Abstract
Background and Aims
Natalizumab is an efficacious agent for induction and maintenance of remission in Crohn’s disease (CD) patients who have failed anti-tumor necrosis factor (TNF) agents. We aimed to assess the efficacy and safety of natalizumab outside of clinical trial at a US tertiary center.
Methods
Retrospective case review of CD patients receiving natalizumab.
Results
Forty-nine CD patients (28 female, median age 33) receiving natalizumab from April 2008 to November 2011 were identified. Median duration of disease was 180 months (range 36–576), and 40 had ileocolonic disease, 1 had ileal disease, and 8 had colonic disease. Twenty-one patients had penetrating disease, and 28 had a history of CD-related surgery. Forty-seven patients previously failed treatment with at least one anti-TNF agent. Median duration of natalizumab treatment was 7 months (interquartile range 3–21.5). Twenty-four patients (49%) were continuing natalizumab at the time of this review, and twenty-five discontinued treatment due to lack of response, side effects, or positive JC virus antibody. Seventeen patients (35%) successfully continued treatment with natalizumab for longer than 12 months, and non-penetrating disease phenotype was identified as a predictor of longer response (vs. penetrating phenotype, p = 0.013). Nine patients (18.4%) experienced adverse effects, five of which were serious, but no case of progressive multifocal leukoencephalopathy occurred.
Conclusion
This is the largest series of natalizumab treated patients with CD. Our results show that natalizumab is an efficacious and safe treatment for patients refractory to anti-TNF agents and that non-penetrating disease phenotype has more durable remission over time.
Keywords: Crohn’s disease, natalizumab, biologic, safety
INTRODUCTION
Crohn’s disease (CD) is a type of chronic inflammatory bowel disease (IBD) characterized by chronic, usually progressive, transmural inflammation1. The precise pathogenesis of CD remains unknown, but is thought to reflect dysregulated intestinal inflammation related to a combination of genetic susceptibility and environmental factors including an altered intestinal flora2–4.
Conventional treatments including 5-aminosalicylic acid (5-ASAs), corticosteroids, and immunomodulators have been used for many decades in the treatment of active CD5. 5-ASAs and immunomodulators are also used for maintenance of remission6, 7. More recently, anti-tumor necrosis factor (TNF) agents have been shown to be effective in inducing and maintaining remission in CD8–10. Natalizumab (Tysabri®, Elan Pharmaceuticals, South San Francisco, CA), a humanized IgG4 anti-á4 -integrin antibody, blocks the adhesion and subsequent migration of leukocytes from the blood vessels into inflamed tissues. Natalizumab treatment was effective and well-tolerated in clinical trials of CD11, 12; however, due to the development of progressive multifocal leukoencephalopathy (PML) in subsequent post marketing surveillance, its labeled use is limited to adult CD patients who have failed or have intolerance to conventional treatments such as anti-TNF agents. Furthermore, the FDA has restricted its use to mono-therapy (along with short-term steroids) without concomitant immunosuppressants or anti-TNF agents.
The present retrospective case review was undertaken to identify the effectiveness of natalizumab in clinical practice and to assess the frequency and the features of adverse events occurring during the course of natalizumab treatment for CD at a US tertiary center.
METHODS
This was a retrospective case review of patients with CD who received natalizumab therapy from April 2008 to November 2011 at the University of Chicago Inflammatory Bowel Disease Center. The study was approved by the institutional review board. Physicians at the University of Chicago IBD Center are registered in the CD TOUCH (TYSABRI Outreach: Unified Commitment to Health) ® Prescribing Program.
Participants
Forty-nine CD patients who received natalizumab treatment were identified by CD TOUCH registry and further chart review. Clinical charts were reviewed for patient demographics, CD characteristics and severity (according to the Harvey Bradshaw index [HBI]), extraintestinal manifestations, previous CD treatments, natalizumab treatment (duration, dosages and adverse events), comorbidities, laboratory parameters, and concomitant systemic CD treatments (duration and dosages).
