Abstract
The effect of probiotics on late-onset sepsis (LOS) in preterm neonates remains controversial. The authors systematically reviewed the literature to investigate whether enteral probiotic supplementation reduced the risk of LOS in preterm neonates in neonatal intensive care units.
PubMed, Embase, and Cochrane Central Register of Controlled Trials were systematically searched for randomized controlled trials (RCTs) regarding the effect of probiotics in preterm neonates. The primary outcome was culture-proven bacterial and/or fungal sepsis. The Mantel–Haenszel method with random-effects model was used to calculate pooled relative risks (RRs) and 95% confidence intervals (CIs).
Twenty-seven trials were included in our review, and 25 trials involving 6104 preterm neonates were statistically analyzed. Pooled analysis indicated that enteral probiotic supplementation significantly reduced the risk of any sepsis (25 RCTs; RR 0.83, 95% CI 0.73–0.94; I2 = 26%), bacterial sepsis (11 RCTs; RR 0.82, 95% CI 0.71–0.95; I2 = 0%), and fungal sepsis (6 RCTs; RR 0.57, 95% CI 0.41–0.78; I2 = 0%). This beneficial effect remains in very low birth weight infants (<1500 g) (19 RCTs; RR 0.86, 95% CI 0.75–0.97; I2 = 18%), but not in extremely low birth weight infants (<1000 g) (3 RCTs; RR 0.73, 95% CI 0.45–1.19; I2 = 53%). All the included trials reported no systemic infection caused by the supplemental probiotic organisms.
Current evidence indicates that probiotic supplementation is safe, and effective in reducing the risk of LOS in preterm neonates in neonatal intensive care units. Further studies are needed to address the optimal probiotic organism, dosing, timing, and duration. High-quality and adequately powered RCTs regarding the efficacy and safety of the use of probiotics in extremely low birth weight infants are still warranted.
INTRODUCTION
In neonatal intensive care units (NICUs), late-onset sepsis (LOS) arising >72 hours after birth is a frequent complication of prematurity, and is associated with increased medical costs, prolonged hospitalization, and significant mortality and morbidity.1–3 Despite the improvements in the quality of neonatal assistance, the reported incidences of LOS are still dramatically high.1,2,4 Preterm neonates are indeed highly prone to develop bacterial and fungal sepsis because of their immature skin/mucosal barrier and immune response, use of invasive procedures and devices, use of broad-spectrum antimicrobial drugs, and exposure to the hospital milieu, which gives rise to gastrointestinal colonization with pathogens.5–9
Probiotics, defined as live microorganisms, confer health benefits to the host when administered at adequate doses,10 and have been suggested to modify the enteric microflora, suppress the overgrowth and translocation of pathogens in the gut, and therefore prevent life-threatening infections.11–14 Although there is no controversy about probiotics reducing the risk of stage II to III necrotizing enterocolitis (NEC) in preterm neonates,15–17 the effect of probiotics on LOS remains a highly live issue. So far, studies reporting the effect of probiotics on LOS conveyed conflicting results. Furthermore, because of small sample sizes, these studies were not adequately powered to detect the effect of probiotics on LOS in preterm neonates. Thus, to provide the latest and most convincing evidence, we systematically reviewed the current available literature to investigate whether enteric probiotic supplementation reduced the risk of LOS in preterm neonates in NICUs.
METHODS
This systematic review and meta-analysis was conducted and reported in adherence to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement,18 and the guidelines of the Cochrane Handbook for Systematic Reviews of Interventions.19 Because our study was a review of previous published studies, ethical approval or patient consent was not required.
Literature Search and Selection Criteria
PubMed, Embase, and Cochrane Central Register of Controlled Trials were searched for records that compared enteral probiotics to placebo or no intervention in preterm neonates in NICUs. The language was restricted to English. The search strategy is shown in Table 1. The last search was conducted on August 11, 2015. The cited references of retrieved articles and previous reviews were also manually checked to identify any additional eligible trials. All citations were imported into a bibliographic database (EndNote X7; Thomson Reuters), and 2 of the authors (G-QZ and H-JH) independently screened the candidate articles to check their eligibility for inclusion.
TABLE 1.
