Abstract
We have traditionally viewed ischemic heart disease in a cardiocentric manner: plaques grow in arteries until they block blood flow, causing acute coronary and other ischemic syndromes. Recent research provides new insight into the integrative biology of inflammation as it contributes to ischemic cardiovascular disease. These results have revealed hitherto unsuspected inflammatory signaling networks at work in these disorders that link the brain, autonomic nervous system, bone marrow, and spleen to the atherosclerotic plaque and to the infarcting myocardium. A burgeoning clinical literature indicates that such inflammatory networks—far from a mere laboratory curiosity—operate in our patients and can influence aspects of ischemic cardiovascular disease that determine decisively clinical outcomes. These new findings enlarge the circle of the traditional “cardiovascular continuum” beyond the heart and vessels to include the nervous system, the spleen, and the bone marrow.
Keywords: acute coronary syndromes, myocardial infarction, white blood cells
Leukocyte functions in atherosclerosis
Several decades of accumulated experimental and clinical evidence have brought inflammation to the fore as a common mechanism that links traditional and emerging risk factors to altered behavior of arterial wall cells, and accumulation and activation of leukocytes within arterial lesions (Table 1). Moreover, we appreciate increasingly the contribution of inflammation and leukocytes to many myocardial diseases (1). Although many gaps remain, our knowledge has progressed to the point where we can begin to gain insight into the integrative biology of inflammation as it contributes to ischemic cardiovascular disease. This review summarizes the experimental evidence that has disclosed a hitherto unsuspected inflammatory signaling network at work in these disorders that links the brain, bone marrow, spleen, the atherosclerotic plaque, and the infarcting myocardium. It will also discuss the burgeoning clinical literature suggesting that such inflammatory networks—far from a mere laboratory curiosity—actually operate in our patients and can influence aspects of ischemic cardiovascular disease that determine decisively clinical outcomes.
Table 1.
Some Triggers and Associated Diseases Implicated in Activating Leukocytes in Cardiovascular Diseases
| Low-density lipoprotein (LDL) |
| Triglyceride-rich lipoproteins (TGRL) |
| Apolipoprotein C3 |
| Angiotensin II |
| Chronic autoimmune conditions |
| Rheumatoid arthritis, systemic lupus erythematosus, inflammatory bowel disease, psoriasis, etc. |
| Chronic autoinflammatory conditions |
| For example, gout, familial Mediterranean fever |
| Chronic infections |
| For example, periodontitis, bronchitis, nonhealing skin ulcers |
| Microbial agents and their products (e.g., from gut microbiota) |
| Pathogen–associated molecular patterns (PAMPs), for example, endotoxins, heat shock proteins |
| Visceral obesity and ectopic adipose tissue |
| Diabetes |
| Major tissue injury |
| For example, myocardial infarction, stroke, trauma |
| Dying cell products |
| Oncosis, apoptosis, pyroptosis |
| Sympathetic nervous stimulation |
| Chronic and acute mental stress |
The study of inflammation biology in cardiovascular disease has focused a great deal on protein mediators, such as cytokines and chemokines, or on small molecules, such as the prostaglandins and reactive oxygen or nitrogen species. This review will deal in greater detail with the cellular protagonists in inflammation: leukocytes, the bulwark of host defenses and the sources for many of the protein and lipid mediators implicated in cardiovascular diseases. These cellular citizens not only orchestrate innate immunity, but serve as messengers that link organ systems implicated in novel inflammatory networks revealed in recent investigations.
Our view of the pathogenesis of atherosclerosis has shifted radically in recent decades. Previously viewed as a lipid storage disease, we now recognize that inflammation drives much of the atherogenic process and mechanisms of clinical complications of this disease. Although lipid risk factors (notably low-density lipoprotein [LDL]) likely play a permissive role in human atherogenesis, many other diseases and their associated triggers to inflammation may aggravate atherosclerotic risk (Table 1).
Pioneering pathologists of the 19th century recognized the foam cell as a hallmark of atherosclerotic lesions (2,3). Studies of increasing rigor identified these cells as lipid-laden macrophages. Smooth muscle cells may also accumulate lipid in atheromata. Recent work has suggested fuzzy borders between macrophages and smooth muscle cells, particularly in experimental lesions in mice (4,5). Yet, most agree that the majority of foam cells in plaques derive from mononuclear phagocytes. Many prior reviews have described how blood monocytes, captured by adhesion molecules on the surface of endothelial cells stimulated by proinflammatory cytokines, enter the arterial wall, beckoned by chemokines (6,7). Scavenger receptors regulated, in part, by inflammatory mediators expressed on the surface of plaque macrophages, permit them to accumulate cholesterol, packing their cytoplasm with lipid droplets that yield the characteristic foamy appearance of the plaque macrophage.
Descriptions of leukocyte recruitment to nascent plaques have generally considered monocytes as a homogenous population. We now appreciate a much more interesting and complex picture of the population of mononuclear phagocytes in atherosclerotic plaques. We postulated the heterogeneity of mononuclear phagocytes in the atherosclerotic plaque in the 1990s on the basis of varied expression of scavenger receptors and markers of inflammatory activation, such as Class II major histocompatibility antigens in plaque macrophages (8). Only more recently have we come to appreciate the role of differential accumulation of various macrophage subsets to the artery wall as one mechanism of this heterogeneity (9,10). Moreover, even before the initiation of atherogenesis, the artery wall contains a population of “resident” mononuclear phagocytes, including dendritic cells (11,12).
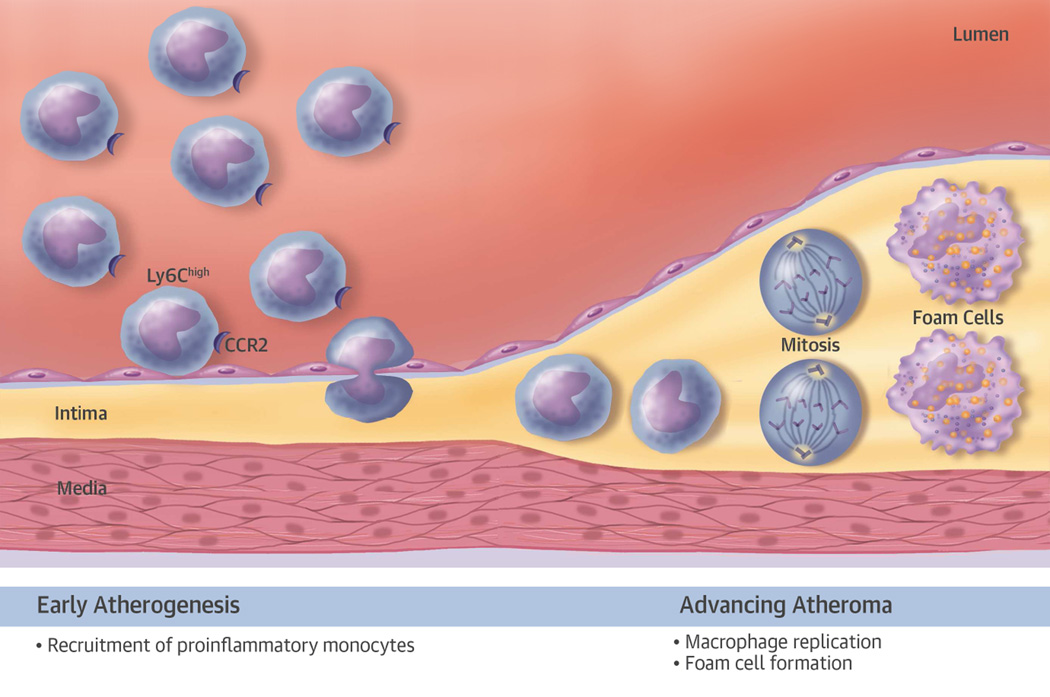
Much of our recent knowledge of the generation of heterogeneity of mononuclear phagocytes during atherogenesis derives from studies on genetically modified mice. Observations in humans have begun to support the relevance of the concepts that have emerged from the study of experimental atheromata to humans. During hypercholesterolemia in mice, a proinflammatory subset of monocytes, marked by high levels of the surface protein Ly6c, accumulate in the peripheral blood. Nascent atherosclerotic plaques selectively recruit these proinflammatory monocytes, primarily mediated by macrophage chemoattractant protein-1 (MCP-1) interacting with its receptor, CC chemokine receptor (CCR)-2 (Figure 1, left side). These cells take up residence in the artery wall where they can elaborate proinflammatory mediators that amplify and sustain inflammation within the early plaque. Mononuclear phagocytes within plaques can replicate in situ.