Treatment
Natalizumab (300 mg) was administered intravenously over one 1 hour, either at the University of Chicago Medical Center or local infusion clinics. Subsequent treatment was scheduled every 4-weeks. Nearly all patients (98.0%) had failed at least one of the three anti-TNF agents (adalimumab, certolizumab pegol or infliximab) or immunomodulators (azathioprine, 6-mercaptopurine, or methotrexate), and, prior to initiation of natalizumab, these agents were discontinued. There was no wash-out period prior to initiating natalizumab therapy. Concomitant corticosteroid or 5-ASA treatment was permitted.
Outcomes
Outcome measures were defined prior to performing the case review. Patients who had received natalizumab outside of clinical trials for any duration prior to November 2011 were included. Clinical and laboratory parameters of patients continuing therapy as of November 2011 were compared to those who had discontinued therapy. Clinical responders and non-responders at 12 months were identified and clinical and laboratory parameters were compared between the two groups. Clinical response was identified when the following criteria were met: improvement of HBI by more than 3 points or improvement of overall Crohn’s symptoms as assessed by the physician, decrease in steroid dose, and not requiring additional CD treatment including surgery.
Adverse events were classified as mild (did not required treatment suspension) or severe (required treatment suspension and/or close monitoring and/or additional treatment). The rate of adverse events and the rate of withdrawals due to severe adverse events were assessed.
Statistical methods
For statistical analysis, data were processed by using Graphpad Prism (La Jolla, CA, USA) and R version 2.14.1 (Vienna, Austria). Standard descriptive statistics, such as mean, median, range and standard deviations were computed for continuous variables. Statsitical analysis was done as noted in each section. Clinical and laboratory parameters between groups were compared by either Fisher's exact test or Mann–Whitney U test. Multivariate discriminant analysis was performed where indicated. Duration of treatment was compared between groups by Kaplan-Meier survival analysis where indicated. All p-values are two-sided and p < 0.05 was considered statistically significant.
RESULTS
Patients
