Abstract
The Precision Medicine Initiative aims to use advances in basic and clinical research to develop therapeutics that selectively target and kill cancer cells. Under the same doctrine of precision medicine, there is an equally important need to visualize these diseased cells to enable diagnosis, facilitate surgical resection and monitor therapeutic response. Therefore, there is a great opportunity for chemists to develop chemically tractable probes that can image cancer in vivo. This review focuses on recent advances in the development of optical probes as well as their current and future applications in the clinical management of cancer. The progress in probe development described here suggests that optical imaging is an important and rapidly developing field of study that encourages continued collaboration between chemists, biologists and clinicians to further refine these tools for interventional surgical imaging, as well as for diagnostic and therapeutic applications.
Introduction
A growing body of basic and clinical research around precision medicine – harnessing biomedical tools to deliver tailored diagnostics and therapeutics for each patient – promises to revolutionize the way translational research and clinical medicine is conducted. In the field of oncology, advances in precision medicine will shift therapeutics away from untargeted chemotherapies that damage healthy cells in addition to cancerous cells, thereby causing serious side effects and poor quality of life. By exploiting specific mutations and pathways unique to cancer cells, therapies will instead target diseased cells while leaving healthy tissue unharmed. This will be a long-term battle, as researchers and clinicians work to discover markers and molecular signatures that subcategorize cancers into specific populations, enabling tailored treatments with improved response rates. In parallel with these therapeutic efforts, there is a significant need to develop tools that will allow necessary classifications of cancer subtypes to be made rapidly, accurately and using minimally invasive methods. Furthermore, the development of agents that assess therapeutic response to guide treatment regimens are likely to alter patient outcomes in a significantly positive way.
With the launch of the Precision Medicine Initiative (PMI) by President Obama in January 2015, the concept of personalized medicine evolved to encompass a wide range of determinants that impact health (Collection), 2015; Collins and Varmus, 2015). Genetic information remains an important component of decision making for the treatment of cancer, but the tenets of precision medicine are expanded to include a wider range of cellular and molecular analyses, environmental and lifestyle choices, as well as an emphasis on disease prevention. The concept of ‘precision’ has particular resonance in the field of surgical oncology, where the gold standard and first step in many patients’ treatment plans is surgery (Institute, 2014; Siegel, et al., 2012; Survey, 2012). Precision at the interventional stage is increased by innovations in techniques and methodologies that help doctors and surgeons to better visualize each patient’s disease. One such breakthrough that is likely to have broad impact on cancer management is the application of imaging tools that enable optical surgical guidance with molecular precision.
The primary challenge for surgical intervention in cancer treatment is finding effective ways to define the boundaries between the tumor and healthy surrounding tissue at the cellular level. Technologies to define the molecular edge between cancer and healthy tissue are designed to exploit characteristics of all cancers, or types of cancers, to precisely identify diseased tissues in the majority of patients. Further, as discussed in this review, these technologies can highlight diseased areas to resect, or they can light up critical structures to preserve, such as ureters and nerves (Park, et al., 2014; Verbeek, et al., 2014) and reviewed in (Orosco, et al., 2013), that cause significant morbidity when accidentally damaged in surgery. Many of these technologies are already reaching clinical trials, and therefore have the potential to positively benefit patients in a shorter time frame than many of the precision therapeutics. In this way, imaging with optical guidance during surgery represents a technology complimentary to the aims of precision medicine in diagnostics and therapeutics, and these technologies should be encompassed in our goals to improve our understanding of the prevention and treatment of human disease. Precision imaging holds additional promise, as many of the tools developed and optimized to image disease can be further harnessed to specifically deliver therapeutics to the target tissue of interest. Together, precision imaging in conjunction with other research aims under the umbrella of the PMI share in the promise to significantly decrease the morbidity and mortality of cancer patients.
There are many modalities available for clinical imaging applications, including radiological, magnetic resonance (MR), ultrasound, and optical imaging. However, while many of these technologies are already integral in early diagnosis and disease monitoring, their direct integration into operating room (OR) workflows remains more challenging. Specifically, the bulky machinery and logistics of visualizing MR or radiological imaging agents make their use during surgery difficult. Optical contrast agents, on the other hand, have the potential to be utilized during a surgeon’s restricted timeframe for resection and can be detected with simple camera systems that enable real-time monitoring of probe signals. However, some of the major challenges facing optical contrast agents include sensitivity, tissue penetrance and the need for a clear pathway for clinical development and approval. Many of these challenges can be addressed with the development of suitable instrumentation and new generations of chemical probes with increased selectivity and in vivo properties. This review will focus on optical imaging agents designed for intraoperative use to provide real-time feedback to surgeons. We will further refine the scope to discuss chemical tools that target enzymatic and metabolic processes upregulated in tumors and the tumor microenvironment.
Small molecule and peptide-based optical contrast agents
We have chosen to focus this review on chemically tractable imaging agents because there are a number of advantages of using such agents for imaging, including cost of production, stability and potential to be topically applied to areas of interest. Clinically, IV administration or topical application of optical contrast agents could be used at multiple stages of surgical intervention. With the current optical probes and imaging devices, optical contrast agents can be used for intraoperative decision-making in vivo or ex vivo when applied to biopsy or surgical tissues (Figure 1). We speculate that these probes will also eventually become an integral technology for pre-operative planning and decision-making stage, as dyes with enhanced properties and imaging devices with more sensitive detection methods become available (Figure 1). Before advances in these technologies become available, the advantage of using the same contrast agent for pre-operative and intraoperative decision making could be proven using analogs of optical probes that also contain a PET tracer. In this scenario, the PET tracer would be employed for pre-operative planning while the optical reporter would be used intraoperatively. Beyond their clinical utility, these chemical tools have a broad range of applications beyond the OR when applied in basic research applications. Importantly, many devices have been designed for the purpose of visualizing optical contrast agents. Currently, a variety of companies manufacture US Food and Drug Administration (FDA)-approved surgical camera systems, such as the Firefly system in the da Vinci Surgical System (Buchs, et al., 2012), the Pinpoint camera (Sherwinter, 2012), the Fluorescence-Assisted Resection and Exploration (FLARE) and Mini-FLARE image-guided surgery systems (Tummers, et al., 2015; Verbeek, et al., 2014; Verbeek, et al., 2013), the Photo Dynamic Eye (PDE) and PDE-neo (Aoki, et al., 2010), the Hyper Eye Medical System (HEMS) (Yoshida, et al., 2012), and the IMAGE1 SPIES near infrared/indocyanine green (NIR/ICG)-system (Schols, et al., 2013). Similarly, there are multiple devices for use in basic science applications and in animal models of disease, including the IVIS, Maestro, Fluobeam, MiroSurge, Lab-FLARE Model R1 Open Space Imaging System and a range of confocal microscopes. While there are a significant number of approved imaging devices, there are no molecularly targeted optical contrast agents that are FDA-approved for clinical use. Thus, all of the current clinical work makes use of non-targeted dyes such as indocyanine green (ICG) (Buchs, et al., 2012; Verbeek, et al., 2014; Yoshida, et al., 2012), methylene blue (MB) (Tummers, et al., 2015; Verbeek, et al., 2013) and fluorescein (Gribar and Hamad, 2007; McGinty, et al., 2003). However, given the availability of imaging devices, efforts to advance optical contrast agents should be a priority, as new agents can be immediately integrated into surgical workflows as they become clinically available. A summary of the optical probes highlighted in this review and their stage of pre-clinical or clinical development is given in Table 1.
Figure 1. Current and Future Clinical applications for optical chemical probes.
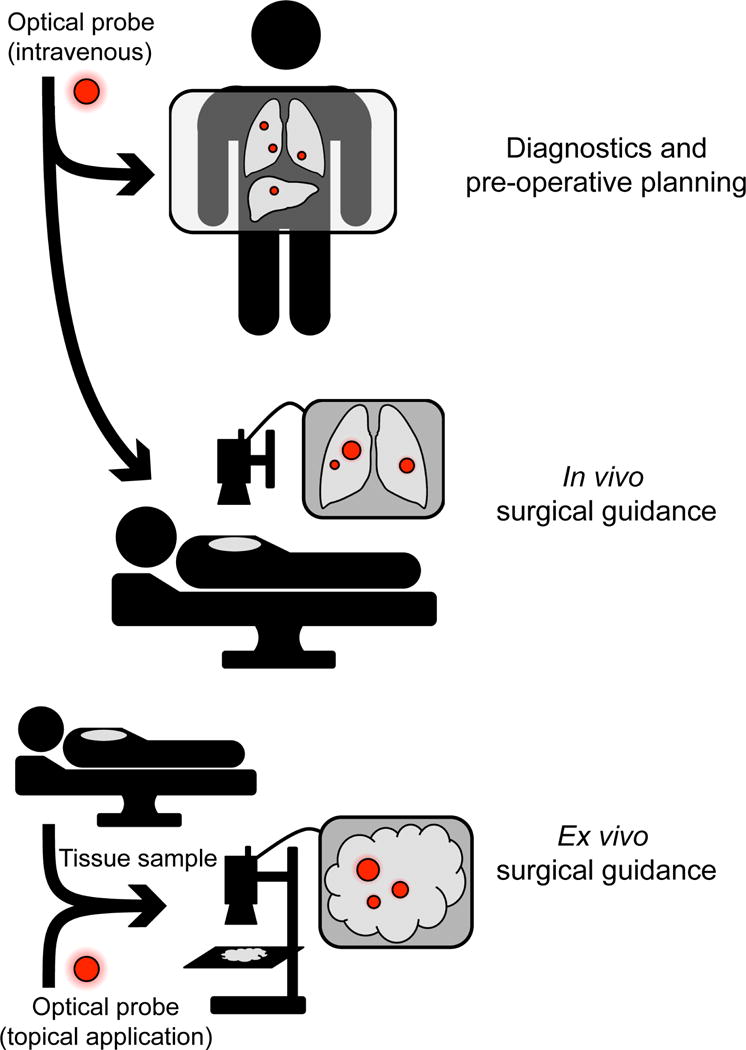
Top: future application of optical contrast agents includes intravenous administration for preoperative diagnostics and planning. Middle: current use of optical contrast agents for in vivo surgical guidance. Bottom: topical probe labeling can be used for ex vivo surgical guidance.
Table 1.
List of probes highlighted in this manuscript and their preclinical or clinical status
| Name | Pre-clinical/clinical trials | Notes |
|---|---|---|
| Tumor Paint (BLZ-100) | Phase 1 clinical trials for adult skin cancer (completed March 2015), sarcoma, pediatric tumors of the central nervous system (CNS), glioma, and other solid tumors | NCT02097875, NCT02464332, NCT02462629, NCT02234297, NCT02496065 |
| CLR1501 and CLR1502 | Pre-clinical animal models | (Swanson, et al., 2015) |
| OTL38 | Phase II clinical trial for FRα-positive ovarian cancer | NCT02317705 |
| cRGD-ZW800-1 | Pre-clinical animal models | (Choi, et al., 2013) |
| YC-27 | Pre-clinical animal models | (Neuman, et al., 2015) |
| PSMA-1-IR800 | Pre-clinical animal models | (Wang, et al., 2014) |
| Fluorocoxib A | Pre-clinical animal models | (Ra, et al., 2015; Uddin, et al., 2010) |
| BMV109 | Pre-clinical animal models | (Segal, et al., 2015; Verdoes, et al., 2013) |
| LUM105 | Phase I clinical trials for sarcomas, soft tissue sarcomas and breast cancer; feasibility clinical trials for breast cancer, GI cancers of the colon, esophagus and pancreas | NCT01626066, NCT02438358, NCT02584244 |
| C-PGC probe | Pre-clinical animal models | (Weissleder, et al., 1999) |
| MMP-2-sensitive NIRF probe | Pre-clinical animal models | (Bremer, et al., 2001) |
| Z-Phe-Arg-HMRG | Pre-clinical animal models | (Fujii, et al., 2014) |
| Lipidated probe 3 | Pre-clinical animal models | (Hu, et al., 2014) |
| 6QCNIR | Pre-clinical animal models | (Ofori, et al., 2015) |
| RACPP AVB-620 | Phase I clinical trial for breast cancer | NCT02391194 |
| C-SNAF | Pre-clinical animal models | (Ye, et al., 2014) |
Considerations for Probe Development
In general, an optical chemical probe consists of three main structural elements: the recognition element, the linker, and the dye (Figure 2A). The recognition element enables targeted delivery of the probe to the cells of interest. Specifically targeting molecular signatures of cancer cells by the recognition element is perhaps the most important component to optimize in the design of an optical probe. The two primary strategies used to induce tumor-specific probe accumulation include optimizing binding affinity for molecular targets that are over-represented in cancer cells or cells associated with a solid tumor (affinity agents), or efficient response to enzymatic signatures found mainly in the cancer cell or tumor-associated cells (substrate and activity-based probes) (Figure 2). These two approaches, affinity-based and enzymatic targeting elements, each have strengths and weaknesses. Recognition elements of affinity-based probes are usually based on the endogenous ligand of an enzyme or receptor that is upregulated in cancerous cells. Alternatively, screening approaches or direct optimization of small molecule ligands can be used to yield probes that have selective affinity for cancer-specific targets. Affinity-based probes largely rely on the accumulation and retention of the dye-containing molecule due to target binding and require removal of the unbound probe to generate contrast. In contrast, enzyme substrates and activity-based probes can be designed to only produce signal or accumulate in the tumor upon processing by a cancer-specific enzyme. This usually involves enzymes that cleave recognition motifs such as proteases, or enzymes that react with the recognition motifs to produce a signal. In both strategies, an important aspect to consider is the localization of the target of interest to the intracellular compartment, extracellular compartment, or specific organelles. Direct studies assessing the challenges and benefits of choosing a target localized to each of these compartments have yet to be carried out. However, one possible challenge to choosing an intracellular target, or a target localized to a specific organelle, is that these probes must cross the cell membrane to interact with their target of interest. Conversely, a possible challenge posed with extracellular targets is signal attenuation due to diffusion.
