Abstract
Although many epidemiological studies have investigated the association between body mass index (BMI) and risk of rheumatoid (RA), the results have been inconsistent. Therefore, we performed a dose-response meta-analysis to quantify the dose-response association between BMI and RA risk.
We systematically searched PubMed, Embase, and Web of Science databases and reference lists of articles for relevant studies published before August 2014 using terms related to BMI and RA. Fixed or random-effects models were used to estimate the pooled relative risk (RR) with 95% confidence interval (CI). Several subgroup analyses, sensitivity analyses, and publication bias tests were performed to explore potential study heterogeneity and bias
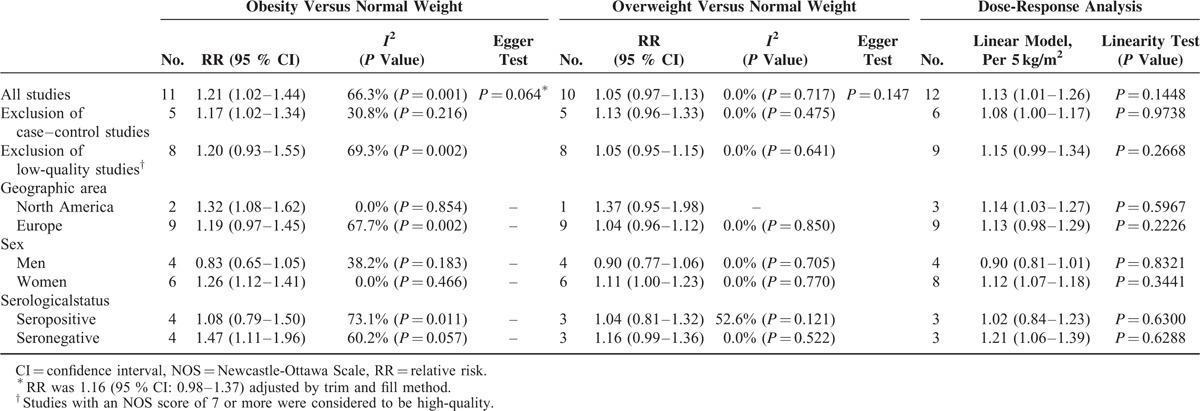
Thirteen studies involving 400,609 participants and 13,562 RA cases were included. The RR of RA was 1.21 (95% CI: 1.02–1.44) for obesity, 1.05 (95% CI: 0.97–1.13) for overweight. The risk of RA increased by 13% (RR: 1.13; 95% CI: 1.01–1.26) for every 5 kg/m2 increase in BMI. The subgroup analyses showed a positive association between BMI and RA risk only in women with an RR of 1.26 (95% CI: 1.12–1.40) for obesity and 1.12(95% CI: 1.07–1.18) for every 5 kg/m2 increase in BMI. Also, an increased risk of RA was found in sero-negative subgroup with an RR of 1.47 (95% CI: 1.11–1.96) for obesity and 1.21 (95% CI: 1.06–1.39) for every 5 kg/m2 increase in BMI.
There is evidence that obesity is a risk factor for developing of RA. Furthermore, the positive association between BMI and RA risk may be stronger among women than men.
INTRODUCTION
Rheumatoid arthritis (RA) is an autoimmune disease characterized by chronic, destructive, debilitating arthritis that affects approximately 1% of the adult population.1,2 An association between excess body weight and various inflammatory/autoimmune conditions has been suggested in many observational studies.3 Excess body weight measured by body mass index (BMI) corresponds to an abnormal accumulation of adipose tissue within the body. Adipose tissue now is considered as an active participant contributing to physiological and pathological processes associated with inflammation and immunity.4 It secretes proinflammatory and antiinflammatory metabolically and hormonally active substances, and produces cytokines and chemokines.5,6 Excess body weight was considered as a potential contributor to the development of RA.7
Although the association between BMI and RA risk has not been widely studied, conflicting results still exist, especially in the subgroup of different sex or serological status.8 To further examine the risk of obesity for the development of RA and summarize the evidence regarding the dose-response association between BMI and risk of RA, we performed a systematic review and meta-analysis of observational studies.