This review included patients treated from April 2008 to November 2011. The characteristics of the 49 patients that received natalizumab are shown in table 1. Twenty-one were male with a median age of 33 years (range 20–62 years). Median duration of disease was 180 months (range 36–576 months). One patient had isolated small bowel (ileal) disease, 8 patients had isolated colonic disease, and 40 patients had ileocolonic disease. Twenty-one patients had a fistulizing disease phenotype and 17 had non-fistulizing disease phenotype. In all patients, the indication for the use of natalizumab was refractory CD failing multiple medical treatments. There were 2 patients with CD and multiple sclerosis who had never received anti-TNF agents, but the remaining patients had failed at least one anti-TNF agent in the past.
Table 1.
Patient characteristics
| Entire patients n = 49 |
Continuing natalizumaba n = 24 |
Discontinuing natalizumabb n = 25 |
p value between a and b |
|
|---|---|---|---|---|
| Gender (male : female) | 21 : 28 | 10 : 14 | 11 : 14 | 0.87† |
| Age (years), median (range) | 33 (20–62) | 34.5 (20–62) | 32 (22–57) | 0.38 |
| Weight (kg), median (IQR) | 68.1 (61.1–85.9) | 68.1 (63.6–86.5) | 68.4 (59.9–85.7) | 0.66 |
| BMI, median (IQR) | 24.9 (20.6–28.4) | 23.2 (20.6–27.1) | 25.89 (20.9–29.8) | 0.42 |
| Age at diagnosis, median (range) | 18 (8–48) | 17 (11–40) | 18 (8–48) | 0.60 |
| Disease duration (months), median (range) | 180 (36–576) | 180 (67–576) | 144 (36–264) | 0.14 |
| Disease location (ileum: colon: ileocolonic) | 1 : 8 : 40 | 1 : 5 : 18 | 0 : 3 : 22 | 0.40† |
| Upper intestinal involvement, n (%) | 5 (10.2) | 2 (8.3) | 3 (12.0) | 0.67† |
| Perianal disease, n (%) | 10 (20.4) | 5 (20.8) | 5 (20.0) | 0.94† |
| Disease behavior (fistulizing : non- fistulizing) | 21 : 27 | 8 : 15 | 13 : 12 | 0.23† |
| Extraintestinal manifestation, n (%) | 21 (42.3) | 8 (33.3) | 12 (48.0) | 0.30† |
| Previous surgery for Crohn’s disease, n (%) | 29 (59.2) | 14 (58.3) | 15 (60.0) | 0.68† |
| Smoker, n (%) | 4 (8.2) | 1 (4.2) | 3 (12.0) | 0.32† |
| Past treatment | ||||
| Corticosteroids, n (%) | 47 (95.9) | 23 (95.8) | 24 (96.0) | 0.98† |
| 5-Aminosalicylates, n (%) | 44 (89.8) | 21 (87.5) | 23 (92.0) | 0.60† |
| Thiopurines, n (%) | 47 (95.9) | 24 (100) | 23 (92.0) | 0.16† |
| Methotrexate, n (%) | 30 (61.2) | 12 (50.0) | 18 (72.0) | 0.11† |
| Anti-TNF, n (%) | 47 (95.9) | 23 (95.8) | 24 (96.0) | 0.98† |
| (3 : 2 : 1 : 0) | (14 : 26 : 7 :2) | (9 : 11 : 3 :1) | (5 : 15 : 4 :1) | 0.60† |
| Concomitant medications | ||||
| Corticosteroids, n (%) | 25 (51.0) | 11 (45.8) | 14 (64.0) | 0.48† |
| 5-Aminosalicylates, n (%) | 3 (6.1) | 2 (8.3) | 1 (4.0) | 0.53† |
| Immunomodulators, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1.00 |
| Harvey-Bradshaw Index, median (IQR) | 12 (9–15.5) | 11 (7.5–13.5) | 13 (10–18.5) | 0.065 |
| C-reactive protein (mg/dl), median (IQR) | 18.5 (7–46.25) | 15 (5.5–26.8) | 34.5 (8–55.5) | 0.066 |
| Albumin (g/dL), median (IQR) | 3.8 (3.4–4.1) | 3.95 (3.6–4.2) | 3.7 (3.2–4) | 0.21 |
