Abstract
Aims
The metabolic syndrome (MetS) is a clustering of low levels of HDL cholesterol, hyperglycaemia, high waist circumference, hypertension and elevated triglycerides, and is associated with cardiovascular disease. Calcified atherosclerotic plaque in the thoracic aorta (TAC), measured by non-contrast cardiac computed tomography (CT) scans, is a marker for atherosclerosis and relates to mortality. We sought to evaluate the independent association of MetS and TAC on cardiac CT scans.
Methods
We examined the relation of the MetS, and each of its components, to the prevalence of TAC, measured from 2000 to 2002 in 6778 white, Chinese, African-American and Hispanic participants in the Multi-Ethnic Study of Atherosclerosis (MESA).
Results
Adjusting for age, gender, race, smoking, LDL cholesterol and lipid-lowering medications, relative risks and 95% confidence intervals (CI) for a TAC score > 0 were: 1.19 (95% CI 1.11 to 1.28) for participants with MetS, 1.34 (95% CI 1.21 to 1.49) for those with diabetes and MetS, and 1.33 (95% CI 1.11, 1.58) for those with diabetes and no MetS compared with participants who were free of the MetS and diabetes. Associations were found for most of the components of the MetS with TAC.
Conclusions
We conclude that in adults without known heart disease, the MetS, most of its components and diabetes are associated with a higher prevalence of calcified atherosclerotic plaque in the thoracic arteries in a multi-ethnic population of men and women.
Introduction
Although calcific aortic disease in the thoracic aorta is common in the elderly, there currently are no medical therapies that have been shown, in prospective, randomized trials, to slow its progression. Population-based prospective studies have shown that the patients with aortic calcification have increased cardiac events and stroke [1–3]. Patients with calcification in the descending thoracic aorta have 3.8 times the relative risk for obstructive coronary artery disease, independent of age [4]. Thoracic aortic calcium is readily apparent on all thoracic computed tomography (CT) scans performed, whether for lung disease, cancer screening or heart disease. Previous studies have shown that the metabolic syndrome (MetS) and diabetes are associated with increased coronary artery calcium scores and aortic valve calcium, as assessed by non-contrast CT [5–7]. However, it is not known whether abnormalities in glucose metabolism/insulin resistance, as typified by the clinical syndromes of MetS and diabetes, are associated with increased likelihood of thoracic aortic calcification (TAC). No previous study has examined the relationship of the MetS and its variables to the likelihood of TAC as detected by CT in a large patient cohort.
We analysed data from the Multi-Ethnic Study of Atherosclerosis (MESA) to determine whether, at baseline, features of the MetS (waist circumference, blood pressure, low HDL cholesterol, high triglycerides and impaired fasting glucose) are associated, either individually or collectively, with increased TAC scores. We also determined the prevalence and quantity of aortic valve calcification among persons with MetS, diabetes and neither condition.
Methods
MESA was designed to investigate the prevalence, correlates and progression of subclinical cardiovascular disease in men and women. Details about the study design have been published elsewhere [8]. In brief, between July 2000 and August 2002, 6814 men and women who identified themselves as white, African American, Hispanic or Chinese and were 45–84 years old and free of clinically apparent cardiovascular disease were recruited from portions of six US communities: Baltimore City and Baltimore County, Maryland; Chicago, Illinois; Forsyth County, North Carolina; Los Angeles County, California; Northern Manhattan and the Bronx, New York; and St. Paul, Minnesota. Each field site recruited from locally available sources, which included lists of residents, lists of dwellings and telephone exchanges. In the last few months of the recruitment period, supplemental sources (lists of Medicare beneficiaries from the Centers for Medicare and Medicaid Services and referrals by participants) were used to ensure adequate numbers of minorities and elderly participants. The institutional review boards at all participating centres approved the study, and all participants gave informed consent.
Standardized questionnaires were used to obtain information about level of education, annual household income, smoking history and medication usage for high blood pressure, high cholesterol or diabetes. Smoking was defined as current, former or never. Waist circumference at the umbilicus was measured to the nearest 0.1 cm using a steel measuring tape (standard 4 oz. tension). Resting brachial systolic and diastolic blood pressure measurements were obtained using the Dinamap® automated blood pressure device (Dinamap Monitor Pro 100®). Three sequential measures were obtained and the average of the second and third measurements was recorded. Total and HDL cholesterol, triglycerides and glucose levels were measured from blood samples obtained after a 12 h fast. LDL cholesterol was calculated with the Friedewald equation [9]. Diabetes was defined as fasting glucose ≥ 7 mmol/l (126 mg/dl) or use of hypoglycaemic medication. Impaired fasting glucose was defined as a fasting glucose of 6.11–7 mmol/l (110–125 mg/dl) [10].
