Abstract
Indocyanine green (ICG) kinetics (PDR/R15) used to quantitatively assess hepatic function in the perioperative period of major resective surgery and liver transplantation have been the object of an extensive, updated and critical review. New, non invasive bedside monitors (pulse dye densitometry technology) make this opportunity widely available in clinical practice. After having reviewed basic concepts of hepatic clearance, we analysed the most common indications ICG kinetic parameters have nowadays in clinical practice, focusing in particular on the diagnostic and prognostic role of PDR and R15 in the perioperative period of major liver surgery and liver transplantation. As recently pointed out, even if of extreme interest, ICG clearance parameters have still some limitations, to be considered when using these tests.
Keywords: Liver function tests, Indocyanine green, Hepatic clearance, Liver surgery, Liver transplantation, Intraabdominal hypertension, Portal hypertension
Core tip: Non invasive monitors for indocyanine green (ICG) clearance (PDR and R15) are now available for a rapid assessment of liver function both in the intensive care unit and in major liver surgery. After having reviewed the basic concepts of hepatic clearance, we have analysed the most common indications of ICG kinetic parameters in clinical practice, focusing on the diagnostic and prognostic role of PDR and R15 in the perioperative period of major resective liver surgery and liver transplantation. Since ICG parameters have still some limitations, we will underline the conditions (mainly hyperbilirubinemia and severe peripheral hypoperfusion) able to alter the reliability of these tests.
INTRODUCTION
ln modern critical care medicine, extensive and accurate liver function assessment has a relevant place while caring for high risk medical patients or candidates to major liver surgery: At the moment, static and dynamic tests are available[1-7]. Static tests, since long included in scores able to quantify acute and chronic (CHILD PUGH, MELD) hepatic dysfunction, assess separately the different functions of the liver and describe the size of the hepatic injury[1-4]. On the contrary, information on the functional aspects of the remnant liver after resective surgery or of the quality of the liver graft recovery after transplantation remain elusive. In other words, available to the clinicians is a “frozen” representation of the hepatocytes integrity and of the (residual) metabolic and synthetic capacities (Figure 1).
Figure 1.
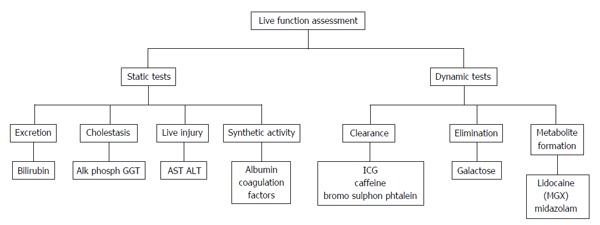
Liver function assessment: Static and dynamic tests (modified from Sakka[3], 2007). AST: Aspartate aminotransferase; ALT: Alanine aminotransferase; ICG: Indocyanine green; GGT: Gamma glutamyl transferase.
STATIC ASSESSMENT OF LIVER FUNCTION
A pivotal role in the amino acids metabolism is played by aspartate aminotransferase (AST) and alanine aminotransferase (ALT). AST, represented at various levels (mainly muscular and cardiac, but not only) are not liver specific and have shorter half life (12-22 h). On the contrary, ALT are liver - specific, have longer half life (30-40 h), are highly expressed in the hepatocytes and largely present in periportal areas. In case of centrilobular hypoxia, ALT show a moderate increase, while in case of acute hepatic injury (acute hepatitis) a significant increase in ALT serum concentration is almost always demonstrated: It is considered a consequence of necrosis or it should be secondary to the increased permeability after a cell membrane damage[2,3]. In case of ischemic injury, the AST and ALT peak may reflect the size of liver damage. As above mentioned, AST/ALT increase (longer for ALT, shorter for AST due to the different half life) does not provide information on the functional impairment of the liver nor, by force, of the (residual) hepatic functional reserve[2,3]. A rather non-specific marker of ischemic damage to the liver (but not only!) is lactate dehydrogenase (mainly fraction 5). Cholestatic alterations are usually described using gamma glutamyl transferase and alkaline phosphatase.
Plasma Bilirubin concentration reflects phase II metabolism and is the indirect expression of uptake, conjugation and excretion functions of the liver. Early (and perhaps self limiting) phases of ischemic injuries have a moderate impact on the phase II processes, defined as “relatively robust”[7]. Among the causes of hyperbilirubinemia (generally speaking due to an increased production or a reduced clearance) relevant are hemolysis, damage of cellular components and reduced intrahepatic bile excretion. One of the main functions of the hepatocytes is protein synthesis. Among synthesized proteins are large part of acute phase proteins, albumin, transport proteins, all the coagulation factors [apart from factor VIII (FVIII) and von Willebrand factor], antithrombin, anticoagulant proteins (protein C, protein S and protein Z), Plasminogen, alpha 2 plasmin inhibitor, complement, lipoproteins[2]. Among coagulation factors, FV and FVII, due to a very short half-life (four to six hours), are included in the Clichy criteria to quantify the synthetic damage of the liver in case of acute liver failure. According to Clichy criteria, in case of acute hepatic failure (so called “fulminant hepatitis”), hepatic encephalopathy grade 3-4 and FV activity below 20% in patients < 30 years (< 30% in patients > 30 years) are the indications for urgent liver transplantation (OLT)[4].
DYNAMIC ASSESSMENT OF LIVER FUNCTION
Since long, dynamic liver function tests[2,3] are considered and used to assess “over time” the liver capacity to metabolize or to eliminate drugs or compounds. Dynamic quantitative liver function tests, unlike conventional (static) tests, rely upon a “quasi” exclusive clearance or metabolization of substances performed by the liver. Being repetible in a short time span, dynamic tests are able to provide a fast and reliable liver functional evaluation, together with a general prognostic assessment (Figure 1). Indocyanine green (ICG) clearance parameters will be described and discussed in this paper, while Caffeine test, Bromsulphalein clearance, Aminoacid clearance, Galactose elimination capacity, Aminopyridine breath test and Monoethylglycinexylidide formation from lignocaine (MEGX test) are beyond the scope of this review (Figure 1).
The hepatic clearance: Matching hepatic perfusion and liver function
According to the clearance principle[5], hepatic clearance (Cl) is the product of liver extraction capacity (Ex) and liver blood flow (Q): Cl = Q × Ex. In general, the dynamic assessment of liver function relies upon this equation: According to the hepatic extraction capacity, the various drugs and compounds are considered at “low” or “high” extraction. Clearance of highly extracted substances approaches hepatic blood flow and is considered an indicator of liver blood flow, extraction rate being limited in case of reduced liver blood flow. Opposite is the case of the clearance of substances at low extraction rate: The clearance of these compounds, not dependent from the hepatic blood flow, becomes a measure of metabolism or elimination processes. A key point of this principle is that the intrinsic hepatic clearance (Clint) becomes a measure of the capacity of the liver to remove substances when blood flow is not limited[5].
ICG CLEARANCE FOR A DYNAMIC ASSESSMENT OF LIVER FUNCTION
Worldwide, ICG clearance is the most common and easy - to - use test for the perioperative dynamic assessment of liver function in case of major liver surgery (resective surgery and liver transplantation) and in the intensive care unit (ICU)[2,6-8]. ICG is an inert, water-soluble, fluorescent tricarbocyanine, with a protein binding close to 95% (mainly, alpha1- and beta-lipoproteins and albumin). In healthy individuals, ICG shows a high hepatic extraction rate, usually above 70%. Toxicity is very low, and very rare are the adverse effects, reported in 1/40000 cases. The presence of Iodine in the ICG molecule constitutes a contraindication to its use in case of thyrotoxicosis and iodine allergy (a reaction due to non-immunological histamine release)[6-8]. Since the early sixties, ICG elimination kinetics were used to measure blood volume and cardiac output, while in recent years an increased interest exists in using ICG clearance parameters for a dynamic assessment of liver function both in medical and surgical settings[6,9-11]. The “standard” determination of ICG clearance (ICGCl) relies upon a rather complex ex vivo photometric analysis of multiple arterial blood samples obtained in a short time frame (15 min) after the intravenous administration: In spite of being so far the gold standard, it is now used for research purposes only. New bedside, easy to use transcutaneous - non-invasive pulse dye densitometry (PDD) devices able to measure ICG concentrations are on the rise for the use in clinical practice[1,6,7]. Among them are LiMon, (Pulsion Medical System, Germany) and DDG 2001 (Nihon Kohden, Japan): ICG elimination is expressed as ICG plasma disappearance rate (ICGPDR) or retention rate at fifteen minutes (ICGR15), assessing relative ICG concentration changes (Figure 2).
