Abstract
Serotonin (5-hydroxytryptamine; C10H12N2O (5-HT)) is produced in the CNS and in some cells of peripheral tissues. In the mammalian male reproductive system, both 5-HT and tryptophan hydroxylase (TPH) have been described in Leydig cells of the testis and in principal cells of the caput epididymis. In capacitated hamster sperm, it has been shown that 5-HT promotes the acrosomal reaction. The aim of this work was to explore the existence of components of the serotoninergic system and their relevance in human sperm physiology. We used both immunocytochemistry and western blot to detect serotoninergic markers such as 5-HT, TPH1, MAOA, 5-HT1B, 5-HT3, and 5HTT; HPLC for TPH enzymatic activity; Computer Assisted Semen Analysis assays to measure sperm motility parameters and pharmacological approaches to show the effect of 5-HT in sperm motility and tyrosine phosphorylation was assessed by western blot. We found the presence of serotoninergic markers (5-HT, TPH1, MAOA, 5-HT1B, 5-HT2A, 5-HT3, 5-HTT, and TPH enzymatic activity) in human sperm. In addition, we observed a significant increase in tyrosine phosphorylation and changes in sperm motility after 5-HT treatment. In conclusion, our data demonstrate the existence of components of a serotoninergic system in human sperm and support the notion for a functional role of 5-HT in mammalian sperm physiology, which can be modulated pharmacologically.
Introduction
Serotonin (5-hydroxytryptamine; C10H12N2O (5-HT)), a highly conserved molecule in the evolution of life, plays important role in almost all living organisms. 5-HT is synthesized from L-tryptophan by tryptophan hydroxylase (TPH) enzyme (Walther & Bader 2003, Adayev et al. 2005).
Walther et al. (2003) reported the existence of two TPH isotypes; while TPH2 is present only in the brain and TPH1 is present in peripheral organs (Walther & Bader 2003, Walther et al. 2003, Zhang et al. 2004). 5-HT acts as a neurotransmitter in the brain and as a neurohormone in peripheral tissues. This indoleamine binds to more than 15 receptors that are pharmacologically classified into seven families, 5-HT1 through 5-HT7 (with their respective subtypes). With the exception of the 5-HT3 receptor subfamily, all the others are G protein-coupled receptors (Hannon & Hoyer 2002, Adayev et al. 2005, Hannon & Hoyer 2008, Pytliaki et al. 2011).
In peripheral organs, 5-HT is found mainly in mast cells, platelet, and neuroendocrine cells, which can reuptake serotonin and release it (Aguilar et al. 1995, Fujita et al. 1995, Abrahamsson 1999, Mayerhofer et al. 1999, Arrighi et al. 2004). At the reproductive level, 5-HT controls libido and sexual behavior in mammals through a hormonal mechanism induced by the neurons of the preoptic area of the hypothalamus (Popova & Amstislavskaya 2002, Hull et al. 2004).
5-HT also plays important roles in reproductive tissues and some imbalances on its metabolism could affect negatively the reproductive status of mammals (see below). 5-HT induces the contraction of the vas deferens (Hay & Wadsworth 1982) and regulates testicular blood flow (Collin et al. 1996), testicular growth, cAMP production, and testosterone production (Tinajero et al. 1993, Frungieri et al. 2002).
Additionally, the LH induces 5-HT release from Leydig cells via a cAMP-dependent pathway, which promotes the secretion of corticotropin-releasing factor, inhibiting androgen production in Leydig cells (Campos et al. 1990, Dufau et al. 1993, Tinajero et al. 1993, Frungieri et al. 1999). Daily injections of 5-HT precursor (5-hydroxytryptophan (5-HTP)) alter reproductive development and age-dependent body weight gain in mice, accompanied by a low testosterone level and highly reduced sperm counts, motility, and viability (Sethi & Chaturvedi 2009). In addition, studies in humans reported that hyperserotonemia derives in azoospermia (Gonzales et al. 1989, 1992). Furthermore, in reproductive pathologies such as varicocele, 5-HT blood levels are elevated and it has also been proven that serotoninergic neurotransmission dysfunctions are involved in premature ejaculation (de Jong et al. 2006, Chan et al. 2008, Motofei 2008). In humans, 5-HT affects the ejaculatory response and sperm transport (Tanrikut & Schlegel 2007, Tanrikut et al. 2010). Even more, in adult rats, it has been demonstrated that 5-HT has two distinct effects on sexual function depending on the receptor subtype that is activated: 5-HT1A agonists decrease intravaginal ejaculatory latency and erection, whereas 5-HT2C agonists increase both erection and ejaculatory latency (Motofei 2008).
We recently found that both 5-HT concentration and TPH activity increase in the epididymis during sexual maturation and in breeding males when compared with naive ones (Jiménez-Trejo et al. 2007) and also that epididymal serotonin concentration correlated with reproductive fitness, offspring number, mating success, and seminal plug volume in copulatory males (Pichardo et al. 2011).
Mammalian sperm seem to express a particularly broad number of neuroreceptors, including 5-HT receptor subtypes (Meizel 2004). Additionally, 5-HT activates motility and increases the rate of fertilization in invertebrate spermatozoa (Parisi et al. 1984). This increased motility induced by 5-HT might result from the activation of flagella through a process of cAMP-dependent dynein phosphorylation (Bandivdekar et al. 1992, Stephens & Prior 1992). Meizel & Turner (1983) have shown that either 5-HT or its agonist 5-methoxytryptamine induces the acrosomal reaction in capacitated sperm, suggesting the existence of a 5-HT-mediated receptor effect in the hamster sperm. In rabbit, binding to the 5-HT2 receptor group was detected in flagella (Young & Laing 1990). We expanded these observations and reported two distinct 5-HT receptor subtypes (5-HT2A and 5-HT3) in immature rat sperm of the caput epididymis (Jiménez-Trejo et al. 2007). Recently, Fujinoki (2011) has shown that 5-HT promotes hyperactivation of hamster sperm through both 5-HT2 and 5-HT4 receptors.
