Abstract
Background
The natural history of prostate-specific antigen (PSA)-defined biochemical recurrence (BCR) of prostate cancer (PCa) after definitive local therapy is highly variable. Validated prediction models for PCa-specific mortality (PCSM) in this population are needed for treatment decision-making and clinical trial design.
Objective
To develop and validate a nomogram to predict the probability of PCSM from the time of BCR among men with rising PSA levels after radical prostatectomy.
Design, setting, and participants
Between 1987 and 2011, 2254 men treated by radical prostatectomy at one of five high-volume hospitals experienced BCR, defined as three successive PSA rises (final value >0.2 ng/ml), single PSA >0.4 ng/ml, or use of secondary therapy administered for detectable PSA >0.1 ng/ml. Clinical information and follow-up data were modeled using competing-risk regression analysis to predict PCSM from the time of BCR.
Intervention
Radical prostatectomy for localized prostate cancer and subsequent PCa BCR.
Outcome measurements and statistical analysis
PCSM.
Results and limitations
The 10-yr PCSM and mortality from competing causes was 19% (95% confidence interval [CI] 16–21%) and 17% (95% CI 14–19%), respectively. A nomogram predicting PCSM for all patients had an internally validated concordance index of 0.774. Inclusion of PSA doubling time (PSADT) in a nomogram based on standard parameters modestly improved predictive accuracy (concordance index 0.763 vs 0.754). Significant parameters in the models were preoperative PSA, pathological Gleason score, extraprostatic extension, seminal vesicle invasion, time to PCa BCR, PSA level at PCa BCR, and PSADT (all p < 0.05).
Conclusions
We constructed and validated a nomogram to predict the risk of PCSM at 10 yr among men with PCa BCR after radical prostatectomy. The nomogram may be used for patient counseling and the design of clinical trials for PCa.
Patient summary
For men with biochemical recurrence of prostate cancer after radical prostatectomy, we have developed a model to predict the long-term risk of death from prostate cancer.
Keywords: Prostatic neoplasms, Prostatectomy, Statistical models
1. Introduction
Approximately 25% of men who undergo radical prostatectomy will experience biochemical recurrence (BCR) of prostate cancer (PCa) [1,2]. In the absence of secondary therapy, the median time from PCa BCR to clinical progression is 5–8 yr, and between 32% and 45% of men will die from PCa within 15 yr [3–5]. Prostate-specific antigen doubling time (PSADT), Gleason score, and time to PCa BCR have been identified as important prognostic parameters for PCa-specific mortality (PCSM) [3,4,6–11]. We endeavored to construct and validate a nomogram for PCSM that can be applied to patients at the time of PCa BCR to guide treatment decision-making.
2. Patients and methods
2.1. Study population
We identified 2254 consecutive men who experienced PCa BCR after initial treatment with radical prostatectomy for localized PCa without neoadjuvant therapy at five US academic medical centers between 1987 and 2011. We excluded 298 men who received adjuvant therapy. The use of secondary therapy was not standardized and was based on individual physician discretion. Overall, 1566 men (69%) received secondary therapy, including radiation therapy (n = 950) and androgen deprivation therapy (n = 1091). No deaths from PCa were observed among the 688 men who received no secondary therapy. All patient information was obtained from prospective databases. All pathological specimens were evaluated by pathologists at each institution.
For the purposes of this study, PCa BCR was defined as three successive rises in PSA level of >0.1 ng/ml at least 6 wk postoperatively with final PSA >0.2 ng/ml (n = 1133), or administration of secondary therapy for evidence of detectable PSA >0.1 ng/ml at least 6 wk postoperatively (n = 337). Patients with PSA ≥0.4 ng/ml at least 6 wk postoperatively were also classified as having PCa BCR, even if they did not have three successive rises (n = 784). Both PSA definitions of BCR are associated with ≥88% probability of subsequent PSA progression [12]. The requirement of three successive PSA rises ≥0.2 ng/ml enabled us to calculate PSADT according to previously published guidelines [3,11]. The date of PCa BCR was the date of the third successive PSA rise without backdating. For patients without three successive PSA rises, the date of PCa BCR was either the date of secondary therapy or the date of the first PSA ≥0.4 ng/ml, whichever occurred first. Different PSA assays were used at different times at each of the institutions over the time period of the study. PSADT was calculated for each patient using all PSA values >0.1 ng/ml at least 6 wk postoperatively until the third successive PSA rise, using the slope for linear regression of the natural log of the patient’s PSA levels versus time of PSA measurement (in mo). PSADT was estimated as 0.693 divided by the slope [3,11].
