Abstract
This study aims to investigate intravitreal injection of Ranibizumab on the surgical outcome for diabetic patients who had tractional retinal detachment but did not receive any preoperative retinal photocoagulation.
Ninety-seven patients (97 eyes) who had diabetic retinopathy with tractional retinal detachment were enrolled to receive 23-G pars plana vitrectomy (PPV). They were assigned to an experimental group (Group I, n = 47 eyes) and a control group (Group II, n = 50 eyes). The patients in Group I were given 1 injection of intravitreal Ranibizumab (Lucentis 0.5 mg/0.05 mL) 1 week before surgery, whereas those in Group II went down to surgery directly. Follow-ups were performed for 6 months to 3 years (16 ± 6 months), and indicators observed included postoperative best-corrected visual acuity, complications, and retinal thickness in the macula measured by optical coherence tomography.
In Group I, BCVA improved from logMAR 1.92 ± 0.49 to logMAR 0.81 ± 0.39 following surgery, whereas in Group II, BCVA improved from logMAR 1.91 ± 0.49 to logMAR 0.85 ± 0.41. There was significant postoperative gain in vision, but there was no significant difference between the 2 groups at postoperative follow-up visits. The mean duration of vitrectomy in Group I and Group II was (40 ± 7) minutes and (53 ± 9) minutes, respectively, with significant difference. Iatrogenic breaks were noted in 5 eyes (11%) in the experimental group and 17 eyes (34%) in the control group; the difference was significant. The retinal thickness in the macula measured by OCT was (256 ± 44) μm and (299 ± 84) μm in Group I and Group II respectively with significant difference. Besides, there were significantly more eyes in Group II that required silicone oil tamponade and postoperative retinal photocoagulation.
23-G PPV combined with intravitreal tamponade and panretinal photocoagulation still remains an effective regimen for the treatment of diabetic retinopathy complicated with tractional retinal detachment. Preoperative intravitreal injection of Ranibizumab could shorten surgical duration, reduce intraoperative complications, and sometimes spare the need for silicone oil tamponade and postoperative retinal photocoagulation, alleviating patients’ suffering from surgery.
INTRODUCTION
Diabetic retinopathy (DR) is a common microvascular complication of diabetes mellitus (DM) and remains a leading cause of vision loss in many developed countries.1 However, in some developing countries such as China, the prevalence rates of DR have been increasing recently due to the growing numbers and life spans of people with diabetes. And it was estimated that 9.2 million Chinese people living in the rural area have DR, including 1.3 million with vision-threatening DR.2 Due to the poor economic status, low literacy level, and inconvenient transportation conditions, these patients could not have effective control of DM, and usually they would not come to the hospital until severe complications such as blindness develop.3 Therefore, their degree of diabetic retinopathy is particularly serious. Proliferative diabetic retinopathy (PDR) is an advanced stage of diabetic microangiopathy that may cause loss of vision from intraocular hemorrhage, traction retinal detachment, and neovascular glaucoma.4 Although panretinal photocoagulation (PRP) reduces the 5-year risk of blindness by 90%,5 there is no doubt that DR with vitreous hemorrhage or tractional retinal detachments needs surgical approach such as vitrectomy, but intraoperative and postoperative bleeding may increase surgical difficulty and compromise surgical effect as well.6
By blocking the effect of vascular endothelial growth factor (VEGF), intravitreal anti-VEGF drugs could decrease vascular permeability and proliferation, thus improving macular edema and reducing the risk of intraocular bleeding in patients with PDR,7 and the most commonly used anti-VEGF drug is Bevacizumab. However, there were reports about it causing tractional retinal detachment (TRD) in cases with pre-existing preretinal fibrosis.8 On the other hand, being licensed as an intravitreal agent for the treatment of wet, age-related macular degeneration (AMD), Ranibizumab is an engineered, humanized, recombinant antibody fragment active against all VEGF-A isoforms and has a shorter half-life than other anti-VEGF agents.9
In this study, we aim to evaluate the effects of pretreatment with intravitreal Ranibizumab (IVR) on the surgical outcome for DR patients with TRD, so as to provide more evidence for the clinical use of Ranibizumab in the treatment of PDR.
