Abstract
Background
Evidence guiding adjuvant chemotherapy (AC) use following lobectomy for stage I NSCLC is limited. This study evaluated the impact of AC use and tumor size on outcomes using a large, nationwide cancer database.
Methods
The effect of AC on long-term survival among patients who underwent lobectomy for margin-negative pathologic T1-2N0M0 NSCLC in the National Cancer Database from 2003–2006 was estimated using the Kaplan-Meier method. The specific tumor-size threshold at which AC began providing benefit was estimated with multivariable Cox proportional hazards modeling.
Results
Overall 3,496 of 34,360 (10.2%) patients who met inclusion criteria were treated with AC, though AC use increased over time from 2003, when only 2.7% of patients with tumors <4cm and 6.2% of patients with tumors ≥4 cm received AC. In unadjusted survival analysis AC was associated with a significant 5-year survival benefit both for patients with tumors <4cm (74.3 vs. 66.9%, p<.0001) and ≥4cm in size (64.8 vs. 49.8%, p<.0001). In sub-analyses of patients grouped by strata of 0.5 cm increments in tumor size, AC was associated with a survival advantage for tumor sizes ranging from 3.0–8.5 cm.
Conclusions
Use of AC among patients with stage I NSCLC has increased over time but remains uncommon. The results of this study support current treatment guidelines that recommend AC use after lobectomy for stage I NSCLC tumors larger than 4cm. These results also suggest that AC use is associated with superior survival for patients with tumors ranging from 3 to 8.5cm in diameter.
Keywords: lung cancer, NSCLC, lobectomy, adjuvant therapy, chemotherapy
Introduction
Adjuvant chemotherapy (AC) after resection of stages II-IIIA non-small cell lung cancer (NSCLC) has repeatedly been shown to improve survival.1–6 The National Comprehensive Cancer Network (NCCN) and the American Society of Clinical Oncology (ASCO) therefore both recommend AC for patients with completely resected stage II or IIIA NSCLC.7, 8 However, indications and benefits of AC for patients with earlier stage NSCLC are less clear. Two large randomized trials failed to show a survival benefit associated with AC for early-stage node-negative NSCLC, while a single-institution retrospective study of 119 patients who had lobectomy for stage IB NSCLC found that adjuvant platinum-based chemotherapy was associated with improved survival. 3, 6, 9, 10, 11 The Cancer and Leukemia Group B (CALGB) 9633 trial demonstrated an early survival advantage to AC for T2N0 patients, however this did not persist with longer follow-up.12 A subset analysis of this study did, however, reveal a survival advantage for tumors 4cm in size or larger, and a similar pooled analysis of two clinical trials demonstrated a tumor size-chemotherapy effect.5, 13
The current NCCN guidelines are based on this data and do not recommend AC for patients with completely resected stage IB NSCLC, with the exception of individuals considered to be high-risk for recurrence, including those who have tumors that are 4 cm or larger in size. However, the use of these exploratory and unplanned subgroup analyses from the CALGB 9633 trial to direct patient care and influence guidelines regarding use of AC for completely resected node-negative NSCLC has been questioned.5, 7, 12 In light of current evidence and recommendations, most patients with early-stage NSCLC do not receive adjuvant chemotherapy.14 The purpose of this current study was to use a nationwide cancer database to provide the largest investigation to date evaluating the use of AC following lobectomy for T1-2N0 NSCLC, in order to better understand current practice patterns and to evaluate the impact of tumor size on outcomes.
Methods
This retrospective analysis of patients with pathologic T1-2N0 NSCLC in the National Cancer Data Base (NCDB) was approved by the Duke University Institutional Review Board. The NCDB is a jointly administered effort by the American College of Surgeons Commission on Cancer (CoC) and the American Cancer Society, and collects data from more than 1,500 CoC-approved facilities around the United States. The NCDB is estimated to capture approximately 70% of all new cancer diagnoses annually, and currently contains more than 30 million patient records.