2.2. Statistical analysis
The primary endpoint of the study was PCSM from the time of PCa BCR. Death was attributed to prostate cancer if there was evidence of metastatic castration-resistant PCa (CRPC) and/or if PCa was listed as the primary cause of death on the death certificate. We used the Fine and Gray competing-risk regression analysis to model clinical parameters and follow-up data. Living patients were censored on the date of last know vital status. The pathological Gleason score was modeled as ≤6, 3+4, 4+3, 8, and 9. PSA at PCa BCR was defined as the PSA level at the time at which the criteria for BCR were fulfilled. The model predictions were adjusted for the year of diagnosis. All decisions with respect to coding of variables were made a priori without knowledge of their association with PCSM. Internal validation of the nomogram was performed using two components. First, a concordance index (c-index) was estimated by subjecting the nomogram to bootstrapping with 200 resamples [13,14]. Second, we compared the predicted probability of PCSM versus actual PCSM at 10 yr (ie, calibration) using 200 bootstrap resamples to reduce overfit bias. To evaluate the impact on predictive accuracy of different patients and treatment practices among institutions, we performed leave-one-out cross-validation in which a model was developed using data for patients from four institutions and applied to the institution that was left out.
All statistical analyses were conducted using S-Plus 2000 Professional statistical software (Insightful Corp., Seattle, WA, USA) with the Design library attached. All p values resulted from the use of two-sided statistical tests, and the level of significance was set at 0.05. Deidentified data sets that did not contain protected health information were acquired following approval by the institutional-review board of each participating institution and handled according to Health Insurance Portability and Accountability Act guidelines.
3. Results
The clinical characteristics of the study population are listed in Table 1. Overall, information to calculate PSADT was available for 1133 (50%) patients and the median PSADT was 9.6 mo (interquartile range [IQR] 4.7–18.7 mo). Clinical information for patients with and without PSADT data is included in Supplementary Table 1. PSADT was calculated using three PSA values ≥0.2 ng/ml for 998 patients (88.1%); only 34 (3%) men had only one PSA value ≥0.2 ng/ml used in the calculation. The median time to PCa BCR was 26 mo (IQR 8–57 mo) and the median PSA level at BCR was 0.56 ng/ml (IQR 0.32–1.3 ng/ml). The median time to PCa BCR significantly differed among the three definitions used in the study (p < 0.001).
Table 1.
Clinical and pathological characteristics of study patients with biochemical recurrence of prostate cancer used for nomogram development
| Parameter | Value |
|---|---|
| Patients | 2254 |
| Median age, yr (IQR) (range) | 62 (57–67) (39–83) |
| Year of diagnosis, n (%) | |
| 1987–1994 | 546 (24) |
| 1995–2000 | 833 (37) |
| 2001–2011 | 875 (39) |
| Median preoperative PSA, ng/ml (IQR) (range) | 8.1 (5.6–13.4) (0.5–97.5) |
| Pathological Gleason score, n (%) | |
| 2–6 | 331 (15) |
| 3+4 | 871 (39) |
| 4+3 | 553 (25) |
| 8 | 244 (11) |
| 9–10 | 255 (11) |
| Extraprostatic extension, n (%) | 1313 (58) |
| Seminal vesicle invasion, n (%) | 545 (24) |
| Positive surgical margins, n (%) | 1060 (47) |
| Lymph node metastasis, n (%) | 275 (12) |
| Median time to BCR, mo (IQR) (range) | 26 (8–57) (1.4–216) |
| Three successive PSA rises, final >0.2 ng/ml | 35 (16–66) (3–216) |
| Secondary therapy for PSA >0.1 ng/ml | 16 (4–35) (1.4–125) |
| PSA ≥0.4 ng/ml | 24 (6–58) (1.5–209) |
| Median PSA at BCR, ng/ml (IQR) (range) | 0.56 (0.32–1.3) (0.12–120) |
| Median PSA doubling time, mo (IQR) (range) | 9.6 (4.7–18.7) (1–110) |
| ≤3 mo, n (%) | 156 (14) |
| 3.1–6 mo, n (%) | 218 (19) |
| 6.1–12 mo, n (%) | 293 (26) |
| >12 mo, n (%) | 466 (41) |
| Median post-BCR follow-up, mo (IQR) | 45 (15–85) |
| Median postoperative follow-up, mo (IQR) | 84 (48–130) |
IQR = interquartile range; PSA = prostate-specific antigen; BCR = biochemical recurrence of prostate cancer.