MATERIALS AND METHODS
Inclusion Criteria
(1) Clinically diagnosed as PDR with TRD; (2) great amount of vitreous hemorrhage or progressive fundus neovascularization detected in ocular examination; (3) no retinal photocoagulation treatment before vitrectomy; (4) no iris neovascularization, and intraocular pressure should be within the range of 10 to 21 mm Hg. No age or gender limits were put to the inclusion criteria, and all the patients enrolled had signed the Informed Consent Form before implementation of the trial.
General Data
Ninety-seven patients (97 eyes) who were admitted to the Department of Ophthalmology, 1st affiliated hospital of ZJU between January 2012 and December 2014 and fulfilled the above-mentioned inclusion criteria were enrolled. The patients receiving IVR were explained the off-label use of the drug, the potential risks of thromboembolic events, endophthalmitis and uveitis. Written informed consent was obtained from all patients before the IVR as well as prior to the PPV. They aged between 24 and 79, with a mean age of (52 ± 9) years. And these patients had been diagnosed with DM for 1 to 30 years (12 ± 9 years on average), with preoperative visual acuity being Hand Movement (HM) to 20/160 (logMAR2–logMAR0.9).
Experiment Method
We performed a retrospective analysis of 97 patients (97 eyes), and they were assigned to an experimental group (n = 47 eyes) and a control group (n = 50 eyes). The patients in the experimental group were given intravitreal injection of Ranibizumab (Lucentis 0.5 mg/0.05 mL, Novartis Ophthalmics, Basel, Switzerland) 1 week before surgery, whereas those in the control group went down to surgery directly. All the patients received 23-G pars plana vitrectomy (PPV) performed by 1 mature vitreoretinal specialist, and the choice of tamponade was made between C3F8 gas or silicone oil on a case-by-case basis depending on the severity of traction and presence of iatrogenic breaks or other intraoperative complications.
Indicators Observed
Follow-ups started from the time when removal of silicone oil was done or C3F8 gas tamponade cleared off, and at the last follow-up visit, all the enrolled patients had transparent refracting media with vitreous cavity filled by normal saline. Follow-ups were performed for 6 months to 3 years (16 ± 6 months), and indicators for comparison between the 2 groups included: (1) best-corrected visual acuity (BCVA) before and after PPV; (2) duration of vitrectomy; (3) intraoperative and postoperative complications such as iatrogenic retinal breaks and iris neovascularization; (4) postoperative retinal thickness measured by optical coherence tomography (OCT).
Statistical Analysis
Continuous numeral values were expressed as mean ± standard deviation (χ±s). For continuous numeral values, descriptive statistical analysis was conducted first to validate the normality and homogeneity of variance of the data. Paired sample or independent sample t test was conducted for data meeting the homogeneity of variance accordingly. Categorical variables were compared using the chi-square test or Fisher exact test wherever applicable. A P value < 0.05 was considered significant.
RESULTS
For intraoperative tamponade, silicone oil was used in 60.8% of the eyes, whereas C3F8 gas was used in 15.5% of the eyes. And postoperative visual acuity of the enrolled patients achieved counting fingers (FC) to 20/25 (logMAR2–logMAR0.1).
Visual Acuity
The BCVA was comparable in both groups at preoperative baseline, but there was no significant difference between the 2 groups at postoperative follow-up visits (Table 1). In both groups, there was significant postoperative gain in vision; in the experimental group, vision improved from logMAR 1.92 ± 0.49 preoperatively to logMAR 0.81 ± 0.39 postoperatively (P < 0.01); in the control group, vision improved from logMAR 1.91 ± 0.49 preoperatively to logMAR 0.85 ± 0.41 postoperatively (P < 0.01). Besides, when reviewing the postoperative BCVA in the 2 groups, we also noticed that the percentage of eyes having postoperative BCVA better than logMAR0.3 in both groups was not significantly different from each other (Table 2).
TABLE 1.
Comparison of Best-Corrected Visual Acuity Between Experimental and Control Groups
TABLE 2.
Comparison of Postoperative Best-Corrected Visual Acuity Between 2 Groups

Intraoperative and Postoperative Complications
The mean duration of vitrectomy in Group I and Group II was (40 ± 7) minutes and (53 ± 9) minutes respectively with significant difference. Iatrogenic breaks were noted in 5 eyes (11%) in the experimental group and 17 eyes (34%) in the control group; the difference was significant (P = 0.006). Only 1 eye (2%) in the control group was noted to have postoperative iris neovascularization, and the difference was not significant between the 2 groups (P = 1) (Table 3).