| White blood cell count (mg/dL), median (IQR) | 8.3 (6.7–11.2) | 8.3 (6.8–11.4) | 8.3 (6.7–10.8) | 0.96 |
| Hemoglobin (mg/dL), median (IQR) | 11.95 (9.9–13.0) | 11.95 (10.2–13.0) | 12.0 (9.8–13.0) | 0.96 |
| Duration of natalizumab treatment (months), median (IQR) | 7 (3–21.5) | 20 (6–32.5) | 4 (2–8) | 0.0004 |
| Adverse effects, n (%) | 9 (18.4) | 3 (12.5) | 6 (24.0) | 0.46† |
Fisher's exact test, all others Mann–Whitney U test.
indicates p value of <0.05.
IQR: interquartile range
Efficacy
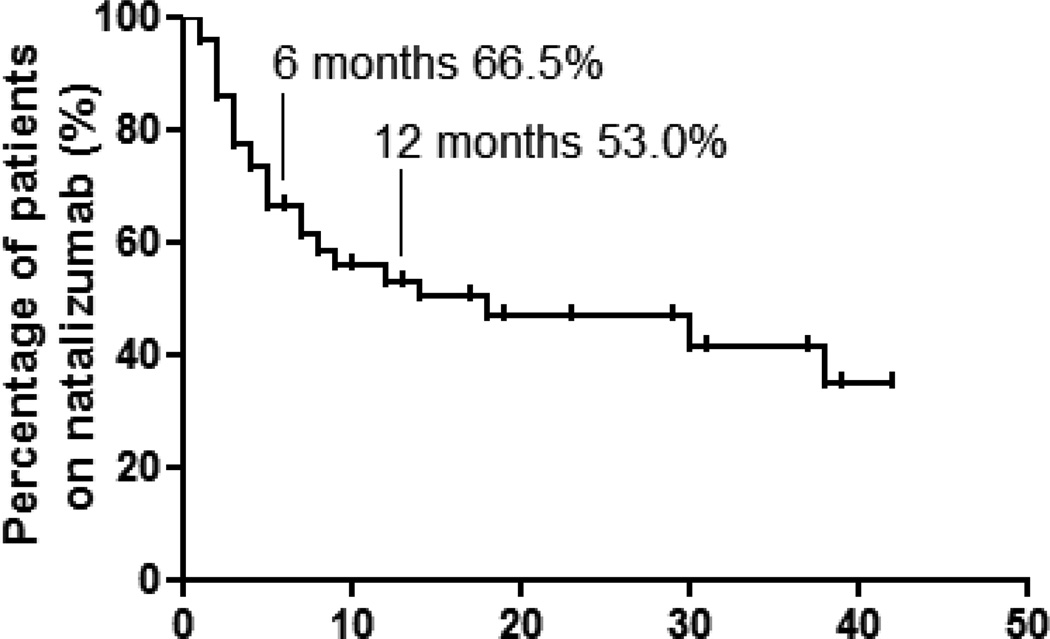
At the time of this review, 24 patients (49.0%) were still continuing natalizumab while 25 had discontinued therapy. Clinical or laboratory parameters were not different between the two groups (table 1). Disease duration was numerically shorter, and baseline CRP level and Harvey-Bradshaw index were numerically greater in those who discontinued natalizumab, but there was no statistical difference between the groups. The maintenance effect of natalizumab was assessed by Kaplan-Meier survival analysis (Figure 1). At 12 months, 53.0% of patients were still receiving natalizumab treatment, and this effect appeared to be stable over the subsequent years.
Figure 1.
Survival analysis of patients receiving natalizumab treatment. Time to discontinuation of natalizumab treatment was analyzed using Kaplan-Meier estimator. Sixty-six and 53% of patients were still on natalizumab at 6 and 12 months respectively. Patients without an outcome of discontinuing natalizumab were regarded as censored and shown as ticks on the graph.
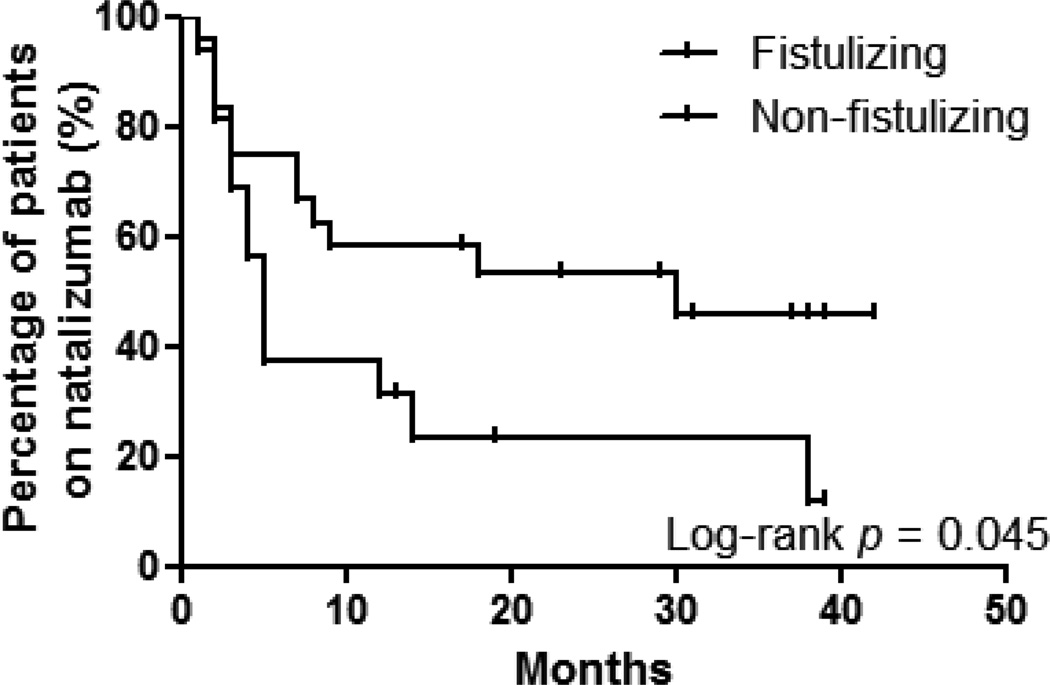
Of the 49 patients nine patients receiving natalizumab had less than 12 months of follow-up. Seventeen of 40 patients (42%) had a clinical response at 12 months and 23 (58%) discontinued treatment either due to lack of response or adverse effect. Comparison of clinical and laboratory parameters between the 12-month responders and failures (table 2) identified that there were more patients with fistulizing disease in the latter group (p = 0.013). Multivariate discriminant analysis of patients with the factors with the three lowest p values showed that fistulizing disease was a negative predictor of response to natalizumab (p = 0.012). Comparison of the maintenance effect of natalizumab between patients with fistulizing disease (n = 24) and non-fistulizing disease (n = 16) by Kaplan-Meier survival analysis demonstrated that patients with non-fistulizing disease were more likely to respond (log-rank test: p = 0.045) (Figure 2). Median time until discontinuation of natalizumab was shorter in patients with fistulizing disease as well (5.0 vs. 30.0 months, ratio 0.17, 95% CI −0.29–0.62).