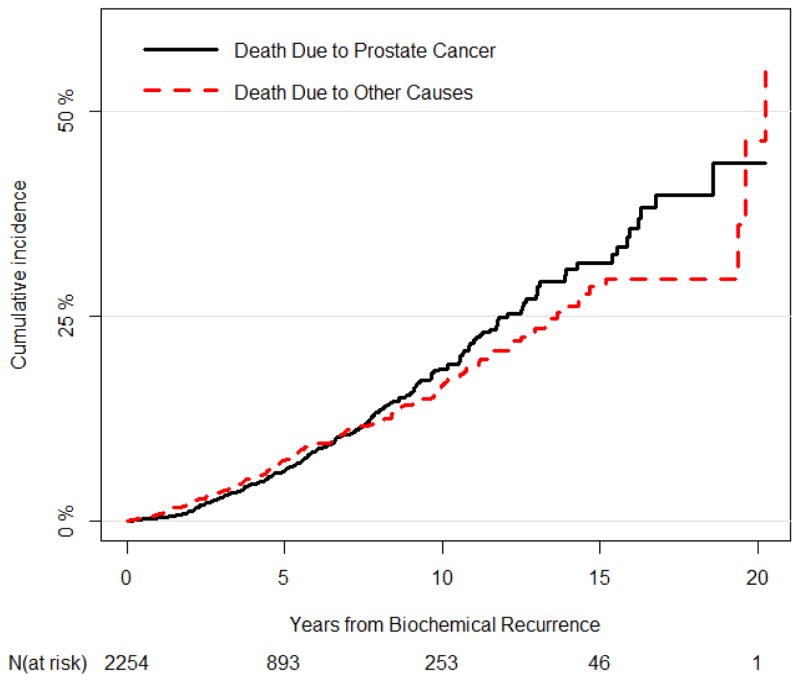
Over a median follow-up of 45 mo (IQR 15–85 mo) from the time of PCa BCR, 208 men died from prostate cancer and 196 died from competing causes of mortality. The 10-yr PCSM and competing-causes mortality were 19% (95% confidence interval [CI] 16–21%) and 17% (95% CI 14–19%), respectively (Fig. 1). The 10-yr PCSM and competing-causes mortality from the time of PCa BCR stratified by important baseline predictors are summarized in Table 2.
Fig. 1.
Prostate cancer–specific mortality and competing-causes mortality among 2254 men with biochemical recurrence of prostate cancer after radical prostatectomy.
Table 2.
PCa-specific mortality and competing-causes mortality stratified by pathological Gleason score, seminal vesicle invasion, time to PCa biochemical recurrence, and PSA doubling time
| 10-yr mortality, % (95% CI)
|
||
|---|---|---|
| PCa-specific | Competing causes | |
| Pathological Gleason score | ||
| Gleason 2–6 | 5.2 (1.7–8.6) | 16.1 (10.3–21.9) |
| Gleason 3+4 | 18.4 (13.7–23.1) | 16.8 (12.5–21.1) |
| Gleason 4+3 | 12.8 (7.6–17.9) | 20.6 (13.4–27.8) |
| Gleason 8 | 27.8 (18.7–36.9) | 13.3 (7.1–19.6) |
| Gleason 9 | 38 (27.8–48.3) | 13.7 (6.7–20.7) |
| Seminal vesicle invasion | ||
| Absent | 13.7 (10.6–16.9) | 17.6 (14.2–21.1) |
| Present | 29.1 (23.3–34.8) | 14.6 (10.4–18.7) |
| Time to BCR | ||
| <12 mo | 21.6 (16.8–26.4) | 12 (8.3–15.6) |
| 12–24 mo | 19.7 (13.5–25.9) | 13 (7.9–18.1) |
| >24 mo | 15.6 (11.3–19.9) | 22.3 (17.6–27.1) |
| PSA doubling time | ||
| <3 mo | 41.9 (30.2–53.6) | 6.7 (2.3–11) |
| 3–6 mo | 17.3 (9–25.6) | 15.6 (7.6–23.6) |
| 6–12 mo | 15.7 (8.6–22.8) | 17.2 (9.9–24.5) |
| >12 mo | 7.3 (2.7–12) | 22.5 (14.9–30.2) |
PCa = prostate cancer; PSA = prostate-specific antigen; BCR = biochemical recurrence of PCa; CI = confidence interval.