Figure 2.
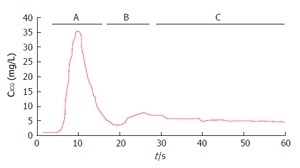
Indocyanine green dilution curve. A: First peak; B: Second peak (re-circulation phase); C: Elimination phase (Modified from Vos et al[6], 2014). ICG: Indocyanine green; CICG: ICG blood concentration.
In hemodynamically stable or unstable ICU patients, in liver transplanted patients and in subjects involved in major liver surgery, good correlation exists between ICG elimination measurements performed with the standard “invasive” method and the PDD technology. In healthy subjects, the intravenous injection of ICG at the dosage of 0.5 mg/kg body weight (BW) generates a plasmatic concentration of 100 mg/mL: In recent experiences, reliable results are also reported with 0.25 mg/kg BW[3]. The K value (rate constant) of the ICG indicator-dilution curve is calculated by both devices applying monoexponential transformation of the ICG concentration and backward dynamic extrapolation of the curve of the elimination phase[6]. With appropriate calculations, functional parameters of extreme interest for the dynamic assessment of liver function are thus available.
After intravenous injection, ICG, almost completely bound to proteins, is distributed in the blood within 2 to 3 min: Volume of distribution is very close to plasma volume and half-life is very short (3 to 5 min[1,3,6], longer in case of hepatic dysfunction). Extraction from the blood occurs almost exclusively by the liver, with selective uptake across the sinusoidal plasma membrane by 1 B3 and Na-taurocholate co-transporting polypeptides. ICG is excreted unchanged and almost completely (97%) into the bile in a non-conjugated form, following a two-compartmental model (excretion from the peripheral and not from the central compartment). The absence of metabolism and of enterohepatic recirculation supports the correlation between ICG elimination kinetics and liver function. Sinusoidal uptake (relevant in humans) and canalicular excretion are the two main processes involved in ICG hepatic clearance. The ATP-dependent-export pump multidrug resistance associated protein 2 (MDRP2) and the multi-drug resistance (MDR3) P-glycoprotein are the specific carriers involved in this process, expression of the liver energy status and of the excretory function[1,3,6,7].
Two peaks and one slope (the latter representing the elimination phase, usually lasting 10-20 min) are easy recognizable in the dye disappearance curve[5]. Of the two peaks, the first is used for the cardiac output determination, while the second is associated with the recirculation phase (elimination peak). Smaller peaks may follow the first two and are used for the estimation of circulating blood volume[6]. According to Imamura et al[5], in the ICG plasma disappearance curve (Figure 3) the initial sharp fall in concentration, (distribution phase, due to the rapid hepatic uptake of ICG from the plasma) is followed by a less steep fall (elimination phase, due to the passage from the liver into the bile). Twenty to 30 min are usually needed for the transition from the distribution to the elimination phase: K value (/min) is derived from the first fifteen minutes component of the disappearance curve.
Figure 3.
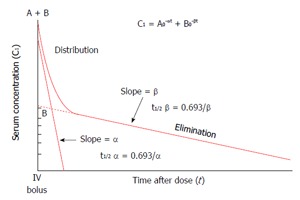
Schematic representation of indocyanine green kinetics (modified from Imamura et al[5], 2005).
In case of liver dysfunction/disease, a consistent prolongation of IGC half - life is usually recorded, as ICG hepatic clearance depends from both carriers capacity and liver blood flow. In individuals suffering for acute liver injury or steatohepatitis, release of cytokines (mainly tumor necrosis factor alpha and interleukine 6) by the reticuloendothelial cells (mainly Kuppfer cells) is able to downregulate the expression of organic anion transporting polypeptide isoforms and sodium-taurocholate co-transporting polypeptide, reducing the hepatic uptake capacity. In contrast, ICG transport capacity is competitively inhibited in case of hyperbilirubinemia[6-8,10-13], due to the same carrier system (ATP - export pump - MDRP2) shared by ICG and bilirubin: In case of hyperbilirubinemia (serum bilirubin > 3 mg/mL), “falsely” reduced ICG clearance values may be recorded due to the carrier competition (vide infra)[6,12,13]. This could be the case of OLT candidates with preoperative hyperbilirubinemia, in which functional recovery of the newly grafted liver is assessed early after transplant: In this specific context, “falsely” poor results may be found, making the ICG test useless and possibly misleading (vide infra)[6,7]. Less common, but indeed not infrequent in the critically ill, is the case of high flow states: False reassuring findings (better than expected) due to “normal or near normal” results might be recorded, masking an altered liver excretory function[7]. In the cirrhotic population, measurements of liver blood flow using ICGCl are not to be considered completely reliable[14]: The hepatic extraction rate in this context is extremely reduced (close to 20%-30%) and ICGCl becomes a measure of the uptake clearance (Cint, as demonstrated by Imamura et al[5])[14]. Interestingly enough, bile elimination constant was not altered, as reported by Kawasaki et al[15]. Using the galactose clearance test to measure liver blood flow, the same AAs were able to demonstrate that in liver cirrhosis a reduced ICGCl, (reported as ICGR15) was dependent from a reduction of both hepatic extraction and hepatic blood flow. Sinusoidal capillarization and intrahepatic shunts, largely represented in cirrhotic patients, are proposed as a possible explanation[6,15,16]. In normal conditions, the diffusion of drugs and substances (including proteins) is free between the sinusoids and the hepatocytes: In presence of sinusoid capillarization due to a barrier-limiting factor, it is impaired. ICG, which is highly protein - bound, is particularly prone to this phenomenon. Then, in cirrhotic patients ICGK and ICGR15 (vide infra) might reflect not only the degree of sinusoidal capillarization and intrahepatic shunts but, at least in part, also the reduction of hepatic blood flow[15]. The logarithmic transformation of the distribution phase of the dye dilution curve is the key passage for the quantitative assessment of ICG removal by the liver cells.
ICG clearance parameters most commonly reported in the literature are[6,7]: (1) Plasma disappearance rate - ICGPDR; (2) Retention rate at 15 min - ICGR15; (3) Disappearance rate constant (or elimination rate constant) (K constant) - ICGK; and (4) ICGCl - ICG clearance.
ICGPDR and ICGR15 are the two kinetic parameters most frequently used in clinical practice for the dynamic assessment of liver function[6-8,17] (Table 1, from Vos et al[6], 2014).
Table 1.
Quantitative indocyanine green kinetics variables (modified from Vos et al[6], 2014)
| Variable | Denomination | Unit | Formula for calculation | Normal value |
| ICGPDR | ICG plasma disappearance rate | % per minute | Backward extrapolation of k, curve fitted as: CICG (t) = C0 × e-k × t | > 18%-24% per minute |
| ICGR15 | ICG retention ratio after 15 min | % | (CICG(15)/CICG(0)) × 100 | < 10% |
| ICGt/2 | ICG half life | min | (In2 × VD) CIICG | 3-5 |
| ClICG | ICG clearance | mL/min per kilogram | K × VD | 6-12 |
e: Euler’s number (approximately 2.718); k: Fractional ICG concentration change per minute; VD: ICG volume of distribution; t: Time; CICG (t): ICG concentration at time point t (min); ClICG: ICG clearance (mL/min per kilogram); ICG: Indocyanine green.
ICGPDR - PDR: Percentage change over time of the reduction of ICG blood concentration starting from a concentration of 100% (> 18% per minute). PDR is automatically calculated according to the time course of the ICG blood concentration using monoexponential transformation of the original ICG concentration curve and backward extrapolation to time point zero. In the critically ill, PDR is an accepted surrogate for clearance, due to the good correlation with ICGCl (r2 = 0.77)[2].
PDR (% per minute) = ln 2/t1/2 × 100 or CICG (t) = C0 × e-k × t
ICGR15 - R15: The ratio between ICG concentration 15 min after injection and initial concentration (normal 0%-10%).
R15 (%) = CICG15/CICGT0 × 100
An initial ICG plasma concentration of 100 mg/mL is usually achieved after the intravenous administration of 0.5 mg/kg BW (considering an average plasma volume of 50 mL/kg). ICGR15 is calculated transforming the ICG concentration curve to a “point zero” (100%) and then describing the decay (at minute fifteen) as a percentage change per time (% per minute) in a logarithmic graph. ICGR15 has been widely used as an alternative to ICGK, being pharmacologically equivalent[5]: It could be considered a surrogate of liver blood flow.
ICG plasma clearance (500-700 mL/min per square): Volume of plasma entirely cleared off of ICG per unit time; plasma clearance is dependent on liver function, hepatic blood flow, bile flow (Table 1).