Table 2.
Comparison of characteristics between 12 months responders and non-responders
| 12 months respondersa n = 17 |
Non-responders b n = 23 |
p value between a and b |
|
|---|---|---|---|
| Gender (male : female) | 6 : 11 | 11 : 12 | 0.43† |
| Age (years), median (range) | 32 (20–59) | 32 (22–62) | 0.76 |
| Weight (kg), median (IQR) | 65.3 (63.0–77.0) | 70.1 (60.5–89.3) | 0.42 |
| BMI, median (IQR) | 22.31(20.2–24.8) | 26.49 (20.6–30.3) | 0.12 |
| Age at diagnosis, median (range) | 15 (12–48) | 18 (8–47) | 0.59 |
| Disease duration (months), median (range) | 132 (67–372) | 144 (36–576) | 0.71 |
| Disease location (ileum: colon: ileocolonic) | 1 : 4 : 13 | 0 : 3 : 20 | 0.36† |
| Upper intestinal involvement, n (%) | 3 (17.6) | 2 (8.7) | 0.40† |
| Perianal disease, n (%) | 3 (17.6) | 5 (21.7) | 0.75† |
| Disease behavior (fistulizing : non–fistulizing) | 3 : 14 | 13 : 10 | 0.013†* |
| Extraintestinal manifestation, n (%) | 6 (35.3) | 12 (52.2) | 0.29† |
| Previous surgery for Crohn’s disease, n (%) | 9 (52.9) | 15 (65.2) | 0.25† |
| Smoker, n (%) | 1 (5.9) | 3 (15.0) | 0.46† |
| Past treatment | |||
| Corticosteroids, n (%) | 17 (100.0) | 22 (95.7) | 0.38† |
| 5-Aminosalicylates, n (%) | 16 (94.1) | 21 (91.3) | 0.74† |
| Thiopurines, n (%) | 17 (100.0) | 22 (95.7) | 0.21† |
| Methotrexate, n (%) | 9 (52.9) | 16 (69.6) | 0.28† |
| Anti-TNF, n (%) | 16 (94.1) | 22 (95.7) | 0.83† |
| (3 : 2 : 1 : 0) | (5 : 8 : 3 :1) | (5 : 14 : 3 :1) | 0.60† |
| Concomitant medications | |||
| Corticosteroids, n (%) | 7 (41.2) | 10 (56.5) | 0.34† |
| 5-Aminosalicylates, n (%) | 1 (5.9) | 1 (4.3) | 0.83† |
| Harvey-Bradshaw Index, median (IQR) | 11 (6–13) | 13 (10–17) | 0.11 |
| C-reactive protein (mg/dl), median (IQR) | 16 (10.5–31.5) | 34.5 (7.75–54.5) | 0.79 |
| Albumin (g/dL), median (IQR) | 4.1 (3.7–4.3) | 3.7 (3.2–4.0) | 0.12 |
| White blood cell count (mg/dL), median (IQR) | 9.4 (7.1–11.9) | 8.3 (6.6–10.7) | 0.49 |
| Hemoglobin (mg/dL), median (IQR) | 11.3 (9.8–12.8) | 11.8 (10.2–13.1) | 0.48 |
| Adverse effects, n (%) | 1 (5.9) | 5 (21.7) | 0.17† |
Fisher's exact test, all others Mann–Whitney U test.
indicates p value of <0.05.