MATERIALS AND METHODS
Literature Search
Two investigators (QC and ZJ) electronically searched the PubMed (from 1965 to August 2014), EmBase (from 1965 to August 2014), and Web of Science (from 1986 to August 2014), using the MeSH terms and free key words “rheumatoid arthritis” combined with “body mass index” or “BMI.” Observational studies examining BMI and RA risk were eligible for inclusion in our meta-analysis, without any restriction on language, publication status, and article type. In addition, we scrutinized reference lists from relevant original and review articles to identify further eligible studies.
Eligibility Criteria
The titles and abstracts of the studies identified in the database were reviewed by 2 investigators (QC and FY) for the identification of studies that met the following criteria: any type of observational study (case–control study, nested case–control study, and cohort study; the exposure of interest was BMI; determination of prevalence of RA, as identified by physicians and/or by use of the record linkage system, as the outcome of interest; and reporting the relative risk (RR) or odds ratio (OR) and its 95% confidence interval (CI) for the association between BMI and RA risk. If more than 1 article reported data from the same population, the most recent and complete articles were included in our meta-analysis. Institutional review board approval and patient consent were not required for this meta-analysis of observational studies.
Data Extraction
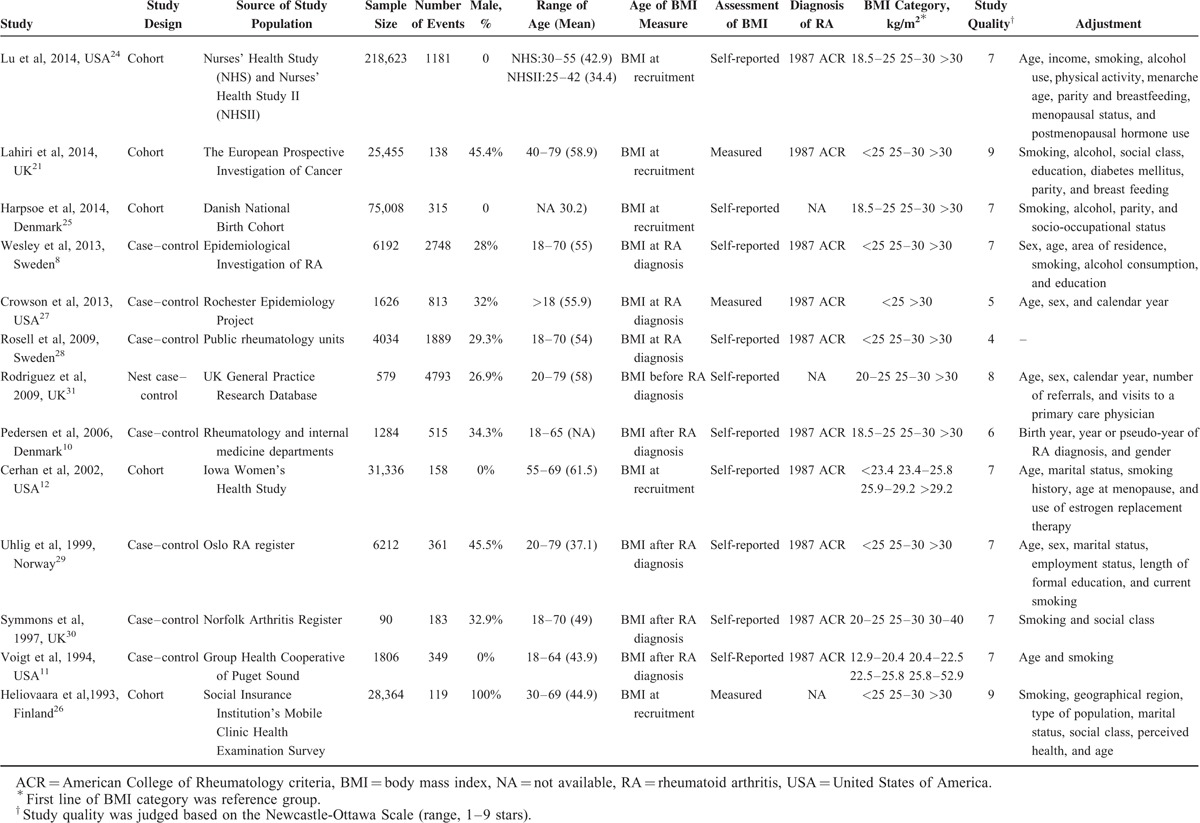
Data extraction was conducted by 2 investigators (QC and FY), and independently checked for accuracy by a 3rd investigator (JH). For each included study, data regarding the author, publication year, country in which it was conducted, study design, source of study population, sample size, number of events, proportion of male, range of age, age of BMI measure, assessment of BMI, diagnosis of RA, BMI category, covariates controlled for by matching or multivariable analysis, the number of cases/noncases or person-year data, and adjusted RR/OR for each BMI category and its 95% CI were extracted. For studies that reported several multivariable adjusted RRs, the effect estimate that was most fully adjusted for potential confounders was extracted. Study quality was assessed using the 9-star Newcastle-Ottawa Scale9 by 2 investigators (QC and FY). For studies that reported several BMI measurements, the BMI that reported at the recruitment was extracted.10–12
Statistical Analysis
We examined the relationship between BMI and risk of developing RA on the basis of the adjusted RRs and 95% CIs reported in each study. Because the incidence of RA is low, the ORs in case–control studies approximate the RRs.13 According to World Health Organization guidelines,14 individuals with a BMI of 30 kg/m2 are classified as obesity and those with a BMI of 25 to 30 kg/m2 were characterized as overweight.