Protein phosphorylation has been associated with sperm capacitation and the acrosome reaction in several species (Duncan & Fraser 1993, Visconti et al. 1995, Pukazhenthi et al. 1998), so it is an interesting question whether 5-HT could promote protein phosphorylation in human sperm.
In the present work, we explored the hypothesis that there is a serotonin system, independent of others, in human sperm by identifying several serotonergic components present in these cells. Furthermore, we show that 5-HT increases motility and induces tyrosine phosphorylation in human sperm.
Results
Expression of serotonergic markers
5-HT1B receptor (Fig. 1A) was observed intensely in postacrosomal region in human sperm (arrows), while 5-HTT (Fig. 1B) was evident in both postacrosomal and principal piece (arrows). 5-HT3 (Fig. 1C) receptor immunoreactivity was detected in the entire length of the sperm mainly in midpiece region and the principal piece (arrows). Sperm cells showed immunoreactivity to MAOA (Fig. 1D), which was evident in both acrosomal zone and midpiece regions (arrows; n=5). Inserts in Fig. 1A, B, C and D show control experiments.
Figure 1.
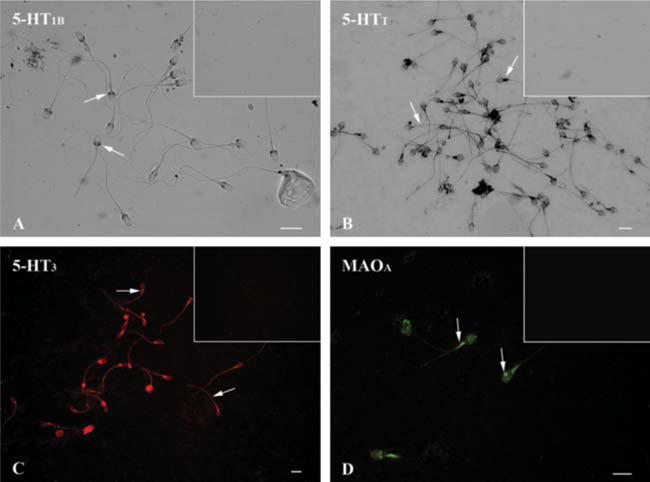
Immunodetection of serotonin-related elements in human sperm. (A) 5-HT1B receptor was detected in postacrosomal region (arrows); 5-HTT transporter (B) was evident in both postacrosomal and principal piece regions (arrows); and the same distribution pattern was found for the 5-HT3 receptor (C, arrows). MAOA (D) were found scarcely in the sperm head and strongly stained in the midpiece (arrows). No staining for any of the antigens analyzed was observed in control cells (insets) when incubated with pre-immune serum or omitted primary antibodies. Scale bar: 10 μm.
Using immunofluorescence, we detected immunoreactivity for 5-HT mainly in the midpiece (arrows, Fig. 2A and B), while TPH was expressed in the acrosomal region (arrows, Fig. 2C). Figure 2B and D and insets therein show control smears. To further validate the expression of these markers, western blots against 5-HT1B (~47 kDa), 5-HT3 (~48 kDa), MAOA (~61 kDa), TPH1 (~51 kDa), and 5-HTT (~64 kDa) proteins were performed (Fig. 3A; n=3); all proteins were found in human smears. Representative Coomassie blue stained gel image corresponding to western blots. Although we found no presence of 5-HT2A receptor using immunocytochemistry, a single band (~53 kDa) using western blot was detected (Fig. 3A).
Figure 2.
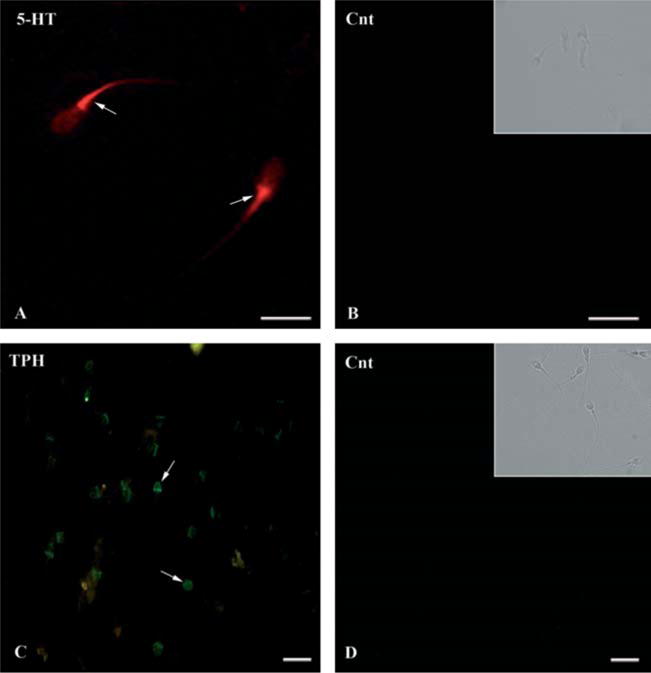
Immunodetection of both 5-HT (A) and TPH in human sperm. Positive staining (arrows) for 5-HT was found at midpiece level of human; tryptophan hydroxylase (TPH) was found strongly stained in the acrosomal zone and lighter in the postacrosomal region of human sperm (C, arrows). No staining for any of the antigens analyzed was observed in control sperm (Cnt: B and D, bright field in insets) when incubated with pre-immune serum or omitted primary antibodies. Scale bar: 10 μm.
Figure 3.
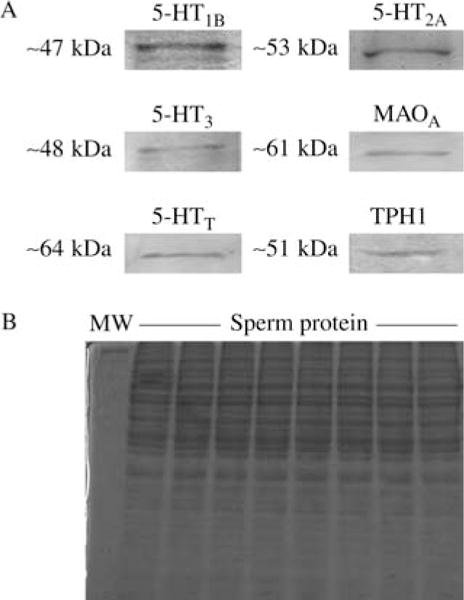
(A) Representative immunoblots of protein extracts from sperm using antibodies related to serotoninergic system. As it can be observed the single bands from different samples of donators per group were positive for 5-HT1B, 5-HT2A, 5-HT3, MAOA, 5-HTT, and TPH1. (B) Control gel showing the presence of sperm protein. Experiments were performed by duplicate.
