Abstract
Despite known limitations of positron emission tomography (PET) for mediastinal staging of non-small cell lung cancer (NSCLC), radiation treatment fields are generally based on PET-identified disease extent. However, no studies have examined the accuracy of FDG-PET/CT on a per-node basis in patients being considered for curative-intent radiotherapy in NSCLC.
In a prospective trial, patients with NSCLC being considered for definitive thoracic radiotherapy (± systemic chemotherapy) underwent minimally invasive systematic mediastinal evaluation with endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) following noninvasive staging with integrated PET-CT.
Thirty patients underwent EBUS-TBNA, with TBNA performed from a mean 2.5 lymph node (LN) stations per patient (median 3, range 1–5). Discordant findings between PET-CT and EBUS-TBNA were observed in 10 patients (33%, 95% CI 19%–51%). PET-occult LN metastases were demonstrated by EBUS in 4 patients, whereas a lesser extent of mediastinal involvement, compared with FDG-PET, was demonstrated by EBUS in 6 patients, including 2 patients downstaged from cN3 to pN2. LNs upstaged by EBUS were significantly smaller than nodes downstaged by EBUS, 7.5 mm (range 7–9) versus 12 mm (range 6–21), P = 0.005.
A significant proportion of patients considered for definitive radiotherapy (+/-chemotherapy) undergoing systematic mediastinal evaluation with EBUS-TBNA in this study have an extent of mediastinal NSCLC involvement discordant with that indicated by PET-CT. Systematic EBUS-TBNA may aid in defining the extent of mediastinal involvement in NSCLC patients undergoing radiotherapy. Systematic EBUS-TBNA has the potential to contribute significantly to radiotherapy planning and delivery, by either identifying occult nodal metastases, or demonstrating FDG-avid LNs to be disease-free.
INTRODUCTION
Accurate mediastinal staging of nonsmall cell lung cancer (NSCLC) is critical for determination of optimal treatment strategies. 18F-fluorodeoxyglucose positron emission tomography (FDG-PET) fused with computed tomography (PET-CT) imaging is routinely used for noninvasive staging of patients with suspected or known NSCLC, although mediastinal abnormalities on PET-CT require invasive confirmation due to the limited diagnostic accuracy of PET-CT.1,2
Thoracic surgical guidelines identify mediastinal sampling as being selective (involving only selected suspicious nodes), or systematic (exploration and biopsy of a standard set of lymph node [LN] stations in each case).3,4 For patients with early-stage (Stage I and II) NSCLC, guidelines recommend systematic intraoperative mediastinal LN sampling or complete mediastinal LN dissection (Grade 1B)3,5 to accurately assess the pathologic stage, which is critical to direct adjuvant therapy. Consequently, at completion of therapy, the pathologic extent of disease is discretely defined for surgical patients.
In contrast, although invasive pathologic confirmation of mediastinal NSCLC disease is recommended before radical intent radiotherapy (± systemic chemotherapy), there is no consensus regarding the extent to which pathologic evaluation of the mediastinum should be performed. Thus, although pathological confirmation of mediastinal LN involvement is recommended before radical radiotherapy (with or without chemotherapy),2 in contrast to surgical candidates, comprehensive staging of the mediastinum is not routinely performed in this patient group. This is potentially clinically significant, as, despite the imperfect negative- and positive-predictive value of PET,2,6–10 radiation treatment fields are generally constructed on the basis of PET-identified disease extent.11 Sensitivity of PET/CT is even poorer when individual nodal stations are considered separately.12 Hence, any false-positive nodal activity will result in an unnecessarily extensive field of radiation with consequent greater risk of toxicity, whereas PET-occult nodal metastases will result in the risk of geographic miss, increasing the likelihood of local disease recurrence.
Thus, accurate pathologic characterization of the mediastinum in patients receiving radical radiotherapy for NSCLC (±chemotherapy) has the potential to improve treatment outcomes both in terms of disease control and treatment toxicity. One prior study has demonstrated EBUS may detect PET-occult LN metastases in patients being considered for stereotactic radiotherapy for clinical Stage I NSCLC13; however, no previous studies have undertaken systematic mediastinal evaluation of patients with locally advanced NSCLC.
We hypothesized that systematic mediastinal evaluation with minimally invasive EBUS-TBNA in NSCLC patients being considered for radical radiation therapy may identify disease extent discrepant of that indicated by PET-CT. This may have significant implications for radiation treatment planning and consequently treatment-related outcomes. We conducted a prospective observational study to examine this hypothesis and findings are presented here.
METHODS
Melbourne Health Institutional review board approval was granted for performance of this prospective observational study. All patients provided written informed consent.
Design and Setting
We performed a prospective multicenter observational cohort study in 3 tertiary centers in Melbourne, Australia.
Patients
Eligible patients were those undergoing mediastinal evaluation with EBUS-TBNA for diagnosis\staging of suspected\known NSCLC wherein noninvasive imaging and\or clinical condition indicated the likely treatment modality would be external beam radiotherapy (±systemic chemotherapy) with curative intent, following discussion at a Lung Cancer Multidisciplinary Meeting. Diagnoses other than NSCLC, and the presence of medical comorbidities precluding bronchoscopy, resulted in exclusion from the cohort.
