Abstract
Anastomotic leakage (AL) is one of the most serious complications of colorectal surgery. It can affect long-term oncologic outcomes, but the impact on long-term survival remains uncertain. The aim of this study is to evaluate the operative characteristics of leakage and no leakage groups and to analyze long-term oncologic outcomes.
We prospectively enrolled 10,477 patients from 2000 to 2011 and retrospectively reviewed the data.
Male sex (odds ratio [OR], 3.90; P < 0.001), intraoperative transfusion (OR, 2.31; P = 0.042), and operative time (OR, 1.73; P = 0.032) were independent risk factors of AL in the colon. In the rectum, male sex (OR, 2.37; P < 0.001), neoadjuvant chemoradiotherapy (OR, 2.26; P < 0.001), and regional lymph node metastasis (OR, 1.43; P = 0.012) were independent risk factors of AL, and diverting stoma (OR, 0.24; P < 0.001) was associated with a deceased risk of AL. AL in the rectum without a diverting stoma was associated with disease-free survival (DFS, OR, 1.47; P = 0.037). Colonic leakage was not associated with 5-year DFS (leakage group vs nonleakage group, 72.4% vs 80.9%, P = 0.084); however, in patients undergoing rectal resection, there was a significant difference in 5-year DFS (67.0% vs 76.6%, P = 0.005, respectively).
AL in the rectum is associated with worse long-term DFS and overall survival. A diverting stoma was shown to protect against this effect and was associated with long-term survival in rectal surgery. Therefore, creating a diverting stoma should be considered in high-risk patients undergoing rectal surgery.
INTRODUCTION
Anastomotic leakage (AL) is one of the most frequent postoperative complications following colorectal surgery. Most AL is considered a major postoperative complication, and its severity is greater than any other complication.1 The rates of AL in colorectal surgery vary widely depending on several factors, particularly whether the anastomosis is intra- or extraabdominal (2.7–8.7% and 3.6–13.3%, respectively).1–13
Several studies have evaluated a variety of risk factors of AL; however, there is no universal agreement regarding the associated risk factors. Although it is generally accepted that variables such as low anastomotic level are associated with leakage,14–16 other risk factors including male sex and neoadjuvant chemoradiotherapy (nCRT) are not widely recognized.8 These risk factors were analyzed in the whole cohort in the present study, regardless of the anastomosis site. Although many studies have investigated risk factors of AL, it remains a life-threatening complication that can arise in patients with no known risk factors.
In addition, the few studies on long-term oncologic outcomes in patients with AL have demonstrated consistent results.1,3,17,18 In rectal cancer patients, AL has been associated with an increased rate of local recurrence, but its relationship with distant metastasis is unclear. In colorectal cancer patients, long-term mortality is related to AL.18 In contrast, AL in rectal surgery has not been shown to associate with overall survival (OS) or disease-free survival (DFS).1 The relationship between AL and long-term prognosis remains unclear.
The aims of this study were to evaluate preoperative and intraoperative risk factors of clinically significant AL in patients undergoing surgery involving colonic and rectal anastomosis. Secondary aims were to investigate the impact of AL on long-term outcome after surgical resection for colonic and rectal cancer.
METHODS
The medical records of 12,066 consecutive patients who underwent surgery for colorectal disease from January 2000 to December 2011 were analyzed. Patients who had an ostomy operation such as Hartmann's operation, diverting ileostomy, colostomy, or abdominoperineal resection were excluded. A total of 10,477 patients who underwent colorectal operations with ileocolonic, colocolonic, colorectal, ileorectal, or colo-anal anastomosis were enrolled in this study. Most patients (97.9%) had colorectal cancer, and only 222 patients (2.1%) had benign disease.
This study was a retrospective analysis of our prospectively collected colorectal surgery database. The following demographic data were collected and analyzed: age, sex, American Society of Anesthesiologists score, body mass index, preoperative carcinoembryonic antigen (CEA) level, underlying disease, previous abdominal surgery, preoperative treatment, clinical bowel obstruction, preoperative stenting, clinical bowel perforation, type of operation, level of anastomosis, route of access, emergency operation, diverting stoma, operative time, transfusion during operation, postoperative mortality, length of hospital stay, readmission rate, pathologic features (benign or malignant, T stage, N stage, M stage, lymphovascular invasion, perineural invasion, and harvested lymph nodes), and adjuvant therapy. Colorectal cancer was staged according to the 7th American Joint Committee on Cancer TNM staging system. Five-year DFS and 5-year OS were assessed. Survival analysis was performed only in patients whose surgery was intended to be curative. Thus, the cohort for survival analysis excluded patients who underwent surgery for benign disease or palliative resection. This study was approved by the Institutional Review Board of Samsung Medical Center.
