Abstract
Our clinical workload as infectious diseases pediatricians in northern Australia is dominated by complicated bone and joint infections in indigenous children. We reviewed the clinical presentation, microbiology, management, and outcomes of children presenting to Royal Darwin Hospital with bone and joint infections between 2010 and 2013, and aimed to compare severity and incidence with other populations worldwide.
A retrospective audit was performed on children aged 0 to 18 years who were admitted to Royal Darwin Hospital between 1 January 2010 and 31 December 2013 with a bone and joint infection.
Seventy-nine patients were identified, of whom 57 (72%) had osteomyelitis ± associated septic arthritis and 22 (28%) had septic arthritis alone. Sixty (76%) were indigenous Australians. The incidence rate of osteomyelitis for indigenous children was 82 per 100,000 children. Staphylococcus aureus was the confirmed pathogen in 43/79 (54%), of which 17/43 (40%) were methicillin resistant. Median length of stay was 17 days (interquartile range: 10–31 days) and median length of IV antibiotics was 15 days (interquartile range: 6–24 days). Fifty-six (71%) required at least 1 surgical procedure. Relapse within 12 months was documented in 12 (15%) patients.
We report 3 key findings: osteomyelitis incidence in indigenous children of northern Australia is amongst the highest reported in the world; methicillin-resistant S aureus accounts for 36% of osteomyelitis with a positive microbiological diagnosis; and the severity of disease requires extended antibiotic therapy. Despite this, 15% of the cohort relapsed within 12 months and required readmission.
INTRODUCTION
The incidence of pediatric osteomyelitis in high-income countries has been estimated at between 3 and 13 per 100,000,1–3 but is thought to be more common in low-income countries.4 However, despite Australia being a high-income country rates of 150 per 100,000 were reported in indigenous Western Australian children in the 1980s compared with 4 to 32 per 100,000 in their nonindigenous counterparts.5 In a more recent Staphylococcus aureus audit at a tertiary children's hospital in southeastern Australia, indigenous children were again identified as having higher rates of osteomyelitis than their nonindigenous peers.6
Little has been published on the epidemiology of bone and joint infections in indigenous children of northern Australia. In our clinical experience, bone and joint infections represent a common and severe reason for prolonged admission of children to hospital in the Northern territory, Australia where 27% of the population is indigenous and predominantly live in very remote communities.7 A clinical audit of infectious diseases consulting children in 2012 identified that these make up at least 25% of current workload (Bowen, personal communication). In comparison, bone and joint infections are less prominent in pediatric infectious diseases consultations in other Australian jurisdictions, comprising 12%.8
Most commonly acute osteomyelitis is caused by hematogenous spread in children, with or without concomitant septic arthritis. Less frequent etiologies include contiguous spread from other sites of infection, and vascular insufficiency.9 In recent international studies, the most common organisms causing acute osteomyelitis are skin and respiratory pathogens, respectively S aureus, Streptococcus pneumoniae, and Haemophilus influenzae4 with S aureus as by far the most common, accounting for up to 80% of pediatric osteomyelitis.4,10Kingella kingae has been increasingly recognized as an osteoarticular pathogen in children.11 Recommendations for treatment of acute osteomyelitis in children are much shorter than in adults, based on recent randomized controlled trials.12,13 Current Australian guidelines14 and international consensus15 now favor a short duration of intravenous antibiotics for acute osteomyelitis and septic arthritis of between 3 and 7 days provided that there is clinical improvement, followed by oral therapy for a further 3 and 4 weeks.12,13 However, the generalizability of these results to our population is uncertain. Our clinical experience of bone and joint infections did not mirror those described in these recent trials and we were concerned that these results may not be applicable to our predominantly indigenous population.
We therefore aimed to formally describe the incidence, epidemiology, clinical presentation, severity, and outcomes of osteoarticular infections in our pediatric population, and compare these against published data of pediatric cohorts worldwide.
METHODS
Setting
Royal Darwin Hospital is a 345-bed general, tertiary hospital with 55 pediatric beds and serves as the only referral center for the “Top End” of the Northern Territory (NT) in Australia. The NT is a vast, scarcely populated region of 1.5 million square kilometers, of which the Top End comprises approximately the northern third7 and has a tropical climate with average daily temperatures of 32 °C.16 An estimated pediatric population of 50,000 children and adolescents under the age of 18 years live in this catchment area,7 of whom 27% are indigenous. Children from the Top End with osteomyelitis are usually admitted to Royal Darwin Hospital, as this is where the only general orthopedic, surgical, intensive care, and pediatric specialist services are located.
Inclusion Criteria and Definitions
In this retrospective audit, patients were included if they were aged less than 18 years when admitted to Royal Darwin Hospital and had a World Health Organization International Classification of Diseases (WHO ICD-10) discharge code for septic arthritis (ICD codes M0.00–0.99) and/or osteomyelitis (ICD codes M86.00–86.99), between January 1, 2010 and December 31, 2013. Data were collected from individual medical records, electronic records for pathology, and radiology results, as well as electronic inpatient medication prescriptions and discharge prescriptions. Patients were included if the clinical diagnosis of bone and joint infection (osteomyelitis, septic arthritis, or both) was made by the treating team based on characteristic clinical, radiological, and microbiological findings. Acute osteomyelitis was defined as a clinical history of less than 2 weeks, and chronic osteomyelitis defined as a clinical history of more than 2 weeks.4 The subgroup of children with both osteomyelitis and septic arthritis has been included in the osteomyelitis group, in order to compare with similar groups in worldwide cohorts published in the literature.10,17 Demographics, diagnostic methods, results of microbiological investigations, treatment, and outcomes were recorded on the case report form. A patient was recorded as being from a remote community if they lived outside of the greater Darwin region (including Darwin city, Darwin suburbs, Litchfield, and Palmerston). “Full compliance” of antibiotics was recorded if the patient reported in the clinic notes that they took all of their discharge antibiotics, “partial noncompliance” if they had taken some, but not all of their antibiotics, and “complete noncompliance” if they reported taking none of their discharge antibiotics. “Relapse” was defined as an ongoing or worsening infection (as determined by the treating clinician) while the patient was still on their discharge antibiotics, or recurrence of infection in the same site by the same microorganism after antibiotics had been ceased.