IQR: interquartile range
Figure 2.
Survival analysis of fistulizing and non-fistulizing patients among 12 months responders and non-responders. Time to discontinuation of natalizumab treatment was analyzed using Kaplan-Meier estimator as in figure 1 in the two groups. Patients with non-fistulizing disease were significantly more likely to respond to natalizumab (log-rank p = 0.031).
Reasons for discontinuation of and adverse reactions to natalizumab
There were 25 patients that discontinued natalizumab treatment. The most common reason for discontinuation was lack of effectiveness/worsening of CD (n = 21). One patient opted to discontinue because of constipation that by the physician’s assessment may have actually been due to improvement of the CD. No patient developed PML. One patient decided to discontinue treatment at 18 months because of fear of PML before the anti-JC virus antibody test became available. Eleven of our patients had anti-JC virus antibody checked. Six out of 11 (54.5%) tested positive which is similar to the rate reported in the general population and multiple sclerosis patients. One patient discontinued natalizumab due to the positive test result after 38 months of treatment. Discussions regarding whether to continue natalizumab are currently ongoing with the rest of the patients. Two patients discontinued natalizumab after developing malignancy, one case with testicular cancer and another with colon cancer (identified during a surveillance colonoscopy where there was no active Crohn’s disease identified in the colon). The former was successfully treated with chemotherapy and the latter with colectomy. There were 2 cases of serious infections. One patient had recurrence of herpes simplex virus meningitis that was successfully treated, and later reinitiated treatment with natalizumab. Another patient developed sepsis due to an infected central venous catheter port and required discontinuation of treatment. Four patients reported mild immediate hypersensitivity infusion reactions such as shortness of breath and itchiness, neither of which could be managed with anti-histamines. No patient discontinued treatment due to infusion reactions.
Therapy for flares during or following discontinuation of natalizumab
Of the 24 patients that were still on natalizumab treatment, there were 4 that required a short course of steroid treatment for transient worsening of Crohn’s symptoms, but all were able to decrease the dose of corticosteroids compared to pretreatment with natalizumab. Two patients required incision and drainage of a perianal abscess, and another 2 had surgery (one with a strictureplasty at 13 months and another with small bowel resection at 14 months); however, all were able to subsequently continue natalizumab with stable symptoms of their CD.
In contrast, fourteen out of 25 patients (56.0%) that discontinued natalizumab subsequently required surgery. Ten required a proctocolectomy with an end-ileostomy procedure. Of the remaining 11 patients that did not have surgery, three experienced improvement of their symptoms with a combination of other immunosuppressive agents, one is stable with 5-ASA, and the others are living with chronic active symptoms despite a trial of other medications, including investigational drugs.
DISCUSSION
The present series demonstrates the effectiveness of natalizumab treatment in CD patients at a tertiary medical center in the US. Natalizumab was administered as an induction as well as a maintenance treatment and showed excellent efficacy and tolerability in nearly half of this population of refractory CD that had previously failed multiple biologics and immunosuppressive agents. The effectiveness of natalizumab was significantly greater in patients with non-fistulizing disease and was maintained over a year.
The natural behavior of Crohn’s disease is characterized by progressive, transmural inflammation with a variable (inductive and maintenance) response to conventional and biological therapies13. A majority of patients with CD require at least one surgery during their lifetime. 5-ASAs, corticosteroids, immunomodulators, and, more recently, biologic agents have been used for medical treatment of CD5. The use of natalizumab is currently restricted to CD patients who have failed treatment with anti-TNF agents, and, due to the rare but fatal side effect of PML, its use requires participation in a national registry in order to qualify to receive its treatment.