Figure 1. Heterogeneity of Mononuclear Phagocytes in the Atheroma.
In early atherosclerotic plaques (shown on the left-hand side of the diagram), proinflammatory leukocytes, characterized by high levels of Ly6c and the chemokine receptor CCR2, preferentially adhere to the activated endothelial monolayer overlying the early plaque. The adherent leukocytes enter the intima by diapedesis. Accumulation of mononuclear phagocytes in the early atherosclerotic plaque occurs primarily by recruitment from blood. In the advancing atheroma (right-hand portion of the diagram), monocyte macrophage replication occurs in response to hematopoietic growth factors, some of which may arise from a specialized set of B lymphocytes known as immune response activator B cells (34). The mitotic figures indicate cellular replication. Mononuclear phagocytes resident in the intima ultimately will accumulate cholesteryl ester, forming foam cells, the hallmark of the atherosclerotic lesion. CCR = CC chemokine receptor.
Whereas the accumulation of these cells in early plaques appears to result from heightened recruitment, their replication predominates as a mechanism underlying their increased number in established plaques (13). The replication of mononuclear phagocytes within the atheroma depends on hematopoietic growth factors, including macrophage colony-stimulating factor (M-CSF/CSF-1) (14). We localized M-CSF and granulocyte-macrophage colony-stimulating factor (GM-CSF) to human and experimental atherosclerotic plaques (15–17). We also found that M-CSF stimulated scavenger receptor expression on these cells and promoted their maturation into macrophages (15).
Once taking up residence and multiplying within the artery wall, mononuclear phagocytes have several fates. Retention factors can retard their transit in the plaque and discourage their egress. These retention factors include neural guidance factors, such as netrin-1 and the leukocyte adhesion molecule vascular cell adhesion molecule-1 (VCAM-1) (18–21). Mononuclear phagocytes can eventually leave the plaque. Although the mechanisms remain controversial, lymph channels provide one apparent portal for egress of these cells (22,23). Some experimental evidence suggests that elevating concentrations of high-density lipoprotein (HDL) or lowering the concentration of low-density lipoprotein (LDL) can promote the efflux of monocytes from plaques (23). In mice, augmented expression of CCR-7 on leukocytes and reduced retention mediators appear to favor monocyte emigration under conditions of lipid lowering (23,24).
Death of mononuclear phagocytes also occurs in atheromata (25). These cells can perish due to “accidental cell death” (oncosis, often erroneously referred to as “necrosis”) (26). They also often die by apoptosis (25,27). Dying macrophages can release microparticles that contain the procoagulant tissue factor. They also elaborate proinflammatory cytokines, such as interleukin-1-alpha (IL-1α). Dying macrophages might also provide a nidus for microcalcifications. Such calcific foci may lead to biomechanical inhomogeneities postulated to favor plaque rupture (28).
Other mononuclear phagocytes usually rapidly engulf dead or dying cells and ferry them from the plaque, a process known as efferocytosis. We noted in the 1990s that, on the basis of arithmetic considerations, clearance of dead cells from plaques appeared impaired (25). Tabas et al. popularized the concept of defective efferocytosis of phagocytes within atheromatous plaques (5,29). They studied the underlying mechanisms that regulate efferocytosis within the plaque, which likely contribute to the formation of a lipid-rich “necrotic” core (30).
Some of the monocyte/macrophages within plaques assume a set of functions that may mute inflammation and promote tissue repair. These cells express low levels of Ly6c and elaborate IL-10, vascular endothelial growth factor (VEGF), a promoter of angiogenesis, and transforming growth factor beta (TGF-β), a stimulator of fibrosis (Figure 2) (31).
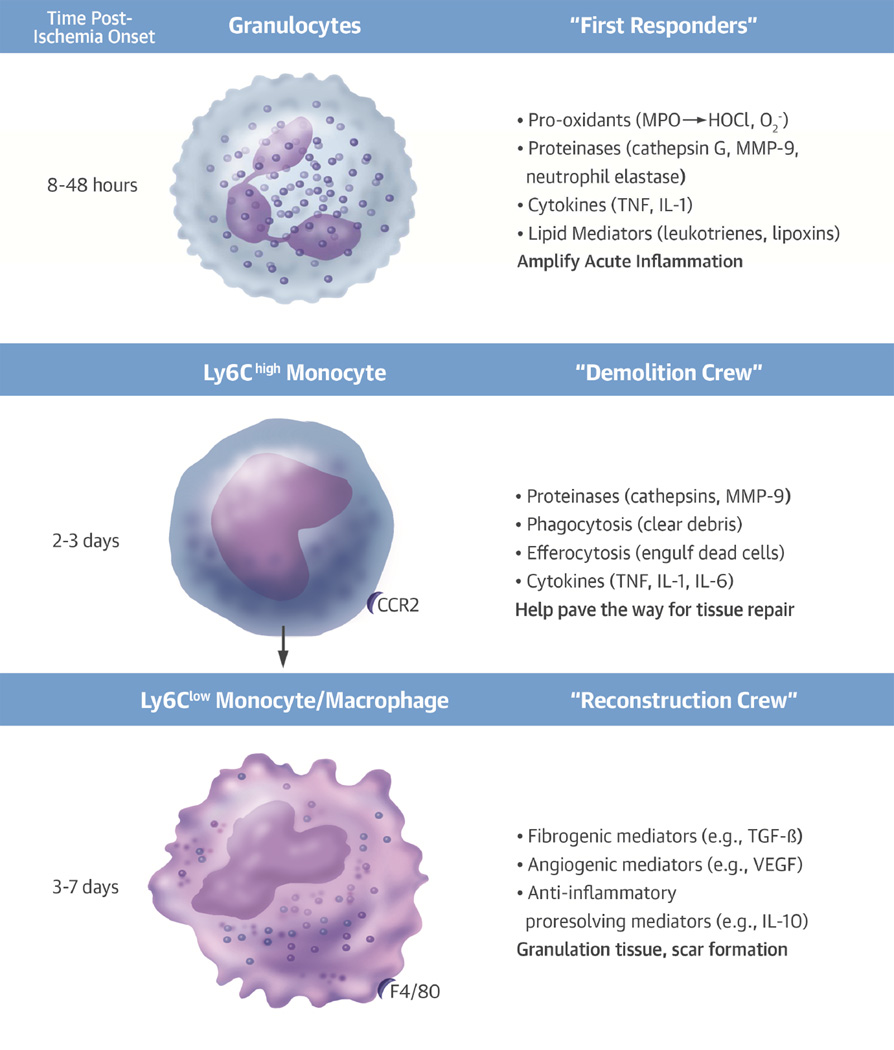
Figure 2. Temporal Sequence and Functions of Leukocytes Localizing in the Infarcting Myocardium.
Granulocytes arrive in the ischemic area within hours of the onset of infarction. The granulocytes, “first responders” to ischemic injury, elaborate pro-oxidants, proteinases, cytokines, and lipid mediators among other substances that amplify the acute inflammatory response. Examples of the mediators are shown in parentheses. In the second and third day following onset of myocardial ischemia, proinflammatory monocytes bearing high levels of the marker Ly6c and the chemokine receptor CCR2 accumulate in the infarcting tissue. These cells perform cleanup functions by secreting proteinases that can digest debris from dead or dying cells. They can phagocytose the debris and engulf dead cells by efferocytosis. These “demolition” functions can help pave the way for tissue repair. From days 4 through 7, monocytes, macrophages that exhibit reparative functions, assume predominance. In mice, these cells arise from the proinflammatory monocytes in response to transcriptional control by Nr4a1. These reparative monocytes elaborate TGF-β, which stimulates the collagen synthesis necessary for scar formation and healthy healing of the ischemic area. Angiogenic mediators, such as VEGF, stimulate microvessels characteristic of granulation tissue in the healing myocardium. These reparative monocytes can also elaborate anti-inflammatory cytokines, such as IL-10, that can quell the inflammatory response after clearance of the acutely injured cells and foster formation of a fibrous scar. IL = interleukin; MMP = matrix metalloproteinase; TGF = transforming growth factor; VEGF = vascular endothelial growth factor.