MetS was defined using the Third Adult Treatment Panel of the National Cholesterol Education Program (ATP III) [11] modified criteria: three or more of the following: large waist circumference (women > 88 cm and men > 102 cm); elevated triglycerides (≥ 8.33 mmol/l [150 mg/dl]); low HDL-cholesterol (men < 40 and women < 50 mg/dl); impaired fasting glucose (6.11–7 mmol/l); and elevated blood pressure (≥ 130/85 mmHg or self-reported use of medications for hypertension).
All participants underwent two CT scans at the same time for evaluation of coronary artery calcium after signing informed consent. Scans were assessed for the presence of thoracic aorta and valvular calcifications, and quantified as described below. CT studies were performed using either an Imatron C-150XL computed tomographic scanner (GE-Imatron, South San Francisco, CA, USA) in three sites, or multidetector CT scanners (four-slice) in three sites. Image slices were obtained with the patient supine, with no couch angulation. A minimum of 35 contiguous images of 2.5 or 3.0 mm slice thickness were obtained starting above the left main coronary artery to the bottom of both ventricles. The exact scanning methodology employed in the MESA study has been reported previously [12]. Each scan was obtained in a single breath-hold. A section thickness of 3 mm, field-of-view of 35 cm and matrix of 512 × 512 were used to reconstruct the raw image data. The nominal section thickness was 3.0 mm for electron-beam CT and 2.5 mm for four-detector row CT. The technologist evaluated the first scan to ensure that the protocol was followed and the entire coronary image was obtained. The participant was then rescanned using the same protocol. The participant remained on the CT couch between cardiac scans and the second scan was completed within minutes of the first scan.
The calcium score of each lesion was calculated by multiplying the lesion area by a density factor derived from the maximal Hounsfield units within this area, as described by Agatston [13]. The density factor was assigned in the following manner: 1 = lesions whose maximal density was 130–199 Hu; 2 = lesions of 200–299 Hu; 3, lesions of 300–399 Hu; and 4, lesions > 400 Hu. A total calcium score (for both Agatston and volume) was determined by summing individual lesion scores at each anatomic site. The volume of calcium was also measured, in mm3 as the volumetric score [14].
TAC was measured and quantified using the same lesion definition as for coronary calcification. The ascending and descending thoracic aorta was visualized from the lower edge of the pulmonary artery bifurcation to the cardiac apex on each cardiac CT. TAC is defined as total calcification in the ascending + descending portions. TAC score (Agatston and volume) was assessed in every patient. The absence of TAC was given a score of 0.
Data analysis
The study population for this analysis includes all MESA participants who had TAC measured as well as no missing data on any component of the MetS. After applying these criteria 6778 individuals remained for analysis.
Participants were classified by the presence or absence of the MetS and diabetes, creating three groups (diabetes, MetS or neither condition). Comparisons between the MetS, diabetes and neither condition (and across number of MetS risk factors) with demographic measures and cardiovascular risk factors are expressed using means and proportions. The chi-squared test for proportions and analysis of variance (ANOVA) for comparing levels of continuous risk factors were used.
Relative risk regression with Poisson error distribution and robust standard errors was used to estimate the cross-sectional association of the MetS with calcification prevalence after adjustment for potential confounding factors. Estimates from these analyses can be interpreted as prevalence ratios. Using this method, we examined the prevalence of TAC > 0 among the three disease groups (MetS, diabetes and neither condition), adjusted for age and ethnicity and additionally for other non-MetS risk factors (LDL cholesterol, lipid-lowering medications and smoking). Among those with detectable calcium, linear regression was used to estimate adjusted associations of the MetS with the extent of calcification among participants with non-zero calcification scores, which were log transformed to stabilize the variance and moderate the influence of outlying values. Estimates from the linear regression models can be interpreted as the relative difference in the geometric mean calcification score. We further examined the number of metabolic risk factors (from none to all five) and their association with prevalence of TAC, and also examined the risk of TAC prevalence and severity by number of MetS risk factors using those with 0 MetS risk factors as the reference group. Two-way interactions between the MetS and age, gender and race with the outcomes of interest were examined.
Statistical analysis was performed with SPSS 16.0.2 software for Windows (SPSS Inc., Chicago, IL, USA) and Stata 10.0 for Windows (Stata Corp., College Station, TX, USA). A P–value < 0.05 was considered statistically significant. Confidence intervals are expressed as 95% confidence intervals (95% CI).
Results
Participant characteristics
The mean age of the cohort was 62 years (range 45–84), 47% were male, 12% were Chinese American, 28% were African American and 22% were Hispanic. In this study, 1769 participants (26%) were defined as having MetS and 856 (13%) met the definition for diabetes, out of the total sample of 6778 participants. There were significant differences in all coronary risk factors between those without disease, those with MetS and those with diabetes (Table 1). For components of the MetS, large waist circumference, low HDL and high triglycerides were more prevalent in those with the MetS (85%, 73% and 65%, respectively) compared with people with diabetes (69%, 51% and 43%, respectively).
