Abstract
Background:
The up-regulation of hepatic Golgi protein 73 (GP73) is associated with the progression of hepatocellular carcinoma (HCC). However, the exact mechanism and clinical values of its diagnosis and prognosis still need to be clarified.
Objectives:
To investigate the clinical values of abnormal liver or circulating GP73 expression and their effect on HCC diagnosis and prognosis.
Materials and Methods:
The expression of GP73 was investigated in 88 cancerous and self-control non-cancerous tissues using tissue microarrays with immunohisto- chemistry and was confirmed by Western blotting. Circulating GP73 levels were detected in the sera of 281 patients with liver diseases using enzyme-linked immunosorbent assay.
Results:
The levels of circulating GP73 expression in the HCC group were higher than those in any group of benign liver diseases or controls. No significant difference was found between GP73 expression and patients’ sex or age, tumor size, or AFP level except for those with hepatitis B virus (HBV) infection or distal metastasis (P < 0.05). The area under the receiver operating characteristic curve, sensitivity, and specificity for HCC diagnosis were 0.881, 78.34%, and 77.59% for GP73 levels over 70 μg/L or 0.754, 71.97%, and 84.48% for alpha-fetoprotein levels over 50 μg/L, respectively. The total incidence of GP73 plus alpha-fetoprotein was up to 87.26% for HCC. A positive GP73 result with brown particles was mainly located in the cytosol, with a few in the nucleus and none in the cell membrane, with abnormal expression in HCC tissues (480.7 ± 148.7) that was significantly higher (t = 10.730, P < 0.001) than those in their non-cancerous tissues (208.0 ± 66.1). The high GP73 expression in HCC was related to lymph node metastasis (χ2 = 6.940, P = 0.008), gross classification (χ2 = 6.311, P = 0.012), HBV (χ2 = 4.803, P = 0.028), tumor node metastasis staging (χ2 = 4.887, P = 0.027), and five-year survival (χ2 = 5.206, P = 0.023).
Conclusions:
Abnormality of hepatic or circulating GP73 expression should be regarded as an emerging biomarker for HCC diagnosis and prognosis.
Keywords: Carcinoma, Hepatocellular, GP73, Diagnosis, Prognosis
1. Background
Hepatocellular carcinoma (HCC) is one of the most common tumors and fatal malignancies worldwide, and its incidence is increasing (1, 2). It is associated with chronic, persistent infection of hepatitis B virus (HBV) or hepatitis C virus (HCV) as well as non-virus etiologies, including alcohol, chemicals, and aflatoxin B1 (3, 4). Most HCC patients are usually asymptomatic until the late stages and therefore are given a dismal prognosis. Because HCC cells are not sensitive to radiotherapy or chemotherapy, hepatic resection or transplantation is the only potential curative treatment (5). In these patients, strategies to detect early HCC are necessary (6). Circulating alpha-fetoprotein (AFP) is considered to be the classical serological marker for screening patients at high risk of HCC, as well as for monitoring their treatment response (7). However, the clinical value of AFP has been questioned due to its low sensitivity and specificity (8-10).
Great progress in understanding the HCC mechanism(s) has been made in recent years, including many gene alterations, such as oncogenes, tumor suppressor genes, apoptosis genes, growth factor genes, and anti-apoptosis genes in hepatocarcinogenesis (11, 12). Therefore, more sensitive diagnostic tools should be investigated. Recently, a novel Golgi protein 73 (GP73) was identified as a resident Golgi-specific membrane protein that is normally expressed in the epithelial cells of many human tissues (13). It is preferentially expressed in biliary epithelial cells, has little or no signal in normal hepatocytes (14, 15), and is upregulated in sera from patients at low levels in hepatitis, with the highest amounts seen in HCC (16).
Abnormal GP73 was first identified in a genetic screen for proteins with differential expression in adult giant-cell hepatitis (17). RNA analysis from multiple tissues revealed a single GP73 mRNA transcript with a size of approximately 3.0 kb that was described as a resident Golgi type II transmembrane protein, with a single, N-terminal transmembrane domain and an extensive, C-terminal coiled-coil domain located on the luminal surface of the Golgi apparatus (17). Their coiled-coil domains function as homotypic or heterotypic protein interaction sites that are essential for the binding, docking, and trafficking of transport vesicles to the cisternal membranes with no homologies to the known glycosyltransferases, are unlikely to have catalytic functions, and have no significant sequence homologies or structural similarities to any of the known nucleotide, sugar, or ATP transporters (18). Many studies have described the application of GP73 as a serum marker for HCC (19, 20); however, the results have been inconsistent and have shown evident heterogeneity. Therefore, in the present study, the goal was to investigate GP73 expression in cancerous tissues and sera of patients with chronic liver diseases to evaluate the diagnostic and prognostic values for HCC.
2. Objectives
In the present study, we aimed to investigate the clinical values of abnormal hepatic or circulating GP73 expression for HCC diagnosis and prognosis.
3. Materials and Methods
3.1. Patient Recruitment
A total of 157 HCC patients were collected (Table 1) from November 2012 to September 2014 at the affiliated hospital of Nantong university in China. The patients’ ages ranged 33 - 85 years. Other cases studied included 58 patients with liver cirrhosis (LC), 66 patients with chronic viral hepatitis (CH), and 47 healthy individuals who were serologically negative for HBV markers (HBsAg, HBcAb, HBV-DNA) or anti-HCV antibody with normal liver function tests from the Nantong Central Blood Bank in China who were used as the control group (26 - 69 years old).
Table 1. Patient Data in the Present Study.
| Group | N | Gender | Age, y | HBsAg, +/- | AFP, μg/L | |||
|---|---|---|---|---|---|---|---|---|
| Male | Female | ≤ 20 | 21 - 399 | ≥ 400 | ||||
| HCC | 157 | 129 | 28 | 30 - 85 | 113/44 | 32 | 69 | 56 |
| Liver cirrhosis | 58 | 36 | 22 | 35 - 82 | 37/21 | 42 | 11 | 5 |
| Chronic hepatitis | 66 | 58 | 8 | 18 - 63 | 61/5 | 57 | 9 | 0 |
| Normal control | 47 | 26 | 21 | 26 - 69 | 0/47 | 47 | 0 | 0 |
Abbreviations: AFP, α-fetoprotein; HBsAg, hepatitis B virus surface antigen; HBsAg (+/-), HBsAg positive/HBsAg negative; HCC, hepatocellular carcinoma.