Firstly, meta-analyses were performed to compare the risk between obesity/overweight and normal BMI. A fixed effects model was used to estimate the pooled RRs with 95% CIs if there was no evidence of heterogeneity; otherwise, a random effect model was used.15 The χ2 test and I2 statistic were used to explore the heterogeneity.16 The Egger regression test was used to assess the publication bias.17 If publication bias existed, we tried to evaluate the effect of publication bias by trim and fill method.18
In addition, we explored the potential nonlinear relationship between BMI and RA, using restricted cubic splines with 3 knots (10%, 50%, and 90%). The P value for nonlinearity was calculated by testing the null hypothesis that the coefficient of the 2nd spline was equal to zero. A linear model was used to estimate linear trends of RR for RA every 5 kg/m2 increase in BMI if without any evidence of nonlinearity. The details of the methods used have been described by Larsson and Orsini.19,20 The numbers of cases and person-years or noncases and the RRs with the variance estimates for at least 3 quantitative exposure categories are required when using this method. However, the numbers of cases for each BMI category were not available in Lahiri study;21 so, we obtained a estimation of the distribution of cases for each category in this study using methods described by Aune et al.22 The median or mean BMI in each category was assigned to the corresponding dose of the BMI. If the highest or lowest category was open ended, we assumed that its amplitude was same as the neighboring category.23
Finally, subgroup analyses by geographic area, sex, and serological status were performed. Sensitivity analyses were performed in 2 ways: 1st, by excluding those studies that met relatively fewer quality criteria of the Newcastle-Ottawa scale (<7 stars); 2nd, by excluding the studies that used a case–control design. Stata Version 12.0 software (Stata Corp, College Station, TX) was used for all analyses and all statistical tests were 2-side. P < 0.05 was considered an indication of statistical significance.
RESULTS
Up to August 20, 2013, 3316 records were retrieved using the search strategy described. Review of the titles and abstracts according to the inclusion and exclusion criteria resulted in exclusion of 3248 articles. Reading of the full text of the remaining 68 articles for further evaluation resulted in the selection of 13 studies, including 5 cohort studies,12,21,24–26 7 case–control studies8,10,11,27–30 and 1 nested case–control study.31 Our study was to investigate the association between obesity, overweight, every 5-unit BMI increase, and RA. We chose to exclude 3 studies32–34 in which RR for RA were calculated per standard deviation of BMI to avoid combining studies that were not comparable. Figure 1 shows the search and exclusion process. Table 1 shows the general characteristics of the 13 included studies, which together had examined 400,609 participants and 13,562 RA cases.
FIGURE 1.
