Abstract
Background and aims
The hypothesis of this study was that microvascular flow-induced dilation (FID) and acetylcholine-induced dilation (AChID) is impaired in visceral (VAT) compared to subcutaneous adipose tissue (SAT) arterioles in morbidly obese women. Additional aim was to determine the mechanisms contributing to FID and AChID in VAT and SAT arterioles.
Methods and results
Arterioles were obtained from SAT and VAT biopsies from women (BMI>35 kg/m2) undergoing bariatric surgery. Microvessels were cannulated for reactivity measurements in response to flow (pressure gradients of 10–100 cmH2O) and to acetylcholine (ACh;10−9–10−4 M) with and without Nω-nitro-L-arginine methyl ester (L-NAME), indomethacin (INDO), and PEG-catalase. Nitric oxide (NO)and hydrogen peroxide (H2O2) generation were detected in arterioles by fluorescence microscopy. FID and AChID of arterioles from VAT were reduced compared to SAT arterioles. In SAT arterioles, L-NAME, INDO, and PEG-catalase significantly reduced FID and AChID but had no effect individually on VAT arterioles’ vasodilator reactivity. INDO+L-NAME reduced FID in VAT arterioles. NO-fluorescence was greater in arterioles from SAT compared to VAT arterioles. Vascular H2O2 generation during flow was similar in both VAT and SAT.
Conclusion
Our results suggest that VAT arterioles display reduced vasodilator reactivity to flow and ACh compared to SAT arterioles, mediated by different regulatory mechanisms in human obesity.
Keywords: Obesity, Adipose, Microcirculation, Blood Flow, Endothelium
Introduction
Obesity is a major global public health concern [1, 2]. It is well established that obesity is a major risk factor for premature mortality from cardiovascular diseases [3–5]. Several mechanisms may contribute to the increased risk of cardiovascular disease in obese individuals including an increased inflammatory state in adipose tissue depots [6, 7]. The endothelium is a key regulator of vascular function and reduced endothelium-dependent vasodilation is an early indicator of atherosclerosis and cardiovascular disease [8]. Microvascular dysfunction can elicit an increase in peripheral resistance and altered glucose homeostasis [9]. In adipose tissue this may be an important initial step in development of cardiovascular disease among obese individuals and therefore, may be a prognostic factor for future cardiovascular events [10, 11].
There is considerable heterogeneity in the risks associated with intra-abdominal adiposity. Previous studies have shown that visceral adipose tissue (VAT) accumulation is associated with impaired endothelial function [12, 13]. VAT also has a strong association with cardiovascular (CV) morbidities such as metabolic syndrome and diabetes mellitus [14, 15]. The physiology of vascular function in different adipose depots in humans is not completely understood. However, our previous studies have found that the dilator mechanisms to flow in visceral adipose during cardiovascular disease appear to be similar to the coronary circulation [16, 17]. Previous studies also indicate that adipose is a regulator of vascular function. For example, Farb et al. showed that VAT appears more pro-atherogenic and exhibited reduced vasodilator reactivity to acetylcholine compared to subcutaneous adipose tissue (SAT) [6]. Further, Virdis et al. reported that that vasodilator reactivity of visceral adipose arterioles to ACh was reduced in obese compared to lean individuals [18]. However, potential differences in the mechanisms regulating endothelium-dependent flow-induced vasodilation between these two adipose depots in human morbid obesity remains to be elucidated. Adipose tissue has been proposed as a source of endocrine and paracrine modulation of vascular function during morbid obesity. Therefore, microvessels from the abdominal region could serve as surrogates for altered endothelial function and could help to explain the progression of early disease phenotype in this population [9, 19]. The primary goals of the present study were to 1) test the hypothesis that microvascular FID and AChID are reduced in VAT compared to SAT in morbidly obese individuals, and 2) determine the regulatory mechanisms contributing to altered vasodilator function in VAT and SAT arterioles in human morbid obesity.
Materials and Methods
Study Population
Fifty-three morbidly obese women, who underwent planned bariatric surgery, were included in the study. Written informed consent was obtained from each patient. The study protocol and procedures conformed to the standards set by the latest revision of the Declaration of Helsinki and were approved by the University of Illinois at Chicago Institutional Review Board. All participants were pre-menopausal between 26–48 years old. Volunteers were excluded if they had diabetes mellitus, cancer, and heart disease, a history of smoking, kidney disease, liver disease, gallbladder disease, rheumatoid arthritis, HIV/AIDS, inflammatory, or bowel disease. Ten patients were taking anti-hypertensive medications and seven subjects were taking medications for gastric reflux prior to surgery.
Anthropometric and Metabolic Measures
Anthropometric measurements (height, weight, BMI, and waist circumference) and blood pressure measurements were determined before the collection of tissue biopsies and blood draws. Fasting blood samples for biochemical parameters (blood glucose, insulin levels, and lipid panels) were collected on the day of surgery.
Tissue Acquisition
Matched samples of SAT and VAT biopsies were collected from each patient during planned bariatric surgery at the University of Illinois Hospital and Health Sciences Center. Subcutaneous adipose tissue was obtained from the lower abdominal wall and VAT was secured from the greater omentum. Biopsies were placed in cold (4°C) HEPES buffer solution. Arterioles were cleaned of fat and connective tissue and prepared for continuous measurement of internal luminal diameter.
Experimental protocol and microvascular preparation
In an organ chamber, arterioles were cannulated and prepared as previously described [28]. The internal luminal diameter of each microvessel was initially measured after 30 minutes of stabilization at 60 cmH2O and following administration of endothelin-1 (ET-1; 100–200 pM) to constrict microvessels to 30–50% of their internal luminal diameter. This was followed by reactivity measurements to flow and to acetylcholine (ACh). Flow was produced by simultaneously changing the heights of the reservoirs in equal and opposite directions to generate a pressure gradient of Δ10, Δ20, Δ40, Δ60, and Δ100 cmH2O. In separate experiments, dilations to ACh (10−9 to 10−4 M) were determined. FID and AChID of arterioles were measured in the absence and presence of: a) the NO synthase (NOS) inhibitor, -nitro-L-arginine methyl ester (L-NAME; 10−4M), b) the cyclooxygenase (COX) inhibitor indomethacin (INDO; 10−5M), and c) the H2O2 scavenger, polyethylene glycol catalase (PEG-CAT; 500 U/ml). Inhibitors were added to the external bathing solution of an organ chamber for 30 minutes prior to application of flow or ACh. Maximal internal luminal diameter of each microvessel was determined in the presence of papaverine (10−4M), at the end of each experiment. In separate experiments, dose responses to the NO donor sodium nitroprusside (SNP; 10−9 M to 10−4 M) were determined in VAT and SAT arterioles.