ICGPDR and ICGR15 might be considered the two faces of the same phenomenon. ICGPDR quantifies ICG disappearance from the plasma over time (% per minute); ICGR15 is the amount of the circulating ICG fifteen minutes after the administration (%). However, at variance of ICGR15, ICGPDR should be associated with ICG uptake by the hepatocytes mass, bile excretion, blood flow - dependent liver metabolism and the energy status[17]. Unfortunately, across the various studies the two parameters are used in a different and possibly confounding manner. ICGR15 is almost always considered for the dynamic assessment of hepatic functional reserve in case of liver resection for hepatocellular carcinoma on cirrhosis (HCC)[5,8]; ICGPDR and ICG R15 to assess liver graft function after liver transplantation[18]; ICGPDR in the critical care setting[2,17].
ICGPDR and ICGR15 are determined using either the high performance liquid chromatography with ultraviolet and fluorescence detection (cumbersome and time consuming methodology) or, as almost always reported nowadays, the modern, non-invasive PDD method (pulse dye densitometry method and spectrophotometry)[6-8]. A first “invasive” tool was available in the early nineties with the COLD System (Pulsion Medical System, Germany): ICGPDR was measured using an arterial fiberoptic catheter inserted in the femoral artery and connected to the COLD system. The system provided a complete and advanced volumetric hemodynamic profile and the ICGPDR[19]. A non invasive, optical transcutaneous pulse spectrophotometric sensor (PDD technology) is instead used by LiMON, (Pulsion Medical System, Germany) and DDG 2001, (Nihon Kohden, Japan) analysers[20-23]. The system measures ICG concentration determining the relative changes in light absorbtion by the arterial ICG at two different wave lengths, 805 nm (frequency of the ICG peak absorbtion) and 905 nm (frequency with no ICG absorption): No interference comes from oxidized or reduced hemoglobin and from bilirubin (peak absorption at 470 nm)[6,7]. PDD has been validated both in stable and unstable hemodynamic settings[18-21]. Purcell et al[22] validated the PDD algorithm comparing ICGR15 values obtained from direct measurement of blood samples and from LiMON. Stable hemodynamic conditions are imperative for reliable data on liver function[6,8]. Systemic or local conditions able to reduce hepatic blood flow (low cardiac output inducing hepatosplanchnic hypoperfusion or hepatic artery thrombosis and abdominal hypertension, respectively) have significant impact on IGC elimination, which is reduced in the above mentioned settings. On the contrary, splanchnic hyperperfusion, increasing ICG extraction, might produce (falsely) high ICGPDR readings. In case of liver dysfunction, true pathological IGCPDR or ICGR15 values are present because of a decreased transport from the systemic circulation to the liver (reduced blood flow) and/or a decreased uptake by the hepatocytes from the sinusoids. In the liver transplant setting, for example, conditions able to negatively impact on liver blood flow and/or extraction capacities are hepatic artery thrombosis (HAT), primary graft non function (PGNF), severe early graft dysfunction, severe rejection[9,10].
Altered IGCPDR and ICGR15 might also be reported in case of elevated serum bilirubin levels: In the active transport process into the hepatocytes, competition between bilirubin and ICG for the same carrier “alters” ICG kinetic results. This specific condition could be quite common in the early postoperative period of liver transplantation in patients with pretransplant hyperbilirubinemia: Pathological results should be attributed to ICG/Bilirubin competition for the same carrier (Na Taurocolate-co-transporting peptide) and not necessarily to a graft dysfunction. Since pathological ICGR15 or IGCPDR values might be recorded with serum bilirubin > 3 mg/dL[6,7], extreme caution has to be used when interpreting IGC clearance results in hyperbilirubinemic patients. According to the available studies, a bilirubin level > 3 mg/dL should be considered the cut-off value. In a series of 76 liver transplanted patients, a higher bilirubin level (6 mg/dL) was found by our group to be the cut-off value able to interfere with ICG kinetics (published in abstract)[24].
IGCPDR and ICGR15 are now used: (1) preoperatively, to assess the liver functional reserve before hepatic resection, particularly in cirrhotic patients[6,23]; (2) in the liver transplant setting, either in sequential assessments during the various phases of liver transplantation (rare) or (most often) to dynamically assess the recovery of the graft early after transplantation; and (3) following hepatic resection for a functional evaluation of the remnant liver both in cirrhotic and non cirrhotic patients and after partial hepatectomy (particularly the right hepatectomy) in case of living related liver donation. As above underlined, caution must be used while interpreting the results in case of hyperbilirubinemia[6,24]. Last but not least, ICG clearance parameters might be altered in case of repeated administrations if intervals between the sequential IGC injections are too short (less than 30 min): Residual ICG may change the baseline drift[6].
In contemporary clinical liver medicine, a temptative list of indications of ICG kinetic parameters could be the following[2,6-8]: (1) Functional definition of the hepatic reserve in cirrhotic and non cirrhotic patients undergoing resective surgery; (2) Morbidity/mortality prediction in the same setting; (3) Functional assessment in cadaveric donors of liver function, particularly in case of extended criteria donors, and in case of living donation (beyond the scope of the review); (4) Non invasive assessment of portal hypertension (PH) and esophageal varices[25]; and (5) Early functional assessment of the newly grafted liver.
THE ROLE OF IGC CLEARANCE KINETICS IN THE PREOPERATIVE ASSESSMENT OF LIVER RESECTION IN CIRRHOTIC PATIENTS
Nowadays, in the clinical management of HCC in cirrhotic and non cirrhotic patients relevant is the role played by the appropriate indication of surgery. Liver resection is considered for cirrhotic patients with compensated hepatic function, as assessed by scores, static or dynamic liver function tests, imaging[26]. In 2003, Imamura et al[27] were able to report zero mortality in a series of 1056 hepatectomies: However, mortality rates ranging from 2% to 5% (and higher) are still reported by others[23,26,27]. Posthepatectomy liver dysfunction or failure remains an extremely feared complication, still reported in up to 30% of the cases: In spite of major innovations in surgical and anesthesiological techniques and in the postoperative care, mortality remains high[27-30]. Postoperative liver dysfunction is more frequent in cirrhotic patients who underwent hepatic resection: According to the literature, major risk factors are inadequate preoperative assessment of liver functional reserve, too “aggressive” resection, perioperative hemorrhagic complications and transfusion needs, postoperative infective complications[30-35].
Usually (but not exclusively), indications and extension of resective surgery are tailored according to: (1) presence or absence of ascites and hepatic encephalopathy in the preoperative period; (2) results of conventional static liver function tests (AST/ALT, serum Bilirubin level); (3) imaging (magnetic resonance imaging/magnetic resonance imaging volumetric imaging to predict the remnant hepatic volume); and (4) CTP and MELD scoring systems[23,33-35].
Scores systems widely used in liver medicine for a comprehensive assessment of liver function are CPT and MELD. The CTP score, proposed in 1964 by Child et al[36], and later modified by Pugh (CTP), was created to predict the morbidity/mortality risk of cirrhotic patients with severe PH admitted to shunt surgery[36,37].
Using serum bilirubin, albumin and prothrombin time (PT), (common biochemical parameters, easy to determine in everyday clinical practice) and clinical findings (presence/absence of ascites and encephalopathy), the AAs defined three classes (A, B, C) able to identify the severity of the chronic liver disease. Within the three classes, Pugh et al[37] later introduced a score for different values of the biochemical and clinical parameters to identify within the same class (A, B, C) subgroups of patients (A 5-6; B 7- 9; C 10-15) at increasing severity. The CTP score, still quite reliable in predicting mortality after general surgery (roughly, CTP A, 10%; CTP B, up to 30%; CTP C, as high as or above 50%)[6], has some important limitations: Insufficient information on regional assessment of liver function (CTP is by definition a sort of broad classification of the severity of liver disease) and the absence of information on the volume of liver parenchima safely resectable are indeed relevant in the surgical setting[6,7].
