Abstract
The extent of inflammatory infiltrates in arteries from patients with giant-cell arteritis (GCA) have been described using different terms and definitions. Studies investigating the relationship between GCA histological features and clinical manifestations have produced controversial results. The aims of this study were to characterize and validate histological patterns in temporal artery biopsies (TABs) from GCA patients, to explore additional histological features, including the coexistence of different patterns, and also to investigate the relationship of the inflammatory patterns with clinical and laboratory features.
We performed histological examination of TAB from patients with GCA consecutively diagnosed between 1992 and 2012. Patterns of inflammation were defined according to the extent and distribution of inflammatory infiltrates within the artery. Clinical and laboratory variables were recorded. Two external investigators underwent a focused, one-day training session and then independently scored 77 cases. Quadratic-weighted kappa was calculated.
TAB from 285 patients (200 female/85 male) were evaluated. Four histological inflammatory patterns were distinguished: 1 – adventitial (n = 16); 2 – adventitial invasive: adventitial involvement with some extension to the muscular layer (n = 21); 3 – concentric bilayer: adventitial and intimal involvement with media layer preservation (n = 52); and 4 – panarteritic (n = 196). Skip lesions were observed in 10% and coexistence of various patterns in 43%. Raw agreement of each external scorer with the gold-standard was 82% and 77% (55% and 46% agreement expected from chance); kappa = 0.82 (95% confidence interval [CI] 0.70–0.95) and 0.79 (95% CI 0.68–0.91). Although abnormalities on temporal artery palpation and the presence of jaw claudication and scalp tenderness tended to occur more frequently in patients with arteries depicting more extensive inflammation, no statistically significant correlations were found between histological patterns and clinical features or laboratory findings.
In conclusion, we have described and validated 4 histological patterns. The presence of different coexisting patterns likely reflects sequential steps in the progression of inflammation and injury. No clear relationship was found between these patterns and clinical or laboratory findings. However, several cranial manifestations tended to occur more often in patients with temporal arteries exhibiting panarteritic inflammation. This validated score system may be useful to standardize stratification of histological severity for immunopathology biomarker studies or correlation with imaging.
INTRODUCTION
Giant-cell arteritis (GCA) is a granulomatous vasculitis preferentially affecting arteries ranging from medium to large in size.1,2 Macrophages and CD4-positive lymphocytes are the main component of the inflammatory infiltrates in all artery layers and multinucleated giant cells are present in about one half of positive biopsies.1–7 Other cell types, such as polymorphonuclear neutrophils, eosinophils, plasma cells, B-lymphocytes, and dendritic cells, may be occasionally observed.1–5 Additional histological characteristics include fragmentation of the internal elastic lamina, intimal hyperplasia, and formation of new capillaries (neoangiogenesis), particularly at the intima and intima-media junction.1–5
After the initial clinical report in 1890 by Hutchinson,8 Horton et al9 first identified the histopathological substrate of GCA in temporal artery biopsies (TABs) in 1932. In 1948, Harrison10 reviewed the histological characteristics of GCA-involved arteries and reported that inflammatory lesions in GCA “vary a good deal in their distribution.” Variability was observed between consecutive sections of the same artery and consisted of uneven density of inflammatory cells through the vessel wall, which included the presence of noninflamed segments and inconstant observation of giant cells.10 These observations still prevail in present times, since other comprehensive studies have recently corroborated that temporal arteries from GCA patients may exhibit a variable extent of inflammatory involvement, ranging from slight adventitial infiltrates to fully developed granulomatous lesions distributed along the entire vessel wall.4,11–13
In addition to the unequivocal inflammation of the temporal artery, other histological patterns may be part of the picture of GCA. Such patterns include (Figure 1):
Normal temporal artery sections intermingled with involved segments: this discontinuous involvement has been referred as skip lesions and occurs in 8% to 28% of biopsy-proven GCA.4,14,15
Vasculitis of collateral vessels surrounding a noninflamed temporal artery: although this picture may be part of the histological spectrum of GCA, it can also be observed in other diseases, including other types of systemic vasculitides.4,16–20
Healing or obsolescent pattern: it is characterized by fibrotic changes in the media and intima layers with connective tissue replacement of damaged portions of the media, internal elastic lamina disruption and scant inflammatory aggregates.4 However, the healing pattern is sometimes difficult to differentiate from aging or atherosclerotic changes.4,11,13,21–24
FIGURE 1.
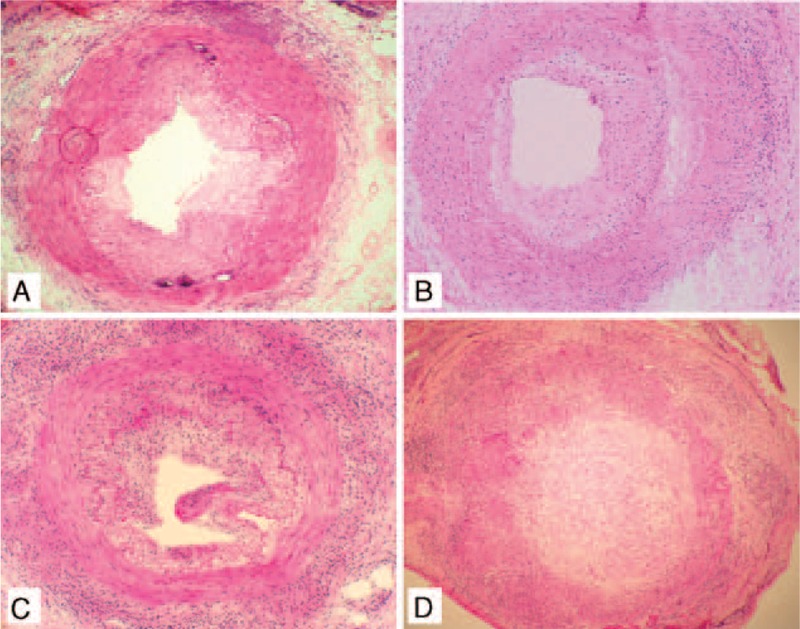
Active histological inflammatory patterns involving temporal arteries in giant cell arteritis (GCA). (A) Adventitial pattern, (B) Adventitial extended pattern, and (C) Concentric bilayer pattern. (D) Panarteritic pattern.
Histological patterns encompassing the whole spectrum of inflammation in GCA have been described using different terms and definitions. On the other hand, studies investigating the relationship between histological features in inflamed arteries from GCA patients and clinical manifestations have produced controversial results.11,19,25–34 The current study was aimed to describe and validate the main histological patterns found in GCA-involved temporal arteries, to examine additional histological features, including the coexistence of different patterns in the same artery that assist in delineating a model of infiltration across the artery wall, and also to investigate the relationship between the extent of the inflammatory infiltrates and clinical and laboratory features at disease onset in a large series of consecutively diagnosed GCA patients.
PATIENTS AND METHODS
Patient Selection and Data Collection
All patients consecutively diagnosed with biopsy-proven GCA according to the database of the Pathology Department (Hospital Clínic, Barcelona) between 1992 and 2012 were considered for inclusion in the study. A TAB was defined as positive when unequivocal presence of mononuclear cells was observed in the temporal artery.4
Biopsies with inflammation limited to small vessels surrounding a spared temporal artery were not analyzed since there is a considerable level of uncertainty regarding diagnosis in this setting.4,16–19Although it is currently considered that inflammatory infiltrates reach and invade the temporal artery through the vasa vasorum and surrounding small vessels,35,36 this histological finding has been repeatedly reported in the context of other systemic vasculitides, usually medium or small vessel vasculitis,4,16–20 malignancy or infection.4,20
Glucocorticoid treatment previous to the TAB excision was not considered an exclusion criterion. This allowed investigation of whether variations in the extent of inflammatory infiltrates could be related to previous glucocorticoid therapy. In treated patients, the prednisone dose taken at the time of TAB and its duration were recorded.