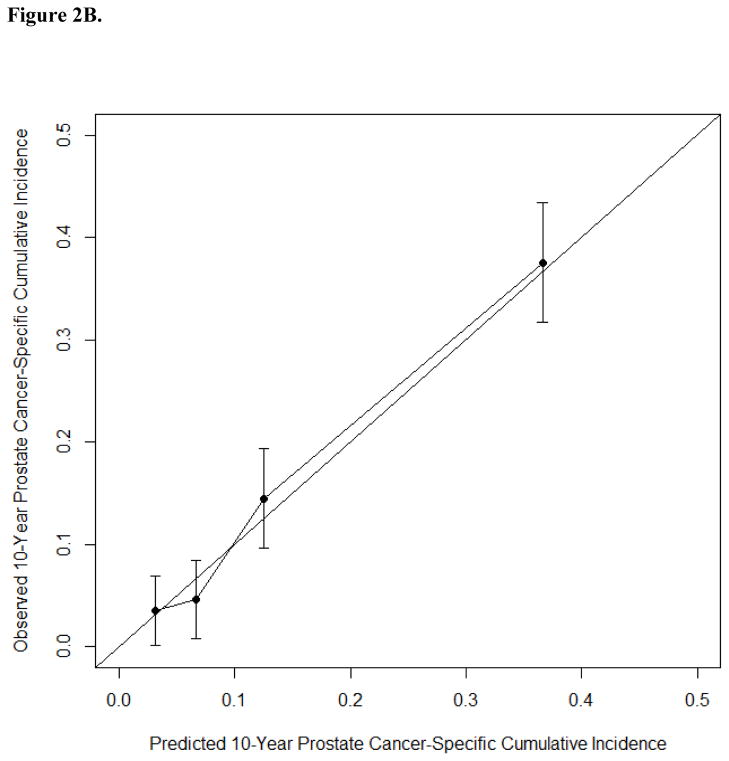
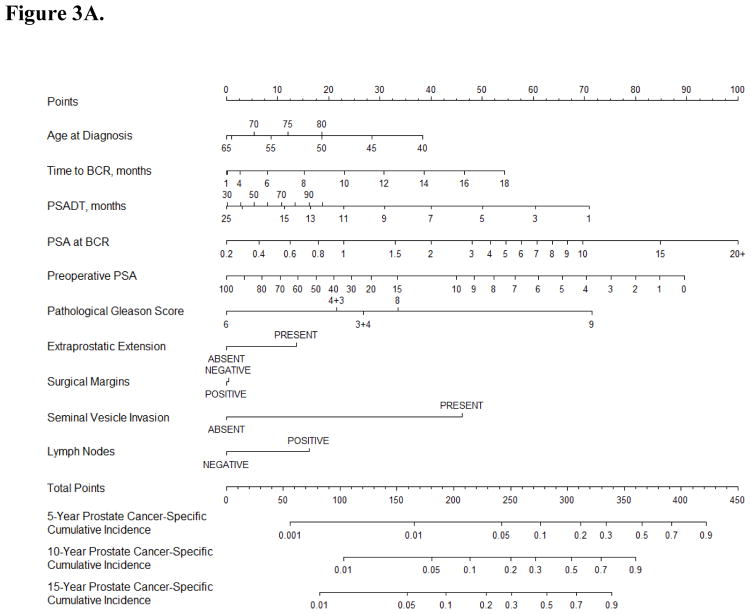
A nomogram predicting 10-yr PCSM after PCa BCR was developed using data for all 2254 patients based on nine standard parameters and year of diagnosis (but not PSADT). The internally validated c-index of this nomogram was 0.774 and mortality estimates were well correlated with observed outcome (Fig. 2, Supplementary Table 2). Statistically significant parameters associated with PCSM in this model included PSA level at the time of PCa BCR (p < 0.001), pathological Gleason score (p < 0.001), seminal vesicle invasion (p < 0.001), extraprostatic extension (p = 0.007), preoperative PSA (p = 0.017), and time to BCR (p = 0.032). In leave-one-out cross-validation, the c-index for models based on data from four institutions applied to the institution left out ranged from 0.741 to 0.846, suggesting similar model performance across different patient groups. Among the patients in our study, 1636 (74%), 1007 (45%), 477 (22%), and 277 (12%) had a predicted 10-yr PCSM greater than 5%, 10%, 20%, and 30%, respectively.
Fig. 2.
(A) Nomogram predicting prostate cancer–specific mortality at 5, 10, and 15 yr after the time of biochemical recurrence of prostate cancer (BCR) among men treated by radical prostatectomy according to ten standard clinical parameters. (B) Calibration of the nomogram at the 10-yr endpoint. PSA = prostate-specific antigen.
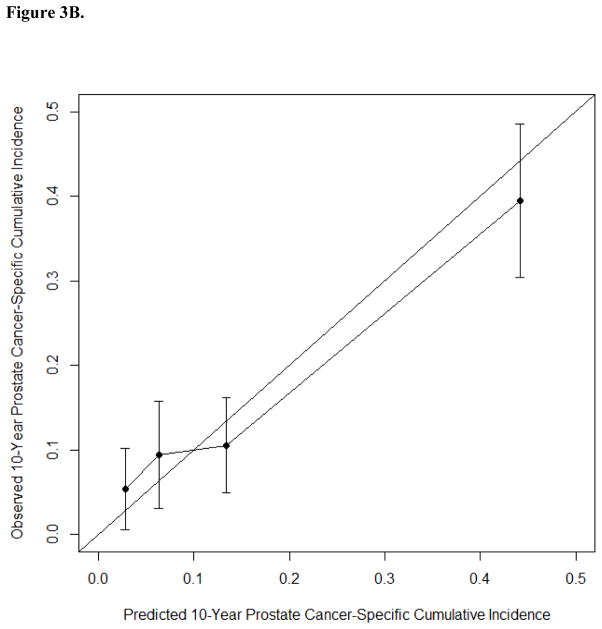
We developed a separate nomogram based on data for 1133 patients with PSADT information (Fig. 3, Supplementary Table 3). The c-index of a model based on the ten standard clinical parameters plus PSADT (0.763) was similar to that of a model based on ten standard clinical parameters without PSADT (0.754). We investigated whether the empiric prognostic value of PSADT was dependent on the PSA level at the time of PCa BCR because 50% of patients had either low (<0.32 ng/ml) or high PSA levels (>1.3 ng/ml) at the time of BCR. This investigation was conducted by modeling the interaction term between the two parameters. Whether modeled as a linear or nonlinear interaction, the interaction term was not significantly associated with PCSM (p = 0.8 and 0.9), and inclusion of this parameter in the model did not improve predictive accuracy (c-index 0.738 and 0.740).