We defined AL as any defect in intestinal wall integrity at the colo-colonic, colorectal, or colo-anal anastomotic site (including suture and staple lines of neorectal reservoirs) leading to a communication between the intra- and extraluminal compartments.19 A pelvic abscess or abscess close to the anastomosis was also considered AL. Clinically, AL was suspected when the patient showed signs or symptoms of clinical peritonitis, including leukocytosis and abdominal pain associated with a pelvic abscess or fecal discharge through the intraabdominal drainage catheter. Abdominopelvic computed tomography (CT) was routinely performed in patients suspected of AL. Additionally, orders for fasting and administration of antibiotics were applied in these patients at our institution.20
nCRT was considered the optimal management strategy for patients with locally advanced (T3/T4 or N positive) rectal cancer located <10 cm from the anal verge, as diagnosed by abdominopelvic CT and/or pelvic MRI for local staging. nCRT was not administered to elderly patients, those with poor performance status, and/or those who refused nCRT. Preoperative radiotherapy was delivered to the whole pelvis at a dose of 45 Gy in 25 fractions. Preoperative chemotherapy consisted of a bolus injection of 5-fluorouracil, 500 mg/m2 per day 3 days per cycle during the first and last weeks of radiotherapy or capecitabine, and 850 mg/m2 per day 5 days per week for 5 weeks.21
Five-fluorouracil-based chemo-regimen was preferentially considered as adjuvant chemotherapy to the patients proven as T3, T4, or node-positive disease after surgical resection. But, it was depended on the patient's general condition, compliance, and physician preference. Patients were underwent postoperative follow-up at 3-month intervals for 2 years, at 6-month intervals for the next 3 years, and annually thereafter. During follow-up period, clinical history, physical examination, serum CEA assay, chest and abdominopelvic CT or MRI, and colonoscopy were evaluated for detecting recurrence. Additional histologic biopsy examination was performed for confirming recurrence, if it would be needed.
Data were analyzed with Statistical Package for the Social Sciences for Windows, version 18.0 (SPSS Inc., Chicago, IL). The clinicopathologic features between 2 groups were compared using a Chi-squared test or t-test. Survival analysis was performed by the Kaplan–Meier method, and prognostic factors and survival curves were compared using log-rank test. A P value ≤0.05 was considered statistically significant. All variables related to the risk of DFS or OS with a P value inferior to 0.1 in univariate analysis were included in a multivariate analysis using the Cox regression model.
RESULTS
From 2000 to 2011, 10,477 patients were included in this study, and the median follow-up period was 39 months (mean follow-up period: 45.4 months). The number of patients undergoing each type of surgery was as follows: right hemicolectomy, 2356 (22.5%); left hemicolectomy, 471 (4.5%); anterior resection, 3260 (31.1%); low anterior resection, 3854 (36.8%); and other operations including segmental colon resection, total or subtotal colectomy, and total proctocolectomy, 536 (5.1%). Most patients (95.9%) underwent elective surgery.
The AL rate of the whole cohort was 2.8% (290 of 10,477). AL occurred in 71 (1.1%) of the 6565 patients whose anastomoses were in the colon (intraperitoneal anastomosis) and in 219 (5.6%) of the 3912 patients whose anastomoses were in the rectum (extraperitoneal anastomosis). Significant associations in the AL group included younger age (57 vs 59, P = 0.002), male sex (80.3% vs 59.7%, P < 0.001), preoperative nCRT (19.4% vs 6.8%, P < 0.001), low anterior resection (73.1% vs 35.8%, P < 0.001), longer operative time (149 vs 139 minutes, P < 0.001), increased mortality rate (1.7% vs 0.3%, P = 0.001), longer hospital stay (18.0 vs 8.0 days, P < 0.001), higher readmission rate (24.5% vs 6.7%, P < 0.001), and increased spread to lymph nodes (50.3% vs 43.8%, P = 0.043). There was no statistically significant difference in local or distant recurrence in the colon or rectum between the 2 groups (Table 1).
TABLE 1.
