Abstract
The antibiotic resistance of Helicobacter pylori (H pylori) is steadily increasing worldwide, resulting in the low efficiency of the current therapeutic approaches for eradication. In this study, we investigated the relationship between antibiotic resistances, the year of sample collection, and the ages of the infected individuals.
A total of 29,034 gastric mucosa biopsy samples were randomly collected from January 1, 2009 to December 9, 2014 in Jiaxing City, Zhejiang Province, China. An antibiotic susceptibility testing was determined using an agar-dilution method. The statistical significance was tested using the chi-squared (χ2) test.
A total of 9687 strains were isolated. The resistance rate to clarithromycin, levofloxacin, and metronidazole were 17.76%, 19.66%, and 95.5%, respectively. Resistance was rare against amoxicillin, gentamicin, and furazolidone. The metronidazole resistance rate stayed at a consistently high level. In contrast, the resistance rates of clarithromycin and levofloxacin increased rapidly from 2009 to 2011, gradually decreased from 2012 to 2013, and then increased again in 2014. Although patients ages 31 to 50 and 71 to 80 years had lower infection rates of H pylori, they also had higher resistance rates to clarithromycin and levofloxacin. The highest antibiotic resistance rate was observed in patients’ ages 71 to 80 years old. Younger patients (below 30 years old) had a lower resistance to levofloxacin. Patients’ ages 51 to 60 years old may thus represent an important category for the future study of H pylori infection.
Age plays a key element in H pylori resistance to clarithromycin and levofloxacin. It is therefore necessary to consider individualized therapy for the optimized treatment of H pylori-infected patients.
INTRODUCTION
Helicobacter pylori (H pylori) is a gram-negative and spiral-shaped microaerophilic bacterium that colonizes the human gastric antrum and duodenal mucosa.1 Several treatments for H pylori infection have been developed over the last 30 years. Nevertheless, the treatment success rate does not exceed 80% globally, and has fallen into an unacceptable range (<70%) in some studies.2,3 Triple therapy, which consists of 1 proton pump inhibitor (PPI) and 2 antibiotics (clarithromycin and amoxicillin or metronidazole), has been commonly used as the first-line treatment regimen for the planned eradication of H pylori.4 Unfortunately, the success of the eradication program has been seriously hampered by increasing antibiotic resistance of H pylori, especially against clarithromycin.5,6 Traditional quadruple therapy consists of a bismuth, tetracycline hydrochloride, metronidazole, and PPI, which is not a good choice in areas where bismuth is not available or high metronidazole resistance is observed, although metronidazole resistance could be partially overcome by increased doses and treatment durations.5,7 Poor success rates have even made nonbismuth-based quadruple (concomitant) therapy unacceptable as an empiric therapy option.8 Sequential therapy consisted of PPI and amoxicillin for the first 5 days, followed by triple therapy to complete the 10-day therapy. However, the cure rate is also insufficiently improved by the presence of infections with multidrug resistances, such as both clarithromycin and metronidazole resistance.9,10
A major cause of treatment failure is the excessive and indiscriminate use of antibiotics.6 Antibiotic resistance rates of H pylori vary in different countries, and between developed and developing countries.11–15 In most countries, clarithromycin resistance has exceeded the minimum value (15%) of the Maastricht IV consensus recommendations. It is reported to be 28% in Japan and 38.5% in Korea.7,11,13 Metronidazole resistance to H pylori is now considered to be ubiquitous, and the global resistance rate ranges from 14.4% to 93.2%.15 Importantly, the increasing rate of levofloxacin resistance in the current H pylori strains has drawn global attention.12
The development of antibiotic resistance in H pylori is an evolving process and varies across different countries and even between the age groups of affected patients. To obtain comprehensive epidemiologic surveillances of H pylori resistance, it is necessary to perform a prevalence survey in a country or city. In this study, we performed the latest survey of H pylori antibiotic resistance from 2009 to 2014 in Jiaxing City, Zhejiang Province, China, and analyzed the pattern of H pylori resistance to currently recommended therapies over a 6-year period and further investigated the relationship between antibiotic resistance and different age groups. The results provide valuable insights into the choice of available treatment strategies for H pylori infections.