TABLE 3.
Comparison of Complication Incidence Between 2 Groups
Postoperative Retinal Thickness
We measured the retinal thickness of each enrolled eye using OCT (Heidelberg Engineering GmbH/Germany) at postoperative follow-ups, and the value was (256 ± 44) μm and (299 ± 84) μm in the experimental group and control group respectively with significant difference (P = 0.002), indicating retinal edema was more severe in eyes of the control group.
Intraoperative Silicone Oil Tamponade and Postoperative Laser Photocoagulation
Silicone oil was used as internal tamponading agent in 22 eyes (47%) of experimental group and 37 eyes (74%) of control group (P = 0.006). On the other hand, postoperative photocoagulation was performed in 16 eyes (34%) of experimental group and 32 eyes (64%) of control group (P = 0.003), respectively (Table 4).
TABLE 4.
Comparison of Internal Tamponade and Postoperative Photocoagulation

DISCUSSION
Recently, there have been many reports regarding the effects of anti-VEGF agents on PDR, saying preoperative intravitreal anti-VEGF injection might be helpful to facilitate vitrectomy in severe PDR cases.10,11 Intravitreal injection of Bevacizumab 1 week before vitrectomy could reduce the incidence of early postoperative hemorrhage in DR patients,12,13 and the need for vitrectomy also may be decreased significantly in these cases.14 Furthermore, this new strategy could reduce retinal and iris neovascularization, thus making surgery easier and safer and improving the anatomical and functional prognosis.15 However, most of these reports focused on the usage of Bevacizumab, and there were still other reports suggesting TRD may occur or progress shortly following administration of intravitreal Bevacizumab (IVB) in patients with severe PDR.16
Researchers discovered that postoperative visual improvement of ≥ 0.3 logMAR units was seen in significantly more eyes with pretreatment of intravitreal Avastin, especially in eyes undergoing vitrectomy within 2 weeks following intravitreal injection.17 However, pooled data demonstrated no significance in the proportion of eyes with improved BCVA at a follow-up of >3 months (range, 3–6 months) when comparing the IVB injection and vitrectomy-alone groups.18
Based on the previous study results above, we conducted this experiment to further investigate the role of preoperative Ranibizumab (Lucentis) injection in the treatment of severe PDR. As a developing country, the health care level in China is limited with uneven distribution, and lack of ophthalmologists is common in remote areas, therefore many patients suffering from eye diseases could not receive effective treatment until late-stage symptoms develop,19 and that is why we are very concerned about visual recovery in these patients. As a result, the patients enrolled in our study had 2 particular features: (1) they all suffered from late-stage PDR complicated by TRD, and (2) none of them had received retinal photocoagulation or any other form of relative treatment before the surgery.
In our study, we discovered that vitrectomy was beneficial to anatomical and functional recovery of retina in PDR patients with TRD, whether there was preoperative injection of Ranizumab or not. However, neither the postoperative BCVA nor the proportion of eyes with BCVA improvement showed significant difference between the 2 groups at postoperative follow-up visits, implying pretreatment of Lucentis before vitrectomy was not a determinant factor for postoperative BCVA, which was in accordance with previous studies.20,21 The reason why our results regarding postoperative BCVA were different from other researchers17 may be that the patients enrolled in our study had more severe PDR, thus neutralizing the effects of Lucentis on visual recovery.
The patients included in our study all had particularly serious diabetic retinopathy; therefore, for patients who did not have Lucentis injection, intraoperative bleeding was more prone to occur and hard to stop, thus extending surgical duration22,23 and leading to iatrogenic breaks due to blurred images of operative field, and retinal edema was also more common in these patients. Compared with direct vitrectomy, patients who received intravitreal injection of Ranizumab 1 week before vitrectomy had shorter surgical duration, fewer intraoperative complications, less use of silicone oil as internal tamponade, and less need for postoperative retinal laser coagulation, alleviating these patients’ suffering from repeated surgical treatment.
Significant increase of vitreous proliferation was not observed in patients who had intravitreal Ranizumab. In our study, we also discovered that in the experimental group, the adhesion between the proliferative membrane and retina became loosened after Lucentis injection, making it easier to be dissected,24 which helped lower the incidence of iatrogenic breaks and silicone oil tamponade. On the other hand, for patients who had Lucentis injection, their retinal edema were milder, so intraoperative pan-retinal photocoagulation was made much easier and more thorough for them, thus reducing the necessity of postoperative photocoagulation (Figures 1–3).