Search Strategy

We developed a PICOS (Patient, Intervention, Comparators, Outcome, and Study design) approach as the eligibility criteria: 1) Population: preterm infants <37 weeks or birth weight <2500 g, or both; 2) Intervention: any species/strains/doses regimen of live probiotics administered for >7 days; 3) Comparators: placebo or no probiotics; 4) Outcome: the primary outcome was any sepsis occurring >72 hours after birth, defined as positive blood/urine/cerebrospinal fluid cultures. The secondary outcome was systemic infection caused by supplemented probiotic organisms; 5) Study design: only randomized controlled trials (RCTs) were eligible. We excluded interventions other than live probiotics, administration of probiotics with prebiotics or other agents, and those conducted in children or adolescents. Discrepancies regarding study inclusion between the 2 authors (G-QZ and H-JH) were resolved through discussion with the correspondence author (Z-YL), as required.
Date Extraction and Quality Assessment
Two of the authors (G-QZ and H-JH) independently extracted relevant data from each included trials by using a unified data form. Extracted data were entered into a standardized Word file. The items included in the data form were as follows: source (first author), number of preterm infants enrolled, strains/doses/duration of probiotics administered, type of milk (human milk or formula), and outcomes of interest (any sepsis/bacterial sepsis/fungal sepsis). Discrepancies between authors were resolved by consensus. Authors were contacted in case of inadequate information to clarify or provide additional information. We adopted the Cochrane Risk-of-Bias Tool to assess the risk of bias for each RCT.20
Statistical Analysis
To evaluate the effect of probiotics, we calculated relative risks (RRs) for the incidence of LOS between intervention and control groups. Trials with uneven distribution of sepsis-related risk factors between study and control groups were not included in our meta-analysis, such as gestational age, birth weight, Apgar score, prenatal steroids, antimicrobial drugs, and use of invasive devices.21 When trials investigated 2 separate probiotic groups versus placebo, data on the 2 probiotic groups were combined into a single RR, which we included in the meta-analysis. Heterogeneity across studies was tested by using the I2 statistic. Studies with an I2 value of >50% were considered to have significant heterogeneity.22 The Mantel–Haenszel method with random-effects model was used to calculate pooled RRs and 95% confidence intervals (CIs). Subgroup analyses were conducted according to type of sepsis, birth weight, probiotic organism, probiotic dose, time of initiation, duration of intervention, type of milk, caesarean delivery rate, and risk of bias. We also investigated the influence of a single study on the overall pooled RR by omitting each study in turn. An assessment of publication bias was performed by visually inspecting funnel plot and by using the Begg's and Egger's tests.23,24 A P value <0.05 was considered as statistically significant, except where otherwise specified. All the statistical analyses were performed using the Stata 12.0 (Stata Corporation, College Station, TX) and RevMan 5.3 (The Nordic Cochrane Centre, Copenhagen, Denmark).
RESULTS
The selection process is detailed in Figure 1. A total of 601 potentially relevant records were identified by our search strategy. Seventy-four records were excluded for duplicates and an additional 497 records were excluded based on the titles and abstracts. The remaining 30 full-text articles were assessed for eligibility, 3 of which14,25,26 were further excluded because incidences of LOS were not reported. Finally, 27 trials were eligible for this review.27–53 Two trials were not included in meta-analysis because of the uneven distribution of birth weight38 and duration of umbilical venous catheter49 between study and control groups. Hence, 25 trials were statistically analyzed.27–37,39–48,50–53 Characteristics of the 27 trials are summarized in Table 2 and the outcome data of each included study are presented in Table 3. The quality of the trials assessed by the Cochrane Risk-of-Bias Tool is summarized in Table 4.
FIGURE 1.

Selection process for the studies included in the meta-analysis.
TABLE 2.
Characteristics of Randomized Controlled Trials Included in Our Meta-Analysis

TABLE 2 (Continued).
Characteristics of Randomized Controlled Trials Included in Our Meta-Analysis

TABLE 3.
Outcome Data of Included Studies

Figure 2 shows the results from each trial and overall, using a random-effects model, for probiotics in the prevention of LOS in preterm neonates. Of the 25 estimates, 20 were <1.0. The summary of RR of LOS was 0.83 (95% CI 0.73–0.94). Results of the studies were homogeneous (I2 = 26%). Furthermore, including the 2 trials with uneven distribution of sepsis-related risk factors between intervention and control groups, the RR was consistent with the main analysis (RR 0.86, 95% CI 0.76–0.98, I2 = 37%). Further exclusion of any single study did not materially alter the overall combined RR, with a range from 0.81 (95% CI 0.72–0.92) to 0.84 (95% CI 0.75–0.95). There was no evidence of significant publication bias by inspection of the funnel plot and formal statistical tests (Egger's test, P = 0.269; Begg's test, P = 0.264; Figure 3). None of the included trials reported any systemic infection caused by the supplemented probiotic organisms.