MATERIALS AND METHODS
Patient and Tissue Samples
Gastric mucosa biopsy samples were collected from 29,034 patients (14,003 males and 15,031 females) who were diagnosed at the First Hospital of Jiaxing City, Zhejiang Province, China, from January 1, 2009 to December 9, 2014. The average age of these patients was 48.18 ± 13.73, and they were subdivided into 7 groups (<20, 21–30, 31–40, 41–50, 51–60, 61–70, and 71–80 years of age). Subsequently, the gastric mucosa biopsy specimens were collected by gastrointestinal endoscopy and were stored immediately in a brain–heart infusion broth (Oxoid, Dardilly, France) with 5% glycerin. They were then sent to the laboratory at Hangzhou Zhiyuan Medical Inspection Institute for antibiotics susceptibility testing. This study was approved by the Ethics Committee of the National Institute for Communicable Disease Control and Prevention. Additionally, each patient wrote his or her informed consent and agreed to H pylori isolation prior to gastrointestinal endoscopy.
Inclusion Criteria and Exclusion Criteria
The inclusion criteria were as follows. Firstly, patients had symptoms of abdominal pain, bloating, acid reflux, belching, nausea, vomiting, heartburn, chest pain, vomiting, melena, etc. Secondly, patients were unused antibiotics, bismuth, H2 receptor antagonists, or PPI in the last 2 weeks before gastrointestinal endoscopy. Thirdly, patients agreed to H pylori culture and sensitivity testing taken by endoscopy gastric biopsy specimens.
The exclusion criteria were as follows. Firstly, patients with severe heart, liver, kidney dysfunction, pregnant, or lactating women were not allowed in this study. Secondly, patients with complications of bleeding, perforation, pyloric obstruction, cancer or esophageal, and gastrointestinal surgery history were not allowed in this study. Thirdly, patients whose could not properly express their complaints, such as psychosis, severe neurosis were not allowed in this study. Fourthly, patients with allergic to penicillin or either drugs of the 6 antibiotic tested by susceptibility testing were not allowed in this study.
Isolation of H pylori Strains
The isolation of H pylori was based on Gram staining and enzyme activity testing as described previously.16 Briefly, a gastric mucosa biopsy sample was ground and inoculated directly onto a Columbia Agar (Oxoid) plate containing 5% defibrinated sheep blood. The plate was then incubated under microaerophilic conditions (5% O2 and 10% CO2) for 3 days at 37°C. Translucent colonies were identified by colony morphology with Gram staining and urease, catalase, and oxidase activity testing. Spiral gram-negative strains that were positive for all 3 enzyme activities were identified as H pylori. Sequentially, these strains were collected in phosphate-buffered saline (PBS, pH 7.4) at a concentration of 4 × 108 CFU/mL for antibiotic susceptibility testing.
Antibiotic Susceptibility Testing
The susceptibility of the isolated H pylori strains to 6 antibiotics (clarithromycin, levofloxacin, metronidazole, amoxicillin, gentamicin, and furazolidone) was determined by the agar dilution method. The resistance breakpoints of the 6 antibiotics were defined for clarithromycin ≥ 1, levofloxacin ≥ 2, metronidazole ≥ 8, amoxicillin ≥ 2, gentamicin ≥ 16, and furazolidone ≥ 2 μg/mL.17 Two microliter suspensions of H pylori were transferred onto Mueller–Hintonagar (Oxoid) supplemented with 5% sheep blood and a single antibiotic. They were then grown in a microaerobic humidified atmosphere at 37°C for 3 days. As a control, a standard H pylori strain (ATCC43504/NCTC11637) was used. All tests were repeated and conducted at the Hangzhou Zhiyuan Medical Inspection Institute.
