Abstract
Endoscopic variceal band ligation (EVL) is an effective procedure to control and prevent variceal bleeding in patients with liver cirrhosis, but it can be complicated by bleeding from post-EVL ulcers. Several studies have reported that proton pump inhibitors (PPIs) decrease the size of post-EVL ulcers. However, evidence are limited as to whether PPIs actually reduce the risk of bleeding after EVL. This study aimed to analyze the factors associated with bleeding after prophylactic EVL and to assess the effect of PPI therapy.
Five hundred and five cirrhotic patients with high risk esophageal varices who received primary prophylactic EVL were included for this retrospective cohort study. Post-EVL bleeding was defined as bleeding after prophylactic EVL within 8 weeks evidenced by the occurrence of melena or hematemesis, or by a decrease of hemoglobin by >2.0 g/dL. If evidence of bleeding from ulceration of the EVL sites was confirmed by endoscopy, we defined it as post-EVL ulcer bleeding.
Fourteen patients developed bleeding after prophylactic EVL. Factors associated with post-EVL bleeding included alcohol as etiology, low albumin, high total bilirubin, high Child-Pugh score, high MELD score, coexistence of gastric varices, and not administrating PPI medication by univariate analysis. In multivariate logistic analysis, Co-existing gastric varix (odds ratio [OR] 5.680, P = 0.005] and not administrating PPIs (OR 8.217, P = 0.002) were associated with bleeding after prophylactic EVL. In the subgroup analysis excluding patients whose gastric varices were treated, not administering PPI medication (OR 8.827, P = 0.008) was the sole factor associated with post-EVL bleeding.
We suggest that PPI therapy needs to be considered in patients receiving prophylactic EVL to reduce the risk of bleeding after prophylactic EVL.
INTRODUCTION
Portal hypertension results from a combination of increased intrahepatic vascular resistance and increased blood flow through the portal venous system in liver cirrhosis.1 Esophageal varices are caused by portal hypertension, and they develop in 50% of patients with liver cirrhosis.2 Variceal bleeding occurs at an annual rate of 5% to 15%, is one of the most serious complications of cirrhosis, and is the second most common cause of mortality among patients with cirrhosis.3 Several studies have shown the effectiveness of preventive options, including endoscopic and pharmacological treatment.4 Endoscopic variceal band ligation (EVL) has been used for the prophylaxis of variceal bleeding, and it significantly reduces the incidence of first variceal hemorrhages.5 However, there are risks of complications associated with EVL, including esophageal laceration or perforation, retrosternal pain, transient dysphagia, esophageal stricture, the transient accentuation of portal hypertensive gastropathy, ulcer bleeding, and bacteremia, and, of particular note, life-threatening post-ligation ulcer bleeding occurs in 2% to 5% of patients after EVL.6,7 The ligated tissue may fall off within a few days following the application of the bands over the esophageal varices. Following the sloughing of varices, shallow esophageal ulcers are ubiquitous at ligated sites of esophageal varices.6,7 In general, ulcers of ligated sites are relatively shallow and heal spontaneously within several weeks. However, bleeding from the sites, which is caused by band slippage and the exposure of the unhealed ulcers on the varices, may be fatal.6,7 Recent controlled trials have demonstrated that subjects who received pantoprazole after elective EVL had significantly smaller post-banding ulcers on follow-up endoscopy than subjects who received placebo.8,9 However, there are only few studies that investigated whether proton pump inhibitors (PPIs) can actually lower the risk of bleeding after EVL. It is very difficult to demonstrate the efficacy of PPIs, because variceal bleeding is more closely associated with an increase in portal pressure and poor hepatic function, particularly in patients who have bled previously.10–12 Therefore, it would be more reasonable to evaluate the role of PPIs in patients never bled before and restrict the follow period until post-EVL ulcers may exist.
We performed this study to analyze predictive factors for bleeding after prophylactic EVL and to assess the effect of PPI therapy during postprocedural follow-up period.
METHODS
Subjects
Patients with liver cirrhosis who received elective EVL for primary prophylaxis of variceal bleeding between January 1998 and April 2011 at a tertiary hospital were included in this retrospective cohort study. Patients were excluded if any of the following criteria were met: underwent emergency endoscopy for acute variceal bleeding, received EVL for secondary prophylaxis of variceal bleeding, underwent EVL for nonvariceal upper gastrointestinal bleeding such as Mallory-Weiss tearing, had hepatocellular carcinoma with portal vein thrombosis, had an allergic reaction to PPIs, had active peptic ulcers, or had a gastric varix only. Clinical variables such as age, sex, medical history, and results of laboratory tests were assessed at baseline before EVL. Occurrence of bleeding was evaluated during 8 weeks’ follow-up period after EVL. This study was approved by the institutional review board of Korea University Ansan Hospital (AS11077) and performed in accordance with the declaration of Helsinki.
Endoscopic Procedures for Varices
EVL for primary prophylaxis of a first variceal bleeding was performed in patients with high-risk esophageal varices by expert endoscopists. In the presence of high-risk varices during the screening, the endoscope was removed and EVL device was attached. The endoscope was reinserted, and EVL was performed on the varix. Follow-up endoscopy was performed after 4 to 8 weeks to check for complete healing of postbanding ulcers. The end-point of the EVL procedure was defined as the eradication of the varix or a decrease in size to smaller than a F1 varix on endoscopic examination according to the classification system of the Japanese Society for Portal Hypertension described below.