Patients diagnosed with pT1-2N0M0 NSCLC from 2003–2006 were identified for inclusion based on International Classification of Diseases for Oncology, 3rd Edition (ICD-O-3) histology codes for NSCLC, as well as Facility Oncology Registry Data Standards (FORDS) procedure codes for lobectomy. This time period was chosen as patients diagnosed in 2007 and later do not currently have long-term survival data available in the NCDB. Pathological stage data was directly extracted based on American Joint Committee on Cancer (AJCC) 6th edition staging criteria. Tumor size data is recorded as the largest dimension of the diameter of the primary tumor, in millimeters. Patients who received induction therapy and those with missing data regarding the use of AC and/or tumor size were excluded from analysis. Only patients with negative margins following lobectomy were studied, as recommendations guiding the management of patients with margin-positive disease are notably different.
Baseline univariate comparisons of patient characteristics between the cohort of patients who received AC and the cohort of patients treated with surgery alone were made using Pearson’s chi-square test for discrete variables and Student’s t-test for continuous variables. Consistent with existing NCCN guidelines, patients were then grouped according to whether their tumors were less than 4 cm or greater than 4 cm in size. Trends in the use of AC among the two size groups were assessed across the study time period. The effect of adjuvant chemotherapy on long-term survival was estimated for each of the two groups. Patients were then grouped into strata by tumor size in increments of 1 cm, and unadjusted median and 5-year survival rates were calculated by stratum. All unadjusted survival analyses were performed using the Kaplan-Meier product limit estimator. To identify the specific tumor size threshold where AC appeared to begin providing benefit, the study cohort was grouped by strata of increasing 5mm in tumor size, and multivariable Cox proportional hazards models were developed within each strata, adjusting for patient age, sex, race, and Charlson/Deyo comorbidity score.