TPH activity
TPH activity was detected in human sperm homogenates. We found that sperm convert L-tryptophan to 5-HTP (0.2715±0.052 nM/mg protein per h, n=10) and that this activity is reduced in human sperm treated with 50 μM of paracholorophenylalanine (pCPA) for 15 min (0.0332±0.005 nM/mg protein per h, n=5; P<0.01, Fig. 4).
Figure 4.
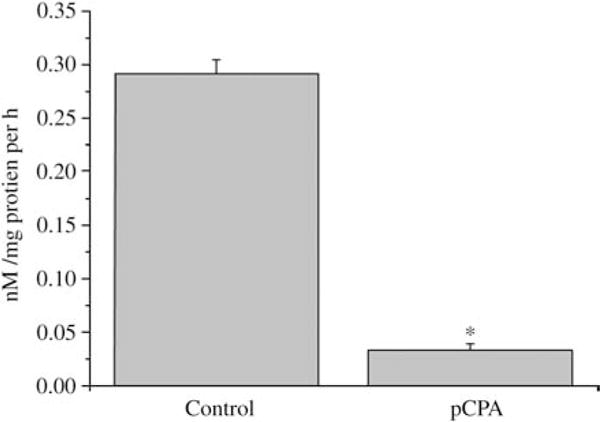
Tryptophan hydroxylase activity monitored through the production of 5-hydroxytryptophan in human sperm using HPLC technique. In vitro pCPA administration produced a drastic decrease in such activity. Asterisk denotes a P<0.001 (Kruskal–Wallis one-way ANOVA followed by Mann–Whitney U test).
Effects of 5-HT on sperm motility
Because progressive motility is an important condition to achieve ovum fertilization in the female reproductive tract during the capacitation (Visconti et al. 1995, Aitken 2006), we decided to evaluate the effect of 5-HT on three parameters of progressive sperm motility using Computer Assisted Semen Analysis (CASA) system. As shown in Fig. 5A, VCL is slightly increased when 5-HT is added to the medium at all concentrations used and throughout time, being significant only in the group of 100 μM 5-HT. The VSL is also increased throughout all the time points tested, being significant only in the groups of 10 and 50 μM (Fig. 5B). As evidenced in Fig. 5C, VAP behaves similar to the VSL (n=5).
Figure 5.
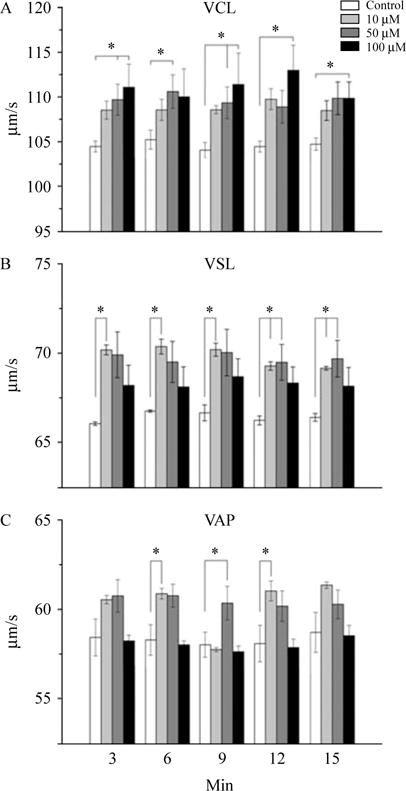
Effect induced by 5-HT on several parameters of human sperm motility at different time points after the in vitro addition of 5-HT. Clear dose-dependent responses in almost all experiments were observed. (A) Curvilinear velocity (VCL); (B) straight-line velocity (VSL); and (C) average path velocity (VAP). Asterisks denote a significant difference vs control group (Kruskal–Wallis one-way ANOVA followed by Mann–Whitney U test).
Effects of 5-HT on tyrosine phosphorylation
We used an antibody that identifies the phosphorylated form of some proteins as a band of ~97 kDa. Figure 6A shows a representative image of the tyrosine phosphorylation in human sperm (n=5); Fig. 6B shows the densitometric analysis of five independent western blot experiments. Sperm incubated with 50 and 100 μM show higher levels of tyrosine phosphorylation (P<0.05, Fig. 6B; n=5). Coomassie blue stained gel images, corresponding to western blots.
Figure 6.
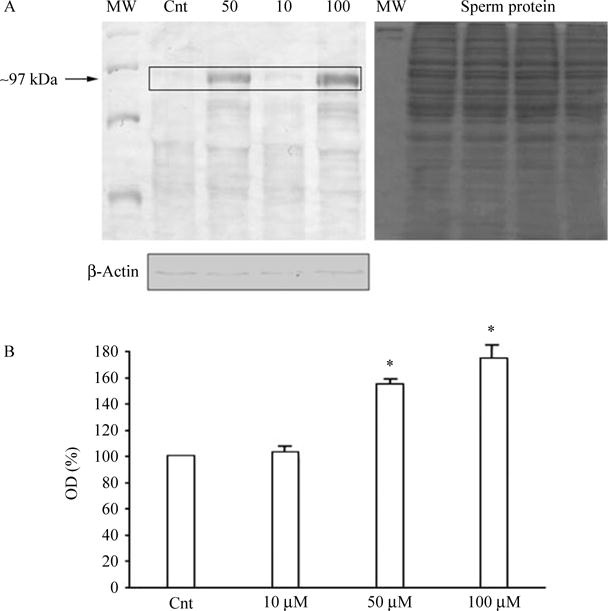
Dose–response curve of 5-HT in the expression of phosphorylated tyrosine after 15 min of incubation with different concentration of 5-HT. (A) Shows the representative western blots of tyrosine phosphorylation and control gel, while densitometric analysis of blots are represented in (B) and show the increase in the optical density of 97 kDa band (box). Asterisks denote significant differences between groups (P<0.05, Kruskal–Wallis one-way ANOVA followed by Mann–Whitney U test).