Figure 2. General considerations for probe design.
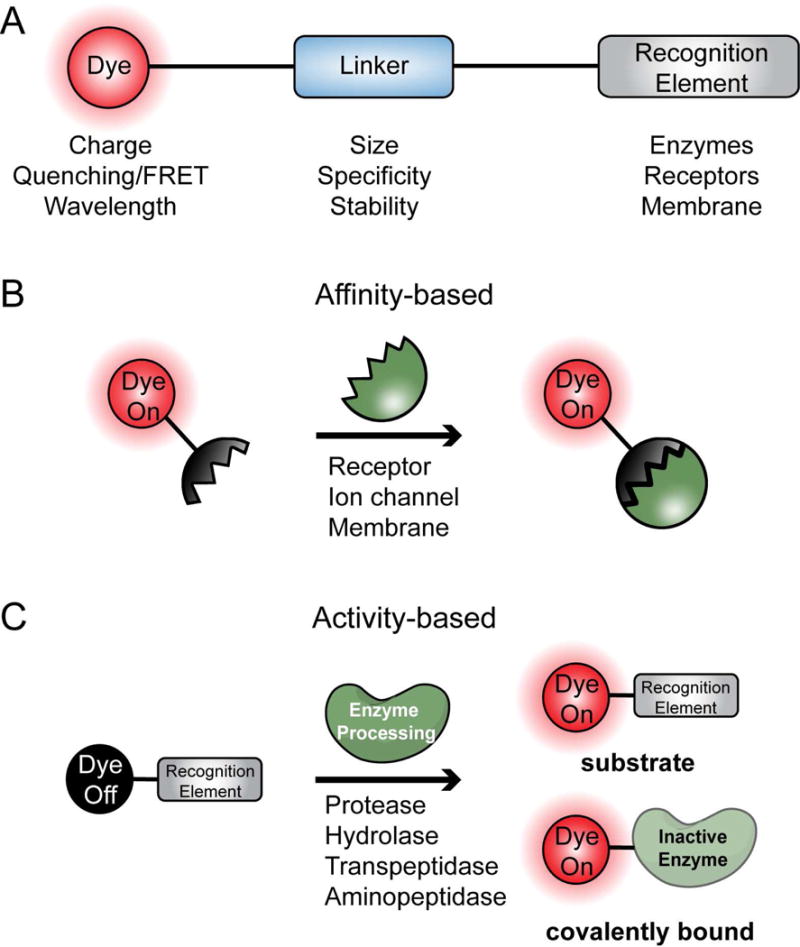
A. Optical chemical probes consist of 3 main structural elements: the dye, linker, and recognition element. B. General model for affinity-based probes. The probe binds to targets such as receptors, ion channels and membranes with high affinity. C. General model for activity-based probes. These probes give off a fluorescent signal upon processing by enzymes such as proteases and peptidases.
In addition to the mechanism of targeting, it is also important to optimize the type of reporter dye used and how these reporters are attached to the targeting group. Selection of an optimal dye is an important aspect of probe design. Because small molecule probes are by their very nature relatively small in size, the attachment of large quenchers and dyes to the core recognition element can dramatically impact the in vivo properties, cell permeability and biodistribution of the probe. Furthermore, the excitation and emission wavelengths of the dye are key considerations. Near-infrared (NIR) light lies outside the range of tissue autofluorescence and can detect tumors up to 5 – 10 millimeters into tissues (Rosenthal, et al., 2015; Schols, et al., 2013; Vahrmeijer, et al., 2013). Thus, virtually all optical imaging agents for clinical applications make use of NIR dyes. There are currently many commercially available dyes that cover the range of NIR wavelengths, and agents with increasingly longer wavelengths are proving to be useful for increased signal and reduced background (Antaris, et al., 2015). In addition to the excitation and emission wavelengths of the dye, the overall balance of charge-to-hydrophobicity of the chemical structure also plays an important role in the signal-to-background ratio output of the probe (Choi, et al., 2013). By employing zwitterionic dyes, it is possible to achieve a well balanced charge distribution over the surface of the chemical probe, allowing for a more hydrophilic molecule with low nonspecific binding properties (Choi, et al., 2013).
Contrast agents can also be optimized in the linker region that attaches the dye to the targeting element, as well as in the dye placement on the probe. Varying the linker (to include PEG or poly amino acids) to control the overall size of the probe influences overall properties of the probe, such as rate of diffusion, cell permeability, and plasma circulation times. Larger probes take advantage of the enhanced permeability and retention effect (EPR), a tendency of larger molecules to accumulate in tumor tissue to a greater extent than in healthy tissue (Matsumura and Maeda, 1986). However, this can come at the price of speed of signal generation, as larger probes tend to penetrate solid tumors at a slower rate than small probes (Blum, et al., 2009). Additionally, the linker can be used to change the overall charge of the molecule or to localize it to specific intracellular locations. Finally, the placement of the dye molecule can dramatically impact the imaging properties of the probe, as will be discussed for several of the probes highlighted in this review.
Affinity-based Probes
Affinity-based probes generally exploit the fact that certain proteins, such as enzymes and cell surface receptors, are upregulated in cancer cells (Figure 3). Furthermore, there are a number of naturally occurring ligands with high intrinsic affinity for cancer targets that can be used as starting points for probe design. In some cases, this can involve the simple attachment of a suitable fluorescent dye to generate the imaging agent.
Figure 3. Affinity-based Probes.
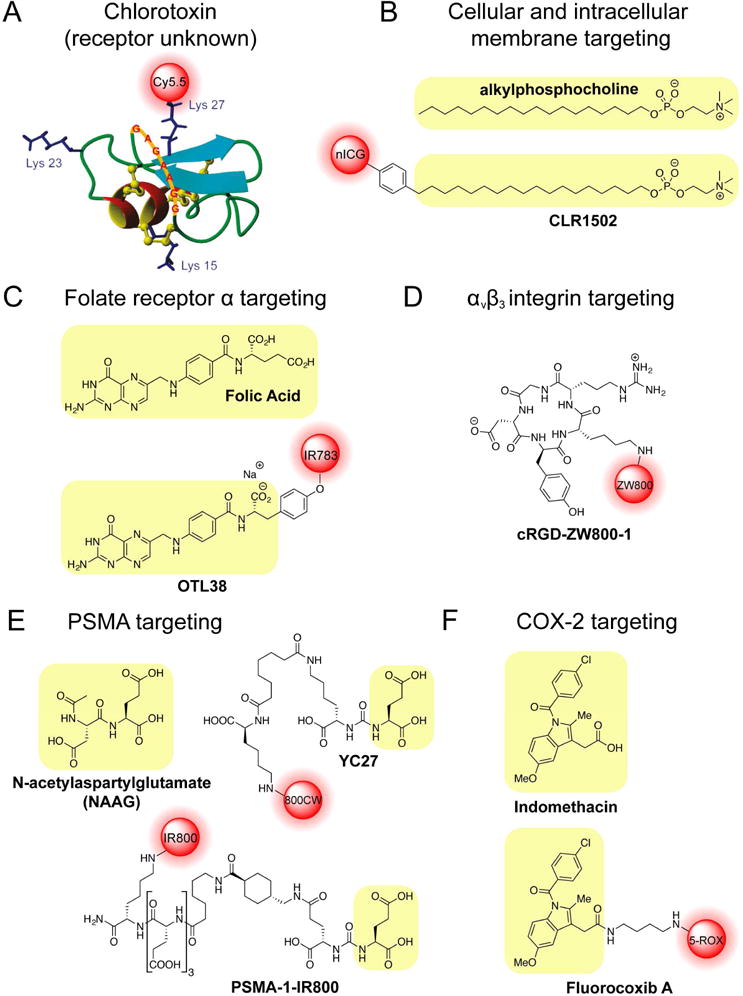
A. Chlorotoxin-based probe BLZ-100. B. Alkylphosphocholine (APC) structure and APC-derived probe CLR1502. C. Folic acid structure and folate receptor α-targeting probe OTL38. D. Cyclic RGD peptides as tumor contrast agents. E. NAAG structure and PSMA targeting probes YC27 and PSMA-1-IR800. F. Structure of Indomethacin and the corresponding labeled probe Fluorocoxib A.
One example of a promising new affinity-based imaging agent is Tumor Paint (BLZ-100) (Figure 3A). This peptidic probe is derived from chlorotoxin venom (CTX) of the Leiurus quinquestriatus scorpion (Lyons, et al., 2002; Soroceanu, et al., 1998; Veiseh, et al., 2007), which binds preferentially to the surface of cancerous glioma cells in the brain (Lyons, et al., 2002; Soroceanu, et al., 1998). Interestingly, the specific molecular target of CTX is not yet clear and reports have suggested multiple possible targets, including the glioma-specific chloride ion channel (GCC), a lipid raft-anchored complex that binds to matrix metalloproteinase-2 (MMP-2), membrane type-1 MMP, transmembrane inhibitor of MMP-2 (TIMP2), and ClC-3 chloride ion channel (Deshane, et al., 2003; Soroceanu, et al., 1998; Veiseh, et al., 2007). CTX is a 36 amino acid peptide that contains four disfulfide bonds, causing it to fold into a compact and well-defined structure (Soroceanu, et al., 1998; Veiseh, et al., 2007). CTX was developed into an optical imaging agent by the Olson group through conjugation with a Cy5.5 NIR fluorophore (Veiseh, et al., 2007). Because CTX contains three lysine residues, Cy5.5 conjugation resulted in a mixture of mono-, di-, and tri-labeled probes (Akcan, et al., 2011). This posed a regulatory and manufacturing problem for translation to the clinic and required re-engineering of the CTX peptide to replace Lys15 and Lys23 with alanine or arginine (Akcan, et al., 2011). Modification of these two residues resulted in specific Lys27 labeling to generate a probe that retained the stability and in vivo half-life properties of the original peptide (Akcan, et al., 2011). Interestingly, cyclization of CTX containing all three lysine residues resulted in only a single Cy5.5 labeled species, but decreased the in vivo half-life to 11 hours as compared to the 14 hour half-life of the linear peptidic probe (Akcan, et al., 2011). Although originally designed based on its glioma-specific targeting, Tumor Paint has now been used in a variety of cancers, including medulloblastoma, sarcoma and carcinomas of the prostate, colon, breast, lung, skin and intestines (Akcan, et al., 2011; Veiseh, et al., 2007). Importantly, the company Blaze Biosciences (http://www.blazebioscience.com), which has a license to the Tumor Paint technology, has five completed and ongoing Phase I clinical trials investigating the use of BLZ-100 in adult skin cancer (completed March 2015), sarcoma, pediatric tumors of the central nervous system (CNS), glioma, and other solid tumors (www.clinicaltrials.gov identifiers: NCT02097875, NCT02464332, NCT02462629, NCT02234297, NCT02496065).