Microbiological Methods
Bacterial cultures of blood, bone, and joint fluid were processed using standard methods. Organisms were identified phenotypically and confirmed using traditional methods or the Vitek2 gram positive card (bioMerieux, NC). Antibiotic susceptibility testing was performed using an automated system (Vitek2 AST-P612 card, bioMerieux) and the Kirby–Bauer disk diffusion method in accordance with the guidelines of the Clinical and Laboratory Standards Institute.18 Nonmultiresistant methicillin-resistant Staphylococcus aureus (nmMRSA) was defined as strains of S aureus that were resistant to cefoxitin, whereas multiresistant methicillin-resistant Staphylococcus aureus (mMRSA) was defined as S aureus that was resistant to ≥3 non-β-lactam antibiotic classes in addition to cefoxitin.19Neisseria gonorrhoeae was detected using traditional culture methods or the commercially available (polymerase chain reaction) PCR assay (VERSANT CT/GC DNA 1.0 Assay kPCR, Siemens, Victoria Australia).
A positive blood culture or joint aspirate was considered microbiological confirmation of the causative pathogen. Where neither of these was available, superficial swab results taken from a draining wound were used to infer the likely causative pathogen. Where more than 1 pathogen was identified, those isolated from blood culture or joint aspirate and those that were cultured more than once were considered significant, compared with those from superficial swabs or cultured only once. In the case of superficial swabs taken from a draining wound that cultured methicillin-resistant Staphylococcus aureus (MRSA) in addition to another microorganism, MRSA was targeted clinically in light of the high rates of MRSA in our region. We therefore identified MRSA as the causative organism in these cases.
Statistical Methods
Descriptive and regression statistics were performed using STATA13 (StataCorp, TX). Categorical data were tested using the χ2 test for dependence and means for continuous data were tested using a single-tailed Student t test, with a P-value <0.05 considered significant.
Population figures from the Australian Bureau of Statistics were used to calculate annual incidence rates of disease.7 We used the population figures given for children aged 0–17 years from the Royal Darwin Hospital catchment regions of Darwin, Daly, Tiwi, West Arnhem, East Arnhem, and Katherine to calculate the denominator data. The percentage indigenous population for each region was then used to calculate the denominator data to determine the incidence rate amongst the indigenous population.
Ethics Statement
Prospective approval for this audit was granted by the Human Research Ethics Committee of the Northern Territory Department of Health and Menzies School of Health Research (Human Research Ethics Committee 13–2020).
RESULTS
Epidemiology
One hundred and eight patients were identified with an ICD-10 discharge code for septic arthritis and/or osteomyelitis between 2010 and 2013. Of these, 11 charts were unavailable, 5 patients were more than 18 years old and 13 patients had an alternative diagnosis.
Seventy-nine patients met the inclusion criteria. Of those, 49 (62%) were male and the median age was 8 years (interquartile range [IQR]: 5–12 years) (Figure 1). Fifty-seven children (72%) had osteomyelitis, including 18 (32%) who had concomitant septic arthritis. Twenty-two patients (28%) had septic arthritis alone. indigenous Australian children accounted for 60/79 (76%) of presentations, of whom 92% were from remote communities (Table 1). The crude incidence of osteomyelitis (including those with contiguous septic arthritis) was 31 per 100,000 children; 90 per 100,000 for indigenous, and 9 per 100,000 for nonindigenous children (incidence rate ratio 10, 95% CI 5–23). The incidence of septic arthritis was 12 per 100,000 overall; 30 per 100,000 for indigenous, and 5 per 100,000 for nonindigenous children (incidence rate ratio 6: 95% CI 2–20) (Figure 2).
FIGURE 1.
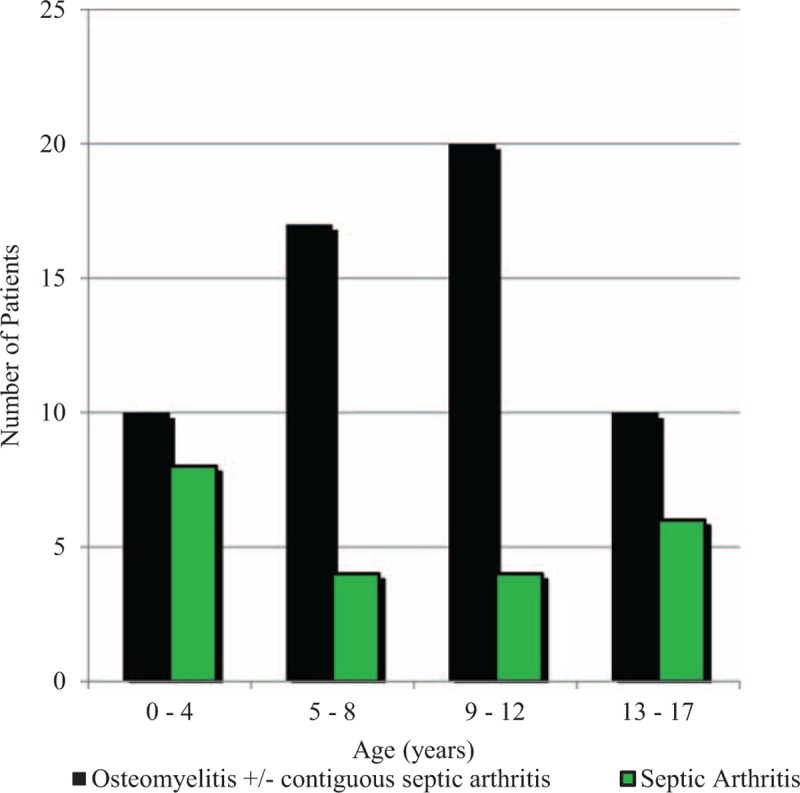
The age distribution of children admitted with bone and joint infections to Royal Darwin Hospital 2010 to 2013.