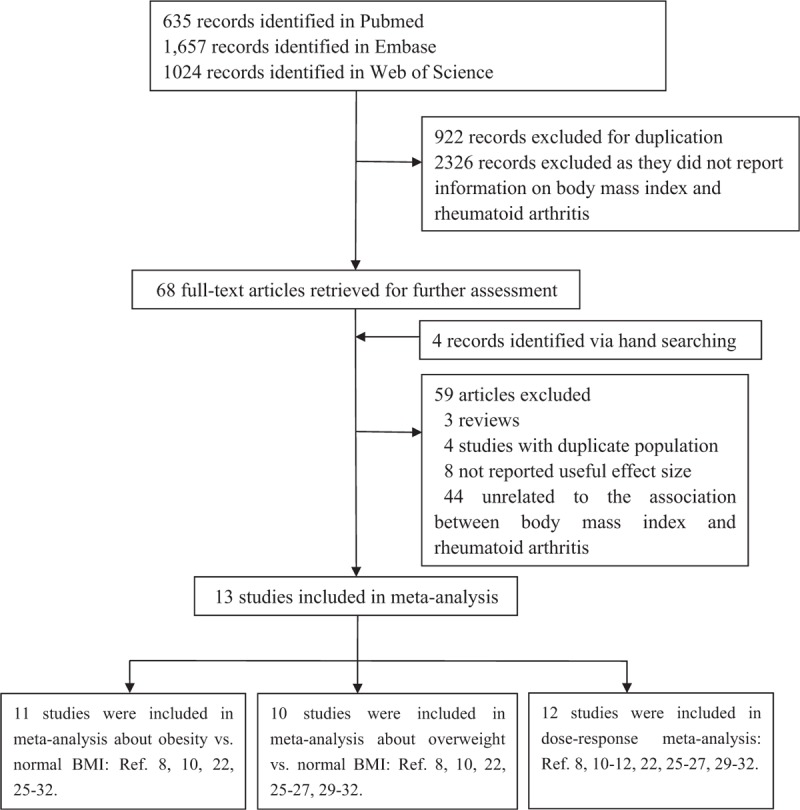
Selection of studies for inclusion in meta-analysis.
TABLE 1.
Characteristic of the Studies With Regard to Body Mass Index and Risk of Rheumatoid Arthritis
Effects of BMI on RA
As shown in Figures 2 and 3, the combined RRs (95% CIs) were 1.21 (1.02–1.44) and 1.05(0.97–1.13) for the category of obesity and overweight, respectively. Evidence of the existence of heterogeneity across studies was identified when comparing the obesity to normal BMI (I2 = 66.3%, P = 0.001). No evidence of a nonlinear relationship between BMI and risk of RA was found (P = 0.145). A statistically significant positive association was observed when linear relationship was modeled [RR: 1.13 (1.01–1.26) for every 5 kg/m2 increase in BMI] (Figure 4).
FIGURE 2.
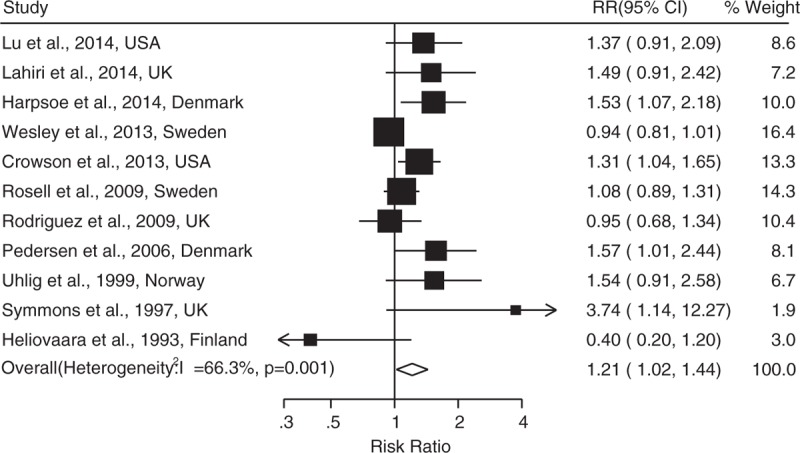
Adjusted relative risks of rheumatoid arthritis for obesity compared to normal weight.
FIGURE 3.

Adjusted relative risks of rheumatoid arthritis for overweight compared to normal weight.
FIGURE 4.