In spite of these reported limitations, in the Western surgical school CTP and the degree of PH (often qualitatively defined), together with imaging are often used to assess liver function in the preoperative period. Liver resective surgery should be considered for patients in class A and, limiting the extent of the resection to reduce the risk of postoperative hepatic dysfunction, in well selected Class B patients[5,27,30]. Controversial is the use of MELD score or its derivates (NaMELD and iMELD) in the surgical context. MELD score, based on bilirubin, creatinine and PT as INR, was originally introduced to predict the outcome of patients candidates to transjugular intrahepatic portosystemic shunt procedure. Nowadays, MELD is mainly considered to define the severity of chronic liver disease and its prognosis, to prioritize the liver transplant procedure, to predict survival in liver transplant candidates[38-40]. However, reliability of MELD to predict mortality after liver resection is still a matter of debate: major concerns arise from the narrow range (9-14) in which the score is used. In patients with MELD score > 10, Cucchetti et al[41] found a high rate of postoperative liver dysfunction. Hepatic resection is contraindicated in CTP class C patients or in patients whose MELD score is above 14. Instead, in well selected class B patients or in subjects whose MELD score ranges from 9 to 14, the surgical option might be considered: Each single case mandates a thorough preoperative evaluation, including the type of liver resection and its feasibility[42,43].
On the contrary, ICG clearance parameters (mainly ICGR15) are since the eighties championed by the Eastern surgical schools[5,32-34]: In particular dynamic tests were strongly supported to assess in advance the maximum extent of the resection of the hepatic parenchima associated with a good functioning remnant liver. In the evidence- based guidelines for the treatment of hepatocellular carcinoma released in Japan in 2009, the use of ICGR15 was recommended (level of evidence B) for the preoperative assessment of liver function[43]. Very recently, ICGR15 was incorporated in a modified functional evaluation score [Liver Damage Grading System (LDGS)] derived from the CTP classification (Table 2). The Japanese Liver Cancer Study Group of Japan proposed the LDGS, instead of the CTP score, as a more accurate and appropriate tool for the functional assessment of the hepatic reserve[8,23].
Table 2.
Liver damage grading system (Mizuguchi et al[8], 2014, modified)
| Parameters | Liver damage grade A | Liver damage grade B | Liver damage grade C |
| Albumin (g/L) | > 3.5 | 3.5-3 | < 3 |
| Bilirubin (mg/dL) | < 2 | 2-3 | < 3 |
| PT (%) | > 80 | 50-80 | < 50 |
| Ascites | None | Small or controlled | Tense |
| IGCR15 (%) | < 15 | 15-40 | > 40 |
PT: Prothrombin activity; ICGR15: Indocyanine green retention ratio at 15 min.
In cirrhotic patients, liver resections should be performed with ICGR15 < 15%: According to authoritative reports, appropriate candidates for right hepatectomies were patients with ICGR15 > 10%, whereas left hepatectomies were considered also in surgical candidates with slightly longer ICGR15 (range 10% to 19%)[43-45]. In other series, major liver resections were successfully performed with longer ICGR15 (range 15% to 20%), if the volume of the residual liver was deemed “sufficient”[44]. The role of ICGR15 in major liver resection became relevant and evident after the publication of the Makuuchi group’s experience: Analyzing the results obtained between 1994 and 2002, the AAs were able to report zero mortality in 1056 hepatectomies[5]. Three variables were particularly highlighted in the preoperative assessment: (1) ascites (presence or absence); (2) bilirubinemia; and (3) ICGR15[5,27].
According to the original decisional tree proposed by Imamura et al[5], key points are: (1) contraindication to hepatic resection in presence of uncontrolled ascites or serum bilirubin > 1.9 mg/dL; (2) minor resections possible with serum bilirubin ranging between 1 and 1.9 mg/dL, the lower the bilirubin level, the larger the resection; and (3) according to ICGR15 intervals different types of hepatic resection possible in case of serum bilirubin < 1.1 mg/dL and no ascites (Figure 4).
Figure 4.
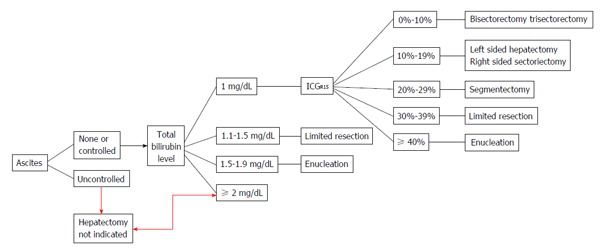
Makuuchi decisional algorithm to select liver resective procedures in cirrhotic patients according to liver functional reserve (from Imamura et al[5], 2005). ICGR15: Indocyanine green retention ratio at 15 min.
Nowadays, preoperative selective portal vein embolization is a challenging option in very well selected subjects candidates to liver resection: An example could be a patient with ICGR15 15%-20% whose remnant liver volume after the planned resection is considered “not sufficient”. The aim of portal vein embolization is to induce hyperplasia of the hepatic lobules perfused by the contralateral portal vein to increase the volume of the “future remnant” liver[6,7,46]. ICGR15 after embolization correlates with both the volumetric changes and the modification of the liver functional reserve: It should allow a sort of functional prediction of the remnant liver before resective surgery[46]. In the original algorithm proposed by Poon and Fan, hepatic hyperplasia and preservation of “total” liver blood flow were the mainstays of this surgical strategy[33]. Definitive implementation of the procedure is still ongoing, even if available results seem promising.
In recent studies, postoperative morbidity [mainly represented by post-hepatectomy liver failure (PHLF)] is reliably predicted by R15 or PDR[44-47]. Still under debate is instead the ability of ICG kinetics to correctly predict mortality: The small number of negative events (death) might represent a possible cause[44,45]. Using intraoperative ICGPDR in a small series of patients, a value of < 9% per minute min predicted postresective liver failure with high sensitivity (88%) and specificity (82%)[44]. In another experience, liver failure occurring on postoperative day (POD) 2-5 was predicted by ICGPDR < 7% per minute on POD 1[45]. Prospectively studying postoperative complications in 100 cirrhotic patients admitted to different liver resections, our group was able to document a significant increase in postresective morbidity associated with ICGR15 > 40%: Interestingly enough, mortality was not influenced by ICGR15 (published in abstract)[47].
The most recent intraoperative application of IGC kinetics (ICGPDR/ICGR15) in major liver surgery was proposed by Thomas et al[48]: Scope of the study was the definition of reliability of an intraoperative simulation of post-resection liver function. In 20 patients undergoing liver resection, ICG kinetics (LiMON, Pulsion Medical System, Germany) was assessed before and after selective arterial and portal venous inflow trial clamping (TC) of the resected liver segments: The aim was to prevent/avoid PHLF. Similar data were recorded under TC (a significant ICGPDR decrease from 16.5% to 10.5% per minute) and after resection (median ICGPDR after resection 10.5% per minute). Thomas et al[48] proposed ICG kinetics as able to reliably simulate post-resection liver function during TC: In their opinion, it might become a useful tool to prevent/avoid PHLF and to reduce hospital length of stay.
In a recent paper, combining the changes of total Bilirubin and INR on POD 1, 3, 5 and 7, Du et al[49] proposed a definition of postoperative liver failure (PLF). An hepatic damage score (HDs) was built up and used after liver resection to define the degree of the liver metabolic functional impairment (0 = mild; 1 = reversible hepatic “dysfunction”; 2 = fatal hepatic failure). Interestingly enough, in the most compromised patients (HDs = 2) a linear relationship was found between ICGR15 and the number of the resected segments, possibly identifying preoperative criteria for the most appropriate and safest selection of hepatic resection to reduce PLF[49].
Preoperative pathological ICGR15 may be wrongly associated with liver dysfunction in case of biliary obstruction. If this is the case, caution should be exerted in interpreting the test results: While the programmed surgical strategy should not be withhold, further and multimodal investigations are to be considered to adapt/optimize the surgical program[6]. In case of hyperbilirubinemia, the South Korean and Japanese surgical schools suggest, as very recently reported by Ge et al[17], Tc - galactosyl serum albumin scintigraphy for a more precise functional assessment of the liver. According to the most updated literature, GSA seems to be the ideal agent to predict the volume of hepatocyte mass and its function, due, at least in part, to track the distribution of asialoglycoprotein receptors[17].
ICGR15 IN PH: A ROLE AS A NON INVASIVE MARKER?
As above discussed, total liver blood flow and hepatic functional reserve are reflected by ICGR15, often used as a prognostic marker in decompensated cirrhotic patients and in candidates to resective liver surgery[50]. In cirrhotic patients admitted to resective surgery, preexisting PH and postoperative parenchymal dysfunction are among the most common causes of PHLF. Recently, Lisotti et al[25] in a cohort of CHILD. A cirrhotic patients with well-preserved liver function evaluated the accuracy of ICGR15 in reflecting the alteration of hepatic blood flow and, indirectly, the presence and grade of PH and esophageal varices (EV). As comparators, the AAs used hepatic vein pressure gradient and upper gastrointestinal endoscopy, actually the gold standards in this setting. Interestingly enough, Lisotti et al[25] documented a good performance of ICGR15 for the diagnosis of both PH and EV. In patients with compensated cirrhosis, ICGR15 < 6.7% and < 6.9% ruled out clinically significant PH and severe PH respectively, while ICGR15 < 10% was able to exclude the presence of EV. The AAs concluded for a role of ICGR15 in identifying patients with advanced liver disease for whom the endoscopic study is warranted.