FIGURE 2.

Effect of probiotics on late-onset sepsis in preterm neonates.
FIGURE 3.
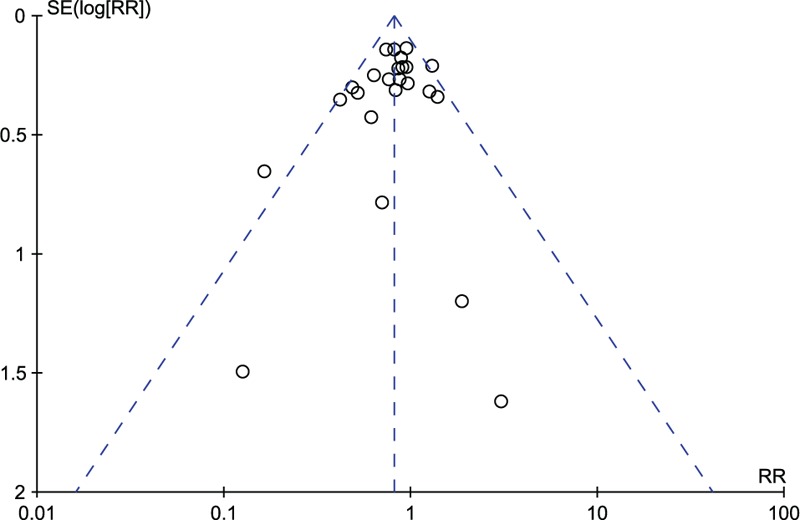
Funnel plot of trials on probiotics and prevention of late-onset sepsis.
Table 5 reports the pooled RRs for probiotic supplementation in the prevention of LOS in preterm neonates in selected subgroups. Probiotic supplementation was consistently associated with reduced incidence of LOS in most subgroups. Significant differences were observed according to birth weight (<2500, <1500, or <1000 g), probiotic organisms, duration of intervention (<6 weeks or ≥6 weeks), and type of milk (human milk or formula milk).
TABLE 4.
Risk-of-Bias Assessment of the Included Randomized Controlled Trials∗

TABLE 5.
Subgroup Analyses for Probiotic Supplementation in the Prevention of Late-Onset Sepsis

DISCUSSION
The results of our meta-analysis indicated that administration of prophylactic probiotics could significantly reduce the incidence of LOS in preterm neonates in NICUs. Low heterogeneity, influence analysis, lack of publication bias, and the consistency of results in most subgroups added robustness to our main findings. Our study also provided robust safety data of probiotics utilization in preterm neonates.
Comparison with Previous Studies
Differences between the current meta-analysis and 2 recent meta-analyses should be noted. A meta-analysis by Bernardo et al16 in 2013 evaluated the effect of probiotics on sepsis in preterm neonates (gestational age <34 weeks or birth weight <1500 g). The authors included 12 RCTs involving 2907 subjects and concluded that enteral administration of probiotics reduced the incidence of sepsis in preterm neonates, although with no significant difference between groups (RD −0.03, 95% CI −0.05 to −0.00, I2 = 52%). In another meta-analysis in 201415 focusing on preterm neonates (gestational age <37 weeks or birth weight <2500 g), AlFaleh et al included 19 RCTs involving 5338 subjects and concluded that there was no evidence of probiotic supplementation reducing the risk of nosocomial sepsis (RR 0.91, 95% CI 0.80–1.03, I2 = 47%). Several limitations, however, should be noted in the 2 meta-analyses. First, not all trials that met their specific eligibility criteria were included, for example, 6 trials27,30,36,45,51,52 for Bernardo et al and 3 trials34,45,52 for AlFaleh et al, which could potentially lead to publication bias. Second, 1 RCT54 should not be included because of ineligible intervention (probiotics administered with bovine lactoferrin). Third, these pooled results were based on an improper model of fixed effects model because of significant clinical/statistical heterogeneity. Overall, both previous meta-analyses had obvious flaws that might threaten the authenticity of their findings. After the 2 meta-analyses, several studies investigating the effect of probiotics in preterm neonates were published. Our updated meta-analysis included 25 RCTs with a total of 6104 subjects. In contrast with the previous meta-analyses, the current 1 suggested that enteral probiotic supplementation significantly reduced the incidence of LOS in preterm neonates in NICUs. Moreover, low heterogeneity, influence analysis, lack of publication bias, and the consistency of results in most subgroups added robustness to our main findings.