In the presence of concomitant large gastric varices, endoscopic variceal obturation (EVO) or endoscopic gastric variceal band ligation (GVL) was performed before EVL during the same session. Our methods of procedures on gastric varices were similar to those described previously.13
Concomitant Medical Therapies
PPIs were initiated once a day at standard doses in the presence of esophagogastric mucosal lesions such as reflux esophagitis, peptic ulcerations, or erosions, which were observed by endoscopy at the time of variceal screening or EVL procedures. Without any mucosal lesions, PPIs were not prescribed, as the drugs were not approved for routine use of post-EVL care. Once medications were prescribed, patients’ compliance to drugs was strictly monitored at every clinic visit.
In the absence of contraindication, propranolol was prescribed after second day of EVL if patients were willing to take the medication after the information on potential risk of adverse effects was given. Otherwise, only endoscopic therapy was performed. Propranolol was titrated from 20 mg 2 times a day to maximal dose at which patients could tolerate or reduction of the heart rates either by 25% from baseline or to 55 beats/minute was achieved.
Definitions
The endoscopic findings of esophageal varices were evaluated according to the grading system by the Japanese Society for Portal Hypertension. The form (F) of the varices was classified as small and straight (F1), enlarged and tortuous (F2), or large and coil-shaped (F3).14
Large esophageal (F2 or F3) varices or any form of varix with red wale markings in liver cirrhosis patients with Child-Pugh B or C liver function were defined as high-risk varices.15,16
Post-EVL bleeding was defined as bleeding within 8 weeks after prophylactic EVL evidenced by melena or hematemesis, or a decrease of hemoglobin by >2.0 g/dL during follow-up. In addition, post-EVL ulcer bleeding was defined if evidence of bleeding from ulceration of the EVL sites was confirmed by endoscopy.
End Point
The primary end point of this study was the occurrence of post-EVL bleeding in patients who underwent primary prophylactic EVL procedures.
Statistical Analyses
The statistical analyses were performed using SPSS for Windows, version 12.5.1 (SPSS Inc, Chicago, IL). The differences in the clinical variables between the patients with post-EVL bleeding and those without post-EVL bleeding were tested using the χ2 test and the independent t test. Cox proportional hazards models were used to assess the influence of the clinical variables on post-EVL bleeding. Covariates with P values <0.05 in the univariate analysis were included in the multivariate analysis. The cumulative rate of bleeding was calculated using the Kaplan-Meier method and censoring the patients who were lost to follow-up. The log-rank test was performed to compare the differences between the groups. The results are expressed as the means ± standard deviations, and a P value <0.05 was considered statistically significant.
RESULTS
Baseline Characteristics
One thousand five patients underwent an EVL procedure at the Korea University Ansan Hospital during the study period. Among the 1005 patients, 500 patients were excluded for the following reasons: emergency EVL (n = 333), secondary prophylactic EVL (n = 102), hepatocellular carcinoma with portal vein thrombosis (n = 51), and Mallory-Weiss tear (n = 14). A total of 505 patients underwent EVL for primary prophylaxis of esophageal variceal bleeding (Figure 1). Table 1 describes the baseline characteristics of patients in this study. Among the 505 enrolled patients (age, 53.6 ± 10.58 years; male, 76.8%), 51.1% had chronic viral hepatitis (B, n = 236 or C, n = 22), 38.6% (n = 195) had alcoholic liver disease, and the rest of them (10.2%, n = 52) had autoimmune liver diseases, nonalcoholic fatty liver disease, cryptogenic or mixed etiology for underlying cause of liver cirrhosis. Patients who belonged to Child-Pugh A class comprised 25.7% (n = 130) of the study population, 61% (n = 312) of patients were Child-Pugh B class, and 12.5% (n = 63) of patients were Child-Pugh C class. The mean Model for end-stage liver disease (MELD) score was 12.24 ± 0.22. Three hundred fifty-nine patients (71.0%) received PPIs after EVL.
FIGURE 1.
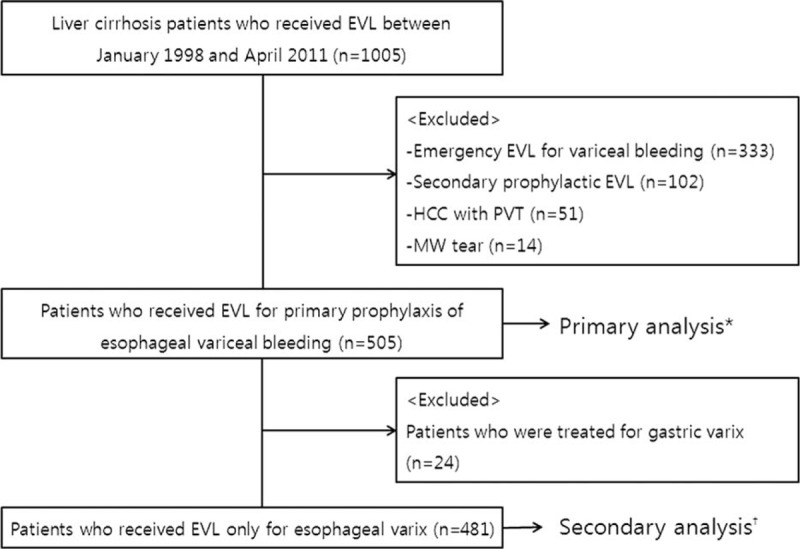
Disposition of the patients enrolled in the study. ∗Results of the primary analysis for the predictors of post-endoscopic variceal band ligation bleeding are shown in Table 3. †Results of the secondary analysis are shown in Table 4. EVL = endoscopic variceal band ligation, MW = Mallory-Weiss, PVT = portal vein thrombosis.
TABLE 1.