TABLE 1.
Clinical Characteristics of the Inpatient Episodes for Osteomyelitis and Septic Arthritis
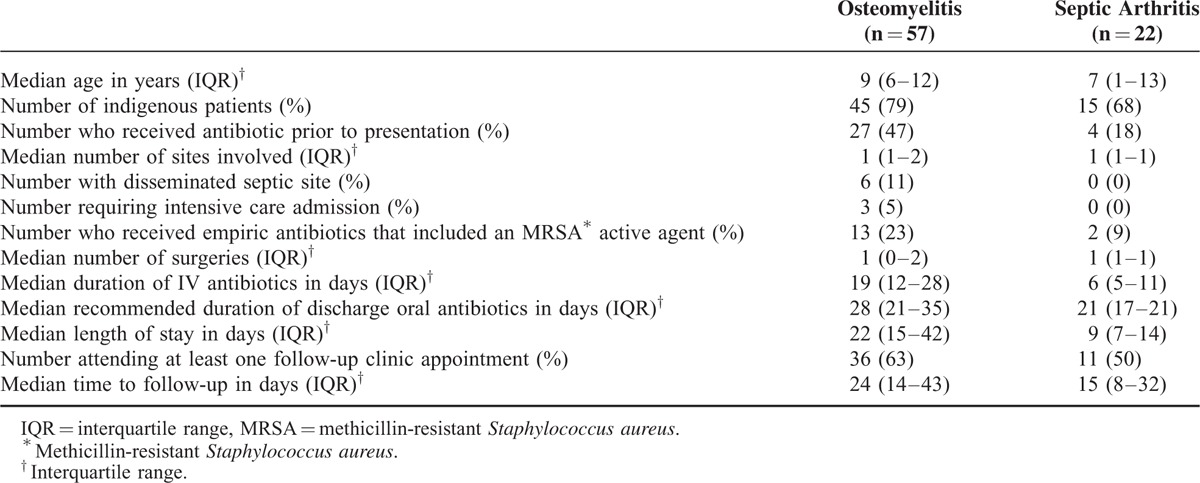
FIGURE 2.
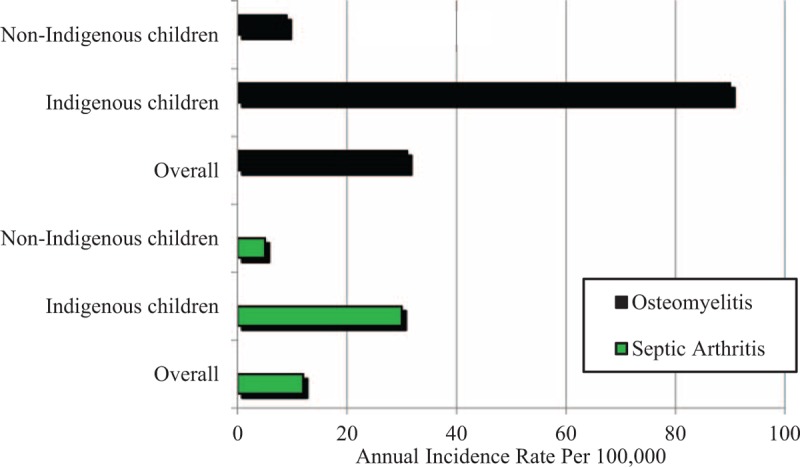
Crude incidence rate of osteomyelitis and septic arthritis per 100,000 population (0–17 year olds) between 2010 and 2013.
Clinical Presentation
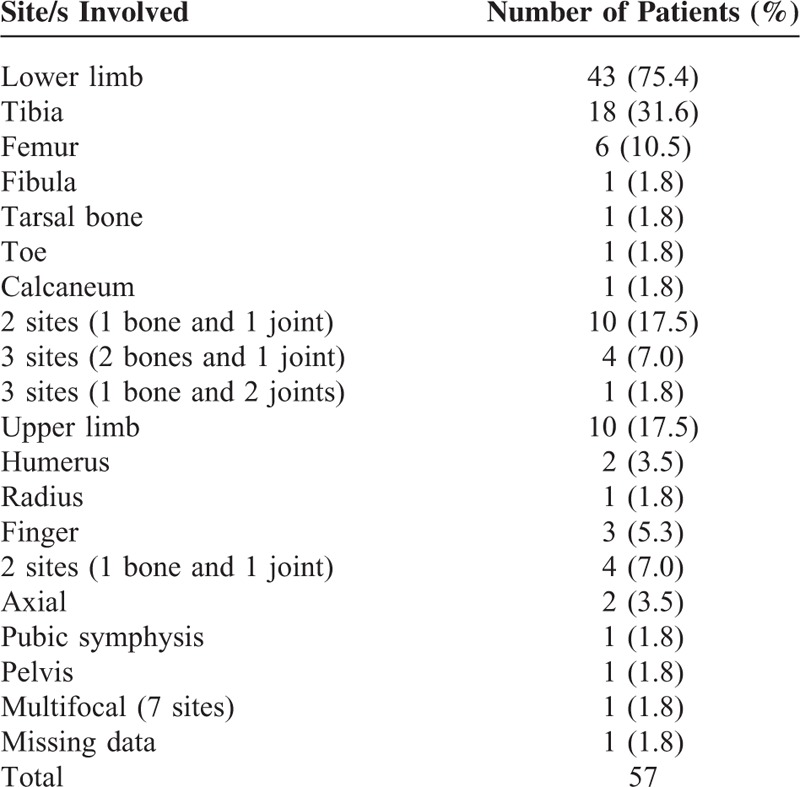
In the osteomyelitis group, 44% were managed by general orthopedic surgeons, and 56% by pediatricians. Median duration of symptoms prior to presentation was 5 days (IQR: 3, 12). Subjective fever was reported in 67%, localized pain in 95%, localized swelling in 72%, inability to weight bear in 54%, and joint immobility in 32%. The majority (56/57, 98%) had acute osteomyelitis. One patient presented with chronic osteomyelitis following acute osteomyelitis treated previously at another institution. All 39 patients with osteomyelitis alone involved a single bone. Eighteen patients with osteomyelitis (32%) had concomitant septic arthritis in at least 1 contiguous joint, the most severe case being 1 patient with no known immunodeficiency disorder who had multifocal bone and joint involvement of 7 separate sites. The lower limbs mainly involved were tibia (49%) and femur (21%) (Table 2). The majority of cases (70%) were secondary to hematogenous seeding, 16% were secondary to trauma, 12% were secondary to an overlying soft-tissue infection, and 2% were iatrogenic.
