Abstract
Dexmedetomidine (DEX) has been reported to have synergistic action with local anesthetics. This prospective, randomized, double-blind clinical study was designed to observe the efficacy of intravenous DEX without loading dose on spinal blockade duration, postoperative sedation, patient-controlled analgesia and its morphine-sparing effect in lower limb surgeries.
Seventy-five patients, scheduled for lower limb surgery under spinal anesthesia, were randomly allocated into 2 groups: group BS (received 15 mg of 0.5% of bupivacaine for subarachnoid anesthesia and continuous intravenous infusion of saline in Ringer solution) and BD group (received 15 mg of 0.5% of bupivacaine for subarachnoid anesthesia and continuous intravenous infusion of DEX in Ringer solution at a rate of 0.25 μg/kg/h). Intravenous infusion started 15 minutes before spinal anesthesia.
The onset time of sensory and motor blockade was shorten, the duration of sensory and motor blockade was significantly prolonged in BD patients when compared to BS patients. The Ramsay sedation score measured immediately after surgery was greater in BD group than BS group. BD patients also shown increased time to the first request of postoperative morphine and decreased total morphine consumption as compared with BS patients.
Collectively, intravenous administration of DEX without loading dose promoted the efficacy of spinal bupivacaine anesthesia and postoperative analgesia in patients undergoing lower limb surgery.
INTRODUCTION
Anesthesia management may modulate surgery-induced anesthesia duration and acute pain.1 Drug regimens have been reported to promote anesthesia and postoperative analgesia during local and general anesthesia.2 DEX was a relatively new alpha 2 adrenergic receptor agonist with a higher α2/α1 selectivity ratio of 1620:1 than clonidine (200:1).1 It was first introduced to clinical as a sedative in intensive care unit. Increasing evidence have shown that DEX could potentiate the anesthesia effect of local anesthetics, promote sedation, and prolong the duration of postoperative analgesia when used as an adjuvant through intrathecal, epidural, or intravenous routes.1 Single intravenous injection and continuous infusion starting with a loading dose were 2 commonly used methods to achieve this synergistic action with local anesthetics.3–6 Recent studies reported that intraoperative administration of intravenous DEX without loading dose provided stable general anesthesia statement, promoted postoperative analgesia and displayed potent morphine-sparing effect in patients undergoing different surgeries.7–10
This prospective, randomized, double-blind, placebo-controlled clinical study was designed to evaluate the efficacy of intravenous dexmedetomidine (DEX) without loading dose on spinal blockade duration, postoperative sedation, patient-controlled analgesia (PCA) and its morphine-sparing effect in lower limb surgeries.
METHODS
Subjects and Allocation
This study was approved by the Institutional Medical Ethics Committee of Yidu Central Hospital of Weifang City, and was in accordance with the approved guidelines. Informed consent was obtained from all subjects. The sample size of the study was calculated using a free software (http://www.statpages.org/#Power) based on a pilot study. Eighty-four of 87 qualified patients (3 patients refused) were enrolled, and randomly assigned into BS (n = 42, 7 patients were lost because of noncooperation), and BD (n = 42, 2 patients were lost because of noncooperation) group using computer-generated randomized table (Figure 1). The BS and BD patients received intravenous saline or DEX infusion, and bupivacaine for local anesthesia, respectively. The infusion syringe pumps were prepared by a different anesthesiologist to make this study a randomized, double-blinded investigation. Patients matching the following criteria were included in this study: between 18 and 65 years old; American Society of Anesthesiologists (ASA) grade I or II; weight 55 to 85 kg. Patients were excluded if they had ischemic heart diseases; opioid addiction, long-term alcohol abuse, long-term smoking history, sedative–hypnotic drug(s); obesity (BMI > 30); postoperative nausea and vomiting history; or neuropsychiatric diseases and related treatment history. Patients were instructed to the use the i.v. PCA pump (50 mg morphine and 10 mg ondansetron in 100 ml saline, every pump press leads to a 2 ml of infusion), the first time of morphine request and total morphine consumption were recorded.