Baseline Characteristics of the Patients
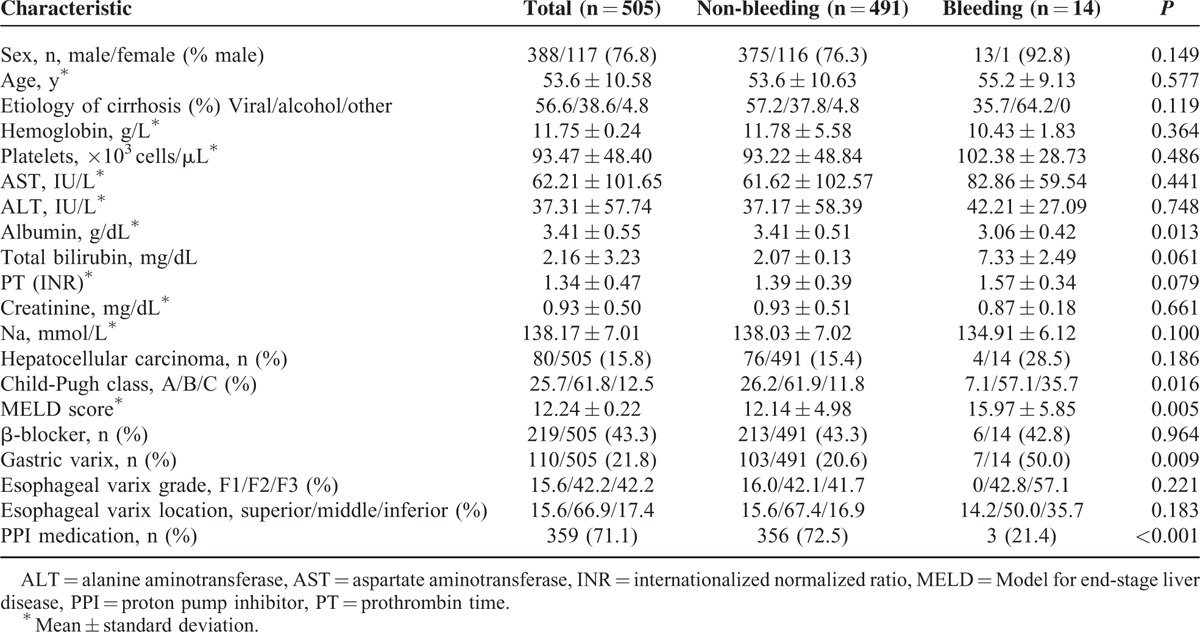
There were several different PPI medications used after EVL in this study: lansoprazole 30 mg (n = 263), pantoprazole 40 mg (n = 22), omeprazole 40 mg (n = 57), and rabeprazole 20 mg (n = 17). Duration of receiving PPIs was >4 weeks.
Clinical Findings During and After EVL
One hundred ten patients had esophageal varices and coexisting gastric varices. Of these, 21 patients underwent EVO and 6 patients were treated with GVL. Three hundred ninety-five patients had esophageal varices only. F1 or F2 varices were observed in 292 patients (57.8%) and F3 varices were observed in 213 patients (42.2%).
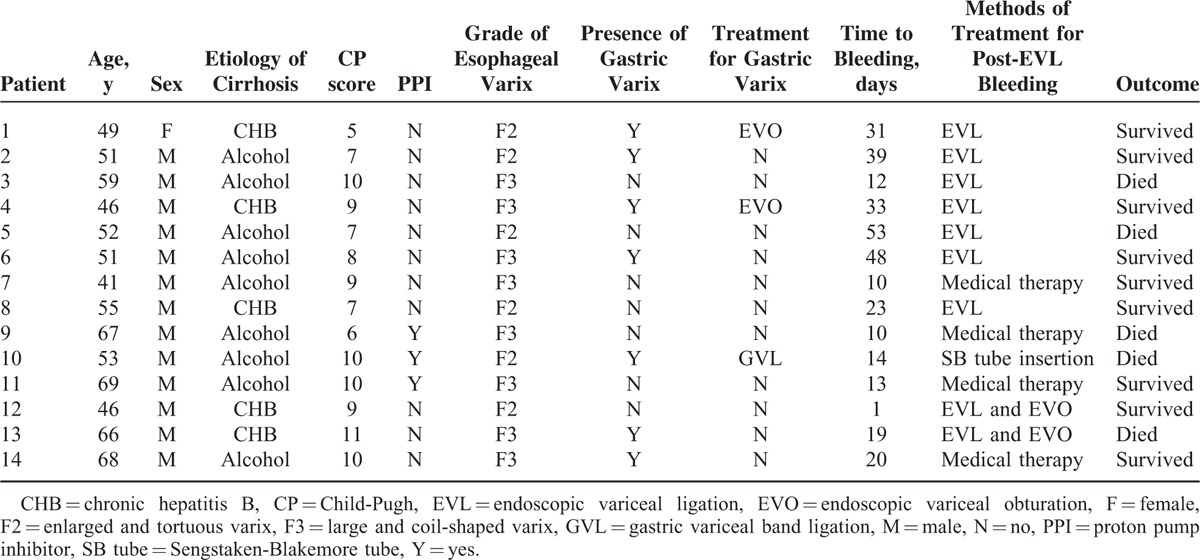
Fourteen patients (2.7%) developed bleeding after prophylactic EVL. Table 2 describes the baseline characteristics and the outcomes of the patients with post-EVL bleeding. Thirteen patients had melena or hematemesis, and 1 patient showed a decrease in their hemoglobin level of >2 g/dL. Post-EVL ulcer bleeding was confirmed by endoscopic examination in 13 patients. The presence or absence of an ulcer could not be confirmed in 1 patient due to cardiac arrest at the emergency room. The patients with bleeding after EVL were treated with additional EVL (n = 7), EVL with EVO (n = 2), Sengstaken-Blakemore tube insertion (n = 1), or medical treatment alone (n = 4).