Demographic, clinical, and laboratory findings were prospectively recorded at the time of diagnosis as part of the data-collection protocol used in our Vasculitis Research Unit. All collected data were later transferred to a standardized database. Recorded clinical manifestations included cranial symptoms, neuro-ophthalmologic ischemic complications, polymyalgia rheumatica (PMR), fever >37 °C, weight loss >4 kg, and duration of clinically apparent disease until GCA diagnosis. Laboratory results, such as erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), hemoglobin, platelet count, and haptoglobin, were analyzed only from patients who did not receive glucocorticoid therapy or were treated less than 3 days prior to the blood extraction. The study was approved by the Ethics Committee of our institution, and all patients signed informed consent.
Histological Characterization
All temporal arteries from biopsy-proven GCA patients were snap-frozen in isopentane prechilled in liquid nitrogen, OCT embedded and stored at −80 °C. Specimens were systematically frozen instead of formalin-fixed, paraffin-embedded, because of the planned use of remaining tissue for molecular pathology studies.35–39 Four to 6 μm cryostat sections were stained with hematoxylin-eosin. Histological stratification was carried out by 4 investigators blinded to clinical data and was repeated in 2 different rounds by 3 of them (JHR, IV, and MCC, the 1st round, and JHR, GM, and MCC, the 2nd round). In cases with divergent opinion, consensus was achieved after a 3rd reevaluation and this was considered the referral standard for subsequent validation.
Histological scoring was based on the distribution of inflammatory infiltrates through the different layers of the artery wall. Absence or presence of multinucleated giant cells (at ×20 magnification), granuloma formation, as well as the sectoral or diffuse inflammatory involvement of the arterial circumference were also assessed. Granuloma was defined as the existence of compact collections of mononuclear cells (lymphocytes and macrophages), with or without the formation of giant cells. Arterial inflammation was considered sectoral when the inflammatory infiltrates involved less than two thirds of the vessel circumference, and diffuse when inflammatory infiltrates occupied at least two thirds of the artery circumference. When different histological patterns occurred in serial sections of the same biopsy, the most extensive pattern was considered for comparison purposes.
The extent of intimal hyperplasia was quantified according to the scoring system previously described as mild (thickness of the intima <50% of the distance spanning from the center of the lumen to the internal elastic lamina) and severe (between 50% and 100% or the lumen virtually occluded).38 When different degrees of intimal hyperplasia were observed along the arterial sections, the highest score was considered. Disruption of the internal elastic lamina, which may also be found in aging or atherosclerosis, is consistently present in GCA inflamed arteries.4,21 Although this fragmentation can be observed in many hematoxylin-eosin stained preparations, this was not evaluated because no specific staining for elastic fibers was performed.
Validation of the Histological Scoring System
To analyze the level of agreement between different investigators for the histological scoring system described, characteristics in 77 TABs from GCA patients were randomly selected and blindly reevaluated by 2 investigators from the University of Leeds (UK), 1 rheumatologist (SLM) and 1 pathologist (AC) after a focused, 1-day training session. Data analyzed included the main histological pattern (or the most extensive, in cases where concomitant patterns were observed), as well as additional findings, such as the presence of giant cells or granuloma.
Statistical Analysis
Results are expressed as mean ± SD or median plus range, where applicable. Chi-square or Fisher exact tests were used for contingency tables. Quantitative differences between groups were analyzed by using Student's unpaired t-test. Data were analyzed with the SPSS PC statistical package (version 18.0). Differences with a value of P < 0.05 were considered statistically significant.