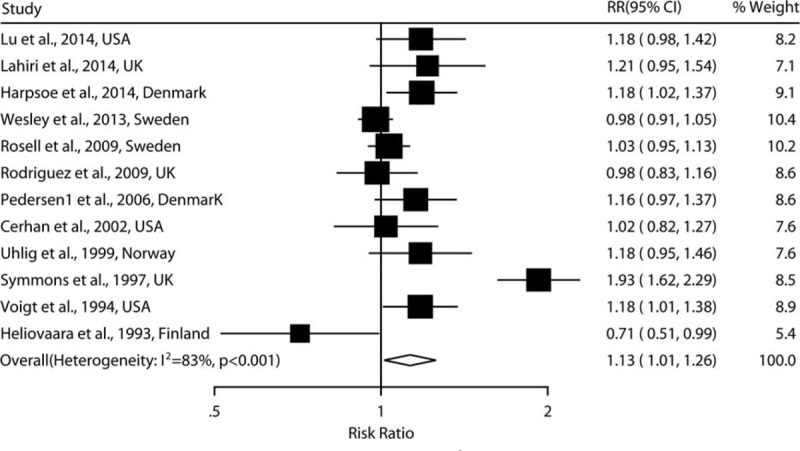
Adjusted relative risks of rheumatoid arthritis for every 5 kg/m2 increase in body mass index.
Subgroup Analysis
As shown in Table 2, women who have the BMI > 30 were found to have a 26% increase in RA risk (RR: 1.26, 95% CI: 1.12–1.40). The association was still statistical significant in the women with overweight (RR: 1.11, 95% CI: 1.00–1.23). Regardless of sex, a consistency of increase of risk was found in sero-negative subgroup, which have a 47% increase in RA risk (RR: 1.47; 95% CI: 1.11–1.96). The subgroup analyses under the dose-response setting showed comparable results, which also found that women with BMI > 30 and persons with sero-negative status had a higher risk of RA than that with normal weight.
TABLE 2.
Meta-Analyses of Association Between Body Mass Index and Risk of Rheumatoid Arthritis
Sensitivity Analysis
To explore whether the results were influenced by study design and quality, 2 ways of sensitivity analyses were carried out. As shown in Table 2, the results were comparable when case–control studies were excluded. The significant positive associations were still observed in obesity population and the positive associations were still nonsignificant in overweight population. The dose-response trend was similar to that identified by analysis of all 11 studies pooled when 6 studies with case–control design were excluded. However, the results of sensitivity analysis performed after excluding the studies with low quality showed a marginal statistical significance.
Publication Bias
The funnel plot (Figure 5) and Egger test (P = 0.064) showed some evidence of publication bias in the comparison between obesity and normal BMI. When trim and fill method was used, the summary estimates was marginally statistically significant (RR: 1.16, 95% CI: 0.98–1.37). No significant asymmetry of the funnel plot was detected in the comparison between overweight and normal BMI.
FIGURE 5.
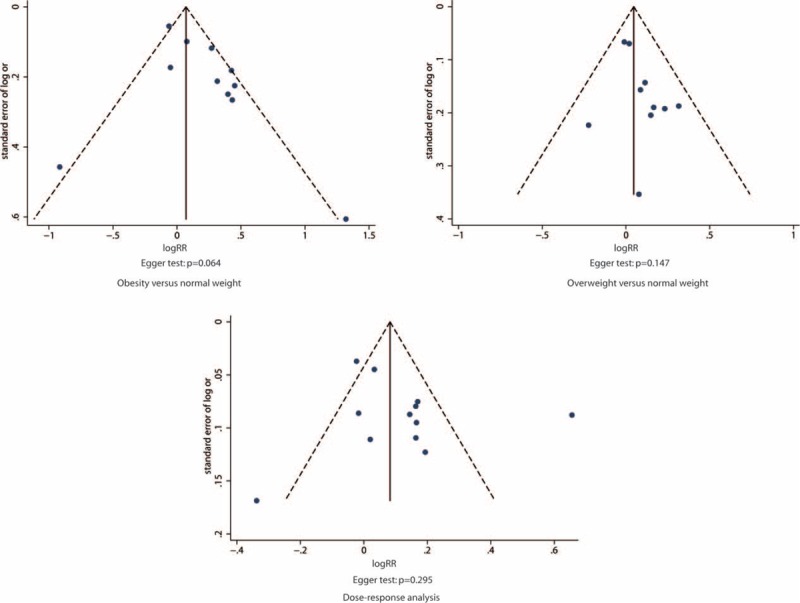
Funnel plot of log relative risk versus standard error of log relative risks.
DISCUSSION
The findings of the meta-analysis described here indicate that obesity is a risk factor for developing of RA and BMI is linearly positively associated with RA risk. Women and sero-negative population were more prone to suffer from RA when comparing those with normal BMI. The likelihood of developing RA increases linearly as the increase of BMI.