Table 1. Characteristics of participants with diabetes, ATP III metabolic syndrome, or neither condition and individual components, in the MESA population.
| Neither condition (n = 4153) | MetS (n = 1769) | Diabetes without MetS (n = 180) | Diabetes with MetS (n = 676) | |
|---|---|---|---|---|
| TAC > 0 | 999 (24%) | 576 (33%) | 68 (38%) | 254 (38%) |
| TAC score (for those with TAC> 0)* | 207 (6) | 255 (6) | 252 (5) | 258 (5) |
| Age | 61 (10) | 63 (10) | 65 (10) | 65 (9) |
| Female | 2129 (51%) | 1042 (59%) | 48 (27%) | 359 (53%) |
| Ethnicity | ||||
| White | 1745 (42%) | 706 (40%) | 29 (16%) | 129 (19%) |
| Chinese American | 534 (13%) | 162 (9%) | 38 (21%) | 67 (10%) |
| African American | 1094 (26%) | 451 (26%) | 71 (39%) | 258 (38%) |
| Hispanic | 780 (19%) | 450 (25%) | 42 (23%) | 222 (33%) |
| Serum Glucose | 87 (9) | 96 (12) | 151 (62) | 151 (55) |
| BMI | 26.8 (4.9) | 30.9 (5.2) | 26.2 (3.7) | 31.7 (5.7) |
| Total cholesterol | 194 (34) | 197 (37) | 183 (34) | 190 (41) |
| HDL cholesterol | 55 (15) | 43 (10) | 53 (12) | 44 (12) |
| LDL cholesterol | 118 (30) | 118 (32) | 112 (30) | 112 (35) |
| Triglycerides | 104 (57) | 181 (91) | 97 (40) | 181 (153) |
| Lipid-lowering meds | 532 (13%) | 324 (18%) | 42 (23%) | 199 (29%) |
| Diastolic BP | 71 (10) | 74 (10) | 72 (11) | 72 (10) |
| Systolic BP | 122 (21) | 134 (21) | 125 (19) | 135 (22) |
| Anti-hypertensive meds | 1046 (25%) | 928 (53%) | 65 (36%) | 482 (71%) |
| Current smoker | 532 (13%) | 237 (14%) | 27 (15%) | 84 (13%) |
| Metabolic syndrome components† | ||||
| Abdominal obesity (large waist) | 1579 (38%) | 1502 (85%) | 25 (14%) | 561 (83%) |
| High blood pressure | 1756 (42%) | 1436 (81%) | 89 (49%) | 583 (86%) |
| Low high-density lipoprotein | 724 (17%) | 1296 (73%) | 18 (10%) | 418 (62%) |
| High triglycerides | 492 (12%) | 1146 (65%) | 11 (6%) | 360 (53%) |
| Impaired fasting glucose | 257 (6%) | 681 (39%) | 180 (100%) | 676 (100%) |
Geometric mean (sd).
Relationships of MetS and diabetes to TAC prevalence and severity
The prevalence of TAC in people with MetS was 33%, compared with 38% in those with diabetes (with and without the MetS) and 24% of those with neither condition (P < 0.001). Among participants with detectable calcium who had neither condition the geometric mean was 207 Agatston units (sd = 6) compared with the geometric mean of those with MetS of 255 (sd = 6) and those with diabetes of 252 (sd = 5) for those without the MetS and 258 (sd = 5) for diabetics with the MetS. The difference between these means was not significant (P = 0.062). Furthermore the prevalence of MetS and diabetes (with and without the MetS) in the top quartile of severity (Agatston score > 834) was 27%, 28% and 22%, respectively, compared with 23% in those with neither condition (P = 0.196). We tested for interactions between the MetS and age, gender and race in the prediction of the presence of TAC and found no significant interactions (all P > 0.10).
Relationship of TAC prevalence to number of MetS components
There was a graded, linear association between the prevalence of TAC and the number of metabolic risk factors. TAC prevalence ranged from 12% in those without any metabolic risk factors to 25%, 30%, 29%, 38% and 46% in those with one, two, three, four and five metabolic risk factors, respectively (P < 0.001 for trend). For severity of TAC, the prevalence of TAC was greatest in the top quartile, and also exhibited a linear association (P = 0.003 for trend).