Fluorescence detection of microvascular NO and H2O2 production
Vascular NO was measured using an NO Detection Kit. The non-fluorescent and cell-permeable NO detection dye reacts with NO in the presence of O2 with high specificity, sensitivity and accuracy, yielding a water-insoluble red fluorescent product. Application of dichlorodehydrofluorescein diacetate (DCF-DA; 2 μM) was used to measure H2O2 with and without PEG-CAT and with and without flow [16]. The NO fluorescent product was excited by a 650 nm wavelength light with an emission spectrum of 670 nm and DCF-DA fluorescence was excited by a 488 nm wavelength of light with an emission spectrum of 527 nm using a krypton/argon fluorescent microscope (Nikon eclipse 80i). Microvessels were cannulated and maintained at 37 °C at an equilibration pressure of 60 cmH2O for 30 minutes and then exposed to flow (pressure gradient of Δ60 cmH2O) in the presence or absence of either L-NAME (10−4) for NO measurements or PEG-CAT (500 U/ml) for H2O2 measurements. Vessels were then exposed to the NO detection dye or DCF-DA dye for the final 30 minutes, rinsed in HEPES buffer, and mounted to slides for image acquisition. Acquired images were analyzed for fluorescence intensity while correcting for background auto fluorescence using NIH image software (Image J).
Materials
The NO detection kit was obtained from Enzo Life Sciences. The DCF-DA dye was obtained from Life Technologies. The remaining chemical agents were obtained from Sigma-Aldrich. Final molar concentrations of the agents in the organ chambers were reported. None of the pharmacological antagonists or inhibitors produced significant changes in baseline arteriolar diameter and resulted in less than a 1% change in total volume (data not shown).
Statistical Analysis
All data are expressed as mean±SEM (except for data in Table 1 which are expressed as mean±SD). Percent dilation was calculated as the percent change from constricted diameter to the diameter after flow or ACh with 100% representing maximal diameter usually in the presence of papaverine (10−4M). Responses to flow and ACh were analyzed with two factor analysis of variance (ANOVA) to determine the effect of depot and treatment on flow or AChID. Significant differences were followed by Scheffe’s Post Hoc analysis. To compare the maximum intraluminal dilation to papaverine between experimental protocols, a Student’s t-test was used. When variables were not normally distributed, the Mann-Whitney-Wilcoxon Rank Sum Test was used. Statistical significance was set to P<0.05 for a two-sided test. Sigma Plot v.12 (Systat Software, Inc, Chicago, USA) was used for statistical analysis.
Table 1.
Anthropometric, biochemical and hemodynamic measurements in study participants (n=53)
| Mean ± SD | |
|---|---|
| Age (years) | 36 ± 6 |
| Height (cm) | 165 ± 6 |
| Weight (kg) | 133 ± 27 |
| BMI (kg/m2) | 48 ± 10 |
| Waist Circumference | 134 ± 17 |
| Glucose (mg/dL) | 87 ± 15 |
| Triglycerides (mg/dL) | 135 ± 74 |
| Total cholesterol (mg/dL) | 173 ± 28 |
| HDL cholesterol (mg/dL) | 42 ± 7 |
| LDL cholesterol (mg/dL) | 111 ± 25 |
| SBP (mmHg) | 125 ± 16 |
| DBP (mmHg) | 73 ± 11 |
| MAP (mmHg) | 91 ± 11 |
BMI - Body Mass Index; HDL - High-Density Lipoprotein;
LDL - Low-Density Lipoprotein; SBP - Systolic Blood Pressure;
DBP - Diastolic Blood Pressure; MAP - Mean Arterial Pressure
Results
Table 1 summarizes anthropometric and biochemical parameters of the study population. There were ten patients taking anti-hypertension medications and seven patients taking medication for acid reflux. There was no difference between the reactivity of blood vessels between patients taking medications and patients without prior pharmacological treatment (not shown). Therefore, all data were pooled for analysis.
Flow- and ACh- induced dilation of resistance arteries from SAT and VAT
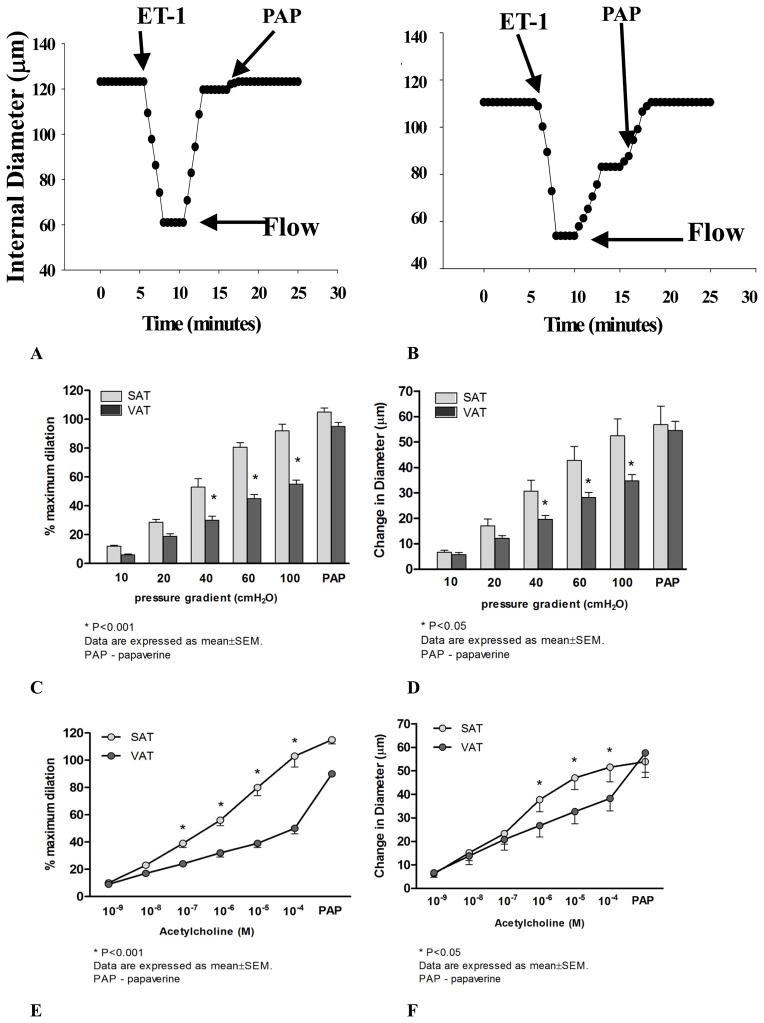
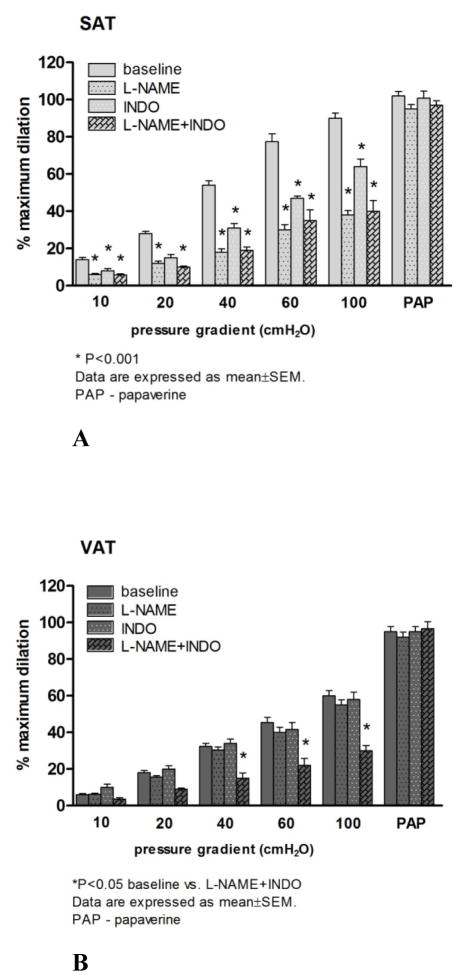
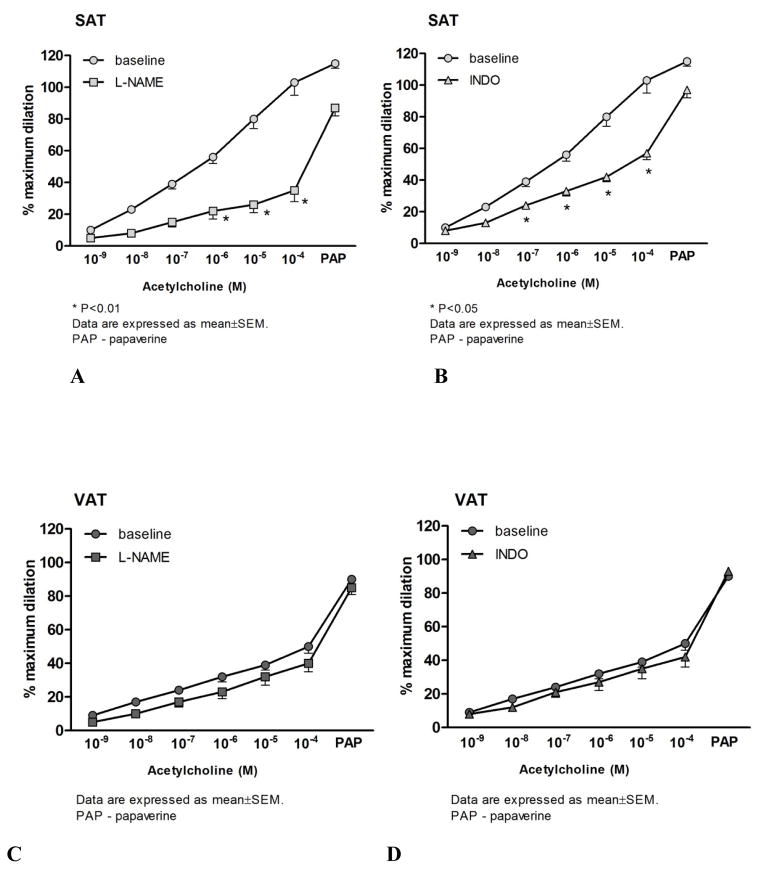