Fig. 3.
(A) Nomogram predicting prostate cancer–specific mortality at 5, 10, and 15 yr after the time of biochemical recurrence of prostate cancer (BCR) among men treated by radical prostatectomy according to ten standard clinical parameters and prostate-specific antigen doubling time (PSADT). (B) Calibration of the nomogram at the 10-yr endpoint. PSA = prostate-specific antigen.
4. Discussion
Treatment of men with post-prostatectomy PCa BCR is complicated by a highly variable natural history, a lack of validated prognostic models for PCSM, and the uncertain benefit of secondary therapy administered before clinical disease progression. We constructed and validated a robust nomogram to predict PCSM among men with PCa BCR after radical prostatectomy treated at several high-volume US hospitals. Inclusion of PSADT using information available at the time of PCa BCR in a model that included standard (and easily measured) parameters modestly improved predictive accuracy. We anticipate that these models will be useful for patient counseling and treatment decision-making purposes and for the design of clinical trials in the PCa BCR population.
The need for validated prediction models of clinical progression and PCSM in the PCa BCR population is evidenced by the variable natural history of PCa. Although PCa BCR universally antedates clinical progression, at 15 yr after BCR, approximately one-third of men are alive, one-third have died from prostate cancer, and one-third have died from competing causes [5]. Our nomogram could be used to inform men with PCa BCR of their risk of PCSM to determine the need for secondary therapy. Men at low risk of PCSM may be managed expectantly to avoid the potential toxicity of salvage therapy. By contrast, those at higher risk of PCSM may be candidates for early salvage therapy (alone or in combination) or newer therapeutics in the setting of clinical trials. Validated nomograms for secondary therapy (such as our salvage radiotherapy nomogram) could be used in the selection and timing of such therapy [15].
Several groups have investigated prognostic factors in the postprostatectomy PCa BCR population. Freedland et al [4] developed risk tables for PCSM using a subset of 379 of 979 men with PCa BCR from a single-surgeon series and identified PSADT, pathological Gleason score, and time to BCR as statistically significant parameters [4]. In this study, PSA data for calculating PSADT were collected for 2 yr after PCa BCR. D’Amico et al [7,10] reported that PSADT was the only significant predictor of PCSM among a subset of 498 and 611 patients with PCa BCR after radical prostatectomy on the basis of PSA values collected for a minimum of 6 mo after BCR (the number of patients excluded for insufficient PSADT information was not reported). In our study, PSADT alone had a c-index of 0.705 (data not shown). Neither of these models has been formally validated and theoretically cannot be applied to patients until a waiting period of 6–24 mo to calculate PSADT. Patients may wish to know their prognosis at the time of PCa BCR because critical decisions about secondary therapy may not permit a lengthy observation period to obtain an accurate measure of PSADT. In our model, PSA at PCa BCR, PSADT, pathological Gleason score, time to BCR, seminal vesicle invasion, extraprostatic extension, and preoperative PSA were all significant predictors. The PSA level at PCa BCR was not considered in these prior studies. It is a measure of overall disease burden and predicts the presence of bone metastases [16]. We previously identified seminal vesicle invasion as a prime determinant of PCSM in a postoperative nomogram [17].
Although several criticisms have been raised against the utility of PSA kinetics in the pretreatment setting [18–20], the general perception is that post-treatment PSADT remains a valuable prognostic marker [11,21]. Thus, an intriguing finding of our study is the lack of substantial prognostic utility of PSADT when added to a model containing standard clinical parameters known at the time of PCa BCR. PSADT appears to add modest (and possibly redundant) prognostic information once the pathological features of PCa, time to PCa BCR, and PSA level at BCR are known. In our patients, short PSADT was correlated with higher PSA levels at PCa BCR, shorter time to BCR, and adverse pathology (all p < 0.05; data not shown). The lack of additive value of PSADT was not related to patients with low or high absolute PSA values at PCa BCR because the interaction term between PSA and PSADT was not significant. Our base model without PSADT had the best predictive accuracy (c-index 0.774); its other advantages are that it does not require complicated PSADT calculations, it is applicable to all patients with PCa BCR, and it can be used to guide treatment decisions at the time of BCR (not after a waiting period of 6–24 mo).