We made an affirmative decision to control for type I error at the level of all comparisons, and p-values less than 0.05 were considered statistically significant. Missing data were handled with complete-case analysis in light of the substantial completeness of the NCDB data for the study population. All analyses were performed using SAS (version 9.3; SAS Institute Inc., Cary, North Carolina).
Results
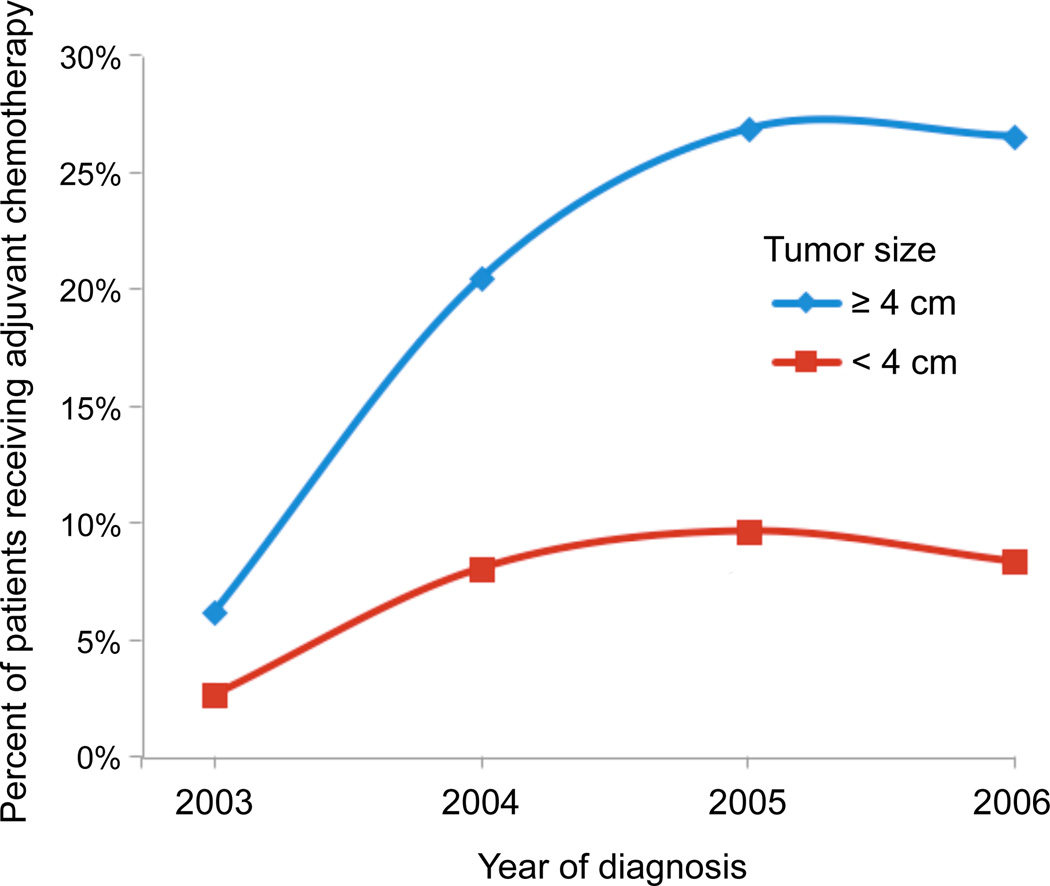
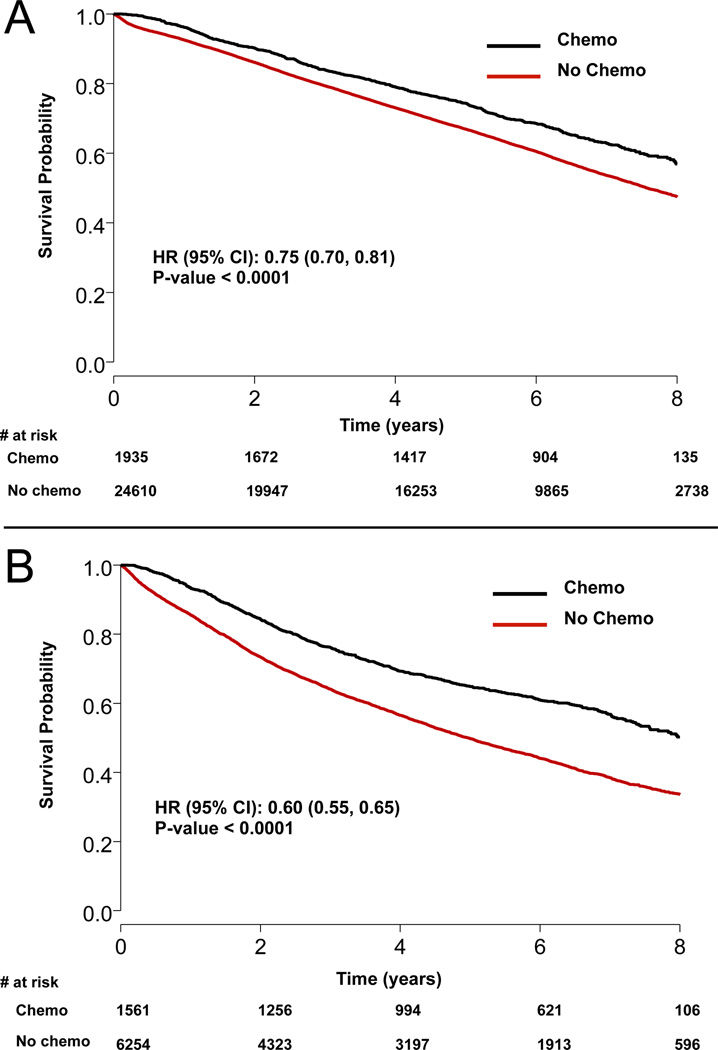
A total of 34,360 patients underwent margin-negative lobectomy for pT1-2N0M0 NSCLC between 2003 and 2006, of which 3,496 (10.2%) were treated with AC while the remaining 30,864 (89.8%) were treated with surgery alone. Patients treated with AC were younger and had slightly lower Charlson comorbidity scores, but had significantly larger tumors and were more likely to have T2 disease (Table 1). Table 2 summarizes survival according to tumor size by 1 cm increments, and demonstrates progressively worse survival with increasing tumor size, ranging from a 75% (95% CI: 72–78%) 5-year survival rate among patients with tumors <1 cm to a rate of 46% (95% CI: 44–49%) for patients with tumors ≥7 cm. Trends over time in the use of AC, grouped according to the currently accepted size threshold of 4 cm, are shown in Figure 1. Use of AC increased for both small (<4 cm) and large (≥4 cm) tumors, and while AC among the smaller tumors increased from 2.7% in 2003 to a peak of 9.7% in 2005, use among patients with tumors ≥4 cm increased from 6.2% in 2003 to a peak of 26.9% in 2005. In unadjusted survival analysis as shown in Table 3 and Figures 2A and 2B, adjuvant chemotherapy was associated with a significant survival benefit both for patients with tumors < 4cm (5-year survival: 74.3 vs. 66.9%, p<.0001) and ≥4 cm in size (5-year survival: 64.8 vs. 49.8%, p<.0001).