An alternate approach to generate affinity-based probes uses modified metabolites and biosynthetic molecules that are known to accumulate in tumors. For example, phospholipid ethers (PLEs) were found to accumulate to higher levels in cancer cells compared to normal tissues as early as the 1960s (Snyder, et al., 1969; Snyder and Wood, 1969). At the same time, PLE and alkylphosphocholine (APC) analogs were shown to exhibit cytostatic and anti-tumor activities (Andreesen, et al., 1978). The anti-cancer activity of phospholipid analogs is thought to occur upon insertion into cellular membranes, which disrupts phospholipid and cholesterol metabolism, the function of lipid rafts, and the homeostasis of important signaling pathways such as inositol triphosphate (IP3) and calcium signaling, the PI3K/Akt pathway, and the MAPK pathway (van Blitterswijk and Verheij, 2013; Weichert, et al., 2014). Taking advantage of the tumor-specific uptake of PLEs and APCs, the APC derivative octadecyl phosphocholine has been conjugated with BODIPY (CLR1501) and new ICG (CLR1502) to generate optical imaging agents (Figure 3B)(Swanson, et al., 2015). Significant SAR analyses have also been performed on PLE analogs to optimize their tumor uptake and pharmacokinetic properties including plasma half-life, biodistribution, and clearance (Pinchuk, et al., 2006). Alkyl chain length was found to be important for tumor targeting and clearance, with the increased hydrophilicity of C7 analogs resulting in decreased tumor targeting and rapid clearance (Pinchuk, et al., 2006). Increasing the alkyl chain length to 18 carbons afforded increased plasma half-life when compared to C15 analogs (Pinchuk, et al., 2006). Derivatization to propanediol analogues resulted in undesirable retention of the probe in hepatocytes, and adding a 2-O-methyl group to the glycerol backbone decreased tumor uptake (Pinchuk, et al., 2006). These SAR studies identified the optimal APC analog as the C18 octadecylphosphocholine, which was made into radiolabeled 18-(p-Iodophenyl)octadecylphsophocholine (CLR1404, formerly NM404), as well as optical agents (CLR1501 and CLR1502). While no clinical trials have been initiated for the optical imaging agents CLR1501 or CLR1502, CLR1404 was advanced into animal proof-of-concept studies as well as multiple Phase I/II clinical trials by Cellectar Biosciences (http://cellectarbiosciences.com) (clinicaltrials.gov identifiers: NCT00582283, NCT00925275, NCT01662284, NCT01516905).
One of the more common strategies for affinity probe design is to exploit cell surface receptors known to be upregulated in cancer. The folate receptor α (FRα), is a promising target for directed diagnosis, imaging, and therapies in cancer treatment. Its high level of overexpression on the surface of many cancer types, including carcinomas of the ovary, breast, lung, kidney, and colon, make it an ideal target for molecularly targeted agents (Parker, et al., 2005; Srinivasarao, et al., 2015). Studies have reported levels of FRα expression on cancer cells as high as 280,000 receptors per cell (Destito, et al., 2007; Saul, et al., 2003). Furthermore, in normal tissues, with the exception of cells in the proximal tubule of the kidney and lung, expression of FRα is confined to the apical surface of cells, rendering it inaccessible to therapeutics or contrast agents due to adherens and tight junctions (O’Shannessy, et al., 2012; Parker, et al., 2005; Weitman, et al., 1992; Yang, et al., 2012). These qualities result in high signal to noise ratios for agents specifically targeting FRα (Srinivasarao, et al., 2015; van Dam, et al., 2011; Weitman, et al., 1992; Yang, et al., 2012). Furthermore, FRα binds folate (vitamin B9) with high affinity to facilitate its uptake for single-carbon metabolism, a process that is particularly important for amino acid metabolism and nucleotide synthesis (Antony, 1996). To generate optical probes based on this cancer specific target, Low et al. created the first generation probe EC17 by conjugating folate to fluorescein isothiocyanate (FITC)(van Dam, et al., 2011). EC17 was used for a small human clinical study in patients with ovarian cancer. The trial confirmed that the probe detected FRα overexpression in a subset of these patients and improved clinicians’ ability to detect cancer lesions (van Dam, et al., 2011). Further optimization led to a related compound, OTL38, which uses a folate analog, pteroic acid-tyrosine conjugated to IR-783 (Figure 3C) (Srinivasarao, et al., 2015). On Target Laboratories (http://www.ontargetlaboratories.com), the company licensing OTL38, is currently investigating this probe in a Phase II clinical trial for intra-operative imaging in patients with FRα-positive ovarian cancer, with an expected completion date in 2015 (clinicaltrials.gov identifier: NCT02317705).
Alternatively, many types of targeted contrast agents have made use of the high expression of cell surface adhesion molecules. The integrins make ideal receptors for imaging agents because they are highly expressed on the surface of many types of cancer. In particular, αvβ3 integrin can be targeted using short peptides that contain an RGD sequence. Using this approach, Frangioni et al. have made cyclic RGD peptides linked to NIR dyes for optical imaging of tumors (Figure 3D) (Choi, et al., 2013). These probes also make use of a novel of zwitterionic dye that has improved signal over background ratios in vitro and in vivo compared to more highly charged, dipole-like commercial dyes (Choi, et al., 2013).
Another cell surface receptor that has generated significant interest as an imaging target due to its high expression levels in prostate cancer cells is the zinc metalloenzyme prostate-specific membrane antigen (PSMA) or glutamate carboxypeptidase II (Banerjee, et al., 2008; Schulke, et al., 2003). Prostate cancer is the most prevalent cancer in men (Group, 2015; Institute, 2014) with an incidence of 196,038 in the US (Henley, et al., 2014), and 138,000 prostatectomies performed in 2010 alone (Survey, 2012). In addition to the high volume of prostatectomy surgeries performed each year, this surgery presents a challenge due to the desire to preserve critical autonomic nerves that run through the prostatic fascia for patients without extracapsular extension (Lepor, 2005). Therefore, optical tools to intraoperatively define whether tumor margins extend beyond the prostate capsule would lead to significant decreases in morbidity and other complications associated with prostatectomy.
Several groups have designed high affinity optical and radiological probes to target PSMA. Some of the most successful and clinically advanced probes use urea-based scaffolds based on the structures of the high affinity PSMA inhibitors 2-(phosphomethyl)pentanedioic acid (PMPA) and phosphonic bis-dicarboxylic acid (Kozikowski, et al., 2001; Pomper, et al., 2002). To generate the optical probe YC-27, the NIR dye IRDye800CW was linked to cysteine-glutamate or lysine-glutamate urea inhibitors, resulting in compounds that retained their high affinity for the PSMA active site and selectively targeted PSMA+ xenografts over PSMA− xenografts in murine and porcine models (Figure 3E) (Banerjee, et al., 2011; Chen, et al., 2009; Neuman, et al., 2015). A dual modality radionuclide- and optically-labeled probe was created using the lysine-glutamate IRDye800CW urea labeled with radioactive 111In (Banerjee, et al., 2011). This dual modality probe retained similar potency to that of the singly labeled NIRF and radionuclide probes, and displayed rapid and specific labeling in mice with PSMA+ xenografts when visualized using sequential Single Photon Emission Computed Tomography and X-ray Computed Tomography (SPECT-CT) and optical imaging (Banerjee, et al., 2011). Furthermore, a fluorine-18 labeled molecule, N-[N-[(S)-1,3-Dicarboxypropyl]carbamoyl]-4-[18F]fluorobenzyl-L-cysteine ([18F]DCFBC) has been used in human studies and early-phase clinical trials to detect primary and metastatic prostate cancer (Cho, et al., 2012; Mease, et al., 2008; Rowe, et al., 2015; Rowe, et al., 2015) (clinicaltrials.gov identifiers: NCT01815515, NCT02190279, NCT01417182, NCT01496157).
An alternative approach created a high affinity ligand, PSMA-1, by attaching a linker element containing three D-glutamic acid residues to the parent cysteine-glutamate urea targeting element (Wang, et al., 2014). The increased negative charge of the probe from the glutamic acid residues decreased background signal, while the use of D-amino acids increased the in vivo stability of the molecule (Huang, et al., 2014; Kozikowski, et al., 2001; Wang, et al., 2014). Furthermore, the linker increased binding affinity 4.3-fold compared to the original Cys-Glu parent molecule (Wang, et al., 2014). Interestingly, the binding affinity of PSMA-1 conjugated to the IR800 dye further improved affinity while the use of the Cy5.5 fluorophore did not, highlighting the importance of dye selection in probe design (Figure 3E) (Wang, et al., 2014). These PSMA-directed optical probes have yet to enter into human clinical trials, however numerous clinical trials utilizing PSMA-directed contrast agents for diagnostic PET or MRI imaging highlights the potential for these types of agents in clinical practice (Barrett, et al., 2013; Cho, et al., 2012; Mease, et al., 2008; Rowe, et al., 2015; Rowe, et al., 2015) (clinicaltrials.gov identifiers: NCT02190279, NCT02488070, NCT02048150, NCT01496157, NCT02420977, NCT01815515, NCT02282137, NCT01173146, NCT00992745, NCT00712829).
In another example of probe based on a high affinity small molecule inhibitor, Marnett and co-workers have engineered fluorescently labeled probes that target the enzyme cyclooxygenase-2 (COX-2). This enzyme is not normally expressed in healthy tissues, but has high expression levels in sites of inflammation and neoplasia (Eberhart, et al., 1994). Thus it is an ideal imaging biomarker that has been shown to be upregulated in colon, prostate, breast, pancreatic, lung and skin cancers (Sobolewski, et al., 2010). Initial design efforts for COX-2 probes involved significant SAR studies to identify selective COX-2 inhibitors as well as an optimal dye for coupling to the parent inhibitor molecule (Uddin, et al., 2010; Uddin, et al., 2013). The authors identified the nonselective COX inhibitor indomethacin as the best affinity scaffold over other COX inhibitors such as celecoxib and nonsteroidal anti-inflammatory drugs (NSAIDs) (Uddin, et al., 2013). Further, fluorescent conjugation to indomethacin scaffolds as well as optimizing the length and electronic properties of the linker could confer selectivity in inhibition to COX-2. For instance, the red fluorophore 5-ROX conferred COX-2 selectivity but was only potent when the ethylenediamide linker was increased from 2 to 4 carbons, illustrating the significant effects that the chemical properties of the dyes and linkers have on overall probe performance (Uddin, et al., 2013). This COX-2 probe, termed fluorocoxib A (Figure 3F), has been used to image colon cancer polyps (Uddin, et al., 2010) as well as non-melanoma skin cancer using non-invasive detection methods (Ra, et al., 2015), however use of the 5-ROX fluorophore (λex = 580 nm, λem = 605 nm) may limit its clinical translatability to human patients.
In addition to these affinity-based probes that are currently advancing to, or are already in, clinical trials, there are a number of commercially available optical imaging tools for basic cancer research and preclinical animal studies. Perkin Elmer markets a folate receptor-specific probe, the FolateRSense 680; the IntegriSense probe, a non-peptidic small molecule designed as an integrin avb3 antagonist; BombesinRSense 680, a 7-amino acid NIR-labeled peptide analog; OsteoSense, a bisphosphonate probe targeting bone growth and resorption; Transferrin-Vivo probe, which utilizes transferrins to target transferrin receptors; and the XenoLight RediJet probe which targets COX-2 using a high affinity inhibitor (http://www.perkinelmer.com/Catalog/Category/ID/Targeted). All of these probes make use of ligands with high affinity for a given target to generate optical imaging probes that follow distinct cell types or enzymatic targets associated with disease.