For the validation study, quadratic-weighted kappa was calculated to assess inter-rater reliability. Significance tests for weighted kappa indicated whether the calculated values exceeded 0.6 (ie, whether agreement was at least “substantial” according to thresholds proposed by Landis and Koch40). Prevalence-adjusted, bias-adjusted kappa was also calculated to assess the impact of these issues on the measured agreement level. Stuart–Maxwell tests of marginal homogeneity were also performed as an additional indicator of rater bias. Kappas for each of the 4 histological patterns were then calculated separately to identify the specific source(s) of any overall disagreement. Unweighted kappas were calculated for the presence/absence of giant cells and granuloma. Three 2-way comparisons were performed (between the original score and each of the 2 external raters, and between the 2 raters). The data were analyzed in Stata 11 IC and in the free program WinPepi available from http://www.brixtonhealth.com/pepi4windows.html.
RESULTS
A total of 285 TABs from GCA patients (200 females and 85 males) were included in the study. All patients fulfilled 1990 American College of Rheumatology classification criteria for GCA.41 Clinical and laboratory features of these patients are listed in Table 1. A mean of 47 ± 34 (standard deviation [SD]) temporal artery sections distributed in 5 ± 2.5 slides per biopsy were examined (Table 2).
TABLE 1.
Clinical and Laboratory Findings in the Whole Series of GCA Patients
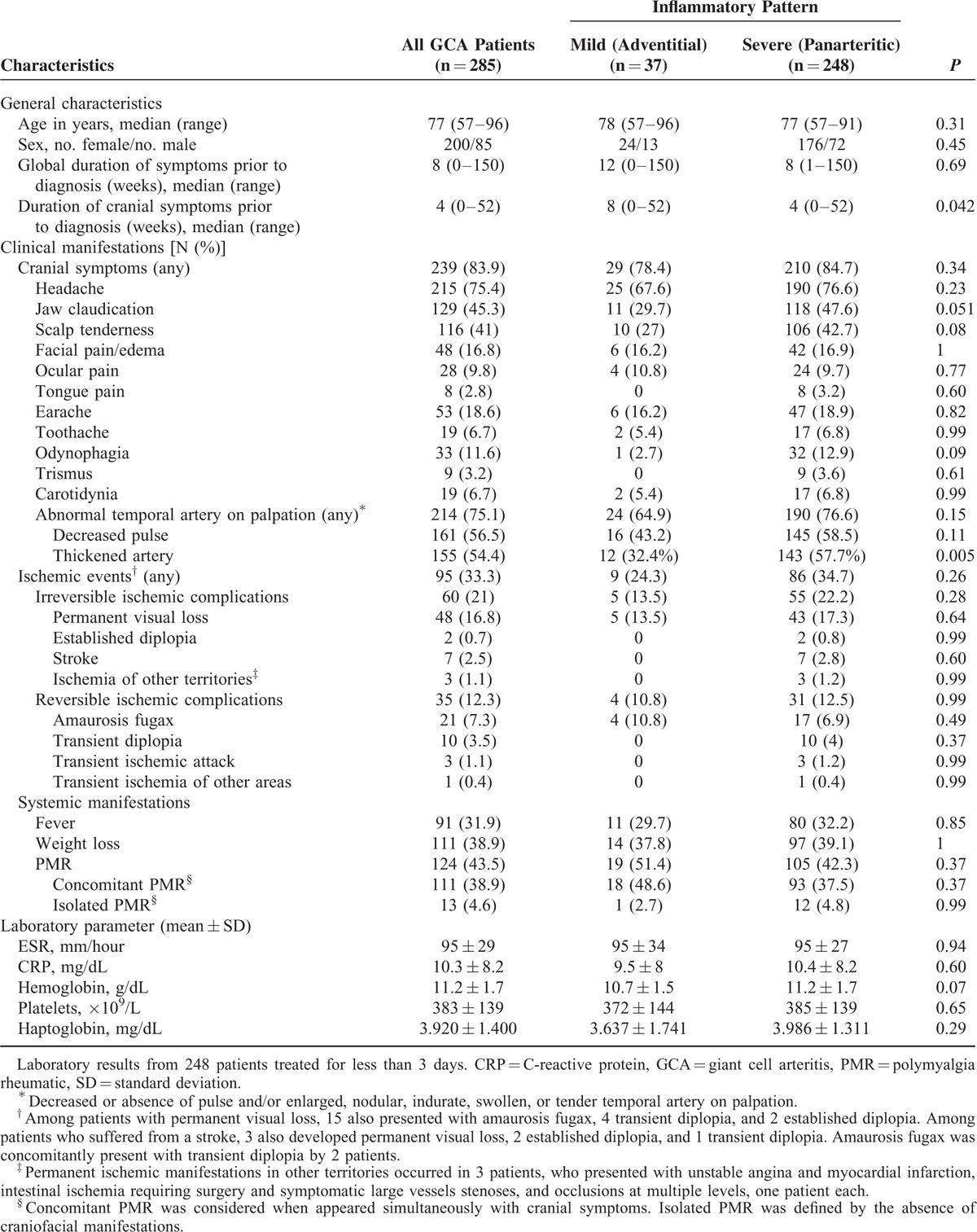
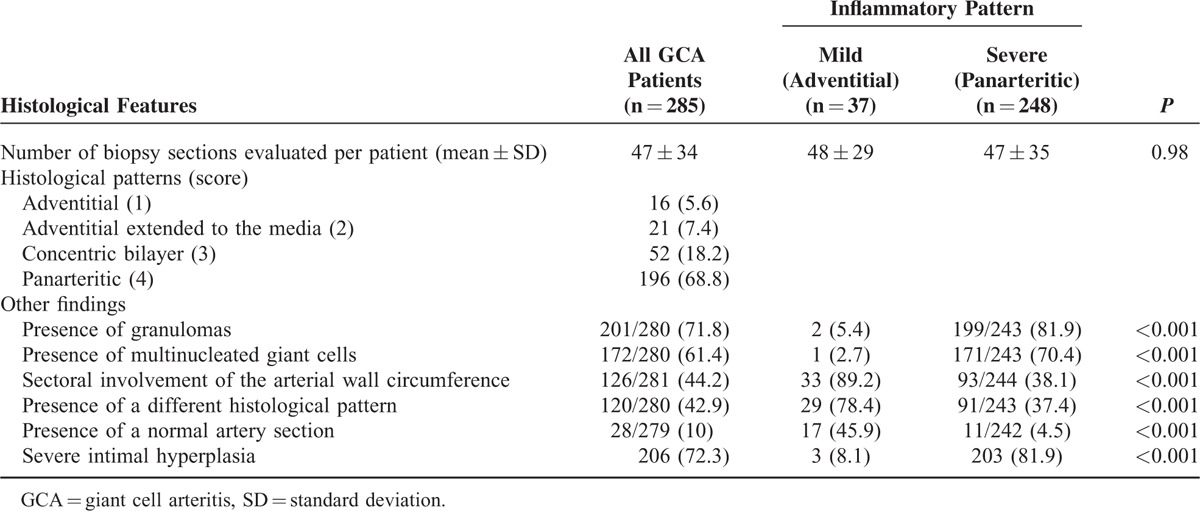
TABLE 2.
Histological Findings in the Whole Series of GCA Patients
Histopathology of GCA
Main Histological Patterns Involving the Temporal Artery in GCA
According to the distribution of inflammatory infiltrates through the artery wall, 4 main histological patterns were differentiated as follows (Figure 1):
Adventitial pattern: when inflammatory cells were restricted to the adventitia, with preservation of media and intima layers (n = 16 biopsies; 5.6%).
Adventitial invasive pattern: when adventitial infiltration was followed by local invasion of the muscular layer, with integrity of the intima (n = 21 biopsies; 7.3%).
Concentric bilayer pattern: when inflammatory cells were infiltrating the adventitia and the intima (or the intima/media junction), with a preserved media (n = 52 biopsies; 18.2%).
Panarteritic pattern: when the inflammatory infiltrates were distributed through the 3 arterial layers (n = 196 biopsies; 68.8%).
In order to increase statistical power to perform clinical and pathologic correlations, these 4 histological patterns were clustered in 2 main histological groups according to their impact on the vessel wall integrity: Mild infiltrative pattern, which encompasses scores 1 and 2; and Extensive infiltrative pattern, encompassing scores 3 and 4. These patterns were observed in 37 (13%) and 248 (87%) of the cases, respectively.
Extent of Intimal Hyperplasia and Other Histological Features
Mild intimal hyperplasia score was observed in 79 arteries and severe intimal hyperplasia in 206 biopsies. Granulomas were detected in 201 (70.5%) samples and multinucleated giant cells in 172 (61.4%) biopsies. Sectoral involvement of the arterial circumference was observed in 126 (45%) of the examined samples. Details of the histological results are listed in Table 2.
Validation Study
Statistical results of the validation study are listed in Table 3. The 4 histological patterns (from 1 to 4) were identified by the pathologist (Rater 1) in 10%, 2.5%, 8%, and 77% of patients (2 cases [2.5%] were evaluated as normal), and by the rheumatologist (Rater 2) in 13%, 4%, 23%, and 60%, respectively. Raw agreement of each UK scorer with the referral-standard for the main histological pattern was 82% for the pathologist and 77% for the rheumatologist (55% and 46% agreement expected from chance, respectively). Quadratic-weighted kappa values of 0.82 and 0.79, respectively, indicated substantial agreement when partial credit was given for agreement ± 1 or 2 categories (both P < 0.001). Significant Stuart–Maxwell tests indicated consistent bias for the pathologist who tended to overestimate the proportion of pattern 1 and underestimate the proportion of pattern 2 relative to the referral score, and relative to the rheumatologist. Prevalence-adjusted, bias-adjusted values (PABAK) indicated that each of the raters almost completely agreed with the referral score when partial credit was given (0.95 for both raters). Agreement tended to be better for patterns 1 and 4 than for patterns 2 and 3. The 2 raters substantially agreed with each other over the main histological pattern. When the scores were collapsed into mild (scores 1 or 2) and extensive (scores 3 or 4), agreement substantially improved; raw agreement for each comparison between raters and the referral score exceeded 90%. Kappa was significantly better than “moderate” (>0.6) when each rater was compared to the referral score; comparing rater 1 to rater 2, kappa values were slightly lower (95% confidence interval 0.56–0.96), but increased when prevalence and bias were accounted for (0.87).
TABLE 3.