The atheroma harbors other leukocytes, in addition to mononuclear phagocytes. T lymphocytes, although less numerous, likely have important regulatory functions instructing macrophages (6,32,33). These adaptive immune cells also display considerable functional heterogeneity. Certain T lymphocytes (Th1 cells) primarily secrete proinflammatory cytokines, such as interferon gamma (IFN-γ), that promote atherosclerosis and activate inflammatory functions of macrophages. A specialized subset of adaptive immune cells, innate response activator (IRA) B cells, can promote Th1 polarization (34). Another T lymphocyte subclass known as Th2 lymphocytes can mute inflammation by secreting anti-inflammatory cytokines, such as IL-10. A distinct T cell subset, regulatory T cells (Treg), can suppress inflammation and promote fibrogenesis by elaborating TGF-β. Controversy surrounds the role in atherogenesis of another T cell subtype, Th17, generally considered proinflammatory.
B lymphocytes may also modulate atherogenesis (34,35). Natural antibodies secreted by B1 lymphocytes appear to protect against atherosclerosis. Many of these antibodies recognize oxidized lipoproteins thought to accumulate in atheromata and associate with dying cells. In contrast, B2 lymphocytes appear to aggravate atherosclerosis, for example, by producing B cell activating factor (BAFF) (36,37). Other leukocyte classes implicated in atherogenesis include mast cells, a cell type that localizes in the normal arterial adventitia (38–40). Cytokines produced by mast cells, such as IL-6 and IFN-γ may aggravate atherogenesis (39). The role of mast cells in human atherogenesis requires further study (41).
The picture that emerges from contemporary studies of leukocytes in atherogenesis highlights a tug of war between proinflammatory subsets of each leukocyte class and their less inflammatory or “reparative” counterparts. This constant competition can contribute to the chronicity of atherosclerotic plaques, a process that progresses over many decades. Not only does the balance between these countervailing forces vary in time during the life of the plaque, but also in space. Different plaques in the same circulatory bed or in the same person may exhibit different states of inflammatory activation, in part attributable to distinct balances between pro- and anti-inflammatory subsets of leukocytes. Recent reviews have considered the manner in which mononuclear phagocytes and crosstalk with adaptive immune cells may exert functions that precipitate lesion complications, such as plaque rupture and thrombosis (42).
Leukocyte functions in Acute Myocardial Infarction
Acute coronary syndromes bring patients with atherosclerosis to the cardiologist’s attention most dramatically. Leukocytes contribute decisively to acute ischemic injury of the myocardium. Pathologists have long recognized the participation of successive waves of different leukocyte classes in the process of myocardial infarction (Figure 2) (43). An initial wave of acute inflammatory cells, the polymorphonuclear leukocytes, arrives within minutes and dominates the leukocyte population in the infarcting myocardium during the first day or so following the onset of ischemia. Mononuclear phagocytes also begin to accumulate within the first hours of ischemia, and predominate by days 3 through 5 in the leukocytes present in infarcting myocardium.
Recent experimental work has resolved the population of mononuclear phagocytes in the plaque into an initial proinflammatory subset, followed some days later by a second wave of less inflammatory monocytes (Figure 2) (44). The initial proinflammatory population of monocytes arises by recruitment from the bone marrow and from a preformed pool located in the spleen in mice (45). This recruitment depends in part on angiotensin II, an observation that may underlie the salutary effect of interruption of angiotensin action on remodeling of the infarcting myocardium (46). The pivot to less inflammatory phagocytes in the latter phase appears to result from a shift in the programming of the mononuclear cells, rather than recruitment of less inflammatory monocytes. A transcription factor, Nr4A1, modulates gene expression towards a reparative set of functions, although this transition doubtless involves other regulatory mechanisms (47).
The proinflammatory monocytes comprise a “demolition crew” with highly active phagocytosis, elaboration of proteolytic enzymes, and proinflammatory cytokines, that participate in clearing of the debris of dead and dying cells in the infarct and paving the way for reconstruction (Central Illustration). The reparative monocytes elaborate angiogenic factors, such as VEGF and the fibrogenic mediator TGF-β, which promote formation of granulation tissue, a provisional matrix, the scaffold for eventual repair of the infarcted myocardium and replacement with fibrotic scar.
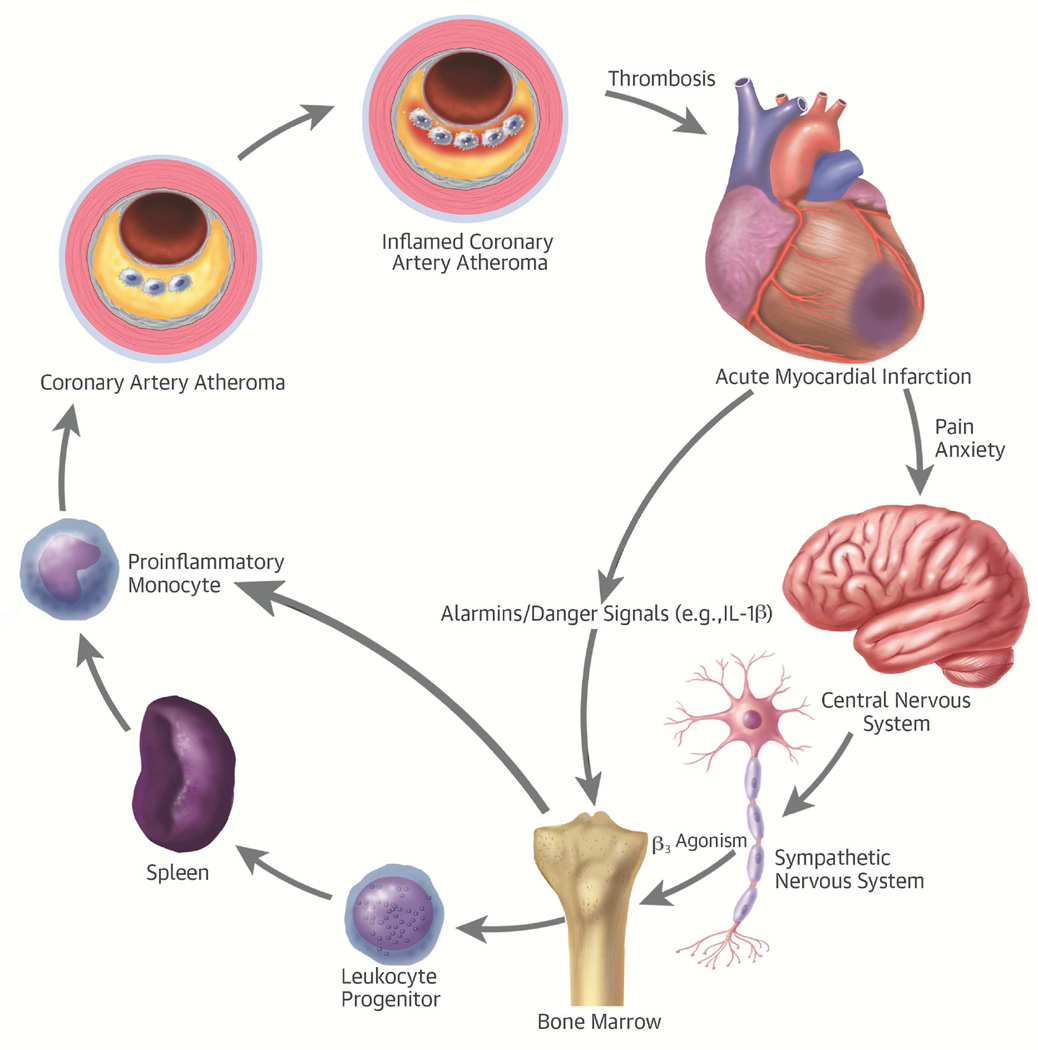
Central Illustration. Leukocytes Link Local and Systemic Inflammation in Ischemic Cardiovascular Disease: The integrative pathophysiology of inflammation in ischemic heart disease.