FIGURE 1.
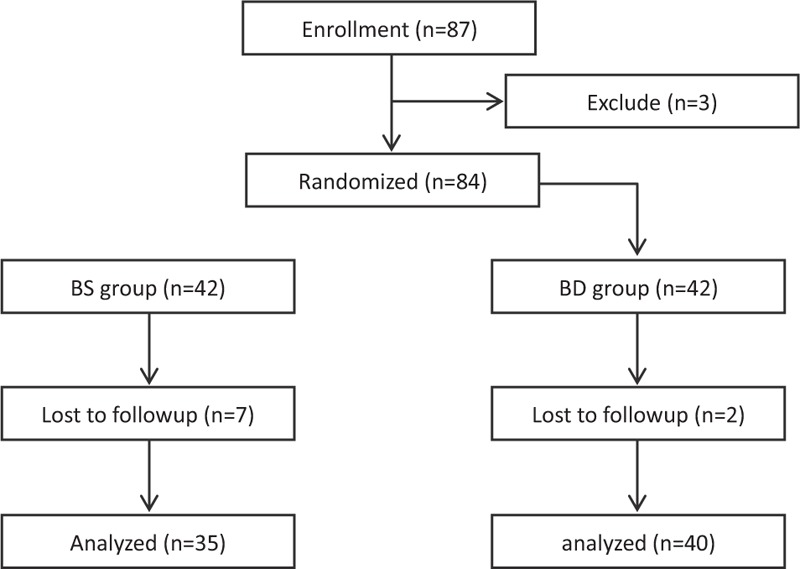
Flow diagram of the study.
Anesthesia
On arrival, electrocardiography, blood pressure, and oxygen saturation were monitored every 5 minutes. Following prehydration with 500 ml of lactated Ringer solution, a lumbar puncture was performed at the L3–L4 level. Patients from the both groups received 15 mg of 0.5% of bupivacaine for subarachnoid anesthesia, and continuous intravenous infusion of saline or DEX in Ringer solution at a rate of 0.25 μg/kg/hour. Intravenous infusion started 15 minutes before spinal anesthesia, and were switched to Ringer solution when the patients were transferred to the postanesthesia care unit (PACU) from the operation room. The patient and the anesthesiologist were double-blinded to the treatment, and all recordings were performed by an anesthesiologist blinded to group allocation. Sensory blockade was assessed using sterile pin prick method in the mid-axillary line on both sides of chest. Immediately after sensory block assessment, motor block was evaluated with a modified Bromage scale as reported in previous studies (grade 0: no paralysis; grade 1: unable to raise; grade 2: unable to flex knees, grade 3: unable to flex ankle).
Data Collection
Patient demographic information was collected on admission. Hemodynamic indexes were recorded during surgery every 5 minutes, and data from selected time points used for analysis. Sedation level was evaluated immediately after surgery with Ramsay sedation score as previously reported.7,8 Time to first request of morphine, morphine consumption, and postoperative adverse effects were recorded. No changes were made after the beginning of this study.
Statistics
All data in the present study were analyzed with GraphPad Prism 5.0 software. Parameters like age, weight, surgery time, first request time, and morphine consumption were compared between the 2 groups with unpaired Student t test. HR and mean blood pressure (MBP) at different time points were compared between the 2 group with 2-way ANOVA followed by Bonferroni posttest. ASA grade and postoperative adverse effects were analyzed with Fisher test. All data with P < 0.05 were considered significant.
RESULTS
Demographic Characteristics
Patients from both groups had comparable demographic and surgery/anesthesia-related variables, including age, weight, BMI, gender, ASA class, surgery time (Table 1). The 2 groups were also comparable with respect to their perioperative MBP and mean heart rate (HR) during their surgeries (Figure 2A and B).