Finally, though this review focuses mainly on chemically tractable optical probes, there are a number of clinically advanced optical probes composed of monoclonal antibodies conjugated to fluorescent reporters. These antibody-based probes utilize the same targeting principle as the recognition elements of affinity-based probes; that is, they exploit targets overexpressed on tumor cells in comparison to healthy tissue. Many of these clinically advanced probes exploit monoclonal antibodies (mAbs) and fluorescent tags that, as separate entities, have already been approved for clinical use. For example, approved mAbs specific to epidermal growth factor receptor (EGFR), the chimeric (human/mouse) mAb cetuximab and the fully human mAb panitumumab, have been conjugated to IRDye800. Both mAb-based probes are currently in Phase I clinical trials for use in head and neck squamous cell carcinoma (HNSCC) (Rosenthal, et al., 2015) (clinicaltrials.gov identifiers: NCT01987375 and NCT 02415881). In addition to EGFR specific mAbs, the mAb bevacizumab specific to vascular endothelial growth factor A (VEGF-A) has been conjugated to IRDye800CW for use in tumor resection. This mAb-based probe has been used in Phase I and II clinical trials to aid in the removal tumors in breast cancer (clinicaltrials.gov identifiers: NCT01508572 and NCT02583568), and is also being investigated in a Phase I clinical trial as an endoscopic contrast agent to detect adenomas in patients with familial adenomatous polyposis (clinicaltrials.gov identifier: NCT 02113202). More detailed discussion of these antibody-based probes and their design strategies can be found in the reviews (Nguyen and Tsien, 2013; Warram, et al., 2014).
There are number of advantages and disadvantages to the affinity-based approach. Because many protein biomarkers of cancer already bind to specific ligands, probes can be built by simply attaching reporter groups to an existing high affinity ligand. Optimization of the linker and dye can further improve affinity and in vivo properties of these tools. A further advantage is that the reporter tag can be readily replaced with other imaging tracers (i.e. for PET- or MR-imaging) or with therapeutic payloads, thus utilizing the specificity of the probe to not only report on the site of tumor cells, but also to selectively deliver therapeutic agents. However, affinity reagents are limited by the binding specificity of the probe and expression levels of the target in the tumor. Target expression levels can differ between different patients with the same type of tumor (van Dam, et al., 2011), as well as among different tumor cells within the same patient due to tumor heterogeneity. This highlights the importance of pre-operative biopsies to ensure the target of interest is expressed before intraoperative use, as well as the need to continue to improve the dyes and technologies in optical contrast agents to allow noninvasive pre-operative planning and diagnostics. Until tools that allow full body scanning with these probes becomes a reailty, dual optical/PET probes could be used for this purpose. Finally, the primary disadvantage of these affinity-based probes is that imaging contrast is only generated by removal of unbound agents, and is therefore controlled by the diffusion rate of the probe and biological clearance in vivo. This generally prevents affinity agents from being used on short time scales or for local delivery in topical application.
Substrate and Activity-based Probes
Activity-based probes (ABPs) and enzyme substrates are molecules that measure the activity of an enzymatic target. ABP and substrate probes exploit the catalytic activity of a target to generate an imaging signal. The main difference between ABPs and substrates is that substrates are processed and released by the target enzyme while ABPs remain covalently bound to the active site. Like affinity probes, ABPs and substrates are typically designed to target specific enzymatic activities associated with a particular disease tissue. To provide specific contrast, they make use of a change in fluorescent signal upon processing or binding to the enzyme target. This includes a quenching mechanism where only the product is fluorescent or a fluorescence energy transfer (FRET) mechanism where fluorescent signals change wavelength upon processing. This ability to report signal only when acted on by a target enzyme allows rapid imaging, as there is no need to remove the unbound agent. A number of strategies have been developed to take advantage of changes in enzyme activities in cancer to generate signal contrast (Figures 4 and 5).
Figure 4. Substrate and activity-based probes.
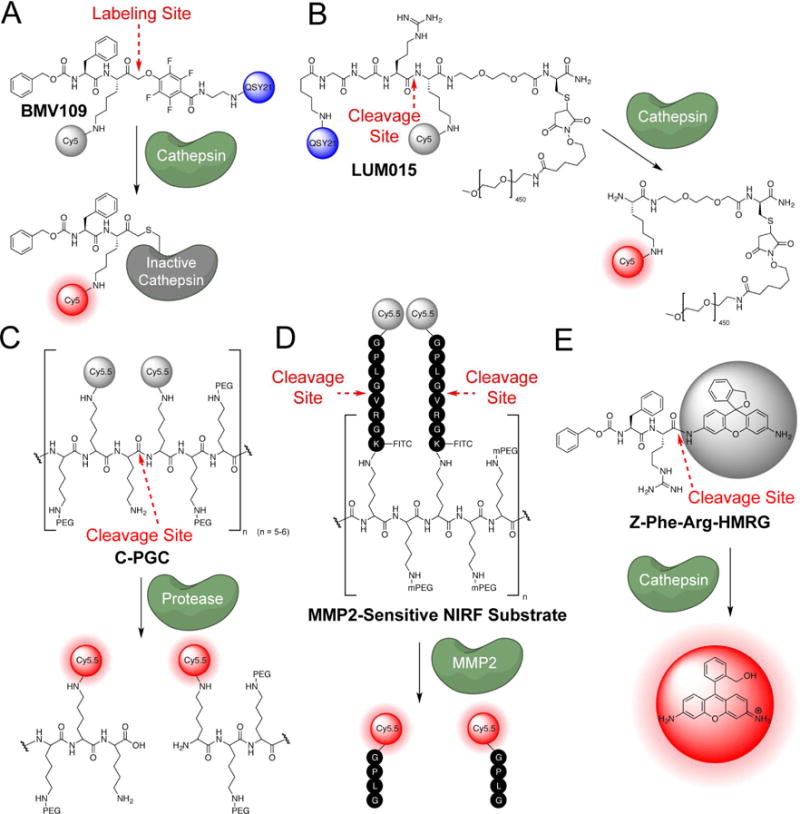
(ABPs) A. The cathespin targeted covalent probe BMV109 B. The cathepsin targeted substrate LUM015 C. The polyer-based protease substrate probe C-PGC probe D. A polymer MMP-2-sensitive NIRF substrate E. The turn-on fluorophore based probe for cathepsins, Z-Phe-Arg-HMRG.
Figure 5. Substrate and activity-based probes (ABPs) structures.
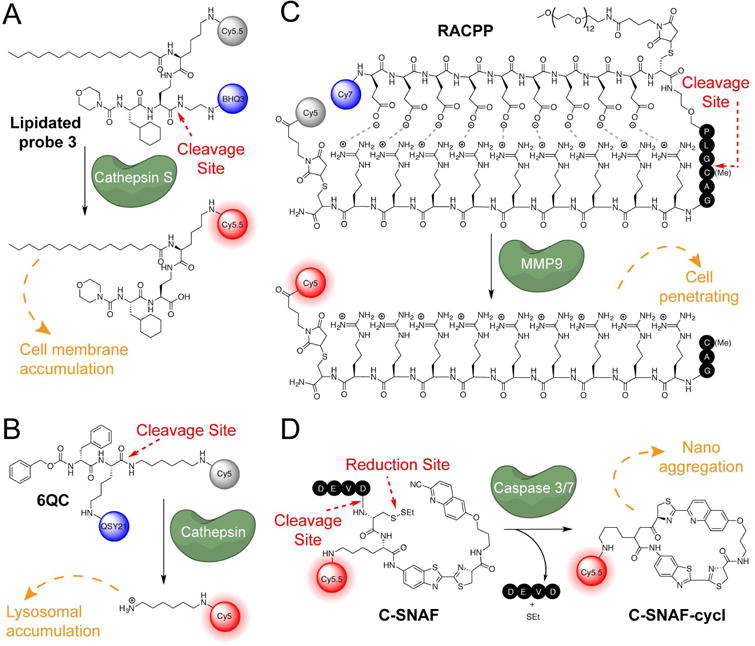
A. Cathespin targeted lipidated probe 3 B. The cathepsin targeting probe, 6QCNIR C. The cell penetrating probe, RACPP D. Nanoaggregating probe for caspases, C-SNAF.
Many groups have developed protease-based ABPs and substrate probes, focusing efforts on specific proteases upregulated in cancers. Specifically, cysteine cathepsins alter the tumor microenvironment by directly cleaving components of the extracellular matrix (ECM) such as laminin and fibronectin as well as adhesion proteins and other proteases, allowing cancer cells to escape and metastasize (Bromme, 2011; Mohamed and Sloane, 2006). They are also highly expressed in activated macrophages making them ideal markers of both cancer and inflammation. Additionally, matrix metalloproteinases (MMPs) and serine proteases degrade the ECM, allowing the cancer to invade surrounding tissues (Bromme, 2011; Mohamed and Sloane, 2006). Further, metabolic acidosis in the tumor microenvironment caused by lactic acid buildup from glycolysis induces the activation of extracellular cysteine cathepsins which are released by both tumor and tumor-associated cells such as macrophages (Bromme, 2011; Mohamed and Sloane, 2006). Due to the high levels of cysteine cathepsins in tumors, a number of covalent ABPs and substrates for this class of proteases have been developed.
Fluorescent ABPs that covalently modify cysteine cathepsins were first described over a decade ago, and made use of reactive electrophiles such as epoxides, diazomethyl ketones (DMKs) and acyloxymethyl ketones (AOMKs) (Powers, et al., 2002). However, all of the original cathepsin ABPs contained a fluorophore that was not quenched and, therefore, like the affinity probes, unbound probe had to be removed to obtain image contrast. A significant improvement came in the form of probes containing a quenching group on the AOMK electrophile that is lost upon covalent target modification (Blum, et al., 2005; Blum, et al., 2007). Further refinement of the electrophile to 2,3,5,6-tetrafluoro phenoxymethyl ketone (PMK) resulted in probes with greatly improved in vivo stability. One such PMK probe, BMV109, is a fluorescently quenched pan-cathepsin probe that is effective at labeling tumors in mouse models of cancer (Figure 4A)(Verdoes, et al., 2013). Cathepsins recognize the Cbz-Phe-Lys peptide sequence of the probe backbone and upon cleavage, the enzyme is inactivated by the PMK electrophile (Edgington, et al., 2013). Because the probe covalently labels target proteases, selectivity of the probe can be directly assessed using mass spectrometry-based approaches. Once the selectivity of the probe is known, SDS-PAGE gel electrophoresis of labeled tissues serves as a direct fluorescent readout to determine which cathepsins have upregulated enzymatic activity in a given tumor tissue. In addition, the covalent nature of the interaction increases signal durability, and the overall small size of the probe provides rapid signal generation. BMV109 has been used for noninvasive optical imaging in animal models of breast and colon cancer and can topically label tissues (Segal, et al., 2015). The general peptide scaffold of the ABP can also be modified to generate non-peptidic analogs with a high degree of selectivity for individual cathepsins, such as cathepsin S-specific probes (Oresic Bender, et al., 2015; Verdoes, et al., 2012). Furthermore, earlier generations of the covalent cathepsin probes have been used for topical labeling of brain cancer cells (Cutter, et al., 2012), suggesting that these small molecule turn-on probes could be used for direct visualization during surgical intervention.
While covalently labeling proteases has the advantage of localizing the probe to the site of proteolysis and thereby attenuating probe clearance, the disadvantage of enzyme inactivation is that it does not allow signal amplification that results from continued processing events after release of the probe. To generate signal amplification, substrate probes have been designed that do not covalently inactivate the protease of interest, but are instead cleaved to produce a fluorescent product. Indeed, many groups have shown the utility of employing substrate probes to image protease activity. One of the earliest fluorescently quenched imaging substrates for proteases was generated by conjugating a NIR dye to a poly-L-lysine backbone modified with additional mPEG side chains (Figure 4C)(Weissleder, et al., 1999). This probe localizes to the lysosome where lysosomal proteolytic systems (which include cysteine proteases, serine proteases, and other hydrolases) enzymatically cleave the probe at Lys-Lys junctions. The NIR Cy5.5 dyes are endogenously quenched due to proximity when conjugated to the poly-L-lysine backbone, but produce a signal as they are released from the polymer (Weissleder, et al., 1999). Using this poly lysine probe as a foundation, additional classes of imaging probes targeting MMPs by inserting a peptide substrate (GPLG*VRGK(FITC)) between the poly-Lys backbone and the fluorophore were developed (Figure 4D)(Bremer, et al., 2001). By conjugating many fluorophore/peptide reporter groups to each probe, sites of upregulated MMPs can be visualized with high sensitivity. These probe scaffolds were subsequently developed into the ProSense and MMPSense probes that are commercially available from Perkin Elmer (http://www.perkinelmer.com/Catalog/Category/ID/Activatable).