Regression analyses of MetS, diabetes and risk of TAC > 0
The relative risk (RR) of TAC (compared with those with neither condition) adjusted for age, gender and race was significantly higher among those with MetS (RR 1.21, 95% CI 1.12 to 1.30) and diabetes (without MetS RR 1.35, 95% CI 1.14 to 1.61; with MetS RR 1.3, 95% CI 1.22 to 1.50). Additional adjustment for LDL cholesterol, lipid-lowering medication use and cigarette smoking showed these relations to persist (MetS RR 1.19, 95% CI 1.11 to 1.28, diabetes without MetS RR 1.33, 95% CI 1.11 to 1.58, diabetes with MetS RR 1.34, 95% CI 1.21 to 1.49) (Table 2). In the fully adjusted models, performed separately for each component of the MetS, all components were significantly associated with an approximate 11–52% increase in TAC (Table 2). {{When all five of the components of the MetS were included in a single fully adjusted model (results not shown), the relative risk for elevated blood pressure, low HDL and impaired fasting glucose were attenuated slightly, with elevated blood pressure still exhibiting the strongest association with TAC relative to the other components (RR 1.49, 95% CI 1.36 to 1.63; RR 1.12, 95% CI 1.04 to 1.22; RR 1.10, 95% CI 1.03 to 1.19 respectively). High triglycerides and abdominal obesity were less strongly associated with TAC in the fully adjusted model (RR 1.03, 95% CI 0.96 to 1.12; RR 1.01, 95% CI 0.93 to 1.09, respectively). To examine the association between the number of components and presence of TAC, models were fit with categories representing the presence of one, two, three, four and five components, with zero components as the referent (Table 2). Participants with five components were associated with 111% increase in the prevalence of TAC compared with participants with zero components. Further, the presence of only one or any two components was positively associated with the presence of TAC (RR 1.44 95% CI 1.22 to 1.71 and RR 1.70, 95% CI 1.44 to 2.01, respectively).
Table 2. Cross-sectional associations of the MetS, components and number of components of the MetS with prevalent TAC.
| % prevalent TAC | Model 1* | Model 2† | Model 3‡ | |
|---|---|---|---|---|
| PR (95% CI) | PR (95% CI) | PR (95% CI) | ||
| MetS and diabetes | ||||
| Neither condition | 24 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| MetS | 33 | 1.35 (1.24, 1.47) | 1.21 (1.12, 1.30) | 1.19 (1.11, 1.28) |
| Diabetes without MetS | 38 | 1.57 (1.29, 1.91) | 1.35 (1.14, 1.61) | 1.33 (1.11, 1.58) |
| Diabetes with MetS | 38 | 1.56 (1.40, 1.75) | 1.35 (1.22, 1.50) | 1.34 (1.21, 1.49) |
| Components of the MetS | ||||
| Elevated BP | 38 | 2.52 (2.29, 2.77) | 1.52 (1.39, 1.67) | 1.52 (1.39, 1.66) |
| Low HDL | 29 | 1.07 (0.99, 1.16) | 1.17 (1.10, 1.26) | 1.17 (1.10, 1.26) |
| High triglycerides | 30 | 1.11 (1.03, 1.22) | 1.16 (1.08, 1.24) | 1.14 (1.06, 1.22) |
| Impaired fasting glucose (≥ 100 mg/dl) | 36 | 1.40 (1.30, 1.52) | 1.21 (1.13, 1.30) | 1.18 (1.10, 1.27) |
| Abdominal obesity | 30 | 1.15 (1.06, 1.24) | 1.11 (1.03, 1.20) | 1.11 (1.03, 1.19) |
| Number of components of the MetS | ||||
| 0 | 12 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| 1 | 25 | 2.04 (1.69, 2.46) | 1.49 (1.26, 1.76) | 1.44 (1.22, 1.71) |
| 2 | 31 | 2.51 (2.09, 3.02) | 1.76 (1.49, 2.08) | 1.70 (1.44, 2.01) |
| 3 | 30 | 2.45 (2.03, 2.96) | 1.75 (1.48, 2.08) | 1.68 (1.42, 1.99) |
| 4 | 37 | 3.01 (2.48, 3.64) | 2.00 (1.68, 2.38) | 1.94 (1.63, 2.31) |
| 5 | 45 | 3.63 (2.92, 4.51) | 2.17 (1.77, 2.65) | 2.11 (1.72, 2.59) |
Unadjusted.
Adjusted for age, gender and ethnicity.
Adjusted for age, gender, ethnicity, LDL, smoking and lipid-lowering medications.
PR, prevalence ratio.
Regression analyses of MetS, diabetes and risk of severity of TAC among those with TAC
Both MetS and diabetes with MetS were associated with a significantly greater TAC prevalence among those with detectable calcium (Table 3). This ranged from 23% (95% CI 3% to 45%) in those with the MetS to 39% (95% CI 9% to 74%) in those with diabetes and MetS. In predicting the prevalence of TAC (> 0 among those with TAC present) with each component separately only elevated blood pressure and impaired fasting glucose showed the strongest significant association (relative difference of 1.46, 95% CI 1.22 to 1.75 and 1.21, 95% CI 1.03 to 1.43, respectively) in the fully adjusted model (Table 3). For those with a positive TAC score, participants with three, four or five components had a relative difference of 1.43 (95% CI 1.02 to 2.01), 1.52 (95% CI 1.06 to 2.16) and 1.64 (95% CI 1.06 to 2.53) compared to those with zero components (Table 3).