Potential underlying mechanisms by which probiotics might prevent sepsis include competitively colonizing the gut, competitive exclusion of potentially pathogenic luminal bacteria and fungi,55 enhanced mucosal immunoglobulin (Ig) A responses,56 modulation of the gut barrier function and permeability,57 production of antimicrobial peptides,58 and upregulation of immune responses.59 We, however, saw a lack of effect of probiotics in extremely low birth weight infants (ELBW; < 1000 g). One probable reason was that our study was not adequately powered to detect its beneficial effect, because only 3 studies27,35,42 involving 771 neonates were included in this subgroup analysis. But, we still cannot exclude the possibility that probiotics may have a lesser effect in ELBW infants, compared with neonates with a birth weight of <1500 g, because of even greater increase in the overall risk of infection.39 In summary, probiotics appear promising for use as prevention strategy for LOS, but there are still insufficient data about the efficacy and safety of the use of probiotics in ELBW infants. Hence, high-quality and adequately powered RCTs in ELBW infants are warranted.
The reason why there was a lack of effect of probiotics on LOS in the 2 trials,38,49 which were excluded from our meta-analysis, should be discussed. Of note, there was uneven distribution of infection-related risk factors between study and control groups. This uneven baseline characteristics between groups (more infants weighing <750 g or longer duration of umbilical venous catheterization in the study group) could probably lead to overturn of the real effects. On the other hand, the pathogens causing sepsis were most often related to catheter-related infections in the 2 trials. It is tempting to speculate that probiotics alone are not capable of preventing the invasive procedures inducing infections, because the effects of orally administered probiotics are primarily in the gastrointestinal tract.
Because different probiotic organisms probably have distinct regulatory effects on the host,60 caution is needed in interpreting our results. Our study indicated that Lactobacillus species or a mixture of 2 or 3 species of probiotics may be more effective in reducing the risk of LOS. A meta-analysis conducted in 2015 also found that Lactobacillus reuteri DSM 17938 could significantly reduce the risk of NEC and LOS.61 To our knowledge, only 1 trial, however, compared the effect of different probiotic strains on LOS in preterm neonates,45 which showed no difference between groups. Therefore, future experimental and clinical studies are still needed to characterize the mechanisms by which specific probiotic organisms influence the development of LOS.
In our study, we observed that preterm neonates fed exclusively human milk benefit more from probiotics. It is well known that human milk contains several substances with putative anti-infective actions, such as lactoferrin, IgA, IgG, and IgM, etc.21,62 The feeding of human milk was also associated with decreased gut permeability,63 which might result in less translocations of pathogens from the gut and ultimately less infections.62 On the other hand, human milk promotes the establishment of beneficial microorganism in the infant gut by providing several substances, such as oligosaccharides, which act as favorable substrates for probiotic organisms.38,64 Also in a European cohort, probiotics were reported to prevent NEC only in preterm neonates fed breast milk not formula.65 Therefore, probiotics and human milk may have synergistic effects to prevent LOS development. Still, further studies investigating the influence of feeding formula or breast milk on the effect of probiotics are needed.
Although none of our included studies reported septicemia caused by probiotic organisms, several cases of systemic infections caused by supplemental probiotics have been reported.66–69 Jenke et al70 also reported Bifidobacterium septicemia in an ELBW infant under probiotic therapy. Owing to concerns about the safety issues, studies regarding the efficacy and safety of probiotics in ELBW infants are scant.71 So, more studies are needed to establish the safety of probiotics in preterm neonates, especially in ELBW neonates.71
Several potential limitations should be taken into consideration when interpreting the results. First, although no statistical heterogeneity was found for the primary outcome, population characteristics, probiotic regimens (various organisms, daily doses, time of initiation, and length of intervention), and type of milk differed across the included studies. We adopted random-effects model to try to account for this variability. Second, to examine the influence of these clinical factors on the overall pooled estimate and to verify the robustness of our findings, subgroup analyses were conducted and the results were consistent in most selected subgroups. We, however, can only analyze covariates that are available to us from the original articles. Moreover, subgroup analyses were susceptible to type II errors because of relatively small sample sizes. Third, our search language was restricted to only English, which could potentially lead to publication bias. We, however, used a very thorough and comprehensive search strategy yielding 27 RCTs, which made our study the largest review to date, and the funnel plot and formal statistical tests also did not show any publication bias. Finally, our results should be viewed with caution because 15 of 25 trials included in our meta-analysis were of low methodological quality, that is, unclear or high risk of bias. We tried to verify the robustness of our findings by subgroup analyses (Table 5). When stratified by risk of bias, the beneficial effects of probiotics remained in the 2 strata, especially with no statistical heterogeneity among the 10 studies with low risk of bias (I2 = 0%).