Statistical Analysis
The statistical significances in the resistance rates among the different collection years, to the different antibiotics and in the different patient ages were analyzed by the chi-squared (χ2) test using the SPSS statistical software package version 19.0 (SPSS Inc., Chicago, IL). Probability (P) values < 0.05 were considered significant.
RESULTS
Distribution of the H pylori Strains
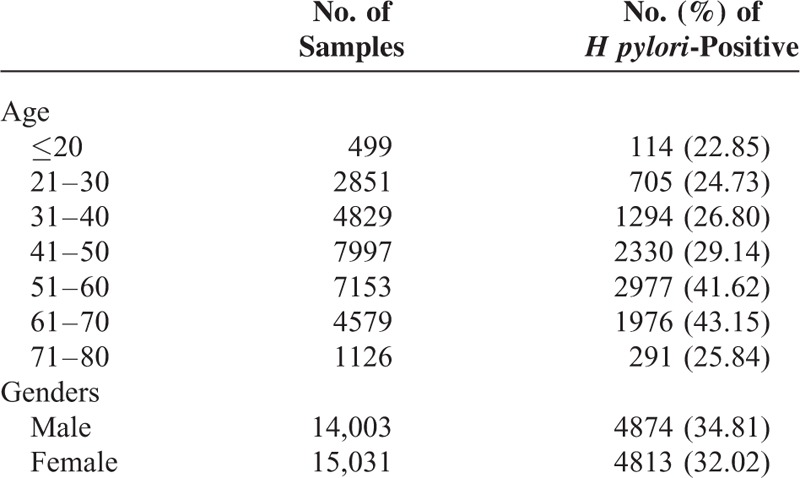
A total of 9687 H pylori strains were isolated. As shown in Table 1, a high percentage of the H pylori isolates was found in the 61- to 70-year-old group (43.15%) and the 51- to 60-year-old group (41.62%), and significant differences were observed between the high-infection group (the 51- to 60-year-old and the 61- to 70-year-old group) and other age groups (χ2 = 723.72, P < 0.001). Moreover, 34.81% and 32.02% of the H pylori isolates were found in male and female patients, respectively (χ2 = 25.315, P < 0.001).
TABLE 1.
Characteristics of the Distribution of Helicobacter pylori-Positive Strains in This Study
Antimicrobial Susceptibility
Six antibiotics (clarithromycin, levofloxacin, metronidazole, amoxicillin, gentamicin, and furazolidone) were chosen to perform the susceptibility testing. As shown in Table 2, of the 9687 H pylori strains isolated, 17.76% showed resistance to clarithromycin, 19.66% showed resistance to levofloxacin, and 95.5% showed resistance to metronidazole. Only a single case of amoxicillin resistance was found in 2014, while a single case of furazolidone resistance was found in 2009. No resistance to gentamicin was found in any of the H pylori strains isolated from 2009 to 2014.
TABLE 2.
Antimicrobial Resistance Rate of H pylori Strains During 2009 to 2014
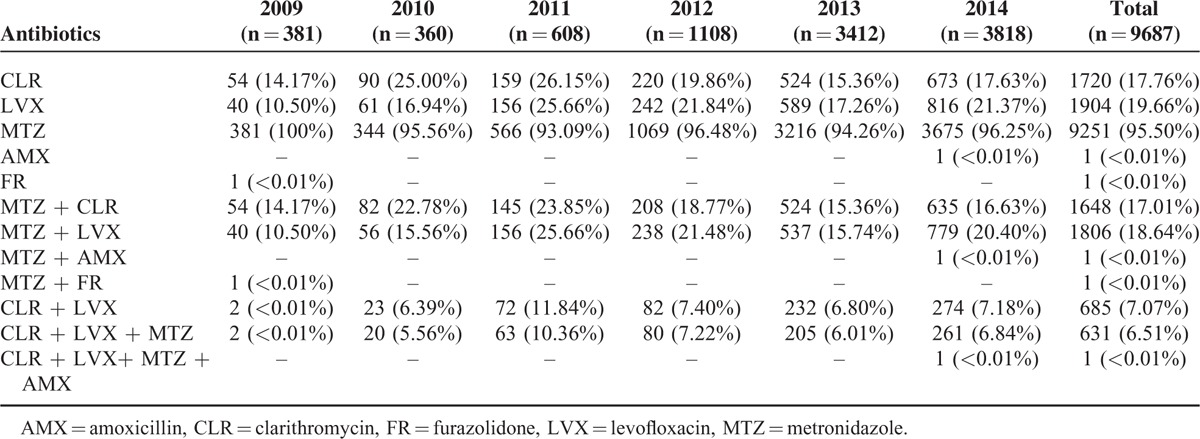
The resistance rates to the different antibiotics varied each year. The rate of metronidazole resistance stayed at a consistently high level, with no noticeable trends from 2009 to 2014. In contrast, the resistance rates of clarithromycin and levofloxacin increased rapidly from 2009 to 2011, reached a maximum in 2011, and then gradually decreased in 2012 and 2013. However, increased antibiotic resistance was observed again in clarithromycin (χ2 = 6.717, P = 0.01) and levofloxacin (χ2 = 19.438, P < 0.01) in 2014 (Table 2).