Clinicopathologic Features (N = 10,477)

The median length of time from operation to AL was 5 days (range, 1–85). Most patients in the leakage group (96.9%) suffered from AL within 30 days after operation. In the AL group, 30 patients (42.3%) who had intraabdominal anastomosis were treated with nonsurgical intervention. In comparison, 181 (82.6%, P < 0.001) patients in the AL group with anastomosis in the rectum were treated with surgical intervention.
Multivariate analysis identified the following patient factors associated with AL in the colon: male sex (P < 0.001), intraoperative transfusion (P = 0.042), and longer operative time (P = 0.032). In the rectal anastomosis group, male sex (P < 0.001), nCRT (P < 0.001), diverting stoma (P < 0.001), and pathologic N stage (P = 0.012) were independent risk factors of AL. In rectal anastomosis patients without diverting stoma, the factors associated with AL included male sex (P < 0.001) and nCRT (P < 0.001). Tumor distance from the anal verge (>5.0/≤5.0 cm) was not associated with AL (odds ratio [OR], 0.98; P = 0.885) (Table 2). We analyzed factors potentially associated with AL in the rectum with diverting stoma, but no significant relationships were identified (the data are not shown).
TABLE 2.
Factors Influencing Anastomotic Leakage in the Whole Cohort and Colon, Rectum With Stoma, and Rectum Without Stoma Cohorts

The factors associated with DFS in the colon were preoperative CEA, cerebrovascular disease, pulmonary disease, preoperative obstruction, preoperative perforation, pathologic N stage, perineural invasion, and venous invasion. For analysis, patients with rectal anastomosis were divided into those with and without diverting stoma. In the rectal group with diverting stoma, preoperative perforation, pathologic T stage, pathologic N stage, and venous invasion were associated with DFS. AL in this group was not associated with DFS (univariate results: OR, 0.69; P = 0.604). On the other hand, in the rectal group without diverting stoma, independent prognostic factors included preoperative CEA, open surgery, pathologic T stage, pathologic N stage, perineural invasion, venous invasion, and AL (Table 3).
TABLE 3.
Factors Influencing DFS in the Whole Cohort and Colon, Rectum With Stoma, and Rectum Without Stoma Cohorts
The prognostic factors of OS in the colonic anastomosis group were age, preoperative CEA, underlying pulmonary disease, preoperative perforation, minimally invasive surgery, diverting stoma, and intraoperative transfusion. In the rectal group with diverting stoma, preoperative CEA, underlying diabetes mellitus, pathologic T stage, and venous invasion were independent prognostic factors of OS. AL in this group was not associated with OS (univariate results: OR, 1.58; P = 0.453). On the other hand, age, body mass index, underlying cerebrovascular disease, preoperative obstruction, minimally invasive surgery, perineural invasion, and AL were independent prognostic factors in the rectal group without diverting stoma (Table 4).