Patients underwent noninvasive staging with PET-CT before bronchoscopy with pretreatment staging established according to the 7th edition of the Lung Cancer Stage Classification, the TNM descriptors for which are reviewed in detail elsewhere.14
Performance of PET-CT
Integrated PET-CT was performed before EBUS-TBNA in all patients at 3 accredited Australian PET centers according to standard institutional protocols using one of GE discovery 690 (GE Medical Systems, Milwaukee, WI), Discovery STE (GE Medical Systems), or Biograph 64/40 (Siemens Medical Solutions, Malvern, PA).
Performance of EBUS-TBNA
EBUS-TBNA was performed under conscious sedation as previously described15,16 with a dedicated linear array bronchoscope (either BF-UC180F-OL8, Olympus, Tokyo, Japan, or EB-1970UK Pentax, Kashiwa, Japan).
Convex probe EBUS evaluation was performed in a systematic fashion, commencing with the highest contralateral mediastinal (N3) LN, as previously described17 LN station anatomy was identified according to endobronchial and sonographic landmarks as previously described.18 Any identified LN ≥6 mm in diameter was sampled via EBUS-TBNA, regardless of sonographic findings.19
Rapid on-site cytologic examination (ROSE) of TBNA aspirates was performed using a rapid Romanowsky stain (Quick Dip; POCD Scientific, Artarmon, Australia), as previously described.20 The aspirate was deemed adequate if lymphocytes were observed, or diagnostic if malignant cells were observed. If inadequate, TBNA was repeated once more. If adequate benign lymphocyte tissue was seen, progression to evaluation of N2 LNs was undertaken, commencing from the most superior PET-negative station, then proceeding as above to inferior N2 stations (eg. 4R\L, 7). Once PET-negative stations were evaluated, we proceeded to EBUS-TBNA of PET-positive LN stations.
For each TBNA, following transfer of initial TBNA material to slides for ROSE, all subsequent materials were placed in formalin solution to allow the preparation of a cell block for histological evaluation and immunohistochemistry (IHC), as previously described.21 Pathology outcomes were based on final histocytologic reports following examination of these specimens.
Review of Discordant Cases
PET-CT imaging for all patients in whom EBUS and PET-CT returned discordant findings regarding the extent of mediastinal involvement underwent independent blinded review to confirm findings from the original PET report. A systematized approach to this was undertaken as follows:
A single PET physician (DG) blinded to the EBUS results and PET-CT reports obtained DICOM image files for the discordant FDG-PET studies and viewed the studies using OsiriX imaging software (Pixmeo, Bermex, Switzerland). Positive nodes were classified as nodes demonstrating FDG uptake greater than mediastinal blood pool uptake. The specific nodal stations assessed were 2R, 2L, 4R, 4L, and 7, and the reviewing PET physician reported each nodal station individually.
The aim of this observational study was to compare diagnostic accuracy of EBUS-TBNA with PET. Where a greater extent of mediastinal involvement was demonstrated by EBUS, this information was incorporated into radiotherapy planning. Despite the high negative predictive value (NPV) of EBUS-TBNA,22,23,46 given the surgical risks of mediastinoscopy,24–26 wherein EBUS suggested a lesser extent of mediastinal disease than was suggested on PET, planning was undertaken on the basis of PET findings alone.
Statistical Analysis
Categorical variables were presented as summary statistics, including simple proportions. All reported confidence intervals are 2-sided. Sensitivity, specificity, and accuracy of the 2 methods were calculated according to standard definition. Continuous data were analyzed with the unpaired t test with Welch correction using GraphPad InStat (GraphPad Software Inc, La Jolla, CA). For all analyses, the level of statistical significance was set at 0.05.
RESULTS
Thirty-five patients undergoing EBUS-TBNA consented to inclusion in the study. All patients underwent EBUS within a maximum of 15 days of performance of PET-CT. Five patients were excluded from the study (Figure 1); therefore, 30 eligible patients with NSCLC form the basis of this report. Male:female ratio was 21:9.
FIGURE 1.
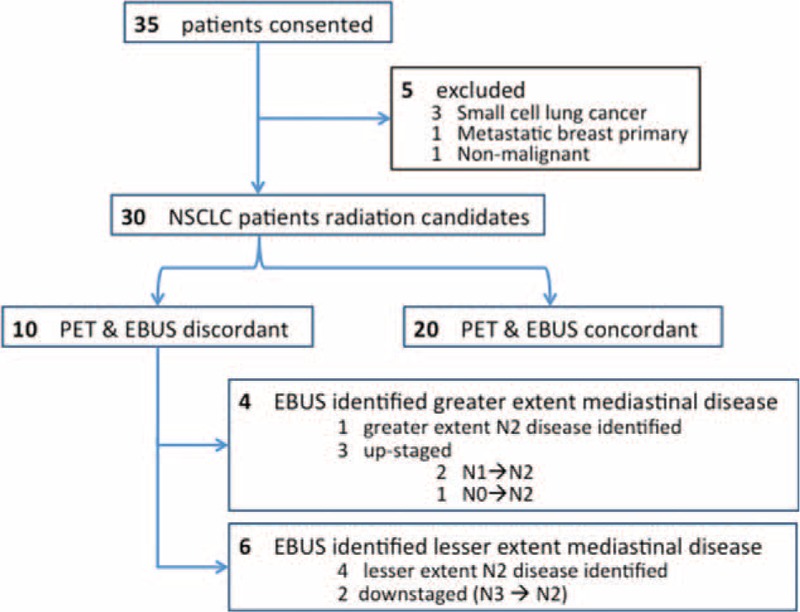
Flowchart of patients enrolled in the study.