Of the 9687 H pylori strains, 28.96% were resistant to more than 1 antibiotic. Overall, 22.32% of the H pylori strains exhibited resistance to 2 classes of antibacterial agents, while 6.52% were resistant to 3 classes. The dual resistance rate to clarithromycin and levofloxacin was 7.07%, and the most prominent type of triple resistance was to metronidazole, levofloxacin, and clarithromycin. Quadruple resistance occurred with metronidazole, levofloxacin, clarithromycin, and amoxicillin. Quadruple resistance was observed in 2014.
Antibiotic Resistance Difference in Different Age Stage
The patient ages associated with resistance to clarithromycin and levofloxacin are presented in Figure 1. For clarithromycin, patients 31 to 50 or 71 to 80 years of age had higher resistance rates. Young patients (below 30 years old) and older patients (from 51 to 70 years old) had lower resistance rates. The higher clarithromycin-resistant group, for example, 31 to 50 or 71 to 80 years of age, was found to have significant differences from the other resistance group (χ2 = 42.681, P < 0.05), except in patients under 20 years of age (χ2 = 5.074, P = 0.166).
FIGURE 1.
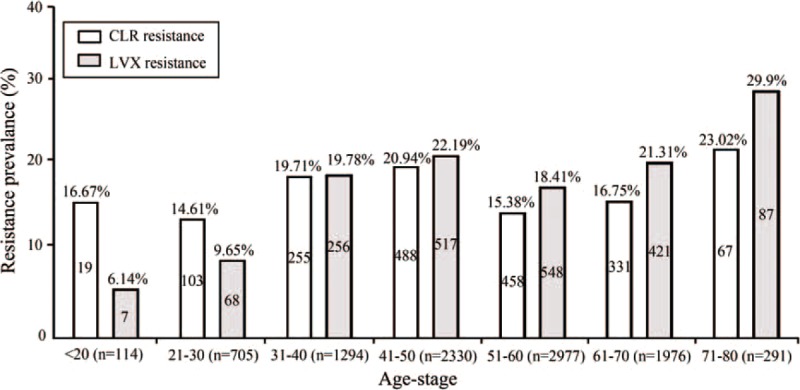
Distribution of the resistance rates among the 9687 Helicobacter pylori strains isolated from patients of different ages. The percentages above the bars represent the resistance rates. The number of antibiotic-resistant H pylori strains is marked in the bars. The numbers under the bars represent the number of H pylori strains isolated from each age bracket. CLR = clarithromycin, LVX = levofloxacin.
For levofloxacin, young patients (below 30 years of age) had a lower resistance rates than those of the other age groups (χ2 = 93.071, P < 0.001), but there was no significant difference between the patients below 20 years of age and those between 21 and 30 years of age (χ2 = 1.449, P = 0.229). The resistance rates against levofloxacin increased rapidly in the 31 to 50 years of age bracket, decreased in the 51 to 60 years of age bracket, and then gradually increased and reached a maximum in the 71 to 80 years of age bracket (29.9%). In patients 51 to 60 years of age, the resistance rate of the isolates to levofloxacin was 18.41%. This was significantly different from the rate observed in the patients 41 to 50 years of age (χ2 = 11.649, P = 0.001) and 61 to 70 (χ2 = 6.338, P = 0.012).