3.2. Liver Specimens and Clinical Data of HCC Patients
Fresh surgical specimens, including self-control cancer and distal non-cancerous tissues, were intra-operatively obtained from 88 patients (68 men and 20 women, aged 30 – 68 years, mean: 51.7 ± 10.8) from November 2005 to September 2007 at the Affiliated Hospital of Nantong University in China. The sizes of the tumors were > 2 cm in 71 cases and < 2 cm in 17 cases. Twenty-four cases had AFP levels ≥ 400 ng/mL, while 64 were < 400 ng/mL. The number of tumors present was one in 72 cases and multiple tumors in 16. Tumor differentiation was graded using the Edmondson grading system with 30 well-, 45 moderately and 13 poorly differentiated tumors. Tumor staging classified 70 cases (79.5%, 70 of 88) at stages I and II and 18 cases (20.5%, 16 of 88) at III and IV based on the 6th edition of the tumor-node-metastasis (TNM) classification of the International Union against Cancer (IUAC). The incidence of hepatitis B surface antigen (HBsAg) was 81.8% (72 of 88) in these HCC tissues, and 71.6% (63 of 88) had a history of LC. All patients attended regular follow-up. The follow-up period was defined as the duration from the date of operation to the date of either death or the last follow-up through October 2013. The overall survival was evaluated using the duration between the dates of surgery and death.
The specimens were snap frozen in liquid nitrogen and stored at -80°C until use. A diagnosis of HCC was made based on the criteria proposed by the National Collaborative Cancer Research of China (21) in addition to Edmondson grading, TNM staging, and histological examination. Each specimen was divided into two parts for the immuno-histochemical analysis and pathological examination (H and E staining). The diagnosis of HCC was confirmed using histology in all patients. Written informed consent was obtained from all patients, and the study was approved by the medical ethics committee (No.: TDFY2005008) of the Affiliated Hospital of Nantong University, China.
3.3. Tissue Microarrays
All HCC tissues were stained using Hematoxylin and Eosin (H&E) and were reviewed by two histopathologists. Representative areas free from necrotic and hemorrhagic materials were marked in paraffin blocks. The TMA slides were constructed from 2.0 mm tissue cores taken from each representative tumor tissue and peritumoral tissue.
3.4. Immunohistochemical Analysis
The tumor tissues used for immunohistochemistry (IHC) were deparaffinized. The peroxidase was quenched with methanol and 3% H2O2 for 15 minutes. For antigen retrieval, the sections were boiled under pressure in a citrate buffer (pH 6.0) for 3 minutes. Then the tissues were incubated with primary mouse anti-GP73 antibody (SC-48010, Santa Cruz Biotechnology, Inc.) for 1 hour, which was diluted in 1% bovine serum albumin (1:300). Following washing with phosphate buffered saline (PBS), the sections were incubated with peroxidase-conjugated goat anti-mouse antibody (Dako Cytomation, USA) for 15 minutes and then washed again with PBS. The color was developed by 15 minutes incubation with diaminobenzidine solution (Kem-En-Tec Diagnostics, Denmark), and the sections were weakly counterstained with hematoxylin. The primary antibodies were omitted for the negative controls.
3.5. Evaluation of the IHC Findings
After immunostaining, the slides were restained with hematoxylin, dehydrated in a series of ethanol solutions, covered with neutral gum, and observed under the microscope (Olympus BX 50). Blinded evaluations of the immunostaining of the aforementioned GP73 and independent observations were carried out simultaneously. HCC tissues with positive GP73 expression served as the positive control. The level of GP73 expression in the livers according to the number of positive cells was separated into weakly positive (+, 10% - 25%), moderately positive (++, 26% - 75%), and strongly positive (+++, > 75%) categories. According to the above criterion, the HCC tissues with GP73 expression were divided into two groups: low with 0–25% (0 ~ +), and over 26% (++ ~ +++) as high expression.
3.6. Liver Protein Extraction and Measurement
The liver tissue (50 mg) was homogenized for 3 min after the addition of 1.0 mL PBS. The slurry was transferred into tubes (1.5 mL) and centrifuged at 5,000 rpm for 4 minutes at 4°C. The supernatant was put into another tube and stored at 4°C. The concentration of total protein was determined using the bicinchoninic acid (BCA) method. Working reagent was prepared by mixing BCA Reagents A: B (50:1), pipetting 10 μL of each protein standard into a 96-well plate, and diluting the solution to 100 μL with 0.9% normal saline. In addition, the standards were added into the 96-well plate as follows: 0, 1, 2, 4, 8, 12, and 20 μL. Each well received 0.9% normal saline up to a total volume of 20 μL with dilution. Then 5 μL of tissue proteins were pipetted into the wells to an adjusted volume of 20 μL with 0.9% normal saline. In the end, 200 μL BCA working reagent was added, and the sample was incubated at 37°C for 30 minutes. The absorbance was measured at 562 nm on a plate reader.
3.7. Quantitative Detection of GP73 and AFP levels
The levels of serum GP73 expression were detected using an enzyme-linked immunosorbent assay (ELISA) kit (R&D, USA) according to the manufacturer’s instructions. Fifty microliters of diluted serum samples (1:1000 in sample dilution solution) or the standard solution were separately put into each well of a 96-well ELISA plate and incubated for 30 minutes at 37°C. Next, 50 mL of horse radish peroxidase (HRP)-conjugate was added to the wells, which were incubated for 1 hour at 37°C. Substrate A (50 mL) and substrate B (50 mL) were added to each well for 15 minutes at 37°C. Then 50 mL of stop solution were added to each well. The absorbance was read at 450 nm. During the procedure, the plate was washed according to the routine ELISA method. The levels of AFP were tested using commercially available immunoassays utilizing enhanced chemiluminescence at our hospital’s central laboratory. The upper limit of the normal level was 40 μg/L.