Table 1.
Patient and tumor characteristics for patients with margin-negative pT1-2N0 NSCLC, by use of adjuvant chemotherapy
| Adjuvant Chemotherapy (N=3,496) |
No Adjuvant Chemotherapy (N=30,864) |
p-value | |
|---|---|---|---|
| Patient Age | <0.0001 | ||
| Median | 63.0 | 69.0 | |
| Q1, Q3 | 56.0, 70.0 | 62.0, 75.0 | |
| Sex | 0.92 | ||
| Male | 1716 (49.1%) | 15121 (49.0%) | |
| Female | 1780 (50.9%) | 15743 (51.0%) | |
| Race | 0.27 | ||
| White | 3143 (89.9%) | 27889 (90.4%) | |
| Black | 277 (7.9%) | 2237 (7.2%) | |
| Other | 76 (2.2%) | 738 (2.4%) | |
| Charlson/Deyo Score | <0.0001 | ||
| 0 | 1991 (57.0%) | 15799 (51.2%) | |
| 1 | 1169 (33.4%) | 11284 (36.6%) | |
| 2 | 336 (9.6%) | 3781 (12.3%) | |
| AJCC Pathologic T | <0.0001 | ||
| 1 | 714 (20.4%) | 18436 (59.7%) | |
| 2 | 2782 (79.6%) | 12428 (40.3%) | |
| Size of Tumor (cm) | <0.0001 | ||
| Mean (SD) | 4.1 (3.1) | 2.9 (2.5) | |
| Range | (0.1–95.0) | (0.1–93.3) | |
| Tumor Size | <0.0001 | ||
| < 4 cm | 1935 (55.3%) | 24610 (79.7%) | |
| >= 4 cm | 1561 (44.7%) | 6254 (20.3%) |
Table 2.
Survival estimates by tumor size
| Size of Tumor (cm) |
5-year survival % (95% CI) |
Median Survival [yrs] (95% CI) |
|---|---|---|
| < 1 | 75 (72, 78) | 8.8 (8.6, NA) |
| 1–1.9 | 73 (72, 74) | 8.6 (8.4, 8.9) |
| 2–2.9 | 65 (64, 66) | 7.4 (7.2, 7.5) |
| 3–3.9 | 61 (60, 62) | 6.5 (6.4, 6.8) |
| 4–4.9 | 56 (54, 57) | 6.0 (5.7, 6.4) |
| 5–5.9 | 53 (50, 55) | 5.6 (5.1, 6.2) |
| 6–6.9 | 52 (49, 55) | 5.2 (4.8, 5.9) |
| ≥ 7 | 46 (44, 49) | 4.4 (3.9, 4.8) |
Figure 1.
Trends in use of adjuvant chemotherapy by tumor size over study period: 2003–2006.
Table 3.
Survival estimates by tumor size and use of adjuvant chemotherapy
| Tumor size |
Treatment | Median survival, years (95% CI) |
5-year Survival % (95% CI) |
Hazard Ratio [chemo vs. no chemo] (95% CI) |
P value |
|---|---|---|---|---|---|
| < 4 cm | Adjuvant Chemotherapy |
8.7 (8.4, NA) | 74.3% (72.3, 76.3) | 0.75 (0.70, 0.81) | < 0.0001 |
| No Adjuvant Chemotherapy |
7.6 (7.5, 7.7) | 66.9% (66.3, 67.5) | |||
| ≥ 4 cm | Adjuvant Chemotherapy |
8 (7.5, NA) | 64.8% (62.3, 67.2) | 0.6 (0.55, 0.65) | < 0.0001 |
| No Adjuvant Chemotherapy |
5 (4.7, 5.1) | 49.8% (48.5, 51.0) |
Figure 2.
Kaplan-Meier survival curves for A) tumors less than 4 cm and B) tumors ≥ 4 cm.