CONCLUSIONS
Current evidence indicates that probiotic supplementation is safe, and effective in reducing the risk of LOS in preterm neonates in NICUs. Further studies are needed to address the optimal probiotic organism, dosing, timing, and duration. High-quality and adequately powered RCTs regarding the efficacy and safety of the use of probiotics in ELBW infants are still warranted.
Acknowledgments
The authors thank Jin-Liang Chen for his technical assistance and full-text articles acquisition.
Footnotes
Abbreviations: CI = confidence interval, ELBW = extremely low birth weight, Ig = immunoglobulin, LOS = late-onset sepsis, NEC = necrotizing enterocolitis, NICU = neonatal intensive care unit, RCT = randomized controlled trial, RR = relative risk.
This study was supported by the Health Department of Chongqing City Foundation (2013-1-023).
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Stoll BJ, Hansen N, Fanaroff AA, et al. Late-onset sepsis in very low birth weight neonates: the experience of the NICHD Neonatal Research Network. Pediatrics 2002; 110:285–291. [DOI] [PubMed] [Google Scholar]
- 2.Hornik CP, Fort P, Clark RH, et al. Early and late onset sepsis in very-low-birth-weight infants from a large group of neonatal intensive care units. Early Hum Dev 2012; 88:S69–S74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Payne NR, Carpenter JH, Badger GJ, et al. Marginal increase in cost and excess length of stay associated with nosocomial bloodstream infections in surviving very low birth weight infants. Pediatrics 2004; 114:348–355. [DOI] [PubMed] [Google Scholar]
- 4.Manzoni P, Rizzollo S, Decembrino L, et al. Recent advances in prevention of sepsis in the premature neonates in NICU. Early Hum Dev 2011; 87:S31–S33. [DOI] [PubMed] [Google Scholar]
- 5.Magne F, Suau A, Pochart P, et al. Fecal microbial community in preterm infants. J Pediatr Gastroenterol Nutr 2005; 41:386–392. [DOI] [PubMed] [Google Scholar]
- 6.Schwiertz A, Gruhl B, Lobnitz M, et al. Development of the intestinal bacterial composition in hospitalized preterm infants in comparison with breast-fed, full-term infants. Pediatr Res 2003; 54:393–399. [DOI] [PubMed] [Google Scholar]
- 7.Goldmann DA, Leclair J, Macone A. Bacterial colonization of neonates admitted to an intensive care environment. J Pediatr 1978; 93:288–293. [DOI] [PubMed] [Google Scholar]
- 8.Polin RA, Denson S, Brady MT. Strategies for prevention of health care-associated infections in the NICU. Pediatrics 2012; 129:e1085–e1093. [DOI] [PubMed] [Google Scholar]
- 9.Cotten CM, Taylor S, Stoll B, et al. Prolonged duration of initial empirical antibiotic treatment is associated with increased rates of necrotizing enterocolitis and death for extremely low birth weight infants. Pediatrics 2009; 123:58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Joint FAO/WHO Working Group Report on Drafting Guidelines for the Evaluation of Probiotics in Food Joint FAO/WHO Working Group Report, Guidelines for the Evaluation of Probiotics in Food. London, Canada:2002. [Google Scholar]
- 11.Mack DR, Michail S, Wei S, et al. Probiotics inhibit enteropathogenic E. coli adherence in vitro by inducing intestinal mucin gene expression. Am J Physiol 1999; 276:G941–G950. [DOI] [PubMed] [Google Scholar]
- 12.Griffiths EA, Duffy LC, Schanbacher FL, et al. In vivo effects of bifidobacteria and lactoferrin on gut endotoxin concentration and mucosal immunity in Balb/c mice. Dig Dis Sci 2004; 49:579–589. [DOI] [PubMed] [Google Scholar]
- 13.Urao M, Fujimoto T, Lane GJ, et al. Does probiotics administration decrease serum endotoxin levels in infants? J Pediatr Surg 1999; 34:273–276. [DOI] [PubMed] [Google Scholar]
- 14.Mohan R, Koebnick C, Schildt J, et al. Effects of Bifidobacterium lactis Bb12 supplementation on intestinal microbiota of preterm infants: a double-blind, placebo-controlled, randomized study. J Clin Microbiol 2006; 44:4025–4031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.AlFaleh K, Anabrees J. Probiotics for prevention of necrotizing enterocolitis in preterm infants. Cochrane Database Syst Rev 2014; 4: CD005496. [DOI] [PubMed] [Google Scholar]