A plausible explanation of an increased risk of RA in obese population is that obesity may promote autoimmunity through variety of mechanisms including the secretion of adipokines.3 Interestingly, the subgroup analysis indicated that there is a positive relationship between obesity and risk of seronegative RA. However, no clear-cut biologic mechanism has been identified to explain this positive association.
Compared with the results of a newly published study that contains 2 large prospective cohorts,21,24 the results of the present study are comparable. Lu et al24 reported a positive effect of overweight or obesity on the development of RA in the Nurses’ Health Survey and the subsequent Nurses’ Health Survey II, which have been used extensively for the risk factors research. Our study also found the highest risk of RA in obese women subgroup. However, this relationship between obesity and RA observed in women may not apply to men directly. Some studies found that men who had a high BMI were at a reduced risk of developing RA.8,33 The subgroup results of men in this study showed that obesity had a neutral effect of developing RA. This suggests that hormone-related factors or other sex-specific exposures modify the impact of obesity in RA.35
There was some evidence of publication bias and heterogeneity in the comparison between obesity and normal BMI. The possibility of publication bias was inevitable as in all meta-analyses of published studies. In the present study, some small studies with inverse association between BMI and risk of RA seemed to be suppressed. Ignoring the suppressed small studies will overestimate the effect. However, the summary estimates were still marginally statistically significant after adjusted by trim and filled method. Heterogeneity may be introduced because of clinical or methodological differences among studies. In this meta-analysis, the sensitivity analyses regarding methodological differences have yielded consistent results after exclusion of case–control studies and marginal statistical significance after exclusion of low-quality studies. The results from subgroup analyses indicated that the source of heterogeneity might mostly come from sex and serological difference.
Strengths of the present meta-analysis were the large number of RA cases, separate analyses by sex and serological status accuracy, assessment of the potential nonlinear relationship between BMI and RA risk, which increased the reliability and validity of the our findings. However, several potential limitations must be considered when interpreting the results. First, a meta-analysis is not able to solve problems with confounding factors that could be inherent in the original studies. Although some major potential confounders had been adjusted in most included studies, residual or unknown confounding cannot be excluded. Peoples with obesity may share a greater number of harmful environmental factors compared to those with normal BMI, such as less ability to engage in physical activity and more like to have an unhealthier diet. RA is considered to result from the interactions between environmental and genetic factors,36 but no data regarding genetic factors were contained in the primary aggregate results. Second limitation is self-reported BMI, which may lead to misclassification of the exposure. However, the accuracy of self-reports of past body weight has been generally supported in epidemiologic studies.37,38 Third, definitions of reference category of BMI differed in the included studies, which made meta-analysis somewhat difficult. As shown in Table 1, some studies defined BMI < 25 as reference group, some defined 18.5 < BMI < 25 as reference group, and others defined 20 < BMI < 25 as reference group. Nevertheless, there were a small number of underweight individuals in studies defined BMI < 25 as reference group, which may not bring bias in calculating summary RRs of RA for obesity/overweight compared to reference group.
CONCLUSIONS
In conclusion, the results of this meta-analysis suggested that obesity may increase the risk of developing RA, possibly in a sex dependent and linear manner. The obese women have the highest risk for RA, emphasizing the public health importance of combating the obesity epidemic. Future work should involve combining genetic and environmental factors in large prospective cohorts to characterize gene–environment interactions in the development of RA.
Acknowledgments
This work was supported by the grants from the leading talents of science in Shanghai 2010(022), the key discipline construction of evidence-based public health in Shanghai (12GWZX0602), the Key Program of Shanghai Soft Science Research (14692101700), and the Natural Science Foundation of Shanghai (15ZR1412300).
Footnotes
Abbreviations: BMI = body mass index, CI = confidence interval, OR = odds ratio, RA = rheumatoid arthritis, RR = relative risk.
JF, QC, FY, SC, and ZJ contributed equally to this work.
QC, JF, and JH discussed and developed the question for this review; QC, ZJ, and SC carried out the searches; QC, FY, and JF assessed the eligibility of the studies for inclusion, extracted data, and carried out all analysis. All authors were involved in interpreted and discussed results. JF wrote the first draft of this paper and it was reviewed by QC and JH. QC, YL and ZW was involved in interpreted and discussed results. All authors agreed on the final draft of this study. JH is the guarantor.