Table 3. Associations of MetS, components and number of components of the MetS with the severity of TAC among participants with prevalent TAC.
| N (%) | Model 1* | Model 2† | Model 3‡ | |
|---|---|---|---|---|
| Exp(β) (95% CI) | Exp(β) (95% CI) | Exp(β) (95% CI) | ||
| MetS and diabetes | ||||
| Neither condition | 999 (61) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| MetS | 576 (26) | 1.24 (1.03, 1.48) | 1.22 (1.03, 1.44) | 1.23 (1.03, 1.45) |
| Diabetes without MetS | 68 (4) | 1.25 (0.79, 1.86) | 1.44 (0.93, 2.12) | 1.43 (0.93, 2.10) |
| Diabetes with MetS | 254 (13) | 1.26 (0.98, 1.59) | 1.42 (1.12, 1.77) | 1.39 (1.09, 1.74) |
| Components of the MetS | ||||
| Elevated BP | 1468 (77) | 1.69 (1.40, 2.04) | 1.46 (1.22,.1.75) | 1.46 (1.22, 1.75) |
| Low HDL | 717 (38) | 1.01 (0.86, 1.19) | 1.07 (0.92, 1.26) | 1.07 (0.90, 1.26) |
| High TG | 607 (32) | 0.98 (0.85, 1.16) | 1.02 (0.86, 1.20) | 0.92 (0.77, 1.09) |
| Impaired fasting glucose (≥100 mg/dl) | 638 (34) | 1.21 (1.02, 1.43) | 1.28 (1.09, 1.50) | 1.21 (1.03, 1.43) |
| Abdominal obesity | 1097 (58) | 1.07 (0.91, 1.27) | 1.07 (0.91, 1.27) | 1.01 (0.85, 1.19) |
| Number of components of the MetS | ||||
| 0 | 118 (6) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| 1 | 406 (21) | 1.38 (0.96, 1.98) | 1.29 (0.92, 1.81) | 1.23 (0.88, 1.72) |
| 2 | 543 (29) | 1.32 (0.93, 1.88) | 1.28 (0.91, 1.78) | 1.22 (0.88, 1.69) |
| 3 | 415 (22) | 1.51 (1.05, 2.17) | 1.48 (1.05, 2.08) | 1.43 (1.02, 2.01) |
| 4 | 301 (16) | 1.63 (1.12, 2.38) | 1.60 (1.12, 2.27) | 1.52 (1.06, 2.16) |
| 5 | 114 (6) | 1.82 (1.15, 2.86) | 1.74 (1.13, 2.67) | 1.64 (1.06, 2.53) |
Unadjusted.
Adjusted for age, gender and ethnicity.
Adjusted for age, gender, ethnicity, LDL, smoking and lipid-lowering medications.
Exp(β), relative difference in geometric mean TAC score compared to the reference group.
Discussion
This investigation is the first to demonstrate, in a population-based multi-ethnic cohort, that the MetS and diabetes are both independently associated with increased prevalence and severity of TAC after adjustment for age, gender and ethnicity. The significance of MetS as a cardiovascular risk marker, particularly its incremental value over its individual components, has remained a matter of considerable debate and that makes it pertinent to learn how well MetS correlates with TAC [15–20]. Several studies have suggested that MetS is incrementally associated with cardiovascular risk, others have pointed out that the cardiovascular risk associated with MetS is no more than the sum of the risk imparted by its individual components [15–20]. On additional adjustment for non-MetS risk factors and individual MetS components, the associations attenuated only slightly and models for MetS retained statistically significance. Moreover, there was a graded, linear relationship between the number of MetS components and RR for the presence and severity of TAC, which further strengthens the association of MetS with TAC. Interestingly, in MESA, we have shown that the risk of cardiovascular events is only modest in the presence of MetS alone, but increases substantially once diabetes develops (MetS plus diabetes) [21]. However, in the current study, the RR for TAC for MetS alone and for diabetes were not much different, nor were the actual TAC scores. Thus, the risk mediated by MetS must be beyond TAC, most likely including inflammation and coronary artery calcium.
Several smaller studies have demonstrated that different atherosclerotic risk factors contribute to the formation or presence of TAC [22,23]. In previous reports from the MESA study, it was shown that risk factors for TAC were similar to cardiovascular risk factors, with the highest prevalence in both white and Chinese populations [24] and significantly lower risk of presence of TAC in African Americans and Hispanics. Similar to the results with MetS and diabetes in the current study, these differences do not appear to be completely accounted for by traditional risk factors [25,26]. Because there is no additional scanning or participant burden to acquire information about TAC, the incremental prognostic information identified from MESA may be more pronounced in subpopulations [27]. TAC was also shown to be associated with coronary artery calcium [28,29], carotid intima media thickness [30] and carotid distensibility [31]. In symptomatic patients with stable angina pectoris, TAC is associated with an increased risk of death and cardiovascular events [32].