TABLE 4.
Factors Influencing OS in the Whole Cohort and Colon, Rectum With Stoma, and Rectum Without Stoma Cohorts

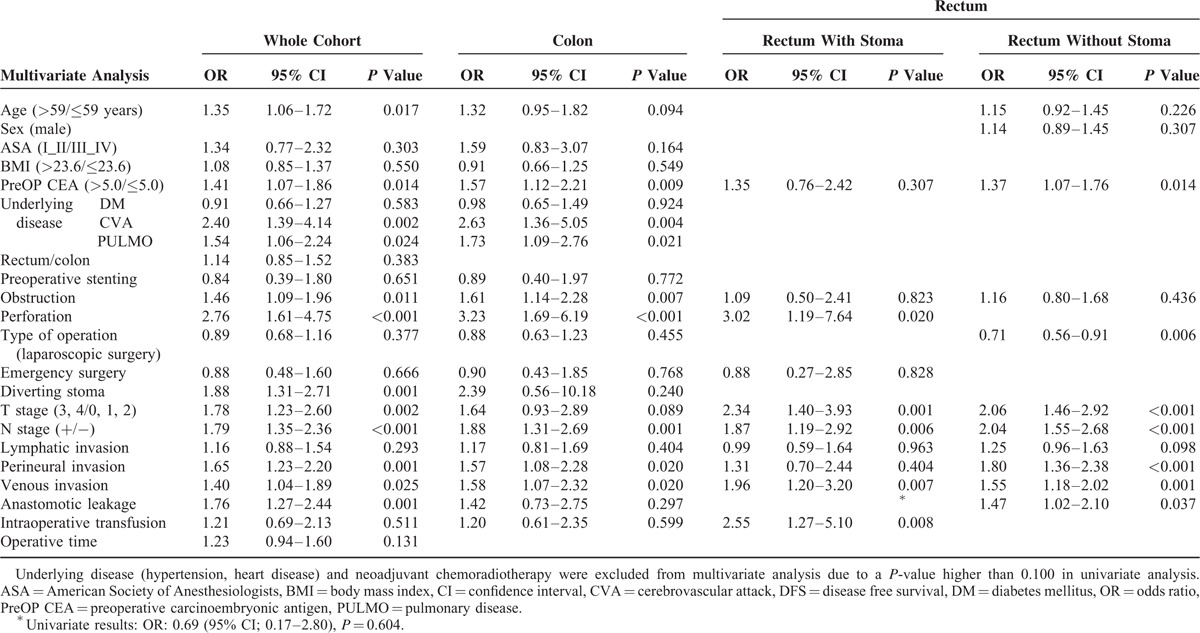
We analyzed comparisons between the leakage and no leakage groups with regard to DFS and OS. Five-year DFS was not different between the leakage (72.4%) and no leakage groups (80.9%) in the colon (P = 0.084); however, a worse prognosis was noted in the leakage group of the whole cohort (68.2% vs 79.3%, P < 0.001) and in the rectal anastomosis group (67.0% vs 76.6%, P = 0.005). There was no significant difference in 5-year OS in the colon group (82.3% vs 88.1%, P = 0.198); however, 5-year OS did vary in the whole cohort (78.4% vs 87.7%, P < 0.001) and rectal anastomosis group (77.3% vs 87.1%, P < 0.001) (Figure 1).
FIGURE 1.
Comparison of disease-free survival and overall survival in leakage and no leakage groups.
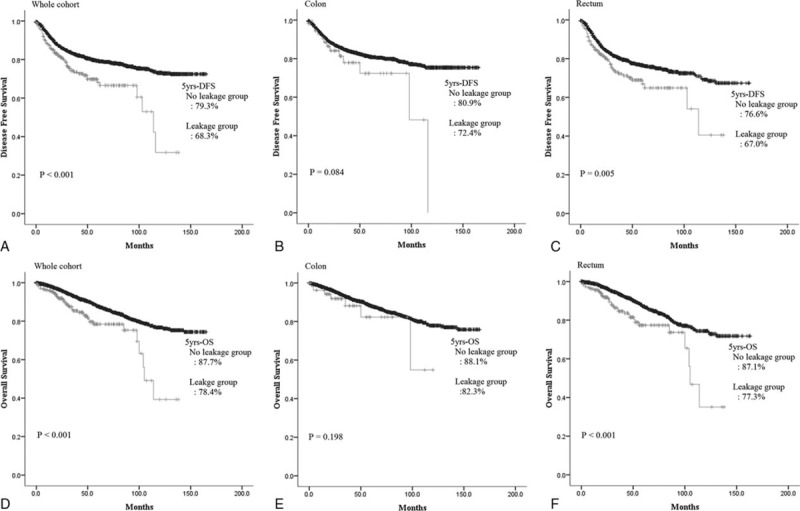
We calculated the leakage rate and OR according to number of risk factors in the colon and rectum. The leakage rate in the colon increased gradually from 0.1% to 3.2% as sex, intraoperative transfusion, and operative time risk factors were added. This trend was also seen in the rectum. The leakage rate increased gradually in the rectum with the addition of male sex, nCRT, and positive regional lymph node metastasis. When the patient had no risk factors, the leakage rate was expected to be 1.6%. When 3 risk factors were present, the leakage rate increased to 11.3% (Figure 2). In addition, the number of risk factors was associated with DFS and OS. Five-year DFS in patients with colonic anastomoses who had no other risk factors was 81.1%, while 5-year DFS in patients who had 3 risk factors was only 64.1% (P < 0.009). The same trend was observed for 5-year OS: 5-year OS was 88.2% without any risk factors, but was only 70.1% in patients with 3 risk factors (P < 0.001). Five-year DFS in the rectal anastomosis group was 87.2% when the patients had no risk factors, but decreased to 59.9% in the presence of 3 risk factors (P < 0.001); 5-year OS showed the same trend. Five-year OS was 95.4% without any risk factors, and 79.7% with 3 risk factors (P < 0.001) (Figure 3).
FIGURE 2.

Leakage rate and odds ratio according to number of risk factors in colon and rectum.
FIGURE 3.