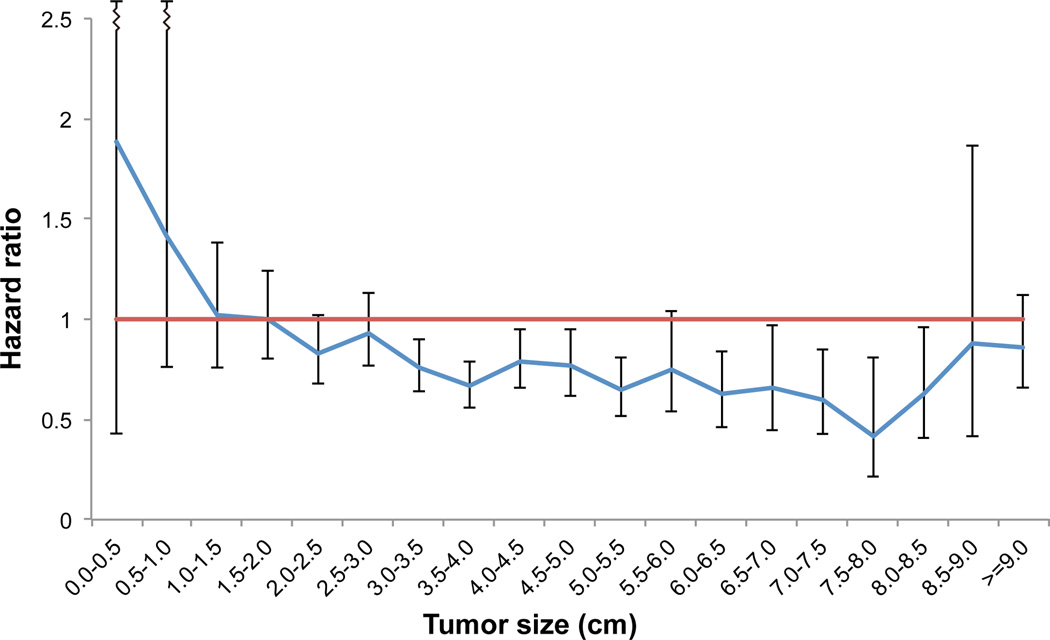
Following grouping into cohorts by tumor size increments of 5 mm and subsequent multivariable Cox proportional hazards modeling, AC was associated with a statistically significant survival advantage for tumors starting at a threshold of 3.0 cm and ranging up to an upper limit of 8.5 cm in size. As shown in Figure 3, patients with small (<3 cm) tumors did not derive a significant adjusted survival benefit from AC, with a trend toward particularly bad outcomes for patients with tumors less than 1 cm who received AC. Similarly, for tumors larger than 8.5 cm, AC did not confer a statistically significant survival advantage following multivariable adjustment.
Figure 3.
Plot of adjusted hazard of death associated with adjuvant chemotherapy after lobectomy for T1-2N0 grouped into strata by tumor size. The hazard ratios were determined from multivariable survival models performed on subsets of patients created by grouping the entire cohort by strata of tumor size in 0.5 cm increments.
Discussion
In this NCDB study of over 34,000 patients treated with lobectomy for pathologic T1-2N0M0 NSCLC in the United States from 2003–2006, we found that approximately 10% of patients were treated postoperatively with AC. Patients who were given AC were younger with fewer comorbidities, but with larger tumors. Survival among patients treated with AC for tumors ranging from 3 to 8.5 cm was significantly better than when chemotherapy was not given (Figure 3). The use of AC for both smaller and larger tumors appeared to increase from 2003–2005, corresponding to the time at which data from the CALGB 9633 study started becoming available and began shaping clinical practice.
Our results are consistent with the subgroup analysis from the prospective randomized CALGB 9633 trial for node-negative NSCLC tumors larger than 4 cm, and are generally in agreement with current NCCN guidelines. Considering some of the concerns for basing clinical care solely on that unplanned subgroup analysis, the current study provides additional evidence to guide treatment and influence guidelines for this clinical situation. However, it should be noted that several other randomized trials have been unable to demonstrate any long-term survival benefit of AC after resection of stage I NSCLC. The International Adjuvant Lung Cancer Trial (IALT) included 1867 patients with surgically-resected lung cancer who were randomly assigned either to cisplatin-based adjuvant chemotherapy or to observation, and reported a statistically significant survival benefit with cisplatin-based adjuvant therapy in patients with completely resected stage I, II, or III NSCLC.2 After 7.5 years of follow-up, however, there were more deaths in the chemotherapy group and the benefit of chemotherapy continued to decrease over time.15 Similar to the CALGB 9366 trial, the JBR.10 and the ANITA trials both compared the effectiveness of adjuvant vinorelbine plus cisplatin versus observation in early-stage NSCLC, and showed benefit for stages II and IIIA patients but not for stage IB patients after long-term follow-up.3, 6, 9 Therefore, while the results of our current study provide a meaningful contribution to the existing literature regarding this unresolved question, they must be interpreted with caution.