Moreover, we investigated the antibiotic resistance across the different ages and years under study (Figure 2). For clarithromycin and levofloxacin, the resistance rate in 2014 was higher than that in 2013, except for clarithromycin resistance in patients 31 to 40 years of age. Interestingly, in patients 71 to 80 years of age, the resistance rate of clarithromycin and levofloxacin had the opposite trend in 2009 to 2012. The patients 31 to 40 years of age also showed the opposite resistance trend from 2012 to 2014. Antibiotic resistance information of patients in different ages were listed in Supplementary Tables 1 and 2.
FIGURE 2.
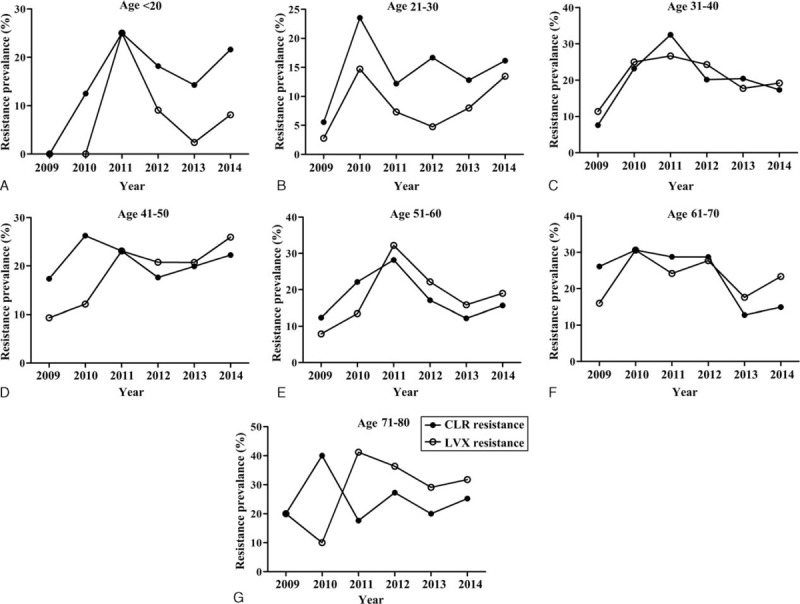
Trends in primary antibiotic resistance to clarithromycin and levofloxacin in Helicobacter pylori strains isolated between 2009 and 2014. CLR = clarithromycin, LVX = levofloxacin.
DISCUSSION
The emergence of antibiotic resistance in H pylori infections has become the biggest obstacle facing current therapeutic regimens.4,5,7,8 Globally, the overall cure rate is below 80%.2–4 To date, the excessive use of antibiotics has mainly involved clarithromycin, levofloxacin, and metronidazole.
Clarithromycin was initially used as a first-line antibiotic in triple therapy; however, its efficacy has been seriously hampered by the increasing proportion of clarithromycin resistance (10–50%).4,5,18 In this study, the overall resistance rate to clarithromycin was found to be 17.76%, with the highest resistance rate (26.15%) detected in 2011 (Table 2). The clarithromycin resistance rate declined to 15.36% in 2013, but it rose again in 2014 (17.63%). The change in antibiotic resistance indicates that the first-line therapy needs to be adjusted according to the current antibiotic resistance prevalence of H pylori. For example, triple therapy should have been abandoned in 2011, but this therapy would have been a reasonable first-line treatment in 2013, given the low levels of clarithromycin resistance at the time (15.36%).