Discussion
This study demonstrated that several serotonergic components, including 5-HT receptors, MAOA, and TPH1 enzymes, 5-HTT and 5-HT, are present in human sperm. Furthermore, a significant increase induced by 5-HT in both sperm motility and tyrosine phosphorylation activity in human sperm was found.
The presence of 5-HT1B, 5-HT2A, and 5-HT3 receptors in human sperm suggests that these cells may be capable of inducing both second messenger cascades (5-HT1B and 5-HT2A) and fast ionic responses through receptor channels (5-HT3) as it occurs in neuronal tissues (Hannon & Hoyer 2002, 2008, Adayev et al. 2005, Pytliaki et al. 2011).
It has been established that 5-HT synthesis is mediated by TPH1 activity in peripheral nervous tissues. This observation is in agreement with our finding showing that 1) TPH immunoreactivity was found in the sperm head and 5-HT was found in the midpiece and 2) both TPH activity and 5-HT were present in human sperm. In addition, the activity of TPH in human sperm was diminished with the inhibitory compound pCPA, which was previously used to evaluate the function of 5-HT in reproductive tissues (Naumenko & Shishkina 1978, Shishkina & Dygalo 2000, Jiménez-Trejo et al. 2007).
Taken together anatomical, biochemical, and pharmacological approaches, our results strongly suggest that at least some fraction of 5-HT present in the sperm is synthesized directly on it and reinforce the idea that 5-HT should participate in sperm physiology.
Interestingly, 5-HT immunoreactivity was located mainly in the midpiece, a region essential for oxidative metabolism and for promoting sperm motility (Volpe et al. 2009). It seems that 5-HT could activate principal piece through a cAMP-dependent dynein phosphorylation process (Stephens & Prior 1992). In this respect, there is growing evidence that ATP supporting sperm motility is generated by glycolysis, which takes place along the entire length of the principal piece (Mukai & Okuno 2004, Miki 2007). It has been demonstrated that the beat frequency of flagella is proportional to the rate of ATP hydrolysis by dynein when the waveform is kept constant (Okuno & Brokaw 1979). In erythrocytes, 5-HT enhances the glycolytic flux through activation of 6-phosphofructo-1-kinase (PFK), which occurs through modulation of the enzyme binding to the membrane cytoskeleton (Assouline-Cohen et al. 1998). In addition, PFK activity correlates with a whole glycolytic pathway in the muscle (Kemp & Foe 1993). 5-HT enhances skeletal muscle glucose consumption and suggesting that this neurohormone may regulate cell energy metabolism (Coelho et al. 2007); thus, in human sperm, it is probable that 5-HT may play a similar role.
5-HT induced an increase only for VCL at high concentrations while it increases both VSL and VAP but not VCL at lower concentrations. As VCL comprises only the sperm head displacement but not the principal piece movement rate (as VSL and VAP do), we reasoned that a high concentration of 5-HT (50 μM) induces abnormally fast sperm head movements, while our observations at lower concentrations suggest an increase in the linearity of sperm displacement (data not show). Because 50 μM 5-HT increases the tyrosine phosphorylation level, in our study, this observation is online with a previous report, indicating that this indoleamine induces hyperphosphorylation of dynein, in the midpiece, resulting in nonphysiological displacement of the sperm (Bandivdekar et al. 1992, Stephens & Prior 1992). However, the effects of 5-HT in different parameters of sperm motility, at different concentrations observed in our experiments, suggest that 5-HT may participate modulating the sperm displacement at different molecular levels, but this aspect require further investigation.
Because the modulation of the intracellular calcium concentration [Ca2+]i is fundamental for some specific aspects of sperm physiology, we explored the intracellular calcium signaling induced by 5-HT in human sperm to evaluate functionality of ion channels associated with some serotonergic receptors using spectrofluorometric methods as described in Torres-Flores et al. (2008), but we did not find functional activity for any of these that was able to induce changes in the opening of calcium channels (data not shown).
5-HT (50 μM) or its analog 5α-methoxytryptamine (5 μM) promotes an acrosomal reaction in hamster sperm 15 min after its incubation (Meizel & Turner 1983). Recently, Fujinoki (2011) reported that 5-HT promotes sperm hyperactivation when added to hamster sperm at concentrations from 1 fmol/l to 1 μmol/l. Furthermore, using agonists and antagonists of 5-HT receptors (5-HT2 and 5-HT4), the sperm motility was modified, supporting that 5-HT could alter sperm physiology.
On the other hand, MAOA was expressed in both head and midpiece of the sperm; this finding suggests that degradation of 5-HT is potentially active in human sperm, as it has been reported in neurons and principal cells of the epididymis (Gaspar et al. 2003, Jiménez-Trejo et al. 2007).
Moreover, it could be possible that 5-HT will be present in the female genital tract, probably promoting physiological responses in human sperm during their transit through it, as suggested in vitro experiments where the precursor-free amino acid L-tryptophan is the natural chemoattractant for sperm of the red abalone Haliotis rufescens (Riffell et al. 2002). This metabolite is rapidly released from eggs at concentrations sufficient to induce changes both in speed and in direction of sperm swimming, whereas that the effect of 5-HTon directional motility of human sperm under in vitro conditions is possible. Because we found both receptors and MAOA, it is tempting to postulate that 5-HT system could be a sensing mechanism for the sperm in their way to the oocyte through female tract.
As 5-HT is a molecule involved in many physiological functions (Popova & Amstislavskaya 2002, Gaspar et al. 2003, Hull et al. 2004, Adayev et al. 2005), and because it is synthesized, degraded, and stored directly in the human sperm, we postulate that 5-HT may participate as a regulator of particular physiological processes in animal sperm. Taken together, the current findings support an important role of 5-HT on the migration of sperm throughout the female tract. The possibility that 5-HT plays a role in sperm physiology may occur at 5-HT receptors and/or 5-HT transporters located on the surface of sperm, responding to the 5-HT contained in the uterine or seminal fluid (Mann et al. 1961, Amenta et al. 1992). However, the surprising finding that some other serotoninergic elements are intracellular remains unclear and requires further investigation. A more precise knowledge of mechanisms involved in serotonin role on mammalian sperm may be useful in the development of improved contraceptives and in the treatment of motility-related sperm infertility.