TABLE 1.
Basic Demographic Data and Surgery Duration (Mean ± SEM)
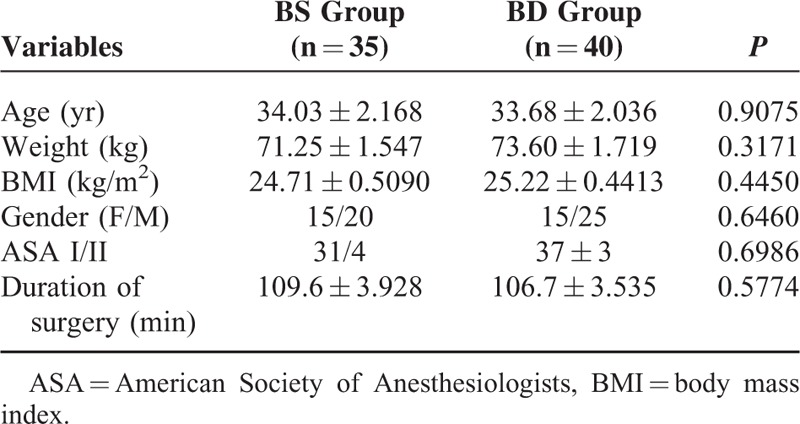
FIGURE 2.
Perioperative HR and MBP at different time points. T0: baseline, T1: nerve blockade (15 min after infusion), T2: surgery on, T3: sugary on 30 minutes, T4: surgery on 60 minutes, T5: surgery on 90 minutes, T6: 2 hours postsurgery, T7: 24 hours postsurgery.
Intraoperative DEX Prolongs Spinal Bupivacaine Anesthesia
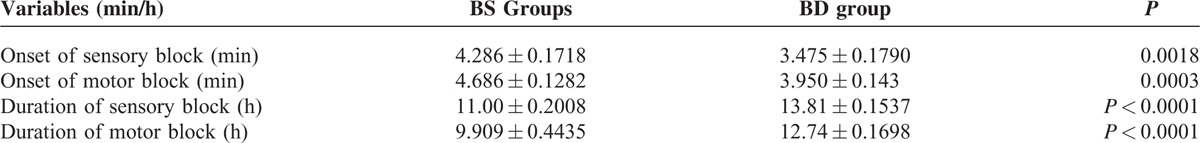
Compared to BS group, the patients in the BD group had a shorter onset time of both sensory and motor blockade, and also shown a longer-lasting duration of sensory and motor blockade (Table 2). These data indicated that intraoperative infusion of DEX prolonged the anesthesia effects of spinal bupivacaine.
TABLE 2.
Onset Time and Duration of Sensory and Motor Block (Mean ± SEM)
Intraoperative DEX Promotes Sedation and Spare Postoperative Morphine Consumption
DEX was first introduced into hospital as a sedative, and we found a higher Ramsay sedation score in the BD group patients when compared to those patients in the BS group (Figure 3A). In the postoperative PCA, DEX increased the first time of request for postoperative analgesic (Figure 3B), and reduced the total consumption of morphine during the first postoperative 24 hours (Figure 3C).
FIGURE 3.
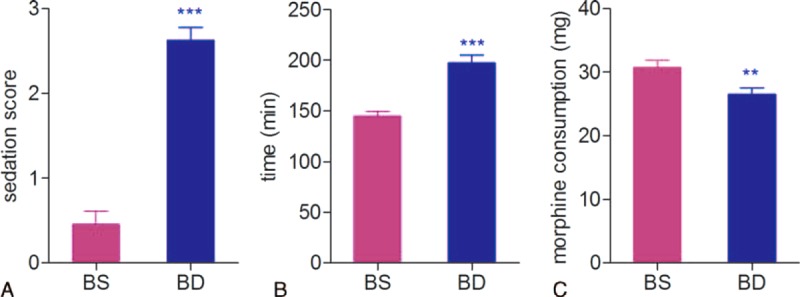
Ramsay sedation score after surgery and 24-hour morphine consumption. (A) Sedation score right after surgery (∗∗∗P < 0.0001). (B) Time to first press of PCA pump (∗∗∗P < 0.0001). (C) Total morphine consumption of the first 24 hours (∗∗P < 0.01).