Levofloxacin, another effective antibiotic has been widely used to treat urinary infections over the last decade. Unfortunately, levofloxacin resistance has been increasing so rapidly that it will soon reach the level of clarithromycin resistance.12,19 As noted above, the resistance rate to levofloxacin reached 19.66%, which has surpassed the rate of clarithromycin resistance, especially between 2012 and 2014 (Table 2). These results are likely associated with alternate first-line treatments, such as the replacement of clarithromycin with levofloxacin. In some countries, the resistance rate to levofloxacin has risen to 20%.20 The Maastricht IV consensus recommends that if the resistance rate is higher than 15% in a particular region, triple therapy should be abandoned unless an antibiotics susceptibility testing is performed prior to treatment.7 Therefore, considering the increased clarithromycin and levofloxacin resistance, susceptibility testing should be a priority in the future treatment of H pylori.
Metronidazole resistance is a reflection of antibiotics overuses, which has resulted in significant differences in the resistance levels between developed and developing countries. In this study, metronidazole resistance was observed at a high level (95.5%) (Table 2), although its resistance rate to metronidazole in Japan is declining from approximately 50% to 30% during 2019 to 2013.21 This phenomenon is likely due to the long-term and indiscriminate use of this cost-effective antibiotic in China. Although the impact of metronidazole resistance on the eradication rate can be partly overcome by increased doses and treatment durations, metronidazole should not be used as a first-line antibiotic in high-resistant circumstance without antibiotics susceptibility testing.5,7
Multiple antibiotics resistance in H pylori has been reported in many countries.12,19,22 In this study, 28.96% of the H pylori strains were found to be resistant to more than 1 antibiotic. The dual and triple antibiotic resistant rates were 22.32% and 6.52%, respectively. Although 1 H pylori strain was resistant to amoxicillin in this study, this strain actually demonstrated quadruple resistance. We must treat the emergence of multiple antibiotic resistances with great caution, because they are usually associated with multiple gastric diseases. According to the antimicrobial susceptibility strategy testing detailed in Table 2, we recommend that the first-line therapy regimen should choose effective and safe antibiotics. Antibiotic usage should be established according to patient antibiotics susceptibility testing. For example, we should select “levofloxacin + amoxicillin + PPI” for clarithromycin-resistant patients, while “amoxicillin + furazolidone + PPI” should be used to treat patients with clarithromycin and levofloxacin resistance.
Age plays an important role in the distribution of antibiotic resistance. Clarithromycin is widely employed for the treatment of respiratory diseases, especially in children.16 In this study, the resistance rate of clarithromycin was 16.67% in patients below 20 years old (Figure 1), and the rate increased from 14.29% in 2013 to 21.62% in 2014 (Figure 2). The highest resistance rate of clarithromycin was 23.02%, which was found in patients’ ages 71 to 80 years old (Figure 2). Nevertheless, the clarithromycin resistance was significantly lower in Jiaxing City than in Beijing, with a percentage of 84.9% in children and 37.2% in adults.12,16 The correlation between outpatient antibiotic consumption and high resistance rates has become increasingly clear, especially when the consumption of antibiotics is much more than standard doses.16 Therefore, with the rapid economic development of the region, the use of clarithromycin should be limited, particularly in younger (21.62% in 2014) and older (25.23% in 2014) patients. In France, to effectively eliminate H pylori, a policy of restricting clarithromycin use was enforced, after which the resistance rate stabilized.23
Old age is a risk factor for levofloxacin resistance, which is probably due to the high incidence of urinary infections in this age group.6 In this study, young patients (those below 30 years of age) had a lower level of resistance than other age groups (P < 0.001) and the highest resistance rate to levofloxacin was observed in the patients 71 to 80 years of age (29.9%). In Beijing, a high levofloxacin resistance rate of 50.3% was also reported in adults.12 Of note, no resistance to levofloxacin was reported in Malaysia.14 Therefore, the use of levofloxacin as a second-line drug should be considered in some regions in China, especially for the treatment of older adults.