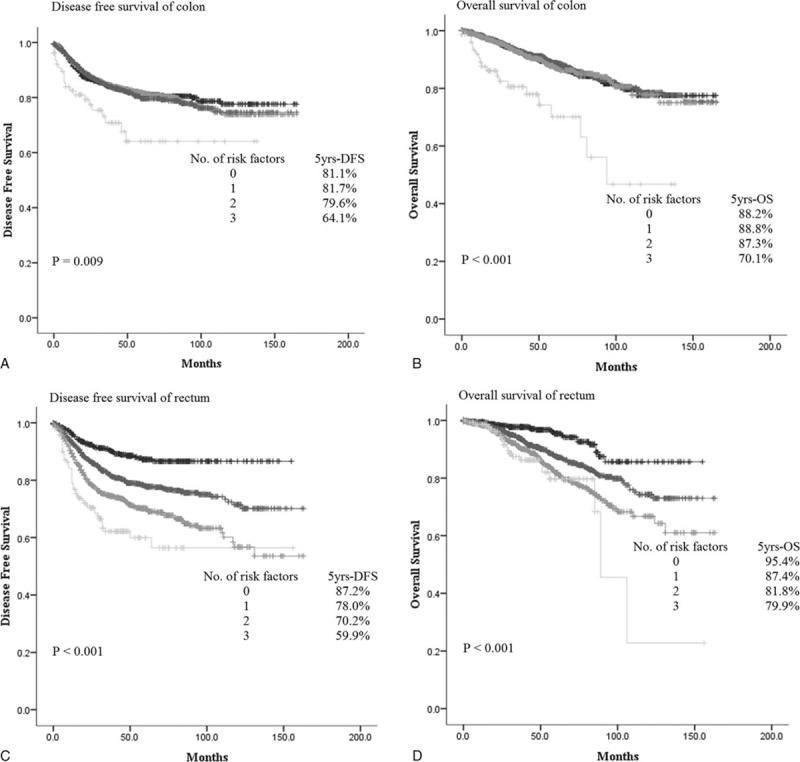
Disease-free survival and overall survival according to number of risk factors in colon and rectum.
DISCUSSION
The postoperative complications of colon or rectal surgery can pose serious problems and can adversely affect mortality.1,22 Moreover, AL has been shown to be associated with poor long-term prognosis.3,17,18,23,24 Although AL can occur in patients without any known risk factors, evaluation of the risk factors associated with AL could contribute to surgical planning related to protection of the anastomosis, such as creating a diverting stoma.
Although this was a single institutional study, its strengths were that it used a large population of 10,477 patients from a prospectively collected database over a long-term period of 12 years. We analyzed characteristics and risk factors associated with AL and survival of the whole cohort. Moreover, these factors were evaluated by group based on the anastomotic site.
Male sex, intraoperative transfusion, and operative time were determined to be independent risk factors of colonic AL in this study. Several studies have demonstrated that intraoperative transfusion and intraoperative blood loss are independently associated with AL.5,14 Blood transfusion and postoperative infection have been shown to synergistically augment postoperative cytokine response.24 Operative time is also related to AL,15 possibly due to intraabdominal adhesion or anastomotic variation. Several studies have reported male sex to be an independent risk factor of AL in rectal resection.8,11,13,22,25,26 Male pelvic dissection is more difficult due to the narrower pelvis; however, in the context of colonic resection, few studies have reported male sex as a risk factor of AL.4 The reason for the difference in AL rates between men and women in colonic resection remains unclear. Further studies are needed to explain this difference.