3.8. Western Blotting
The proteins in the supernatants were subjected to Western blotting as described previously. Protein (100 μg) was purified from liver tissue lysed in an RIPA lysis buffer containing PMSF, separated on 10% SDS polyacrylamide gel, and electro- transferred to a PVDF membrane. After blocking with nonfat dry milk in a TBST buffer, the membrane was probed with the anti-GP73 antibody (1:500, Abcam, UK), followed by incubation with the HRP-conjugated anti-goat IgG secondary antibody (1:1000, Shanghai Beyotime Institute of Biotechnology, China) and then visualized with an enhanced chemiluminescence reagent. Anti-GAPDH antibody was used for the purposes of normalization. The band images detected by the ChemiDoc XRS+ system (Bio-Rad, USA) were analyzed using Quantity One (ver. 4.62) software.
3.9. Statistical Analysis
Descriptive statistics for GP73 in the different groups were compared by an examination of the histograms. All values were reported as mean ± SD. Comparisons between any two groups were performed using the Kruskal-Wallis one-way analysis of variance (ANOVA) test and the two-tailed Student’s t-test, assuming equal variances. Survival curves were calculated using the Kaplan-Meier method and compared with the log-rank test. Factors shown to be of prognostic significance with the univariate Cox regression model were subsequently evaluated using the multivariate Cox regression model. To determine the optimal cut-off value for GP73 in the diagnosis of HCC and liver diseases, receiver-operating characteristic (ROC) curves were constructed using the area under the curves (AUC) determined. All analyses were performed using the Statistical Package for the Social Sciences (SPSS) version 18.0 software (SPSS Inc., Chicago, IL, USA) and GraphPad Prism 5. A P value < 0.05 was considered statistically significant.
4. Results
4.1. Circulating GP73 Expression in Different Liver Diseases
The levels of circulating GP73 expression in patients with different liver diseases are shown in Table 2. The average levels of serum GP73 or AFP expression were 66.75 μg/L or 1432.71 μg/L in HCC, 62.30 μg/L or 167.35 μg/L in LC, 49.86 μg/L or 71.36 μg/L in CH, and 28.83 μg/L or 8.31 μg/L in the control group, respectively. Both values in each group of liver diseases were higher than those in the control group. Serum GP73 is significantly increased in HCC patients compared with healthy, chronic hepatitis, and cirrhosis controls. The correlations between serum GP73 levels and other pathological characteristics are shown in Table 3. No significant differences were found between GP73 expression and patients’ sex or age, tumor size, or AFP level except for HBV infection or the distal metastasis group (P < 0.05).
Table 2. Levels of GP73 and AFP (μg/L) in the Sera of Patients With Different Liver Diseases.
| Group | N | GP73a | P Valueb | AFPa | P Valueb | ||
|---|---|---|---|---|---|---|---|
| Normal control | 47 | 0.58 - 58.31 | 28.83 ± 21.00 | 0.21 - 18.97 | 8.31 ± 5.56 | ||
| Chronic hepatitis | 66 | 3.25 - 154.27 | 49.86 ± 40.46 | 0.002 | 1.59 - 261.96 | 71.36 ± 101.78 | < 0.001 |
| Liver cirrhosis | 58 | 6.94 - 290.02 | 62.30 ± 56.30 | < 0.001 | 1.11 - 493.56 | 167.35 ± 159.04 | < 0.001 |
| HCC | 157 | 14.04 ~ 383.26 | 66.75 ± 69.97 | < 0.001 | 1.14 ~ 5783.55 | 1432.71 ± 1895.33 | < 0.001 |
Abbreviations: AFP, α-fetoprotein; GP73, Golgi protein 73; HCC, hepatocellular carcinoma.
aData are presented as The range and mean ± SD.
bP value vs. the normal control group.
Table 3. Clinical Features of GP73 Expression in the Sera of HCC Patients.
| Variables | N | GP73, μg/La | T Value | P-Value | GP73 Positiveb | χ2 | P-Value |
|---|---|---|---|---|---|---|---|
| HCC | 157 | 66.75 ± 69.97 | NA | 123 (78.34) | NA | ||
| Age, y | 1.731 | 0.085 | 2.801 | 0.094 | |||
| ≤ 50 | 96 | 61.81 ± 62.50 | 71 (73.96) | ||||
| > 50 | 61 | 78.64 ± 54.06 | 52 (85.25) | ||||
| Tumor, cm | 1.236 | 0.218 | 0.125 | 0.724 | |||
| ≤ 5.0 | 56 | 58.32 ± 48.97 | 43 (76.79) | ||||
| > 5.0 | 101 | 71.99 ± 74.26 | 80 (79.21) | ||||
| AFP, μg/L | 0.781 | 0.436 | 2.966 | 0.085 | |||
| ≤ 400.0 | 94 | 72.29 ± 70.05 | 78 (82.98) | ||||
| > 400.0 | 63 | 64.11 ± 54.73 | 45 (71.43) | ||||
| HBV | 2.340 | 0.021 | 7.794 | 0.005 | |||
| Yes | 113 | 91.67 ± 78.01 | 95 (84.07) | ||||
| No | 44 | 61.04 ± 60.85 | 28 (63.64) | ||||
| Metastasis | 2.167 | 0.032 | 5.862 | 0.016 | |||
| With | 64 | 59.13 ± 52.77 | 44 (68.75) | ||||
| Without | 93 | 73.49 ± 69.69 | 79 (84.95) |
Abbreviations: AFP, α-fetoprotein; GP73, Golgi protein 73; HBV, hepatitis B virus infection; NA, not available.
aData are presented as mean ± SD.
bData are presented as No. (%).
4.2. Diagnostic Value of Circulating GP73 for HCC
The GP73 level showed an increasing trend with the progression of liver disease. The incidence was 78.34% in HCC, 37.93% in LC, 22.73% in CH, and none in controls at a level more than 70 μg/l. The GP73 level in HCC patients (66.75 ± 69.97 μg/L) was three times higher than that in the control group (P < 0.001). The relationship between circulating GP73 and AFP expression and the areas under the receiver operating characteristic curve (AUROC) for HCC are shown in Figure 1. There was no significant relationship between GP73 expression and AFP level (Figure 1A). The AUROC was 0.881 (95% CI: 0.824 - 0.939) for the GP73 level with 70 μg/L as an optimal cutoff point and 0.754 (95% CI: 0.673 - 0.836) for the AFP level with 50 μg/L as a cutoff value (Figure 1B).