TABLE 2.
Site of Osteomyelitis (With and Without Concomitant Septic Arthritis)
Mean (± standard deviation) peak erythrocyte sedimentation rate (ESR) of those with osteomyelitis was 68 ± 37 mm/Hr, (n = 44) and mean (± standard deviation) peak C-reactive protein (CRP) was 143 ± 109 mg/L (n = 57).
At presentation, 6/57 (11%) patients with osteomyelitis had another disseminated site of septic involvement apart from bone or joint such as pneumonia (2), muscle abscess (2), pneumonia and muscle abscess (1) endocarditis (1), and one in which the distal septic site was not recorded. Two had nmMRSA, 1 had MSSA, 1 had Burkholderia pseudomallei, 1 had Group A Streptococcus, and 1 did not have an organism identified after extensive investigation (including mycobacterial and fungal cultures). Three patients with osteomyelitis (5%) required intensive care unit admission at presentation.
Of the patients presenting with septic arthritis alone 45% were looked after by a general orthopedic surgeon, 50% by a pediatrician, and 5% by an adult general medical team. Median duration of symptoms prior to presentation was 6 days (IQR: 4, 8). Subjective fever was reported in 64%, localized pain in 91%, localized swelling in 68%, inability to weight bear in 82%, and joint immobility in 27%. Monoarticular septic arthritis occurred in 17/22 (77%) patients and was most common in the knee (35%) (Table 3). Mean (± standard deviation) peak ESR was 49 ± 30 mm/Hr (n = 15) and mean (± standard deviation) peak CRP was 100 ± 76 mg/L (n = 22). No patients with septic arthritis had other septic foci, or required intensive care unit admission.
TABLE 3.
Site of Septic Arthritis

Microbiological Diagnosis
Overall, 57/79 patients (72%) had a positive microbiological diagnosis, including 44/57 (77%) of those with osteomyelitis. Culture of blood was the most common confirmatory specimen in the osteomyelitis group (n = 26, 59%) followed by bone puncture (n = 14, 31%) and superficial swabs taken from a draining wound (n = 4, 9%). There were 8 patients who cultured more than 1 organism. One patient who grew nmMRSA and group G Streptococcus from superficial swabs, and group G Streptococcus from a deep operative specimen was, however, analyzed in the nmMRSA group, as nmMRSA was targeted in the treatment regimen and group G Streptococcus is a very rare cause of pediatric bone and joint disease.20S aureus was the dominant causative organism (40/57, 70%), with 40% (16/40) of these cases being nmMRSA (Figure 3).
FIGURE 3.
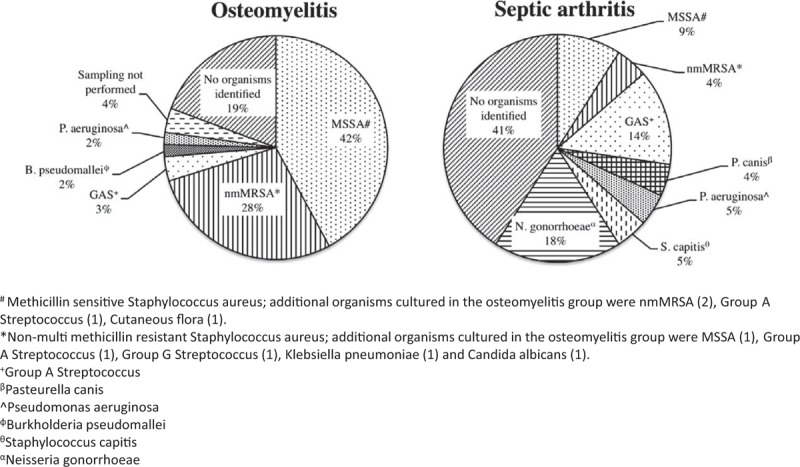
Microbiological causes of osteomyelitis and septic arthritis. #Methicillin-sensitive Staphylococcus aureus; additional organisms cultured in the osteomyelitis group were nonmultimethicillin-resistant S aureus (2), group A. Streptococcus (1), cutaneous flora (1). ∗Nonmultimethicillin-resistant S aureus; additional organisms cultured in the osteomyelitis group were methicillin-sensitive S aureus (1), group. A Streptococcus (1), group G Streptococcus (1), Klebsiella pneumoniae (1), and Candida albicans (1). +Group A Streptococcus. βPasteurella canis. ^Pseudomonas aeruginosa. ϕBurkholderia pseudomallei. θStaphylococcus capitis. αNeisseria gonorrhoeae.
Of 22 patients with septic arthritis alone, 13/22 (59%) had a positive microbiological diagnosis (Figure 3). The most common site for microbiological yield was in sampling of the affected joint in 10/13 (77%) of patients, whereas the remaining 3/13 (23%) had positive blood cultures.