Male sex and nCRT have been accepted as AL risk factors in rectal cancer surgery.8,11,22,25 In one study, preoperative radiotherapy was an independent risk factor of AL, while creation of a defunctioning stoma was not routinely recommended in preoperatively radiated patients who received 25 Gy.22 However, nCRT was performed with 45 Gy in our institution. Additionally, a meta-analysis showed that preoperative radiotherapy might be a risk factor of AL.27 Few studies have reported a relationship between regional lymph node stage and AL, but there have been studies on the correlation between Union for International Cancer Control stage and AL.7,28 The anastomosis site should be closer to the anus in order to obtain an appropriate resection margin. The distance between the anastomosis site and the anal verge is known to be a risk factor of AL.7,13,22,25 In our study, tumor distance from anal verge was not associated with AL; however, this relationship may have been weak due to missing data (1134 of 3912 rectal anastomosis cases). Further studies will be needed to resolve this issue. In accordance with previous studies, this study showed a diverting stoma to protect against AL.7,8
In our study, there were no differences between local or distant recurrence in colonic and rectal surgeries. These results are consistent with several prior studies.29–31 In contrast, 1 meta-analysis found an association between AL and local recurrence in rectal surgery.18 This association might be related to the release of a variety of acute-phase reactants and proinflammatory mediators;18 however, the mechanism by which AL might affect tumor recurrence remains uncertain. Another study found that AL was significantly associated with an increased rate of distant recurrence, which could have been due to canceled or delayed administration of adjuvant chemotherapy.17
Anastomotic leaks were not associated with worse long-term survival in patients who underwent resection for colon cancer. Furthermore, in multivariate analysis, AL was not found to be a negative independent prognostic factor in colonic resection. In accordance with previous studies, our survival analysis based on cancer stage between the AL group and non-AL group showed significantly worse outcomes in the stage I/II leakage group compared with the stage III/IV group (data not shown). It might be related to associated comorbid medical conditions of the patients.23 In addition, 42.3% of patients who suffered from AL improved with conservative management. The number of patients with delayed or canceled administration of adjuvant chemotherapy, a known risk factor of decreased survival, might be relatively small.17 The median hospital stay for patients with colonic leakage was relatively short (16 days). Although it was previously reported that rectal leakage made no difference in long-term survival,1 we found that AL in the rectum was an independent factor of poor OS.18,23,24,32 This indicates that OS can be affected by both cancer-related and noncancer-related factors.32 Although there was no difference in the use of temporary stoma in patients with or without AL in the rectum,22 many other reports have demonstrated that a defunctioning diverting stoma effectively reduces the clinical consequences of AL and reoperation.7,8,13,33–35 Thus, if patients have risk factors for AL, the use of a defunctioning stoma is recommended.14,15 In a randomized multicenter trial, 10.3% of patients with a defunctioning stoma had symptomatic leakage, while leakage was observed in 28.0% of those without a stoma (P < 0.001).34 In a study of patients who underwent preoperative radiotherapy, a defunctioning stoma was found to be an independent risk factor for AL.8 The results of our study also revealed that a diverting stoma could reduce AL in extraperitoneal anastomosis. Furthermore, rectal AL was a risk factor associated with poor OS in patients without a stoma. Therefore, creation of a diverting stoma should be considered for males, patients who have undergone nCRT, and those with positive regional lymph node metastasis in order to prevent AL.
The main limitations of this study were that it was conducted at a single institution, and the retrospective study design, which might result in bias. Additionally, there was a lack of information regarding adjuvant chemotherapy and the height of anastomosis in rectal surgery, which are known to be associated with prognosis or AL. One study reported that worse long-term prognosis in patients with AL might partly account for canceled or delayed administration of adjuvant chemotherapy.17 The inclusion of this information might have explained the difference in prognosis between the 2 rectal groups.
In conclusion, the AL rate in the colon increased with the added risk factors of male sex, intraoperative transfusion, and operative time, while male sex, nCRT, and regional lymph node metastasis increased the leakage rate in the rectum. AL in the rectum is associated with long-term OS. Additionally, a diverting stoma was shown to be protective against AL and was associated with longer OS. Therefore, creation of a diverting stoma should be recommended for patients with risk factors for extraperitoneal anastomosis.
Acknowledgments
The authors thank the support from the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (grant number 2015R1A1A1A05001160).
Footnotes
Abbreviations: AL = anastomotic leakage, CEA = carcinoembryonic antigen, CT = computed tomography, DFS = disease-free survival, nCRT = neoadjuvant chemoradiotherapy, OR = odds ratio, OS = overall survival.
JSP contributed to data collection, analysis, and drafting of the manuscript. JWH developed the study design and proposal and performed data analysis and final revision of the manuscript and is responsible for correspondence. Patients were enrolled by YAP, YBC, SHY, HCK, and WYL. All authors approved the final version of the manuscript.
This study was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (grant number 2015R1A1A1A05001160).