Statistical Data From the Validation Study
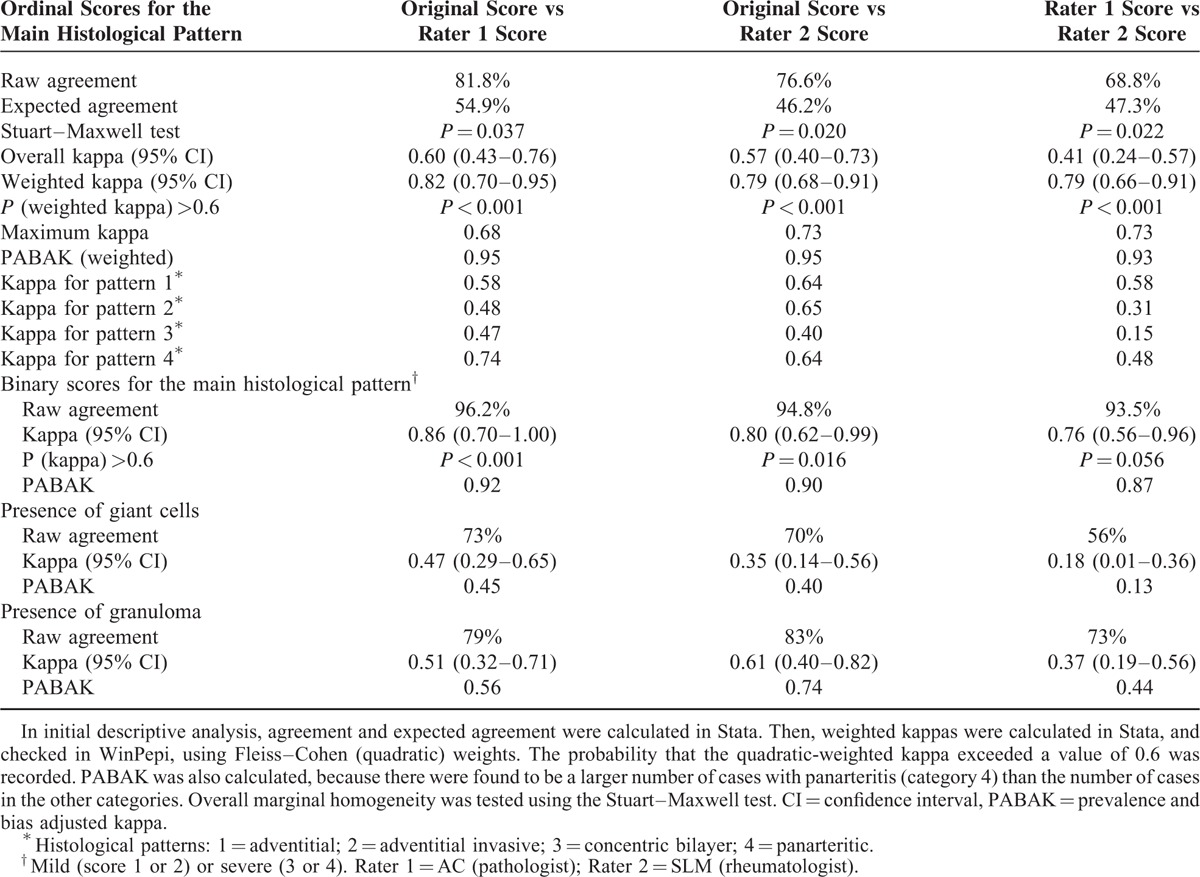
Regarding specific histological features, for which no specific training was given, kappa coefficients for the observation of presence/absence of giant cells relative to the referral score were 0.47 and 0.35, and for granuloma were 0.51 and 0.61, respectively. Adjusting for prevalence and bias did not substantially alter these values, which represent only moderate agreement with the referral standard, and agreement between the 2 raters was relatively poor.
Other Histological Patterns and Coexisting Lesions
Although only samples showing inflammation involving the temporal artery were considered for validation and comparison purposes in the current study, other histological patterns different from the main patterns described above and always coexisting with them were also observed. These additional patterns included: normal temporal artery section/s; inflammation of the small vessels surrounding a normal temporal artery (in consecutive sections of arteries with adventitial involvement only); and healing pattern (Figure 2A–C).
FIGURE 2.
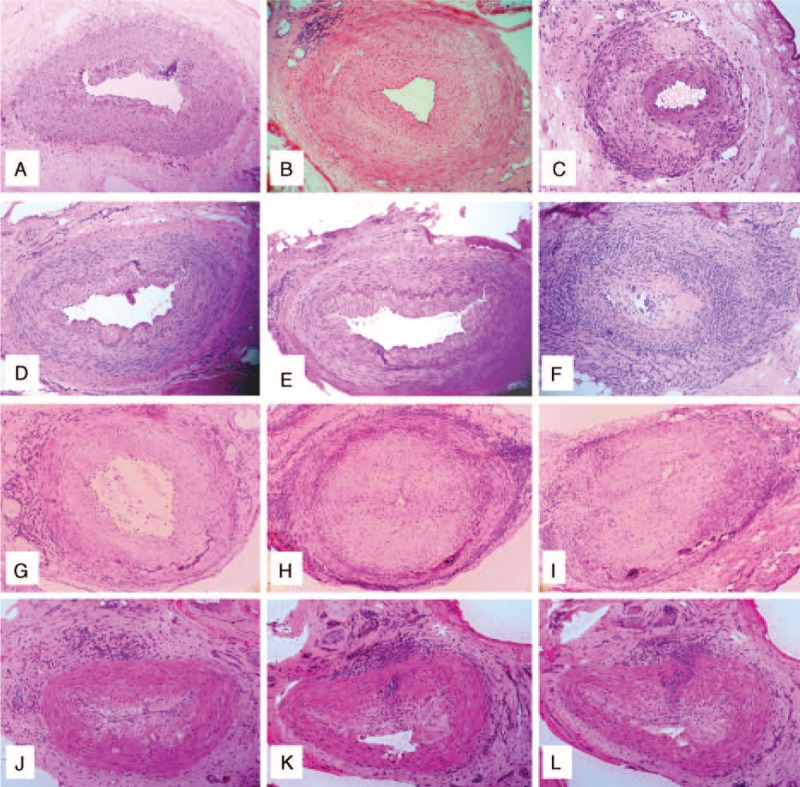
(A–C) Additional histological patterns observed in temporal artery biopsies from patients with giant cell arteritis (GCA). Normal temporal artery section (A). Vasculitis of small vessels surrounding a spared temporal artery (B). Healing or obsolescent pattern (C). (D–L) Coexistence of different histological patterns in consecutive sections of the same temporal artery biopsy in different patients with GCA: Patient 1 (D–F): (D) normal section; (E) focal adventitial inflammation; and (F) panarteritic involvement; Patient 2 (G–I): (G) adventitial inflammatory infiltrates; (H) concentric bilayer pattern; and (I) panarteritic inflammation; Patient 3 (J–L): (J) adventitial inflammation; (K) inflammatory cells crossing from the adventitia to the intima, leaving a preserved muscular layer (concentric bilayer pattern); and (L) cells crossing from de adventitia to the intima along the media in a subsequent section (focal panarteritic infiltration).
In 120 of 280 (43%) cases, a lesion different from the main pattern was concurrently observed in other sections of the same biopsy sample. In 72 of 196 (36.7%) arteries with panarteritic pattern, coexisting patterns included concentric bilayer pattern in 54 (75%) cases, adventitial and adventitial invasive pattern, in 9 and 2 cases, respectively, healing pattern in 6 and a normal pattern in 1. In 19 of 52 (36.5%) arteries with a concentric bilayer pattern as the most severe pattern, 14 and 4 included sections with an adventitial or adventitial invasive pattern, respectively, and 1 a normal pattern. Among 16 of 21 (76%) arteries showing an adventitial invasive pattern, 15 also showed coexisting adventitial lesions and in 1, a healing pattern. In 13 of 16 (81%) arteries with adventitial inflammation only, 3 and 10 cases had a coexisting healing or normal pattern, respectively
Two or more coexisting patterns were observed in 24 temporal arteries. Overall, healing pattern and normal sections were detected in 10 and 28 cases, respectively. Examples of coexisting different histological patterns in sequential temporal artery sections are illustrated in Figure 2D–L.
Correlations Between Histopathological Features
The number of temporal artery sections evaluated in groups with mild or extensive infiltrative patterns was similar (Table 2). The presence of extensive artery inflammation strongly correlated with severe intimal hyperplasia (P < 0.001). Other parameters indicating more developed lesions, such as the presence of granulomas or giant cells, were more frequently observed in arteries with the most extended patterns. On the contrary, sectoral involvement of the arterial wall circumference and the coexistence of different histological patterns in serial sections of the same artery, including a normal pattern, occurred more commonly in arteries with the mildest inflammatory scores. All the histological associations are depicted in Table 2.