Figure 1. The Receiver Operating Characteristic (ROC) Curves of Circulating GP73 and AFP Levels in HCC Patients.
The ROC curves were created according to the levels of serum GP73 and AFP in patients with hepatocellular carcinoma. Sensitivity: the true-positive rate. Specificity: the false-positive rate. The AUROC was 0.881 for GP73 and 0.754 for AFP.
4.3. Complementary Diagnostic Value of Serum GP73 for HCC
A comprehensive evaluation of serum GP73 and AFP levels for HCC diagnosis are shown in Table 4. When the cutoff value is over 70 μg/L for GP73 or 50 μg/L for AFP, to evaluate the diagnostic efficiency, the GP73 or AFP marker had to be 78.34% or 71.97% in sensitivity and 77.59% or 84.48% in specificity for HCC, respectively. However, the total incidence combining the GP73 and AFP detection could rise up to 87.26% (137 of 157).
Table 4. Comprehensive Evaluation of Serum GP73 and AFP (μg/L) for HCC Diagnosis.
| GP73 > 70 | AFP > 50 | GP7370 + AFP50 | |
|---|---|---|---|
| Sensitivity | 78.34 | 71.97 | 87.26 |
| Specificity | 77.59 | 84.48 | 74.14 |
| Positive expectations | 90.44 | 92.62 | 90.13 |
| Negative expectations | 56.96 | 52.69 | 68.25 |
| Accuracy | 78.14 | 75.35 | 83.72 |
Abbreviations: AFP, α-fetoprotein; GP73, Golgi protein 73; GP7370 + AFP50 μg/L (%), total incidence of HCC cases that were GP73 level more than 70 μg/L plus AFP level more than 50 μg/L.
4.4. Cellular Distribution of Liver GP73 Expression
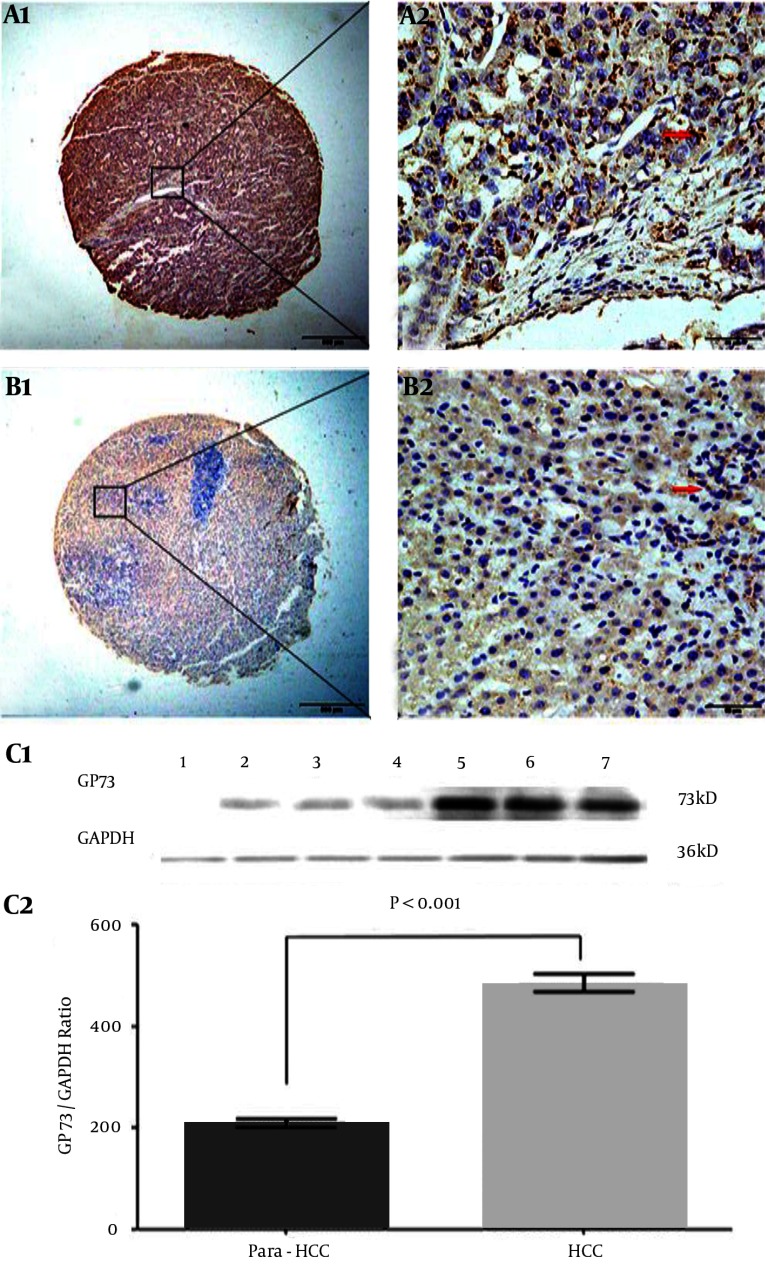
The features, cellular distribution, and relative quantitative levels of hepatic GP73 expression in HCC and their non-cancerous tissues are shown in Figure 2. The positive brown particles of GP73 expression in the liver tissue microarray by immunohistochemical staining were mainly located in the cytosol, with a few in the nucleus and none in the cell membrane in poorly differentiated (Figure 2 A1 and A2) or well-differentiated (Figure 2 B1 and B2) HCC tissue. The distribution of positive cells was clear and heterogeneous, with the cytoplasmic staining being significantly higher in HCC than in the non-cancerous tissues. The GP73 expressions in different liver tissues were confirmed by Western blotting (Figure 2 C1). The stripe grey values of GP73 protein were relatively quantified in HCC or their non-cancerous tissues (Figure 2 C2). More positive diffuse staining was localized in the cancerous tissues, and the ratio of GP73/GAPDH was significantly higher (t = 10.730, P < 0.001) in the cancerous group (480.7 ± 148.7) than that in the non-cancerous group (208.0 ± 66.1).
Figure 2. Characteristics of Liver GP73 Expression and Cellular Distribution.