The mean internal luminal diameter was 151 ± 20μm and 161 ± 13μm for SAT and VAT (P=0.5), respectively. The average ET-1 dose used to pre-constrict arterioles was similar between the two groups (SAT: 159±4 pMvs. VAT: 165±4 pM; P>0.05). There was no statistical difference between the final constricted diameters in SAT (101.9±10 μm) and VAT (89.6 ±10 μm; P=0.4). However, dilator responses to flow were blunted in VAT microvessels compared to those from SAT (Figure 1C). Similarly, VAT arterioles were less sensitive to ACh compared to those from SAT (Figure 1E). The presence of L-NAME significantly reduced FID and AChID in SAT microvessels (Figure 2A). In contrast, L-NAME had no effect on vasodilator reactivity to flow and ACh in VAT arterioles compared to baseline (Figures 2B and Figure 3C).
Figure 1.
Flow- induced dilation (FID) and acetylcholine -induced dilation (AChID) in visceral and subcutaneous of human adipose. Representative time course data showing the internal diameter changes to flow (intraluminal pressure gradient of 100 cmH2O) in arterioles from visceral adipose tissue (VAT, A) and subcutaneous adipose tissue (SAT, B). FID (n=21) was reduced in VAT compared to SAT (C and D, n=21). AChID was reduced in VAT compared to SAT (E and F, n=17). There is a significant group by dose effect for flow (P=0.004) and ACh (P=0.001). *p<0.001 SAT vs. VAT at each level of flow or ACh. PAP: papavarine (10−4 M)
Figure 2.
The effects of nitric oxide synthase (NOS) and cyclooxygenase (COX)inhibition on flow-induced dilation (FID) in subcutaneous adipose tissue (SAT) and visceral adipose tissue (VAT) arterioles. NOS inhibition with L-NAME, significantly reduced FID in SAT (A). Indomethacin (INDO) reduced FID in SAT, but had no additional effect in the presence of L-NAME (A). The presence of L-NAME or INDO had no effect on FID in VAT resistance arteries (B). However, the combination of L-NAME and INDO reduced dilation at higher flow rates in VAT vessels (B). There is a significant group by dose effect for flow (P=0.035) *p<0.001 baseline vs. inhibitors in SAT or VAT at each level of flow or ACh (n=17–21). PAP: papavarine (10−4 M)
Figure 3.
The effects of nitric oxide synthase (NOS) and cyclooxygenase (COX) inhibition on acetylcholine-induced dilation (AChID) in visceral (VAT) and subcutaneous adipose tissue (SAT) arterioles. Panel C presents the effect of nitric oxide synthase (NOS) inhibition on AChID in visceral adipose tissue (VAT) resistance arteries. Panel D presents the effect of cyclooxygenase (COX) inhibition on AChID in VAT resistance arteries. In the presence of NOS, L-NAME, the AChID was reduced in SAT at 10−6, 10−5 and 10−4M doses of ACh (n=10, A). Cyclooxygenase inhibition with INDO reduced AChID of SAT resistance arteries at 10−7–10−4 doses of ACh (n=9, B). L-NAME and indomethacin had no effect on acetylcholine-induced dilation in VAT resistance arteries compared to baseline (n=10) (C and D). PAP: papavarine (10−4 M)
Effects of cyclooxygenase and catalase on flow- and ACh-induced dilations
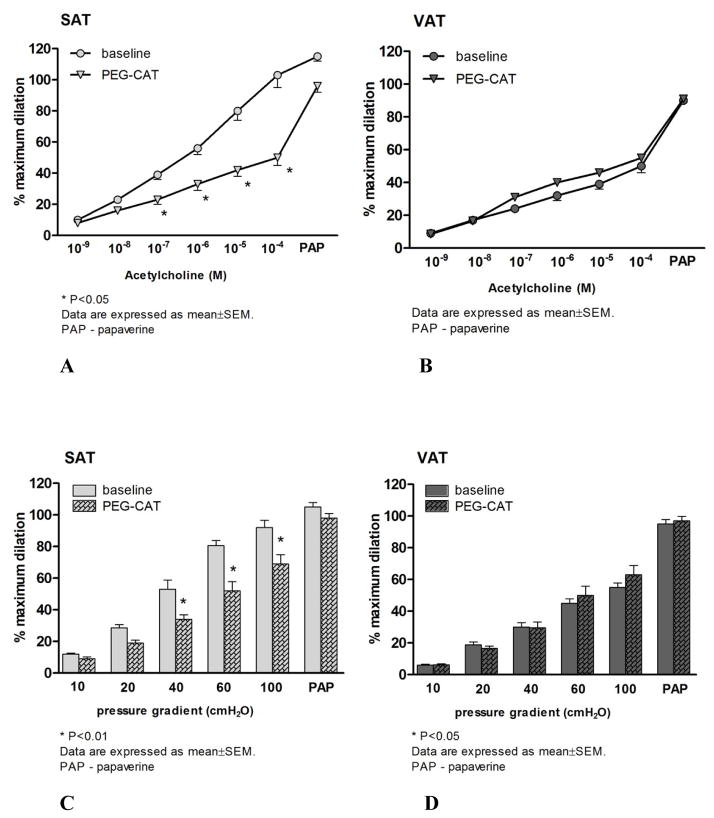
Similar to L-NAME, the COX inhibitor INDO significantly reduced FID in SAT microvessels compared to the baseline (Figure 2A). FID in the presence of both L-NAME and INDO was similar to L-NAME alone in SAT microvessels (Figure 2A). The addition of L-NAME to INDO reduced dilation at higher flow rates in SAT arterioles (Figure 2B). While there was no effect of INDO on FID in VAT microvessels, the addition of L-NAME and INDO significantly reduced the FID response in this tissue (Figure 2B). Similarly, the H2O2 scavenger, PEG-CAT, significantly reduced FID of SAT microvessels compared to their baseline response (Figure 4C) but did not affect impaired vasodilation in response to flow increments in VAT microvessels (Figure 4D). Treatment of microvessels from SAT with INDO or PEG-CAT reduced AChID compared to the baseline measures (Figure 3B and Figure 4A, respectively), while incubation of VAT microvessels with INDO or PEG-CAT did not change AChID compared to their baseline response (Figure 3C and Figure 4B, respectively). However, the combination of L-NAME and INDO reduced AChID in VAT arterioles (data not shown).