Natalizumab has been shown to be effective for induction and maintenance of CD in several randomized controlled trials. Gosh et al. evaluated the effect of natalizumab in inducing remission of CD in 248 patients with moderate-to-severe CD12. Two doses of 3 or 6 mg/kg of natalizumab, one dose of 3 mg/kg of natalizumab, or 2 doses of placebo were given 4 weeks apart. Only the 3 mg/kg group met the primary end-point which was clinical remission at week 6, but both groups showed positive results over placebo at multiple time points between weeks 4 to 12. Sandborn et al. reported that 300 mg of natalizumab given at weeks 0, 4, and 8 was not significantly superior to placebo in inducing remission of moderately to severely active CD patients11. However, patients who had a response at weeks 10/12 had significantly increased rates of sustained response and remission over 60 weeks. During the open-label phase of this study, one patient died of PML. Post-hoc analysis of this study demonstrated that patients with an elevated C reactive protein (CRP) of above the upper limit of normal showed statistically significant improvement over placebo at the induction phase. A subsequent study enrolling 509 patients with active CD and an elevated CRP demonstrated that natalizumab given at weeks 0, 4, and 8 was superior to placebo at inducing clinical remission of CD at week 1214.
In the above clinical trials, combination treatment with an immunomodulator was permitted, and about one-third was taking either azathioprine, mercaptopurine or methotrexate. Due to the subsequent recognition of the risk of PML, the use of concomitant immunomodulator was restricted when the FDA approved natalizumab therapy for CD as post hoc assessment of the clinical trials did not demonstrate an efficacy benefit for concomitant therapies. However, no study has demonstrated the effectiveness (efficacy and safety) of natalizumab in the current, regulatory-restricted, clinical setting. In our patient population, about half of patients were receiving steroids at the time of initiation of natalizumab, but none were on immunomodulators or anti-TNF agents. Irrespective of these limitations, natalizumab showed excellent clinical benefit in nearly half of our treatment refractory CD patients.
We sought to determine predictors of response to natalizumab and identified that patients with a history of fistulizing disease were less likely to respond to natalizumab. Fistulizing disease has been a risk factor for CD recurrence in many studies, and we presume that this population has already manifest as a more aggressive and treatment refractory disease. Furthermore, patients who did not respond to natalizumab were likely to require surgery, most often, colectomy. This is not surprising since natalizumab has been positioned as a treatment to be used after failure of all medical therapies and when surgery is being otherwise recommended. As opposed to the results of the clinical trials, CRP was not an indicator of clinical response in our study as the majority of patients had a CRP level above normal range.
Adverse effects were seen in 18.4% of patients, with 2 cases of malignancy. No case of PML was observed. The risk of PML has been shown to be highest (11/1,000) in patients with longer treatment duration, prior immunosuppressive use, and presence of anti-JC virus antibody [15]. Recently, testing for plasma JC virus antibody has become clinically available and 11 of our patients were assayed at the time the database was “locked”. Six out of 11 (54.5%) tested positive, and one patient has already discontinued natalizumab due to the test result. Discussions regarding whether to continue natalizumab are currently ongoing with the remainder of the patients.
The major shortcomings of our study are the retrospective nature and the rather small sample size that would make it difficult to detect significant findings. However, natalizumab is currently only approved in the US for CD, and not many institutions have experience such as ours. It has been a decade since the first biologic was introduced to the market, and we believe that the findings of our study is important as the numbers of anti-TNF failures are increasing.
To date, this is the largest study confirming the efficacy and tolerability of natalizumab in real world clinical practice. The results of our study represent its utility in selected CD patients at a major tertiary center, and it may not be reflective of its effect and safety in general clinical practice among primary GI physicians. However, since its use is still limited to major centers in US, we believe that the results of our study should be a reasonable representation of its use.
Collectively, we have shown that natalizumab treatment was effective and safe in clinical practice for CD patients with a history of refractory disease. Our clinical experience supports and extends the utilities noted in induction and maintenance clinical trials and supports the long-term effectiveness, in particular in patients with luminal disease not encumbered by fistula.
Acknowledgements
AS was supported by the Foreign Clinical Pharmacology Training Program of the Japanese Society of Clinical Pharmacology and Therapeutics.
RDC and SBH have received consulting fee from Elan Pharmaceuticals. DTR has received consulting fees and research support (Safety Registry) from Elan Pharmaceuticals. This study was not supported by any pharmaceutical industry.