TABLE 2.
Characteristics and Outcomes of Patients With Postendoscopic Variceal Ligation Bleeding
Adverse Events and Mortality
Of the 5 patients who died of post-EVL bleeding during the 8-week follow-up period, 3 patients did not receive PPIs after EVL and 2 patients had gastric varices. Other causes of death during the 8-week follow-up period that were not related to the EVL procedures included complications associated with liver disease (n = 6), infections (n = 2) that caused a brain abscess and pneumonia, and an intracranial hemorrhage (n = 1).
Impact of Clinical and Endoscopic Factors on Post-Procedural Bleeding
Univariate analysis showed that alcohol as the etiology (odds ratio [OR] = 2.952, 95% confidence interval [CI]: 0.974–8.941, P = 0.056), low albumin levels (OR = 0.264, 95% CI: 0.087–0.799, P = 0.019), high total bilirubin levels (OR = 4.014, 95% CI: 1.323–12.179, P = 0.014), Child-Pugh scores ≥9 (OR = 6.192, 95% CI: 2.095–18.297, P = 0.001), and MELD scores ≥18 (OR = 4.315, 95% CI: 1.397–13.334, P = 0.011) were the clinical factors, which were independently associated with post-EVL bleeding. In addition, the endoscopic and treatment factors independently associated with post-EVL bleeding were the presence of gastric varices (OR = 3.767, 95% CI: 1.292–10.981, P = 0.015) and no PPI medication (OR = 9.669, 95% CI: 2.657–35.192, P = 0.001). Multivariate analysis showed that the presence of coexisting gastric varices (OR = 5.680, 95% CI: 0.670–19.317, P = 0.005) and not administering PPIs (OR = 8.217, 95% CI: 2.126–31.767, P = 0.002) were associated with post-EVL bleeding (Table 3). The bleeding-free survival rate was significantly better in patients who had been administered PPIs compared with those who were not administered PPIs (P < 0.001), and in those patients who did not have gastric varices compared with those who had gastric varices (P = 0.010) (Figure 2); bleeding events occurred in 3 of 359 patients who received PPIs and in 11 of 146 patients who did not receive PPIs, and they occurred in 7 of 110 patients who had gastric varices and in 7 of 395 patients who did not have gastric varices.
TABLE 3.
Univariate and Multivariate Analyses of Predictors for Bleeding in all Patients
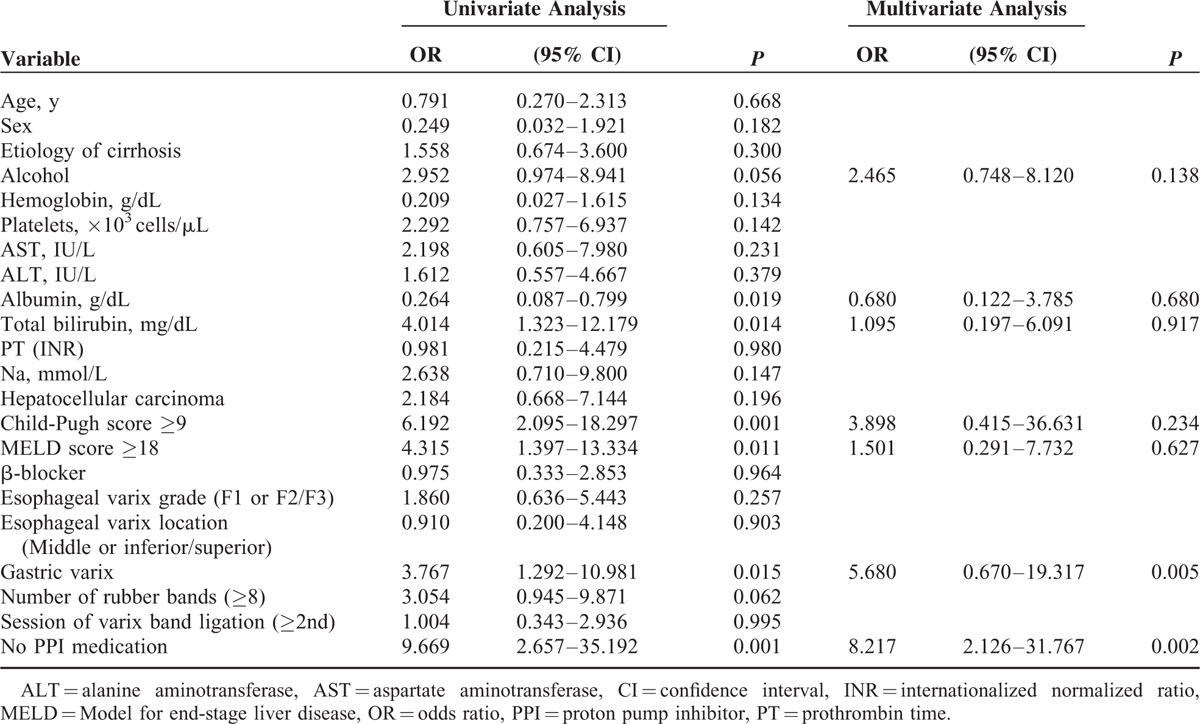
FIGURE 2.