FIGURE 1.
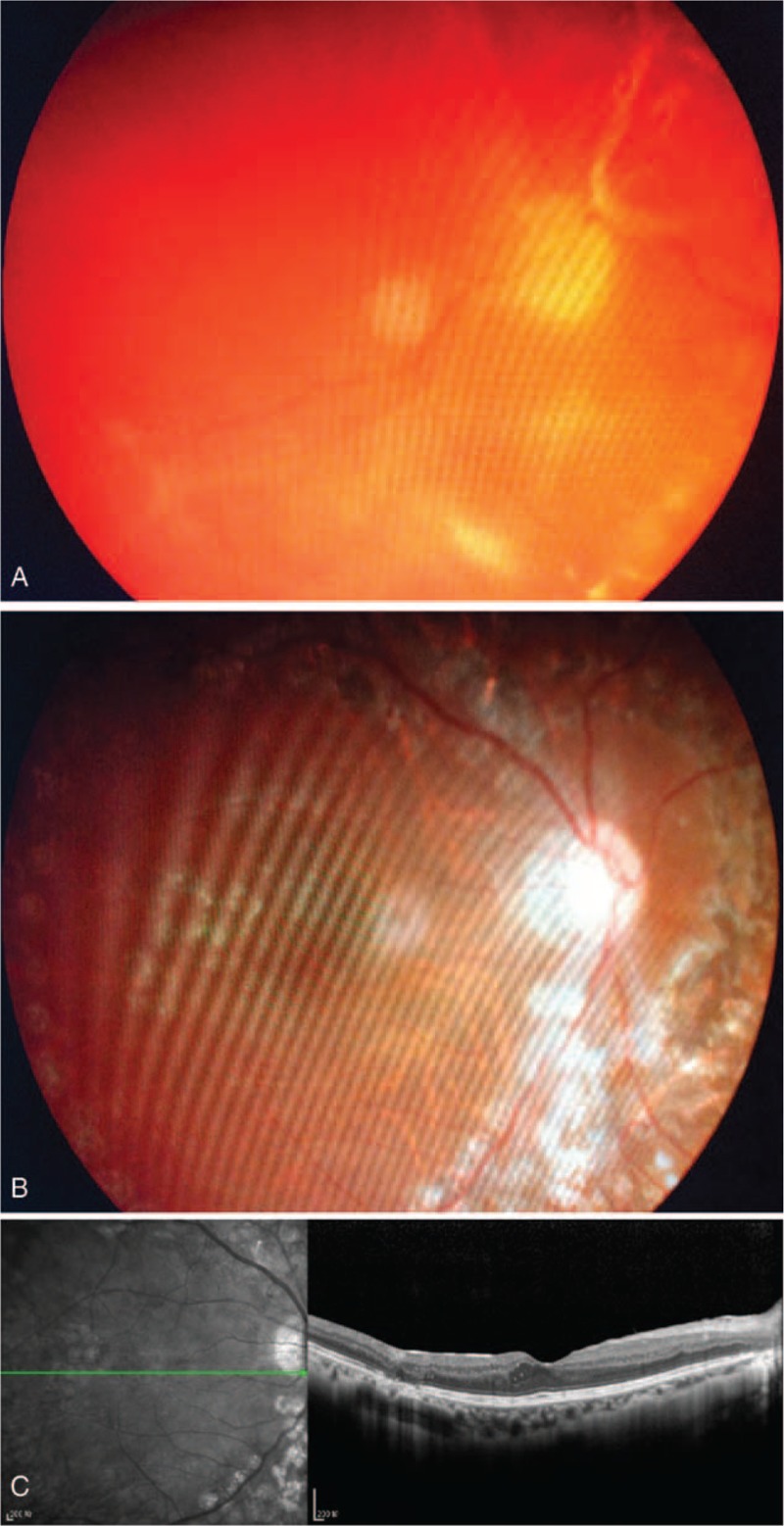
(A) A 28-year-old woman with type I diabetes showing extensive tractional retinal detachment and fibrovascular proliferation in the right eye and visual acuity of hand motions. (B) At 1-year follow-up after vitrectomy, her retina remained attached with disappearance of neovascularization, and her postoperative BCVA was 20/30. (C) The OCT image of macular area at 1-year follow-up. BCVA = best-corrected visual acuity, OCT = optical coherence tomography.