Influence of Glucocorticoid Treatment on Histological Changes
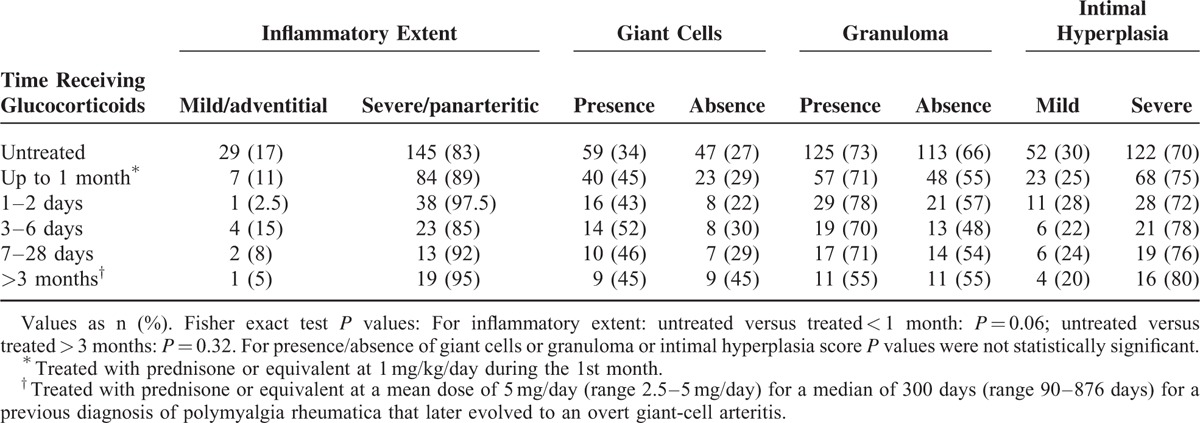
A total of 174 (61%) patients were glucocorticoid naive at the time of the TAB and 91 (32%) patients had received prednisone at 1 mg/kg/day, for 1 to 2 days (n = 39; 13.7%), for 3 to 6 days (n = 27; 9.5%), or for 7 to 28 days (n = 25; 8.8%). In addition, 20 (7%) patients had been treated for a median of 300 days (range 90–876 days) with a median dose of prednisone of 5 mg/day (range 2.5–5 mg/day) for a previous diagnosis of isolated PMR that later evolved to an overt GCA. No significant differences in the proportion of the different infiltrative patterns were found between patients untreated (adventitial/mild 17%, panarteritic/extensive 83%), and those receiving full doses of prednisone during the 1st month (mild 11%, extensive 89%), or those treated with low doses for more than 3 months (mild 5%, extensive 95%). No clear differences were either found with regard to the presence of giant cells or granuloma and intimal hyperplasia degree between untreated and treated patients (see detailed results in Table 4).
TABLE 4.
Data of Histological Features With Regard to Glucocorticoid Therapy
Clinical-Histological Correlations
No clear relationship was found between the extent of inflammatory infiltrates and age, gender, cranial manifestations or neuro-ophthalmic ischemic complications, presence of systemic manifestations, such as fever, weight loss or PMR, or any of the laboratory findings analyzed (Table 1). However, patients with TAB disclosing a panarteritic pattern tended to present more often with jaw claudication and scalp tenderness than those with arteries showing adventitial involvement. These patients also had more abnormalities on temporal artery palpation, mainly temporal artery thickening, and into a lesser extent, decreased pulse than those with adventitial involvement (Table 1). Whereas duration of systemic symptoms were similar in both groups, cranial symptoms had longer duration in patients with arteries showing adventitial inflammation.
A significant correlation was found between pulse reduction on temporal artery palpation and the degree of intimal hyperplasia. A decreased or absent pulse in temporal arteries was observed in 44.9% and 60.9% of patients with mild and severe intimal hyperplasia, respectively (P = 0.016). The presence or absence of giant cells and the extent of intimal hyperplasia did not correlate with the remaining clinical symptoms, including cranial and neuro-ophthalmic ischemic manifestations, or laboratory parameters (data not shown).
DISCUSSION
In 1932, Horton et al9 reported the 1st histological description of GCA in 2 patients. Since then, histopathological features observed in temporal arteries from patients with GCA have ranged from slight cellular inflammatory aggregates in the outer layer to a granulomatous inflammation involving the entire artery wall, with or without multinucleated giant cells.4,11–13,42
Variations in the spectrum of inflammatory changes observed in the temporal arteries from patients with GCA have been reported with different names. Terms such as “typical or classical GCA”4,43 or “granulomatous arteritis”27 illustrated TABs with a panarteritic granulomatous inflammation, including the frequent presence of multinucleated giant cells. “Atypical GCA”4,43 or “GCA variant with predominantly intimal changes”27 have been reported to define lighter inflammatory infiltrates without granuloma formation, mainly confined to the adventitia. Lie4 confirmed the coexistence of both (typical and atypical) patterns in the same biopsy after multiple-block serial sections in a previous study of 1109 TABs. Cavazza et al19 recently analyzed clinical and histological features of 317 inflamed temporal arteries from patients with GCA. Although 14.5% of TAB revealed small-vessel and vasa vasorum vasculitis with a spared temporal artery, in the remaining 85.5% of samples, the 2 main histological patterns involving the temporal artery were categorized as “transmural inflammation” and “inflammation limited to the adventitia,” which according to our classification, accounted for extensive and mild infiltrative involvement, respectively. These patterns were found in 93.4% and 6.6% of biopsies,19 a similar proportion to that found in our study.
The concentric bilayer pattern has been seldom reported, sometimes as atypical GCA.4,11,27,43 Cavazza et al19 recently reported that this pattern (named as “concentric rings”) frequently occurred in arteries with transmural inflammation. Nevertheless, no information was provided about the frequency of this pattern with respect to the others or its specific correlations with clinical features.19 In the present series, a concentric bilayer pattern was observed in 106 arteries, accounting for 37.2% of all TAB. In 52 (18.2%) cases, concentric bilayer pattern was the most intense pattern and in 54 (18.9%) of them, it coexisted with adjacent panarteritic involvement.
In our series, skip lesions were observed in 10% of the samples. A healing pattern was also observed along with the whole spectrum of active lesions in 3.6% of arteries. Because a healing pattern had been previously reported in patients with delayed biopsies, with or without long-term glucocorticoid treatment,13,22–24,39,44,45 the novel finding of healing lesions close to sections showing any type of inflammatory pattern suggests that these scarring changes are part of the evolving reparative process. Healing changes were similarly observed in sequential sections from arteries with panarteritic and adventitial pattern. Therefore, its presence does not seem to be clearly associated with any specific active pattern.
Based on the different histological patterns observed in arteries from GCA patients, and on the fact that different inflammatory patterns may coexist with normal sections and/or a healing pattern in sequential sections of the same temporal artery, a dynamic model of arterial inflammation in GCA has been postulated and depicted in Figure 3. The steps of this invasive process would be as follows:
the first contact of inflammatory cells with the artery would occur through the small vessels of the adventitia, as supported by previous studies35,38 and by the fact that the adventitial involvement invariably occurs in all GCA patients
lymphocytes and macrophages would subsequently penetrate through the muscular layer to reach the intima38
once in the inner layer, the inflammatory cells might continue their progression by horizontal spreading through the intima and the adventitia, with preservation of the media layer, which has been considered an immunoprivileged site46 (concentric bilayer pattern)
panarteritic involvement may eventually result from progression of the previous stages with massive infiltration of the vessel wall and finally
a reparative process would occur in the disrupted vessel wall with intimal thickening and fibrotic changes in the media (healing or obsolescent pattern)
FIGURE 3.