ICG KINETICS IN LIVER TRANSPLANT SURGERY
An increased demand of grafts due to the expanded liver transplant (OLT) indications has to face organ shortage, perhaps the most relevant restraint when dealing with solid organ transplant surgery. To expand the donors pool, extended criteria donors and/or suboptimal (“marginal”) grafts are ever and ever harvested to match the increasing transplant demand. Early after OLT, the results of conventional “static” liver function tests may raise doubts or uncertainties when used to assess the functional recovery of the liver grafts[6]. Recently, few, small single center studies reported on ICGPDR to assist and (more objectively support) the decision to harvest livers from suboptimal donors. ICG clearance kinetics, mainly expressed as ICGPDR or K constant of elimination, have been used in cadaveric donors before organ harvesting for a quantitative assessment of liver function[6]. Unfortunately, the value of ICGPDR to assist graft suitability assessment before harvesting deserves further studies, as values < 15% per minute during donor observation were consistently associated with a poor outcome of the graft[6].
IGC kinetics have since long a place in the liver transplant setting. ICG kinetics were recently incorporated in the MELD score for a fine tuning of survival prediction in transplant candidates: As a matter of fact, in candidates whose MELD score ranged from 10 to 30, the ICG-MELD score further improved the prediction performance[50]. ICG kinetics into the MELD score add an estimation of liver blood flow, making the new score more accurate than the “simple” MELD and Na MELD in predicting survival in moderate to severe cirrhosis. The role played by hyperbilirubinemia, if present, has of course to be considered. Much more extensively studied is the use of ICG kinetics to predict early perioperative complications and graft and patient survival after OLT. Among the most feared complications in the early postoperative period are HAT and PGNF, conditions which warrant early diagnosis and a timely and appropriate treatment: Urgent retransplantation is mandatory in case of PGNF and very often is the only solution to avoid fatalities in cases of HAT. In the mid nineties, a number of relevant studies[51-53] strongly supported the use of ICG clearance parameters for an early assessment of graft function and to predict patient and graft survival. Jalan et al[51], using ICG clearance, correctly predicted both the immediate functional recovery of the new liver and the good graft function three months after OLT when ICGCl on POD 1 was > 200 mL/min. More recently, “low” ICGPDR values (5% to 12% per minute) early after OLT were associated with graft malfunction/failure. In the liver transplant setting, the definition of a reproducible and reliable “low” cut-off value is, even if eagerly awaited, still ill - defined: ideally, this value should not be affected by conditions able to create falsely pathological results. Unfortunately, no consensus exists in the literature on this critical point, so far. Faybik et al[54], studying IGCPDR using COLD System (Pulsion, Germany) and LiMon (Pulsion, Germany) in a series of patients who underwent OLT found ICGPDR < 10% per minute as a predictor of postoperative complications. Hori et al[55], using ICGK (Nihon Kohden DDG 2001, Japan) in a cohort of thirty patients admitted to living donor liver transplant, assessed graft function daily for the first 14 postoperative days, and then on POD 21 and 28. The early outcome was defined “unfavourable” in case of increased morbidity or mortality. According to this definition, the AAs retrospectively allocated the transplanted patients to two groups, A (favourable outcome, 24 subjects) and B (unfavourable outcome, 6 subjects). ICGK < 0.180 on POD 1 correctly predicted the poor outcome of the six patients of group B.
Levesque et al[56,57] using LiMON (Pulsion Medical System, Germany) from POD 1 to POD 5 defined an ICGPDR value able to predict early postoperative complications. In a first study[56], in a series of 70 consecutive procedures, the transplanted patients were divided in two groups according to the early outcome: In the group of patients who did well, had immediate good graft function, favourable postoperative course and positive outcome, ICGPDR was 24.4% ± 6.8% per minute. Instead, the patients who had postoperative complications were retrospectively subdivided into two subgroups: The first group was composed by subjects who experienced PGNF, HAT, and hemorrhagic or septic shock (early complications); the second included patients who had rejection (late complications). While ICGPDR was low (8.8% ± 4.5% per minute) during the first 5 d in the first subgroup, in the second the ICGPDR, initially normal, decreased significantly within 3 to 5 d (ICGPDR 10.3% ± 2.5% per minute). Levesque et al[56] proposed ICGPDR < 12.85% per minute as a marker of very early postoperative complications (mainly severe hepatocellular dysfunction, such as PGNF). In a second paper, the same AAs retrospectively reviewing ICGPDR in patients who had HAT in the early post OLT period found a significantly lower ICGPDR when HAT was documented (range 0.4 to 9.5, mean 5.8% ± 4.3% vs non HAT, range 15.3% to 32.9%, median 23.8% ± 7.4% per minute): ICGPDR increased significantly after the revascularization (mean 15.6% ± 3.5% per minute). The AAs concluded defining IGCPDR as an interesting diagnostic tool in the early posttransplant period to manage patients suspected for acute HAT[57]. The major concern that could be raised on this specific item is the absence of a clear cutoff value in the presence of HAT (see the wide range of ICGPDR in the HAT patients). As a matter of fact, this item is quite controversial in the literature. ICG kinetic parameters were used by Olmedilla et al[58] at the end of OLT or on POD 1 to assess early graft function. In patients who suffered early severe hepatic dysfunction and had an increased mortality rate, ICGPDR was < 10.8% per minute. Instead, a favorable outcome was recorded in transplanted patients who had ICGPDR > 10.8% per minute: In the same study the AAs were also able to document a very high (99%) negative predictive value. In the most recent study coming from the same group, ICGPDR and INR were used to build a risk score to predict short term outcome after OLT. Cut-off values were ≥ 2.2 for INR (1 point) and < 10% per minute for PDR (2 points). The AAs defined four categories (points 0 to 3) in which the risk of early death or retransplantation was described by the score, the higher the score, the higher the risk of adverse outcome (point 0, 4.4%; point 1, 6.5%; points 2, 12%; points 3, 50%). A similar trend was reported also for ICU length of stay and duration of mechanical ventilation. In a validation cohort of 70 patients the score had a good diagnostic performance with sensitivity 60%; specificity 95.5%; positive predictive value (PPV), 66.7%; negative predictive value (NPV) 94.1%. The AAs concluded for a simple and useful tool to be considered for the selection of diagnostic and therapeutic strategies in the early postoperative period[59]. Different result were proposed by Escorsell et al[60]. In their experience, ICGPDR was not a predictor of liver dysfunction and short term outcome. Using a cut off of 8.8% per minute the AAs subdivided the transplanted patients in two groups (A < 8.8% per minute; B > 8.8% per minute). Interestingly enough, outcome of patients in group A was similar to outcome of patients in group B: Since transplanted patients in group A showed significantly higher bilirubin levels, a false “low” reading of the ICGPDR might have occurred. The most probable explanation should be a non proper categorization of a graft as “malfunctioning” because of hyperbilirubinemia and not because of a real dysfunction. Confirmation of this interpretation comes from the reported outcome. Very similar were the results we proposed (in abstract) studying a cohort of 76 consecutive liver transplants[24]: ICGPDR < 10% per minute was not associated with a poor outcome of the patient and of the graft in the early postoperative period. Interestingly enough, serum bilirubin > 6 mg/dL was always present when ICGPDR was < 8% per minute[24]. We speculated that in this specific condition (hyperbilirubinemia), ICGPDR should be considered, as above underlined, unreliable[6,7,12,13]. This point is unfortunately not completely addressed, in our opinion, by Levesque et al[61] in the most recent review on this item. The last two studies are, in our opinion, a further strong argument to support the relevant alteration introduced by hyperbilirubinemia, not infrequently observed early after OLT, on ICG kinetics. In both studies, ICGPDR falsely predicted an early hepatic dysfunction, not confirmed by the early and medium term outcome of both patients and grafts. Instead, Escorsell et al[60] showed a strong correlation between lactate clearance and the functional recovery of the newly grafted livers, further stressing the high PPV of this test: A further confirmation of very similar results we obtained in an earlier study[62]. Last but not least, ICG kinetics might be altered by other factors or conditions quite common in the early post transplant period: Among them, the impact of different values of total proteins and hematocrit[63].