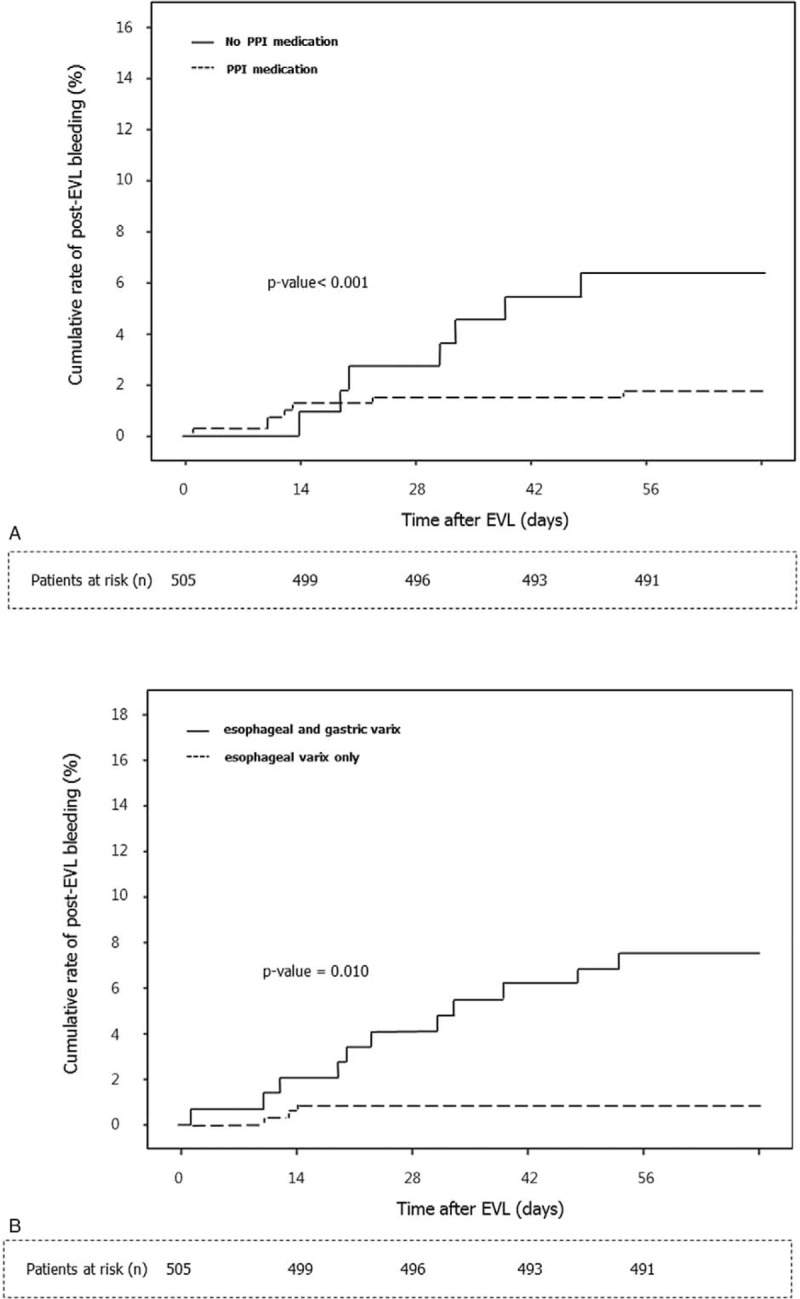
Kaplan-Meier estimates of bleeding within 8 weeks of esophageal varix ligation in all 505 patients. (A) Stratification according to PPI therapy. Bleeding events occurred in 3 out of 359 patients who received PPIs and in 11 of 146 patients who did not receive a PPI. (B) Stratification according to the presence of gastric varices. Bleeding events occurred in 7 of 110 patients who had gastric varices and in 7 of 395 patients who did not have gastric varices. EVL = endoscopic variceal band ligation, PPI = proton pump inhibitor.
Subgroup Analysis
We separately evaluated the prognostic impact of clinical and endoscopic factors excluding patients who were treated for gastric varix (n = 481). Eleven patients developed bleeding after EVL among the 481 patients. Univariate analysis showed that that alcohol as the etiology (OR = 4.335, 95% CI: 0.1.135–16.555, P = 0.032), high total bilirubin levels (OR = 3.884, 95% CI: 1.119–13.473, P = 0.033), Child-Pugh scores ≥9 (OR = 5.514, 95% CI: 1.644–18.493, P = 0.006), MELD scores ≥18 (OR = 4.496, 95% CI: 1.274–15.871, P = 0.019), low albumin levels (OR = 0.263, 95% CI: 0.076–0.911, P = 0.035), and not administering PPIs (OR = 11.284, 95% CI: 2.406–52.908, P = 0.002) were independently associated with post-EVL bleeding. Multivariate analysis showed that not administering PPIs (OR = 8.827, 95% CI: 1.770–44.019, P = 0.008) was independently associated with post-EVL bleeding after adjusting for the alcohol as the etiology, albumin level, total bilirubin level, Child-Pugh score, and the MELD score (Table 4). The bleeding-free survival rate was significantly better in patients who were administered PPIs compared with those who were not administered PPIs (P < 0.001) (Figure 3); bleeding events occurred in 2 of 338 patients who received PPIs and in 9 of 143 patients who did not receive PPIs.
TABLE 4.