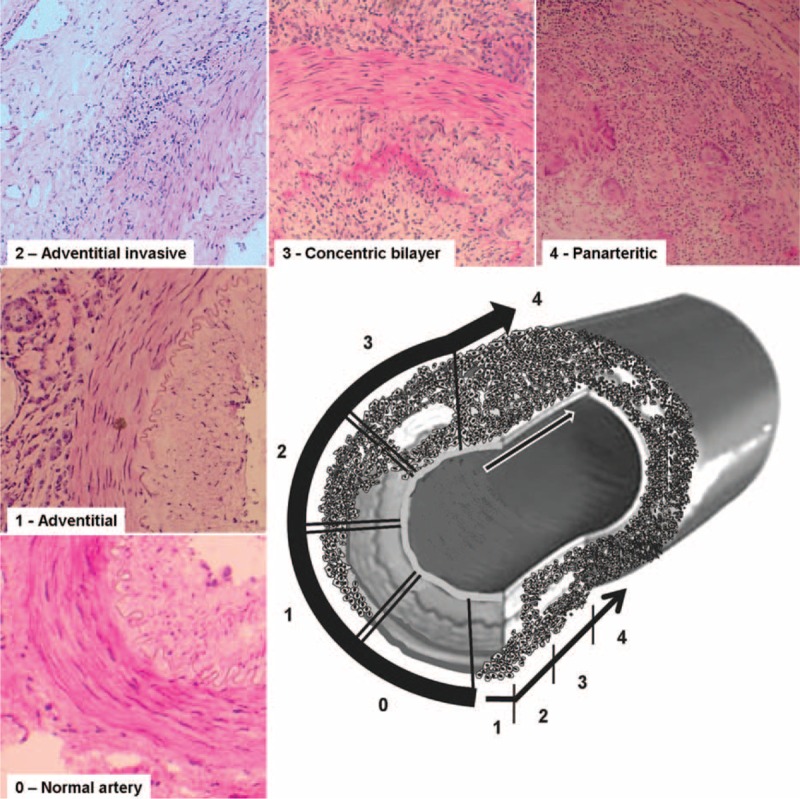
Hypothetical model of the sequential invasion of the artery by Inflammatory cells according to our histological findings.
As in our series, other studies have observed a remarkable intimal hyperplasia in all histological categories.4,27,43 However, our results corroborate a striking correlation between the extent of inflammatory infiltrates and the severity of intimal thickening, as previously described in a morphometric study of inflamed temporal arteries.47 Several investigators have observed that additional structural changes, such as internal elastic lamina disruption also correlate with the extent of intima hyperplasia and the density of artery wall inflammatory infiltrates.38 In this regard, multiple inflammatory pathways, in which infiltrating lymphocytes, macrophages, and their products (cytokines, metalloproteinases, growth and angiogenic factors) participate, may lead to tissue damage, neoangiogenesis, and intimal proliferation.33,35–39 Therefore, the extension of inflammatory infiltrates in the artery wall might be considered an important determinant of the development of intimal hyperplasia.
We have also confirmed that glucocorticoid therapy at full-doses, at least during the first month, do not seem to influence the morphology and extent of the inflammatory infiltrates.3,48 Indeed, low doses (ie, 2.5–10 mg/day) of prednisone do not preclude the development of full-blown vascular inflammatory infiltrates in GCA in some patients receiving low-dose prednisone for apparently isolated PMR.48 In this study, we did not perform biopsies from patients treated with high doses of prednisone beyond 1 month, and therefore, we could not assess the impact of treatment on morphology beyond this period. In this regard, previous studies have shown that, overtime, biopsies performed in long-term treated patients, are more likely to disclose healing lesions.49 In addition, in a small series of biopsies performed before and after 1 year of treatment, the panarteritic initial pattern evolved into healing changes in all cases.39 Although, as shown in the present study, healing lesions may develop early and spontaneously, it is likely that glucocorticoid therapy helps this process since increase in the expression of fibrogenic growth factors in temporal arteries was observed after 1 year of treatment.39
The relationship between clinical findings or blood test results and the density of inflammatory infiltrates within the temporal artery has been repeatedly analyzed with conflicting results (Table 5). Similarly to previous studies,11,25–29 we did not find clear correlations between inflammatory changes in temporal arteries and the clinical and biological parameters analyzed, including the presence of neuro-ophthalmic ischemic complications.11,27,28 No apparent explanation can be provided for the finding of longer duration of cranial symptoms in patients with arteries showing adventitial pattern. Furthermore, no correlations were found between the magnitude of intimal hyperplasia or the presence of giant cells and GCA clinical symptoms, including the development of ischemic complications. However, intimal hyperplasia degree was positively associated with decreased pulse in temporal arteries on palpation. Such finding might reflect a local effect without necessarily conveying distal consequences in smaller vessels supplying ophthalmic territories.
TABLE 5.
Studies in GCA Describing Histological Inflammatory Patterns and Intimal Hyperplasia Degree, and Their Correlations With Clinical Features
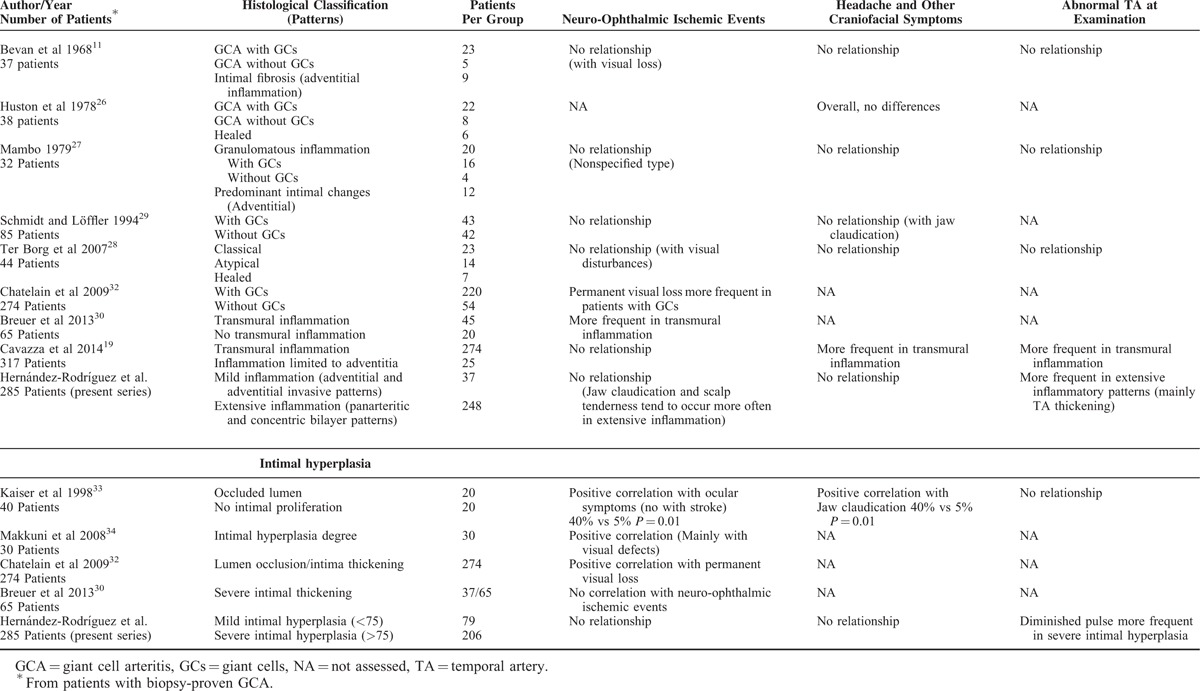
On the other hand, several studies have reported a relationship between histological changes at temporal artery level and GCA disease-related manifestations. Cavazza et al19 found that GCA patients with transmural inflammation present more frequently with cranial manifestations, in particular headache and abnormalities of the temporal arteries, than those showing an arterial inflammation limited to adventitia. In the same sense, in our study, patients with temporal arteries showing panarteritic involvement had more frequently an abnormal artery palpation and tended to present more often with jaw claudication and scalp tenderness. Another study showed that PMR was more frequently presented by GCA patients with atypical (adventitial) inflammatory infiltrates in the temporal artery than by those with a transmural granulomatous reaction.28 Several studies, not always including consecutively selected patients, have reported an association between the development of neuro-ophthalmic ischemic complications and the presence of transmural inflammation,30 giant cells,31,32 or intimal thickening (Table 5).32–34 Two studies reported that GCA patients with biopsies showing nongranulomatous infiltrates tended to have higher ESR and C-reactive protein, and lower hemoglobin levels than those with granulomatous inflammatory lesions.11,28 In contrast, other authors found that patients with transmural inflammation had a more intense systemic inflammatory response (including anemia and higher ESR values) than those without transmural inflammation.30
Overall, the discrepancies between our results and those with positive correlations between clinical manifestations and histological lesions might in part be explained by the fact that we identified coexisting patterns in 43% of TAB after an extensive examination (a mean of 47 temporal artery sections per biopsy). Variability in results or trends is probably related to the number of the examined sections and the search for concomitant patterns, which may have been missed in smaller studies. Moreover, studies in which patients were not consecutively included may have been subjected to selection bias.