This incremental measure of atherosclerosis, as detected by calcified plaque in the thoracic aorta, may help explain the incremental risk of MetS over individual risk factors. In the current study, in adults without known heart disease, the MetS, most of its components and diabetes are associated with a higher prevalence of calcified atherosclerotic plaque in the thoracic arteries in a multi-ethnic population of men and women. These data, along with other cohort studies demonstrating adverse outcome, continue to reinforce the value of quantitative reporting of extracoronary calcification on cardiac scans.
Further applications of TAC assessment now extend to ungated low-dose CT used commonly for assessment of lung nodules. Prior studies have demonstrated that these ungated studies are highly reliable for prediction and quantification of TAC, as the aorta does not move and thus minimizes any motion artefacts avoided with gating. Concordance between gated studies and ungated studies for TAC is high [33]. This measure should allow for atherosclerotic disease risk stratification among patients undergoing ungated lung CT evaluation without requiring additional scanning. Currently, the events in this cohort in MESA with metabolic syndrome are still too infrequent to assess the prognostic importance of this measure in this cohort, but future studies will focus on the independent prognostic significance of this measure.
Figure 1.
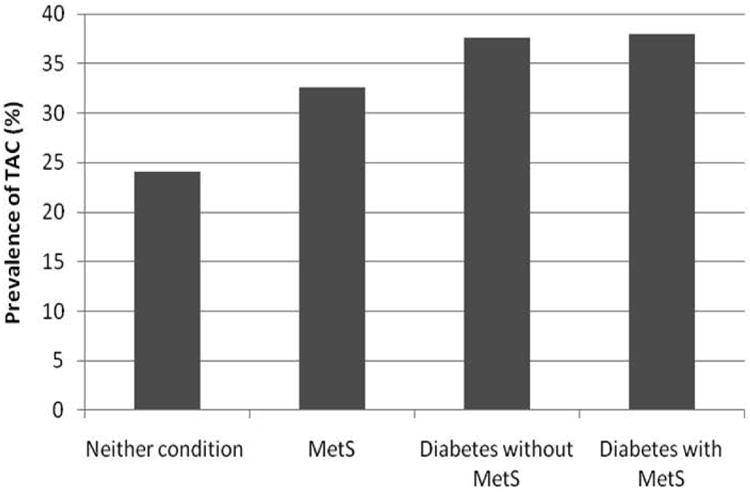
Prevalence of TAC by diabetes, metabolic syndrome or neither condition.
Figure 2.
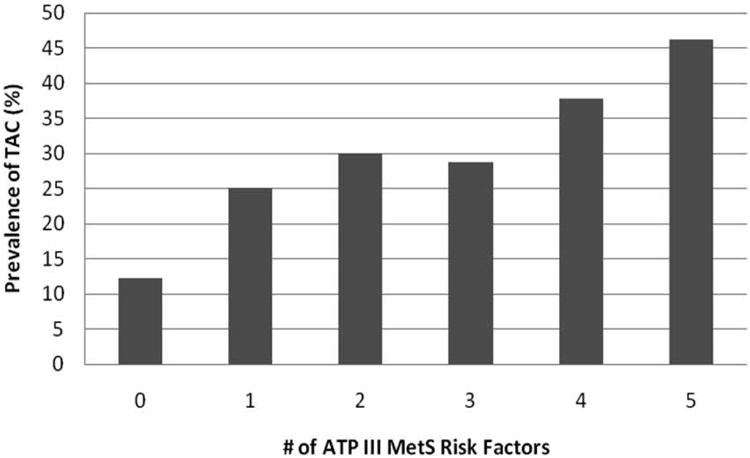
Prevalence of TAC > 0 by the number of ATP III metabolic syndrome risk factors.
What's new?
Thoracic aortic calcium is an emerging risk marker, readily available on all chest and cardiac computed tomography scans.
This is the first article evaluating the metabolic syndrome and it's association with thoracic aortic calcification.
The study demonstrated an independent relationship of metabolic syndrome and thoracic aortic calcification.
Acknowledgments
Matthew Budoff guarantees the integrity of this data. This research was supported by R01 HL071739 and contracts N01-HC-95159 through N01-HC-95165 and N01 HC 95169 from the National Heart, Lung, and Blood Institute. The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Funding sources: This research was supported by R01 HL071739 and contracts N01-HC-95159 through N01-HC-95165 and N01 HC 95169 from the National Heart, Lung, and Blood Institute.
Footnotes
Author contributions: M.B. researched data. R.K. wrote manuscript, researched data. K.N. reviewed/edited manuscript. N.D. contributed to discussion, reviewed/edited manuscript. K.O. researched data, contributed discussion.