Four adolescent patients presented with polyarticular septic arthritis and were found to have disseminated gonococcal infection. All 4 joints were culture negative and the diagnosis was confirmed using urine PCR (n = 3) or urine culture (n = 1) for N gonorrhoeae. N gonorrhoeae was in fact the most common microbiological diagnosis for septic arthritis alone.
MANAGEMENT
Clinical management of patients with osteomyelitis and septic arthritis, including duration of intravenous and discharge antibiotics, surgical management and length of stay is summarized in Table 1. Flucloxacillin was the most commonly used empiric antibiotic in both groups. It was used in 44/57 (77%) of osteomyelitis patients (25% in combination with at least 1 other agent), and in 15/22 (68%) septic arthritis patients (13% with at least 1 other agent). An MRSA active agent was used empirically in 23% of osteomyelitis patients overall, in 50% of those with MRSA osteomyelitis and 9% of those with septic arthritis. For those with MRSA who were initially started on a β-lactam, there was a median of 1 day (IQR: 1, 2) of ineffective therapy before being placed on an MRSA active agent.
The most common discharge antibiotics prescribed for those with osteomyelitis were trimethoprim–sulfamethoxazole (53%) and flucloxacillin (21%), and for septic arthritis were amoxicillin (18%), flucloxacillin (14%), trimethoprim/sulfamethoxazole (14%), and amoxicillin/clavulanic acid (14%). Hospital in the Home has previously been shown to be both feasible and effective in our population21 and was used for 13/79 (16%) of patients.
Almost 70% of those with osteomyelitis underwent at least 1 surgery for washout and debridement, with 79% of those with MSSA, 88% of those with nmMRSA, 100% of those with B pseudomallei and 0% of those with group A Streptococcus undergoing at least 1 surgical procedure. Likewise, surgical drainage and washout were performed in 17/22 (77%) of patients with septic arthritis. No patients with N gonorrhoeae underwent surgery, whereas all of the patients with MSSA, nmMRSA, group A Streptococcus, Pasteurella canis, and Pseudomonas aeruginosa septic arthritis underwent at least 1 surgical washout.
Follow-Up
For those with osteomyelitis, 36/57 (63%) attended at least 1 follow-up clinic appointment. Median time from discharge to follow-up was 24 days (IQR, 14–43 days). At follow-up, 19 (53%) were assessed as having normal function of the affected limb or region, 4 (11%) had difficulty weight bearing, another 4 (11%) had limited range of movement, and 9/36 (25%) did not have any documentation of their functional status. For these 8 patients with abnormal function documented at follow-up, 2/8 (25%) were recorded as being fully compliant with their discharge antibiotics, 3/8 (38%) used Hospital in the Home to continue their intravenous antibiotics, 5/8 (63%) were indigenous, and 5/8 (63%) relapsed. Causative pathogens were: nmMRSA (4/8, 50%), MSSA (3/8, 38%), and B pseudomallei (1/8, 12%). Of the 19 patients in whom there was documentation of medication compliance, 12/19 (63%) reported full compliance with their discharge antibiotics, 3/19 (16%) reported partial noncompliance, and 4/19 (21%) reported complete noncompliance.
Half of the patients (11/22) with septic arthritis were seen in follow-up clinic, with a median time post discharge to follow-up of 15 days (IQR, 8–32). Function was assessed as normal in 10/11 (91%) and 1/11 (9%) had difficulty weight bearing. Full compliance with discharge antibiotics was seen in 6/11 (55%), 1/11 had partial noncompliance (9%), and 4/11 (36%) did not have compliance status recorded.
Relapsed Versus Nonrelapsed Group
Despite lengthy admissions for intravenous antibiotics, 15% (12/79) of the cohort had a documented relapse of their bone and joint infection, all of whom required a further course of antibiotics, and 75% (9/12) of these relapsed patients required readmission to hospital. Seven patients had acute osteomyelitis and septic arthritis, 2 had acute osteomyelitis, 1 had chronic osteomyelitis, and 2 had septic arthritis. All relapses occurred within 13 months, with half of these (6/12) relapsing within 30 days of hospital discharge. Omitting 3 patients in whom ethnicity details were not recorded, there was no statistical difference (P value = 0.58) in relapse rates for indigenous (13%) and nonindigenous children (19%). Of those who relapsed 33% were documented to have been compliant with their previously prescribed course of discharge oral antibiotics, 25% were partially compliant, 25% were fully noncompliant, and 17% did not have compliance data recorded.
The clinical characteristics of those who relapsed compared with the nonrelapsed group are presented in Table 4. Bacteremia at presentation, >1 site of bone and joint infection, another site of disseminated foci of infection, and >1 surgical procedure were all found to significantly increase the chance of relapse in our population. We were unable to analyze whether documented compliance with discharge antibiotics increased this risk as only 33% of those who did not relapse had compliance data recorded in their notes.
TABLE 4.
Clinical Characteristics of Those Who Relapsed Versus Nonrelapsed Group
DISCUSSION
Our study reveals one of the highest incidences of osteoarticular infections reported in the world. In particular, the calculated incidence rate of osteomyelitis among indigenous children (90 per 100,000) well surpasses other reported incidence rates of 3 to 13 per 100,000 in high-income countries.4 Subgroup analysis of the nonindigenous children reveals an incidence rate (9 per 100,000) on par with other international studies. This implies that the excess disease burden lies firmly among our indigenous children, which is a finding that has been repeatedly reported in previous studies of this population.5,22
The exact cause of such high incidence rates of bone and joint infections among our indigenous pediatric population is not definitively known. We propose hematogenous seeding from recurrent skin infections as a possible link.4 Skin infections are endemic within Australian indigenous children,23 with prevalence rates of skin sores and scabies up to 70% and 50%, respectively in some communities.24 Impetigo has been identified as a risk factor for bacteremia and bone and joint infections in indigenous people living in the NT.25–27 The prevalence of impetigo in indigenous children of Australia, is among the highest reported in the world with median prevalence in children of 43% (IQR 40%–46%).28 Unfortunately, documentation of skin infection at the time of admission was poorly recorded in our study. This may represent the normalization of high rates of skin infection in indigenous Australians and a lack of recognition by medical staff of the entry point for bone and joint infections.