FIGURE 3.
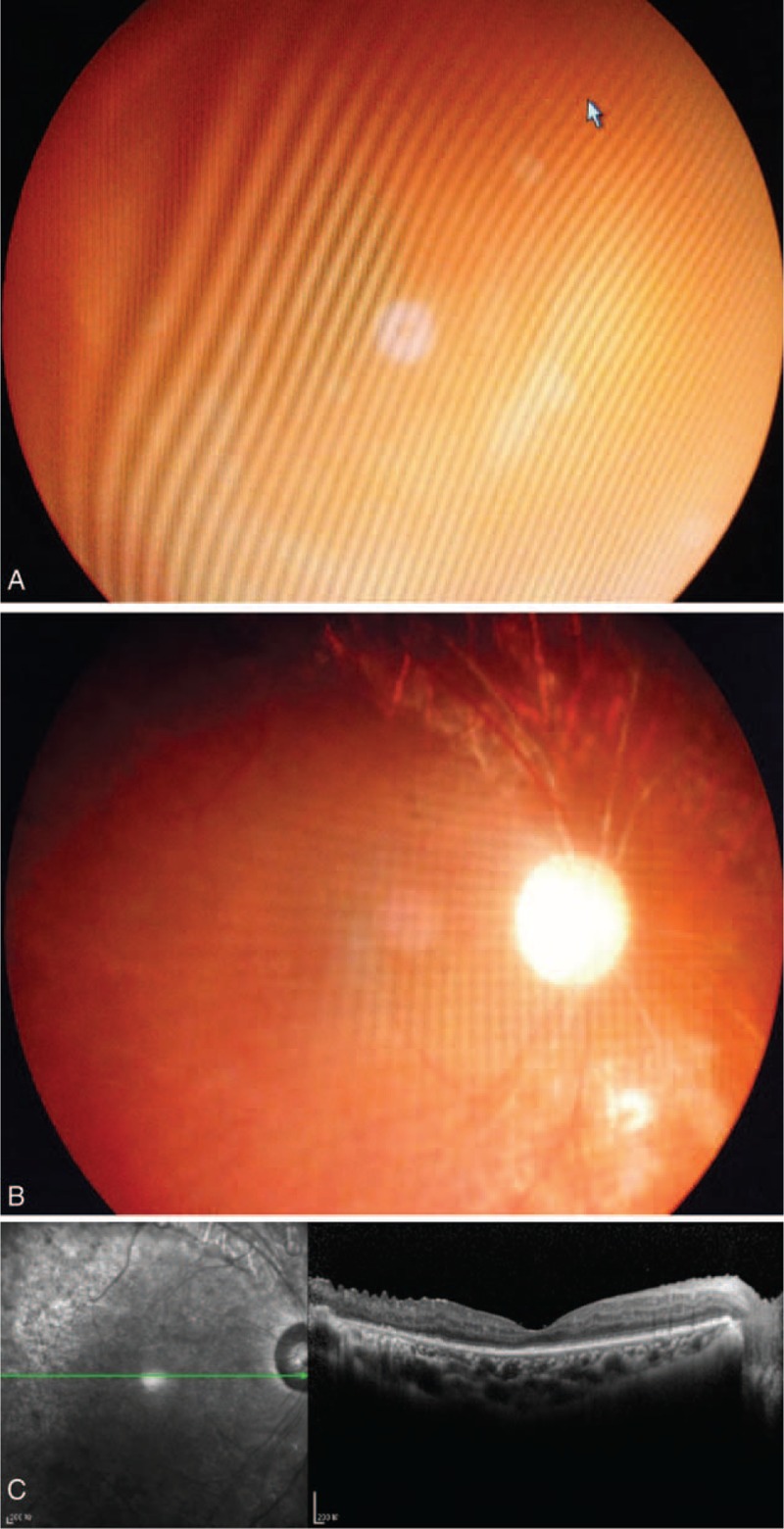
(A) A 52-year-old woman with type II diabetes showing vitreous hemorrhage and tractional retinal detachment in the right eye and visual acuity of hand motions, and she received Lucentis 1 week before vitrectomy. (B) At 1-year follow-up after surgery, her retina remained attached without neovascularization, and her BCVA was 20/160. (C) The OCT image of macular area at 1-year follow-up. BCVA = best-corrected visual acuity, OCT = optical coherence tomography.