Nevertheless, our study also demonstrates that long-term survival for patients treated with current optimal treatment (lobectomy) for early-stage NSCLC remains disappointingly low nationally, with 5-year survival rates of only 75% [95% CI 72% –78%] following lobectomy for even the earliest stage I NSCLC measuring less than 1 cm in size. These survival data suggest that continued investigation is necessary to improve outcomes for patients diagnosed with what should be the most curable situation of NSCLC, as surgery alone may not be adequate in up to a quarter of cases. Indeed, chemotherapy now has a category 2A recommendation as adjuvant treatment for patients with high-risk features, including not only tumors larger than 4 cm but also poorly differentiated tumors, vascular invasion, wedge resection, visceral pleural involvement, and incomplete lymph node sampling (Nx).5, 10 Interestingly, multivariate survival analysis in this current study also shows that AC is significantly associated with improved survival for tumors 3–3.9 cm in size and supports the consideration of AC for smaller tumors in some situations. However, analysis in our study also found that the association of AC with improved survival was no longer statistically significant for tumors bigger than 8.5 cm (Figure 3). While this may simply represent a lack of statistical power due to a relative rarity of such large tumors, there may also be an underlying biological mechanism related to tumors that grow to very large sizes. Therefore the risk of treatment failure with current strategies appears to be much more significant as tumors exceed 8 cm in size, and future trials that develop and study new treatment regimens specifically for very large tumors should be considered.
Although this study showed that the use of AC for larger tumors increased from 6.2% in 2003 to approximately 27% in 2005 and 2006, clearly AC was still used in a minority of patients. This finding is consistent with other studies that have investigated the utilization of AC for NSCLC. Although several recent randomized studies and meta-analyses have demonstrated a survival benefit for AC, guideline adherence following resection has been shown to be only 61%.16 Barriers to the use of AC in non-trial settings likely include opinions of both physicians and patients regarding ability to tolerate chemotherapy and whether the potential benefits of adjuvant therapy outweigh the risks, particularly considering that the benefits of AC are generally relatively modest.4 Counseling patients on the potential benefits for their specific situation can be somewhat difficult, as the phase III studies included a relatively heterogeneous group of stages.2, 3, 6 Previous studies have shown that as many as 26% of patients decline further treatment after surgery.14 It is likely that some patients did not feel the risks of AC were worth the potentially increased chance of long-term survival. The current study provides more accurate estimates of the potential benefits of AC use for patients who have undergone resection of early-stage node-negative NSCLC in a non-trial setting, which can improve the shared decision-making process and better inform treatment recommendations.
In this study, 900 patients who were given AC were aged 70 years or older. While there are not specific guidelines for treatment of NSCLC based on age, counseling elderly patients on the potential benefits of AC may be more difficult compared to younger patients as most randomized studies either excluded older participants or enrolled very limited numbers.2, 3, 6, 17, 18 Even though NSCLC is generally a disease of the elderly and the median age at diagnosis is 70 years, only 9% of patients in the meta-analysis of randomized trials were older than 70 years.19 In that meta-analysis, patients older than 70 were shown to have had a survival benefit from AC similar to younger patients despite receiving lower doses and fewer cycles, having lower performance status, and having more non-lung cancer related causes of death.18 Other retrospective analyses of both randomized study data and registry data have also demonstrated benefits of AC use among patients older than 65 years.20, 21 In our study, AC was an independent predictor of improved survival even when age was considered.
The NCDB does also have some inherent limitations related to the availability of clinical information related to the decision on whether to administer adjuvant chemotherapy after surgery. Even though multivariable adjusted analysis can correct for measured covariates, the NCDB does not contain important clinical variables such as overall performance status, pulmonary function, specific comorbidities and smoking status. AC may have been preferentially selected for patients who did well after surgery or who had better functional status, better pulmonary function, and less significant current and past smoking use, which are all factors that can impact both treatment selection as well as outcomes such as survival. This study has other limitations due to its retrospective nature and reliance on an administrative database in which some data may be missing. Additionally, we were unable to investigate disease-specific survival among our patient cohorts, as this is not currently available in the NCDB.