Lower socioeconomic status has previously been associated with higher rates of osteomyelitis,29 although this link has not been consistently shown in subsequent studies.3 Factors associated with lower socioeconomic status, such as overcrowding, poor hygiene, and sanitation have been proposed as causes of increased infection rates, and have been repeatedly documented in Australian indigenous communities.24,30,31 A recent local study demonstrated a link between the incidence of S aureus bacteremia and socioeconomic status.32
Genetic predisposition to increased susceptibility for infection has been suggested as a possible explanation of the higher infection rates seen in indigenous populations32,33; however, current published evidence is lacking to confirm or disprove this, and further studies are required.
We describe a cohort of children with more severe disease than those reported in other settings. Total 30% of children had more than 1 bone or joint involved, compared with 4.5% in a New Zealand series.10 Total 32% had osteomyelitis with contiguous septic arthritis, compared with 18.1% with contiguous septic arthritis in a study from Finland.13 Mean maximum CRP in our cohort was 143 ± 109 mg/L, which is higher than the maximum CRP of approximately 100 mg/L that was reported in the study from Finland.13 Median duration of intravenous antibiotics was 19 days, which is considerably longer than the 1 to 4 days recommended in other studies.2,13 We believe that this represents severity of disease rather than clinician preference as the decision to switch to oral therapy is usually based on clinical and biochemical response to treatment. Surgical management of osteomyelitis at our institution is recommended when ongoing fevers, pain, and/or imaging confirm a purulent collection in need of drainage. Total 68% of our osteomyelitis group underwent at least 1 surgical washout or debridement, compared with 44% of the New Zealand series10 and 34% of the series from Sydney.17 Although 76% of the participants from the Finland trial had a surgical procedure performed a proportion of those reported (26%) were purely diagnostic procedures.13 Despite the intensive surgical and antibiotic treatment provided, we report a relatively high relapse rate of 15%, compared with 6.8% in New Zealand,10 1.5% in Finland13, and 25% in Cambodia.34 This may relate to the late stage at presentation. Our reported median duration of symptoms of 5 days prior to admission is longer than other published reports.17,35 It may also reflect the low rate of early use of MRSA active antibiotics, despite the high rate of detection of MRSA in this cohort. We did not, however, find a higher relapse rate in the group identified to have an MRSA infection.
Among our staphylococcal osteomyelitis cases, 40% were due to nmMRSA (28% of osteomyelitis cases overall). This is a significantly higher rate than 9% reported in a study from Sydney, Australia in 2005,17 2% from a New Zealand study in 2014,10 and 13% to 24% from Taiwan in 2009.36 Our rates approach those reported in parts of the United States, where MRSA accounted for more than 50% of staphylococcal bone and joint infections seen in their pediatric population.37
Community-associated MRSA rates have been increasing over recent years in Australia,38 and have consistently been reported as higher within the indigenous population.32,39,40 There is a high prevalence of risk factors for nmMRSA in the indigenous communities,31 similarly described in other indigenous populations around the world where high rates of nmMRSA carriage are also found.41 Our high reported rates of nmMRSA may herald emergence of nmMRSA as an important causative pathogen for pediatric bone and joint infections elsewhere in Australia. This has important implications for the national guidelines regarding empirical antibiotics, which currently recommend flucloxacillin alone.14 There are also important clinical implications, as bone and joint infections with nmMRSA have been reported as causing more severe disease.37
N gonorrhoeae was the causative organism in 5% of bone and joint infections in our cohort, and accounted for 80% of polyarticular septic arthritis seen, all in adolescents. The differential diagnosis for the presentation of painful, swollen joints in this cohort is acute rheumatic fever (ARF), as northern Australia has one of the highest reported rates of ARF in the world.42 High incidence rates of N gonorrhoeae have also previously been described in the adolescent indigenous population,43 and our results highlight the importance of having a high index of suspicion for gonorrhoeae, particularly when adolescents present with polyarticular disease and ARF may be the first suspected diagnosis.
We had 1 case of osteomyelitis secondary to B pseudomallei, which is endemic in our region and known to cause bone and joint infections.44 The patient had primary involvement of the hip and pubic symphysis, required 3 surgical washouts and developed an associated muscle abscess.
There were several limitations to our study. Our study included small numbers, and derives from a population, which is unique in its tropical climate, ethnic mix, and socioeconomic demographic, which may limit the generalizability of our findings. Despite this, our findings were in contrast to the pediatric literature on severity, length of treatment, and relapse. Being a retrospective study in design, some data were incomplete or missing, including much of the follow-up data pertaining to compliance with discharge antibiotics. We were, therefore, unable to draw correlations between outcomes and the oral antibiotic used, or total duration of antibiotics.