- 16.Bernardo WM, Aires FT, Carneiro RM, et al. Effectiveness of probiotics in the prophylaxis of necrotizing enterocolitis in preterm neonates: a systematic review and meta-analysis. J Pediatr 2013; 89:18–24. [DOI] [PubMed] [Google Scholar]
- 17.Deshpande G, Rao S, Patole S, et al. Updated meta-analysis of probiotics for preventing necrotizing enterocolitis in preterm neonates. Pediatrics 2010; 125:921–930. [DOI] [PubMed] [Google Scholar]
- 18.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med 2009; 151:W65–W94. [DOI] [PubMed] [Google Scholar]
- 19.Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available at: www.cochrane-handbook.org. [Google Scholar]
- 20.Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. Br Med J 2011; 343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manzoni P, De Luca D, Stronati M, et al. Prevention of nosocomial infections in neonatal intensive care units. Am J Perinatol 2013; 30:81–88. [DOI] [PubMed] [Google Scholar]
- 22.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. Br Med J 2003; 327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994; 50:1088–1101. [PubMed] [Google Scholar]
- 24.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. Br Med J 1997; 315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reuman PD, Duckworth DH, Smith KL, et al. Lack of effect of Lactobacillus on gastrointestinal bacterial colonization in premature infants. Pediatr Infect Dis 1986; 5:663–668. [DOI] [PubMed] [Google Scholar]
- 26.Agarwal R, Sharma N, Chaudhry R, et al. Effects of oral Lactobacillus GG on enteric microflora in low-birth-weight neonates. J Pediatr Gastroenterol Nutr 2003; 36:397–402. [DOI] [PubMed] [Google Scholar]
- 27.Al-Hosni M, Duenas M, Hawk M, et al. Probiotics-supplemented feeding in extremely low-birth-weight infants. J Perinatol 2012; 32:253–259. [DOI] [PubMed] [Google Scholar]
- 28.Bin-Nun A, Bromiker R, Wilschanski M, et al. Oral probiotics prevent necrotizing enterocolitis in very low birth weight neonates. J Pediatr 2005; 147:192–196. [DOI] [PubMed] [Google Scholar]
- 29.Braga TD, da Silva GA, de Lira PI, et al. Efficacy of Bifidobacterium breve and Lactobacillus casei oral supplementation on necrotizing enterocolitis in very-low-birth-weight preterm infants: a double-blind, randomized, controlled trial. Am J Clin Nutr 2011; 93:81–86. [DOI] [PubMed] [Google Scholar]
- 30.Costalos C, Skouteri V, Gounaris A, et al. Enteral feeding of premature infants with Saccharomyces boulardii. Early Hum Dev 2003; 74:89–96. [DOI] [PubMed] [Google Scholar]
- 31.Dani C, Biadaioli R, Bertini G, et al. Probiotics feeding in prevention of urinary tract infection, bacterial sepsis and necrotizing enterocolitis in preterm infants. A prospective double-blind study. Biol Neonate 2002; 82:103–108. [DOI] [PubMed] [Google Scholar]
- 32.Demirel G, Erdeve O, Celik IH, et al. Saccharomyces boulardii for prevention of necrotizing enterocolitis in preterm infants: a randomized, controlled study. Acta Paediatr 2013; 102:e560–565. [DOI] [PubMed] [Google Scholar]
- 33.Dilli D, Aydin B, Fettah ND, et al. The propre-save study: effects of probiotics and prebiotics alone or combined on necrotizing enterocolitis in very low birth weight infants. J Pediatr 2015; 166:545–551. [DOI] [PubMed] [Google Scholar]
- 34.Fernandez-Carrocera LA, Solis-Herrera A, Cabanillas-Ayon M, et al. Double-blind, randomised clinical assay to evaluate the efficacy of probiotics in preterm newborns weighing less than 1500 g in the prevention of necrotising enterocolitis. Arch Dis Child Fetal Neonatal Ed 2013; 98:F5–F9. [DOI] [PubMed] [Google Scholar]
- 35.Jacobs SE, Tobin JM, Opie GF, et al. Probiotic effects on late-onset sepsis in very preterm infants: a randomized controlled trial. Pediatrics 2013; 132:1055–1062. [DOI] [PubMed] [Google Scholar]