The brown GP73 expressions of liver tissues were stained by tissue microarray using immunohistochemical technology. A1 and A2, the GP73 expression in poorly differentiated HCC tissue; B1 and B2, the GP73 expression in well-differentiated HCC tissue. Original magnification × 40 in A1 and B1 or × 400 in A2 and B2; as a negative control, the second goat anti-rabbit antibody without pre-staining for specific GP73 monoclonal antibody did not give any significant background staining. In HCC tissues, the positive cells were often localized in periportal areas. GP73: Golgi protein 73; HCC: hepatocellular carcinoma. C1, the GP73 expression of different tissues was confirmed by Western blotting. Lane 1: the control liver tissue; Lanes 2–4: the non-cancerous tissues; Lanes 5 - 7: the HCC tissues; GP73, GP73 protein (73 kD); GAPDH, glyceraldehyde-phosphate dehydrogenase (36 kD) as a control. C2, the above stripe grey values of GP73 protein were relatively quantitated in HCC or their non-cancerous tissues.
4.5. Clinicopathologic Characteristics of High GP73 Expression in HCC
The correlation between high GP73 levels and the clinicopathological parameters in the tissues of 88 HCC patients is shown in Table 5. A high GP73 expression was associated with regional lymph node metastasis (TNM, χ2 = 6.940, P = 0.008), gross classification (χ2 = 6.311, P = 0.012), higher TNM staging (χ2 = 4.887, P = 0.027), HBV infection (χ2 = 4.803, P = 0.028), and 5-year survival (χ2 = 5.206, P = 0.023). By contrast, there were no significant associations between high GP73 expression and other clinico-pathological features, such as HCC patients’ gender or age, tumor number or size, AFP level, liver cirrhosis, and differentiation degree.
Table 5. Clinicopathological Characteristics of High GP73 Expression in HCC Tissuesa.
| Variables | N | GP73 High Expression | χ2 | P Value |
|---|---|---|---|---|
| Tumor diameter | 0.052 | .819 | ||
| ≤ 2.0 cm | 17 (19.32) | 11 (64.71) | ||
| > 2.0 cm | 71 (80.68) | 48 (67.61) | ||
| Differentiation | 0.209 | .647 | ||
| Well-Moderate | 75 (85.23) | 51 (68.00) | ||
| Poor | 13 (14.77) | 8 (61.54) | ||
| AFP, ng/mL | 3.695 | .055 | ||
| ≤ 400 | 64 (72.73) | 40 (62.50) | ||
| > 400 | 24 (27.27) | 19 (79.17) | ||
| Liver cirrhosis | 0.147 | .702 | ||
| Yes | 63 (71.59) | 43 (68.25) | ||
| No | 25 (28.41) | 16 (64.00) | ||
| Lymph node metastasis | 6.940 | .008 | ||
| Yes | 25 (28.41) | 22 (88.00) | ||
| No | 63 (71.59) | 37 (58.73) | ||
| Gross classification | 6.311 | .012 | ||
| Multifocal | 16 (18.18) | 15 (93.75) | ||
| Unifocal | 72 (81.82) | 44 (61.11) | ||
| HBV infection | 4.803 | .028 | ||
| Positive | 72 (81.81) | 52 (72.22) | ||
| Negative | 16 (18.18) | 7 (43.75) | ||
| TNM Staging | 4.887 | .027 | ||
| Stage I - II | 70 (79.55) | 43 (61.43) | ||
| Stage III - IV | 18 (20.45) | 16 (88.89) | ||
| Five-year survival | 5.206 | .023 | ||
| Yes | 23 (26.14) | 11 (47.83) | ||
| No | 65 (73.86) | 48 (73.85) |
Abbreviations: AFP, α-fetoprotein; GP73, Golgi protein 73; HBV, hepatitis B virus infection; TNM, tumor node metastasis.
aData are presented as No. (%).
4.6. Prognostic Values of High GP73 Expression for HCC Patients’ Survival
The univariate and multivariable analysis of prognostic variables for the 5-year survival rate of HCC is shown in Table 6. The results of univariate Cox regression analyses for all variables revealed that up-regulated GP73 was a significant prognostic factor for HCC (hazard ratio [HR]: 1.008, 95% CI: 1.002 - 1.014, P = 0.005). Kaplan-Meier survival analysis of GP73 further confirmed that increasing the GP73 level was significantly associated with a shortened overall survival of HCC, which was gradually decreased with the increasing GP73 expression (Figure 3). The survival curve corresponding to different GP73 levels showed a good distinction without too much overlap, presenting the success of score standard for evaluating the GP73 staining and the reliability of GP73 as a prognostic factor.
Table 6. Univariate and Multivariable Analysis of Prognostic Variables.
| Variables | N | Univariate Analysis | Multivariable Analysis | ||||
|---|---|---|---|---|---|---|---|
| HR | P Value | 95% CI | HR | P Value | 95% CI | ||
| Tumor diameter | 0.990 | 0.978 | 0.506 - 1.938 | NA | NA | NA | |
| ≤ 2 cm | 17 | ||||||
| > 2 cm | 71 | ||||||
| Differentiation | 1.112 | 0.795 | 0500 - 2.473 | NA | NA | NA | |
| Well–Moderate | 75 | ||||||
| Poor | 13 | ||||||
| Liver cirrhosis | 0.840 | 0.580 | 0.452 - 1.559 | NA | NA | NA | |
| Yes | 63 | ||||||
| No | 25 | ||||||
| Portal vein invasion | 0.198 | < 0.001 | 0.086 - 0.458 | 0.195 | < 0.001 | 0.084 - 0.454 | |
| Yes | 16 | ||||||
| No | 72 | ||||||
| Lymph node metastasis | 0.139 | 0.002 | 0.040 - 0.478 | 0.138 | 0.002 | 0.039 - 0.495 | |
| Yes | 25 | ||||||
| No | 63 | ||||||
| Gross classification | 0.410 | 0.009 | 0.211 - 0.797 | 0.389 | 0.008 | 0.194 - 0.777 | |
| Multifocal | 16 | ||||||
| Unifocal | 72 | ||||||
| HBV infection | 0.356 | 0.035 | 0.136 - 0.929 | 0.348 | 0.035 | 0.130 - 0.929 | |
| Positive | 72 | ||||||
| Negative | 16 | ||||||
| TNM Stage | 0.231 | < 0.001 | 0.121 - 0.440 | 0.221 | < 0.001 | 0.114 - 0.426 | |
| I - II | 70 | ||||||
| III - IV | 18 | ||||||
| GP73 intensity | 1.008 | 0.005 | 1.002 - 1.014 | 0.477 | 0.010 | 0.272 - 0.837 | |
| High | 59 | ||||||
| Low | 29 | ||||||
Abbreviations: CI, confidence interval; GP73, Golgi protein 73; HBV, hepatitis B virus infection; HR, hazard ratio; NA, not available; TNM, tumor node metastasis.