In another example of protease substrates, Turk et al. designed probes based on the structures of highly selective inhibitors by converting them into substrates of specific cathepsin proteases (Hu, et al., 2014; Watzke, et al., 2008). Using this ‘reverse-design’ approach, they engineered a substrate-based probe that is highly selective for cathepsin S (Link and Zipfel, 2006). The probe was designed from a non-peptidic, high affinity inhibitor to control selectivity and benefit from the optimized in vivo properties of the drug scaffold. These substrate probes produce a high signal in transplanted tumors in a short timeframe, generating signal as soon as 30 minutes after probe injection (Hu, et al., 2014).
One of the major issues with using substrate probes is that the product of the cleavage reaction is usually a small molecule fragment with the potential to diffuse away from the site of interest. This is particularly apparent with larger polymer-based probes, where the intact non-fluorescent imaging agent is slow to enter tissues and the cleavage products have more rapid diffusion out of the tumor, resulting in slow turn on and rapid signal loss (Blum, et al., 2009; Edgington, et al., 2009). To address this issue, several groups have used chemical appendages such as lipids (Hu, et al., 2014) and PEG linkers (Lee, et al., 2014) to increase signal retention. In a recent example, Turk et al. optimized the substrate-based cathepsin probe from Watzke et al. mentioned above by adding a palmitoyl lipid chain to promote cell membrane localization (termed lipidated probe 3; Figure 5A) (Hu, et al., 2014). In a 4T1 grafted tumor mouse model, the cleavable substrate functionalized with palmitic acid on the reporter end achieved a signal twice as bright as the previous non-lipidated probe after 24 hours, with continued signal accumulation for 8 days post-injection (Hu, et al., 2014). The authors attribute the long-term retention of the probe as well as the high signal-to-noise ratio to the lipid attachment and hypothesize that the lipid functionalization promotes binding of the probe to albumin in the blood, increasing circulation times. Additionally, the authors speculate that lipidation localizes the probe predominantly to the cell surface, allowing the signal to accumulate after cleavage and blocking diffusion out of the cell. The rapid signal production of these agents compared to the original ProSense polymer probes is likely due to the fact that the relatively small size of the reverse-designed probes allows rapid diffusion into the tumor tissues.
There are examples of successful molecular probes that are currently in clinical trials that make use of a large polymer attached to the cleaved substrate. LUM015 (Lee, et al., 2014), a chemical probe developed by Lumicell (http://lumicell.com), has shown promising results in mice and dogs (Lee, et al., 2014). It is a fluorescently quenched substrate probe consisting of a PEGylated (average 450, MW = 22kDa) NIR Cy5 fluorophore attached to a QSY21 quencher (Figure 4B). A pan-cathepsin Gly-Gly-Arg recognition sequence was chosen to allow its use in imaging a variety of cancers. LUM015 has been tested in mouse models that were genetically engineered to develop sarcomas (Kirsch, et al., 2007), as well as in xenograft (MMTV) breast cancer mouse models. When injected 6–24 hours prior to surgery, no adverse effects were seen (even at 25x normal dose) in mice, and the Lumicell group were able to achieve 12.8x (sarcoma) and 8.3x (xenograft) signal to noise ratios in their mouse models. Additionally, Lumicell has used their probe in a preclinical study of canines with naturally occurring cancers. LUM015 showed no related adverse effects in these 12 canines and intraoperative imaging of 49 tissue samples showed 100% correlation with the pathology results confirming cancer (Lee, et al., 2014). LUM015 is currently in phase 1 clinical trials for soft tissue sarcomas and breast cancer, as well as feasibility clinical trials for breast cancer and gastrointestinal cancers of the colon, esophagus and pancreas (clinicaltrials.gov identifiers: NCT01626066, NCT02438358, NCT02584244).
While some probes take advantage of the benefits of large probe size in their design, this strategy may increase background in undesired tissues as well as slow uptake into tumors. To address the problem of rapid diffusion of small molecule substrate probes while avoiding major modifications to the probe scaffold, the fluorescent reporter can be positioned to exploit the acidic environment of the lysosome. Since cleavage of the nascent amide bond by a protease target yields a fragment containing a free amine group, placement of the fluorophore on this part of the probe results in a reporter fragment that can be protonated and therefore trapped in the lysosome. This latent lysosomotropic effect (LLE) has been used to design quenched cathepsin protease probes that produce fluorescent fragments with long half-lives after cleavage. One such probe, 6QCNIR (Figure 5B), produced rapid fluorescent signals in cancers of the lung, breast and colon, and was compatible with the FDA-approved da Vinci® imaging system equipped with the Firefly detection system (Ofori, et al., 2015). Therefore, this class of probes could be readily advanced into clinical studies.
A similar approach to address the problem of signal loss by diffusion is to design probes that expose a cell penetrating peptide upon cleavage. Therefore, processing by a protease exposes a peptide sequence carrying a reporter dye that rapidly enters cells and becomes trapped. Tsien and Nguyen et al. have designed a ratiometric activatable cell penetrating peptide (RACPP) that enters cells and is retained intracellularly upon cleavage by proteases associated with the tumor microenvironment (Savariar, et al., 2013). The probe contains an ionic zipper comprised of the cell penetrating poly-D-arginine sequence modified with a Cy5 dye as well as a stretch of poly-D-glutamates linked to a Cy7 dye (Figure 5C). These two peptides ‘stick’ to each other due to ionic charge interactions and prevent cell penetration by the poly-Arg cell penetrating peptide. By linking the two peptides with a protease cleavage site the probe only produces a cell-permeant dye fragment when cleaved by the protease to release the poly-Glu sequence (Savariar, et al., 2013). This strategy has been used to generate thrombin-, MMP-, and elastase-specific probes (Jiang, et al., 2004; Olson, et al., 2009; Savariar, et al., 2013; Whitney, et al., 2010; Whitney, et al., 2013). Furthermore, FRET interaction of the two dyes decreases background signal from incomplete quenching and increases overall specific signal by measuring a ratio of one dye fluorescence to the other. This eliminates biases in the overall signal strength that result from differential accumulation of the probe, which can dramatically alter overall signal strength when using quenched probes. Sponsored by Avelas Biosciences, the RACPP probe AVB-620 is currently in Phase 1 clinical trials for surgical removal of breast cancer (clinicaltrials.gov identifier: NCT02391194).
As an alternative to the quenched and FRET-based probes described so far, some probes contain a caged fluorophore, which generates a fluorescent signal upon processing by the target enzyme. Acetylated hydroxymethyl rhodamine green (Ac-HMRG) is a closed spirocyclic, non-fluorescent molecule. However, the non-acetylated HMRG, exists as an open molecule that is highly fluorescent. Urano et al. have functionalized this fluorophore with various substrate moieties to produce a fluorescent signal when removed by a target enzyme. Targets include leucine aminopeptidase (Leu-HMRG) (Sakabe, et al., 2013), fibroblast activation protein (Ac-GlyPro-HMRG) (Sakabe, et al., 2013), β-galactosidase (βGal-HMRG) (Kamiya, et al., 2011), γ-glutamyltransferase (gGlu-HMRG) (Urano, et al., 2011), and most recently cathepsins (ZFR-HMRG) (Figure 4E) (Fujii, et al., 2014). This type of turn-on dye has great promise, as it is relatively small and does not require additional large quencher groups. Recent work has demonstrated the feasibility of such turn-on dyes in clinically relevant human samples from breast-conserving surgery. In freshly excised human breast specimens, application of the gGlu-HMRG probe for 5 minutes allowed discrimination of tumors as small as 1 mm in size from normal mammary tissues with 92% sensitivity and 94% specificity (Ueo, et al., 2015). The HMRG fluorophore has an emission wavelength of 521 nm, which may limit its use in vivo due to high background and low penetrance of light at this wavelength. It is likely that further design will lead to turn-on dyes with higher wavelength emissions in the NIR range.
Another unique mechanism for generation of turn-on optical probes makes use of an enzyme-induced chemical reaction leading to microaggregate formation that promotes retention of the fluorescent signals inside target cells. Rao et al. designed a chemical probe fitted with an NIR dye that forms microaggregates under specific tumor microenvironmental conditions. The probe contains a cleavage site for caspases -3 and -7 and an additional disulfide linked thioethyl group. In the reducing tumor microenvironment with upregulated caspases, the chemical probe C-SNAF undergoes an intramolecular cyclization to produce C-CNAF-cycl that then self assembles into aggregates in situ (Figure 5D)(Ye, et al., 2014). This aggregation can be attributed to the pi stacking of C-SNAF-cycl and requires the macrocylization event. With this probe, Rao et al. were able to non-invasively image apoptosis in HeLa tumor-bearing mice that had undergone three rounds of doxorubicin chemotherapy. Activation by this two-step bioorthogonal mechanism improves specificity, and its adaptation to target cancer-specific enzymes could be used to improve signal to noise signal in cancer imaging.
Outlook and future directions for optical chemical probes
Despite a growing body of basic research and promising evidence gathered in early-phase clinical trials supporting the utility of optical contrast agents for surgical guidance (Figure 6), significant hurdles remain before molecularly targeted contrast agents are approved by the FDA and included in standard surgical workflows. Currently, the only FDA-approved optical imaging agents include ICG, MB and fluorescein. Even though none of these agents provide any kind of directed targeting, they are finding widespread use in various aspects of surgical care, confirming the clinical value of optical guidance.
Figure 6. Examples of optical chemical probes being used in animal models for noninvasive cancer diagnostics, in vivo, and ex vivo surgical guidance.
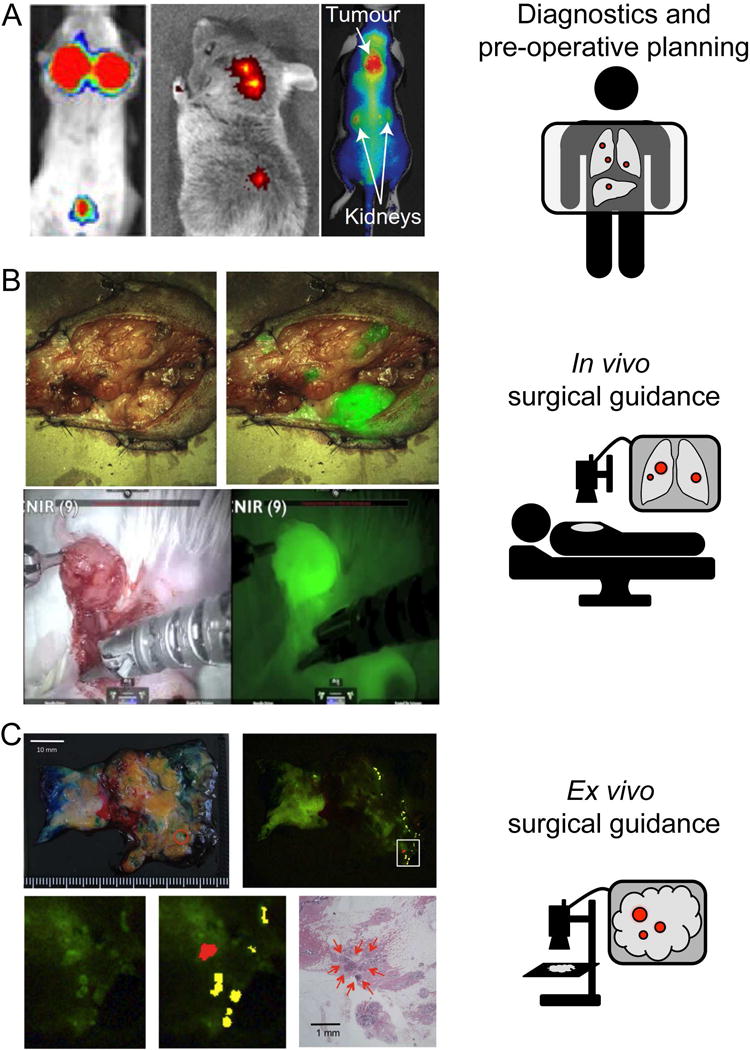
A. LEFT: 6QC (20 nmol IV), imaged after 4 hr in 4T1 Breast cancer mouse model, MIDDLE: Folate-Dylight680 (10 nmol IV), imaged after 4 hr in FR-expressing L1210A tumor mouse model, RIGHT: C-SNAF (5 nmol IV), imaged after 1 hr in three times DOX treated tumor-bearing mice. B. TOP: CTX:Cy5.5. Image shows white light and white light with NIR overlay on a canine tumor tissue sample. BOTTOM: 6QCNIR injected 6h prior to surgery. Image shows white light on the left and fluorescence using the da Vinci® surgical system on right. C. gGlu-HMRG (3 mL of 5 μM in 0.5% v/v DMSO in RPMI1640) topically applied to patient specimen diagnosed with invasive ductal carcinoma (papillotubular) and imaged after 5 min.