The low mortality rate observed after radical prostatectomy for screen-detected PCa has posed a substantial challenge in the design and execution of clinical trials in the adjuvant setting for clinically meaningful endpoints because of the low rate of events [22,23]. Although there is great interest in evaluating novel compounds active against CRPC in the hormone-naïve, nonmetastatic setting, the study size and long follow-up period required in the neoadjuvant or adjuvant setting might render such trials impractical. The PCa BCR population may be more suitable for evaluating new therapeutics and our nomogram could be used to identify a high-risk subset and to balance treatment arms.
Our study has important strengths, including its large size, multicenter composition, and prospective data collection. All patients were treated at high-volume hospitals and all pathological specimens were reviewed by genitourinary pathologists at each institution. However, the performance of our model may differ when applied to patients treated at low-volume hospitals or in regions where PSA screening is not widely practiced. Few patients treated at these hospitals received neoadjuvant or adjuvant therapy over the time course of this study. Thus, our cohort is likely to be representative of the overall PCa BCR population treated at high-volume US hospitals.
Several limitations also exist. The timing of PSA measurements, the assays used, and the use of secondary therapy were not standardized. However, it is unlikely that such information would be available outside of a study protocol. Unlike the studies of Pound et al [3] and Freedland et al [4], many patients in our study received secondary therapy before the onset of metastatic disease, which may have affected the performance and external validity of the model; indeed, the use of postoperative radiation therapy varied between 41% and 48% among centers. Despite these differences, the model performed well across all institutions in leave-one-out cross-validation. In addition, there are conflicting data on the impact of early postoperative radiation therapy [23–26] and early androgen deprivation therapy [27–30] on PCSM from randomized trials and observational studies. Our model predictions may not be applicable to contemporary patients because of substantial changes in the treatment of CRPC over the time period of the study; indeed, 53% of our PCa deaths occurred in the pre-docetaxel era and virtually no patient received abiraterone acetate and enzalutamide [31–36]. Given that the survival benefit associated with these agents is several months, it is unlikely the survival predictions of our model will differ substantially from expected survival and model discrimination may be unaffected. Nevertheless, it is appropriate to counsel patients that the model predictions are based on conventional secondary therapy administered at or before clinical progression. Changes to tumor grading have occurred over the time course of this study, and these have not been accounted for. However, there was no significant association between year of treatment and PCSM, suggesting that the impact of the modified Gleason scoring system may be slight. Lastly, our study cohort consisted of patients who underwent radical prostatectomy and our model cannot be applied to patients treated with radiation therapy. However, such models rely on clinical parameters only. Because the prostatectomy grade and stage provide important prognostic information, it is likely that a nomogram based on clinical parameters only for surgical patients will yield an inferior model.
In calculating PSADT, we attempted to adhere to published guidelines (minimum of three PSA values at > 0.2 ng/ml at least 3 mo apart) while excluding as few patients as possible [11]. We did not specify a minimum of 3 mo between the first and last PSA values or a minimum of 4 wk between each measurement, and 11.9% of patients with one or two PSA values between 0.11 and 0.19 ng/ml were used in calculating PSADT. We might have observed a different effect of PSADT if we had used longer time periods to measure PSA. However, such a model could only be applied to patients after a waiting period, which might be impractical for treatment decision-making. We also lacked PSADT information for half of our patients, although this is less than in prior studies [3,4].
5. Conclusions
We constructed and validated a nomogram based on the PSA value at the time of PCa BCR, the time to BCR, preoperative PSA, and the pathological features of PCa that accurately predicts the risk of PCSM among men with PCa BCR after radical prostatectomy. PSADT using information available at the time of PCa BCR had modest prognostic utility when modeled with other standard parameters. Our nomogram can be used to counsel patients and guide treatment decisions at the time of PCa BCR. Web-based versions of these models are available for free use in the public domain at http://www.r-calc.com.
Supplementary Material
Take Home Message.
Using a multi-institutional cohort of 2254 men with rising prostate-specific antigen (PSA) after radical prostatectomy, we developed and validated a robust nomogram to predict the long-term risk of prostate cancer–specific mortality from the time of biochemical recurrence. PSA doubling time contributed little to the accuracy of the model.
Acknowledgments
Funding/Support and role of the sponsor: None.
Footnotes
Author contributions: Andrew J. Stephenson had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Stephenson, Klein, Kattan, Scardino, Wood, Rabah, Brockman, Vickers, Alanee, Ciezki, Gao, Lin, Kibel, Bianco.
Acquisition of data: Stephenson, Klein, Wood, Scardino, Kibel, Lin.
Analysis and interpretation of data: Stephenson, Klein, Kattan, Scardino, Wood, Rabah, Brockman, Vickers, Alanee, Ciezki, Gao, Lin, Kibel, Bianco.