Figure 4.
The effect of the polyethylene glycol catalase (PEG-CAT), on acetylcholine-induced dilation (AChID) in subcutaneous adipose tissue (SAT) arterioles (panel A) and in visceral adipose tissue (VAT) arterioles (panel B). In the presence of PEG-CAT, AChID is reduced at 10−7–10−4M doses of ACh in the vessels from SAT compared to baseline (N=6). PEG-CAT has no effect on AChID in the vessels from VAT (n=8; *p<0.05 baseline vs PEG CAT in SAT vessels). The effects of PEG-CAT on flow-induced dilation (FID) in subcutaneous adipose tissue (SAT) resistance arteries are shown in panel C. In the presence of PEG-CAT, FID of SAT resistance arteries was significantly reduced at Δ40, Δ60 and Δ100 cmH2O compared to baseline. The effects of PEG-CAT on FID in VAT arterioles are shown in panel D. The H2O2 scavenger PEG-CAT has no effect on FID in the vessels from VAT. PAP: papavarine (10−4 M)
Endothelium- independent dilator responses
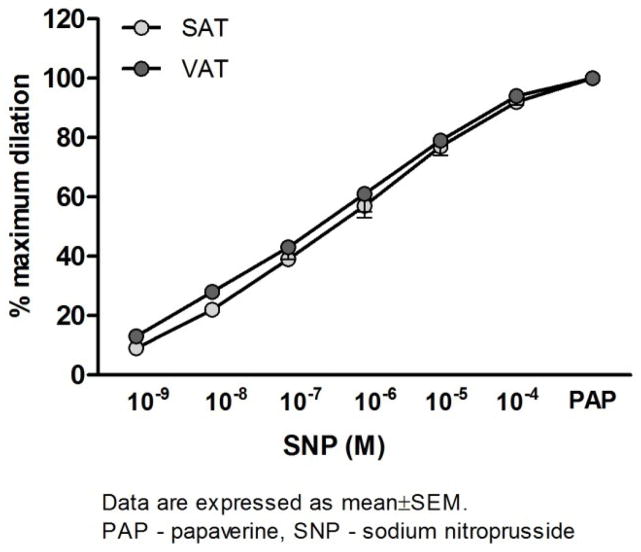
Figure 5 presents the dose-response curves to the endothelium-independent NO donor, sodium-nitroprusside (SNP). Vasodilation in response to SNP of both VAT and SAT microvessels were preserved and there were no differences in responsiveness to SNP between groups.
Figure 5.
Vasodilator responses to incremental doses of sodium nitroprusside (SNP) in arterioles from visceral (VAT) and subcutaneous (SAT) adipose tissue. There are no differences between the VAT and SAT dose-response curves to SNP. PAP: papavarine (10−4 M), (n=10).
Fluorescence detection of NO and H2O2 production
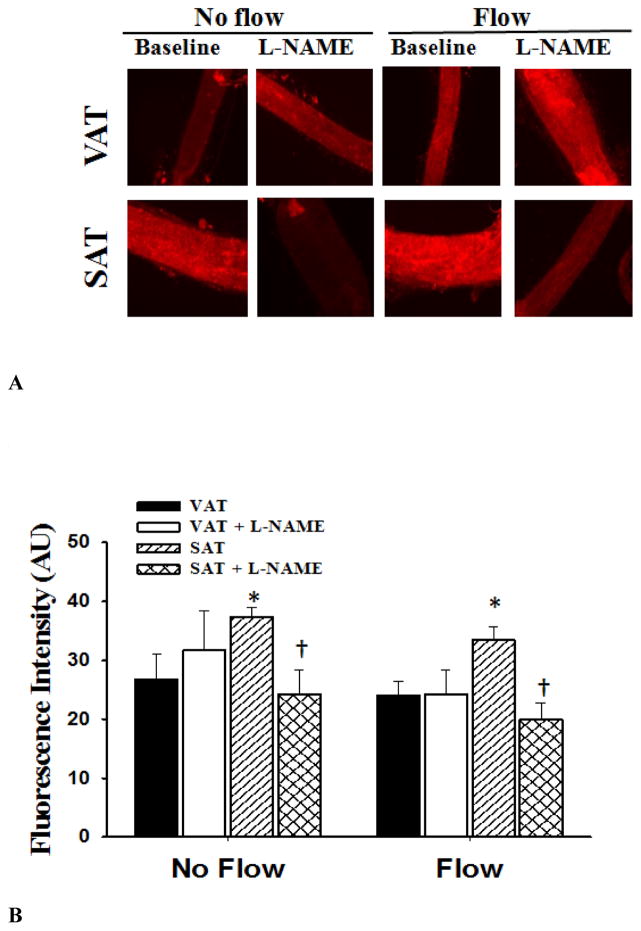
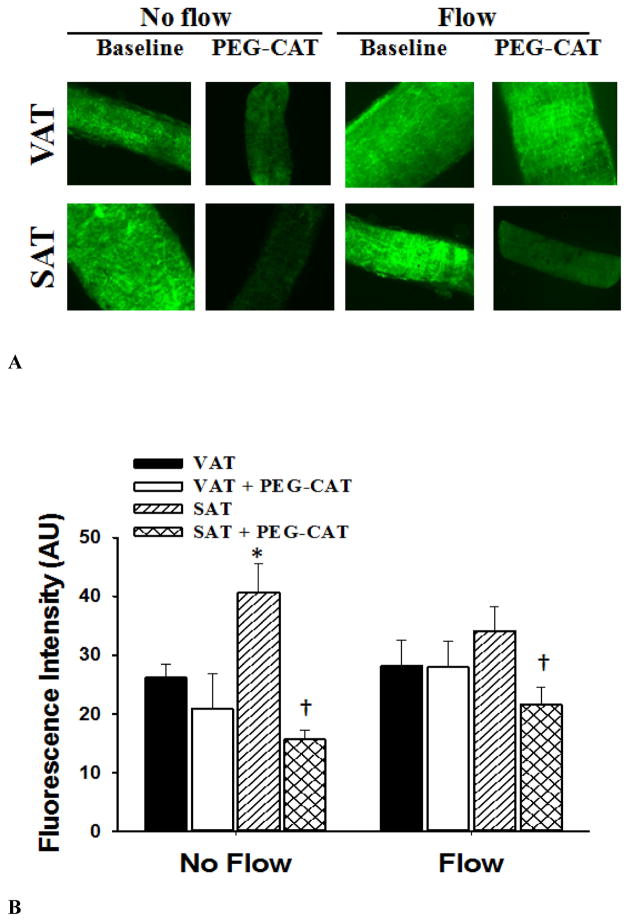
Determination of NO and H2O2 production in arterioles were made by fluorescence microscopy in conditions with and without flow. In the absence of flow, NO production was lower in arterioles from VAT compared to those from SAT (Figure 6). Incubation with L-NAME reduced NO production in microvessels from SAT (vs. no L-NAME) in no flow and in intraluminal flow conditions. There was no effect of L-NAME on NO production in VAT arterioles (Figure 6). There was no effect of flow on H2O2 fluorescence compared to no flow conditions. However, the presence of PEG-CAT reduced H2O2 fluorescence in arterioles from SAT(Figure 7).