Figure 3. Survival Curves of HCC Patients Postoperatively.
The survival curves of 88 patients with hepatocellular carcinoma were created according to hepatic GP73 expression using the Kaplan-Meier method. The green line is the high GP73 expression group, and the blue line is the low- or no-GP73 expression group.
Besides, portal vein invasion (HR: 0.198, 95% CI: 0.086 - 0.458, P < 0.001), lymph node metastasis (HR: 0.139, 95% CI: 0.040 - 0.478, P = 0.002), gross classification (HR: 0.410, 95% CI: 0.211 - 0.797, P = 0.009), HBV infection (HR: 0.356, 95% CI: 0.136 - 0.929, P = 0.035), and TNM staging (HR: 0.231, 95% CI: 0.121 - 0.440, P < 0.001) had a significantly poor prognosis. Multivariate Cox regression analysis of the same set of HCC (Table 6) further demonstrated that the up-regulation of GP73 was found to be an independent poor prognostic marker for the patient’s survival (HR: 0.477, 95% CI: 0.272 - 0.837, P = 0.010). In addition, portal vein invasion (HR: 0.195, 95% CI: 0.084 - 0.454, P < 0.001), lymph node metastasis (HR: 0.138, 95% CI: 0.039 - 0.495, P = 0.002), gross classification (HR: 0.389, 95% CI: 0.194 - 0.777, P < 0.008), HBV infection (HR: 0.348, 95% CI: 0.130 - 0.929, P = 0.035), and TNM staging (HR: 0.221, 95% CI: 0.114 - 0.426, P < 0.001) were also independent prognostic factors of the patient’s overall survival.
5. Discussion
HCC is one of the most frequently occurring tumors worldwide. GP73 is a resident Golgi-specific membrane protein expressed by biliary epithelial cells in the normal liver and is up-regulated in patients with CH, LC, and HCC (22, 23). Accumulating data have indicated that abnormal hepatic GP73 expression is associated with tumor progression by interacting with the microenvironment and has been related to a worse outcome. However, the results of the previous studies have been inconsistent and have shown evident heterogeneity. In this present study, therefore, the alterations of GP73 expression in tumor tissues of HCC patients and sera of patients with benign or malignant liver diseases were investigated; it was confirmed that GP3 expression could be a useful biomarker for HCC prognosis and prognosis.
Although GP73 was introduced as a new potential candidate to identify HCC, several studies have described GP73 as an HCC-specific marker (24, 25). According to the present data, the GP73 levels of HCC patients were significantly higher than those in patients with cirrhosis. Compared with healthy controls, the GP73 level in patients with liver disease was significantly increased. Of HCC patients, the GP73 level showed a positive skewness distribution, and there is a closely positive relationship between GP73 expression and HBV infection or distal metastasis. No significant difference was found between the GP73 level and sex or age, tumor size, and AFP level. With the development of liver disease, GP73 showed a significantly increasing trend, suggesting that abnormal GP73 might be used as a useful biomarker to monitor the progression of HBV-related liver diseases (26, 27).
The current research approaches are directed at the discovery of early sensitive diagnostic markers and the identification of new tools to predict recurrence (28). Up to now, none of these markers have been sufficiently validated, and their development is limited by routine adaptation of techniques and cost (29, 30). Comprehensive evaluations that combined GP73 and AFP detection were shown in, and both could increase the accuracy (83.7%) and sensitivity (87.3%) of HCC diagnoses. The circulating GP73 and AFP levels have a complementary value for HCC diagnosis with a higher AUROC curve. Although the serological GP73 level is not as specific as GPC-3 or HIF-1α (31, 32), it could be an emerging biomarker for the diagnosis of HCC (33, 34).
The poor prognosis of HCC is attributed to its insidious onset and late presentation at diagnosis (35, 36). Effective screening systems to detect HCC at an early stage may result in more effective treatment to extend patient survival (37). The level of GP73 expression in cancerous tissue is higher than in non-cancerous liver tissue, with positive GP73 staining appearing as brown particles mainly located in the cytosol, with a few in the nucleus and none in the cell membrane; its expression in HCC tissues was clearly heterogeneous, with cytoplasmic GP73 staining in HCC being significantly higher than that observed in the non-cancerous tissues. In contrast, a high level of expression of GP73 was present in diseased hepatocytes, regardless of the cause of liver disease (18). GP73 was expressed at low levels in inflammatory cells or in cells within the cirrhotic septa. This striking difference suggests that GP73 might participate in an important cellular pathway of liver disease (19).
The association between GP73 expression and prognosis during the 5-year follow-up was analyzed using the Kaplan-Meier method and the log-rank test. The overall survival of higher GP73 patients was significantly shorter than that of patients with low GP73 expression. In the Cox proportional hazard model, it was confirmed that GP73 expression in the biopsy samples, tumor stage, HBV infection, and venous infiltration were predictors of a poor prognosis for HCC patients. However, the age, gender, tumor size, and AFP level were not independent predictors for HCC patient survival, indicating that a higher GP73 level in cancerous cells might be related to cell survival and could play a role in preventing the cells from undergoing apoptosis (19, 20).
In conclusion, the abnormality of GP73 expression as a useful biomarker has been confirmed in the diagnosis of and prognosis for HCC. Early diagnosis of HCC or careful monitoring for malignant transformation of hepatocytes still remains an important objective for improving the prognosis and justifying screening programs of patients at risk, such as chronic carriers of HBV or individuals with cirrhotic HCV. However, further studies will permit us to analyze why the expression of GP73 is abnormal in the tissues and sera of HCC or to use global genomic or proteomic technologies to explore the molecular mechanism of GP73 gene transcription alteration in the progression of HCC (38).