No procedural complications occurred during performance of EBUS-TBNA. LNs were visualized by EBUS at a mean 2.9 LN stations per patient. Sampling of visualized LNs was precluded because of size <6 mm (n = 9) or positive ROSE specimen at superior mediastinal LN station (n = 2). Thus, LN sampling was performed from a mean 2.5 LN stations per patient (median 3, range 1–5).
Adequate samples were obtained from all sites examined by EBUS-TBNA. Mean long-axis size of sampled LN was 16 ± 7.8 mm (median 13 mm, range 5–36 mm). Twenty-four percent of sampled LNs were ≤10 mm.
Comparison of PET and EBUS Findings
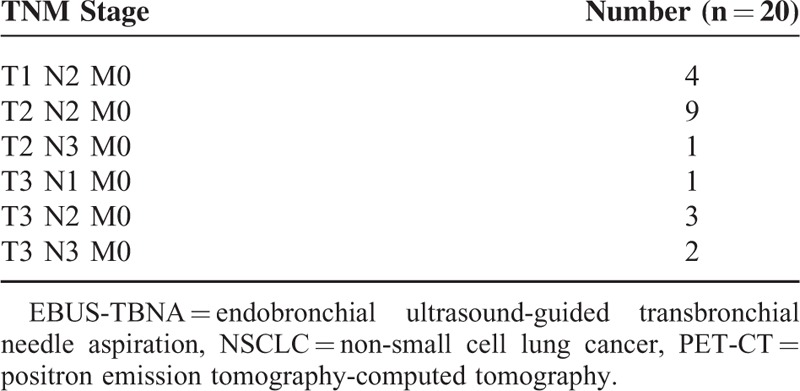
Findings regarding the extent of mediastinal disease on PET-CT and EBUS were concordant in 20 of 30 participants (67%, 95% CI 0.49–0.81). T-, and N-stage of these participants are recorded in Table 1.
TABLE 1.
T-, N-, M-stage of patients in whom EBUS-TBNA and PET-CT demonstrated concordant results regarding mediastinal extent of NSCLC involvement
Discordant findings were observed in 10 of 30 patients (33%, 95% CI 0.19–0.51) Detailed information regarding CT-, PET-, and EBUS-identified stage, and findings at the LN stations returning discrepant findings are presented in Table 2.
TABLE 2.
Clinicoradiologic Features of Patients With Discordant Lymph Node Staging Results Between PET and EBUS
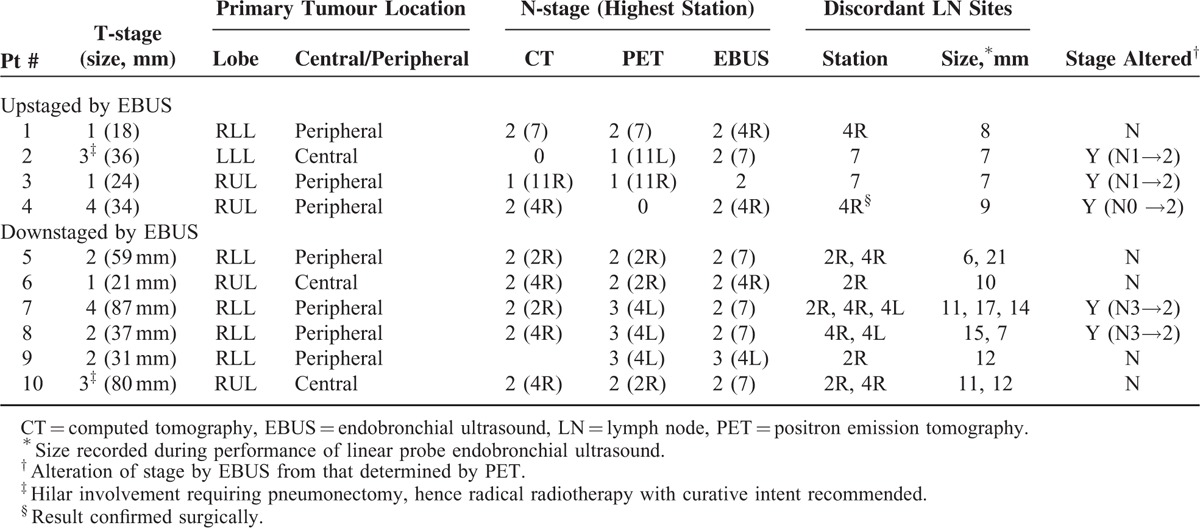
EBUS-TBNA identified malignancy in 4 LNs wherein PET-CT had not detected disease, thus identifying a greater extent of mediastinal involvement in 4 patients (false negative on PET). Three patients were upstaged by EBUS-TBNA and in 1 further patient, extent of disease was greater than noted on PET because of more proximal involvement of LN disease not resulting in stage advancement. Median size of LN upstaged by EBUS was 7.5 mm (range 7–9).