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Mrak K, Eberl T, Laske A, et al. Impact of postoperative complications on long-term survival after resection for rectal cancer. Dis Colon Rectum 2013; 56:20–28. [DOI] [PubMed] [Google Scholar]
- 2.Veyrie N, Ata T, Muscari F, et al. Anastomotic leakage after elective right versus left colectomy for cancer: prevalence and independent risk factors. J Am Coll Surg 2007; 205:785–793. [DOI] [PubMed] [Google Scholar]
- 3.Marra F, Steffen T, Kalak N, et al. Anastomotic leakage as a risk factor for the long-term outcome after curative resection of colon cancer. Eur J Surg Oncol 2009; 35:1060–1064. [DOI] [PubMed] [Google Scholar]
- 4.Frasson M, Flor-Lorente B, Rodriguez JL, et al. Risk factors for anastomotic leak after colon resection for cancer: multivariate analysis and nomogram from a multicentric, prospective, national study with 3193 patients. Ann Surg 2015; 262:321–330. [DOI] [PubMed] [Google Scholar]
- 5.Leichtle SW, Mouawad NJ, Welch KB, et al. Risk factors for anastomotic leakage after colectomy. Dis Colon Rectum 2012; 55:569–575. [DOI] [PubMed] [Google Scholar]
- 6.Akiyoshi T, Ueno M, Fukunaga Y, et al. Incidence of and risk factors for anastomotic leakage after laparoscopic anterior resection with intracorporeal rectal transection and double-stapling technique anastomosis for rectal cancer. Am J Surg 2011; 202:259–264. [DOI] [PubMed] [Google Scholar]
- 7.Warschkow R, Steffen T, Thierbach J, et al. Risk factors for anastomotic leakage after rectal cancer resection and reconstruction with colorectostomy. A retrospective study with bootstrap analysis. Ann Surg Oncol 2011; 18:2772–2782. [DOI] [PubMed] [Google Scholar]
- 8.Wang L, Gu J. Risk factors for symptomatic anastomotic leakage after low anterior resection for rectal cancer with 30 Gy/10 f/2 w preoperative radiotherapy. World J Surg 2010; 34:1080–1085. [DOI] [PubMed] [Google Scholar]
- 9.Lee WS, Yun SH, Roh YN, et al. Risk factors and clinical outcome for anastomotic leakage after total mesorectal excision for rectal cancer. World J Surg 2008; 32:1124–1129. [DOI] [PubMed] [Google Scholar]
- 10.Eckmann C, Kujath P, Schiedeck TH, et al. Anastomotic leakage following low anterior resection: results of a standardized diagnostic and therapeutic approach. Int J Colorectal Dis 2004; 19:128–133. [DOI] [PubMed] [Google Scholar]
- 11.Bennis M, Parc Y, Lefevre JH, et al. Morbidity risk factors after low anterior resection with total mesorectal excision and coloanal anastomosis: a retrospective series of 483 patients. Ann Surg 2012; 255:504–510. [DOI] [PubMed] [Google Scholar]
- 12.Matthiessen P, Lindgren R, Hallbook O, et al. Symptomatic anastomotic leakage diagnosed after hospital discharge following low anterior resection for rectal cancer. Colorectal Dis 2010; 12 (7 Online):e82–e87. [DOI] [PubMed] [Google Scholar]
- 13.Rullier E, Laurent C, Garrelon JL, et al. Risk factors for anastomotic leakage after resection of rectal cancer. Br J Surg 1998; 85:355–358. [DOI] [PubMed] [Google Scholar]
- 14.Alves A, Panis Y, Trancart D, et al. Factors associated with clinically significant anastomotic leakage after large bowel resection: multivariate analysis of 707 patients. World J Surg 2002; 26:499–502. [DOI] [PubMed] [Google Scholar]
- 15.Buchs NC, Gervaz P, Secic M, et al. Incidence, consequences, and risk factors for anastomotic dehiscence after colorectal surgery: a prospective monocentric study. Int J Colorectal Dis 2008; 23:265–270. [DOI] [PubMed] [Google Scholar]
- 16.Kim JS, Cho SY, Min BS, et al. Risk factors for anastomotic leakage after laparoscopic intracorporeal colorectal anastomosis with a double stapling technique. J Am Coll Surg 2009; 209:694–701. [DOI] [PubMed] [Google Scholar]
- 17.Krarup PM, Nordholm-Carstensen A, Jorgensen LN, et al. Anastomotic leak increases distant recurrence and long-term mortality after curative resection for colonic cancer: a nationwide cohort study. Ann Surg 2014; 259:930–938. [DOI] [PubMed] [Google Scholar]