Materials and Methods
Sperm purification
Human sperm was obtained from a panel of 12 healthy volunteers between the ages of 20 and 28 years old with 3–5 days of sexual abstinence. All volunteers agreed to participate in this study after reading and signing a letter of informed consent, approved by the Ethics Committee of the Faculty of Medicine (UNAM) in accordance with the principles of research involving human subjects expressed in the Declaration of Helsinki. Sperm cells (≈100×106/ml) were separated from the seminal plasma using isotonic Percoll gradients (75/50% Percoll; Linares-Hernández et al. 1998). After this, they were washed in Hepes-buffered human sperm medium for its conservation and further assays (González-Martínez et al. 2002).
Immunocytochemistry
After separating human sperm from seminal liquid, smears of sperm were mounted onto gelatin-coated slides and fixed in 4% paraformaldehyde dissolved in phosphate buffer (0.1 mol/l, pH 7.4). Sperm preparations were used for detecting 5-HT, TPH1, MAOA, 5-HTT, 5-HT1B, 5-HT2A, and 5-HT3 receptors as described previously (Jiménez-Trejo et al. 2007). Commercial antibodies used were 1) mouse monoclonal anti-5-HT (1:100, Genetex, Irvine, CA, USA), 2) goat polyclonal anti-TPH (C-20; 1:100), 3) rabbit polyclonal anti-5-HT transporter (1:250, Millipore, Billerica, MA, USA), 4) goat polyclonal anti-5-HT1B receptor (1:200), 5) goat polyclonal anti-5-HT2A receptor (1:100), 6) goat polyclonal anti-5-HT3 receptor antibody (1:200), and 7) rabbit polyclonal anti-MAOA (1:200). Antibodies for MAOA, TPH (C-20), 5-HT1B, and 5-HT3 subtype receptors were supplied by Santa Cruz Biotechnology, Inc. Control slides were incubated with pre-immune serum or the incubation with primary antibodies was omitted.
The sections were visualized and images acquired using an Olympus BX51 epifluorescence microscope equipped with a digital camera Olympus DP70 (Olympus America, Inc.). Images were digitized and figures elaborated using Adobe Photoshop Software (Adobe Systems, Inc.).
Western blot
Sperm samples were processed for SDS–PAGE as described previously (Torres-Flores et al. 2008). Samples were prepared for western blot analysis and immunoblots were performed using the following antibodies: rabbit monospecific polyclonal anti-TPH1 (1:500), which was produced by Dr D M Kuhn using peptides corresponding to the sequence L435ARVSRWPSV444 in the C-terminal region of TPH1, rabbit anti-5HTT, goat anti-5-HT1B, goat anti-5HT2A, goat anti-5HT3, or rabbit anti-MAOA (n=5 for each treatment) whose characteristics were described earlier.
TPH activity
After identifying the presence of TPH on sperm cell, we evaluated the physiological activity of this enzyme in vitro using HPLC system. The activity of TPH in the human sperm was estimated by measuring the production of 5-HTP following the protocol described previously (Johansen et al. 1991, Jiménez-Trejo et al. 2007). Accordingly, an aliquot of ~85×106 sperms was used to measure the production of 5-HTP as described by Jiménez-Trejo et al. (2007). Results are expressed in nanomoles of product/milligram of protein per hour (nmol/mg protein per h). Chromatograms were recorded online and the peak heights measured using Millennium 32 Software (Waters Co., Milford, MA, USA). In another set, TPH activity was analyzed in sperm treated with 50 μM of a competitive inhibitor of the serotonin (5-HT) synthesis enzyme TPH, pCPA, incubated 15 min in culture medium (Ham’s F-10), and immediately centrifuged and freezing until use (n=5 for each treatment).
Effects of 5-HT on sperm motility
Sperm samples were immersed in Ham’s F-10 medium supplemented with 10% synthetic substitute serum. After 5 min of stabilization, different concentrations (10, 50, and 100 μM) of 5-HT were added to the medium. The effect in sperm motility was evaluated at different times (3, 6, 9, 12, and 15 min) with the aid of CASA (Hamilton Thorne Motility Analyzer; HTM-IVOS v.12; Hamilton Thorne, Inc., Beverly, MA, USA). We measured three parameters: curvilinear velocity (VCL), straight-line velocity (VSL), and average path velocity (VAP). After incubation, the samples were processed to detect phosphotyrosine proteins by western blot (n=5 for each treatment).
Effects of 5-HT on tyrosine phosphorylation
Sperm were preincubated with different concentrations of 5-HT (0, 10, 50, and 100 μM, ~4×107 cells) in Ham’s F-10 medium supplemented with 10% serum synthetic substitute. Total proteins from human sperm were used to detect tyrosine phosphorylation using western blot assays (Luconi et al. 1995, Torres-Flores et al. 2008). The immunoblots were incubated with primary antibody mouse anti-phosphotyrosine coupled with peroxidase (Sigma Chemical Co.). A chemiluminescent HRP substrate kit (ECL, Amersham-Pharmacia-Biotech) was used to detect immunoreactivity. Images of these films were captured, digitized, and analyzed in the same conditions (Software Quantity One 4.4.1, Bio-Rad) using a computer-based imaging analysis system (Fluor S Multimager, Bio-Rad). All measurements were corrected based on the average value of each film’s background (n=5 for each treatment). All assays were carried out by duplicated.
Statistical analysis
Normality tests (Shapiro–Wilk and Anderson–Darling test) and variance homogeneity test (Levene’s test) were used for all experimental data groups. For comparing the TPH1 activity, a Student’s t-test was used. Other comparisons were done using Kruskal–Wallis ANOVA followed by Mann–Whitney U test. A value of P<0.05 was considered to be statistically significant.