EBUS-TBNA demonstrated only benign lymphocytes in 11 mediastinal LNs (6 patients) wherein PET-CT had indicated the presence of disease. Thus, in 6 patients, EBUS identified a lesser extent of mediastinal disease than PET, including 2 patients downstaged from N3 to N2 (50% of all patients staged N3 by PET-CT, 95% CI 15%–85%). Median size of LN downstaged by EBUS was 12 mm (range 6–21).
LNs upstaged were significantly smaller than those downstaged (P = 0.005)
DISCUSSION
Our report is the first to describe discrepancies in mediastinal staging of NSCLC patients between invasive and noninvasive methods in patients with locoregional NSCLC before radical radiotherapy (±systemic chemotherapy). Our results suggest a significant proportion of this group has an extent of mediastinal nodal involvement different to that indicated by noninvasive PET-CT, with clear implications for radiotherapy planning performed on the basis of imaging alone. We identify 33% of such patients have inaccurate characterization of the mediastinum by PET-CT, with EBUS-TBNA demonstrating a greater extent of disease in 4 patients and a lesser extent of disease in 6 (including 2 patients downstaged from N3 to N2.
EBUS-TBNA is a minimally invasive technique with high diagnostic accuracy in mediastinal staging of NSCLC.16,27 It has supplanted invasive surgical staging for mediastinal assessment given its equivalent\superior diagnostic performance, and its beneficial safety/morbidity16,27 and cost28 profiles. It is recommended as the best first test for invasive mediastinal evaluation,2 and recent studies confirm sensitivity of EBUS-TBNA is equivalent to, or exceeds, mediastinoscopy.22,29 Multiple studies have confirmed the ability of EBUS-TBNA to detect PET-occult LN metastases in clinical stage I NSCLC, and our findings indicate EBUS-TBNA may also identify PET-occult LN metastases within the mediastinum of patients with clinical Stage III NSCLC.
Previous reports suggest a very high NPV of EBUS-TBNA, varying from 0.91 to 0.99.22,23,46 In comparison, sensitivity and specificity of PET-CT for detection of mediastinal metastases are estimated at 77% and 86%,2 and just 53% and 91%, respectively, when examined on a per-nodal basis.12 Consequently, invasive mediastinal staging of FDG-avid lymph nodes is recommended to ensure operable (Stage I and II) patients are not wrongly excluded from potentially curative resection. Such a recommendation regarding FDG-avid nodes in patients in which N2 disease has been confirmed at an alternate site is lacking. Our results indicate that in patients with FDG-avid LN metastases, other sites of FDG-avidity may be false-positive results.
Our observation of variation between EBUS- and PET-determined mediastinal disease extent is consistent with surgical literature, although this variation has not previously been demonstrated in pN2 NSCLC patients undergoing nonsurgical treatment. EBUS detected PET-occult nodal involvement in 13% of our cohort, consistent with the 5% to 16% upstaging rate among patients PET-staged cN0 following surgical6,7 or minimally invasive8–10 lymph node sampling. Similarly, false-positive PET-CT findings were suggested by EBUS-TBNA in 20% of our cohort, consistent with previously reported specificity of 83% to 87% for PET-CT detection of mediastinal disease wherein prevalence of disease is >20%.2
Published reviews have reported a sensitivity of integrated PET-CT of just 62% for detection of mediastinal lymph node metastases.2 Importantly, postsurgical studies suggest that the median size of metastatic foci in mediastinal lymph nodes involved with NSCLC is 7 mm.30 This is less than the accepted limit of detection of PET/CT12,31; therefore, almost certainly a proportion of patients have a mediastinal disease extent greater than that indicated by PET/CT. Such lesions may only be detected by invasive means. Multiple studies have demonstrated the ability of EBUS-TBNA to identify PET-occult N2 disease,8–10 with high sensitivity preserved even in evaluation of subcentimeter disease.10 Therefore, our finding of EBUS-detected PET-occult disease occurring in nodes with median size 7.5 mm is unsurprising.