Univariate and Multivariate Analyses of Predictors for Bleeding, Excluding Patients Treated for Gastric Varices
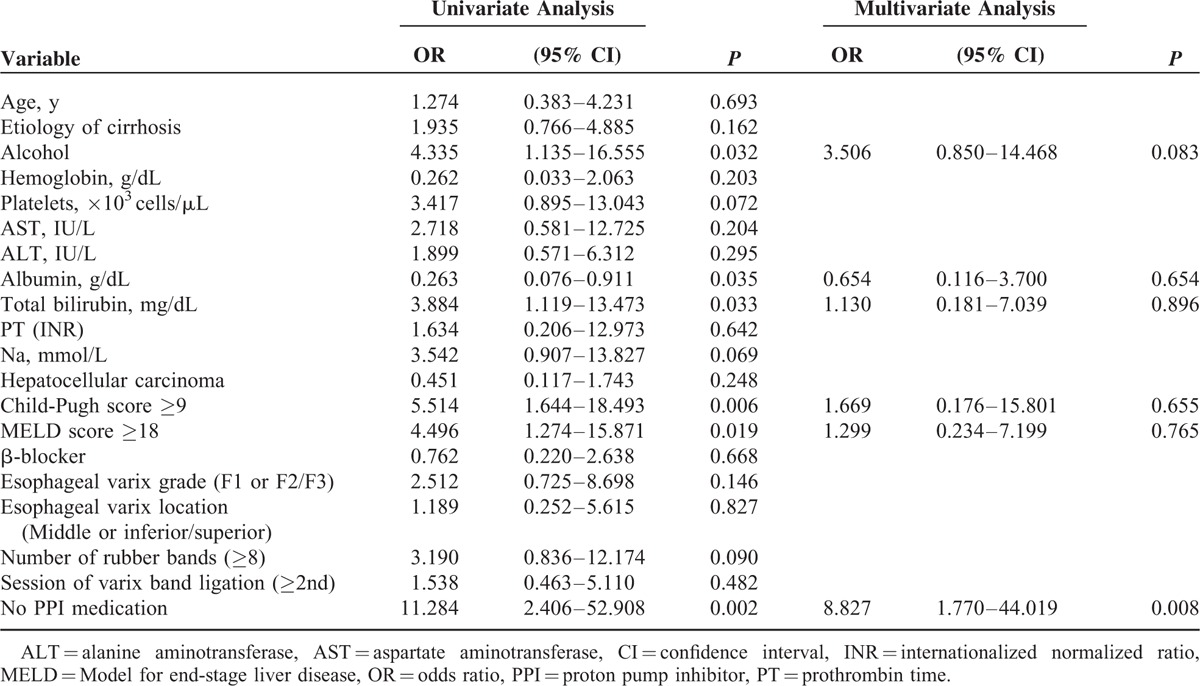
FIGURE 3.
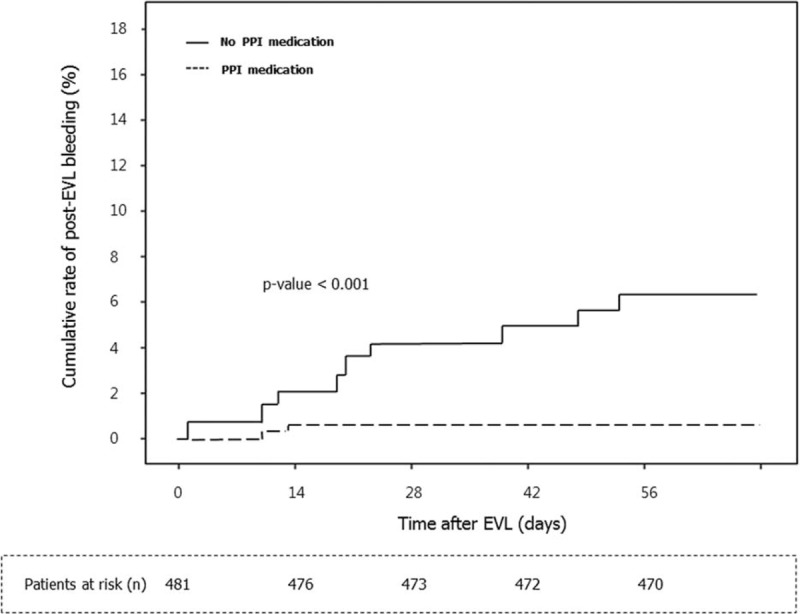
Kaplan-Meier estimates of bleeding within 8 weeks of esophageal varix ligation stratified according to PPI therapy in subgroups of endoscopic variceal band ligation-only patients, excluding those who were treated for gastric varix (n = 481). Bleeding events occurred in 2 of 338 patients who received PPIs and in 9 of 143 patients who did not receive a proton pump inhibitor. EVL = endoscopic variceal band ligation, PPI = proton pump inhibitor.
DISCUSSION
EVL is recommended for the prevention of first variceal bleeding episodes in patients with liver cirrhosis. The procedure is probably more effective than β-blockers, although debates still exist.4,17 However, it can be associated with serious adverse events during and after the procedure including bleeding from band-induced ulcerations.6,7 Although it is assumed that acid suppression may play a role in the prevention of postprocedural bleeding, data on the effect of gastric acid secretion inhibitors on ulceration after EVL are limited.
A previous study has shown omeprazole to be effective for promoting ulcer healing after endoscopic injection sclerotherapy.18–20 However, a difference was not demonstrated in a subsequent double-blind randomized controlled trial, which compared omeprazole and placebo.21 Therefore, the effect of omeprazole on the healing of esophageal ulcers after sclerotherapy is inconclusive.
Recently, a majority of medical centers have abandoned injection sclerotherapy in favor of EVL therapy for the prevention of esophageal variceal bleeding owing to the ease of ligation, more rapid onset of therapeutic benefit, and lower rate of complications.22–24 Therefore, the role of PPIs as an adjunctive treatment with EVL must be clarified. A randomized trial performed in 42 subjects after elective EVL treated with either pantoprazole or placebo showed that patients receiving pantoprazole had significantly smaller post-banding ulcers than those receiving placebo.9 However, the study did not demonstrate any relationship between the use of PPIs and the risk of postprocedural bleeding after prophylactic EVL. A more recent study reported that long-term administration of PPIs reduced the risk of treatment failure after EVL.25 However, all the patients in the study were randomized after confirmation of post-EVL ulcer healing, so the effects of PPI therapy on active state of postbanding ulcerations were not evaluated. Moreover, the study may have been underpowered as enrollment was not completed; only 21 patients for rabeprazole and 22 patients for placebo were included.