Drafting of the manuscript: Stephenson, Brockman.
Critical revision of the manuscript for important intellectual content: Stephenson, Klein, Kattan, Scardino, Wood, Rabah, Brockman, Vickers, Alanee, Ciezki, Gao, Lin, Kibel, Bianco.
Statistical analysis: Stephenson, Gao, Kattan.
Obtaining funding: Klein, Stephenson, Scardino, Wood, Kibel, Lin.
Administrative, technical, or material support: Stephenson.
Supervision: None.
Other (specify): None.
Financial disclosures: Andrew J. Stephenson certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stephenson AJ, Scardino PT, Eastham JA, et al. Preoperative nomogram predicting the 10-year probability of prostate cancer recurrence after radical prostatectomy. J Natl Cancer Inst. 2006;98:715–7. doi: 10.1093/jnci/djj190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Han M, Partin AW, Pound CR, et al. Long-term biochemical disease-free and cancer-specific survival following anatomic radical retropubic prostatectomy. The 15-year Johns Hopkins experience. Urol Clin North Am. 2001;28:555–65. doi: 10.1016/s0094-0143(05)70163-4. [DOI] [PubMed] [Google Scholar]
- 3.Pound CR, Partin AW, Eisenberger MA, et al. Natural history of progression after PSA elevation following radical prostatectomy. JAMA. 1999;281:1591–7. doi: 10.1001/jama.281.17.1591. [DOI] [PubMed] [Google Scholar]
- 4.Freedland SJ, Humphreys EB, Mangold LA, et al. Risk of prostate cancer–specific mortality following biochemical recurrence after radical prostatectomy. JAMA. 2005;294:433–9. doi: 10.1001/jama.294.4.433. [DOI] [PubMed] [Google Scholar]
- 5.Bianco FJ, Jr, Scardino PT, Eastham JA. Radical prostatectomy: long-term cancer control and recovery of sexual and urinary function (“trifecta”) Urology. 2005;66:83–94. doi: 10.1016/j.urology.2005.06.116. [DOI] [PubMed] [Google Scholar]
- 6.D’Amico AV, Cote K, Loffredo M, et al. Determinants of prostate cancer–specific survival after radiation therapy for patients with clinically localized prostate cancer. J Clin Oncol. 20:4567–73. doi: 10.1200/JCO.2002.03.061. 202. [DOI] [PubMed] [Google Scholar]
- 7.Zhou P, Chen MH, McLeod D, et al. Predictors of prostate cancer-specific mortality after radical prostatectomy or radiation therapy. J Clin Oncol. 2005;23:6992–8. doi: 10.1200/JCO.2005.01.2906. [DOI] [PubMed] [Google Scholar]
- 8.Dotan ZA, Bianco FJ, Jr, Rabbani F, et al. Pattern of prostate-specific antigen (PSA) failure dictates the probability of a positive bone scan in patients with an increasing PSA after radical prostatectomy. J Clin Oncol. 2005;23:1962–8. doi: 10.1200/JCO.2005.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Slovin SF, Wilton AS, Heller G, et al. Time to detectable metastatic disease in patients with rising prostate-specific antigen values following surgery or radiation therapy. Clin Cancer Res. 2005;11:8669–73. doi: 10.1158/1078-0432.CCR-05-1668. [DOI] [PubMed] [Google Scholar]
- 10.D’Amico AV, Moul JW, Carroll PR, et al. Surrogate end point for prostate cancer–specific mortality after radical prostatectomy or radiation therapy. J Natl Cancer Inst. 2003;95:1376–83. doi: 10.1093/jnci/djg043. [DOI] [PubMed] [Google Scholar]
- 11.Arlen PM, Bianco F, Dahut WL, et al. Prostate Specific Antigen Working Group guidelines on prostate specific antigen doubling time. J Urol. 2008;179:2181–5. doi: 10.1016/j.juro.2008.01.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stephenson AJ, Kattan MW, Eastham JA, et al. Defining biochemical recurrence of prostate cancer after radical prostatectomy: a proposal for a standardized definition. J Clin Oncol. 2006;24:3973–8. doi: 10.1200/JCO.2005.04.0756. [DOI] [PubMed] [Google Scholar]
- 13.Harrell FE, Jr, Califf RM, Pryor DB, et al. Evaluating the yield of medical tests. JAMA. 1982;247:2543–6. [PubMed] [Google Scholar]
- 14.Efron B, Tibshirani RJ. An introduction to the bootstrap. New York, NY: Chapman & Hall; 1993. [Google Scholar]
- 15.Stephenson AJ, Scardino PT, Kattan MW, et al. Predicting the outcome of salvage radiation therapy for recurrent prostate cancer after radical prostatectomy. J Clin Oncol. 2007;25:2035–41. doi: 10.1200/JCO.2006.08.9607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dotan ZA, Bianco FJ, Jr, Scardino PT, et al. Predicting time to metastatic progression from biochemical recurrence after radical prostatectomy. Proc Am Soc Clin Oncol. 2004;22(Suppl):4642. [Google Scholar]