Interestingly, 2 of 4 patients upstaged by EBUS were patients with cN1 disease (based on PET-CT). Postoperative pathologic upstaging may occur in over 25% of patients staged cN1,32 which likely reflects the increased biologic propensity of tumour to spread to mediastinal nodes given it has already acquired the capacity to metastasize to hilar nodes. Consequently, both ACCP and ESTS guidelines on NSCLC staging recommend (Grade 1C) that invasive staging be performed before resection in those staged cN1 by PET.2,33
Accurate characterization of extent of mediastinal disease may have major ramifications for treatment outcomes. In a significant proportion of patients who demonstrate treatment failure following radiotherapy, disease is seen to recur “out-of-field,”34,35 thai is, in a location not subjected to radiotherapy. Rates of local disease recurrence are significantly higher in lymph node stations receiving suboptimal radiation doses.36 This indicates the importance of accurately defining the extent of disease before radiotherapy for treatment of NSCLC.
The results of this study suggest that systematic mediastinal staging with EBUS may impact the delivery of radiotherapy by either identifying occult nodal metastases (thereby reducing the risk of geographic miss) or demonstrating FDG-avid lymph nodes to be disease-free (thereby allowing reduction in field size and potentially reducing toxicity risks) in a proportion of patients. The dosimetric consequences of discordance of PET/CT- versus EBUS-defined mediastinal staging need to be evaluated in this context. Our findings will be important in informing future studies on systematic mediastinal pathologic staging before radical radiotherapy (±chemotherapy).
Evidence for the potential impact on treatment outcomes for more accurate pathologic staging of the mediastinum may be inferred from studies examining outcomes in patients receiving stereotactic ablative radiotherapy (SABR) for early stage I NSCLC. Higher than expected regional LN failure rates are seen in patients receiving SABR in which staging was performed on the basis of PET alone, with the authors suggesting these outcomes indicate the potential utility of minimally invasive EBUS-TBNA for LN staging before radiation therapy.37
We have enrolled a consecutive sample of patients undergoing EBUS-TBNA mediastinal assessment. Previous studies have identified clinical factors (eg, central tumour, cN1, adenocarcinoma histology),2 sonographic features,19 risk stratification models,38 or used artificial neural networks39 to identify patients at higher risk of postsurgical upstaging. Larger studies will be required to examine the applicability of such tools to cohorts similar to ours, or to identify specific clinicoradiologic features predictive of a higher rate of detection of occult disease (or false-positive PET findings).
Limitations
This was a prospective observational pilot study. Surgical confirmation of discrepant results was not performed. Previous meta-analyses have suggested a 0% false-positive rate with EBUS-TBNA,27 indicating EBUS-detected disease is a reliable true-positive result. Negative EBUS-TBNA at FDG-avid sites is associated with a NPV of 0.91 to 0.99,23,40,41,42 and negative EBUS-TBNA in NSCLC surgical candidates predicts a very low prevalence of metastatic disease in sampled nodes, sufficient to obviate the need for confirmatory mediastinoscopy preoperatively.40 Previous studies have confirmed that pathologic staging is the criterion standard for mediastinal staging, with FDG-avidity on PET not associated with risk of recurrence in patients histologically negative mediastinal lymph nodes.43 Nevertheless, future studies may consider surgical confirmation of negative EBUS-TBNA results before excluding FDG-avid LN from radiation treatment fields.
We have undertaken systematic staging of the mediastinum. It is unclear whether selective lymph node targeting (eg, the next echelon above LN involved on PET-CT) would identify the same proportion of disease not accurately characterized by PET-CT. “Skip” metastases are a well-recognized phenomena,44,45 suggesting that such an approach may reduce accuracy of complete characterization of mediastinal nodes in NSCLC patients before radiation. Potential benefits of selective sampling (eg, reduced procedure time) may be offset by reduced diagnostic performance, although this remains to be examined.
Not all stations were sampled in each patient, with a mean 2.5 LN stations sampled per patient. This is in part because of termination of the procedure following a positive ROSE result, but also is because of no lymph nodes being identified, or only LN <5 mm at a particular station. Although systematic LN sampling was not performed, a systematic EBUS mediastinal examination was performed in each patient.
We have used only EBUS-TBNA in minimally invasive staging. Diagnostic accuracy in mediastinal staging may be improved when both EBUS-TBNA and EUS-FNA are performed,46–48 as EUS allows sampling at sites not amenable to EBUS-TBNA (eg. Station 8, 9)18; however, this requires an additional procedure. More practically, the linear array EBUS bronchoscope may allow performance of EBUS-TBNA and transoesophageal sampling (EUS-B-FNA) by a single operator. Such an approach may further improve diagnostic accuracy of minimally invasive mediastinal assessment42,49,50 and should be considered for future studies.
CONCLUSIONS
Our pilot study demonstrates that systematic mediastinal staging with EBUS-TBNA in nonsurgical NSCLC patients being considered for radical radiotherapy idenitifes a significant proportion of patients with an extent of mediastinal disease differing from that indicated by non-invasive PET-CT. These results suggest systematic minimally invasive staging should be considered for all patients before definitive thoracic radiotherapy to accurately assess pathologic stage of disease, and to ensure treatment fields most accurately encompass all sites of disease.
Comprehensive mediastinal staging with EBUS may improve the delivery of radiotherapy by either identifying occult nodal metastases (thereby reducing the risk of geographic miss) or demonstrating FDG-avid lymph nodes to be disease-free (thereby allowing reduction in field size and potentially reducing toxicity risks) in a proportion of patients.