The stress of acute myocardial infarction produces an “echo” in atherosclerotic plaques. Acute myocardial infarction causes pain and anxiety that triggers sympathetic outflow from the central nervous system. β-3-adrenergic stimulation mobilizes leukocyte progenitors from their bone marrow niche. These progenitor cells can migrate to the spleen, where they can multiply in response to hematopoietic growth factors. The proinflammatory monocytes then leave the spleen and enter the atherosclerotic plaque, where they promote inflammation that can render a plaque more likely to provoke thrombosis and hence acute myocardial infarction. IL = interleukin.
The sequencing of leukocytes in the atheroma has profound functional consequences for healing of the infarct (48). Under hypercholesterolemic conditions, an excess of proinflammatory monocytes in blood impairs the healing of infarcts (49). Interference with inflammatory functions of monocytes by therapeutic small interfering ribonucleic acid (siRNA) silencing can improve the function of the infarcted ventricle. Interference with CCR2 promotes repair of the infarcted ventricle and yields improvement in ventricle function following coronary ligation (50). Silencing of the transcription factor IRF-5 also reprograms the mononuclear phagocytes in the infarct and improves healing (51).
The preformed pool in the spleen of proinflammatory monocytes undergoes replenishment due to “emergency” extramedullary hematopoiesis (Central Illustration) (52). Recruitment of precursor cells for myelocytic lineage leukocytes furnishes the precursors of leukocytes that multiply in the spleen. The proinflammatory monocytes in the spleen lie in wait for mobilization to sites of tissue injury, such the acutely ischemic myocardium.
These various observations have opened up new vistas into the mechanisms by which inflammatory cells can “clean up” damage of the ischemically damaged myocardium and modulate the healing process. Healthy healing of the ventricle can forestall adverse remodeling of the infarcted left ventricle and preserve ventricular function. These insights provide the opportunity of tuning the function of mononuclear phagocytes during myocardial infarction in a manner that can lessen the likelihood of ischemic cardiomyopathy as a critically important long-term consequence of an ischemic insult to the myocardium.
Integrative Pathophysiology of Inflammation in Ischemic Heart Disease
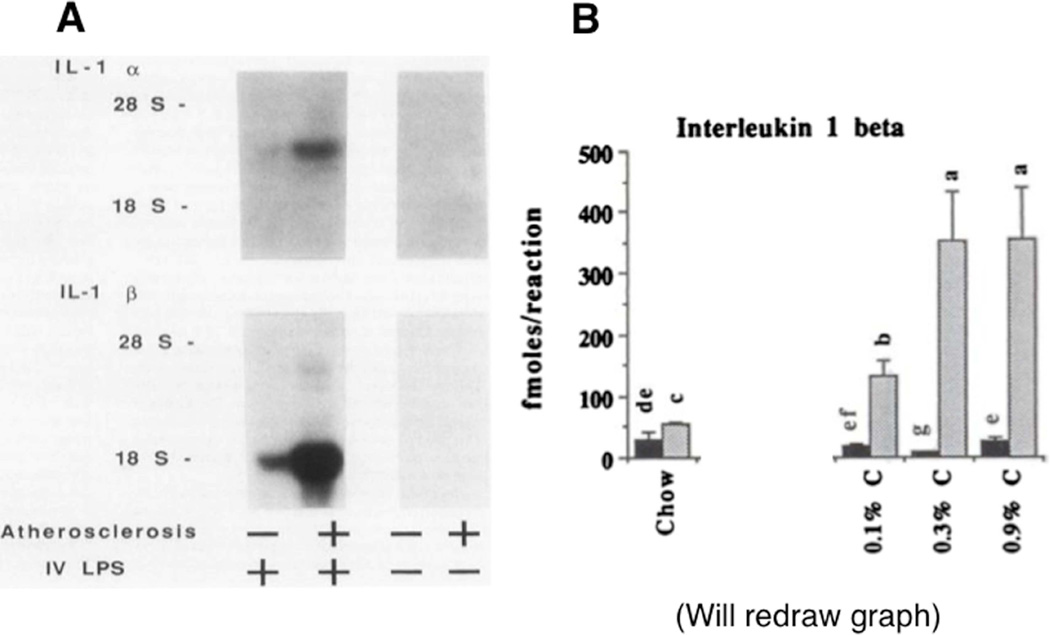
The foregoing sections have emphasized the mechanisms of local inflammatory responses mediated by leukocyte subclasses in the atherosclerotic plaque itself and in the myocardium. Recent work has also shed new light on crosstalk between inflammation in various regions and systemic inflammatory responses. In the early days of exploring the molecular bases of inflammatory signaling in atherosclerosis, we proposed the hypothesis that systemic inflammation could elicit an “echo” in the prepared soil of the atherosclerotic plaque. Soon after its purification and cloning, we found that the prototypical proinflammatory cytokine, IL-1, could be induced in the artery wall by systemic administration of Gram-negative bacterial endotoxin (lipopolysaccharide) (Figure 3A) (53). In the late 1980s, we chose this stimulus as a powerful proinflammatory trigger for activation of inflammatory functions. We further found that rabbit arteries affected by atherosclerosis displayed an accentuated response to intravenous endotoxin, as gauged by a substantial overproduction of IL-1 isoforms (53). Indeed, arteries with greater degrees of atherosclerosis showed increasing amounts of proinflammatory cytokine expression (Figure 3B) (54).
Figure 3. An “Echo” of Systemic Inflammation in the Atheroma.
(A) The left-hand panel shows northern blots for IL-1α-(top) and IL-1β (bottom) in the aortas of nonatherosclerotic and atherosclerotic animals without or with intravenous administration of Gram-negative bacterial endotoxin (lipopolysaccharide [LPS]). Note the striking increase in mRNA encoding both isoforms of IL-1 in the atherosclerotic artery following LPS administration. (B) mRNA concentrations measured by reverse transcriptase-polymerase chain reaction in extracts of the aortas of rabbits that consumed a chow diet or graded increases in concentrations of dietary cholesterol from 0.1% to 0.9 %. Note the marked increase in IL-1β mRNA concentrations in the animals treated with intravenous endotoxin for 1 h (gray bars) versus control. These early experiments illustrate the principle that the resting atheroma can undergo inflammatory activation in response to a systemic proinflammatory stimulus. LPS = lipopolysaccharide; mRNA = messenger ribonucleic acid; RNA = ribonucleic acid. Other abbreviations as in Figure 2.
At the time, we considered bacterial endotoxin injection an experimental artifice that we employed to explore the hypothesis that a systemic inflammatory stimulus could evoke a local inflammatory response. Yet, with greater appreciation of the importance of the intestinal microbiome and potential defects in the barrier function of the intestinal epithelium, we now recognize that low-level leakage of bacterial products may pertain to human pathophysiology much more than we appreciated when we performed these early experiments (Table 1) (55). When we considered the mechanisms by which infectious agents might promote atherogenesis, we hypothesized that, beyond direct infection within the atheroma, local responses to products of microbial invaders could augment local inflammation of the plaque, another instance of an “echo” phenomenon linking systemic inflammation to enhanced local responses at the level of the atherosclerotic lesion (56). Beyond infectious stimuli for leukocyte activation in the context of cardiovascular disease, a variety of autoimmune and autoinflammatory diseases likely trigger innate immune activation (Table 1). Indeed, patients with rheumatoid arthritis, systemic lupus erythematosus, and other autoimmune diseases have elevated cardiovascular risk and likely accelerated atherogenesis, as recently reviewed (57).