An important challenge in translating the chemical tools from academic research laboratories into the clinic involves the regulation and approval process from the FDA. Currently, there is no defined pathway for approval of optical contrast agents, which fall in between categories designated for microdose (defined as less than 100 μg total dose) imaging agents and therapeutics. At the moment, the doses necessary for optical agents to achieve adequate signal are above the microdose level, yet the agents are only administered once before surgery (Rosenthal, et al., 2015). Additionally, there are regulatory and approval challenges associated with the design of human clinical trials for these agents, as the definition of desired outcomes for phase II or III clinical trials are not clear. Specifically, randomization would pose a challenge, as the surgeon could not be blinded to the arm of the study in which each patient was placed. Further, there are many confounding factors in assessing the success of surgery or improving overall survival of a given patient or disease, making it difficult to assess efficacy of the contrast agent alone. Given the significant unmet clinical need for surgical guidance tools coupled with the obvious value of improved visual contrast for many aspects of surgery, we speculate that a unique pathway will be required that straddles the requirements for microdose compounds and therapeutics. In much the same way that the FDA is now being called upon to work with scientists to develop pathways for the efficient regulation of new genomic technologies (Collins and Varmus, 2015), we similarly hope it will define a path for optical contrast agents to move into the clinic in a way that preserves efficiency but ensures efficacy and safety.
One potential alternate route for rapid generation of human clinical data is to make dual optical/PET agents, or analogs of optical probes functionalized with PET radiolabels in place of the fluorescent dyes. This strategy would allow them to first enter into clinical trials and gain FDA approval using microdosing, which provides adequate contrast for PET modalities, and leverages the microdosing regulatory procedures of PET contrast agents to pave the way for an optical imaging analog to follow(Rosenthal, et al., 2015) (Rosenthal, et al., 2015). Ultimately, however, these agents will have to move through alternate routes to become FDA-approved at doses that are adequate for optical-only imaging applications.
Beyond FDA approval, the cost of the agents compared to the potential for reimbursement from insurance companies poses another challenge to the adoption of optical contrast agents into surgical workflows. While it remains unclear what the cost will be to implement optical contrast agents, and costs may also be somewhat probe- and application-dependent, an improvement in patient outcomes coupled with a decrease in re-excision surgery argues for the reimbursement of these tools. As a representative example, we consider the cost-benefit analysis of re-excision surgeries in the treatment of breast cancer using breast conservation surgery, or lumpectomy procedures. The percentage of patients that are post-operatively identified to have positive surgical margins requiring repeated surgeries falls in a wide range, between 17.7 – 72% (Brown, et al., 2013; Huston, et al., 2006; Jacobs, 2008; Mendez, et al., 2006). The cost of re-excision surgeries are high, with the total annual cost for repeated lumpectomy procedures reported by one hospital to be $273,800, or $4721 per patient (Arora, 2015). Further, reimbursement rates for re-excision surgeries are low and are not always fully covered by insurance companies, with the same hospital reporting institutional losses from Medicare reimbursements of $540 per re-excision surgery (Arora, 2015). Re-excision surgery represents a high cost burden on the healthcare system and highlights the potential for optical contrast agents to decrease overall healthcare costs while simultaneously improving patient outcomes.
Precision imaging is a promising interventional technology that complements the advances of biomedical and clinical medicine in the diagnosis and treatment of human cancers. Many of the chemical probes detailed here have the potential to become clinically available in the next 5 – 10 years and decrease morbidity associated with surgical procedures. Regardless of the time and cost required to develop such contrast agents, current success with both pre-clinical and clinically applied optical probes suggests that this technology will have a significant positive impact in the quality of healthcare in the future.
Acknowledgments
This work was funded by National Institutes of Health Grant R01 EB005011 (to M.B.), the Stanford Medical Scientist Training Program (to M.G.) and by a National Science Foundation Graduate Research Fellowship grant DGE-114747 (to J.J.Y.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akcan M, Stroud MR, Hansen SJ, Clark RJ, Daly NL, Craik DJ, Olson JM. Chemical re-engineering of chlorotoxin improves bioconjugation properties for tumor imaging and targeted therapy. J Med Chem. 2011;54:782–787. doi: 10.1021/jm101018r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreesen R, Modolell M, Weltzien HU, Eibl H, Common HH, Lohr GW, Munder PG. Selective destruction of human leukemic cells by alkyl-lysophospholipids. Cancer Res. 1978;38:3894–3899. [PubMed] [Google Scholar]
- Antaris AL, Chen H, Cheng K, Sun Y, Hong G, Qu C, Diao S, Deng Z, Hu X, Zhang B, et al. A small-molecule dye for NIR-II imaging. Nat Mater. 2015 doi: 10.1038/nmat4476. [DOI] [PubMed] [Google Scholar]
- Antony AC. Folate receptors. Annu Rev Nutr. 1996;16:501–521. doi: 10.1146/annurev.nu.16.070196.002441. [DOI] [PubMed] [Google Scholar]
- Aoki T, Murakami M, Yasuda D, Shimizu Y, Kusano T, Matsuda K, Niiya T, Kato H, Murai N, Otsuka K, et al. Intraoperative fluorescent imaging using indocyanine green for liver mapping and cholangiography. J Hepatobiliary Pancreat Sci. 2010;17:590–594. doi: 10.1007/s00534-009-0197-0. [DOI] [PubMed] [Google Scholar]
- Arora D, H S, Eneida M, Abid S, Eneida M, Abid R, Ord C, Dauway E. Cost analysis of re-excisions for breast conserving surgery in Central Texas. Journal of Clinical Oncology, 2015 ASCO Annual Meeting. 2015;33:e11534. [Google Scholar]
- Banerjee SR, Foss CA, Castanares M, Mease RC, Byun Y, Fox JJ, Hilton J, Lupold SE, Kozikowski AP, Pomper MG. Synthesis and evaluation of technetium-99m- and rhenium-labeled inhibitors of the prostate-specific membrane antigen (PSMA) J Med Chem. 2008;51:4504–4517. doi: 10.1021/jm800111u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee SR, Pullambhatla M, Byun Y, Nimmagadda S, Foss CA, Green G, Fox JJ, Lupold SE, Mease RC, Pomper MG. Sequential SPECT and optical imaging of experimental models of prostate cancer with a dual modality inhibitor of the prostate-specific membrane antigen. Angew Chem Int Ed Engl. 2011;50:9167–9170. doi: 10.1002/anie.201102872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett JA, Coleman RE, Goldsmith SJ, Vallabhajosula S, Petry NA, Cho S, Armor T, Stubbs JB, Maresca KP, Stabin MG, et al. First-in-man evaluation of 2 high-affinity PSMA-avid small molecules for imaging prostate cancer. J Nucl Med. 2013;54:380–387. doi: 10.2967/jnumed.112.111203. [DOI] [PubMed] [Google Scholar]
- Blum G, Mullins SR, Keren K, Fonovic M, Jedeszko C, Rice MJ, Sloane BF, Bogyo M. Dynamic imaging of protease activity with fluorescently quenched activity-based probes. Nat Chem Biol. 2005;1:203–209. doi: 10.1038/nchembio728. [DOI] [PubMed] [Google Scholar]
- Blum G, von Degenfeld G, Merchant MJ, Blau HM, Bogyo M. Noninvasive optical imaging of cysteine protease activity using fluorescently quenched activity-based probes. Nat Chem Biol. 2007;3:668–677. doi: 10.1038/nchembio.2007.26. [DOI] [PubMed] [Google Scholar]
- Blum G, Weimer RM, Edgington LE, Adams W, Bogyo M. Comparative assessment of substrates and activity based probes as tools for non-invasive optical imaging of cysteine protease activity. PLoS One. 2009;4:e6374. doi: 10.1371/journal.pone.0006374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremer C, Tung CH, Weissleder R. In vivo molecular target assessment of matrix metalloproteinase inhibition. Nat Med. 2001;7:743–748. doi: 10.1038/89126. [DOI] [PubMed] [Google Scholar]
- Bromme D, W S. Role of Cysteine Cathepsins in Extracellular Proteolysis. In: W.C.P.a.R.P. cMecham, editor. Extracellular Matrix Degradation. Springer; Berlin Heidelberg: 2011. pp. 23–51. [Google Scholar]
- Brown JQ, Bydlon TM, Kennedy SA, Caldwell ML, Gallagher JE, Junker M, Wilke LG, Barry WT, Geradts J, Ramanujam N. Optical spectral surveillance of breast tissue landscapes for detection of residual disease in breast tumor margins. PLoS One. 2013;8:e69906. doi: 10.1371/journal.pone.0069906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchs NC, Hagen ME, Pugin F, Volonte F, Bucher P, Schiffer E, Morel P. Intra-operative fluorescent cholangiography using indocyanin green during robotic single site cholecystectomy. Int J Med Robot. 2012;8:436–440. doi: 10.1002/rcs.1437. [DOI] [PubMed] [Google Scholar]
- Chen Y, Dhara S, Banerjee SR, Byun Y, Pullambhatla M, Mease RC, Pomper MG. A low molecular weight PSMA-based fluorescent imaging agent for cancer. Biochem Biophys Res Commun. 2009;390:624–629. doi: 10.1016/j.bbrc.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho SY, Gage KL, Mease RC, Senthamizhchelvan S, Holt DP, Jeffrey-Kwanisai A, Endres CJ, Dannals RF, Sgouros G, Lodge M, et al. Biodistribution, tumor detection, and radiation dosimetry of 18F-DCFBC, a low-molecular-weight inhibitor of prostate-specific membrane antigen, in patients with metastatic prostate cancer. J Nucl Med. 2012;53:1883–1891. doi: 10.2967/jnumed.112.104661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi HS, Gibbs SL, Lee JH, Kim SH, Ashitate Y, Liu F, Hyun H, Park G, Xie Y, Bae S, et al. Targeted zwitterionic near-infrared fluorophores for improved optical imaging. Nat Biotechnol. 2013;31:148–153. doi: 10.1038/nbt.2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collection, L.o.C.C. ABC News special. President Obama’s State of the Union address; 2015. [2015-01-20] [Google Scholar]
- Collins FS, Varmus H. A new initiative on precision medicine. N Engl J Med. 2015;372:793–795. doi: 10.1056/NEJMp1500523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutter JL, Cohen NT, Wang J, Sloan AE, Cohen AR, Panneerselvam A, Schluchter M, Blum G, Bogyo M, Basilion JP. Topical application of activity-based probes for visualization of brain tumor tissue. PLoS One. 2012;7:e33060. doi: 10.1371/journal.pone.0033060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshane J, Garner CC, Sontheimer H. Chlorotoxin inhibits glioma cell invasion via matrix metalloproteinase-2. J Biol Chem. 2003;278:4135–4144. doi: 10.1074/jbc.M205662200. [DOI] [PubMed] [Google Scholar]
- Destito G, Yeh R, Rae CS, Finn MG, Manchester M. Folic acid-mediated targeting of cowpea mosaic virus particles to tumor cells. Chem Biol. 2007;14:1152–1162. doi: 10.1016/j.chembiol.2007.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberhart CE, Coffey RJ, Radhika A, Giardiello FM, Ferrenbach S, DuBois RN. Up-regulation of cyclooxygenase 2 gene expression in human colorectal adenomas and adenocarcinomas. Gastroenterology. 1994;107:1183–1188. doi: 10.1016/0016-5085(94)90246-1. [DOI] [PubMed] [Google Scholar]