Footnotes
Abbreviations: EBUS = endobronchial ultrasound, EUS = endoscopic ultrasound, FDG-PET = fluorodeoxyglucose positron emission tomography, LN = lymph node, NPV = negative predictive value, NSCLC = non-small cell lung cancer, TBNA = transbronchial needle aspiration.
DPS is supported by the David Bickart Clinician Researcher Fellowship
The authors report no conflicts of interest.
REFERENCES
- 1.Schmidt-Hansen M, Baldwin DR, Hasler E, et al. PET-CT for assessing mediastinal lymph node involvement in patients with suspected resectable non-small cell lung cancer. Cochrane Database Syst Rev 2014; 11: CD009519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Silvestri GA, Gonzalez AV, Jantz MA, et al. Methods for staging non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013; 143 5 Suppl:e211S–250S. [DOI] [PubMed] [Google Scholar]
- 3.Howington JA, Blum MG, Chang AC, et al. Treatment of stage I and II non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013; 143 5 Suppl:e278S–e313S. [DOI] [PubMed] [Google Scholar]
- 4.Lardinois D, De Leyn P, Van Schil P, et al. ESTS guidelines for intraoperative lymph node staging in non-small cell lung cancer. Eur J Cardiothorac Surg 2006; 30:787–792. [DOI] [PubMed] [Google Scholar]
- 5.Ramnath N, Dilling TJ, Harris LJ, et al. Treatment of stage III non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013; 143 5 Suppl:e314S–e340S. [DOI] [PubMed] [Google Scholar]
- 6.Cerfolio RJ, Bryant AS, Eloubeidi MA. Routine mediastinoscopy and esophageal ultrasound fine-needle aspiration in patients with non-small cell lung cancer who are clinically N2 negative: a prospective study. Chest 2006; 130:1791–1795. [DOI] [PubMed] [Google Scholar]
- 7.Al-Sarraf N, Aziz R, Gately K, et al. Pattern and predictors of occult mediastinal lymph node involvement in non-small cell lung cancer patients with negative mediastinal uptake on positron emission tomography. Eur J Cardiothorac Surg 2008; 33:104–109. [DOI] [PubMed] [Google Scholar]
- 8.Yasufuku K, Nakajima T, Waddell T, et al. Endobronchial ultrasound-guided transbronchial needle aspiration for differentiating N0 versus N1 lung cancer. Ann Thorac Surg 2013; 96:1756–1760. [DOI] [PubMed] [Google Scholar]
- 9.Sarwate D, Sarkar S, Krimsky WS, et al. Optimization of mediastinal staging in potential candidates for stereotactic radiosurgery of the chest. J Thorac Cardiovasc Surg 2012; 144:81–86. [DOI] [PubMed] [Google Scholar]
- 10.Herth FJ, Ernst A, Eberhardt R, et al. Endobronchial ultrasound-guided transbronchial needle aspiration of lymph nodes in the radiologically normal mediastinum. Eur Respir J 2006; 28:910–914. [DOI] [PubMed] [Google Scholar]
- 11.Lehman M. Clinical_question:What_are_the_principles_of_radiation_therapy_in_the_definitive_management_of_stage_III_inoperable_NSCLC. Clinical practice guidelines for the treatment of lung cancer 2015 [cited 2015 Apr 16]; Available from: http://wiki.cancer.org.au/australia/Clinical_question:What_are_the_principles_of_radiation_therapy_in_the_definitive_management_of_stage_III_inoperable_NSCLC%3F. [Google Scholar]
- 12.Bille A, Okiror L, Skanjeti A, et al. Evaluation of integrated positron emission tomography and computed tomography accuracy in detecting lymph node metastasis in patients with adenocarcinoma vs squamous cell carcinoma. Eur J Cardiothorac Surg 2013; 43:574–579. [DOI] [PubMed] [Google Scholar]
- 13.Nakajima T, Yasufuku K, Nakajima M, et al. Endobronchial ultrasound-guided transbronchial needle aspiration for lymph node staging in patients with non-small cell lung cancer in non-operable patients pursuing radiotherapy as a primary treatment. J Thorac Oncol 2010; 5:606–611. [DOI] [PubMed] [Google Scholar]
- 14.Detterbeck FC, Postmus PE, Tanoue LT. The stage classification of lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013; 143 5 Suppl:e191S–210S. [DOI] [PubMed] [Google Scholar]
- 15.Steinfort DP, Irving LB. Patient satisfaction during endobronchial ultrasound-guided transbronchial needle aspiration performed under conscious sedation. Respir Care 2010; 55:702–706. [PubMed] [Google Scholar]
- 16.Steinfort DP, Hew MJ, Irving LB. Bronchoscopic evaluation of the mediastinum using endobronchial ultrasound: a description of the first 216 cases carried out at an Australian tertiary hospital. Intern Med J 2011; 41:815–824. [DOI] [PubMed] [Google Scholar]