FIGURE 2.
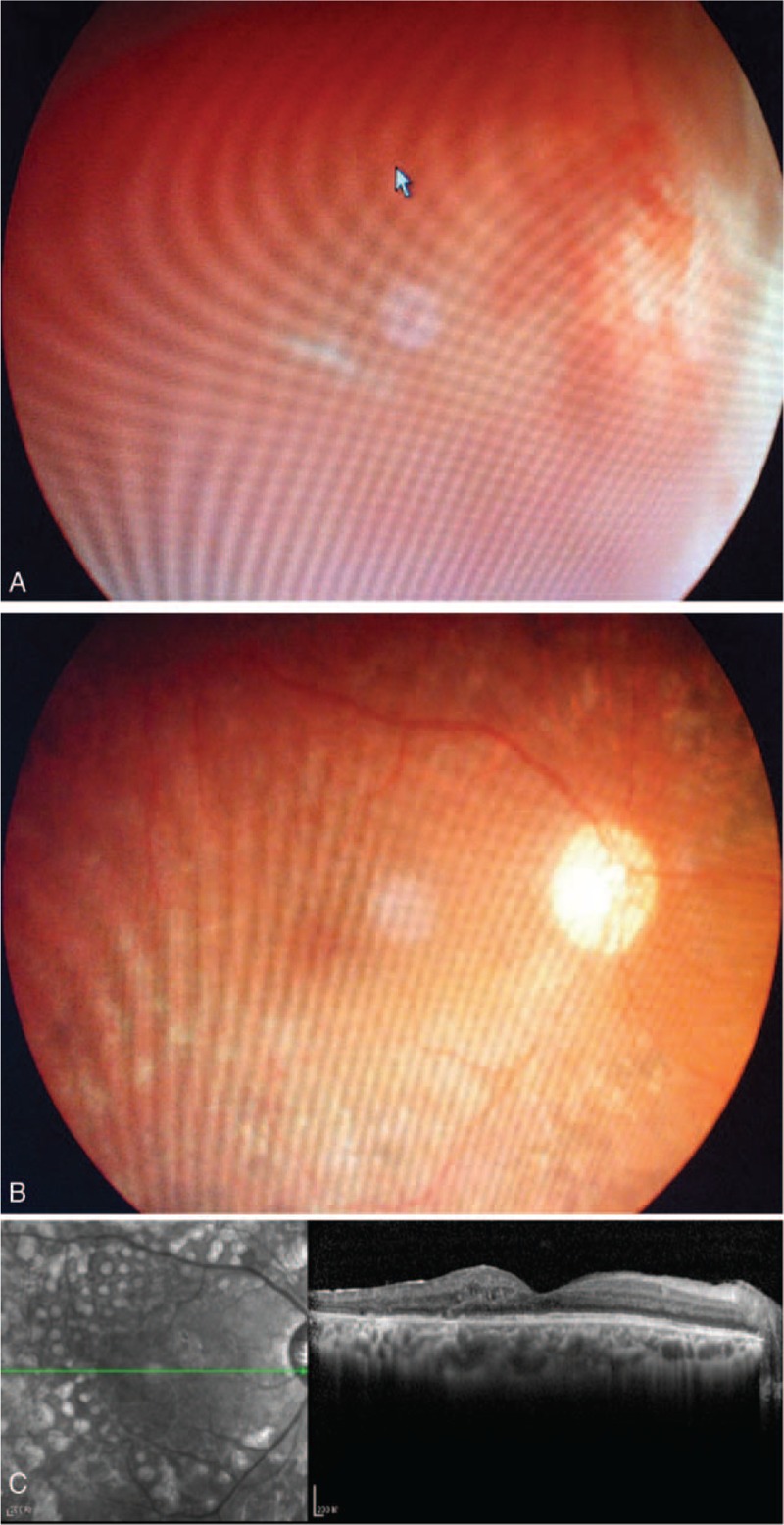
(A) A 30-year-old man with diagnosis of type I diabetes for 1 year showing neovascularization in front of the optic disc, and ultrasonography showed retinal detachment. He received Lucentis 1 week before vitrectomy. (B) One and a half year after surgery, his retina remained attached without neovascularization, and his BCVA was 20/60. (C) The OCT image of macular area at 1.5-year follow-up.BCVA = best-corrected visual acuity, OCT = optical coherence tomography.