Acknowledgments
The authors thank Luis Martínez Méndez, Patricia Padilla, and Alan Larsen. They also thank Dr Miguel Ángel León-Galván for her valuable technical assistance and Dr Gabriel Gutiérrez-Ospina for helpful criticisms.
Funding
This study was partially supported by CONACYT-80338, PAPIIT IN219710-2, PAPIIT IN210412, COFAA-SIP-IPN, and DGAPA/UNAM.
Footnotes
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
References
- Abrahamsson PA. Neuroendocrine cells in tumor growth of the prostate. Endocrine-Related Cancer. 1999;6:503–519. doi: 10.1677/erc.0.0060503. [DOI] [PubMed] [Google Scholar]
- Adayev T, Ranasinghe B, Banerjee P. Transmembrane signaling in the brain by serotonin, a key regulator of physiology and emotion. Bioscience Reports. 2005;25:363–384. doi: 10.1007/s10540-005-2896-3. [DOI] [PubMed] [Google Scholar]
- Aguilar R, Anton F, Bellido C, Aguilar E, Gaytan F. Testicular serotonin is related to mast cells but not to Leydig cells in the rat. Journal of Endocrinology. 1995;146:15–21. doi: 10.1677/joe.0.1460015. [DOI] [PubMed] [Google Scholar]
- Aitken RJ. Sperm function tests and fertility. International Journal of Andrology. 2006;29:69–75. doi: 10.1111/j.1365-2605.2005.00630.x. [DOI] [PubMed] [Google Scholar]
- Amenta F, Vega JA, Ricci A, Collier WL. Localization of 5-hydroxytryptamine-like immunoreactive cells and nerve fibers in the rat female reproductive system. Anatomical Record. 1992;233:478–484. doi: 10.1002/ar.1092330315. [DOI] [PubMed] [Google Scholar]
- Arrighi S, Cremonesi F, Bosi G, Domeneghini C. Endocrine–paracrine cells of the male urogenital apparatus: a comparative histochemical and immunohistochemical study in some domestic ungulates. Anatomia, Histologia, Embryologia. 2004;33:225–232. doi: 10.1111/j.1439-0264.2004.00541.x. [DOI] [PubMed] [Google Scholar]
- Assouline-Cohen M, Ben-Porat H, Beitner R. Activation of membrane skeleton-bound phosphofructokinase in erythrocytes induced by serotonin. Molecular Genetics and Metabolism. 1998;63:235–238. doi: 10.1006/mgme.1997.2673. [DOI] [PubMed] [Google Scholar]
- Bandivdekar AH, Segal SJ, Koide SS. Binding of 5-hydroxytryptamine analogs by isolated Spisula sperm membrane. Invertebrate Reproduction & Development. 1992;21:43–46. doi: 10.1080/07924259.1992.9672218. [DOI] [Google Scholar]
- Campos MB, Vitale ML, Calandra RS, Chiocchio SR. Serotoninergic innervation of the rat testis. Journal of Reproduction and Fertility. 1990;88:475–479. doi: 10.1530/jrf.0.0880475. [DOI] [PubMed] [Google Scholar]
- Chan JS, Olivier B, de Jong TR, Snoeren EM, Kooijman E, van Hasselt FN, Limpens JH, Kas MJ, Waldinger MD, Oosting RS. Translational research into sexual disorders: pharmacology and genomics. European Journal of Pharmacology. 2008;585:426–435. doi: 10.1016/j.ejphar.2008.02.098. [DOI] [PubMed] [Google Scholar]
- Coelho WS, Costa KC, Sola-Penna M. Serotonin stimulates mouse skeletal muscle 6-phosphofructo-1-kinase through tyrosinephosphorylation of the enzyme altering its intracellular localization. Molecular Genetics and Metabolism. 2007;92:364–370. doi: 10.1016/j.ymgme.2007.07.010. [DOI] [PubMed] [Google Scholar]
- Collin O, Damber JE, Bergh A. 5-Hydroxytryptamine a local regulator of testicular blood flow and vasomotion in rats. Journal of Reproduction and Fertility. 1996;106:17–22. doi: 10.1530/jrf.0.1060017. [DOI] [PubMed] [Google Scholar]
- Dufau ML, Tinajero JC, Fabbri A. Corticotropin-releasing factor: an antireproductive hormone of the testis. FASEB Journal. 1993;7:299–307. doi: 10.1096/fasebj.7.2.8382638. [DOI] [PubMed] [Google Scholar]
- Duncan AE, Fraser LR. Cyclic AMP-dependent phosphorylation of epididymal mouse sperm proteins during capacitation in vitro: identification of an M(r) 95,000 phosphotyrosine-containing protein. Journal of Reproduction and Fertility. 1993;97:287–299. doi: 10.1530/jrf.0.0970287. [DOI] [PubMed] [Google Scholar]
- Fujinoki M. Serotonin-enhanced hyperactivation of hamster sperm. Reproduction. 2011;142:255–266. doi: 10.1530/REP-11-0074. [DOI] [PubMed] [Google Scholar]
- Fujita T, Iwananga T, Lee SH, Terada M. Sensor cells and nerve terminals in some gut-derived organs: a review. Italian Journal of Anatomy and Embryology. 1995;100:197–204. [PubMed] [Google Scholar]
- Frungieri MB, González-Calvar SI, Rubio M, Ozu M, Lustig L, Calandra RS. Serotonin in golden hamster testes: testicular levels, immunolocalization and role during sexual development and photoperiodic regresion-recrudescence transition. Neuroendocrinology. 1999;69:299–308. doi: 10.1159/000054431. [DOI] [PubMed] [Google Scholar]
- Frungieri MB, Zitta K, Pignataro OP, Gonzalez-Calvar SI, Calandra RS. Interactions between testicular serotoninergic, catecholaminergic, and corticotropin-releasing hormone systems modulating cAMP and testosterone production in the golden hamster. Neuroendocrinology. 2002;76:35–46. doi: 10.1159/000063682. [DOI] [PubMed] [Google Scholar]
- Gaspar P, Cases O, Maroteaux L. The developmental role of serotonin: news from mouse molecular genetics. Nature Reviews Neuroscience. 2003;4:1002–1012. doi: 10.1038/nrn1256. [DOI] [PubMed] [Google Scholar]