References
- 1.Witteman JC, Kok FJ, van Saase JL, Valkenburg HA. Aortic calcification as a predictor of cardiovascular mortality. Lancet. 1986 Nov 15;2(8516):1120–1122. doi: 10.1016/s0140-6736(86)90530-1. [DOI] [PubMed] [Google Scholar]
- 2.Hollander M, Hak AE, Koudstaal PJ, Bots ML, Grobbee DE, Hofman A, et al. Comparison between measures of atherosclerosis and risk of stroke: the Rotterdam Study. Stroke. 2003;34:2367–2372. doi: 10.1161/01.STR.0000091393.32060.0E. [DOI] [PubMed] [Google Scholar]
- 3.Witteman JC, Kannel WB, Wolf PA, Grobbee DE, Hofman A, D'Agostino RB, et al. Aortic calcified plaques and cardiovascular disease (the Framingham Study) Am J Cardiol. 1990;66:1060–1064. doi: 10.1016/0002-9149(90)90505-u. [DOI] [PubMed] [Google Scholar]
- 4.Takasu J, Mao S, Budoff MJ. Aortic atherosclerosis detected with electron-beam CT as a predictor of obstructive coronary artery disease. Acad Radiol. 2003;10:631–637. doi: 10.1016/s1076-6332(03)80081-8. [DOI] [PubMed] [Google Scholar]
- 5.Arad Y, Newstein D, Cadet F, Roth M, Guerci AD. Association of multiple risk factors and insulin resistance with increased prevalence of asymptomatic coronary artery disease by an electron-beam computed tomographic study. Arterioscler Thromb Vasc Biol. 2001;21:2051–2058. doi: 10.1161/hq1201.100257. [DOI] [PubMed] [Google Scholar]
- 6.Wong ND, Sciammarella MG, Polk D, Gallagher A, Miranda-Peats L, Whitcomb B, et al. The metabolic syndrome, diabetes, and subclinical atherosclerosis assessed by coronary calcium. J Am Coll Cardiol. 2003;41:1547–1553. doi: 10.1016/s0735-1097(03)00193-1. [DOI] [PubMed] [Google Scholar]
- 7.Katz R, Wong ND, Kronmal R, Takasu J, Shavelle DM, Probstfield JL, et al. Features of the metabolic syndrome and diabetes mellitus as predictors of aortic valve calcification in the Multi-Ethnic Study of Atherosclerosis. Circulation. 2006;113:2113–2119. doi: 10.1161/CIRCULATIONAHA.105.598086. [DOI] [PubMed] [Google Scholar]
- 8.Bild DE, Bluemke DA, Burke GL, Detrano R, Roux AV, Folsom AR, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 9.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 10.Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 2003;26(Suppl 1):S5–S20. doi: 10.2337/diacare.26.2007.s5. [DOI] [PubMed] [Google Scholar]
- 11.Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 12.Carr JJ, Nelson JC, Wong ND, Nitt-Gray M, Arad Y, Jacobs DR, Jr, et al. Calcified coronary artery plaque measurement with cardiac CT in population-based studies: standardized protocol of Multi-Ethnic Study of Atherosclerosis (MESA) and Coronary Artery Risk Development in Young Adults (CARDIA) study. Radiology. 2005;234:35–43. doi: 10.1148/radiol.2341040439. [DOI] [PubMed] [Google Scholar]
- 13.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 14.Callister TQ, Cooil B, Raya SP, Lippolis NJ, Russo DJ, Raggi P. Coronary artery disease: improved reproducibility of calcium scoring with an electron-beam CT volumetric method. Radiology. 1998;208:807–814. doi: 10.1148/radiology.208.3.9722864. [DOI] [PubMed] [Google Scholar]
- 15.Sattar N, Gaw A, Scherbakova O, et al. Metabolic syndrome with and without C-reactive protein as a predictor of coronary heart disease and diabetes in the West of Scotland Coronary Prevention Study. Circulation. 2003;108:414–419. doi: 10.1161/01.CIR.0000080897.52664.94. [DOI] [PubMed] [Google Scholar]
- 16.Gami AS, Witt BJ, Howard DE, et al. Metabolic syndrome and risk of incident cardiovascular events and death: a systematic review and meta-analysis of longitudinal studies. J Am Coll Cardiol. 2007;49:403–414. doi: 10.1016/j.jacc.2006.09.032. [DOI] [PubMed] [Google Scholar]
- 17.Najarian RM, Sullivan LM, Kannel WB, Wilson PW, D'Agostino RB, Wolf PA. Metabolic syndrome compared with type 2 diabetes mellitus as a risk factor for stroke: the Framingham Offspring Study. Arch Intern Med. 2006;166:106–111. doi: 10.1001/archinte.166.1.106. [DOI] [PubMed] [Google Scholar]
- 18.Wannamethee SG, Shaper AG, Lennon L, Morris RW. Metabolic syndrome vs Framingham Risk Score for prediction of coronary heart disease, stroke, and type 2 diabetes mellitus. Arch Intern Med. 2005;165:2644–2650. doi: 10.1001/archinte.165.22.2644. [DOI] [PubMed] [Google Scholar]