The use of the NCDB does have the significant strength of being able to investigate a specific cancer sub-stage with high power due to its population-based nature. Any prospective study would be very unlikely to accrue similar numbers of patients as analyzed in this study. In addition, randomized trials generally enroll selected participants with good functional status and a low number of comorbidities, and either exclude or tend to have limited number of older participants.2, 3, 6, 17, 18 The study of patients in a non-trial setting using the NCDB may improve the generalizability of the findings and provide more appropriate expectations to base treatment guidelines on. The results of our current study support current treatment guidelines regarding AC use after lobectomy for stage I NSCLC. Surgeons and medical oncologists should carefully consider the use of AC after lobectomy for node-negative NSCLC tumors larger than 3 cm, and particularly when tumors are larger than 4 cm. However considering the promising but not definitively proven benefit of AC in this setting, the thoracic oncology community should focus on studies that investigate this topic to continue to try to improve outcomes after treatment for early-stage NSCLC.
Acknowledgments
The data used in this study are derived from a de-identified National Cancer Data Base file. The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for the analytic or statistical methodology employed, or the conclusions drawn from these data by the investigators.
Author roles: study conception and design – PJS, MGH, TAD, MFB; data collection and analysis – PJS, LG, XW; Data interpretation – PJS, LG, XW, MGH, TAD, MFB; Drafting of manuscript – PJS, LG; Critical revision of manuscript and final approval: PJS, LG, XW, MGH, TAD, MFB. Guarantors of paper: PJS and MFB.
Funding: This work was supported by the NIH funded Cardiothoracic Surgery Trials Network (M.G.H and M.F.B), 5U01HL088953-05.
Abbreviations
- AC
adjuvant chemotherapy
- NSCLC
non-small cell lung cancer
- FORDS
Facility Oncology Registry Data Standards
- CoC
Commission on Cancer
- ASCO
American Society of Clinical Oncology
- CALGB
Cancer and Leukemia Group B
Footnotes
Disclosures: One of the authors (T.A.D.) serves as a consultant for Scanlan International, Inc.
References
- 1.Arriagada R, Auperin A, Burdett S, et al. Adjuvant chemotherapy, with or without postoperative radiotherapy, in operable non-small-cell lung cancer: two meta-analyses of individual patient data. Lancet. 2010;375:1267–1277. doi: 10.1016/S0140-6736(10)60059-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arriagada R, Bergman B, Dunant A, et al. Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N Engl J Med. 2004;350:351–360. doi: 10.1056/NEJMoa031644. [DOI] [PubMed] [Google Scholar]
- 3.Douillard J-Y, Rosell R, De Lena M, et al. Adjuvant vinorelbine plus cisplatin versus observation in patients with completely resected stage IB-IIIA non-small-cell lung cancer (Adjuvant Navelbine International Trialist Association [ANITA]): a randomised controlled trial. Lancet Oncology. 2006;7:719–727. doi: 10.1016/S1470-2045(06)70804-X. [DOI] [PubMed] [Google Scholar]
- 4.Pignon J-P, Tribodet H, Scagliotti GV, et al. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol. 2008;26:3552–3559. doi: 10.1200/JCO.2007.13.9030. [DOI] [PubMed] [Google Scholar]
- 5.Strauss GM, Herndon JE, Maddaus MA, et al. Adjuvant paclitaxel plus carboplatin compared with observation in stage IB non-small-cell lung cancer: CALGB 9633 with the Cancer and Leukemia Group B Radiation Therapy Oncology Group, and North Central Cancer Treatment Group Study Groups. J Clin Oncol. 2008;26:5043–5051. doi: 10.1200/JCO.2008.16.4855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Winton T, Livingston R, Johnson D, et al. Vinorelbine plus cisplatin vs. observation in resected non-small-cell lung cancer. N Engl J Med. 2005;352:2589–2597. doi: 10.1056/NEJMoa043623. [DOI] [PubMed] [Google Scholar]