Recent work has considerably expanded this notion by exploring the hypothesis that acute local inflammation in the myocardium could augment local inflammation in atherosclerotic plaques. Indeed, after coronary artery ligation, atherosclerotic lesions in mice show augmented protease activity (58). Thinning of the fibrous cap and increase in the lipid core of pre-existing atherosclerotic plaques also follows coronary ligation. These features of plaques associate with a greater propensity to rupture and provoke thrombotic events. These experimental observations may explain, in part, the high rate of early recurrence of ischemic events in patients following an ACS.
An increase in recruitment of leukocytes to pre-existing atherosclerotic plaque occurs post-coronary ligation in mice. The augmented trafficking of leukocytes to the plaque following acute myocardial injury depends on recruitment of progenitor cells from the bone marrow, a precursor to extramedullary hematopoiesis and ongoing leukocyte accumulation with the atherosclerotic lesion itself. Acute myocardial injury evokes a CCR2-dependent mobilization of precursor stem cells from the bone marrow of mice (59). IL-1β participates in the augmented leukopoiesis following experimental myocardial infarction (60). The activation of bone marrow progenitor cells depends on β3-adrenergic stimulation, linking the sympathetic nervous system to the systemic and local inflammatory response in the plaque following myocardial infarction (Central Illustration) (58). The pain and stress of the acute infarction promote local catecholamine synthesis in the bone marrow, as well as the likely systemic release of β3-adrenergic stimulants. These observations support the operation of critical links between pain perception and stress in the central nervous system, adrenergic signaling through the sympathetic nervous system, release of progenitor cells and leukocytes from the bone marrow, and their trafficking to sites of extramedullary hematopoiesis or local inflammation, respectively (Central Illustration). These observations provide a mechanistic explanation for the oft-invoked link between mental stress, a hyperadrenergic state, and the complications of atherosclerosis. Beyond the stress due to myocardial infarction, other forms of chronic mental stress can activate these pathways that accentuate local inflammation. Mice exposed to chronic psychosocial stress show similar activation of myeloid progenitor cells and heightened inflammation in the plaque (61).
Sites of tissue injury and ischemic damage beyond the myocardium can elicit an “echo” at the level of the atherosclerotic plaque and unleash a remote inflammatory response. For example, cerebral ischemia-reperfusion injury, produced in mice by transient middle cerebral artery occlusion, can activate both medullary and splenic myelopoiesis, and augment the leukocyte number and population of proinflammatory Ly6Chigh monocytes in atheromata (58,62).
Clinical Translation of Recent Advances in the Inflammation Biology of Atherosclerosis and Myocardial Infarction
Astute clinical observers have long noted that fever and leukocytosis, cardinal signs of inflammation, accompany acute myocardial infarction. Samuel A. Levine, a pioneer of contemporary cardiology, stated, “the extent of fever and leukocytosis probably depends on the amount of infarcted cardiac tissue involved.” (63). In 1929, he further stated, “infarcted tissue…probably liberates toxic products that produce leukocytosis and fever…The leukocyte count is apt to run hand in hand with the fever…The presence of a leukocytosis is one of the most constant findings in coronary thrombosis.” Today we have a much better mechanistic understanding of the pathophysiological mechanisms that produce fever and leukocytosis. Release of IL-1β from proinflammatory mononuclear phagocytes recruited to the infarct furnishes one of the “toxic products” hypothesized by Levine. Dying leukocytes release IL-1α, the other isoform of IL-1. IL-1, previously known as an “endogenous pyrogen,” provides a major stimulus for fever and can augment the production of hematopoietic growth factors that stimulate leukopoiesis, both in the bone marrow and at extramedullary sites (60).
Epidemiological observational studies have long shown greater risk of coronary events in people with higher circulating leukocyte counts at baseline. Increasing clinical data support the importance of mononuclear phagocyte heterogeneity in ischemic heart disease and atherosclerosis. Subjects followed in the Malmö cohort who had the highest tertile of CD14++ CD16− monocytes had the lowest event-free survival during a 17-year follow-up period (64). Other studies have shown relationships between metrics of atherosclerosis and risk of cardiovascular events with the presence of circulating proinflammatory monocytes (65,66). Supporting the involvement of adrenergic signaling in leukocyte biology in ischemic heart disease, patients undergoing ACS who were nonrandomly allocated to beta-blocker use before the ACS had significantly lower total leukocyte and monocyte counts than those who had never used beta-blockers (58). Autopsy studies in subjects who succumbed during acute myocardial infarction show an increased number of proliferating splenic myeloid progenitor cells in their parenchyma compared with spleens of people dying noncardiac deaths (58).
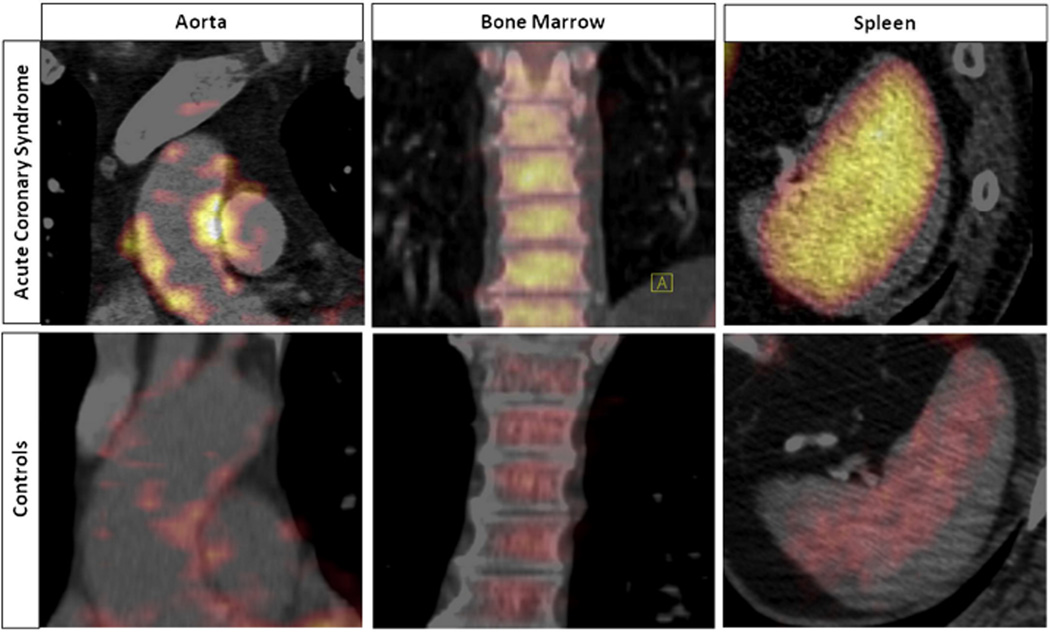
Recent imaging studies underscore the clinical translation of the “splenocardiac” axis revealed by mouse experiments. Patients in the throes of an ACS show increased 18F-fluorodeoxyglucose (18F-FdG) uptake in the bone marrow and spleen compared with those having 18F-FdG-positron emission scans in other clinical circumstances (67). The increased metabolic activity in the spleens of ACS patients, as evidenced by augmented 18F-FdG uptake, correlates with clinical outcomes (Figure 4) (67). Moreover, in medical residents rotating between intensive care unit duty and time off, psychological stress (as measured by formal psychometric testing) increases during call periods in conjunction with an increase in peripheral leukocytosis (61). These clinical observations endorse the translatability of recent findings regarding links between stress and local and systemic inflammation in ischemic heart disease.
Figure 4. Activation of Glucose Uptake in the Arterial Wall, Spleen, and Bone Marrow in Patients With Acute Coronary Syndromes.
18F-fluorodeoxyglucose (18F-FdG) uptake increased significantly in the arterial wall (aorta), bone marrow, and spleen in patients with acute coronary syndrome (ACS) versus control subjects. Reprinted from Emami et al. (67).