Further confirmations for a cautious interpretation of low ICGPDR values while assessing liver function both after liver resection and OLT come from a series of recent studies performed with the Maximal Enzymatic Liver Function (LiMax test), a test which relies upon 13C methacetin metabolism[64-67]. In patients who underwent liver resective surgery, Lock et al[64] compared ICGPDR and Limax to identify patients at risk for postoperative liver failure: Limax showed a better predictive power, once again emphasizing how relevant could be the potential interference of various parameters on the ICG clearance variables.
In a cohort of liver transplant candidates suffering for chronic liver disease, patients who experienced six months liver-related death (primary end point of the study) had, when compared to survivors, significantly lower median Limax values. On the contrary, ICGPDR findings were similar in survivors and non survivors. In the same study LiMAx showed a slightly higher NPV (if compared to ICGPDR and MELD) when six months risk of death was considered[65].
Acute liver failure (ALF) is one of the most challenging conditions in liver medicine. Preliminary results on the use of ICG kinetic parameters were recently reported in small series of patients[7,61,65]: However hyperbilirubinemia, always present in patients with hyperacute, acute (“fulminant”) or subacute hepatic failure, should impact on ICG elimination kinetics, making problematic at best their interpretation. Lock et al[67] recently tested the use of LiMAx in ALF. Remarcably, LiMAx values, contrary to MELD, were significantly lower in patients who had unfavourable outcome. If confirmed, the AAs concluded for an interesting relevant role of LiMax in ALF in predicting the individual prognosis, possibly supporting in the decision for urgent liver transplant[67].
CONCLUSION
In recent years reliable and easy-to-use non-invasive bedside analysers using the PDD technology, (LiMon and Nihon Kohden) have boosted the use of ICG kinetic parameters in hepatic surgery and, in general, while caring for the critically ill. Since long, the Eastern surgical schools have supported an extensive application of this technology, particularly when major surgical options are considered in patients affected by hepatocellular carcinoma on liver cirrhosis. The most relevant results, worth to be considered also by the Western surgical community, deal with liver cancer resectability and the potentials for preventing or avoiding postresective hepatic dysfunction/failure. In liver resective surgery, while firm results are available when dealing with morbidity, concern still exists in predicting mortality. In spite of the initial enthusiasms and some very recent results, the use of post OLT ICG kinetics to predict morbidity and mortality are to be considered, at least in our opinion, still under scrutiny. Notwithstanding the results proposed by the most recent publication[59], mixed results or “false pathological findings” (false positives) are present in the literature: To be specifically addressed in the liver transplant setting is the presence of hyperbilirubinemia. In this context, according to ICGPDR, newly grafted liver might be falsely classified as severely dysfunctioning or at consistent risk of unfavourable outcome, when opposite is the real final outcome. In spite of the most recent evidence[59], no consensus exists on the cut-off value of PDR/R15 below which a reliable assessment of early graft dysfunction is confidently available. In liver transplanted patients, the negative predictive value of ICG kinetics is indeed relevant: Good graft and patients outcome are almost always associated with “normal” IGC clearance parameters. Into our opinion, in this setting “low” or pathological values are still in a gray zone and caution in interpreting results is needed. As appropriately pointed out by Levesque et al[61] when defining severity of complex and evolving diseases, a multistep dynamic approach (instead of single time point static result) should become the rule. Ending up their review, Vos et al[6] proposed a wise and prudent comment on the routine use of IGC kinetics in clinical practice, pushing for further large, prospective, randomized trials: A challenge worth to be considered, particularly in the field of liver transplantation, if gray has to turn to green.
Footnotes
Conflict-of-interest statement: De Gasperi A had fees for serving as a speaker for lectures and travel reimbursements from Astellas, Pfizer, Edwards, SEDA Italia Gilead, MSD, Fresenius Kabi, Grifols; Mazza E and Prosperi M have no conflicts of interest.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: March 25, 2015
First decision: May 18, 2015
Article in press: February 24, 2016
P- Reviewer: Lisotti A, Waisberg J S- Editor: Ji FF L- Editor: A E- Editor: Liu SQ
References
- 1.Wagener G. Assessment of hepatic function, operative candidacy, and medical management after liver resection in the patient with underlying liver disease. Semin Liver Dis. 2013;33:204–212. doi: 10.1055/s-0033-1351777. [DOI] [PubMed] [Google Scholar]
- 2.Hoekstra LT, de Graaf W, Nibourg GA, Heger M, Bennink RJ, Stieger B, van Gulik TM. Physiological and biochemical basis of clinical liver function tests: a review. Ann Surg. 2013;257:27–36. doi: 10.1097/SLA.0b013e31825d5d47. [DOI] [PubMed] [Google Scholar]
- 3.Sakka SG. Assessing liver function. Curr Opin Crit Care. 2007;13:207–214. doi: 10.1097/MCC.0b013e328012b268. [DOI] [PubMed] [Google Scholar]
- 4.Slack A, Ladher N, Wendon J. Acute hepatic failure. In Wagener G, editor. Liver Anesthesiology and Critical care Medicine. New York, Heidelberg, Dordrecht, London: Springer; 2012. pp. 21–42. [Google Scholar]
- 5.Imamura H, Sano K, Sugawara Y, Kokudo N, Makuuchi M. Assessment of hepatic reserve for indication of hepatic resection: decision tree incorporating indocyanine green test. J Hepatobiliary Pancreat Surg. 2005;12:16–22. doi: 10.1007/s00534-004-0965-9. [DOI] [PubMed] [Google Scholar]
- 6.Vos JJ, Wietasch JK, Absalom AR, Hendriks HG, Scheeren TW. Green light for liver function monitoring using indocyanine green? An overview of current clinical applications. Anaesthesia. 2014;69:1364–1376. doi: 10.1111/anae.12755. [DOI] [PubMed] [Google Scholar]
- 7.Halle BM, Poulsen TD, Pedersen HP. Indocyanine green plasma disappearance rate as dynamic liver function test in critically ill patients. Acta Anaesthesiol Scand. 2014;58:1214–1219. doi: 10.1111/aas.12406. [DOI] [PubMed] [Google Scholar]
- 8.Mizuguchi T, Kawamoto M, Meguro M, Hui TT, Hirata K. Preoperative liver function assessments to estimate the prognosis and safety of liver resections. Surg Today. 2014;44:1–10. doi: 10.1007/s00595-013-0534-4. [DOI] [PubMed] [Google Scholar]
- 9.Leevy CM, Mendenhall CL, Lesko w, Howard MM. Estimation of hepatic blood flow with indocyanine green. J Clin Invest. 1962;41:1169–1179. doi: 10.1172/JCI104570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pessayre D, Lebrec D, Descatoire V, Peignoux M, Benhamou JP. Mechanism for reduced drug clearance in patients with cirrhosis. Gastroenterology. 1978;74:566–571. [PubMed] [Google Scholar]
- 11.Lau H, Man K, Fan ST, Yu WC, Lo CM, Wong J. Evaluation of preoperative hepatic function in patients with hepatocellular carcinoma undergoing hepatectomy. Br J Surg. 1997;84:1255–1259. [PubMed] [Google Scholar]
- 12.Shinohara H, Tanaka A, Kitai T, Yanabu N, Inomoto T, Satoh S, Hatano E, Yamaoka Y, Hirao K. Direct measurement of hepatic indocyanine green clearance with near-infrared spectroscopy: separate evaluation of uptake and removal. Hepatology. 1996;23:137–144. doi: 10.1053/jhep.1996.v23.pm0008550033. [DOI] [PubMed] [Google Scholar]