Previous attempts to study the level of agreement by different investigators in scoring some histological features in biopsy-proven GCA, such as the extent of inflammation and quantification of giant cells, have been difficult.23,50 In this regard, a poor inter-rater agreement regarding presence/absence of giant cells and granuloma was also found in the validation study. However, our scoring system has demonstrated to be reliable and reproducible when investigators underwent a brief training session of TAB examination.
Currently, investigation of surrogate diagnostic modalities based on temporal artery imaging (ultrasound or MRI) is in successful progress.51 It is unclear whether imaging studies may detect incomplete or mild histopathological changes, such as inflammation limited to the adventitia or small-vessel vasculitis surrounding a spared temporal artery.19,52 Our scoring system may be useful for imaging/histology correlations.
In conclusion, in this large series of patients with GCA, 4 histological patterns have been described and validated. A dynamic model of arterial invasion, likely reflecting sequential steps in the progression of inflammation and injury, has been postulated. Although no clear relationship was found between the described patterns and clinical or laboratory findings at disease diagnosis, abnormalities on temporal artery palpation and several cranial symptoms, such as jaw claudication and scalp tenderness, tended to occur more frequently in patients with TAB disclosing a panarteritic pattern. The description of these patterns and its validation may be useful for homogenization and comparison of histological data from different sources and for stratification of histological severity for immunopathologic biomarker studies or correlation with imaging.
Acknowledgments
The authors thank the other members of the Vasculitis Research Unit of the Hospital Clínic of Barcelona for their invaluable contribution to this study: Ana García-Martínez, Carme Vilardell, Marta Segarra, Ester Lozano, Marc Corbera-Bellalta, Ester Planas-Rigol, Nekane Terrades, Marco A. Alba, M. Dolores Cano, and Montse Sánchez.
Footnotes
Abbreviations: ESR = erythrocyte sedimentation rate, GCA = giant-cell arteritis, PMR = polymyalgia rheumatica, TAB = temporal artery biopsy.
This study is supported by Ministerio de Economía y Competitividad (SAF 14/57708-R), Instituto de Salud Carlos III and Fondo Europeo de desarrollo regional (FEDER) (PIE 13/00033) and by the Erdheim Travelling Scholarship and the National Institute for Health Research Biomedical Research Unit and Clinician Scientist awards (SLM).
Results partially presented at 12th International Vasculitis and ANCA Workshop, Heidelberg, Germany, June 2005, and 69th American College of Rheumatology Meeting, San Diego CA, November 2005, and the 17th International Vasculitis and ANCA Workshop, London, UK, April 2015.
“No two positive biopsies of GCA are exactly alike, nor are two adjacent arterial segments of the same positive biopsy; this is what makes the interpretation of temporal artery biopsies so interesting, challenging, and exasperating.” J.T. Lie, 1996.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Salvarani C, Cantini F, Hunder GG. Polymyalgia rheumatica and giant-cell arteritis. Lancet 2008; 372:234–245. [DOI] [PubMed] [Google Scholar]
- 2.Cid MC, Font C, Coll-Vinent B, et al. Large vessel vasculitides. Curr Opin Rheumatol 1998; 10:18–28. [DOI] [PubMed] [Google Scholar]
- 3.Cid MC, Campo E, Ercilla G, et al. Immunohistochemical analysis of lymphoid and macrophage cell subsets and their immunologic activation markers in temporal arteritis. Influence of corticosteroid treatment. Arthritis Rheum 1989; 32:884–893. [PubMed] [Google Scholar]
- 4.Lie JT. Temporal artery biopsy diagnosis of giant cell arteritis: lessons from 1109 biopsies. Anat Pathol 1996; 1:69–97. [PubMed] [Google Scholar]
- 5.Parker F, Healey LA, Wilske KR, et al. Light and electron microscopic studies on human temporal arteries with special reference to alterations related to senescence, atherosclerosis and giant cell arteritis. Am J Pathol 1975; 79:57–80. [PMC free article] [PubMed] [Google Scholar]
- 6.Font RL, Prabhakaran VC. Histological parameters helpful in recognising steroid-treated temporal arteritis: an analysis of 35 cases. Br J Ophthalmol 2007; 91:204–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou L, Luneau K, Weyand CM, et al. Clinicopathologic correlations in giant cell arteritis: a retrospective study of 107 cases. Ophthalmology 2009; 116:1574–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hutchinson J. On a peculiar form of thrombotic arteritis of the aged which is sometimes productive of gangrene. Arch Surg 1890; 1:323–329. [Google Scholar]
- 9.Horton B, Magath T, Brown G. An undescribed form of arteritis of the temporal vessels. Proc Staff Meet Mayo Clin 1932; 7:700–701. [Google Scholar]
- 10.Harrison CV. Giant-cell or temporal arteritis: a review. J Clin Pathol 1948; 1:197–211. [PubMed] [Google Scholar]
- 11.Bevan AT, Dunnill MS, Harrison MJ. Clinical and biopsy findings in temporal arteritis. Ann Rheum Dis 1968; 27:271–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nordborg E, Nordborg C. The inflammatory reaction in giant cell arteritis: an immunohistochemical investigation. Clin Exp Rheumatol 1998; 16:165–168. [PubMed] [Google Scholar]
- 13.Ostberg G. Temporal arteritis in a large necropsy series. Ann Rheum Dis 1971; 30:224–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klein RG, Campbell RJ, Hunder GG, et al. Skip lesions in temporal arteritis. Mayo Clin Proc 1976; 51:504–510. [PubMed] [Google Scholar]
- 15.Poller DN, van Wyk Q, Jeffrey MJ. The importance of skip lesions in temporal arteritis. J Clin Pathol 2000; 53:137–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Esteban MJ, Font C, Hernández-Rodríguez J, et al. Small-vessel vasculitis surrounding a spared temporal artery: clinical and pathological findings in a series of twenty-eight patients. Arthritis Rheum 2001; 44:1387–1395. [DOI] [PubMed] [Google Scholar]
- 17.Genereau T, Lortholary O, Pottier MA, et al. Temporal artery biopsy: a diagnostic tool for systemic necrotizing vasculitis. French Vasculitis Study Group. Arthritis Rheum 1999; 42:2674–2681. [DOI] [PubMed] [Google Scholar]
- 18.Restuccia G, Cavazza A, Boiardi L, et al. Small-vessel vasculitis surrounding an uninflamed temporal artery and isolated vasa vasorum vasculitis of the temporal artery: two subsets of giant cell arteritis. Arthritis Rheum 2012; 64:549–556. [DOI] [PubMed] [Google Scholar]
- 19.Cavazza A, Muratore F, Boiardi L, et al. Inflamed temporal artery: histologic findings in 354 biopsies, with clinical correlations. Am J Surg Pathol 2014; 38:1360–1370. [DOI] [PubMed] [Google Scholar]