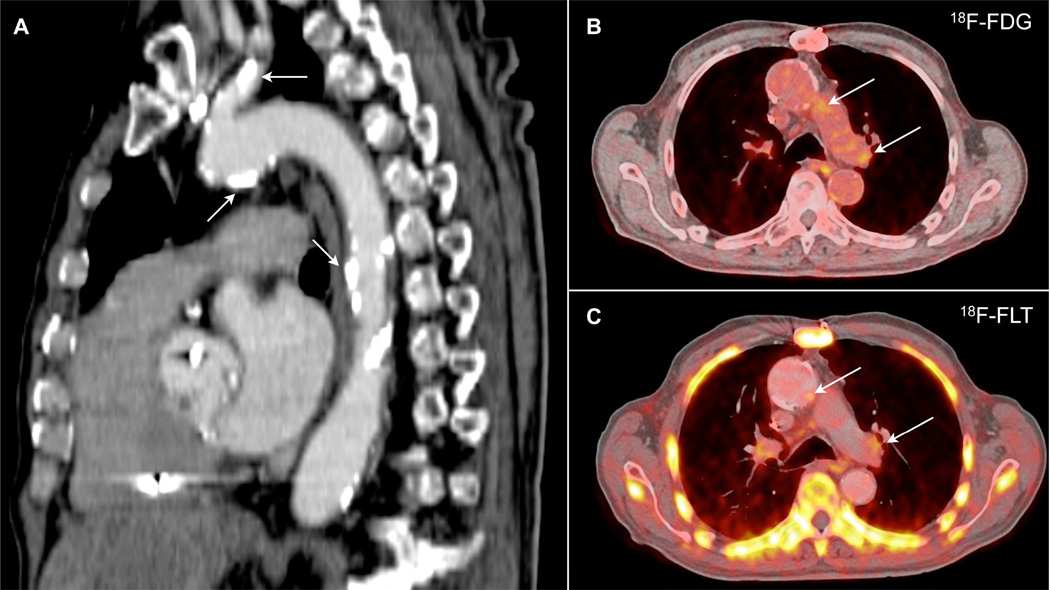
Emerging imaging modalities promise to foster further translational extension of experimental work on leukocytes and inflammation biology in cardiovascular disease. A variety of molecular imaging approaches including near infrared fluorescence, magnetic resonance, computed tomographic, ultrasound, and radionuclide targeted probes have undergone preclinical validation, as recently reviewed (68–74). Despite substantial barriers to translation to humans, initial clinical studies imaging leukocyte functions beyond glucose transport provide a proof of principle that support the continued development of molecular imaging tools to study inflammation. A recent study used 18F-fluorothymidine (18F-FLT) as a probe to visualize cellular proliferation in experimental and human atherosclerosis (75). This approach permitted visualization of proliferation of macrophages in aortic lesions and of leukocyte progenitors in the spleen and bone marrow in mice. This proliferation marker also produced signals in human carotid artery and aortic atherosclerotic lesions, consistent with visualizing leukocyte replication noninvasively (Figure 5). Thus, we look forward to continued translation to human patients of recent advances in leukocyte involvement in cardiovascular diseases that have emerged from experimental studies. Although molecular imaging will not likely prove clinically or cost-effective for routine screening, it promises to provide a new pathway for clinical research, and may prove useful for developing and evaluating normal therapies that target inflammation and leukocyte functions in cardiovascular diseases.
Figure 5. Regions of the Atherosclerotic Human Aorta Accumulate Imaging Reporters of Glucose Uptake (18F-FdG) and Cell Proliferation (18F-FLT).
(A) Sagittal image disclosing extensive calcification in a human aorta and carotid artery (arrows). (B) 18F-fluorodeoxyglucose (18F-FdG) and (C) 18F-fluorothymidine (18F-FLT) images show accumulation of these positron emission tomography (PET) tracers in the aortic arch (arrows). Reprinted from Ye et al. (75).
Conclusion and Clinical Consequences
The traditional “Cardiovascular Continuum” formulated by Dzau and Braunwald focused on the cycle that links arteries and the heart (76). The recent research recounted here has enlarged the circle of this continuum beyond the myocardium and blood vessels to embrace the nervous system, the spleen, and the bone marrow (Central Illustration).
Reductionist research conducted in experimental laboratories over the last decades has opened new windows into truly integrative pathophysiology. Study of fundamental inflammation biology has revealed these heretofore unsuspected mechanistic links between a variety of organ systems intertwined to coordinate the organism’s response to stress and injury. The multiple inflammatory mediators, leukocyte subclasses, and their interplay provide a panoply of novel potential targets for fine-tuning the inflammatory response during acute myocardial infarction and the more chronic smoldering inflammation underway in the atherosclerotic plaque.
The results of this recent research have uncovered new mechanisms by which proven pharmacological interventions may ameliorate the consequences of acute myocardial infarction, for example, β-adrenergic blocking agents and angiotensin-converting enzyme inhibitors. These results offer perspectives on how tipping the balance between proinflammatory leukocytes and their reparative counterparts might augment healthy healing of the infarcted myocardium and quell the echo of inflammation in the plaque in response to acute insults, such as myocardial infarction. Animal experiments have furnished proof of principle of these concepts in laboratory studies. These laboratory observations pave the way for clinical studies that aim to alter inflammatory responses mediated by leukocyte subsets to improve patient outcomes following ACS and forestall the development of chronic ischemic cardiomyopathy, a challenging and growing burden in the contemporary era of cardiology and a major contributor to morbidity and strain on the health care system.
Acknowledgments
Dr. Libby is involved in consulting clinical trials and receives sponsored research support from Novartis. Dr. Libby receives funding from the National Heart, Lung, and Blood Institute (R01 HL080472).
Abbreviations
- CCR
CC chemokine receptor
- FdG
fluorodeoxyglucose
- FLT
fluorothymidine
- IFN-γ
interferon gamma
- IL
interleukin
- LDL
low-density lipoprotein
- M-CSF/CSF-1
macrophage colony stimulating factor
- TGF-β
transforming growth factor beta
- Th1 cells
T lymphocytes
- VEGF
vascular endothelial growth factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: The other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
References
- 1.Libby P. History of discovery: inflammation in atherosclerosis. Arterioscler Thromb Vasc Biol. 2012;32:2045–2051. doi: 10.1161/ATVBAHA.108.179705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Virchow R. Cellular Pathology. London, UK: John Churchill; 1858. [Google Scholar]
- 3.Virchow R. Cellular pathology. As based upon physiological and pathological histology. Lecture XVI--Atheromatous affection of arteries. 1858. Nutr Rev. 1989;47:23–25. doi: 10.1111/j.1753-4887.1989.tb02747.x. [DOI] [PubMed] [Google Scholar]
- 4.Nguyen AT, Gomez D, Bell RD, et al. Smooth muscle cell plasticity: fact or fiction? Circ Res. 2013;112:17–22. doi: 10.1161/CIRCRESAHA.112.281048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tabas I, Garcia-Cardeña G, Owens GK. Recent insights into the cellular biology of atherosclerosis. J Cell Biol. 2015;209:13–22. doi: 10.1083/jcb.201412052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Puri R, Nissen SE, Shao M, et al. Coronary atheroma volume and cardiovascular events during maximally intensive statin therapy. Eur Heart J. 2013;34:3182–3190. doi: 10.1093/eurheartj/eht260. [DOI] [PubMed] [Google Scholar]
- 7.Gerhardt T, Ley K. Monocyte trafficking across the vessel wall. Cardiovasc Res. 2015;107:321–330. doi: 10.1093/cvr/cvv147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Libby P, Hansson GK, Pober JS. Atherogenesis and Inflammation. In: Chien KR, Breslow JL, Leiden JM, editors. Molecular Basis of Heart Disease: A Companion to Braunwald’s Heart Disease. Philadelphia, PA: W.B. Saunders Company; 1998. pp. 349–366. [Google Scholar]
- 9.Swirski FK, Libby P, Aikawa E, et al. Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J Clin Invest. 2007;117:195–205. doi: 10.1172/JCI29950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tacke F, Alvarez D, Kaplan TJ, et al. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J Clin Invest. 2007;117:185–194. doi: 10.1172/JCI28549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cybulsky MI, Jongstra-Bilen J. Resident intimal dendritic cells and the initiation of atherosclerosis. Curr Opin Lipidol. 2010;21:397–403. doi: 10.1097/MOL.0b013e32833ded96. [DOI] [PubMed] [Google Scholar]
- 12.Ensan S, Li A, Degousee N, et al. The origin and maintenance of resident arterial macrophages. Nat Immunol. 2015 in press. [Google Scholar]