- 17.van der Heijden EH, Casal RF, Trisolini R, et al. World Association for Bronchology and Interventional Pulmonology, Task force on Specimen Guidelines. Guideline for the acquisition and preparation of conventional and endobronchial ultrasound-guided transbronchial needle aspiration specimens for the diagnosis and molecular testing of patients with known or suspected lung cancer. Respiration 2014; 88:500–517. [DOI] [PubMed] [Google Scholar]
- 18.Tournoy KG, Annema JT, Krasnik M, et al. Endoscopic and endobronchial ultrasonography according to the proposed lymph node map definition in the seventh edition of the tumor, node, metastasis classification for lung cancer. J Thorac Oncol 2009; 4:1576–1584. [DOI] [PubMed] [Google Scholar]
- 19.Fujiwara T, Yasufuku K, Nakajima T, et al. The utility of sonographic features during endobronchial ultrasound-guided transbronchial needle aspiration for lymph node staging in patients with lung cancer: a standard endobronchial ultrasound image classification system. Chest 2010; 138:641–647. [DOI] [PubMed] [Google Scholar]
- 20.Steinfort DP, Leong TL, Laska IF, et al. Diagnostic utility and accuracy of rapid on-site evaluation of bronchoscopic brushings. Eur Respir J 2015; 45:1653–1660. [DOI] [PubMed] [Google Scholar]
- 21.Steinfort DP, Russell PA, Tsui A, et al. Interobserver agreement in determining non-small cell lung cancer subtype in specimens acquired by EBUS-TBNA. The European respiratory journal 2012; 40:699–705. [DOI] [PubMed] [Google Scholar]
- 22.Yasufuku K, Pierre A, Darling G, et al. A prospective controlled trial of endobronchial ultrasound-guided transbronchial needle aspiration compared with mediastinoscopy for mediastinal lymph node staging of lung cancer. J Thorac Cardiovasc Surg 2011; 142:1393–1400.e1391. [DOI] [PubMed] [Google Scholar]
- 23.Clementsen PF, Skov BG, Vilmann P, et al. Endobronchial ultrasound-guided biopsy performed under optimal conditions in patients with known or suspected lung cancer may render mediastinoscopy unnecessary. J Bronchol Interv Pulmonol 2014; 21:21–25. [DOI] [PubMed] [Google Scholar]
- 24.Reich JM, Brouns MC, O’Connor EA, et al. Mediastinoscopy in patients with presumptive stage I sarcoidosis: a risk/benefit, cost/benefit analysis. Chest 1998; 113:147–153. [DOI] [PubMed] [Google Scholar]
- 25.Hammoud ZT, Anderson RC, Meyers BF, et al. The current role of mediastinoscopy in the evaluation of thoracic disease. J Thorac Cardiovasc Surg 1999; 118:894–899. [DOI] [PubMed] [Google Scholar]
- 26.Hujala KT, Sipila JI, Grenman R. Mediastinoscopy—its role and value today in the differential diagnosis of mediastinal pathology. Acta Oncol 2001; 40:79–82. [DOI] [PubMed] [Google Scholar]
- 27.Gu P, Zhao YZ, Jiang LY, et al. Endobronchial ultrasound-guided transbronchial needle aspiration for staging of lung cancer: a systematic review and meta-analysis. Eur J Cancer 2009; 45:1389–1396. [DOI] [PubMed] [Google Scholar]
- 28.Steinfort DP, Liew D, Conron M, et al. Cost-benefit of minimally invasive staging of non-small cell lung cancer: a decision tree sensitivity analysis. J Thorac Oncol 2010; 5:1564–1570. [DOI] [PubMed] [Google Scholar]
- 29.Ernst A, Anantham D, Eberhardt R, et al. Diagnosis of mediastinal adenopathy-real-time endobronchial ultrasound guided needle aspiration versus mediastinoscopy. J Thorac Oncol 2008; 3:577–582. [DOI] [PubMed] [Google Scholar]
- 30.Nomori H, Watanabe K, Ohtsuka T, et al. The size of metastatic foci and lymph nodes yielding false-negative and false-positive lymph node staging with positron emission tomography in patients with lung cancer. J Thorac Cardiovasc Surg 2004; 127:1087–1092. [DOI] [PubMed] [Google Scholar]
- 31.Wang Y, Ma S, Dong M, et al. Evaluation of the factors affecting the maximum standardized uptake value of metastatic lymph nodes in different histological types of non-small cell lung cancer on PET-CT. BMC Pulm Med 2015; 15:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cerfolio RJ, Bryant AS, Ojha B, et al. Improving the inaccuracies of clinical staging of patients with NSCLC: a prospective trial. Ann Thorac Surg 2005; 80:1207–1213. [DOI] [PubMed] [Google Scholar]
- 33.De Leyn P, Dooms C, Kuzdzal J, et al. Revised ESTS guidelines for preoperative mediastinal lymph node staging for non-small-cell lung cancer. Eur J Cardiothorac Surg 2014; 45:787–798. [DOI] [PubMed] [Google Scholar]