Acknowledgments
We are grateful to Professor Zhizhen Dong of the Department of Diagnostics at the Affiliated Hospital of Nantong University for the clinical analysis. This work was supported in part by grants from the Jiangsu Provincial Special Programs of Medical Science (BL2012053, HK201102), the Nantong Undertaking and Technological Innovation (BK2013048, HS2014078), the Priority Academic Program Development (PADA) of Higher Education Institution of Jiangsu Province, the Chinese National Natural Science Foundation (No.81200634), and the International S and T Cooperation Program (2013DFA32150) of China. We thank T. FitzGibbon, M.D., for comments on earlier drafts of the manuscript.
Footnotes
Authors’ Contribution:Wenli Sai and Li Wang contributed equally to this work. Wenli Sai and Li Wang conceived and designed the experiments. Wenjie Zheng, Liuhong Pan and Wei Wu performed the experiments. Liwei Qiu and Junling Yang analyzed the data. Haijian Zhang and Ying Cai contributed to the reagents. Wenli Sai and Dengfu Yao wrote the manuscript and then revised the manuscript.
Funding/Support:This work was supported in part by grants from the Jiangsu provincial special programs of medical science (BL2012053, HK201102), the Nantong undertaking and technological innovation (BK2013048, HS2014078), the priority academic program development (PADA) of Higher education institution of Jiangsu province, the Chinese national natural science foundation (No.81200634), and the International S&T cooperation program (2013DFA32150) of China.
References
- 1.Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379(9822):1245–55. doi: 10.1016/s0140-6736(11)61347-0. [DOI] [PubMed] [Google Scholar]
- 2.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 3.El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365(12):1118–27. doi: 10.1056/NEJMra1001683. [DOI] [PubMed] [Google Scholar]
- 4.El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142(6):1264–1273 e1. doi: 10.1053/j.gastro.2011.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bruix J, Gores GJ, Mazzaferro V. Hepatocellular carcinoma: clinical frontiers and perspectives. Gut. 2014;63(5):844–55. doi: 10.1136/gutjnl-2013-306627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yao M, Yao DF, Bian YZ, Zhang CG, Qiu LW, Wu W, et al. Oncofetal antigen glypican-3 as a promising early diagnostic marker for hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 2011;10(3):289–94. doi: 10.1016/s1499-3872(11)60048-9. [DOI] [PubMed] [Google Scholar]
- 7.Wu CS, Lee TY, Chou RH, Yen CJ, Huang WC, Wu CY, et al. Development of a highly sensitive glycan microarray for quantifying AFP-L3 for early prediction of hepatitis B virus-related hepatocellular carcinoma. PLoS One. 2014;9(6):e32918. doi: 10.1371/journal.pone.0099959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yao D, Jiang D, Huang Z, Lu J, Tao Q, Yu Z, et al. Abnormal expression of hepatoma specific γ‐glutamyl transferase and alteration of γ‐glutamyl transferase gene methylation status in patients with hepatocellular carcinoma. Cancer. 2000;88(4):761–9. doi: 10.1002/(sici)1097-0142(20000215)88:4<761::aid-cncr5>3.3.co;2-x. [DOI] [PubMed] [Google Scholar]
- 9.Guichard C, Amaddeo G, Imbeaud S, Ladeiro Y, Pelletier L, Maad IB, et al. Integrated analysis of somatic mutations and focal copy-number changes identifies key genes and pathways in hepatocellular carcinoma. Nat Genet. 2012;44(6):694–8. doi: 10.1038/ng.2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma L, Ji L, Yu Y, Wang J. Novel molecular targets for diagnosis and treatment of hepatocellular carcinoma. Discov Med. 2015;19(102):7–14. [PubMed] [Google Scholar]
- 11.Yao M, Yao DF, Bian YZ, Wu W, Yan XD, Yu DD, et al. Values of circulating GPC-3 mRNA and alpha-fetoprotein in detecting patients with hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 2013;12(2):171–9. doi: 10.1016/s1499-3872(13)60028-4. [DOI] [PubMed] [Google Scholar]
- 12.Papatheodoridis GV, Chan HL, Hansen BE, Janssen HL, Lampertico P. Risk of hepatocellular carcinoma in chronic hepatitis B: assessment and modification with current antiviral therapy. J Hepatol. 2015;62(4):956–67. doi: 10.1016/j.jhep.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 13.Iftikhar R, Kladney RD, Havlioglu N, Schmitt-Graff A, Gusmirovic I, Solomon H, et al. Disease- and cell-specific expression of GP73 in human liver disease. Am J Gastroenterol. 2004;99(6):1087–95. doi: 10.1111/j.1572-0241.2004.30572.x. [DOI] [PubMed] [Google Scholar]
- 14.Li QW, Chen HB, Li ZY, Shen P, Qu LL, Gong LL, et al. Preparation and characterization of anti-GP73 monoclonal antibodies and development of double-antibody sandwich ELISA. Asian Pac J Cancer Prev. 2015;16(5):2043–9. doi: 10.7314/apjcp.2015.16.5.2043. [DOI] [PubMed] [Google Scholar]
- 15.Wang F, Long Q, Gong Y, Hu L, Zhang H, Oettgen P, et al. Epithelium-Specific ETS (ESE)-1 upregulated GP73 expression in hepatocellular carcinoma cells. Cell Biosci. 2014;4(1):76. doi: 10.1186/2045-3701-4-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Y, Yang H, Xu H, Lu X, Sang X, Zhong S, et al. Golgi protein 73, not Glypican-3, may be a tumor marker complementary to alpha-Fetoprotein for hepatocellular carcinoma diagnosis. J Gastroenterol Hepatol. 2014;29(3):597–602. doi: 10.1111/jgh.12461. [DOI] [PubMed] [Google Scholar]
- 17.Block TM, Comunale MA, Lowman M, Steel LF, Romano PR, Fimmel C, et al. Use of targeted glycoproteomics to identify serum glycoproteins that correlate with liver cancer in woodchucks and humans. Proc Natl Acad Sci U S A. 2005;102(3):779–84. doi: 10.1073/pnas.0408928102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Norton PA, Comunale MA, Krakover J, Rodemich L, Pirog N, D'Amelio A, et al. N-linked glycosylation of the liver cancer biomarker GP73. J Cell Biochem. 2008;104(1):136–49. doi: 10.1002/jcb.21610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kladney RD, Cui X, Bulla GA, Brunt EM, Fimmel CJ. Expression of GP73, a resident Golgi membrane protein, in viral and nonviral liver disease. Hepatology. 2002;35(6):1431–40. doi: 10.1053/jhep.2002.32525. [DOI] [PubMed] [Google Scholar]
- 20.Yang J, Li J, Dai W, Wang F, Shen M, Chen K, et al. Golgi protein 73 as a biomarker for hepatocellular carcinoma: A diagnostic meta-analysis. Exp Ther Med. 2015;9(4):1413–20. doi: 10.3892/etm.2015.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.The Liver Cancer Committee of Chinese Anticancer Association. Diagnostic criteria of primary hepatocellular carcinoma. Zhonghua Ganzangbing Zazhi. 2001.