- 36.Kitajima H, Sumida Y, Tanaka R, et al. Early administration of Bifidobacterium breve to preterm infants: randomised controlled trial. Arch Dis Child Fetal Neonatal Ed 1997; 76:F101–F107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin HC, Su BH, Chen AC, et al. Oral probiotics reduce the incidence and severity of necrotizing enterocolitis in very low birth weight infants. Pediatrics 2005; 115:1–4. [DOI] [PubMed] [Google Scholar]
- 38.Lin HC, Hsu CH, Chen HL, et al. Oral probiotics prevent necrotizing enterocolitis in very low birth weight preterm infants: a multicenter, randomized, controlled trial. Pediatrics 2008; 122:693–700. [DOI] [PubMed] [Google Scholar]
- 39.Manzoni P, Mostert M, Leonessa ML, et al. Oral supplementation with Lactobacillus casei subspecies rhamnosus prevents enteric colonization by Candida species in preterm neonates: a randomized study. Clin Infect Dis 2006; 42:1735–1742. [DOI] [PubMed] [Google Scholar]
- 40.Mihatsch WA, Vossbeck S, Eikmanns B, et al. Effect of Bifidobacterium lactis on the incidence of nosocomial infections in very-low-birth-weight infants: a randomized controlled trial. Neonatology 2010; 98:156–163. [DOI] [PubMed] [Google Scholar]
- 41.Millar MR, Bacon C, Smith SL, et al. Enteral feeding of premature infants with Lactobacillus GG. Arch Dis Child 1993; 69:483–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oncel MY, Sari FN, Arayici S, et al. Lactobacillus reuteri for the prevention of necrotising enterocolitis in very low birthweight infants: a randomised controlled trial. Arch Dis Child Fetal Neonatal Ed 2014; 99:F110–F115. [DOI] [PubMed] [Google Scholar]
- 43.Patole S, Keil AD, Chang A, et al. Effect of Bifidobacterium breve M-16 V supplementation on fecal bifidobacteria in preterm neonates: a randomised double blind placebo controlled trial. PLoS One 2014; 9:e89511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rojas MA, Lozano JM, Rojas MX, et al. Prophylactic probiotics to prevent death and nosocomial infection in preterm infants. Pediatrics 2012; 130:e1113–e1120. [DOI] [PubMed] [Google Scholar]
- 45.Romeo MG, Romeo DM, Trovato L, et al. Role of probiotics in the prevention of the enteric colonization by Candida in preterm newborns: incidence of late-onset sepsis and neurological outcome. J Perinatol 2011; 31:63–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rouge C, Piloquet H, Butel MJ, et al. Oral supplementation with probiotics in very-low-birth-weight preterm infants: a randomized, double-blind, placebo-controlled trial. Am J Clin Nutr 2009; 89:1828–1835. [DOI] [PubMed] [Google Scholar]
- 47.Roy A, Chaudhuri J, Sarkar D, et al. Role of enteric supplementation of probiotics on late-onset sepsis by Candida species in preterm low birth weight neonates: a randomized, double blind, placebo-controlled trial. N Am J Med Sci 2014; 6:50–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Samanta M, Sarkar M, Ghosh P, et al. Prophylactic probiotics for prevention of necrotizing enterocolitis in very low birth weight newborns. J Trop Pediatr 2009; 55:128–131. [DOI] [PubMed] [Google Scholar]
- 49.Sari FN, Dizdar EA, Oguz S, et al. Oral probiotics: Lactobacillus sporogenes for prevention of necrotizing enterocolitis in very low-birth weight infants: a randomized, controlled trial. Eur J Clin Nutr 2011; 65:434–439. [DOI] [PubMed] [Google Scholar]
- 50.Serce O, Benzer D, Gursoy T, et al. Efficacy of Saccharomyces boulardii on necrotizing enterocolitis or sepsis in very low birth weight infants: a randomised controlled trial. Early Hum Dev 2013; 89:1033–1036. [DOI] [PubMed] [Google Scholar]
- 51.Stratiki Z, Costalos C, Sevastiadou S, et al. The effect of a bifidobacter supplemented bovine milk on intestinal permeability of preterm infants. Early Hum Dev 2007; 83:575–579. [DOI] [PubMed] [Google Scholar]
- 52.Hikaru U, Yayoi S, Hiromichi S, et al. Bifidobacteria prevents preterm infants from developing infection and sepsis. Int J Probiotics Prebiotics 2010; 5:33–36. [Google Scholar]
- 53.Saengtawesin V, Tangpolkaiwalsak R, Kanjanapattankul W. Effect of oral probiotics supplementation in the prevention of necrotizing enterocolitis among very low birth weight preterm infants. J Med Assoc Thai 2014; 97:S20–S25. [PubMed] [Google Scholar]