- Gonzales GF, García-Hjarles MA, Napuri R, Coyotupa J, Guerra-Garcia R. Blood serotonin levels and male infertility. Archives of Andrology. 1989;22:85–89. doi: 10.3109/01485018908986755. [DOI] [PubMed] [Google Scholar]
- Gonzales GF, García-Hjarles MA, Velasquez G. Hyperprolactinaemia and hyperserotoninaemia: their relationship to seminal quality. Andrologia. 1992;24:95–100. doi: 10.1111/j.1439-0272.1992.tb02617.x. [DOI] [PubMed] [Google Scholar]
- González-Martínez MT, Bonilla-Hernandez MA, Guzmán-Grenfell AM. Stimulation of voltage-dependent calcium channels during capacitation and by progesterone in human sperm. Archives of Biochemistry and Biophysics. 2002;408:205–210. doi: 10.1016/S0003-9861(02)00587-8. [DOI] [PubMed] [Google Scholar]
- Hannon J, Hoyer D. Serotonin receptor and systems: endless diversity? Acta Biologica Szegediensis. 2002;46:1–12. [Google Scholar]
- Hannon J, Hoyer D. Molecular biology of 5-HT receptors. Behavioral Brain Research. 2008;195:198–213. doi: 10.1016/j.bbr.2008.03.020. [DOI] [PubMed] [Google Scholar]
- Hay DW, Wadsworth RM. The contractile effects of 5-hydroxytryptamine on the rat isolated vas deferens. British Journal of Pharmacology. 1982;77:605–613. doi: 10.1111/j.1476-5381.1982.tb09338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull EM, Muschamp JW, Sato S. Dopamine and serotonin: influences on male sexual behavior. Physiology & Behavior. 2004;83:291–307. doi: 10.1016/j.physbeh.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Jiménez-Trejo F, Tapia-Rodríguez M, Queiroz DB, Padilla P, Avellar MC, Rivas-Manzano PR, Manjarrez-Gutiérrez G, Gutiérrez-Ospina G, Manjarrez-Gutiérrez G, Gutiérrez-Ospina G. Serotonin concentration, synthesis, cell origin, and targets in the rat caput epididymis during sexual maturation and variations associated with adult mating status: morphological and biochemical studies. Journal of Andrology. 2007;28:136–149. doi: 10.2164/jandrol.106.000653. [DOI] [PubMed] [Google Scholar]
- Johansen PA, Wolf WA, Kuhn DM. Inhibition of tryptophan hydroxylase by benserazide and other catechols. Biochemical Pharmacology. 1991;41:625–628. doi: 10.1016/0006-2952(91)90636-J. [DOI] [PubMed] [Google Scholar]
- de Jong TR, Veening JG, Waldinger MD, Cools AR, Olivier B. Serotonin and the neurobiology of the ejaculatory threshold. Neuroscience and Biobehavioral Reviews. 2006;30:893–907. doi: 10.1016/j.neubiorev.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Kemp RG, Foe LG. Allosteric regulatory properties of muscle phosphofructokinase. Molecular and Cellular Biochemistry. 1993;57:147–154. doi: 10.1007/BF00849191. [DOI] [PubMed] [Google Scholar]
- Linares-Hernández L, Guzmán-Grenfell AM, Hicks-Gómez JJ, González-Martínez MT. Voltage-dependent calcium influx in human sperm assessed by simultaneous optical detection of [Ca2+]i and membrane potential. Biochimica et Biophysica Acta. 1998;1372:1–12. doi: 10.1016/S0005-2736(98)00035-2. [DOI] [PubMed] [Google Scholar]
- Luconi M, Bonaccorsi L, Krausz C, Gervasi G, Forti G, Baldi E. Stimulation of protein tyrosine phosphorylation by platelet-activating factor and progesterone in human spermatozoa. Molecular and Cellular Endocrinology. 1995;108:35–42. doi: 10.1016/0303-7207(95)92576-A. [DOI] [PubMed] [Google Scholar]
- Mann T, Seamark RF, Sharman DF. On the question of the occurrence and metabolism of 5-hydroxytryptamine and related indole compounds in mammalian semen. British Journal of Pharmacology. 1961;17:208–217. doi: 10.1111/j.1476-5381.1961.tb01280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayerhofer A, Frungieri MB, Bulling A, Fritz S. Sources and function of neuronal signalling molecules in the gonads. Medicina. 1999;59:542–545. [PubMed] [Google Scholar]
- Meizel S. The sperm, a neuron with a tail: “neuronal” receptors in mammalian sperm. Biological Reviews of the Cambridge Philosophical Society. 2004;79:713–732. doi: 10.1017/S1464793103006407. [DOI] [PubMed] [Google Scholar]
- Meizel S, Turner KO. Serotonin or its agonist 5-methoxytryptamine can stimulate hamster sperm acrosome reactions in a more direct manner than catecholamines. Journal of Experimental Zoology. 1983;226:171–174. doi: 10.1002/jez.1402260120. [DOI] [PubMed] [Google Scholar]
- Miki K. Energy metabolism and sperm function. Society of Reproduction and Fertility Supplement. 2007;65:309–325. [PubMed] [Google Scholar]
- Motofei IG. A dual physiological character for sexual function: the role of serotonergic receptors. British Journal of Urology International. 2008;101:531–534. doi: 10.1111/j.1464-410X.2007.07255.x. [DOI] [PubMed] [Google Scholar]
- Mukai C, Okuno M. Glycolysis plays a major role for adenosine triphosphate supplementation in mouse sperm flagellar movement. Biology of Reproduction. 2004;71:540–547. doi: 10.1095/biolreprod.103.026054. [DOI] [PubMed] [Google Scholar]