- 20.Disdier P, Pellissier JF, Harle JR, et al. Significance of isolated vasculitis of the vasa vasorum on temporal artery biopsy. J Rheumatol 1994; 21:258–260. [PubMed] [Google Scholar]
- 21.Cox M, Gilks B. Healed or quiescent temporal arteritis versus senescent changes in temporal artery biopsy specimens. Pathology 2001; 33:163–166. [DOI] [PubMed] [Google Scholar]
- 22.Lie JT, Brown AL, Jr, Carter ET. Spectrum of aging changes in temporal arteries. Its significance, in interpretation of biopsy of temporal artery. Arch Pathol 1970; 90:278–285. [PubMed] [Google Scholar]
- 23.McDonnell PJ, Moore GW, Miller NR, et al. Temporal arteritis. A clinicopathologic study. Ophthalmology 1986; 93:518–530. [DOI] [PubMed] [Google Scholar]
- 24.Lee YC, Padera RF, Noss EH, et al. Clinical course and management of a consecutive series of patients with “healed temporal arteritis”. J Rheumatol 2012; 39:295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wadman B, Werner I. Observations on temporal arteritis. Acta Med Scand 1972; 192:377–383. [DOI] [PubMed] [Google Scholar]
- 26.Huston KA, Hunder GG, Lie JT, et al. Temporal arteritis: a 25-year epidemiologic, clinical, and pathologic study. Ann Intern Med 1978; 88:162–167. [DOI] [PubMed] [Google Scholar]
- 27.Mambo NC. Temporal (granulomatous) arteritis: a histopathological study of 32 cases. Histopathology 1979; 3:209–221. [DOI] [PubMed] [Google Scholar]
- 28.ter Borg EJ, Haanen HC, Seldenrijk CA. Relationship between histological subtypes and clinical characteristics at presentation and outcome in biopsy-proven temporal arteritis. Identification of a relatively benign subgroup. Clin Rheumatol 2007; 26:529–532. [DOI] [PubMed] [Google Scholar]
- 29.Schmidt D, Loffler KU. Temporal arteritis. Comparison of histological and clinical findings. Acta Ophthalmol (Copenh) 1994; 72:319–325. [DOI] [PubMed] [Google Scholar]
- 30.Breuer GS, Nesher R, Reinus K, et al. Association between histological features in temporal artery biopsies and clinical features of patients with giant cell arteritis. Isr Med Assoc J 2013; 15:271–274. [PubMed] [Google Scholar]
- 31.Armstrong AT, Tyler WB, Wood GC, et al. Clinical importance of the presence of giant cells in temporal arteritis. J Clin Pathol 2008; 61:669–671. [DOI] [PubMed] [Google Scholar]
- 32.Chatelain D, Duhaut P, Schmidt J, et al. Pathological features of temporal arteries in patients with giant cell arteritis presenting with permanent visual loss. Ann Rheum Dis 2009; 68:84–88. [DOI] [PubMed] [Google Scholar]
- 33.Kaiser M, Weyand CM, Bjornsson J, et al. Platelet-derived growth factor, intimal hyperplasia, and ischemic complications in giant cell arteritis. Arthritis Rheum 1998; 41:623–633. [DOI] [PubMed] [Google Scholar]
- 34.Makkuni D, Bharadwaj A, Wolfe K, et al. Is intimal hyperplasia a marker of neuro-ophthalmic complications of giant cell arteritis? Rheumatology (Oxford) 2008; 47:488–490. [DOI] [PubMed] [Google Scholar]
- 35.Cid MC, Cebrian M, Font C, et al. Cell adhesion molecules in the development of inflammatory infiltrates in giant cell arteritis: inflammation-induced angiogenesis as the preferential site of leukocyte-endothelial cell interactions. Arthritis Rheum 2000; 43:184–194. [DOI] [PubMed] [Google Scholar]
- 36.Foell D, Hernández-Rodríguez J, Sánchez M, et al. Early recruitment of phagocytes contributes to the vascular inflammation of giant cell arteritis. J Pathol 2004; 204:311–316. [DOI] [PubMed] [Google Scholar]
- 37.Hernandez-Rodriguez J, Segarra M, Vilardell C, et al. Tissue production of pro-inflammatory cytokines (IL-1beta, TNFalpha and IL-6) correlates with the intensity of the systemic inflammatory response and with corticosteroid requirements in giant-cell arteritis. Rheumatology (Oxford) 2004; 43:294–301. [DOI] [PubMed] [Google Scholar]
- 38.Segarra M, García-Martínez A, Sánchez M, et al. Gelatinase expression and proteolytic activity in giant-cell arteritis. Ann Rheum Dis 2007; 66:1429–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Visvanathan S, Rahman MU, Hoffman GS, et al. Tissue and serum markers of inflammation during the follow-up of patients with giant-cell arteritis – a prospective longitudinal study. Rheumatology (Oxford) 2011; 50:2061–2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977; 33:159–174. [PubMed] [Google Scholar]
- 41.Hunder GG, Bloch DA, Michel BA, et al. The American College of Rheumatology 1990 criteria for the classification of giant cell arteritis. Arthritis Rheum 1990; 33:1122–1128. [DOI] [PubMed] [Google Scholar]
- 42.Corcoran GM, Prayson RA, Herzog KM. The significance of perivascular inflammation in the absence of arteritis in temporal artery biopsy specimens. Am J Clin Pathol 2001; 115:342–347. [DOI] [PubMed] [Google Scholar]
- 43.Allsop CJ, Gallagher PJ. Temporal artery biopsy in giant-cell arteritis. A reappraisal. Am J Surg Pathol 1981; 5:317–323. [DOI] [PubMed] [Google Scholar]
- 44.Harrison RJ, Harrison CV, Kopelman H. Giant-cell arteritis with aneurysms; effects of hormone therapy. Br Med J 1955; 2:1593–1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hernández-Rodríguez J, García-Martínez A, Espígol-Frigolé G, et al. Sustained spontaneous clinical remission in giant cell arteritis: report of two cases with long-term followup. Arthritis Rheum 2006; 55:160–162. [DOI] [PubMed] [Google Scholar]
- 46.Dal Canto AJ, Swanson PE, O’Guin AK, et al. IFN-gamma action in the media of the great elastic arteries, a novel immunoprivileged site. J Clin Invest 2001; 107:R15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nordborg C, Petursdottir V. Vessel wall morphometry in giant cell arteritis. Arthritis Care Res 2000; 13:286–290. [DOI] [PubMed] [Google Scholar]
- 48.Narváez J, Bernad B, Roig-Vilaseca D, et al. Influence of previous corticosteroid therapy on temporal artery biopsy yield in giant cell arteritis. Semin Arthritis Rheum 2007; 37:13–19. [DOI] [PubMed] [Google Scholar]
- 49.Fauchald P, Rygvold O, Oystese B. Temporal arteritis and polymyalgia rheumatica. Clinical and biopsy findings. Ann Intern Med 1972; 77:845–852. [DOI] [PubMed] [Google Scholar]
- 50.Bharadwaj A, Dasgupta B, Wolfe K, et al. Difficulties in the development of histological scoring of the inflamed temporal arteries in giant cell arteritis. Rheumatology (Oxford) 2005; 44:1579–1580. [DOI] [PubMed] [Google Scholar]
- 51.Bley TA, Reinhard M, Hauenstein C, et al. Comparison of duplex sonography and high-resolution magnetic resonance imaging in the diagnosis of giant cell (temporal) arteritis. Arthritis Rheum 2008; 58:2574–2578. [DOI] [PubMed] [Google Scholar]
- 52.Muratore F, Boiardi L, Restuccia G, et al. Comparison between colour duplex sonography findings and different histological patterns of temporal artery. Rheumatology (Oxford) 2013; 52:2268–2274. [DOI] [PubMed] [Google Scholar]