Interestingly, patients ages 31 to 50 and 71 to 80 years old were found to have lower infection rates of H pylori (Table 1), but higher resistance rates to clarithromycin and levofloxacin, especially in senior patients (71–80 years of age). Moreover, we found that patients 51 to 60 years of age had higher H pylori infection rate; however, the patients 51 to 60 years of age had lower levels of clarithromycin and levofloxacin resistance. The 51 to 60 age bracket may represent an important group for the further study of H pylori treatment because antibiotic resistance was lower than that of the 31 to 50 and 71 to 80 age groups. Ideally, if the pattern of antibiotic susceptibility is well known in a region or country, a treatment regimen based on up-to-date data will reliably produce high cure rates of at least 90%.1,24 Studies in Singapore, where clarithromycin use has been limited, have revealed a cure rate of 90% or greater in response to triple therapy.25 In Malaysia, levofloxacin has been recommended as a first-line therapy due to the local levofloxacin resistance rate (0%).14 Although healthy individuals were not included in this study, the different resistance rates of H pylori suggested that it is necessary to understand the pattern of antibiotic resistance in a region or a country for the targeted eradication of H pylori infection.
CONCLUSIONS
In summary, patient age plays an important role in the distribution of H pylori antibiotic resistance to clarithromycin and levofloxacin. We therefore conclude that it is important to adopt individualized therapy for the treatment of H pylori infection based on the spectrum of antibiotic resistance in the region.
Footnotes
Abbreviations: AMX = amoxicillin, CFU = colony forming units, CLR = clarithromycin, FR = furazolidone, LVX = levofloxacin, MTZ = metronidazole, PBS = phosphate-buffered saline, PPI = proton pump inhibitor.
This study is supported by the Science and Technology Program of Zhejiang Province, China (No. 2001C23140), the grant from National Technology R&D Program in the 12th Five-Year Plan of China (No. 2012BAI06B02), the Major Technology Project as part of “Prevention and Control of Major Infectious Diseases including AIDS and Viral Hepatitis” (No. 2013ZX10004216-002), and the grant from National Key Scientific Instrument and Equipment Development Project (No. 2012YQ180117).
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
REFERENCES
- 1.Lopes D, Nunes C, Martins MC, et al. Eradication of Helicobacter pylori: past, present and future. J Control Release 2014; 12:S0168–S3659. [DOI] [PubMed] [Google Scholar]
- 2.Graham DY, Lu H, Yamaoka Y. A report card to grade Helicobacter pylori therapy. Helicobacter 2007; 12:275–278. [DOI] [PubMed] [Google Scholar]
- 3.Fischbach L, Evans EL. Meta-analysis: the effect of antibiotic resistance status on the efficacy of triple and quadruple first-line therapies for Helicobacter pylori. Aliment Pharmacol Ther 2007; 26:343–357. [DOI] [PubMed] [Google Scholar]
- 4.George LL, Borody TJ, Andrews P, et al. Cure of duodenal ulcer after eradication of Helicobacter pylori. Med J Aust 1990; 153:145–149. [DOI] [PubMed] [Google Scholar]
- 5.Graham DY, Shiotani A. New concepts of resistance in the treatment of Helicobacter pylori infections. Nat Clin Pract Gastroenterol Hepatol 2008; 5:321–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Megraud F, Coenen S, Versporten A, et al. Helicobacter pylori resistance to antibiotics in Europe and its relationship to antibiotic consumption. Gut 2013; 62:34–42. [DOI] [PubMed] [Google Scholar]
- 7.Malfertheiner P, Megraud F, O’Morain CA, et al. Management of Helicobacter pylori infection the Maastricht IV/Florence Consensus Report. Gut 2012; 61:646–664. [DOI] [PubMed] [Google Scholar]