Figure 6.
Nitric oxide (NO) production in visceral (VAT) and subcutaneous adipose tissue (SAT) arterioles. Fluorescence detection of NO was determined with and without L-NAME (10−4 M) under no flow and after 30 minutes of intraluminal flow (pressure gradient of 60 cmH2O). Panel A shows representative images of SAT and VAT vessels under no flow and flow conditions and in the presence and absence of L-NAME. NO production was increased in SAT compared to VAT resistance arteries. Panel B shows the summarized data describing the fluorescence intensity in VAT and SAT. L-NAME decreases NO production in SAT but not in VAT arterioles. n=6 in each group.*P<0.05 vs. visceral fat without flow. †P<0.05 vs. baseline without L-NAME.
Figure 7.
Hydrogen peroxide (H2O2) production in visceral (VAT) and subcutaneous adipose tissue (SAT) arterioles. Fluorescence detection of H2O2 assessed by dichlorodehydrofluorescein diacetate (DCF-DA; 2 μM) was determined in the presence and absence of PEG-CAT under no flow and after 30 minutes of intraluminal flow (pressure gradient of 60 cmH2O). Panel A shows representative images of SAT and VAT vessels under no flow and flow conditions and in the presence and absence of (PEG-CAT, 500 U/ml). During flow, the presence of PEG-CAT significantly reduced DCF-DA fluorescence in SAT arterioles, but had no effect on DCF-DA fluorescence in VAT arterioles. There was no effect of flow on H2O2 production compared to baseline. Panel B shows the summarized data describing the fluorescence intensity in vessels with and without flow. Data are presented as mean ± SEM. *P<0.05 vs. visceral fat without flow. †P<0.01 vs. baseline without PEG-CAT.
Discussion
The primary findings of this study are that 1) in morbidly obese women, microvessels from VAT demonstrated reduced dilator responses to flow and ACh compared to SAT; 2) L-NAME, INDO, and PEG-catalase reduced FID in microvessels from SAT but not in microvessels from VAT of the same patients; 3) baseline and flow- induced NO generation is lower in vessels from visceral compared to subcutaneous adipose tissue, and 4) during flow, the presence of PEG-CAT significantly reduced vascular H2O2 production in SAT arterioles. In addition, there was no effect of flow on H2O2 fluorescence compared to baseline (P=0.6).
Relationship between Visceral Fat and Vascular Dysfunction
Recent studies investigating the relationship between obesity and vascular function have shown that VAT is associated with impaired endothelial function and large artery stiffness [12, 20]. Romero-Corral et al. demonstrated that modest fat gain in healthy normal-weight individuals resulted in endothelial dysfunction and that increased VAT as opposed to SAT predicts such effect [14]. Consistent with our study, a previous study showed reduced endothelium-dependent, ACh- dependent vasodilation in VAT arterioles, compared to those from SAT[6]. Other studies have shown that the pro-inflammatory state and oxidative stress accompanying weight gain alters vascular function by disrupting the balance of vasoconstrictor and vasodilator agents [21]. Like previous studies, our current study showed reduced vasodilator sensitivity to ACh in VAT compared to SAT.
Numerous studies indicate that vascular dysfunction in the obese state is related to increased ROS and inflammation which play a vital role in the pathogenesis of atherosclerosis [22, 23]. Postprandial lipid accumulation and peroxidation occurs in individuals with high visceral fat content [12], an effect that might be related to differences in inflammatory cytokine release from different adipose depots [24]. On the other hand, perivascular adipose tissue (PVAT) may serve as an important modulator of endothelium-dependent vasodilator mechanisms [25]. Future studies are needed to determine if perivascular adipose tissue may account for differences in FID between VAT and SAT and the cardiovascular disease risk associated with excess VAT in morbid obesity.
Mechanisms Mediating Vasodilator Function in Adipose Tissue
Vasodilation to flow is an important physiological regulator of tissue perfusion. The mediator of FID is vessel- and species- dependent, but typically involves the release of NO, prostaglandins (PGI2) and/or endothelial derived hyperpolarizing factor (EDHF)[26–28]. However, this mechanism of FID has not been fully examined in adipose arterioles from obese individuals. In this study we show that microvessel dilator sensitivity to flow is reduced in VAT compared to SAT. Moreover SAT, not VAT, dilator responses to flow are NO-dependent in morbid obesity without the presence of multiple confounding CV risk factors. Whereas the obesity is associated with greater cardiovascular risk, and reduced NO-dependent vasodilation by elevating the production of reactive oxygen species (ROS) [18], other evidence indicates that in the presence of disease, other endothelium-derived dilator substances compensate for the lack of NO release during flow or agonist activation [16, 17]. For example, we previously reported that H2O2 contributes to FID in visceral adipose of patients with coronary disease [16]. Virdis and colleagues reported that the superoxide dismutase (SOD) mimetic Tempol increased AChID in VAT arterioles of obese individuals [18]. While we do not report the effects of superoxide dismutation here, our evidence indicates that the end product of SOD activity, H2O2 may serve as a vasodilator during physiologic and pathologic conditions [28, 29]. Consistent with these studies, we found that PEG-CAT reduces FID and H2O2 generation during flow in SAT arterioles (Figures 3C and 7) suggesting that H2O2 may contribute to dilator function in morbidly obese humans.