- Edgington LE, Berger AB, Blum G, Albrow VE, Paulick MG, Lineberry N, Bogyo M. Noninvasive optical imaging of apoptosis by caspase-targeted activity-based probes. Nat Med. 2009;15:967–973. doi: 10.1038/nm.1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgington LE, Verdoes M, Ortega A, Withana NP, Lee J, Syed S, Bachmann MH, Blum G, Bogyo M. Functional imaging of legumain in cancer using a new quenched activity-based probe. J Am Chem Soc. 2013;135:174–182. doi: 10.1021/ja307083b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii T, Kamiya M, Urano Y. In vivo imaging of intraperitoneally disseminated tumors in model mice by using activatable fluorescent small-molecular probes for activity of cathepsins. Bioconjug Chem. 2014;25:1838–1846. doi: 10.1021/bc5003289. [DOI] [PubMed] [Google Scholar]
- Gribar SC, Hamad GG. Ischemic bowel after laparoscopic Roux-en-Y gastric bypass: limited resection based on fluorescein assessment of bowel viability. Surg Obes Relat Dis. 2007;3:561–563. doi: 10.1016/j.soard.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Group, U.S.C.S.W. Incidence and Mortality Web-based Report. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute; Atlanta: 2015. United States Cancer Statistics: 1999–2012. [Google Scholar]
- Henley SJ, Singh S, King J, Wilson R, Ryerson B, Centers for Disease, C., and Prevention Invasive cancer incidence – United States, 2010. MMWR Morb Mortal Wkly Rep. 2014;63:253–259. [PMC free article] [PubMed] [Google Scholar]
- Hu HY, Vats D, Vizovisek M, Kramer L, Germanier C, Wendt KU, Rudin M, Turk B, Plettenburg O, Schultz C. In vivo imaging of mouse tumors by a lipidated cathepsin S substrate. Angew Chem Int Ed Engl. 2014;53:7669–7673. doi: 10.1002/anie.201310979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang SS, Wang X, Zhang Y, Doke A, DiFilippo FP, Heston WD. Improving the biodistribution of PSMA-targeting tracers with a highly negatively charged linker. Prostate. 2014;74:702–713. doi: 10.1002/pros.22789. [DOI] [PubMed] [Google Scholar]
- Huston TL, Pigalarga R, Osborne MP, Tousimis E. The influence of additional surgical margins on the total specimen volume excised and the reoperative rate after breast-conserving surgery. Am J Surg. 2006;192:509–512. doi: 10.1016/j.amjsurg.2006.06.021. [DOI] [PubMed] [Google Scholar]
- Howlader N, Krapcho M, Garshell J, Miller D, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA, editors. Institute, N.C. SEER Cancer Statistics Review, 1975–2012. National Cancer Institute; 2014. N.A. [Google Scholar]
- Jacobs L. Positive margins: the challenge continues for breast surgeons. Ann Surg Oncol. 2008;15:1271–1272. doi: 10.1245/s10434-007-9766-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang T, Olson ES, Nguyen QT, Roy M, Jennings PA, Tsien RY. Tumor imaging by means of proteolytic activation of cell-penetrating peptides. Proc Natl Acad Sci U S A. 2004;101:17867–17872. doi: 10.1073/pnas.0408191101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya M, Asanuma D, Kuranaga E, Takeishi A, Sakabe M, Miura M, Nagano T, Urano Y. beta-Galactosidase fluorescence probe with improved cellular accumulation based on a spirocyclized rhodol scaffold. J Am Chem Soc. 2011;133:12960–12963. doi: 10.1021/ja204781t. [DOI] [PubMed] [Google Scholar]
- Kirsch DG, Dinulescu DM, Miller JB, Grimm J, Santiago PM, Young NP, Nielsen GP, Quade BJ, Chaber CJ, Schultz CP, et al. A spatially and temporally restricted mouse model of soft tissue sarcoma. Nat Med. 2007;13:992–997. doi: 10.1038/nm1602. [DOI] [PubMed] [Google Scholar]
- Kozikowski AP, Nan F, Conti P, Zhang J, Ramadan E, Bzdega T, Wroblewska B, Neale JH, Pshenichkin S, Wroblewski JT. Design of remarkably simple, yet potent urea-based inhibitors of glutamate carboxypeptidase II (NAALADase) J Med Chem. 2001;44:298–301. doi: 10.1021/jm000406m. [DOI] [PubMed] [Google Scholar]
- Lee WD, Bawendi MG, Ferrer J. Imaging agent for detection of diseased cells. Lumicell, Inc.; USA: 2014. [Google Scholar]
- Lepor H. A review of surgical techniques for radical prostatectomy. Rev Urol. 2005;7(Suppl 2):S11–17. [PMC free article] [PubMed] [Google Scholar]
- Link JO, Zipfel S. Advances in cathepsin S inhibitor design. Curr Opin Drug Discov Devel. 2006;9:471–482. [PubMed] [Google Scholar]
- Lyons SA, O’Neal J, Sontheimer H. Chlorotoxin, a scorpion-derived peptide, specifically binds to gliomas and tumors of neuroectodermal origin. Glia. 2002;39:162–173. doi: 10.1002/glia.10083. [DOI] [PubMed] [Google Scholar]
- Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986;46:6387–6392. [PubMed] [Google Scholar]
- McGinty JJ, Jr, Hogle N, Fowler DL. Laparoscopic evaluation of intestinal ischemia using fluorescein and ultraviolet light in a porcine model. Surg Endosc. 2003;17:1140–1143. doi: 10.1007/s00464-001-8255-y. [DOI] [PubMed] [Google Scholar]
- Mease RC, Dusich CL, Foss CA, Ravert HT, Dannals RF, Seidel J, Prideaux A, Fox JJ, Sgouros G, Kozikowski AP, et al. N-[N-[(S)-1,3-Dicarboxypropyl]carbamoyl]-4-[18F]fluorobenzyl-L-cysteine, [18F]DCFBC: a new imaging probe for prostate cancer. Clin Cancer Res. 2008;14:3036–3043. doi: 10.1158/1078-0432.CCR-07-1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez JE, Lamorte WW, de Las Morenas A, Cerda S, Pistey R, King T, Kavanah M, Hirsch E, Stone MD. Influence of breast cancer margin assessment method on the rates of positive margins and residual carcinoma. Am J Surg. 2006;192:538–540. doi: 10.1016/j.amjsurg.2006.06.009. [DOI] [PubMed] [Google Scholar]
- Mohamed MM, Sloane BF. Cysteine cathepsins: multifunctional enzymes in cancer. Nat Rev Cancer. 2006;6:764–775. doi: 10.1038/nrc1949. [DOI] [PubMed] [Google Scholar]
- Neuman BP, Eifler JB, Castanares M, Chowdhury WH, Chen Y, Mease RC, Ma R, Mukherjee A, Lupold SE, Pomper MG, et al. Real-time, near-infrared fluorescence imaging with an optimized dye/light source/camera combination for surgical guidance of prostate cancer. Clin Cancer Res. 2015;21:771–780. doi: 10.1158/1078-0432.CCR-14-0891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen QT, Tsien RY. Fluorescence-guided surgery with live molecular navigation–a new cutting edge. Nat Rev Cancer. 2013;13:653–662. doi: 10.1038/nrc3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Shannessy DJ, Yu G, Smale R, Fu YS, Singhal S, Thiel RP, Somers EB, Vachani A. Folate receptor alpha expression in lung cancer: diagnostic and prognostic significance. Oncotarget. 2012;3:414–425. doi: 10.18632/oncotarget.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofori LO, Withana NP, Prestwood TR, Verdoes M, Brady JJ, Winslow MM, Sorger J, Bogyo M. Design of Protease Activated Optical Contrast Agents That Exploit a Latent Lysosomotropic Effect for Use in Fluorescence-Guided Surgery. ACS Chem Biol. 2015;10:1977–1988. doi: 10.1021/acschembio.5b00205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson ES, Aguilera TA, Jiang T, Ellies LG, Nguyen QT, Wong EH, Gross LA, Tsien RY. In vivo characterization of activatable cell penetrating peptides for targeting protease activity in cancer. Integr Biol (Camb) 2009;1:382–393. doi: 10.1039/b904890a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oresic Bender K, Ofori L, van der Linden WA, Mock ED, Datta GK, Chowdhury S, Li H, Segal E, Sanchez Lopez M, Ellman JA, et al. Design of a highly selective quenched activity-based probe and its application in dual color imaging studies of cathepsin S activity localization. J Am Chem Soc. 2015;137:4771–4777. doi: 10.1021/jacs.5b00315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orosco RK, Tsien RY, Nguyen QT. Fluorescence imaging in surgery. IEEE Rev Biomed Eng. 2013;6:178–187. doi: 10.1109/RBME.2013.2240294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park MH, Hyun H, Ashitate Y, Wada H, Park G, Lee JH, Njiojob C, Henary M, Frangioni JV, Choi HS. Prototype nerve-specific near-infrared fluorophores. Theranostics. 2014;4:823–833. doi: 10.7150/thno.8696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker N, Turk MJ, Westrick E, Lewis JD, Low PS, Leamon CP. Folate receptor expression in carcinomas and normal tissues determined by a quantitative radioligand binding assay. Anal Biochem. 2005;338:284–293. doi: 10.1016/j.ab.2004.12.026. [DOI] [PubMed] [Google Scholar]
- Pinchuk AN, Rampy MA, Longino MA, Skinner RW, Gross MD, Weichert JP, Counsell RE. Synthesis and structure-activity relationship effects on the tumor avidity of radioiodinated phospholipid ether analogues. J Med Chem. 2006;49:2155–2165. doi: 10.1021/jm050252g. [DOI] [PubMed] [Google Scholar]
- Pomper MG, Musachio JL, Zhang J, Scheffel U, Zhou Y, Hilton J, Maini A, Dannals RF, Wong DF, Kozikowski AP. 11C-MCG: synthesis, uptake selectivity, and primate PET of a probe for glutamate carboxypeptidase II (NAALADase) Mol Imaging. 2002;1:96–101. doi: 10.1162/15353500200202109. [DOI] [PubMed] [Google Scholar]
- Powers JC, Asgian JL, Ekici OD, James KE. Irreversible inhibitors of serine, cysteine, and threonine proteases. Chem Rev. 2002;102:4639–4750. doi: 10.1021/cr010182v. [DOI] [PubMed] [Google Scholar]
- Ra H, Gonzalez-Gonzalez E, Uddin MJ, King BL, Lee A, Ali-Khan I, Marnett LJ, Tang JY, Contag CH. Detection of non-melanoma skin cancer by in vivo fluorescence imaging with fluorocoxib A. Neoplasia. 2015;17:201–207. doi: 10.1016/j.neo.2014.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal EL, Warram JM, Bland KI, Zinn KR. The status of contemporary image-guided modalities in oncologic surgery. Ann Surg. 2015;261:46–55. doi: 10.1097/SLA.0000000000000622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal EL, Warram JM, de Boer E, Basilion JP, Biel MA, Bogyo M, Bouvet M, Brigman BE, Colson YL, DeMeester SR, et al. Successful Translation of Fluorescence Navigation During Oncologic Surgery: A Consensus Report. J Nucl Med. 2015 doi: 10.2967/jnumed.115.158915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal EL, Warram JM, de Boer E, Chung TK, Korb ML, Brandwein-Gensler M, Strong TV, Schmalbach CE, Morlandt AB, Agarwal G, et al. Safety and Tumor Specificity of Cetuximab-IRDye800 for Surgical Navigation in Head and Neck Cancer. Clin Cancer Res. 2015;21:3658–3666. doi: 10.1158/1078-0432.CCR-14-3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe SP, Gage KL, Faraj SF, Macura KJ, Cornish TC, Gonzalez-Roibon N, Guner G, Munari E, Partin AW, Pavlovich CP, et al. (1)(8)F-DCFBC PET/CT for PSMA-Based Detection and Characterization of Primary Prostate Cancer. J Nucl Med. 2015;56:1003–1010. doi: 10.2967/jnumed.115.154336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe SP, Macura KJ, Ciarallo A, Mena E, Blackford A, Nadal R, Antonarakis E, Eisenberger M, Carducci M, Ross A, et al. Comparison of PSMA-based 18F-DCFBC PET/CT to Conventional Imaging Modalities for Detection of Hormone-Sensitive and Castration-Resistant Metastatic Prostate Cancer. J Nucl Med. 2015 doi: 10.2967/jnumed.115.163782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakabe M, Asanuma D, Kamiya M, Iwatate RJ, Hanaoka K, Terai T, Nagano T, Urano Y. Rational design of highly sensitive fluorescence probes for protease and glycosidase based on precisely controlled spirocyclization. J Am Chem Soc. 2013;135:409–414. doi: 10.1021/ja309688m. [DOI] [PubMed] [Google Scholar]