In the present study, we evaluated effect of PPIs on post-EVL ulcer bleeding in a large number of patients, and the bleeding-free survival rate during 8 weeks after EVL was significantly better in patients who received PPI therapy. By multivariate analysis using Cox proportional hazard models, not administrating PPIs and the presence of gastric varices were independent endoscopic and treatment risk factors for bleeding after endoscopic therapy. It is assumed that acid suppression contributed to early healing of the postbanding ulceration by reducing the gastroesophageal acid reflux in the cirrhotic patients.26
As the risk of bleeding after EVL could have been affected by other types of combined endoscopic therapy, we separately analyzed 481 patients excluding those who received concomitant therapeutic procedures for gastric varix. The result confirmed that not administrating PPI medication was the sole risk factor of post-EVL bleeding after primary endoscopic prophylaxis for esophageal variceal hemorrhage. Also, even when we analyzed 395 patients who had esophageal varix only, after exclusion of patients with co-existing gastric varices, the result was the same (data not shown). We speculate that it is important to start PPI therapy after EVL as soon as possible to prevent postprocedural bleeding.
Several studies have demonstrated that the severity of liver disease is an important factor for the occurrence of early variceal rebleeding; Child-Pugh class C or MELD score ≥18 were suggested to be associated with post-EVL bleeding.10,12,27 In the present study, a significant association between high Child-Turcott-Pugh or MELD score and an increased risk of bleeding after primary EVL was observed in univariate analysis, but the effect was not confirmed after correcting for confounding factors in multivariate analysis. As cirrhotic patients with a previous variceal bleeding episode should have worse liver function, severer coagulopathy, higher portal pressure, and larger varices with or without stigmata of bleeding, such factors might be more influential. However, in patients without a history of previous variceal bleeding, the most important factor appears to be associated with postprocedural management such as administrating PPIs.
Larger esophageal varices and the use of more rubber bands were also reported as independent risk factors for bleeding after EVL in a previous study.27 However, there was no difference in bleeding rates associated with particular endoscopic findings or procedure-related factors in the present study.
The strengths of the present study include the fact that the data were obtained from a relatively large number of patients. In addition, the patient population was homogenous; all patients in this study were treated with primary prophylactic EVL, excluding patients who underwent secondary prophylactic EVL and emergency EVL for acute variceal bleeding.
This study does have several limitations. Firstly, we did not evaluate the changes of the postbanding ulcer size and number by endoscopy. This is because that, in the clinical practice, we usually do not perform follow-up endoscopy very shortly as re-inserting an endoscope soon after EVL may increase the risk of untoward events from postbanding ulcers. Indeed, a previous study recommends performing EVL bimonthly rather than at shorter intervals for this reason.28 Second, we did not assess long-term outcomes after EVL. As this study focused on occurrence and prevention of postbanding ulcer bleeding after EVL, we restricted the follow-up period to 8 weeks. However, we admit that future long-term follow study will be ultimately needed. Last, there were various kinds of PPI medications administered. It would be a better idea to include 1 or 2 type of PPI comparing with placebo for future studies. Nevertheless, our data would still be acceptable for interpretation of the results as there is no evidence that the effect on post-EVL ulcer healing differs according to the type of PPIs used.
In conclusion, not administrating PPIs and the presence of gastric varices were significantly associated with an increased risk of bleeding after prophylactic EVL. In particular, not initiating PPI therapy was the only positive predictive factor for a bleeding complication in patients who received EVL without gastric varix therapy. We suggest PPIs to be considered in patients receiving EVL to reduce the risk of post-EVL ulcer bleeding.
Footnotes
Abbreviations: EVL = endoscopic variceal band ligation, EVO = endoscopic variceal obturation, GVL = gastric variceal band ligation, MELD = Model for End-stage Liver Disease, PPIs = proton pump inhibitors.
This study was partly funded by a grant from the Korea Healthcare Technology R&D Project, Ministry of Health and Welfare, Republic of Korea (HI10C2020), and partly by a research grant from Jeil Pharmaceutical Co., Ltd.
The authors report no conflicts of interest.