In the absence of pathology, ACh induces vasodilation through the release of endothelium-derived NO[30, 31]. In present study, the dilation of vessels to ACh from VAT is reduced compared to SAT, showing that physiologic (flow) and pharmacologic stimulation of NOS may be altered. The results of previous studies showed greater reductions in AChID from VAT than our results [6]. Given the similarity in design of the microvascular experiments from the latter study with the current study, it is possible that this difference may be due to differences in the severity of obesity and associated co-morbidities (our population was free of diabetes mellitus and dyslipidemia). We did not find that waist circumference (a common anthropometric measure of abdominal obesity) was related to the magnitude of FID in this study (data not shown). Further studies are needed to determine if the degree of obesity and/or the obesity associated co-morbidities impact arteriolar sensitivity to endothelium-dependent vasodilator stimuli in adipose depots.
It is also possible that vascular PGI2 production is altered in the obese state. Endothelial production of the superoxide anion contributes to enhanced COX expression [32]. The link between the NO and COX pathways is well established. It has been previously demonstrated that the enhanced release of PGI2 is NO-dependent [33]. The COX pathway may be up-regulated after sustained NOS inhibition [34, 35] suggesting thatPGI2 may compensate for the loss of NO. In addition, the inhibition of NOS also augments the contribution of CYP450 metabolites to vasodilation [36], providing further evidence that EDHF may function as a compensatory mechanism when NO synthesis is impaired in obesity. Indomethacin and catalase reduced FID in subcutaneous fat (Figure 2B and 4C), suggesting that COX enzymes are potential sources of superoxide which may be further metabolized to H2O2 in this tissue [37]. Taken together, these results suggest that relaxing factors (in addition to NO) may contribute to FID of subcutaneous adipose tissue in morbid obesity. In contrast to SAT arterioles, VAT arterioles that exhibit impaired vasodilation in response to increment flow and ACh were not reduced in the presence of either L-NAME, PEG-catalase, or INDO alone. However, in combination, INDO and L-NAME significantly reduced FID in VAT arteriole (Figure 2B). These data may suggest that even in the reduced vasodilator state of VAT, that cyclooxygenase metabolites or NO may serve to compensate when either of the other vasodilator pathways is blocked
It is possible that an endothelium-derived contracting factor (EDCF, e.g. 20-HETE or isoprostanes) could reduce FID in VAT. For example, plasma and urinary 20-HETE and F (2)-isoprostanes were significantly elevated in the obese patients with metabolic syndrome [38]. While the exact mechanism of this depot- specific difference in vascular NO is not known. It is possible that inflammatory cytokines responsible for generating ROS are increased in VAT compared to SAT [6]. However, the data presented in Figure 7 indicated that baseline ROS (measured as H2O2) was higher in SAT than VAT. The explanation for this difference in ROS and NO generation between tissues is not entirely clear. The conversion of superoxide to H2O2 by SOD isoforms could be tissue specific and expression of SOD may be altered in obesity [39]. Previous studies have found that H2O2 may be increased during disease in the absence of NO [27]. However, other data suggest that H2O2 is a stimulus for exercise induced eNOS expression [40] and that H2O2 increases NO generation in endothelial cells [41]. Taken together with our data, it is possible that H2O2 serves to maintain NO generation and endothelium-dependent vasodilation in subcutaneous microcirculation but that this mechanism is absent in visceral microcirculation during human obesity.
Structural changes in small subcutaneous arteries have prognostic significance in hypertension and other cardiovascular disease [42]. In the current study maximum dilations to papaverine tended to be reduced in VAT compared to SAT arterioles (Figure 1). This may suggest a divergence in mechanical properties between visceral and subcutaneous that might explain a reduced dilation in SAT arterioles. Others have reported increased arterial stiffness in resistance arteries of obese adults [43]. While the present study was not designed to determine the effects of obesity on vascular stiffness and compliance that might impact vasodilator function; there was no difference in the dilation to the NO donor sodium nitroprusside (Figure 5). These data suggest that the differences in dilator responses to ACh and flow in SAT arterioles were not related to differences in sensitivity of the blood vessel to nitric oxide. Future studies may address any potential difference in the mechanical properties between SAT and VAT arterioles.
This study has several limitations. First, our study was limited to young, morbidly obese (BMI >35 kg/m2) female population limiting the generalizability of the findings. Future studies will need to address the possible potential differences in vascular reactivity between men and women. Second, tissue was obtained from SAT and VAT biopsies during planned bariatric surgery. Therefore, it was not possible to collect longitudinal physiologic data after weight loss with this design to determine the effects of significant weight loss on visceral fat vasodilator reactivity. These findings cannot be extrapolated to individuals who are mildly or moderately overweight. Third, due to technical limitations, fluorescence measurements were not made longitudinally before and then during flow conditions making it difficult to discern the time course of H2O2 and NO generation. Finally, our study design did not control for menstrual cycle phase which could confound endothelial function in this population. However, we excluded patients with confounders that could alter the mechanisms or magnitude of vasodilation independent of obesity (i.e. cardiovascular disease and diabetes). Future studies to determine the effects of more moderate levels of obesity and weight loss interventions on microvascular FID appear warranted.
Conclusions
This study found that microvessels from VAT demonstrate reduced vasodilator reactivity to flow and ACh compared to SAT and that baseline and flow induced NO generation is lower in VAT compared to SAT vessels during morbid obesity. The FID in SAT microvessels can be reduced by L-NAME, INDO, or PEG-catalase an effect that was not observed in VAT vessels from the same patients. Other studies have found that endothelium-dependent (acetylcholine) and independent (SNP) vasodilation in the forearm resistance arteries, but not flow mediated dilations in the brachial artery, were reduced in elderly subjects with visceral adiposity. This result suggests that alterations in microvascular function may occur prior to or in the absence of large artery vascular dysfunction [44] which may help to explain the divergent responses between vascular beds (visceral vs. subcutaneous). Taken together, the reduced FID in the visceral fat that does not extend to subcutaneous microvessels in young morbidly obese patients may be an early step in the progression to more overt and systemic vascular dysfunction.
Perspective
Flow induced NO or other vasodilator metabolites in adipose tissue may represent a mechanistic link between obesity, adipose tissue accumulation, and metabolism. Decreased perfusion to the VAT may evoke inflammation and other deleterious paracrine factors associated with excess VAT accumulation [45]. Therefore, excess VAT coupled with impaired dilation to flow in the microcirculation may serve as an important initial step in development of cardiovascular disease.
Acknowledgments
Dr. Phillips is supported by National Institutes of Health (NIH) grants K23HL85614, R01HL095701, and R01HL095701-01A2S. Drs. Cavka and Grizelj were supported through the Republic of Croatia (Ministry of Science, Education and Sports) UKF connectivity program.