- 54.Manzoni P, Rinaldi M, Cattani S, et al. Bovine lactoferrin supplementation for prevention of late-onset sepsis in very low-birth-weight neonates: a randomized trial. J Am Med Assoc 2009; 302:1421–1428. [DOI] [PubMed] [Google Scholar]
- 55.Jacobsen CN, Rosenfeldt Nielsen V, Hayford AE, et al. Screening of probiotic activities of forty-seven strains of Lactobacillus spp. by in vitro techniques and evaluation of the colonization ability of five selected strains in humans. Appl Environ Microbiol 1999; 65:4949–4956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gronlund MM, Arvilommi H, Kero P, et al. Importance of intestinal colonisation in the maturation of humoral immunity in early infancy: a prospective follow up study of healthy infants aged 0-6 months. Arch Dis Child Fetal Neonatal Ed 2000; 83:F186–F192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ahrne S, Hagslatt ML. Effect of lactobacilli on paracellular permeability in the gut. Nutrients 2011; 3:104–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.De Weirdt R, Crabbe A, Roos S, et al. Glycerol supplementation enhances L. reuteri's protective effect against S. typhimurium colonization in a 3-D model of colonic epithelium. PLoS One 2012; 7:e37116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kukkonen K, Nieminen T, Poussa T, et al. Effect of probiotics on vaccine antibody responses in infancy: a randomized placebo-controlled double-blind trial. Pediatr Allergy Immunol 2006; 17:416–421. [DOI] [PubMed] [Google Scholar]
- 60.Shanahan F. Molecular mechanisms of probiotic action: it's all in the strains!. Gut 2011; 60:1026–1027. [DOI] [PubMed] [Google Scholar]
- 61.Athalye-Jape G, Rao S, Patole S. Lactobacillus reuteri DSM 17938 as a probiotic for preterm neonates: a strain-specific systematic review. J Parenter Enteral Nutr 2015. [DOI] [PubMed] [Google Scholar]
- 62.Pammi M, Abrams SA. Oral lactoferrin for the prevention of sepsis and necrotizing enterocolitis in preterm infants. Cochrane Database Syst Rev 2015; 2: CD007137. [DOI] [PubMed] [Google Scholar]
- 63.Shulman RJ, Schanler RJ, Lau C, et al. Early feeding, antenatal glucocorticoids, and human milk decrease intestinal permeability in preterm infants. Pediatr Res 1998; 44:519–523. [DOI] [PubMed] [Google Scholar]
- 64.Bergmann H, Rodriguez JM, Salminen S, et al. Probiotics in human milk and probiotic supplementation in infant nutrition: a workshop report. Br J Nutr 2014; 112:1119–1128. [DOI] [PubMed] [Google Scholar]
- 65.Repa A, Thanhaeuser M, Endress D, et al. Probiotics (Lactobacillus acidophilus and Bifidobacterium bifidum) prevent NEC in VLBW infants fed breast milk but not formula. Pediatr Res 2015; 77:381–388. [DOI] [PubMed] [Google Scholar]
- 66.Ohishi A, Takahashi S, Ito Y, et al. Bifidobacterium septicemia associated with postoperative probiotic therapy in a neonate with omphalocele. J Pediatr 2010; 156:679–681. [DOI] [PubMed] [Google Scholar]
- 67.Land MH, Rouster-Stevens K, Woods CR, et al. Lactobacillus sepsis associated with probiotic therapy. Pediatrics 2005; 115:178–181. [DOI] [PubMed] [Google Scholar]
- 68.Kunz AN, Noel JM, Fairchok MP. Two cases of Lactobacillus bacteremia during probiotic treatment of short gut syndrome. J Pediatr Gastroenterol Nutr 2004; 38:457–458. [DOI] [PubMed] [Google Scholar]
- 69.Bertelli C, Pillonel T, Torregrossa A, et al. Bifidobacterium longum bacteremia in preterm infants receiving probiotics. Clin Infect Dis 2015; 60:924–927. [DOI] [PubMed] [Google Scholar]
- 70.Jenke A, Ruf EM, Hoppe T, et al. Bifidobacterium septicaemia in an extremely low-birthweight infant under probiotic therapy. Arch Dis Child Fetal Neonatal Ed 2012; 97:F217–F218. [DOI] [PubMed] [Google Scholar]
- 71.Abrahamsson TR, Rautava S, Moore AM, et al. The time for a confirmative necrotizing enterocolitis probiotics prevention trial in the extremely low birth weight infant in North America is now!. J Pediatr 2014; 165:389–394. [DOI] [PubMed] [Google Scholar]


