.2014.949698. [DOI] [PubMed] [Google Scholar]
- 31.Saito S, Kondo S, Mishima S, Ishiguro N, Hasegawa Y, Sandell L, et al. Analysis of cartilage-derivedretinoic-acid-sensitive protein (CD-RAP) in synovial fluid from patients with osteoarthritis and rheumatoid arthritis. J Bone Joint Surg Br. 2002;84:1066–9. doi: 10.1302/0301-620x.84b7.12177. [DOI] [PubMed] [Google Scholar]
- 32.Kondo S, Cha SH, Xie W, Sandell LJ. Cytokine regulation of cartilage-derived retinoic acid-sensitive protein (CD-RAP) in primary articular chondrocytes: suppression by IL-1, bfGF, TGFβ and stimulation by IGF-1. J Orthop Res. 2001;19:712–9. doi: 10.1016/S0736-0266(00)00068-1. [DOI] [PubMed] [Google Scholar]
- 33.Nelson F, Dahlberg L, Laverty S, Reiner A, Pidoux I, Ionescu M, et al. Evidence for altered synthesis of type II collagen in patients with osteoarthritis. J Clin Invest. 1998;102:2115. doi: 10.1172/JCI4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Trumble TN, Trotter GW, Oxford JRT, McIlwraith CW, Cammarata S, Goodnight JL, et al. Synovial fluid gelatinase concentrations and matrix metalloproteinase and cytokine expression in naturally occurring joint disease in horses. Am J Vet Res. 2001;62:1467–77. doi: 10.2460/ajvr.2001.62.1467. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.