- 18.Mirnezami A, Mirnezami R, Chandrakumaran K, et al. Increased local recurrence and reduced survival from colorectal cancer following anastomotic leak: systematic review and meta-analysis. Ann Surg 2011; 253:890–899. [DOI] [PubMed] [Google Scholar]
- 19.Rahbari NN, Weitz J, Hohenberger W, et al. Definition and grading of anastomotic leakage following anterior resection of the rectum: a proposal by the International Study Group of Rectal Cancer. Surgery 2010; 147:339–351. [DOI] [PubMed] [Google Scholar]
- 20.Yun JA, Cho YB, Park YA, et al. Clinical manifestations and risk factors of anastomotic leakage after low anterior resection for rectal cancer. ANZ J Surg 2015; 29: (in press) doi: 10.1111/ans. 13143. [DOI] [PubMed] [Google Scholar]
- 21.Park JS, Huh JW, Park YA, et al. A circumferential resection margin of 1 mm is a negative prognostic factor in rectal cancer patients with and without neoadjuvant chemoradiotherapy. Dis Colon Rectum 2014; 57:933–940. [DOI] [PubMed] [Google Scholar]
- 22.Matthiessen P, Hallbook O, Andersson M, et al. Risk factors for anastomotic leakage after anterior resection of the rectum. Colorectal Dis 2004; 6:462–469. [DOI] [PubMed] [Google Scholar]
- 23.Eberhardt JM, Kiran RP, Lavery IC. The impact of anastomotic leak and intra-abdominal abscess on cancer-related outcomes after resection for colorectal cancer: a case control study. Dis Colon Rectum 2009; 52:380–386. [DOI] [PubMed] [Google Scholar]
- 24.Boccola MA, Buettner PG, Rozen WM, et al. Risk factors and outcomes for anastomotic leakage in colorectal surgery: a single-institution analysis of 1576 patients. World J Surg 2011; 35:186–195. [DOI] [PubMed] [Google Scholar]
- 25.Lipska MA, Bissett IP, Parry BR, et al. Anastomotic leakage after lower gastrointestinal anastomosis: men are at a higher risk. ANZ J Surg 2006; 76:579–585. [DOI] [PubMed] [Google Scholar]
- 26.Lee CM, Huh JW, Yun SH, et al. Laparoscopic versus open reintervention for anastomotic leakage following minimally invasive colorectal surgery. Surg Endosc 2015; 29:931–936. [DOI] [PubMed] [Google Scholar]
- 27.Pommergaard HC, Gessler B, Burcharth J, et al. Preoperative risk factors for anastomotic leakage after resection for colorectal cancer: a systematic review and meta-analysis. Colorectal Dis 2014; 16:662–671. [DOI] [PubMed] [Google Scholar]
- 28.Ptok H, Marusch F, Meyer F, et al. Impact of anastomotic leakage on oncological outcome after rectal cancer resection. Br J Surg 2007; 94:1548–1554. [DOI] [PubMed] [Google Scholar]
- 29.Eriksen MT, Wibe A, Norstein J, et al. Anastomotic leakage following routine mesorectal excision for rectal cancer in a national cohort of patients. Colorectal Dis 2005; 7:51–57. [DOI] [PubMed] [Google Scholar]
- 30.den Dulk M, Marijnen CA, Collette L, et al. Multicentre analysis of oncological and survival outcomes following anastomotic leakage after rectal cancer surgery. Br J Surg 2009; 96:1066–1075. [DOI] [PubMed] [Google Scholar]
- 31.Bertelsen CA, Andreasen AH, Jorgensen T, et al. Anastomotic leakage after curative anterior resection for rectal cancer: short and long-term outcome. Colorectal Dis 2010; 12 (7 Online):e76–e81. [DOI] [PubMed] [Google Scholar]
- 32.Law WL, Choi HK, Lee YM, et al. The impact of postoperative complications on long-term outcomes following curative resection for colorectal cancer. Ann Surg Oncol 2007; 14:2559–2566. [DOI] [PubMed] [Google Scholar]
- 33.Gu WL, Wu SW. Meta-analysis of defunctioning stoma in low anterior resection with total mesorectal excision for rectal cancer: evidence based on thirteen studies. World J Surg Oncol 2015; 13:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matthiessen P, Hallbook O, Rutegard J, et al. Defunctioning stoma reduces symptomatic anastomotic leakage after low anterior resection of the rectum for cancer: a randomized multicenter trial. Ann Surg 2007; 246:207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Poon RT, Chu KW, Ho JW, et al. Prospective evaluation of selective defunctioning stoma for low anterior resection with total mesorectal excision. World J Surg 1999; 23:463–467.discussion 467–468. [DOI] [PubMed] [Google Scholar]