- 8.Georgopoulos S, Papastergiou V, Xirouchakis E, et al. Evaluation of a four-drug, three-antibiotic, nonbismuth-containing “concomitant” therapy as first-line Helicobacter pylori eradication regimen in Greece. Helicobacter 2012; 17:49–53. [DOI] [PubMed] [Google Scholar]
- 9.Zullo A, De Francesco V, Hassan C, et al. The sequential therapy regimen for Helicobacter pylori eradication: a pooled-data analysis. Gut 2007; 56:1353–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vaira D, Zullo A, Vakil N, et al. Sequential therapy versus standard triple-drug therapy for Helicobacter pylori eradication: a randomized trial. Ann Intern Med 2007; 146:556–563. [DOI] [PubMed] [Google Scholar]
- 11.Kobayashi I, Murakami K, Kato M, et al. Changing antimicrobial susceptibility epidemiology of Helicobacter pylori strains in Japan between 2002 and 2005. J Clin Microbiol 2007; 45:4006–4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gao W, Cheng H, Hu FL, et al. The evolution of Helicobacter pylori antibiotics resistance over 10 years in Beijing. Helicobacter 2010; 15:460–466. [DOI] [PubMed] [Google Scholar]
- 13.Hwang TJ, Kim N, Kim HB, et al. Change in antibiotic resistance of Helicobacter pylori strains and the effect of A2143G point mutation of 23S rRNA on the eradication of H. pylori in a single center of Korea. J Clin Gastroenterol 2010; 44:536–543. [DOI] [PubMed] [Google Scholar]
- 14.Goh KL, Navaratnam P. High Helicobacter pylori resistance to metronidazole but zero or low resistance to clarithromycin, levofloxacin, and other antibiotics in Malaysia. Helicobacter 2011; 16:241–245. [DOI] [PubMed] [Google Scholar]
- 15.De Francesco V, Giorgio F, Hassan C, et al. Worldwide H. pylori antibiotic resistance: a systematic review. J Gastrointestin Liver Dis 2010; 19:409–414. [PubMed] [Google Scholar]
- 16.Liu G, Xu X, He L, et al. Primary antibiotic resistance of Helicobacter pylori isolated from Beijing children. Helicobacter 2011; 16:356–362. [DOI] [PubMed] [Google Scholar]
- 17.Su P, Li Y, Li H, et al. Antibiotic resistance of Helicobacter pylori isolated in the Southeast Coastal Region of China. Helicobacter 2013; 18:274–279. [DOI] [PubMed] [Google Scholar]
- 18.European Medicines Agency Note for Guidance on Evaluation of Medicinal Products Indicated for Treatment of Bacterial Infections. 2004; London:European Medicines Agency, EMEA CPMP/EWP/558/95 rev 1. [Google Scholar]
- 19.Glocker E, Stueger HP, Kist M. Quinolone resistance in Helicobacter pylori isolates in Germany. Antimicrob Agents Chemother 2007; 51:346–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hung IF, Chan P, Leung S, et al. Clarithromycin-amoxicillin-containing triple therapy: a valid empirical first-line treatment for Helicobacter pylori eradication in Hong Kong? Helicobacter 2009; 14:505–511. [DOI] [PubMed] [Google Scholar]
- 21.Okamura T, Suga T, Nagaya T, et al. Antimicrobial resistance and characteristics of eradication therapy of Helicobacter pylori in Japan: a multi-generational comparison. Helicobacter 2014; 19:214–220. [DOI] [PubMed] [Google Scholar]
- 22.Kim N, Kim JM, Kim CH, et al. Institutional difference of antibiotic resistance of Helicobacter pylori strains in Korea. J Clin Gastroenterol 2006; 40:683–687. [DOI] [PubMed] [Google Scholar]
- 23.Raymond J, Lamarque D, Kalach N, et al. High level of antimicrobial resistance in French Helicobacter pylori isolates. Helicobacter 2010; 15:21–27. [DOI] [PubMed] [Google Scholar]
- 24.Eurogast Study Group An international association between Helicobacter pylori infection and gastric cancer. Lancet 1993; 341:1359–1362. [PubMed] [Google Scholar]
- 25.Ang TL, Wang L, Ang D, et al. Is there still a role for empiric first-line triple therapy using proton pump inhibitor, amoxicillin and clarithromycin for Helicobacter pylori infection in Singapore? Results of a time trend analysis. J Dig Dis 2013; 14:100–104. [DOI] [PubMed] [Google Scholar]