Intraoperative DEX Has No Effects on Postoperative Adverse Effects
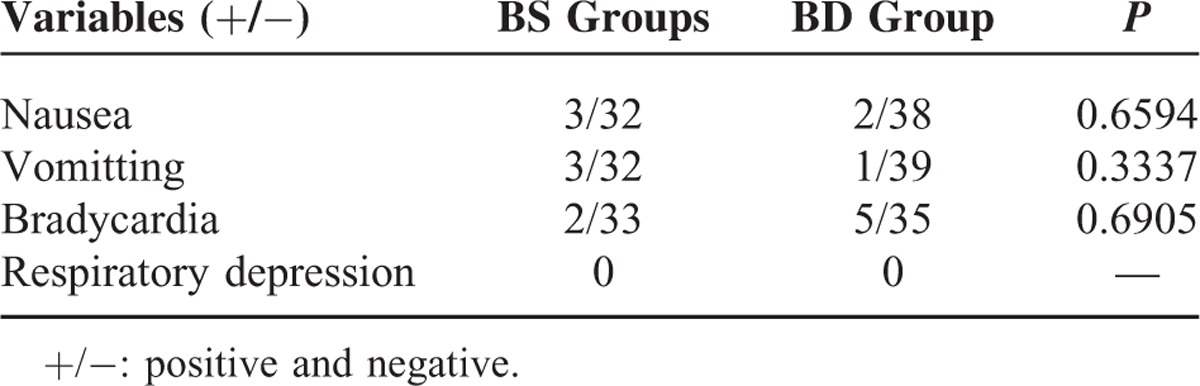
No difference was observed in the postoperative adverse effects between the 2 groups during the first 24 hours (Table 3).
TABLE 3.
Adverse Effects
DISCUSSION
In the present study, we found that intravenous administration of a low dose of DEX without loading dose promoted the anesthesia property during bupivacaine spinal anesthesia, prolonged the duration of first request of postoperative analgesics and induced a potent morphine-sparing effect.
Alpha 2 receptor agonists, like clonidine (α2R:α1R ratio of 200:1), have been used as pain treatments for decades.11,12 A recent study reported that α1 receptor activation encountered α2R-related analgesia and suggested that an agonist with higher α2 R selectivity would show a more potent analgesic effect and would be more suitable for pain treatment.13 DEX is a α2R agonist developed in the 1990s, and it was first used as a short-term sedative in the intensive care units.1 Clinical studies have confirmed its potential as an adjuvant for pain treatment, mostly in acute perioperative settings. Anesthesia studies indicate that it could prolong the analgesic effects of local anesthetics. For example, DEX potentiates neuraxial local anesthetics, decreases intraoperative anesthetic requirements, and improves postoperative analgesia, regardless of the neuraxial route of administration (eg, epidural, caudal, or spinal).14–16 These evidence suggest that DEX might be a new drug promoting the effect of local anesthesia, and could be used for surgery-induced acute pain control.17 Generally, single dose intravenous injection and continuous infusion starting with a loading dose were 2 widely used methods to achieve its effects on anesthesia and analgesia during local or general anesthesia.3–6,18 DEX induces hemodynamic changes, such as hypertension, hypotension, and bradycardia, especially after a loading dose. Thus, in the present study, we administered a continuous infusion without a loading dose. Using this continuous infusion, we observed no significant difference in HR or MBP between the 2 groups, but a significant decrease of onset time and increase of durations in sensory and motor blockade. These data indicated that intravenous infusion of DEX without loading dose could successfully shorten the onset time of bupivacaine, and prolonged the lasting duration of its anesthesia property. Our results was consistent with previous studies that described DEX infusion without the administration of the loading dose avoided the undesirable hemodynamic effects.7–10,19
DEX has been reported to have morphine-sparing effect following intraoperative use during general anesthesia.7,8,20–22 Here, in the present study, we further found that intravenous infusion of DEX without loading dose was successful to prolong the first-request time for analgesics (morphine) and the total consumption of morphine. DEX was first used as a sedative, and we also observed a significant sedation property when we evaluated its sedative effect immediately after surgery. Though the long-lasting proanalgesic effects of DEX was largely unknown, we believe that, beside recently accepted reasons,7,8 the sedative effect might be another contributor.