This work was supported by the grants from the leading talents of science in Shanghai 2010(022), the key discipline construction of evidence-based public health in Shanghai (12GWZX0602), the Key Program of Shanghai Soft Science Research (14692101700), and the Natural Science Foundation of Shanghai (15ZR1412300).
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Gabriel SE, Crowson CS, O’Fallon WM. The epidemiology of rheumatoid arthritis in Rochester, Minnesota, 1955–1985. Arthr Rheum 1999; 42:415–420. [DOI] [PubMed] [Google Scholar]
- 2.Alamanos Y, Drosos AA. Epidemiology of adult rheumatoid arthritis. Autoimmun Rev 2005; 4:130–136. [DOI] [PubMed] [Google Scholar]
- 3.Versini M, Jeandel PY, Rosenthal E, et al. Obesity in autoimmune diseases: not a passive bystander. Autoimmun Rev 2014; 13:981–1000. [DOI] [PubMed] [Google Scholar]
- 4.Fantuzzi G. Adipose tissue, adipokines, and inflammation. J Allergy Clin Immunol 2005; 115:9–911.quiz 20. [DOI] [PubMed] [Google Scholar]
- 5.Touyz RM. Endothelial cell IL-8, a new target for adiponectin: implications in vascular protection. Circ Res 2005; 97:1216–1219. [DOI] [PubMed] [Google Scholar]
- 6.Trayhurn P, Wood IS. Signalling role of adipose tissue: adipokines and inflammation in obesity. Biochem Soc Trans 2005; 33 (Pt 5):1078–1081. [DOI] [PubMed] [Google Scholar]
- 7.Symmons DP. Looking back: rheumatoid arthritis–aetiology, occurrence and mortality. Rheumatology (Oxford, England) 2005; 44 (Suppl 4):iv14–iv17. [DOI] [PubMed] [Google Scholar]
- 8.Wesley A, Bengtsson C, Elkan A-C, et al. Association between body mass index and anti-citrullinated protein antibody-positive and anti-citrullinated protein antibody-negative rheumatoid arthritis: results from a population-based case-control study. Arthritis Care Res 2013; 65:107–112. [DOI] [PubMed] [Google Scholar]
- 9.Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa: Ottawa Hospital Research Institute 2009. [Google Scholar]
- 10.Pedersen M, Jacobsen S, Klarlund M, et al. Environmental risk factors differ between rheumatoid arthritis with and without auto-antibodies against cyclic citrullinated peptides. Arthr Res Ther 2006; 8:R133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Voigt LF, Koepsell TD, Nelson JL, et al. Smoking, obesity, alcohol consumption, and the risk of rheumatoid arthritis. Epidemiology (Cambridge, Mass) 1994; 5:525–532. [PubMed] [Google Scholar]
- 12.Cerhan JR, Saag KG, Criswell LA, et al. Blood transfusion, alcohol use, and anthropometric risk factors for rheumatoid arthritis in older women. J Rheumatol 2002; 29:246–254. [PubMed] [Google Scholar]
- 13.Greenland S. Quantitative methods in the review of epidemiologic literature. Epidemiol Rev 1987; 9:1–30. [DOI] [PubMed] [Google Scholar]
- 14.Series W H O T R. Obesity: preventing and managing the global epidemic. Report of a WHO consultation.[J]. World Health Organization Technical Report, 2000, 894:i-xii, 1-253. [PubMed] [Google Scholar]
- 15.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986; 7:177–188. [DOI] [PubMed] [Google Scholar]
- 16.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ (Clin Res Ed) 2003; 327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ (Clin Res Ed) 1997; 315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taylor S, Tweedie R. Practical estimates of the effect of publication bias in meta-analysis. Australas Epidemiol 1998; 5:14–17. [Google Scholar]
- 19.Larsson SC, Orsini N, Wolk A. Dietary magnesium intake and risk of stroke: a meta-analysis of prospective studies. Am J Clin Nutr 2012; 95:362–366. [DOI] [PubMed] [Google Scholar]
- 20.Larsson SC, Orsini N. Coffee consumption and risk of stroke: a dose-response meta-analysis of prospective studies. Am J Epidemiol 2011; 174:993–1001. [DOI] [PubMed] [Google Scholar]