- 13.Cui Y, König J, Leier I, Buchholz U, Keppler D. Hepatic uptake of bilirubin and its conjugates by the human organic anion transporter SLC21A6. J Biol Chem. 2001;276:9626–9630. doi: 10.1074/jbc.M004968200. [DOI] [PubMed] [Google Scholar]
- 14.Keiding S. Hepatic clearance and liver blood flow. J Hepatol. 1987;4:393–398. doi: 10.1016/s0168-8278(87)80552-4. [DOI] [PubMed] [Google Scholar]
- 15.Kawasaki S, Sugiyama Y, Iga T, Hanano M, Sanjo K, Beppu T, Idezuki Y. Pharmacokinetic study on the hepatic uptake of indocyanine green in cirrhotic patients. Am J Gastroenterol. 1985;80:801–806. [PubMed] [Google Scholar]
- 16.Huet PM, Goresky CA, Villeneuve JP, Marleau D, Lough JO. Assessment of liver microcirculation in human cirrhosis. J Clin Invest. 1982;70:1234–1244. doi: 10.1172/JCI110722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ge PL, Du SD, Mao YL. Advances in preoperative assessment of liver function. Hepatobiliary Pancreat Dis Int. 2014;13:361–370. doi: 10.1016/s1499-3872(14)60267-8. [DOI] [PubMed] [Google Scholar]
- 18.Faybik P, Krenn CG, Baker A, Lahner D, Berlakovich G, Steltzer H, Hetz H. Comparison of invasive and noninvasive measurement of plasma disappearance rate of indocyanine green in patients undergoing liver transplantation: a prospective investigator-blinded study. Liver Transpl. 2004;10:1060–1064. doi: 10.1002/lt.20205. [DOI] [PubMed] [Google Scholar]
- 19.Kisch H, Leucht S, Lichtwarck-Aschoff M, Pfeiffer UJ. Accuracy and reproducibility of the measurement of actively circulating blood volume with an integrated fiberoptic monitoring system. Crit Care Med. 1995;23:885–893. doi: 10.1097/00003246-199505000-00017. [DOI] [PubMed] [Google Scholar]
- 20.Iijima T, Aoyagi T, Iwao Y, Masuda J, Fuse M, Kobayashi N, Sankawa H. Cardiac output and circulating blood volume analysis by pulse dye-densitometry. J Clin Monit. 1997;13:81–89. doi: 10.1023/a:1007339924083. [DOI] [PubMed] [Google Scholar]
- 21.Sakka SG, Reinhart K, Meier-Hellmann A. Comparison of invasive and noninvasive measurements of indocyanine green plasma disappearance rate in critically ill patients with mechanical ventilation and stable hemodynamics. Intensive Care Med. 2000;26:1553–1556. doi: 10.1007/s001340000639. [DOI] [PubMed] [Google Scholar]
- 22.Purcell R, Kruger P, Jones M. Indocyanine green elimination: a comparison of the LiMON and serial blood sampling methods. ANZ J Surg. 2006;76:75–77. doi: 10.1111/j.1445-2197.2006.03643.x. [DOI] [PubMed] [Google Scholar]
- 23.Seyama Y, Kokudo N. Assessment of liver function for safe hepatic resection. Hepatol Res. 2009;39:107–116. doi: 10.1111/j.1872-034X.2008.00441.x. [DOI] [PubMed] [Google Scholar]
- 24.Mazza E, Prosperi M, DeGasperi A Reggiori G, Corti A, Grugni C, Roselli E, Marchesi M, Amici O, Nichelatti M, Pavani M. Plasma disappearance rate of indocyanine green after liver transplantation: always a reliable tool to predict graft function and out come? Liver Transpl. 2008;14:S201: LB476. [Google Scholar]
- 25.Lisotti A, Azzaroli F, Buonfiglioli F, Montagnani M, Cecinato P, Turco L, Calvanese C, Simoni P, Guardigli M, Arena R, et al. Indocyanine green retention test as a noninvasive marker of portal hypertension and esophageal varices in compensated liver cirrhosis. Hepatology. 2014;59:643–650. doi: 10.1002/hep.26700. [DOI] [PubMed] [Google Scholar]
- 26.Manizate F, Hiotis SP, Labow D, Roayaie S, Schwartz M. Liver functional reserve estimation: state of the art and relevance for local treatments: the Western perspective. J Hepatobiliary Pancreat Sci. 2010;17:385–388. doi: 10.1007/s00534-009-0228-x. [DOI] [PubMed] [Google Scholar]
- 27.Imamura H, Seyama Y, Kokudo N, Maema A, Sugawara Y, Sano K, Takayama T, Makuuchi M. One thousand fifty-six hepatectomies without mortality in 8 years. Arch Surg. 2003;138:1198–1206; discussion 1206. doi: 10.1001/archsurg.138.11.1198. [DOI] [PubMed] [Google Scholar]
- 28.Bellavance EC, Lumpkins KM, Mentha G, Marques HP, Capussotti L, Pulitano C, Majno P, Mira P, Rubbia-Brandt L, Ferrero A, et al. Surgical management of early-stage hepatocellular carcinoma: resection or transplantation? J Gastrointest Surg. 2008;12:1699–1708. doi: 10.1007/s11605-008-0652-2. [DOI] [PubMed] [Google Scholar]
- 29.Jarnagin WR, Gonen M, Fong Y, DeMatteo RP, Ben-Porat L, Little S, Corvera C, Weber S, Blumgart LH. Improvement in perioperative outcome after hepatic resection: analysis of 1,803 consecutive cases over the past decade. Ann Surg. 2002;236:397–406; discussion 406-407. doi: 10.1097/01.SLA.0000029003.66466.B3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fan ST. Liver functional reserve estimation: state of the art and relevance for local treatments: the Eastern perspective. J Hepatobiliary Pancreat Sci. 2010;17:380–384. doi: 10.1007/s00534-009-0229-9. [DOI] [PubMed] [Google Scholar]
- 31.Bruix J, Castells A, Bosch J, Feu F, Fuster J, Garcia-Pagan JC, Visa J, Bru C, Rodés J. Surgical resection of hepatocellular carcinoma in cirrhotic patients: prognostic value of preoperative portal pressure. Gastroenterology. 1996;111:1018–1022. doi: 10.1016/s0016-5085(96)70070-7. [DOI] [PubMed] [Google Scholar]
- 32.Lee SG, Hwang S. How I do it: assessment of hepatic functional reserve for indication of hepatic resection. J Hepatobiliary Pancreat Surg. 2005;12:38–43. doi: 10.1007/s00534-004-0949-9. [DOI] [PubMed] [Google Scholar]
- 33.Poon RT, Fan ST. Assessment of hepatic reserve for indication of hepatic resection: how I do it. J Hepatobiliary Pancreat Surg. 2005;12:31–37. doi: 10.1007/s00534-004-0945-0. [DOI] [PubMed] [Google Scholar]
- 34.Nonami T, Nakao A, Kurokawa T, Inagaki H, Matsushita Y, Sakamoto J, Takagi H. Blood loss and ICG clearance as best prognostic markers of post-hepatectomy liver failure. Hepatogastroenterology. 1999;46:1669–1672. [PubMed] [Google Scholar]
- 35.Capussotti L, Viganò L, Giuliante F, Ferrero A, Giovannini I, Nuzzo G. Liver dysfunction and sepsis determine operative mortality after liver resection. Br J Surg. 2009;96:88–94. doi: 10.1002/bjs.6429. [DOI] [PubMed] [Google Scholar]
- 36.Child CG, Turcotte JG. Surgery and portal hypertension. Major Probl Clin Surg. 1964;1:1–85. [PubMed] [Google Scholar]
- 37.Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646–649. doi: 10.1002/bjs.1800600817. [DOI] [PubMed] [Google Scholar]
- 38.Malinchoc M, Kamath PS, Gordon FD, Peine CJ, Rank J, ter Borg PC. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology. 2000;31:864–871. doi: 10.1053/he.2000.5852. [DOI] [PubMed] [Google Scholar]
- 39.Dutkowski P, Oberkofler CE, Béchir M, Müllhaupt B, Geier A, Raptis DA, Clavien PA. The model for end-stage liver disease allocation system for liver transplantation saves lives, but increases morbidity and cost: a prospective outcome analysis. Liver Transpl. 2011;17:674–684. doi: 10.1002/lt.22228. [DOI] [PubMed] [Google Scholar]
- 40.Cholongitas E, Marelli L, Shusang V, Senzolo M, Rolles K, Patch D, Burroughs AK. A systematic review of the performance of the model for end-stage liver disease (MELD) in the setting of liver transplantation. Liver Transpl. 2006;12:1049–1061. doi: 10.1002/lt.20824. [DOI] [PubMed] [Google Scholar]
- 41.Cucchetti A, Ercolani G, Vivarelli M, Cescon M, Ravaioli M, La Barba G, Zanello M, Grazi GL, Pinna AD. Impact of model for end-stage liver disease (MELD) score on prognosis after hepatectomy for hepatocellular carcinoma on cirrhosis. Liver Transpl. 2006;12:966–971. doi: 10.1002/lt.20761. [DOI] [PubMed] [Google Scholar]