REFERENCES
- 1.Bari K, Garcia-Tsao G. Treatment of portal hypertension. World J Gastroenterol 2012; 18:1166–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garcia-Tsao G, Bosch J. Management of varices and variceal hemorrhage in cirrhosis. N Engl J Med 2010; 362:823–832. [DOI] [PubMed] [Google Scholar]
- 3.Grace ND, Groszmann RJ, Garcia-Tsao G, et al. Portal hypertension and variceal bleeding: an AASLD single topic symp∗∗osium. Hepatology 1998; 28:868–880. [DOI] [PubMed] [Google Scholar]
- 4.Gluud LL, Klingenberg S, Nikolova D, et al. Banding ligation versus beta-blockers as primary prophylaxis in esophageal varices: systematic review of randomized trials. Am J Gastroenterol 2007; 102:2842–2848. [DOI] [PubMed] [Google Scholar]
- 5.Khuroo MS, Khuroo NS, Farahat KL, et al. Meta-analysis: endoscopic variceal ligation for primary prophylaxis of oesophageal variceal bleeding. Aliment Pharmacol Ther 2005; 21:347–361. [DOI] [PubMed] [Google Scholar]
- 6.Garcia-Pagán JC, Bosch J. Endoscopic band ligation in the treatment of portal hypertension. Nat Clin Pract Gastroenterol Hepatol 2005; 2:526–535. [DOI] [PubMed] [Google Scholar]
- 7.Vanbiervliet G, Giudicelli-Bornard S, Piche T, et al. Predictive factors of bleeding related to post-banding ulcer following endoscopic variceal ligation in cirrhotic patients: a case-control study. Aliment Pharmacol Ther 2010; 32:225–232. [DOI] [PubMed] [Google Scholar]
- 8.Boo GB, Oh JC, Lee BJ, et al. The effect of proton pump inhibitor on healing of post-esophageal variceal ligation ulcers [Korean]. Korean J Gastroenterol 2008; 51:232–240. [PubMed] [Google Scholar]
- 9.Shaheen NJ, Stuart E, Schmitz SM, et al. Pantoprazole reduces the size of postbanding ulcers after variceal band ligation: a randomized, controlled trial. Hepatology 2005; 41:588–594. [DOI] [PubMed] [Google Scholar]
- 10.Bambha K, Kim WR, Pedersen R, et al. Predictors of early re-bleeding and mortality after acute variceal haemorrhage in patients with cirrhosis. Gut 2008; 57:814–820. [DOI] [PubMed] [Google Scholar]
- 11.D’Amico G, De Franchis R. Cooperative Study Group. Upper digestive bleeding in cirrhosis. Post-therapeutic outcome and prognostic indicators. Hepatology 2003; 38:599–612. [DOI] [PubMed] [Google Scholar]
- 12.Yang MT, Chen HS, Lee HC, et al. Risk factors and survival of early bleeding after esophageal variceal ligation. Hepatogastroenterology 2007; 54:1705–1709. [PubMed] [Google Scholar]
- 13.Tan PC, Hou MC, Lin HC, et al. A randomized trial of endoscopic treatment of acute gastric variceal hemorrhage: N-butyl-2-cyanoacrylate injection versus band ligation. Hepatology 2006; 43:690–697. [DOI] [PubMed] [Google Scholar]
- 14.Tajiri T, Yoshida H, Obara K, et al. General rules for recording endoscopic findings of esophagogastric varices (2nd edition). Dig Endosc 2010; 22:1–9. [DOI] [PubMed] [Google Scholar]
- 15.Sarin SK, Kumar A, Angus PW, et al. Primary prophylaxis of gastroesophageal variceal bleeding: consensus recommendations of the Asian Pacific Association for the Study of the Liver. Hepatol Int 2008; 2:429–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suk KT, Baik SK, Yoon JH, et al. Revision and update on clinical practice guideline for liver cirrhosis. Korean J Hepatol 2012; 18:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sala M, Llovet JM, Vilana R, et al. Initial response to percutaneous ablation predicts survival in patients with hepatocellular carcinoma. Hepatology 2004; 40:1352–1360. [DOI] [PubMed] [Google Scholar]
- 18.Johlin FC, Labrecque DR, Neil GA. Omeprazole heals mucosal ulcers associated with endoscopic injection sclerotherapy. Dig Dis Sci 1992; 37:1373–1376. [DOI] [PubMed] [Google Scholar]
- 19.Gimson A, Polson R, Westaby D, et al. Omeprazole in the management of intractable esophageal ulceration following injection sclerotherapy. Gastroenterology 1990; 99:1829–1831. [DOI] [PubMed] [Google Scholar]
- 20.Jaspersen D, Körner T, Schorr W, et al. Omeprazole in the management of sclerotherapy-induced esophageal ulcers resistant to H2 blocker treatment. J Gastroenterol 1995; 30:128–130. [DOI] [PubMed] [Google Scholar]
- 21.Garg PK, Sidhu SS, Bhargava DK. Role of omeprazole in prevention and treatment of postendoscopic variceal sclerotherapy esophageal complications. Double-blind randomized study. Dig Dis Sci 1995; 40:1569–1574. [DOI] [PubMed] [Google Scholar]
- 22.Avgerinos A, Armonis A, Stefanidis G, et al. Sustained rise of portal pressure after sclerotherapy, but not band ligation, in acute variceal bleeding in cirrhosis. Hepatology 2004; 39:1623–1630. [DOI] [PubMed] [Google Scholar]
- 23.Sarin SK, Govil A, Jain AK, et al. Prospective randomized trial of endoscopic sclerotherapy versus variceal band ligation for esophageal varices: influence on gastropathy, gastric varices and variceal recurrence. J Hepatol 1997; 26:826–832. [DOI] [PubMed] [Google Scholar]
- 24.Svoboda P, Kantorová I, Ochmann J, et al. A prospective randomized controlled trial of sclerotherapy vs ligation in the prophylactic treatment of high-risk esophageal varices. Surg Endosc 1999; 13:580–584. [DOI] [PubMed] [Google Scholar]
- 25.Hidaka H, Nakazawa T, Wang G, et al. Long-term administration of PPI reduces treatment failures after esophageal variceal band ligation: a randomized, controlled trial. J Gastroenterol 2012; 47:118–126. [DOI] [PubMed] [Google Scholar]
- 26.Ahmed AM, al Karawi MA, Shariq S, et al. Frequency of gastroesophageal reflux in patients with liver cirrhosis. Hepatogastroenterology 1993; 40:478–480. [PubMed] [Google Scholar]
- 27.Xu L, Ji F, Xu QW, et al. Risk factors for predicting early variceal rebleeding after endoscopic variceal ligation. World J Gastroenterol 2011; 17:3347–3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yoshida H, Mamada Y, Taniai N, et al. A randomized control trial of bi-monthly versus bi-weekly endoscopic variceal ligation of esophageal varices. Am J Gastroenterol 2005; 100:2005–2009. [DOI] [PubMed] [Google Scholar]