- Saul JM, Annapragada A, Natarajan JV, Bellamkonda RV. Controlled targeting of liposomal doxorubicin via the folate receptor in vitro. J Control Release. 2003;92:49–67. doi: 10.1016/s0168-3659(03)00295-5. [DOI] [PubMed] [Google Scholar]
- Savariar EN, Felsen CN, Nashi N, Jiang T, Ellies LG, Steinbach P, Tsien RY, Nguyen QT. Real-time in vivo molecular detection of primary tumors and metastases with ratiometric activatable cell-penetrating peptides. Cancer Res. 2013;73:855–864. doi: 10.1158/0008-5472.CAN-12-2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schols RM, Bouvy ND, van Dam RM, Masclee AA, Dejong CH, Stassen LP. Combined vascular and biliary fluorescence imaging in laparoscopic cholecystectomy. Surg Endosc. 2013;27:4511–4517. doi: 10.1007/s00464-013-3100-7. [DOI] [PubMed] [Google Scholar]
- Schols RM, Bouvy ND, van Dam RM, Stassen LP. Advanced intraoperative imaging methods for laparoscopic anatomy navigation: an overview. Surg Endosc. 2013;27:1851–1859. doi: 10.1007/s00464-012-2701-x. [DOI] [PubMed] [Google Scholar]
- Schulke N, Varlamova OA, Donovan GP, Ma D, Gardner JP, Morrissey DM, Arrigale RR, Zhan C, Chodera AJ, Surowitz KG, et al. The homodimer of prostate-specific membrane antigen is a functional target for cancer therapy. Proc Natl Acad Sci U S A. 2003;100:12590–12595. doi: 10.1073/pnas.1735443100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal E, Prestwood TR, van der Linden WA, Carmi Y, Bhattacharya N, Withana N, Verdoes M, Habtezion A, Engleman EG, Bogyo M. Detection of intestinal cancer by local, topical application of a quenched fluorescence probe for cysteine cathepsins. Chem Biol. 2015;22:148–158. doi: 10.1016/j.chembiol.2014.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwinter DA. Identification of anomolous biliary anatomy using near-infrared cholangiography. J Gastrointest Surg. 2012;16:1814–1815. doi: 10.1007/s11605-012-1945-z. [DOI] [PubMed] [Google Scholar]
- Siegel R, DeSantis C, Virgo K, Stein K, Mariotto A, Smith T, Cooper D, Gansler T, Lerro C, Fedewa S, et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin. 2012;62:220–241. doi: 10.3322/caac.21149. [DOI] [PubMed] [Google Scholar]
- Snyder F, Blank ML, Morris HP. Occurrence and nature of O-alkyl and O-alk-I-enyl moieties of glycerol in lipids of Morris transplanted hepatomas and normal rat liver. Biochim Biophys Acta. 1969;176:502–510. doi: 10.1016/0005-2760(69)90217-3. [DOI] [PubMed] [Google Scholar]
- Snyder F, Wood R. Alkyl and alk-1-enyl ethers of glycerol in lipids from normal and neoplastic human tissues. Cancer Res. 1969;29:251–257. [PubMed] [Google Scholar]
- Sobolewski C, Cerella C, Dicato M, Ghibelli L, Diederich M. The role of cyclooxygenase-2 in cell proliferation and cell death in human malignancies. Int J Cell Biol. 2010;2010:215158. doi: 10.1155/2010/215158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soroceanu L, Gillespie Y, Khazaeli MB, Sontheimer H. Use of chlorotoxin for targeting of primary brain tumors. Cancer Res. 1998;58:4871–4879. [PubMed] [Google Scholar]
- Srinivasarao M, Galliford CV, Low PS. Principles in the design of ligand-targeted cancer therapeutics and imaging agents. Nat Rev Drug Discov. 2015;14:203–219. doi: 10.1038/nrd4519. [DOI] [PubMed] [Google Scholar]
- Survey CNNHD. Number of all-listed procedures for discharges from short-stay hospitals, by procedure category and age: United States, 2010. CDC; 2012. [Google Scholar]
- Swanson KI, Clark PA, Zhang RR, Kandela IK, Farhoud M, Weichert JP, Kuo JS. Fluorescent cancer-selective alkylphosphocholine analogs for intraoperative glioma detection. Neurosurgery. 2015;76:115–123. doi: 10.1227/NEU.0000000000000622. discussion 123–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tummers QR, Boonstra MC, Frangioni JV, van de Velde CJ, Vahrmeijer AL, Bonsing BA. Intraoperative near-infrared fluorescence imaging of a paraganglioma using methylene blue: A case report. Int J Surg Case Rep. 2015;6C:150–153. doi: 10.1016/j.ijscr.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin MJ, Crews BC, Blobaum AL, Kingsley PJ, Gorden DL, McIntyre JO, Matrisian LM, Subbaramaiah K, Dannenberg AJ, Piston DW, et al. Selective visualization of cyclooxygenase-2 in inflammation and cancer by targeted fluorescent imaging agents. s. 2010;70:3618–3627. doi: 10.1158/0008-5472.CAN-09-2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin MJ, Crews BC, Ghebreselasie K, Marnett LJ. Design, synthesis, and structure-activity relationship studies of fluorescent inhibitors of cycloxygenase-2 as targeted optical imaging agents. Bioconjug Chem. 2013;24:712–723. doi: 10.1021/bc300693w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueo H, Shinden Y, Tobo T, Gamachi A, Udo M, Komatsu H, Nambara S, Saito T, Ueda M, Hirata H, et al. Rapid intraoperative visualization of breast lesions with gamma-glutamyl hydroxymethyl rhodamine green. Sci Rep. 2015;5:12080. doi: 10.1038/srep12080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urano Y, Sakabe M, Kosaka N, Ogawa M, Mitsunaga M, Asanuma D, Kamiya M, Young MR, Nagano T, Choyke PL, et al. Rapid cancer detection by topically spraying a gamma-glutamyltranspeptidase-activated fluorescent probe. Sci Transl Med. 2011;3:110ra119. doi: 10.1126/scitranslmed.3002823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahrmeijer AL, Hutteman M, van der Vorst JR, van de Velde CJ, Frangioni JV. Image-guided cancer surgery using near-infrared fluorescence. Nat Rev Clin Oncol. 2013;10:507–518. doi: 10.1038/nrclinonc.2013.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Blitterswijk WJ, Verheij M. Anticancer mechanisms and clinical application of alkylphospholipids. Biochim Biophys Acta. 2013;1831:663–674. doi: 10.1016/j.bbalip.2012.10.008. [DOI] [PubMed] [Google Scholar]
- van Dam GM, Themelis G, Crane LM, Harlaar NJ, Pleijhuis RG, Kelder W, Sarantopoulos A, de Jong JS, Arts HJ, van der Zee AG, et al. Intraoperative tumor-specific fluorescence imaging in ovarian cancer by folate receptor-alpha targeting: first in-human results. Nat Med. 2011;17:1315–1319. doi: 10.1038/nm.2472. [DOI] [PubMed] [Google Scholar]
- Veiseh M, Gabikian P, Bahrami SB, Veiseh O, Zhang M, Hackman RC, Ravanpay AC, Stroud MR, Kusuma Y, Hansen SJ, et al. Tumor paint: a chlorotoxin:Cy5.5 bioconjugate for intraoperative visualization of cancer foci. Cancer Res. 2007;67:6882–6888. doi: 10.1158/0008-5472.CAN-06-3948. [DOI] [PubMed] [Google Scholar]
- Verbeek FP, Schaafsma BE, Tummers QR, van der Vorst JR, van der Made WJ, Baeten CI, Bonsing BA, Frangioni JV, van de Velde CJ, Vahrmeijer AL, et al. Optimization of near-infrared fluorescence cholangiography for open and laparoscopic surgery. Surg Endosc. 2014;28:1076–1082. doi: 10.1007/s00464-013-3305-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbeek FP, van der Vorst JR, Schaafsma BE, Swijnenburg RJ, Gaarenstroom KN, Elzevier HW, van de Velde CJ, Frangioni JV, Vahrmeijer AL. Intraoperative near infrared fluorescence guided identification of the ureters using low dose methylene blue: a first in human experience. J Urol. 2013;190:574–579. doi: 10.1016/j.juro.2013.02.3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbeek FP, van der Vorst JR, Tummers QR, Boonstra MC, de Rooij KE, Lowik CW, Valentijn AR, van de Velde CJ, Choi HS, Frangioni JV, et al. Near-infrared fluorescence imaging of both colorectal cancer and ureters using a low-dose integrin targeted probe. Ann Surg Oncol. 2014;21(Suppl 4):S528–537. doi: 10.1245/s10434-014-3524-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdoes M, Edgington LE, Scheeren FA, Leyva M, Blum G, Weiskopf K, Bachmann MH, Ellman JA, Bogyo M. A nonpeptidic cathepsin S activity-based probe for noninvasive optical imaging of tumor-associated macrophages. Chem Biol. 2012;19:619–628. doi: 10.1016/j.chembiol.2012.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdoes M, Oresic Bender K, Segal E, van der Linden WA, Syed S, Withana NP, Sanman LE, Bogyo M. Improved quenched fluorescent probe for imaging of cysteine cathepsin activity. J Am Chem Soc. 2013;135:14726–14730. doi: 10.1021/ja4056068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Huang SS, Heston WD, Guo H, Wang BC, Basilion JP. Development of targeted near-infrared imaging agents for prostate cancer. Mol Cancer Ther. 2014;13:2595–2606. doi: 10.1158/1535-7163.MCT-14-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warram JM, de Boer E, Sorace AG, Chung TK, Kim H, Pleijhuis RG, van Dam GM, Rosenthal EL. Antibody-based imaging strategies for cancer. Cancer Metastasis Rev. 2014;33:809–822. doi: 10.1007/s10555-014-9505-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watzke A, Kosec G, Kindermann M, Jeske V, Nestler HP, Turk V, Turk B, Wendt KU. Selective activity-based probes for cysteine cathepsins. Angew Chem Int Ed Engl. 2008;47:406–409. doi: 10.1002/anie.200702811. [DOI] [PubMed] [Google Scholar]
- Weichert JP, Clark PA, Kandela IK, Vaccaro AM, Clarke W, Longino MA, Pinchuk AN, Farhoud M, Swanson KI, Floberg JM, et al. Alkylphosphocholine analogs for broad-spectrum cancer imaging and therapy. Sci Transl Med. 2014;6:240ra275. doi: 10.1126/scitranslmed.3007646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissleder R, Tung CH, Mahmood U, Bogdanov A., Jr In vivo imaging of tumors with protease-activated near-infrared fluorescent probes. Nat Biotechnol. 1999;17:375–378. doi: 10.1038/7933. [DOI] [PubMed] [Google Scholar]
- Weitman SD, Lark RH, Coney LR, Fort DW, Frasca V, Zurawski VR, Jr, Kamen BA. Distribution of the folate receptor GP38 in normal and malignant cell lines and tissues. Cancer Res. 1992;52:3396–3401. [PubMed] [Google Scholar]
- Whitney M, Crisp JL, Olson ES, Aguilera TA, Gross LA, Ellies LG, Tsien RY. Parallel in vivo and in vitro selection using phage display identifies protease-dependent tumor-targeting peptides. J Biol Chem. 2010;285:22532–22541. doi: 10.1074/jbc.M110.138297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney M, Savariar EN, Friedman B, Levin RA, Crisp JL, Glasgow HL, Lefkowitz R, Adams SR, Steinbach P, Nashi N, et al. Ratiometric activatable cell-penetrating peptides provide rapid in vivo readout of thrombin activation. Angew Chem Int Ed Engl. 2013;52:325–330. doi: 10.1002/anie.201205721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Vlashi E, Low P. Folate-linked drugs for the treatment of cancer and inflammatory diseases. Subcell Biochem. 2012;56:163–179. doi: 10.1007/978-94-007-2199-9_9. [DOI] [PubMed] [Google Scholar]
- Ye D, Shuhendler AJ, Cui L, Tong L, Tee SS, Tikhomirov G, Felsher DW, Rao J. Bioorthogonal cyclization-mediated in situ self-assembly of small-molecule probes for imaging caspase activity in vivo. Nat Chem. 2014;6:519–526. doi: 10.1038/nchem.1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M, Kubota K, Kuroda J, Ohta K, Nakamura T, Saito J, Kobayashi M, Sato T, Beck Y, Kitagawa Y, et al. Indocyanine green injection for detecting sentinel nodes using color fluorescence camera in the laparoscopy-assisted gastrectomy. J Gastroenterol Hepatol. 2012;27(Suppl 3):29–33. doi: 10.1111/j.1440-1746.2012.07067.x. [DOI] [PubMed] [Google Scholar]