- 21.Lahiri M, Luben RN, Morgan C, et al. Using lifestyle factors to identify individuals at higher risk of inflammatory polyarthritis (results from the European Prospective Investigation of Cancer-Norfolk and the Norfolk Arthritis Register-the EPIC-2-NOAR Study). Ann Rheum Dis 2014; 73:219–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aune D, Greenwood DC, Chan DS, et al. Body mass index, abdominal fatness and pancreatic cancer risk: a systematic review and non-linear dose-response meta-analysis of prospective studies. Ann Oncol 2012; 23:843–852. [DOI] [PubMed] [Google Scholar]
- 23.Jin Z, Xiang C, Cai Q, et al. Alcohol consumption as a preventive factor for developing rheumatoid arthritis: a dose-response meta-analysis of prospective studies. Ann Rheum Dis 2014; 23 11:1962–1967. [DOI] [PubMed] [Google Scholar]
- 24.Lu B, Hiraki LT, Sparks JA, et al. Being overweight or obese and risk of developing rheumatoid arthritis among women: a prospective cohort study. Ann Rheum Dis 2014; 73:1914–1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harpsoe MC, Basit S, Andersson M, et al. Body mass index and risk of autoimmune diseases: a study within the Danish National Birth Cohort. Int J Epidemiol 2014; 43:843–855. [DOI] [PubMed] [Google Scholar]
- 26.Heliovaara M, Aho K, Aromaa A, et al. Smoking and risk of rheumatoid arthritis. J Rheumatol 1993; 20:1830–1835. [PubMed] [Google Scholar]
- 27.Crowson CS, Matteson EL, Davis JM, 3rd, et al. Contribution of obesity to the rise in incidence of rheumatoid arthritis. Arthr Care Res 2013; 65:71–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosell M, Wesley A-M, Rydin K, et al. Dietary fish and fish oil and the risk of rheumatoid arthritis. Epidemiology (Cambridge, Mass) 2009; 20:896–901. [DOI] [PubMed] [Google Scholar]
- 29.Uhlig T, Hagen KB, Kvien TK. Current tobacco smoking, formal education, and the risk of rheumatoid arthritis. J Rheumatol 1999; 26:47–54. [PubMed] [Google Scholar]
- 30.Symmons DPM, Bankhead CR, Harrison BJ, et al. Blood transfusion, smoking, and obesity as risk factors for the development of rheumatoid arthritis: results from a primary care-based incident case-control study in Norfolk, England. Arthritis Rheum 1997; 40:1955–1961. [DOI] [PubMed] [Google Scholar]
- 31.Rodriguez LA, Tolosa LB, Ruigomez A, et al. Rheumatoid arthritis in UK primary care: incidence and prior morbidity. Scand J Rheumatol 2009; 38:173–177. [DOI] [PubMed] [Google Scholar]
- 32.Pikwer M, Giwercman A, Bergstrom U, et al. Association between testosterone levels and risk of future rheumatoid arthritis in men: a population-based case-control study. Ann Rheum Dis 2014; 73:573–579. [DOI] [PubMed] [Google Scholar]
- 33.Turesson C, Bergström U, Pikwer M, et al. FRI0097 High body mass index is associated with a reduced risk of developing rheumatoid arthritis in men[J]. Annals of the Rheumatic Diseases 2014; 72:A402–A403. [Google Scholar]
- 34.Heliovaara M, Aho K, Knekt P, et al. Coffee consumption, rheumatoid factor, and the risk of rheumatoid arthritis. Ann Rheum Dis 2000; 59:631–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Finckh A, Turesson C. The impact of obesity on the development and progression of rheumatoid arthritis. Ann Rheum Dis [Editorial] 2014; 73:1911–1913. [DOI] [PubMed] [Google Scholar]
- 36.Padyukov L, Silva C, Stolt P, et al. A gene-environment interaction between smoking and shared epitope genes in HLA-DR provides a high risk of seropositive rheumatoid arthritis. Arthritis Rheum 2004; 50:3085–3092. [DOI] [PubMed] [Google Scholar]
- 37.Kovalchik S. Validity of adult lifetime self-reported body weight. Pub Health Nutr 2009; 12:1072–1077. [DOI] [PubMed] [Google Scholar]
- 38.Perry GS, Byers TE, Mokdad AH, et al. The validity of self-reports of past body weights by U.S. adults. Epidemiology (Cambridge, Mass) 1995; 6:61–66. [DOI] [PubMed] [Google Scholar]