This study indicated that the combining administration of intravenous DEX without loading dose with bupivacaine might be useful for spinal anesthesia and postoperative analgesia, and provide useful information for considering the regimens of intravenous DEX with other local anesthetics to achieve elevated anesthesia during, and postoperative analgesia following local anesthesia. One of the limitations in the present study is that the sedation statement was not completely come from the effect of DEX: all the subjects underwent scheduled lower limb surgery, those patient after acute injuries have different waiting time from their injuries to surgeries, injury-induced acute pain and fatigue might induce insomnia which depending very much on the waiting time. When they received local anesthesia, these patients would fall asleep very easily, and shown a deep sedation. It is hard to identify how much DEX contributed to this sedation state.
Taken together, the present study found that the regimen of intravenous DEX without loading dose and bupivacaine produced a long-lasting sensory and motor blockade during local anesthesia, and promoted the postoperative analgesia.
Footnotes
Abbreviations: ASA = American Society of Anesthesiologists, BMI = body mass index, DEX = dexmedetomidine, HR = heart rate, MBP = mean blood pressure, PACU = postanesthesia care unit, PCA = patient-controlled analgesia.
HZ and ML contributed equally to this study.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Grosu I, Lavand’homme P. Use of dexmedetomidine for pain control. F1000 Med Rep 1000; 2:M2–M90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bekker A, Haile M, Kline R, et al. The effect of intraoperative infusion of dexmedetomidine on the quality of recovery after major spinal surgery. J Neurosurg Anesthesiol 2013; 25:16–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al-Mustafa MM, Badran IZ, Abu-Ali HM, et al. Intravenous dexmedetomidine prolongs bupivacaine spinal analgesia. Middle East J Anaesthesiol 2009; 20:225–231. [PubMed] [Google Scholar]
- 4.Reddy VS, Shaik NA, Donthu B, et al. Intravenous dexmedetomidine versus clonidine for prolongation of bupivacaine spinal anesthesia and analgesia: a randomized double-blind study. J Anaesthesiol Clin Pharmacol 2013; 29:342–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wahlander S, Frumento RJ, Wagener G, et al. A prospective, double-blind, randomized, placebo-controlled study of dexmedetomidine as an adjunct to epidural analgesia after thoracic surgery. J Cardiothorac Vasc Anesth 2005; 19:630–635. [DOI] [PubMed] [Google Scholar]
- 6.Ohtani N, Yasui Y, Watanabe D, et al. Perioperative infusion of dexmedetomidine at a high dose reduces postoperative analgesic requirements: a randomized control trial. J Anesth 2011; 25:872–878. [DOI] [PubMed] [Google Scholar]
- 7.Ge DJ, Qi B, Tang G, et al. Intraoperative dexmedetomidine promotes postoperative analgesia and recovery in patients after abdominal colectomy: a CONSORT-prospective, randomized, controlled clinical trial. Medicine 2015; 94:0000000000001727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ge DJ, Qi B, Tang G, et al. Intraoperative dexmedetomidine promotes postoperative analgesia in patients after abdominal colectomy: a consort-prospective, randomized, controlled clinical trial. Medicine 2015; 94:0000000000001514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ren C, Chi M, Zhang Y, et