Reference List
- 1.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA. 2012;307:491–497. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 2.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity among adults: United States, 2011–2012. NCHS Data Brief. 2013:1–8. [PubMed] [Google Scholar]
- 3.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, et al. Heart disease and stroke statistics--2012 update: a report from the American Heart Association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Selthofer-Relatic K, Divkovic D, Radic R, Vizjak V, Selthofer R, Steiner R, Bosnjak I. Overweight - early stage of “adipokines related cardiovascular diseases”: leptin and adiponectin relation to anthropometric parametars. Med Glas (Zenica) 2012;9:198–203. [PubMed] [Google Scholar]
- 5.Bray GA. Medical consequences of obesity. J Clin Endocrinol Metab. 2004;89:2583–2589. doi: 10.1210/jc.2004-0535. [DOI] [PubMed] [Google Scholar]
- 6.Farb MG, Ganley-Leal L, Mott M, Liang Y, Ercan B, Widlansky ME, Bigornia SJ, Fiscale AJ, Apovian CM, Carmine B, et al. Arteriolar function in visceral adipose tissue is impaired in human obesity. Arterioscler Thromb Vasc Biol. 2012;32:467–473. doi: 10.1161/ATVBAHA.111.235846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fantuzzi G, Mazzone T. Adipose tissue and atherosclerosis: exploring the connection. Arterioscler Thromb Vasc Biol. 2007;27:996–1003. doi: 10.1161/ATVBAHA.106.131755. [DOI] [PubMed] [Google Scholar]
- 8.Heitzer T, Schlinzig T, Krohn K, Meinertz T, Munzel T. Endothelial dysfunction, oxidative stress, and risk of cardiovascular events in patients with coronary artery disease. Circulation. 2001;104:2673–2678. doi: 10.1161/hc4601.099485. [DOI] [PubMed] [Google Scholar]
- 9.Jonk AM, Houben AJ, de Jongh RT, Serne EH, Schaper NC, Stehouwer CD. Microvascular dysfunction in obesity: a potential mechanism in the pathogenesis of obesity-associated insulin resistance and hypertension. Physiology (Bethesda) 2007;22:252–260. doi: 10.1152/physiol.00012.2007. [DOI] [PubMed] [Google Scholar]
- 10.Gokce N, Keaney JF, Jr, Hunter LM, Watkins MT, Menzoian JO, Vita JA. Risk stratification for postoperative cardiovascular events via noninvasive assessment of endothelial function: a prospective study. Circulation. 2002;105:1567–1572. doi: 10.1161/01.cir.0000012543.55874.47. [DOI] [PubMed] [Google Scholar]
- 11.Jambrik Z, Venneri L, Varga A, Rigo F, Borges A, Picano E. Peripheral vascular endothelial function testing for the diagnosis of coronary artery disease. Am Heart J. 2004;148:684–689. doi: 10.1016/j.ahj.2004.04.016. [DOI] [PubMed] [Google Scholar]
- 12.Jang Y, Kim OY, Ryu HJ, Kim JY, Song SH, Ordovas JM, Lee JH. Visceral fat accumulation determines postprandial lipemic response, lipid peroxidation, DNA damage, and endothelial dysfunction in nonobese Korean men. J Lipid Res. 2003;44:2356–2364. doi: 10.1194/jlr.M300233-JLR200. [DOI] [PubMed] [Google Scholar]
- 13.de Vigili KS, Kiwanuka E, Tiengo A, Avogaro A. Visceral obesity is characterized by impaired nitric oxide-independent vasodilation. Eur Heart J. 2003;24:1210–1215. doi: 10.1016/s0195-668x(03)00206-9. [DOI] [PubMed] [Google Scholar]
- 14.Romero-Corral A, Sert-Kuniyoshi FH, Sierra-Johnson J, Orban M, Gami A, Davison D, Singh P, Pusalavidyasagar S, Huyber C, Votruba S, et al. Modest visceral fat gain causes endothelial dysfunction in healthy humans. J Am Coll Cardiol. 2010;56:662–666. doi: 10.1016/j.jacc.2010.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bray GA, Bellanger T. Epidemiology, trends, and morbidities of obesity and the metabolic syndrome. Endocrine. 2006;29:109–117. doi: 10.1385/ENDO:29:1:109. [DOI] [PubMed] [Google Scholar]
- 16.Phillips SA, Hatoum OA, Gutterman DD. The mechanism of flow-induced dilation in human adipose arterioles involves hydrogen peroxide during CAD. Am J Physiol Heart Circ Physiol. 2007;292:H93–100. doi: 10.1152/ajpheart.00819.2006. [DOI] [PubMed] [Google Scholar]
- 17.Miura H, Bosnjak JJ, Ning G, Saito T, Miura M, Gutterman DD. Role for hydrogen peroxide in flow-induced dilation of human coronary arterioles. Cir cRes. 2003;92:e31–e40. doi: 10.1161/01.res.0000054200.44505.ab. [DOI] [PubMed] [Google Scholar]
- 18.Virdis A, Santini F, Colucci R, Duranti E, Salvetti G, Rugani I, Segnani C, Anselmino M, Bernardini N, Blandizzi C, et al. Vascular generation of tumor necrosis factor-alpha reduces nitric oxide availability in small arteries from visceral fat of obese patients. J Am Coll Cardiol. 2011;58:238–247. doi: 10.1016/j.jacc.2011.01.050. [DOI] [PubMed] [Google Scholar]
- 19.Meyers MR, Gokce N. Endothelial dysfunction in obesity: etiological role in atherosclerosis. Curr Opin Endocrinol Diabetes Obes. 2007;14:365–369. doi: 10.1097/MED.0b013e3282be90a8. [DOI] [PubMed] [Google Scholar]
- 20.Orr JS, Gentile CL, Davy BM, Davy KP. Large artery stiffening with weight gain in humans: role of visceral fat accumulation. Hypertension. 2008;51:1519–1524. doi: 10.1161/HYPERTENSIONAHA.108.112946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seals DR, Gates PE. Stiffening our resolve against adult weight gain. Hypertension. 2005;45:175–177. doi: 10.1161/01.HYP.0000152199.18202.a9. [DOI] [PubMed] [Google Scholar]
- 22.Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, Nakayama O, Makishima M, Matsuda M, Shimomura I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004;114:1752–1761. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kelly AS, Steinberger J, Kaiser DR, Olson TP, Bank AJ, Dengel DR. Oxidative stress and adverse adipokine profile characterize the metabolic syndrome in children. J Cardiometab Syndr. 2006;1:248–252. doi: 10.1111/j.1559-4564.2006.05758.x. [DOI] [PubMed] [Google Scholar]
- 24.Phillips SA, Ciaraldi TP, Oh DK, Savu MK, Henry RR. Adiponectin secretion and response to pioglitazone is depot dependent in cultured human adipose tissue. Am J Physiol Endocrinol Metab. 2008;295:E842–E850. doi: 10.1152/ajpendo.90359.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Greenstein AS, Khavandi K, Withers SB, Sonoyama K, Clancy O, Jeziorska M, Laing I, Yates AP, Pemberton PW, Malik RA, Heagerty AM. Local inflammation and hypoxia abolish the protective anticontractile properties of perivascular fat in obese patients. Circulation. 2009;119:1661–1670. doi: 10.1161/CIRCULATIONAHA.108.821181. [DOI] [PubMed] [Google Scholar]