- 13.Robbins CS, Hilgendorf I, Weber GF, et al. Local proliferation dominates lesional macrophage accumulation in atherosclerosis. Nat Med. 2013;19:1166–1172. doi: 10.1038/nm.3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Swirski FK, Hilgendorf I, Robbins CS. From proliferation to proliferation: monocyte lineage comes full circle. Semin Immunopathol. 2014;36:137–148. doi: 10.1007/s00281-013-0409-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clinton S, Underwood R, Sherman M, et al. Macrophage-colony stimulating factor gene expression in vascular cells and in experimental and human atherosclerosis. Am J Pathol. 1992;140:301–316. [PMC free article] [PubMed] [Google Scholar]
- 16.Rosenfeld M, Ylä-Herttuala S, Lipton B, et al. Macrophage colony-stimulating factor mRNA and protein in atherosclerotic lesions of rabbits and humans. Am J Pathol. 1992;140:291–300. [PMC free article] [PubMed] [Google Scholar]
- 17.Sugiyama S, Okada Y, Sukhova GK, et al. Macrophage myeloperoxidase regulation by granulocyte macrophage colony-stimulating factor in human atherosclerosis and implications in acute coronary syndromes. Am J Pathol. 2001;158:879–891. doi: 10.1016/S0002-9440(10)64036-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Gils JM, Derby MC, Fernandes LR, et al. The neuroimmune guidance cue netrin-1 promotes atherosclerosis by inhibiting the emigration of macrophages from plaques. Nat Immunol. 2012;13:136–143. doi: 10.1038/ni.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wanschel A, Seibert T, Hewing B, et al. Neuroimmune guidance cue Semaphorin 3E is expressed in atherosclerotic plaques and regulates macrophage retention. Arterioscler Thromb Vasc Biol. 2013;33:886–893. doi: 10.1161/ATVBAHA.112.300941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Swirski FK, Nahrendorf M, Libby P. The ins and outs of inflammatory cells in atheromata. Cell Metab. 2012;15:135–136. doi: 10.1016/j.cmet.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 21.Sheedy FJ, Grebe A, Rayner KJ, et al. CD36 coordinates NLRP3 inflammasome activation by facilitating intracellular nucleation of soluble ligands into particulate ligands in sterile inflammation. Nat Immunol. 2013;14:812–820. doi: 10.1038/ni.2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Randolph GJ. Mechanisms that regulate macrophage burden in atherosclerosis. Circ Res. 2014;114:1757–1771. doi: 10.1161/CIRCRESAHA.114.301174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moore KJ, Sheedy FJ, Fisher EA. Macrophages in atherosclerosis: a dynamic balance. Nat Rev Immunol. 2013;13:709–721. doi: 10.1038/nri3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feig JE, Shang Y, Rotllan N, et al. Statins promote the regression of atherosclerosis via activation of the CCR7-dependent emigration pathway in macrophages. PLoS One. 2011;6:e28534. doi: 10.1371/journal.pone.0028534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Geng YJ, Libby P. Evidence for apoptosis in advanced human atheroma. Colocalization with interleukin-1β-converting enzyme. Am J Pathol. 1995;147:251–266. [PMC free article] [PubMed] [Google Scholar]
- 26.Majno G, Joris I. Apoptosis, oncosis, and necrosis. An overview of cell death. Am J Pathol. 1995;146:3–15. [PMC free article] [PubMed] [Google Scholar]
- 27.Littlewood TD, Bennett MR. Apoptotic cell death in atherosclerosis. Curr Opin Lipidol. 2003;14:469–475. doi: 10.1097/00041433-200310000-00007. [DOI] [PubMed] [Google Scholar]
- 28.Vengrenyuk Y, Carlier S, Xanthos S, et al. A hypothesis for vulnerable plaque rupture due to stress-induced debonding around cellular microcalcifications in thin fibrous caps. Proc Natl Acad Sci U S A. 2006;103:14678–14683. doi: 10.1073/pnas.0606310103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tabas I. Macrophage death and defective inflammation resolution in atherosclerosis. Nat Rev Immunol. 2010;10:36–46. doi: 10.1038/nri2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thorp E, Cui D, Schrijvers DM, et al. Mertk receptor mutation reduces efferocytosis efficiency and promotes apoptotic cell accumulation and plaque necrosis in atherosclerotic lesions of Apoe−/− mice. Arterioscler Thromb Vasc Biol. 2008;28:1421–1428. doi: 10.1161/ATVBAHA.108.167197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Libby P, Nahrendorf M, Swirski FK. Monocyte heterogeneity in cardiovascular disease. Semin Immunopathol. 2013;35:553–562. doi: 10.1007/s00281-013-0387-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Andersson J, Libby P, Hansson GK. Adaptive immunity and atherosclerosis. Clin Immunol. 2010;134:33–46. doi: 10.1016/j.clim.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 33.Lahoute C, Herbin O, Mallat Z, et al. Adaptive immunity in atherosclerosis: mechanisms and future therapeutic targets. Nat Rev Cardiol. 2011;8:348–358. doi: 10.1038/nrcardio.2011.62. [DOI] [PubMed] [Google Scholar]
- 34.Hilgendorf I, Theurl I, Gerhardt LM, et al. Innate response activator B cells aggravate atherosclerosis by stimulating TH1 adaptive immunity. Circulation. 2014;129:1677–1687. doi: 10.1161/CIRCULATIONAHA.113.006381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsiantoulas D, Diehl CJ, Witztum JL, et al. B Cells and humoral immunity in atherosclerosis. Circ Res. 2014;114:1743–1756. doi: 10.1161/CIRCRESAHA.113.301145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sage AP, Tsiantoulas D, Baker L, et al. BAFF receptor deficiency reduces the development of atherosclerosis in mice--brief report. Arterioscler Thromb Vasc Biol. 2012;32:1573–1576. doi: 10.1161/ATVBAHA.111.244731. [DOI] [PubMed] [Google Scholar]
- 37.Kyaw T, Cui P, Tay C, et al. BAFF Receptor mAb treatment ameliorates development and progression of atherosclerosis in hyperlipidemic ApoE−/− mice. PLoS One. 2013;8:e60430. doi: 10.1371/journal.pone.0060430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kovanen PT. Mast cells: multipotent local effector cells in atherothrombosis. Immunol Rev. 2007;217:105–122. doi: 10.1111/j.1600-065X.2007.00515.x. [DOI] [PubMed] [Google Scholar]
- 39.Sun J, Sukhova GK, Wolters PJ, et al. Mast cells promote atherosclerosis by releasing proinflammatory cytokines. Nat Med. 2007;13:719–724. doi: 10.1038/nm1601. [DOI] [PubMed] [Google Scholar]
- 40.Bot I, de Jager SC, Zernecke A, et al. Perivascular mast cells promote atherogenesis and induce plaque destabilization in apolipoprotein E-deficient mice. Circulation. 2007;115:2516–2525. doi: 10.1161/CIRCULATIONAHA.106.660472. [DOI] [PubMed] [Google Scholar]
- 41.Otsuka F, Finn AV, Virmani R. Do vulnerable and ruptured plaques hide in heavily calcified arteries? Atherosclerosis. 2013;229:34–37. doi: 10.1016/j.atherosclerosis.2012.12.032. [DOI] [PubMed] [Google Scholar]
- 42.Libby P. Mechanisms of acute coronary syndromes and their implications for therapy. N Engl J Med. 2013;368:2004–2013. doi: 10.1056/NEJMra1216063. [DOI] [PubMed] [Google Scholar]
- 43.Mallory GK, White PD, Salcedo-Salgar J. The speed of healing of myocardial infarction: A study of the pathologic anatomy in seventy-two cases. Am Heart J. 1939;18:647–671. [Google Scholar]