- 34.Byhardt RW, Scott C, Sause WT, et al. Response, toxicity, failure patterns, and survival in five Radiation Therapy Oncology Group (RTOG) trials of sequential and/or concurrent chemotherapy and radiotherapy for locally advanced non-small-cell carcinoma of the lung. Int J Radiat Oncol Biol Phys 1998; 42:469–478. [DOI] [PubMed] [Google Scholar]
- 35.Chien CR, Chen SW, Hsieh CY, et al. Intra-thoracic failure pattern and survival status following 3D conformal radiotherapy for non-small cell lung cancer: a preliminary report. Jpn J Clin Oncol 2001; 31:55–60. [DOI] [PubMed] [Google Scholar]
- 36.Kimura T, Togami T, Nishiyama Y, et al. Impact of incidental irradiation on clinically uninvolved nodal regions in patients with advanced non-small-cell lung cancer treated with involved-field radiation therapy: does incidental irradiation contribute to the low incidence of elective nodal failure? Int J Radiat Oncol Biol Phys 2010; 77:337–343. [DOI] [PubMed] [Google Scholar]
- 37.Heal C, Ding W, Lamond J, et al. Definitive treatment of early-stage non-small cell lung cancer with stereotactic ablative body radiotherapy in a community cancer center setting. Front Oncol 2015; 5:146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Evison M, Morris J, Martin J, et al. Nodal staging in lung cancer: a risk stratification model for lymph nodes classified as negative by EBUS-TBNA. J Thorac Oncol 2015; 10:126–133. [DOI] [PubMed] [Google Scholar]
- 39.Wnuk P, Kowalewski M, Malkowski B, et al. PET-CT derived Artificial Neural Network can predict mediastinal lymph nodes metastases in Non-Small Cell Lung Cancer patients. Preliminary report and scoring model. Q J Nucl Med Mol Imag 2014; [Epub ahead of print]. [PubMed] [Google Scholar]
- 40.Annema JT, van Meerbeeck JP, Rintoul RC, et al. Mediastinoscopy vs endosonography for mediastinal nodal staging of lung cancer: a randomized trial. JAMA 2010; 304:2245–2252. [DOI] [PubMed] [Google Scholar]
- 41.Sanz-Santos J, Andreo F, Castella E, et al. Representativeness of nodal sampling with endobronchial ultrasonography in non-small-cell lung cancer staging. Ultrasound Med Biol 2012; 38:62–68. [DOI] [PubMed] [Google Scholar]
- 42.Taverner J, See K, Irving LB, et al. Negative EBUS-TBNA predicts very low prevalence of mediastinal disease in staging of NSCLC. J Bronchol Interv Pulmonol 2015; 8: in press. [DOI] [PubMed] [Google Scholar]
- 43.Tandberg DJ, Gee NG, Chino JP, et al. Are discordant positron emission tomography and pathological assessments of the mediastinum in non-small cell lung cancer significant? J Thorac Cardiovasc Surg 2013; 146:796–801. [DOI] [PubMed] [Google Scholar]
- 44.Ilic N, Petricevic A, Arar D, et al. Skip mediastinal nodal metastases in the IIIa/N2 non-small cell lung cancer. J Thorac Oncol 2007; 2:1018–1021. [DOI] [PubMed] [Google Scholar]
- 45.Benoit L, Anusca A, Ortega-Deballon P, et al. Analysis of risk factors for skip lymphatic metastasis and their prognostic value in operated N2 non-small-cell lung carcinoma. Eur J Surg Oncol 2006; 32:583–587. [DOI] [PubMed] [Google Scholar]
- 46.Vilmann P, Krasnik M, Larsen SS, et al. Transesophageal endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) and endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) biopsy: a combined approach in the evaluation of mediastinal lesions. Endoscopy 2005; 37:833–839. [DOI] [PubMed] [Google Scholar]
- 47.Zhang R, Ying K, Shi L, et al. Combined endobronchial and endoscopic ultrasound-guided fine needle aspiration for mediastinal lymph node staging of lung cancer: a meta-analysis. Eur J Cancer 2013; 49:1860–1867. [DOI] [PubMed] [Google Scholar]
- 48.Oki M, Saka H, Ando M, et al. Endoscopic ultrasound-guided fine needle aspiration and endobronchial ultrasound-guided transbronchial needle aspiration: Are two better than one in mediastinal staging of non-small cell lung cancer? J Thorac Cardiovasc Surg 2014; 148:1169–1177. [DOI] [PubMed] [Google Scholar]
- 49.Herth FJ, Krasnik M, Kahn N, et al. Combined endoscopic-endobronchial ultrasound-guided fine-needle aspiration of mediastinal lymph nodes through a single bronchoscope in 150 patients with suspected lung cancer. Chest 2010; 138:790–794. [DOI] [PubMed] [Google Scholar]
- 50.Hwangbo B, Lee GK, Lee HS, et al. Transbronchial and transesophageal fine-needle aspiration using an ultrasound bronchoscope in mediastinal staging of potentially operable lung cancer. Chest 2010; 138:795–802. [DOI] [PubMed] [Google Scholar]