In conclusion, we report an ethnic discrepancy in incidence of bone and joint infections in our populations, with a 10-fold higher rate of osteomyelitis in indigenous compared with nonindigenous children. It is unclear whether environmental or genetic factors account for this, but it is likely the high skin disease burden contributes.28 The disease entity we describe appears to be more aggressive and severe than those found in other studies, requiring prolonged treatment and multiple surgical interventions. Despite this, we found high relapse rates compared with some other studies. Nonmultiresistant methicillin-resistant S aureus rates were also high, leading to concerns regarding its emergence as a causative pathogen in the rest of Australia, and appropriate empirical antibiotic choice. Overall, this is different from the clinical experience reported in the recent literature10,13,17 and suggests changes to guidelines recommending shorter durations of IV antibiotics may not be applicable in this context. We, therefore, conclude that adopting the recent recommendation of using only 3 to 7 days of IV antibiotics in our population would result in even higher morbidity than reported here. We recommend continuing to use IV antibiotics as currently practiced in our population, and to consider longer durations in those patients with more than 1 site of bone and joint infection, who are bacteremic at presentation, with another site of septic focus or who have more than 1 surgical procedure who are identified as being at higher risk of relapse. Further longitudinal studies are recommended to determine the long-term outcomes of these children, and strategies to reduce the high morbidity of this disease in our population are needed. More research is needed to define the appropriate length of therapy in this context.
Acknowledgments
The authors thank Bart Currie and Steve Tong for their critical review of this article, and our statistician Michael Shakhovskoy.
Footnotes
Abbreviations: IQR = interquartile range, mMRSA = multiresistant methicillin-resistant Staphylococcus aureus, MRSA = methicillin-resistant Staphylococcus aureus, MSSA = methicillin-sensitive Staphylococcus aureus, nmMRSA = nonmultiresistant methicillin-resistant Staphylococcus aureus, NT = Northern Territory, SD = Standard deviation, WHO ICD-10 = World Health Organization International Classification of Diseases.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Riise Ø, Kirkhus E, Handeland KS, et al. Childhood osteomyelitis-incidence and differentiation from other acute onset musculoskeletal features in a population-based study. BMC Pediatr 2008; 8:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dartnell J, Ramachandran M, Katchburian M. Haematogenous acute and subacute paediatric osteomyelitis: a systematic review of the literature. J Bone Joint Surg Br 2012; 94:584–595. [DOI] [PubMed] [Google Scholar]
- 3.Blyth MJ, Kincaid R, Craigen MA, et al. The changing epidemiology of acute and subacute haematogenous osteomyelitis in children. J Bone Joint Surg Br 2001; 83:99–102. [DOI] [PubMed] [Google Scholar]
- 4.Pääkkönen M, Peltola H. Acute osteomyelitis in children. N Engl J Med 2014; 370:1365–1366. [DOI] [PubMed] [Google Scholar]
- 5.Gillespie WJ. The epidemiology of acute haematogenous osteomyelitis of childhood. Int J Epidemiol 1985; 14:600–606. [DOI] [PubMed] [Google Scholar]
- 6.Britton PN, Andresen DN. Paediatric community-associated Staphylococcus aureus: a retrospective cohort study. J Paediatr Child Health 2013; 49:754–759. [DOI] [PubMed] [Google Scholar]
- 7.Australian Bureau of Statistics-Census Data 2011. Available at: http://www.censusdata.abs.gov.au/census_services/getproduct/census/2011 Accessed 17 February, 2015 [Google Scholar]
- 8.Gwee A, Carapetis JR, Buttery J, et al. Formal infectious diseases consultations at a tertiary pediatric hospital: a 14-year review. Pediatr Infect Dis J 2014; 33:411–413. [DOI] [PubMed] [Google Scholar]
- 9.Gutierrez K. Bone and joint infections in children. Pediatr Clin North Am 2005; 52:779–794.vi. [DOI] [PubMed] [Google Scholar]
- 10.Street M, Puna R, Huang M, et al. Pediatric acute hematogenous osteomyelitis. J Pediatr Orthop 2015; 35:634–639. [DOI] [PubMed] [Google Scholar]
- 11.Kiang KM, Ogunmodede F, Juni BA, et al. Outbreak of osteomyelitis/septic arthritis caused by Kingella kingae among child care center attendees. Pediatrics 2005; 116:e206–e213. [DOI] [PubMed] [Google Scholar]
- 12.Jagodzinski NA, Kanwar R, Graham K, et al. Prospective evaluation of a shortened regimen of treatment for acute osteomyelitis and septic arthritis in children. J Pediatr Orthop 2009; 29:518–525. [DOI] [PubMed] [Google Scholar]
- 13.Peltola H, Pääkkönen M, Kallio P, et al. Group O-SAS Short- versus long-term antimicrobial treatment for acute hematogenous osteomyelitis of childhood: prospective, randomized trial on 131 culture-positive cases. Pediatr Infect Dis J 2010; 29:1123–1128. [DOI] [PubMed] [Google Scholar]
- 14.Therapeutic Guidelines: Antibiotic Version 15. 15th ed.Melbourne: Therapeutic Guidelines Limited; 2014. [Google Scholar]
- 15.Steer AC, Carapetis JR. Acute hematogenous osteomyelitis in children: recognition and management. Paediatr Drugs 2004; 6:333–346. [DOI] [PubMed] [Google Scholar]
- 16.Australian Bureau of Meteorology-Northern Territory in 2014: Year of Extremes, but Average Overall, 2014. Available at: http://www.bom.gov.au/climate/current/annual/nt/summary.shtml, Accessed 3 August, 2015 [Google Scholar]
- 17.Goergens ED, McEvoy A, Watson M, et al. Acute osteomyelitis and septic arthritis in children. J Paediatr Child Health 2005; 41:59–62. [DOI] [PubMed] [Google Scholar]
- 18.Cockerill FR, Wikler MA, Alder J, et al. , Performance standards for antimicrobial susceptibility testing, Twenty-Second Informational Supplement. Clinical and Laboratory Standards Institute. 12010: 23–24. [Google Scholar]