- 26.Larsen BT, Campbell WB, Gutterman DD. Beyond vasodilatation: non-vasomotor roles of epoxyeicosatrienoic acids in the cardiovascular system. Trends Pharmacol Sci. 2007;28:32–38. doi: 10.1016/j.tips.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 27.Liu Y, Bubolz AH, Mendoza S, Zhang DX, Gutterman DD. H2O2 is the transferrable factor mediating flow-induced dilation in human coronary arterioles. Circ Res. 2011;108:566–573. doi: 10.1161/CIRCRESAHA.110.237636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matoba T, Shimokawa H, Kubota H, Morikawa K, Fujiki T, Kunihiro I, Mukai Y, Hirakawa Y, Takeshita A. Hydrogen peroxide is an endothelium-derived hyperpolarizing factor in human mesenteric arteries. Biochem Biophys Res Commun. 2002;290:909–913. doi: 10.1006/bbrc.2001.6278. [DOI] [PubMed] [Google Scholar]
- 29.Matoba T, Shimokawa H, Nakashima M, Hirakawa Y, Mukai Y, Hirano K, Kanaide H, Takeshita A. Hydrogen peroxide is an endothelium-derived hyperpolarizing factor in mice. J Clin Invest. 2000;106:1521–1530. doi: 10.1172/JCI10506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dharmashankar K, Welsh A, Wang J, Kizhakekuttu TJ, Ying R, Gutterman DD, Widlansky ME. Nitric oxide synthase-dependent vasodilation of human subcutaneous arterioles correlates with noninvasive measurements of endothelial function. Am J Hypertens. 2012;25:528–534. doi: 10.1038/ajh.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Erdei N, Toth A, Pasztor ET, Papp Z, Edes I, Koller A, Bagi Z. High-fat diet-induced reduction in nitric oxide-dependent arteriolar dilation in rats: role of xanthine oxidase-derived superoxide anion. Am J Physiol Heart Circ Physiol. 2006;291:H2107–H2115. doi: 10.1152/ajpheart.00389.2006. [DOI] [PubMed] [Google Scholar]
- 32.Cosentino F, Sill JC, Katusic ZS. Role of superoxide anions in the mediation of endothelium-dependent contractions. Hypertension. 1994;23:229–235. doi: 10.1161/01.hyp.23.2.229. [DOI] [PubMed] [Google Scholar]
- 33.Salvemini D. Regulation of cyclooxygenase enzymes by nitric oxide. Cell Mol Life Sci. 1997;53:576–582. doi: 10.1007/s000180050074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sun D, Huang A, Smith CJ, Stackpole CJ, Connetta JA, Shesely EG, Koller A, Kaley G. Enhanced release of prostaglandins contributes to flow-induced arteriolar dilation in eNOS knockout mice. Circ Res. 1999;85:288–293. doi: 10.1161/01.res.85.3.288. [DOI] [PubMed] [Google Scholar]
- 35.Wu Y, Huang A, Sun D, Falck JR, Koller A, Kaley G. Gender-specific compensation for the lack of NO in the mediation of flow-induced arteriolar dilation. Am J Physiol Heart Circ Physiol. 2001;280:H2456–H2461. doi: 10.1152/ajpheart.2001.280.6.H2456. [DOI] [PubMed] [Google Scholar]
- 36.Fichtlscherer S, Dimmeler S, Breuer S, Busse R, Zeiher AM, Fleming I. Inhibition of cytochrome P450 2C9 improves endothelium-dependent, nitric oxide-mediated vasodilatation in patients with coronary artery disease. Circulation. 2004;109:178–183. doi: 10.1161/01.CIR.0000105763.51286.7F. [DOI] [PubMed] [Google Scholar]
- 37.Fleming I, Michaelis UR, Bredenkotter D, Fisslthaler B, Dehghani F, Brandes RP, Busse R. Endothelium-Derived Hyperpolarizing Factor Synthase (Cytochrome P450 2C9) Is a Functionally Significant Source of Reactive Oxygen Species in Coronary Arteries. Circ Res. 2001;88:44–51. doi: 10.1161/01.res.88.1.44. [DOI] [PubMed] [Google Scholar]
- 38.Tsai IJ, Croft KD, Puddey IB, Beilin LJ, Barden A. 20-Hydroxyeicosatetraenoic acid synthesis is increased in human neutrophils and platelets by angiotensin II and endothelin-1. Am J Physiol Heart Circ Physiol. 2011;300:H1194–1200. doi: 10.1152/ajpheart.00733.2010. [DOI] [PubMed] [Google Scholar]
- 39.Roberts CK, Barnard RJ, Sindhu RK, Jurczak M, Ehdaie A, Vaziri ND. Oxidative stress and dysregulation of NAD(P)H oxidase and antioxidant enzymes in diet-induced metabolic syndrome. Metabolism. 2006;55:928–934. doi: 10.1016/j.metabol.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 40.Lauer N, Suvorava T, Ruther U, Jacob R, Meyer W, Harrison DG, Kojda G. Critical involvement of hydrogen peroxide in exercise-induced up-regulation of endothelial NO synthase. Cardiovasc Res. 2005;65:254–262. doi: 10.1016/j.cardiores.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 41.Zhou X, Yuan D, Wang M, He P. H2O2-induced endothelial NO production contributes to vascular cell apoptosis and increased permeability in rat venules. Am J Physiol Heart Circ Physiol. 2013;304:H82–93. doi: 10.1152/ajpheart.00300.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rizzoni D, Porteri E, Boari GE, de CC, Sleiman I, Muiesan ML, Castellano M, Miclini M, Agabiti-Rosei E. Prognostic significance of small-artery structure in hypertension. Circulation. 2003;108:2230–2235. doi: 10.1161/01.CIR.0000095031.51492.C5. [DOI] [PubMed] [Google Scholar]
- 43.Grassi G, Seravalle G, Scopelliti F, Dell’Oro R, Fattori L, Quarti-Trevano F, Brambilla G, Schiffrin EL, Mancia G. Structural and functional alterations of subcutaneous small resistance arteries in severe human obesity. Obesity (Silver Spring) 2010;18:92–98. doi: 10.1038/oby.2009.195. [DOI] [PubMed] [Google Scholar]
- 44.Lind L, Johansson L, Hulthe J, von Below C, Ahlstrom H. Vasodilation and visceral fat in elderly subjects: the Prospective Investigation of the Vasculature in Uppsala Seniors (PIVUS) study. Atherosclerosis. 2007;194:e64–71. doi: 10.1016/j.atherosclerosis.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 45.Goossens GH, Bizzarri A, Venteclef N, Essers Y, Cleutjens JP, Konings E, Jocken JW, Cajlakovic M, Ribitsch V, Clement K, Blaak EE. Increased adipose tissue oxygen tension in obese compared with lean men is accompanied by insulin resistance, impaired adipose tissue capillarization, and inflammation. Circulation. 2011;124:67–76. doi: 10.1161/CIRCULATIONAHA.111.027813. [DOI] [PubMed] [Google Scholar]