- 42.Teh SH, Christein J, Donohue J, Que F, Kendrick M, Farnell M, Cha S, Kamath P, Kim R, Nagorney DM. Hepatic resection of hepatocellular carcinoma in patients with cirrhosis: Model of End-Stage Liver Disease (MELD) score predicts perioperative mortality. J Gastrointest Surg. 2005;9:1207–1215; discussion 1215. doi: 10.1016/j.gassur.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 43.Kokudo N, Makuuchi M. Evidence-based clinical practice guidelines for hepatocellular carcinoma in Japan: the J-HCC guidelines. J Gastroenterol. 2009;44 Suppl 19:119–121. doi: 10.1007/s00535-008-2244-z. [DOI] [PubMed] [Google Scholar]
- 44.Ohwada S, Kawate S, Hamada K, Yamada T, Sunose Y, Tsutsumi H, Tago K, Okabe T. Perioperative real-time monitoring of indocyanine green clearance by pulse spectrophotometry predicts remnant liver functional reserve in resection of hepatocellular carcinoma. Br J Surg. 2006;93:339–346. doi: 10.1002/bjs.5258. [DOI] [PubMed] [Google Scholar]
- 45.Greco E, Nanji S, Bromberg IL, Shah S, Wei AC, Moulton CA, Greig PD, Gallinger S, Cleary SP. Predictors of peri-opertative morbidity and liver dysfunction after hepatic resection in patients with chronic liver disease. HPB (Oxford) 2011;13:559–565. doi: 10.1111/j.1477-2574.2011.00329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shindoh J, D Tzeng CW, Vauthey JN. Portal vein embolization for hepatocellular carcinoma. Liver Cancer. 2012;1:159–167. doi: 10.1159/000343829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mazza E, Kroeller D, Prosperi M, Grugni MC, Amici O, Rosellli E, De Carlis L, Nichelatti M, De Gasperi A. Does ICG clearance (ICGR15) predict morbidity and mortality after hepatic resection for hepatocellular carcinoma in cirrhotic patients? Intensive Care Med. 2012;38:S169, Abs 609. [Google Scholar]
- 48.Thomas MN, Weninger E, Angele M, Bösch F, Pratschke S, Andrassy J, Rentsch M, Stangl M, Hartwig W, Werner J, et al. Intraoperative simulation of remnant liver function during anatomic liver resection with indocyanine green clearance (LiMON) measurements. HPB (Oxford) 2015;17:471–476. doi: 10.1111/hpb.12380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Du ZG, Wei YG, Chen KF, Li B. An accurate predictor of liver failure and death after hepatectomy: a single institution’s experience with 478 consecutive cases. World J Gastroenterol. 2014;20:274–281. doi: 10.3748/wjg.v20.i1.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zipprich A, Kuss O, Rogowski S, Kleber G, Lotterer E, Seufferlein T, Fleig WE, Dollinger MM. Incorporating indocyanin green clearance into the Model for End Stage Liver Disease (MELD-ICG) improves prognostic accuracy in intermediate to advanced cirrhosis. Gut. 2010;59:963–968. doi: 10.1136/gut.2010.208595. [DOI] [PubMed] [Google Scholar]
- 51.Jalan R, Plevris JN, Jalan AR, Finlayson ND, Hayes PC. A pilot study of indocyanine green clearance as an early predictor of graft function. Transplantation. 1994;58:196–200. [PubMed] [Google Scholar]
- 52.Plevris JN, Jalan R, Bzeizi KI, Dollinger MM, Lee A, Garden OJ, Hayes PC. Indocyanine green clearance reflects reperfusion injury following liver transplantation and is an early predictor of graft function. J Hepatol. 1999;30:142–148. doi: 10.1016/s0168-8278(99)80018-x. [DOI] [PubMed] [Google Scholar]
- 53.Tsubono T, Todo S, Jabbour N, Mizoe A, Warty V, Demetris AJ, Starzl TE. Indocyanine green elimination test in orthotopic liver recipients. Hepatology. 1996;24:1165–1171. doi: 10.1002/hep.510240531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Faybik P, Hetz H. Plasma disappearance rate of indocyanine green in liver dysfunction. Transplant Proc. 2006;38:801–802. doi: 10.1016/j.transproceed.2006.01.049. [DOI] [PubMed] [Google Scholar]
- 55.Hori T, Iida T, Yagi S, Taniguchi K, Yamamoto C, Mizuno S, Yamagiwa K, Isaji S, Uemoto S. K(ICG) value, a reliable real-time estimator of graft function, accurately predicts outcomes in adult living-donor liver transplantation. Liver Transpl. 2006;12:605–613. doi: 10.1002/lt.20713. [DOI] [PubMed] [Google Scholar]
- 56.Levesque E, Saliba F, Benhamida S, Ichaï P, Azoulay D, Adam R, Castaing D, Samuel D. Plasma disappearance rate of indocyanine green: a tool to evaluate early graft outcome after liver transplantation. Liver Transpl. 2009;15:1358–1364. doi: 10.1002/lt.21805. [DOI] [PubMed] [Google Scholar]
- 57.Levesque E, Hoti E, Azoulay D, Adam R, Samuel D, Castaing D, Saliba F. Non-invasive ICG-clearance: a useful tool for the management of hepatic artery thrombosis following liver transplantation. Clin Transplant. 2011;25:297–301. doi: 10.1111/j.1399-0012.2010.01252.x. [DOI] [PubMed] [Google Scholar]
- 58.Olmedilla L, Pérez-Peña JM, Ripoll C, Garutti I, de Diego R, Salcedo M, Jiménez C, Bañares R. Early noninvasive measurement of the indocyanine green plasma disappearance rate accurately predicts early graft dysfunction and mortality after deceased donor liver transplantation. Liver Transpl. 2009;15:1247–1253. doi: 10.1002/lt.21841. [DOI] [PubMed] [Google Scholar]
- 59.Olmedilla L, Lisbona CJ, Pérez-Peña JM, López-Baena JA, Garutti I, Salcedo M, Sanz J, Tisner M, Asencio JM, Fernández-Quero L, et al. Early Measurement of Indocyanine Green Clearance Accurately Predicts Short-Term Outcomes After Liver Transplantation. Transplantation. 2016;100:613–620. doi: 10.1097/TP.0000000000000980. [DOI] [PubMed] [Google Scholar]
- 60.Escorsell À, Mas A, Fernández J, García-Valdecasas JC. Limitations of use of the noninvasive clearance of indocyanine green as a prognostic indicator of graft function in liver transplantation. Transplant Proc. 2012;44:1539–1541. doi: 10.1016/j.transproceed.2012.05.023. [DOI] [PubMed] [Google Scholar]
- 61.Levesque E, Martin E, Dudau D, Lim C, Dhonneur G, Azoulay D. Current use and perspective of indocyanine green clearance in liver diseases. Anaesth Crit Care Pain Med. 2016;35:49–57. doi: 10.1016/j.accpm.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 62.De Gasperi A, Mazza E, Corti A, Zoppi F, Prosperi M, Fantini G, Scaiola A, Colella G, Amici O, Notaro P, et al. Lactate blood levels in the perioperative period of orthotopic liver transplantation. Int J Clin Lab Res. 1997;27:123–128. doi: 10.1007/BF02912446. [DOI] [PubMed] [Google Scholar]
- 63.Kim GY, Bae KS, Noh GJ, Min WK. Estimation of indocyanine green elimination rate constant k and retention rate at 15 min using patient age, weight, bilirubin, and albumin. J Hepatobiliary Pancreat Surg. 2009;16:521–528. doi: 10.1007/s00534-009-0097-3. [DOI] [PubMed] [Google Scholar]
- 64.Lock JF, Schwabauer E, Martus P, Videv N, Pratschke J, Malinowski M, Neuhaus P, Stockmann M. Early diagnosis of primary nonfunction and indication for reoperation after liver transplantation. Liver Transpl. 2010;16:172–180. doi: 10.1002/lt.21973. [DOI] [PubMed] [Google Scholar]
- 65.Jara M, Malinowski M, Lüttgert K, Schott E, Neuhaus P, Stockmann M. Prognostic value of enzymatic liver function for the estimation of short-term survival of liver transplant candidates: a prospective study with the LiMAx test. Transpl Int. 2015;28:52–58. doi: 10.1111/tri.12441. [DOI] [PubMed] [Google Scholar]
- 66.Merle U, Sieg O, Stremmel W, Encke J, Eisenbach C. Sensitivity and specificity of plasma disappearance rate of indocyanine green as a prognostic indicator in acute liver failure. BMC Gastroenterol. 2009;9:91. doi: 10.1186/1471-230X-9-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lock JF, Kotobi AN, Malinowski M, Schulz A, Jara M, Neuhaus P, Stockmann M. Predicting the prognosis in acute liver failure: results from a retrospective pilot study using the LiMAx test. Ann Hepatol. 2013;12:556–562. [PubMed] [Google Scholar]