al. Dexmedetomidine in postoperative analgesia in patients undergoing hysterectomy: a CONSORT-prospective, randomized, controlled trial. Medicine 2015; 94:0000000000001348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ren C, Zhang X, Liu Z, et al. Effect of intraoperative and postoperative infusion of dexmedetomidine on the quality of postoperative analgesia in highly nicotine-dependent patients after thoracic surgery: a CONSORT-prospective, randomized, controlled trial. Medicine 2015; 94:0000000000001329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Engelman E, Marsala C. Efficacy of adding clonidine to intrathecal morphine in acute postoperative pain: meta-analysis. Br J Anaesth 2013; 110:21–27. [DOI] [PubMed] [Google Scholar]
- 12.Lambert P, Cyna AM, Knight N, et al. Clonidine premedication for postoperative analgesia in children. Cochrane Database Syst Rev 2014; 28: 10.1002/14651858.CD009633.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gil DW, Cheevers CV, Kedzie KM, et al. Alpha-1-adrenergic receptor agonist activity of clinical alpha-adrenergic receptor agonists interferes with alpha-2-mediated analgesia. Anesthesiology 2009; 110:401–407. [DOI] [PubMed] [Google Scholar]
- 14.Elhakim M, Abdelhamid D, Abdelfattach H, et al. Effect of epidural dexmedetomidine on intraoperative awareness and post-operative pain after one-lung ventilation. Acta Anaesthesiol Scand 2010; 54:703–709. [DOI] [PubMed] [Google Scholar]
- 15.Schnaider TB, Vieira AM, Brandao AC, et al. [Intraoperative analgesic effect of epidural ketamine, clonidine or dexmedetomidine for upper abdominal surgery]. Rev Bras Anestesiol 2005; 55:525–531. [DOI] [PubMed] [Google Scholar]
- 16.El-Hennawy AM, Abd-Elwahab AM, Abd-Elmaksoud AM, et al. Addition of clonidine or dexmedetomidine to bupivacaine prolongs caudal analgesia in children. Br J Anaesth 2009; 103:268–274. [DOI] [PubMed] [Google Scholar]
- 17.Movafegh A, Shoeibi G, Ansari M, et al. Naloxone infusion and post-hysterectomy morphine consumption: a double-blind, placebo-controlled study. Acta Anaesthesiol Scand 2012; 56:1241–1249. [DOI] [PubMed] [Google Scholar]
- 18.Harsoor SS, Rani DD, Lathashree S, et al. Effect of intraoperative dexmedetomidine infusion on sevoflurane requirement and blood glucose levels during entropy-guided general anesthesia. J Anaesthesiol Clin Pharmacol 2014; 30:25–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ickeringill M, Shehabi Y, Adamson H, et al. Dexmedetomidine infusion without loading dose in surgical patients requiring mechanical ventilation: haemodynamic effects and efficacy. Anaesth Intensive Care 2004; 32:741–745. [DOI] [PubMed] [Google Scholar]
- 20.Gupta N, Rath GP, Prabhakar H, et al. Effect of intraoperative dexmedetomidine on postoperative recovery profile of children undergoing surgery for spinal dysraphism. J Neurosurg Anesthesiol 2013; 25:271–278. [DOI] [PubMed] [Google Scholar]
- 21.Jones JS, Cotugno RE, Singhal NR, et al. Evaluation of dexmedetomidine and postoperative pain management in patients with adolescent idiopathic scoliosis: conclusions based on a retrospective study at a tertiary pediatric hospital. Pediatr Crit Care Med 2014; 15:0000000000000119. [DOI] [PubMed] [Google Scholar]
- 22.McQueen-Shadfar LA, Megalla SA, White WD, et al. Impact of intraoperative dexmedetomidine on postoperative analgesia following gynecologic surgery. Curr Med Res Opin 2011; 27:2091–2097. [DOI] [PubMed] [Google Scholar]