Our study also showed that the incidence of iris neovascularization following surgery was quite low and not significantly different between the experimental group and the control group. This phenomenon indicated that pretreatment with intravitreal Lucentis injection might not be a determinant factor for the occurrence of iris neovascularization,25 and the key point lies in the implementation of sufficient pan-retinal photocoagulation.
Regarding postoperative BCVA, although there was no significant difference between the 2 groups, the difference of postoperative retinal thickness in the macular area exhibited statistical significance, which suggested that intravitreal injection of Lucentis before surgery could reduce macular edema in DR patients26 and that visual acuity might be more closely related to the integrity of retinal structure (ie continuity of OS/IS layer) than the thickness of fovea. But this theory still requires more large-sample trials to confirm.
In conclusion, this study suggested that in a developing country such as China, DR patients living in rural areas usually could not receive early and effective treatment due to inconvenient transportation conditions and inadequate community health care services; therefore it is essential to establish a comprehensive follow-up system, so as to identify DR patients in the early stage. And for PDR patients, timely pan-retinal photocoagulation should be provided; if the condition progresses to TRD, vitrectomy performed by a skilled specialist would help promote patients’ life quality, whereas intravitreal injection of Lucentis 1 week before surgery would serve to alleviating patients’ suffering from surgical treatment.
Footnotes
Abbreviations: AMD = age-related macular degeneration, BCVA = best-corrected visual acuity, DM = diabetes mellitus, DR = diabetic retinopathy, IVB = intravitreal Bevacizumab, IVR = intravitreal Ranibizumab, logMAR = logarithm of the minimal angle of resolution, OCT = optical coherence tomography, PDR = proliferative diabetic retinopathy, PPV = pars plana vitrectomy, PRP = panretinal photocoagulation, TRD = tractional retinal detachment, VEGF = vascular endothelial growth factor.
Funding: the research was supported by a research grant from the Health Bureau of Zhejiang Province 201475932.
The authors have no conflicts of interest to disclose.
This manuscript is a unique submission and is not being considered for publication by any other source in any medium. And the manuscript has not been published, in part or in full, in any form.
REFERENCES
- 1.Cheung N, Mitchell P, Wong TY. Diabetic retinopathy. Lancet 2010; 376:124–136. [DOI] [PubMed] [Google Scholar]
- 2.Wang FH, Liang YB, Zhang F, et al. Prevalence of diabetic retinopathy in rural China: the Handan Eye Study. Ophthalmology 2009; 116:461–467. [DOI] [PubMed] [Google Scholar]
- 3.Wang YX, Zhang JS, You QS, et al. Ocular diseases and 10-year mortality: the Beijing Eye Study 2001/2011. Acta Ophthalmol 2014; 92:e424–428. [DOI] [PubMed] [Google Scholar]
- 4.Mohamed Q, Gillies MC, Wong TY. Management of diabetic retinopathy: a systematic review. JAMA 2007; 298:902–916. [DOI] [PubMed] [Google Scholar]
- 5.Lang GE. Laser treatment of diabetic retinopathy. Dev Ophthalmol 2007; 39:48–68. [DOI] [PubMed] [Google Scholar]
- 6.Oyakawa RT, Schachat AP, Michels RG, et al. Complications of vitreous surgery for diabetic retinopathy. I. Intraoperative complications. Ophthalmology 1983; 90:517–521. [DOI] [PubMed] [Google Scholar]
- 7.Martinez-Zapata MJ, Martí-Carvajal AJ, Solà I, et al. Anti-vascular endothelial growth factor for proliferative diabetic retinopathy. Cochrane Database Syst Rev 2014; 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jonas JB, Schmidbauer M, Rensch F. Progression of tractional retinal detachment following intravitreal bevacizumab. Acta Ophthalmol 2009; 87:571–572. [DOI] [PubMed] [Google Scholar]