- 17.Eggener SE, Scardino PT, Walsh PC, et al. Predicting 15-year prostate cancer specific mortality after radical prostatectomy. J Urol. 2011;185:869–75. doi: 10.1016/j.juro.2010.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vickers AJ, Till C, Tangen CM, et al. An empirical evaluation of guidelines on prostate-specific antigen velocity in prostate cancer detection. J Natl Cancer Inst. 2011;103:462–9. doi: 10.1093/jnci/djr028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vickers AJ, Savage C, O’Brien MF, et al. Systematic review of pretreatment prostate-specific antigen velocity and doubling time as predictors for prostate cancer. J Clin Oncol. 2009;27:398–403. doi: 10.1200/JCO.2008.18.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stephenson AJ, Kattan MW, Eastham JA, et al. Prostate cancer–specific mortality after radical prostatectomy for patients treated in the prostate-specific antigen era. J Clin Oncol. 2009;27:4300–5. doi: 10.1200/JCO.2008.18.2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scher HI, Eisenberger M, D’Amico AV, et al. Eligibility and outcomes reporting guidelines for clinical trials for patients in the state of a rising prostate-specific antigen: recommendations from the Prostate-Specific Antigen Working Group. J Clin Oncol. 2004;22:537–56. doi: 10.1200/JCO.2004.07.099. [DOI] [PubMed] [Google Scholar]
- 22.Dorff TB, Flaig TW, Tangen CM, et al. Adjuvant androgen deprivation for high-risk prostate cancer after radical prostatectomy: SWOG S9921 study. J Clin Oncol. 2011;29:2040–5. doi: 10.1200/JCO.2010.32.2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bolla M, van Poppel H, Tombal B, et al. Postoperative radiotherapy after radical prostatectomy for high-risk prostate cancer: long-term results of a randomised controlled trial (EORTC trial 22911) Lancet. 2012;380:2018–27. doi: 10.1016/S0140-6736(12)61253-7. [DOI] [PubMed] [Google Scholar]
- 24.Thompson IM, Tangen CM, Paradelo J, et al. Adjuvant radiotherapy for pathological T3N0M0 prostate cancer significantly reduces risk of metastases and improves survival: long-term followup of a randomized clinical trial. J Urol. 2009;191:956–62. doi: 10.1016/j.juro.2008.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trock BJ, Han M, Freedland SJ, et al. Prostate cancer–specific survival following salvage radiotherapy vs observation in men with biochemical recurrence after radical prostatectomy. JAMA. 2008;299:2760–9. doi: 10.1001/jama.299.23.2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stephenson AJ, Eggener SE, Hernandez AV, et al. Do margins matter? The influence of positive surgical margins on prostate cancer-specific mortality. Eur Urol. 2014;65:675–80. doi: 10.1016/j.eururo.2013.08.036. [DOI] [PubMed] [Google Scholar]
- 27.Crook JM, O’Callaghan CJ, Duncan G, et al. Intermittent androgen suppression for rising PSA level after radiotherapy. N Engl J Med. 2012;367:895–903. doi: 10.1056/NEJMoa1201546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Messing EM, Manola J, Yao J, et al. Immediate versus deferred androgen deprivation treatment in patients with node-positive prostate cancer after radical prostatectomy and pelvic lymphadenectomy. Lancet Oncol. 2006;7:472–9. doi: 10.1016/S1470-2045(06)70700-8. [DOI] [PubMed] [Google Scholar]
- 29.Schroder FH, Kurth KH, Fossa SD, et al. Early versus delayed endocrine treatment of T2-T3 pN1-3 M0 prostate cancer without local treatment of the primary tumour: final results of European Organisation for the Research and Treatment of Cancer protocol 30846 after 13 years of follow-up (a randomised controlled trial) Eur Urol. 2009;55:14–22. doi: 10.1016/j.eururo.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 30.Walsh PC, DeWeese TL, Eisenberger MA. A structured debate: immediate versus deferred androgen suppression in prostate cancer-evidence for deferred treatment. J Urol. 2001;166:508–15. doi: 10.1016/s0022-5347(05)65972-1. [DOI] [PubMed] [Google Scholar]
- 31.Petrylak DP, Tangen CM, Hussain MH, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513–20. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 32.Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–12. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 33.de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364:1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ryan CJ, Smith MR, de Bono JS, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013;368:138–48. doi: 10.1056/NEJMoa1209096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367:1187–97. doi: 10.1056/NEJMoa1207506. [DOI] [PubMed] [Google Scholar]
- 36.Beer TM, Armstrong AJ, Rathkopf DE, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014;371:424–33. doi: 10.1056/NEJMoa1405095. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.