- 19.Stern MP, Williams K, Gonzalez-Villalpando C, Hunt KJ, Haffner SM. Does the metabolic syndrome improve identification of individuals at risk of type 2 diabetes and/or cardiovascular disease? Diabetes Care. 2004;27:2676–2681. doi: 10.2337/diacare.27.11.2676. [DOI] [PubMed] [Google Scholar]
- 20.Grundy SM, Brewer HB, Jr, Cleeman JI, Smith SC, Jr, Lenfant C for the Conference Participants. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109:433–438. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 21.Malik S, Budoff MJ, Katz R, Blumenthal RS, Bertoni AG, Nasir K, et al. Impact of subclinical atherosclerosis on cardiovascular disease events in individuals with metabolic syndrome and diabetes: the Multi-Ethnic Study of Atherosclerosis. Diabetes Care. 2011;34:2285–2290. doi: 10.2337/dc11-0816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Witteman JC, Kannel WB, Wolf PA, et al. Aortic calcified plaques and cardiovascular disease (the Framingham Study) Am J Cardiol. 1990;66:1060–1064. doi: 10.1016/0002-9149(90)90505-u. [DOI] [PubMed] [Google Scholar]
- 23.Hollander M, Hak AE, Koudstaal PJ, et al. Comparison between measures of atherosclerosis and risk of stroke: the Rotterdam Study. Stroke. 2003;34:2367–2372. doi: 10.1161/01.STR.0000091393.32060.0E. [DOI] [PubMed] [Google Scholar]
- 24.Takasu J, Katz R, Nasir K, et al. Relationships of thoracic aortic wall calcification to cardiovascular risk factors: the Multi-Ethnic Study of Atherosclerosis (MESA) Am Heart J. 2008;155:765–771. doi: 10.1016/j.ahj.2007.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takasu J, Katz R, Shavelle DM, O'Brien K, Mao S, Carr JJ, et al. Inflammation and descending thoracic aortic calcification as detected by computed tomography: The Multi-Ethnic Study of Atherosclerosis. Atherosclerosis. 2008;199:201–206. doi: 10.1016/j.atherosclerosis.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 26.Allison MA, Budoff MJ, Nasir K, et al. Ethnic-specific risks for atherosclerotic calcification of the thoracic and abdominal aorta (from the Multi-Ethnic Study of Atherosclerosis) Am J Cardiol. 2009;104:812–817. doi: 10.1016/j.amjcard.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Budoff MJ, Nasir K, Katz R, et al. Thoracic aortic calcification and coronary heart disease events: the Multi-Ethnic Study of Atherosclerosis (MESA) Atherosclerosis. 2011;215:196–202. doi: 10.1016/j.atherosclerosis.2010.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takasu J, Budoff MJ, O'Brien KD, Shavelle DM, Probstfield JL, Carr JJ, et al. Relationship between coronary artery and descending thoracic aortic calcification as detected by computed tomography: The Multi-Ethnic Study of Atherosclerosis. Atherosclerosis. 2009;204:440–446. doi: 10.1016/j.atherosclerosis.2008.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rivera JJ, Nasir K, Katz R, Takasu J, Allison M, Wong ND, et al. Relationship of thoracic aortic calcium to coronary calcium and its progression (from the Multi-Ethnic Study of Atherosclerosis [MESA]) Am J Cardiol. 2009;103:1562–1567. doi: 10.1016/j.amjcard.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takasu J, Budoff MJ, Katz R, Rivera JJ, O'Brien KD, Shavelle DM, et al. Relationship between common carotid intima-media thickness and thoracic aortic calcification: the Multi-Ethnic Study of Atherosclerosis. Atherosclerosis. 2010;209:142–146. doi: 10.1016/j.atherosclerosis.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blaha MJ, Budoff MJ, Rivera JJ, Katz R, O'Leary DH, Polak JF, et al. Relationship of carotid distensibility and thoracic aorta calcification. Multi-Ethnic Study of Atherosclerosis. Hypertension. 2009;54:1408–1415. doi: 10.1161/HYPERTENSIONAHA.109.138396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eisen A, Tenenbaum A, Koren-Morag N, et al. Calcification of the thoracic aorta as detected by spiral computed tomography among stable angina pectoris patients: association with cardiovascular events and death. Circulation. 2008;118:1328–1334. doi: 10.1161/CIRCULATIONAHA.107.712141. [DOI] [PubMed] [Google Scholar]
- 33.Budoff MJ, Nasir K, Kinney GL, Hokanson JE, Barr RG, Steiner R, et al. Coronary artery and thoracic calcium on noncontrast thoracic CT scans: comparison of ungated and gated examinations in patients from the COPD Gene cohort. J Cardiovasc Comput Tomogr. 2011;5:113–118. doi: 10.1016/j.jcct.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]