- 9.Osaadon P, Fagan XJ, Lifshitz T, et al. A review of anti-VEGF agents for proliferative diabetic retinopathy. Eye (Lond) 2014; 28:510–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Avery RL, Pearlman J, Pieramici DJ, et al. Intravitreal bevacizumab (Avastin) in the treatment of proliferative diabetic retinopathy. Ophthalmology 2006; 113:1695. [DOI] [PubMed] [Google Scholar]
- 11.Chen E, Park CH. Use of intravitreal bevacizumab as a preoperative adjunct for tractional retinal detachment repair in severe proliferative diabetic retinopathy. Retina 2006; 26:699–700. [DOI] [PubMed] [Google Scholar]
- 12.Ahn J, Woo SJ, Chung H, et al. The effect of adjunctive intravitreal bevacizumab for preventing postvitrectomy hemorrhage in proliferative diabetic retinopathy. Ophthalmology 2011; 118:2218–2226. [DOI] [PubMed] [Google Scholar]
- 13.Farahvash MS, Majidi AR, Roohipoor R, et al. Preoperative injection of intravitreal bevacizumab in dense diabetic vitreous hemorrhage. Retina 2011; 31:1254–1260. [DOI] [PubMed] [Google Scholar]
- 14.Ahmadieh H, Shoeibi N, Entezari M, et al. Intravitreal bevacizumab for prevention of early postvitrectomy hemorrhage in diabetic patients: a randomized clinical trial. Ophthalmology 2009; 116:1943–1948. [DOI] [PubMed] [Google Scholar]
- 15.Di Lauro R, De Ruggiero P, di Lauro R, et al. Intravitreal bevacizumab for surgical treatment of severe proliferative diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol 2010; 248:785–791. [DOI] [PubMed] [Google Scholar]
- 16.Arevalo JF, Maia M, Flynn HW, Jr, et al. Tractional retinal detachment following intravitreal bevacizumab (Avastin) in patients with severe proliferative diabetic retinopathy. Br J Ophthalmol 2008; 92:213–216. [DOI] [PubMed] [Google Scholar]
- 17.Gupta A, Bansal R, Gupta V, et al. Six-month visual outcome after pars plana vitrectomy in proliferative diabetic retinopathy with or without a single preoperative injection of intravitreal bevacizumab. Int Ophthalmol 2012; 32:135–144. [DOI] [PubMed] [Google Scholar]
- 18.Zhang ZH, Liu HY, Hernandez-Da Mota SE, et al. Vitrectomy with or without preoperative intravitreal bevacizumab for proliferative diabetic retinopathy: a meta-analysis of randomized controlled trials. Am J Ophthalmol 2013; 156:106–115. [DOI] [PubMed] [Google Scholar]
- 19.Zheng Y, He M, Congdon N. The worldwide epidemic of diabetic retinopathy. Indian J Ophthalmol 2012; 60:428–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.El-Batarny AM. Intravitreal bevacizumab as an adjunctive therapy before diabetic vitrectomy. Clin Ophthalmol 2008; 2:709–716. [PMC free article] [PubMed] [Google Scholar]
- 21.Modarres M, Nazari H, Falavarjani KG, et al. Intravitreal injection of bevacizumab before vitrectomy for proliferative diabetic retinopathy. Eur J Ophthalmol 2009; 19:848–852. [DOI] [PubMed] [Google Scholar]
- 22.Hernandez-Da Mota SE, Nunez-Solorio SM. Experience with intravitreal bevacizumab as a preoperative adjunct in 23-G vitrectomy for advanced proliferative diabetic retinopathy. Eur J Ophthalmol 2010; 20:1047–1052. [DOI] [PubMed] [Google Scholar]
- 23.Rizzo S, Genovesi-Ebert F, Di Bartolo E, et al. Injection of intravitreal bevacizumab (Avastin) as a preoperative adjunct before vitrectomy surgery in the treatment of severe proliferative diabetic retinopathy (PDR). Graefes Arch Clin Exp Ophthalmol 2008; 246:837–842. [DOI] [PubMed] [Google Scholar]
- 24.El-Sabagh HA, Abdelghaffar W, Labib AM, et al. Preoperative intravitreal bevacizumab use as an adjuvant to diabetic vitrectomy: histopathologic findings and clinical implications. Ophthalmology 2011; 118:636–641. [DOI] [PubMed] [Google Scholar]
- 25.Figueroa MS, Contreras I, Noval S. Anti-angiogenic drugs as an adjunctive therapy in the surgical treatment of diabetic retinopathy. Curr Diabetes Rev 2009; 5:52–56. [DOI] [PubMed] [Google Scholar]
- 26.Nicholson BP, Schachat AP. A review of clinical trials of anti-VEGF agents for diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol 2010; 248:915–930. [DOI] [PubMed] [Google Scholar]