- 7.Ettinger DS, Akerley W, Borghaei H, et al. Non-small cell lung cancer. J Natl Compr Canc Netw. 2012;10:1236–1271. doi: 10.6004/jnccn.2012.0130. [DOI] [PubMed] [Google Scholar]
- 8.Pisters KMW, Evans WK, Azzoli CG, et al. Cancer Care Ontario and American Society of Clinical Oncology adjuvant chemotherapy and adjuvant radiation therapy for stages I-IIIA resectable non small-cell lung cancer guideline. J Clin Oncol. 2007;25:5506–5518. doi: 10.1200/JCO.2007.14.1226. [DOI] [PubMed] [Google Scholar]
- 9.Butts CA, Ding K, Seymour L, et al. Randomized phase III trial of vinorelbine plus cisplatin compared with observation in completely resected stage IB and II non-small-cell lung cancer: updated survival analysis of JBR-10. J Clin Oncol. 2010;28:29–34. doi: 10.1200/JCO.2009.24.0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park SY, Lee JG, Kim J, et al. Efficacy of platinum-based adjuvant chemotherapy in T2aN0 stage IB non-small cell lung cancer. J Cardiothorac Surg. 2013;8:151. doi: 10.1186/1749-8090-8-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roselli M, Mariotti S, Ferroni P, et al. Postsurgical chemotherapy in stage IB nonsmall cell lung cancer: Long-term survival in a randomized study. Int J Cancer. 2006;119(4):955–60. doi: 10.1002/ijc.21933. [DOI] [PubMed] [Google Scholar]
- 12.Katz A, Saad ED. CALGB 9633: an underpowered trial with a methodologically questionable conclusion. J Clin Oncol. 2009;27:2300–2301. doi: 10.1200/JCO.2008.21.1565. author reply 2301-2302. [DOI] [PubMed] [Google Scholar]
- 13.Cuffe S, Bourredjem A, Graziano S, et al. A pooled exploratory analysis of the effect of tumor size and KRAS mutations on survival benefit from adjuvant platinum-based chemotherapy in node-negative non-small cell lung cancer. J Thorac Oncol. 2012;7:963–972. doi: 10.1097/JTO.0b013e31824fe9e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zornosa C, Mamet R, Reid M, et al. Utilization of adjuvant therapy among completely resected non-small cell lung cancer (NSCLC) patients in the National Comprehensive Cancer Network (NCCN) Outcomes Database Project. J Clin Oncol. 2010;28:7s. [Google Scholar]
- 15.Arriagada R, Dunant A, Pignon J-P, et al. Long-term results of the International Adjuvant Lung Cancer Trial evaluating adjuvant Cisplatin-based chemotherapy in resected lung cancer. J Clin Oncol. 2010;28:35–42. doi: 10.1200/JCO.2009.23.2272. [DOI] [PubMed] [Google Scholar]
- 16.Wang Z, Askamit I, Tuscher L, et al. Rates of guideline adherence among US community oncologists treating NSCLC. Am J Manag Care. 2013;19:185–192. [PubMed] [Google Scholar]
- 17.Scagliotti GV, Fossati R, Torri V, et al. Randomized study of adjuvant chemotherapy for completely resected stage I, II, or IIIA non-small-cell Lung cancer. J Natl Cancer Inst. 2003;95:1453–1461. doi: 10.1093/jnci/djg059. [DOI] [PubMed] [Google Scholar]
- 18.Waller D, Peake MD, Stephens RJ, et al. Chemotherapy for patients with non-small cell lung cancer: the surgical setting of the Big Lung Trial. Eur J Cardiothorac Surg. 2004;26:173–182. doi: 10.1016/j.ejcts.2004.03.041. [DOI] [PubMed] [Google Scholar]
- 19.Früh M, Rolland E, Pignon J-P, et al. Pooled analysis of the effect of age on adjuvant cisplatin-based chemotherapy for completely resected non-small-cell lung cancer. J Clin Oncol. 2008;26:3573–3581. doi: 10.1200/JCO.2008.16.2727. [DOI] [PubMed] [Google Scholar]
- 20.Pepe C, Hasan B, Winton TL, et al. Adjuvant vinorelbine and cisplatin in elderly patients: National Cancer Institute of Canada and Intergroup Study JBR.10. J Clin Oncol. 2007;25:1553–1561. doi: 10.1200/JCO.2006.09.5570. [DOI] [PubMed] [Google Scholar]
- 21.Wisnivesky JP, Smith CB, Packer S, et al. Survival and risk of adverse events in older patients receiving postoperative adjuvant chemotherapy for resected stages II-IIIA lung cancer: observational cohort study. BMJ. 2011;343:d4013. doi: 10.1136/bmj.d4013. [DOI] [PMC free article] [PubMed] [Google Scholar]