Footnotes
Author contribution: AS, concept, study design, analysis of data, and writing of manuscript; KK, CC and JM, analysis of data and approval of final manuscript; RDC and SBH, patient recruitment and approval of final manuscript; DTR, supervision, patient recruitment, and approval of final manuscript.
Conflict of interest: AS, KK, CC and JM, no conflict of interest exists.
References
- 1.Hanauer SB. Inflammatory bowel disease: epidemiology, pathogenesis, and therapeutic opportunities. Inflamm Bowel Dis. 2006;12(Suppl 1):S3–S9. doi: 10.1097/01.mib.0000195385.19268.68. [DOI] [PubMed] [Google Scholar]
- 2.Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–434. doi: 10.1038/nature06005. [DOI] [PubMed] [Google Scholar]
- 3.Hibi T, Ogata H. Novel pathophysiological concepts of inflammatory bowel disease. J Gastroenterol. 2006;41:10–16. doi: 10.1007/s00535-005-1744-3. [DOI] [PubMed] [Google Scholar]
- 4.Sakuraba A, Sato T, Kamada N, et al. Th1/Th17 immune response is induced by mesenteric lymph node dendritic cells in Crohn's disease. Gastroenterology. 2009;137:1736–1745. doi: 10.1053/j.gastro.2009.07.049. [DOI] [PubMed] [Google Scholar]
- 5.Hanauer SB, Present DH. The state of the art in the management of inflammatory bowel disease. Rev Gastroenterol Disord. 2003;3:81–92. [PubMed] [Google Scholar]
- 6.Hanauer SB. Review article: aminosalicylates in inflammatory bowel disease. Aliment Pharmacol Ther. 2004;20(Suppl 4):60–65. doi: 10.1111/j.1365-2036.2004.02048.x. [DOI] [PubMed] [Google Scholar]
- 7.Guessous I, Juillerat P, Pittet V, et al. Evaluating appropriateness of treatment for Crohn's disease: feasibility of an explicit approach. Digestion. 2007;75:46–52. doi: 10.1159/000101566. [DOI] [PubMed] [Google Scholar]
- 8.Rutgeerts P, Feagan BG, Lichtenstein GR, et al. Comparison of scheduled and episodic treatment strategies of infliximab in Crohn's disease. Gastroenterology. 2004;126:402–413. doi: 10.1053/j.gastro.2003.11.014. [DOI] [PubMed] [Google Scholar]
- 9.Colombel JF, Sandborn WJ, Rutgeerts P, et al. Adalimumab for maintenance of clinical response and remission in patients with Crohn's disease: the CHARM trial. Gastroenterology. 2007;132:52–65. doi: 10.1053/j.gastro.2006.11.041. [DOI] [PubMed] [Google Scholar]
- 10.Hanauer SB, Sandborn WJ, Rutgeerts P, et al. Human anti-tumor necrosis factor monoclonal antibody (adalimumab) in Crohn's disease: the CLASSIC-I trial. Gastroenterology. 2006;130:323–333. doi: 10.1053/j.gastro.2005.11.030. quiz 591. [DOI] [PubMed] [Google Scholar]
- 11.Sandborn WJ, Colombel JF, Enns R, et al. Natalizumab induction and maintenance therapy for Crohn's disease. N Engl J Med. 2005;353:1912–1925. doi: 10.1056/NEJMoa043335. [DOI] [PubMed] [Google Scholar]
- 12.Ghosh S, Goldin E, Gordon FH, et al. Natalizumab for active Crohn's disease. N Engl J Med. 2003;348:24–32. doi: 10.1056/NEJMoa020732. [DOI] [PubMed] [Google Scholar]
- 13.Hanauer SB. Inflammatory bowel disease. N Engl J Med. 1996;334:841–848. doi: 10.1056/NEJM199603283341307. [DOI] [PubMed] [Google Scholar]
- 14.Targan SR, Feagan BG, Fedorak RN, et al. Natalizumab for the treatment of active Crohn's disease: results of the ENCORE Trial. Gastroenterology. 2007;132:1672–1683. doi: 10.1053/j.gastro.2007.03.024. [DOI] [PubMed] [Google Scholar]