- 44.Nahrendorf M, Swirski FK, Aikawa E, et al. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med. 2007;204:3037–3047. doi: 10.1084/jem.20070885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Swirski FK, Nahrendorf M, Etzrodt M, et al. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science. 2009;325:612–616. doi: 10.1126/science.1175202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Leuschner F, Panizzi P, Chico-Calero I, et al. Angiotensin-converting enzyme inhibition prevents the release of monocytes from their splenic reservoir in mice with myocardial infarction. Circ Res. 2010;107:1364–1373. doi: 10.1161/CIRCRESAHA.110.227454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hilgendorf I, Gerhardt LM, Tan TC, et al. Ly-6Chigh monocytes depend on Nr4a1 to balance both inflammatory and reparative phases in the infarcted myocardium. Circ Res. 2014;114:1611–1622. doi: 10.1161/CIRCRESAHA.114.303204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Swirski FK, Nahrendorf M. Leukocyte behavior in atherosclerosis, myocardial infarction, and heart failure. Science. 2013;339:161–166. doi: 10.1126/science.1230719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Panizzi P, Swirski FK, Figueiredo JL, et al. Impaired infarct healing in atherosclerotic mice with Ly-6Chi monocytosis. J Am Coll Cardiol. 2010;55:1629–1638. doi: 10.1016/j.jacc.2009.08.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Leuschner F, Dutta P, Gorbatov R, et al. Therapeutic siRNA silencing in inflammatory monocytes in mice. Nat Biotechnol. 2011;29:1005–1010. doi: 10.1038/nbt.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Courties G, Heidt T, Sebas M, et al. In vivo silencing of the transcription factor IRF5 reprograms the macrophage phenotype and improves infarct healing. J Am Coll Cardiol. 2014;63:1556–1566. doi: 10.1016/j.jacc.2013.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Leuschner F, Rauch PJ, Ueno T, et al. Rapid monocyte kinetics in acute myocardial infarction are sustained by extramedullary monocytopoiesis. J Exp Med. 2012;209:123–137. doi: 10.1084/jem.20111009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Clinton SK, Fleet JC, Loppnow H, et al. Interleukin-1 gene expression in rabbit vascular tissue in vivo. Am J Pathol. 1991;138:1005–1014. [PMC free article] [PubMed] [Google Scholar]
- 54.Fleet JC, Clinton SK, Salomon RN, et al. Atherogenic diets enhance endotoxin-stimulated interleukin-1 and tumor necrosis factor gene expression in rabbit aortae. J Nutr. 1992;122:294–305. doi: 10.1093/jn/122.2.294. [DOI] [PubMed] [Google Scholar]
- 55.Koren O, Spor A, Felin J, et al. Human oral, gut and plaque microbiota in patients with atherosclerosis. Proc Natl Acad Sci U S A. 2011;108(Suppl 1):4592–4598. doi: 10.1073/pnas.1011383107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Libby P, Egan D, Skarlatos S. Roles of infectious agents in atherosclerosis and restenosis: an assessment of the evidence and need for future research. Circulation. 1997;96:4095–4103. doi: 10.1161/01.cir.96.11.4095. [DOI] [PubMed] [Google Scholar]
- 57.Mason JC, Libby P. Cardiovascular disease in patients with chronic inflammation: mechanisms underlying premature cardiovascular events in rheumatologic conditions. Eur Heart J. 2015;36:482c–489c. doi: 10.1093/eurheartj/ehu403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dutta P, Courties G, Wei Y, et al. Myocardial infarction accelerates atherosclerosis. Nature. 2012;487:325–329. doi: 10.1038/nature11260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dutta P, Sager HB, Stengel KR, et al. Myocardial infarction activates CCR2+ hematopoietic stem and progenitor cells. Cell Stem Cell. 2015;16:477–487. doi: 10.1016/j.stem.2015.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sager HB, Heidt T, Hulsmans M, et al. Targeting interleukin-1β reduces leukocyte production after acute myocardial infarction. Circulation. 2015;132:1880–1890. doi: 10.1161/CIRCULATIONAHA.115.016160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Heidt T, Sager HB, Courties G, et al. Chronic variable stress activates hematopoietic stem cells. Nat Med. 2014;20:754–758. doi: 10.1038/nm.3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Courties G, Herisson F, Sager HB, et al. Ischemic stroke activates hematopoietic bone marrow stem cells. Circ Res. 2015;116:407–417. doi: 10.1161/CIRCRESAHA.116.305207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Levine SA. Coronary Thrombosis: Its Various Clinical Features. Baltimore, MD: The Williams & Wilkins Company; 1929. [Google Scholar]
- 64.Berg KE, Ljungcrantz I, Andersson L, et al. Elevated CD14++CD16− monocytes predict cardiovascular events. Circ Cardiovasc Genet. 2012;5:122–131. doi: 10.1161/CIRCGENETICS.111.960385. [DOI] [PubMed] [Google Scholar]
- 65.Heine GH, Ulrich C, Seibert E, et al. CD14++CD16+ monocytes but not total monocyte numbers predict cardiovascular events in dialysis patients. Kidney Int. 2008;73:622–629. doi: 10.1038/sj.ki.5002744. [DOI] [PubMed] [Google Scholar]
- 66.Rogacev KS, Cremers B, Zawada AM, et al. CD14++CD16+ monocytes independently predict cardiovascular events: a cohort study of 951 patients referred for elective coronary angiography. J Am Coll Cardiol. 2012;60:1512–1520. doi: 10.1016/j.jacc.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 67.Emami H, Singh P, MacNabb M, et al. Splenic metabolic activity predicts risk of future cardiovascular events: demonstration of a cardiosplenic axis in humans. J Am Coll Cardiol Img. 2015;8:121–130. doi: 10.1016/j.jcmg.2014.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Libby P, Di Carli MF, Weissleder R. The vascular biology of atherosclerosis and imaging targets. J Nucl Med. 2010;51(Suppl 1):33S–37S. doi: 10.2967/jnumed.109.069633. [DOI] [PubMed] [Google Scholar]
- 69.Quillard T, Croce K, Jaffer FA, et al. Molecular imaging of macrophage protease activity in cardiovascular inflammation in vivo. Thromb Haemost. 2011;105:828–836. doi: 10.1160/TH10-09-0589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Libby P, Nahrendorf M, Weissleder R. Molecular imaging of atherosclerosis: a progress report. Tex Heart Inst J. 2010;37:324–327. [PMC free article] [PubMed] [Google Scholar]
- 71.Rudd JH, Narula J, Strauss HW, et al. Imaging atherosclerotic plaque inflammation by fluorodeoxyglucose with positron emission tomography: ready for prime time? J Am Coll Cardiol. 2010;55:2527–2535. doi: 10.1016/j.jacc.2009.12.061. [DOI] [PubMed] [Google Scholar]
- 72.Majmudar MD, Nahrendorf M. Cardiovascular molecular imaging: the road ahead. J Nucl Med. 2012;53:673–676. doi: 10.2967/jnumed.111.099838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Taqueti VR, Di Carli MF, Jerosch-Herold M, et al. Increased microvascularization and vessel permeability associate with active inflammation in human atheromata. Circ Cardiovasc Imaging. 2014;7:920–929. doi: 10.1161/CIRCIMAGING.114.002113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tarkin JM, Rudd JH. Techniques for noninvasive molecular imaging of atherosclerotic plaque. Nat Rev Cardiol. 2015;12:79. doi: 10.1038/nrcardio.2014.80-c2. [DOI] [PubMed] [Google Scholar]
- 75.Ye YX, Calcagno C, Binderup T, et al. Imaging macrophage and hematopoietic progenitor proliferation in atherosclerosis. Circ Res. 2015;117:835–845. doi: 10.1161/CIRCRESAHA.115.307024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dzau V, Braunwald E. Resolved and unresolved issues in the prevention and treatment of coronary artery disease: a workshop consensus statement. Am Heart J. 1991;121:1244–1263. doi: 10.1016/0002-8703(91)90694-d. [DOI] [PubMed] [Google Scholar]