- Naumenko EV, Shishkina GT. Role of serotonin in feedback control of hypothalamic–pituitary–testicular complex in male rats. Neuroendocrinology. 1978;26:359–366. doi: 10.1159/000122791. [DOI] [PubMed] [Google Scholar]
- Okuno M, Brokaw CJ. Inhibition of movement of tritondemembranated sea-urchin sperm flagella by Mg2+, ATP4−, ADP and P1. Journal of Cell Science. 1979;38:105–123. doi: 10.1242/jcs.38.1.105. [DOI] [PubMed] [Google Scholar]
- Parisi E, De Prisco P, Capasso A, del Prete M. Serotonin and sperm motility. Cell Biology International Reports. 1984;8:95. doi: 10.1016/0309-1651(84)90075-4. [DOI] [PubMed] [Google Scholar]
- Pichardo AI, Tlachi-López J, Jiménez-Trejo F, Fuentes-Farías A, Baez-Saldaña A, Molina-Cerón M, Manjarrez-Gutierrez G, Gutiérrez-Ospina G, Lucio A. Increased serotonin concentration and tryptophan hydroxylase activity in reproductive organs of copulator males: a case of adaptive plasticity. Advances in Bioscience and Biotechnology. 2011;2:75–83. doi: 10.4236/abb.2011.22012. [DOI] [Google Scholar]
- Popova NK, Amstislavskaya TG. 5-HT2A and 5-HT2C serotonin receptors differentially modulate mouse sexual arousal and the hypothalamo-pituitary–testicular response to the presence of a female. Neuroendocrinology. 2002;76:28–34. doi: 10.1159/000063681. [DOI] [PubMed] [Google Scholar]
- Pukazhenthi BS, Long JA, Wildt DE, Ottinger MA, Armstrong DL, Howard J. Regulation of sperm function by protein tyrosine phosphorylation in diverse wild feud species. Journal of Andrology. 1998;19:675–685. [PubMed] [Google Scholar]
- Pytliak M, Vargova V, Mechírova V, Felsöci M. Serotonin receptors –from molecular biology to clinical applications. Physiological Research. 2011;60:15–25. doi: 10.33549/physiolres.931903. [DOI] [PubMed] [Google Scholar]
- Riffell JA, Krug PJ, Zimmer RK. Fertilization in the sea: the chemical identity of an abalone sperm attractant. Journal of Experimental Zoology. 2002;205:1439–1450. doi: 10.1242/jeb.205.10.1439. [DOI] [PubMed] [Google Scholar]
- Sethi S, Chaturvedi CM. Temporal sinergism of neurotransmitters (serotonin and dopamine) affects testicular development in mice. Zoology. 2009;112:461–470. doi: 10.1016/j.zool.2009.03.002. [DOI] [PubMed] [Google Scholar]
- Shishkina GT, Dygalo NN. Role of the serotoninergic system in the acceleration of sexual maturation in wild Norway rats selected for reduced aggressiveness toward humans. Comparative Biochemistry and Physiology Toxicology & Pharmacology. 2000;125:45–51. doi: 10.1016/S0742-8413(99)00092-4. [DOI] [PubMed] [Google Scholar]
- Stephens RE, Prior G. Dynein from serotonin-activated cilia and flagella: extraction characteristics and distinct sites for cAMP-dependent protein phosphorylation. Journal of Cell Science. 1992;103:999–1012. doi: 10.1242/jcs.103.4.999. [DOI] [PubMed] [Google Scholar]
- Tanrikut C, Schlegel PN. Antidepressant-associated changes in semen parameters. Urology. 2007;69:185e5–185e7. doi: 10.1016/j.urology.2006.10.034. [DOI] [PubMed] [Google Scholar]
- Tanrikut C, Feldman AS, Altemus M, Paduch DA, Schlegel PN. Adverse effect of paroxetine on sperm. Fertility and Sterility. 2010;94:1021–1026. doi: 10.1016/j.fertnstert.2009.04.039. [DOI] [PubMed] [Google Scholar]
- Tinajero JC, Fabbri A, Ciocca DR, Dufau ML. Serotonin secretion from rat Leydig cells. Endocrinology. 1993;133:3026–3029. doi: 10.1210/en.133.6.3026. [DOI] [PubMed] [Google Scholar]
- Torres-Flores V, Hernández-Rueda YL, Neri-Vidaurri P, del C, Jiménez-Trejo F, Calderón-Salinas V, Molina-Guarneros JA, González-Martínez MT. Activation of protein kinase A stimulates the progesterone-induced calcium influx in human sperm exposed to the phosphodiesterase inhibitor papaverine. Journal of Andrology. 2008;29:549–557. doi: 10.2164/jandrol.107.004614. [DOI] [PubMed] [Google Scholar]
- Visconti PE, Bailey JL, Moore GD, Pan D, Olds-Clarke P, Kopf GS. Capacitation of mouse spermatozoa. I. Correlation between the capacitation state and protein tyrosine phosphorylation. Development. 1995;121:1129–1137. doi: 10.1242/dev.121.4.1129. [DOI] [PubMed] [Google Scholar]
- Volpe S, Leoci R, Aiudi G, Lacalandra GM. Relationship between motility and mitochondrial functional status in canine spermatozoa. Reproduction in Domestic Animals. 2009;44(Supplement 2):275–278. doi: 10.1111/j.1439-0531.2009.01457.x. [DOI] [PubMed] [Google Scholar]
- Walther DJ, Bader M. A unique central tryptophan hydroxylase isoform. Biochemical Pharmacology. 2003;66:1673–1680. doi: 10.1016/S0006-2952(03)00556-2. [DOI] [PubMed] [Google Scholar]
- Walther DJ, Peter JU, Bashammakh S, Hortnagl H, Voits M, Fink H, Bader M. Synthesis of serotonin by a second tryptophan hydroxylase isoform. Science. 2003;299:76. doi: 10.1126/science.1078197. [DOI] [PubMed] [Google Scholar]
- Young RJ, Laing JC. Biogenic amine binding sites in rabbit spermatozoa. Biochemistry International. 1990;4:781–787. [PubMed] [Google Scholar]
- Zhang X, Beaulieu JM, Sotnikova TD, Gainetdinov RR, Caron MG. Tryptophan hydroxylase-2 controls brain serotonin synthesis. Science. 2004;5681:217. doi: 10.1126/science.1097540. [DOI] [PubMed] [Google Scholar]