- 19.Tong SY, Bishop EJ, Lilliebridge RA, et al. Community-associated strains of methicillin-resistant Staphylococcus aureus and methicillin-susceptible S. aureus in indigenous Northern Australia: epidemiology and outcomes. J Infect Dis 2009; 199:1461–1470. [DOI] [PubMed] [Google Scholar]
- 20.Ferroni A, Al Khoury H, Dana C, et al. Prospective survey of acute osteoarticular infections in a French paediatric orthopedic surgery unit. Clin Microbiol Infect 2013; 19:822–828. [DOI] [PubMed] [Google Scholar]
- 21.White HA, Davis JS, Kittler P, et al. Outpatient parenteral antimicrobial therapy-treated bone and joint infections in a tropical setting. Intern Med J 2011; 41:668–673. [DOI] [PubMed] [Google Scholar]
- 22.Morgan DS, Fisher D, Merianos A, et al. An 18 year clinical review of septic arthritis from tropical Australia. Epidemiol Infect 1996; 117:423–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clucas DB, Carville KS, Connors C, et al. Disease burden and health-care clinic attendances for young children in remote aboriginal communities of northern Australia. Bull World Health Organ 2008; 86:275–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Currie BJ, Carapetis JR. Skin infections and infestations in aboriginal communities in northern Australia. Australas J Dermatol 2000; 41:139–143.quiz 144-135. [DOI] [PubMed] [Google Scholar]
- 25.Skull SA, Krause V, Coombs G, et al. Investigation of a cluster of Staphylococcus aureus invasive infection in the top end of the Northern Territory. Aust N Z J Med 1999; 29:66–72. [DOI] [PubMed] [Google Scholar]
- 26.Gear RJ, Carter JC, Carapetis JR, et al. Changes in the clinical and epidemiological features of group A streptococcal bacteraemia in Australia's Northern territory. Trop Med Int Health 2015; 20:40–47. [DOI] [PubMed] [Google Scholar]
- 27.Carapetis JR, Walker AM, Hibble M, et al. Clinical and epidemiological features of group A streptococcal bacteraemia in a region with hyperendemic superficial streptococcal infection. Epidemiol Infect 1999; 122:59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bowen AC, Mahé A, Hay RJ, et al. The Global epidemiology of impetigo: a systematic review of the population prevalence of impetigo and pyoderma. PLoS One 2015; 10:e0136789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.White M, Dennison WM. Acute haematogenous osteitis in childhood. J Bone Joint Surg Br 1952. 608–623.34-B. [DOI] [PubMed] [Google Scholar]
- 30.McDonald MI, Towers RJ, Andrews RM, et al. Low rates of streptococcal pharyngitis and high rates of pyoderma in Australian aboriginal communities where acute rheumatic fever is hyperendemic. Clin Infect Dis 2006; 43:683–689. [DOI] [PubMed] [Google Scholar]
- 31.Bailie RS, Stevens MR, McDonald E, et al. Skin infection, housing and social circumstances in children living in remote Indigenous communities: testing conceptual and methodological approaches. BMC Public Health 2005; 5:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tong SY, van Hal SJ, Einsiedel L, et al. Impact of ethnicity and socio-economic status on Staphylococcus aureus bacteremia incidence and mortality: a heavy burden in indigenous Australians. BMC Infect Dis 2012; 12:249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gillespie WJ. Racial and environmental factors in acute haematogenous osteomyelitis in New Zealand. N Z Med J 1979; 90:93–95. [PubMed] [Google Scholar]
- 34.Stoesser N, Pocock J, Moore CE, et al. The epidemiology of pediatric bone and joint infections in Cambodia. J Trop Pediatr 2013; 59:36–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mitha A, Boutry N, Nectoux E, et al. Community-acquired bone and joint infections in children: a 1-year prospective epidemiological study. Arch Dis Child 2015; 100:126–129. [DOI] [PubMed] [Google Scholar]
- 36.Chen WL, Chang WN, Chen YS, et al. Acute community-acquired osteoarticular infections in children: high incidence of concomitant bone and joint involvement. J Microbiol Immunol Infect 2010; 43:332–338. [DOI] [PubMed] [Google Scholar]
- 37.Arnold SR, Elias D, Buckingham SC, et al. Changing patterns of acute hematogenous osteomyelitis and septic arthritis: emergence of community-associated methicillin-resistant Staphylococcus aureus. J Pediatr Orthop 2006; 26:703–708. [DOI] [PubMed] [Google Scholar]
- 38.Coombs GW, Daly DA, Pearson JC, et al. Community-onset Staphylococcus aureus Surveillance Programme annual report. Commun Dis Intell Q Rep 2014; 38:E59–E69. [PubMed] [Google Scholar]
- 39.Tong SY, Varrone L, Chatfield MD, et al. Progressive increase in community-associated methicillin-resistant Staphylococcus aureus in Indigenous populations in northern Australia from 1993 to. Epidemiol Infect 2015; 143:1519–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Williamson DA, Coombs GW, Nimmo GR. Staphylococcus aureus ’Down Under’: contemporary epidemiology of S. aureus in Australia, New Zealand, and the South West Pacific. Clin Microbiol Infect 2014; 20:597–604. [DOI] [PubMed] [Google Scholar]
- 41.Ofner-Agostini M, Simor AE, Mulvey M, et al. Methicillin-resistant Staphylococcus aureus in Canadian aboriginal people. Infect Control Hosp Epidemiol 2006; 27:204–207. [DOI] [PubMed] [Google Scholar]
- 42.Parnaby MG, Carapetis JR. Rheumatic fever in indigenous Australian children. J Paediatr Child Health 2010; 46:527–533. [DOI] [PubMed] [Google Scholar]
- 43.Miller PJ, Law M, Torzillo PJ, et al. Incident sexually transmitted infections and their risk factors in an aboriginal community in Australia: a population based cohort study. Sex Transm Infect 2001; 77:21–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morse LP, Smith J, Mehta J, et al. Osteomyelitis and septic arthritis from infection with Burkholderia pseudomallei: a 20-year prospective melioidosis study from northern Australia. J Orthop 2013; 10:86–91. [DOI] [PMC free article] [PubMed] [Google Scholar]