- 22.Qiao Y, Chen J, Li X, Wei H, Xiao F, Chang L, et al. Serum gp73 is also a biomarker for diagnosing cirrhosis in population with chronic HBV infection. Clin Biochem. 2014;47(16-17):216–22. doi: 10.1016/j.clinbiochem.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 23.Liu Y, Zou Z, Zhu B, Hu Z, Zeng P. CXCL10 decreases GP73 expression in hepatoma cells at the early stage of hepatitis C virus (HCV) infection. Int J Mol Sci. 2013;14(12):24230–41. doi: 10.3390/ijms141224230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wei H, Zhang J, Li H, Ren H, Hao X, Huang Y. GP73, a new marker for diagnosing HBV-ACLF in population with chronic HBV infections. Diagn Microbiol Infect Dis. 2014;79(1):19–24. doi: 10.1016/j.diagmicrobio.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 25.Broker ME, Ijzermans JN, Witjes CD, van Vuuren HJ, de Man RA. The predictive value of Golgi protein 73 in differentiating benign from malignant liver tumors. PLoS One. 2014;9(7):e32918. doi: 10.1371/journal.pone.0100187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shan SG, Gao YT, Xu YJ, Huang Y, Zhang Q, Zhai DK, et al. Gradually increased Golgi protein 73 expression in the progression of benign liver diseases to precancerous lesions and hepatocellular carcinoma correlates with prognosis of patients. Hepatol Res. 2013;43(11):1199–210. doi: 10.1111/hepr.12078. [DOI] [PubMed] [Google Scholar]
- 27.Sun Y, Yang H, Mao Y, Xu H, Zhang J, Li G, et al. Increased Golgi protein 73 expression in hepatocellular carcinoma tissue correlates with tumor aggression but not survival. J Gastroenterol Hepatol. 2011;26(7):1207–12. doi: 10.1111/j.1440-1746.2011.06733.x. [DOI] [PubMed] [Google Scholar]
- 28.Wang M, Long RE, Comunale MA, Junaidi O, Marrero J, Di Bisceglie AM, et al. Novel fucosylated biomarkers for the early detection of hepatocellular carcinoma. Cancer Epidemiol Biomarkers Prev. 2009;18(6):1914–21. doi: 10.1158/1055-9965.EPI-08-0980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hu B, Tian X, Sun J, Meng X. Evaluation of individual and combined applications of serum biomarkers for diagnosis of hepatocellular carcinoma: a meta-analysis. Int J Mol Sci. 2013;14(12):23559–80. doi: 10.3390/ijms141223559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang L, Yao M, Dong Z, Zhang Y, Yao D. Circulating specific biomarkers in diagnosis of hepatocellular carcinoma and its metastasis monitoring. Tumour Biol. 2014;35(1):9–20. doi: 10.1007/s13277-013-1141-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang L, Yao M, Pan L, Qian Q, Yao D. Glypican-3 is a biomarker and a therapeutic target of hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 2015;14(4):361–6. doi: 10.1016/s1499-3872(15)60396-4. [DOI] [PubMed] [Google Scholar]
- 32.Li S, Yao D, Wang L, Wu W, Qiu L, Yao M, et al. Expression characteristics of hypoxia-inducible factor-1alpha and its clinical values in diagnosis and prognosis of hepatocellular carcinoma. Hepat Mon. 2011;11(10):821–8. doi: 10.5812/kowsar.1735143X.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang NY, Wang C, Li W, Wang GJ, Cui GZ, He H, et al. Prognostic value of serum AFP, AFP-L3, and GP73 in monitoring short-term treatment response and recurrence of hepatocellular carcinoma after radiofrequency ablation. Asian Pac J Cancer Prev. 2014;15(4):1539–44. doi: 10.7314/apjcp.2014.15.4.1539. [DOI] [PubMed] [Google Scholar]
- 34.Xu WJ, Guo BL, Han YG, Shi L, Ma WS. Diagnostic value of alpha-fetoprotein-L3 and Golgi protein 73 in hepatocellular carcinomas with low AFP levels. Tumour Biol. 2014;35(12):12069–74. doi: 10.1007/s13277-014-2506-8. [DOI] [PubMed] [Google Scholar]
- 35.Bao YX, Cao Q, Yang Y, Mao R, Xiao L, Zhang H, et al. Expression and prognostic significance of golgiglycoprotein73 (GP73) with epithelial-mesenchymal transition (EMT) related molecules in hepatocellular carcinoma (HCC). Diagn Pathol. 2013;8:197. doi: 10.1186/1746-1596-8-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tian L, Wang Y, Xu D, Gui J, Jia X, Tong H, et al. Serological AFP/Golgi protein 73 could be a new diagnostic parameter of hepatic diseases. Int J Cancer. 2011;129(8):1923–31. doi: 10.1002/ijc.25838. [DOI] [PubMed] [Google Scholar]
- 37.Ozkan H, Erdal H, Tutkak H, Karaeren Z, Yakut M, Yuksel O, et al. Diagnostic and prognostic validity of Golgi protein 73 in hepatocellular carcinoma. Digestion. 2011;83(1-2):83–8. doi: 10.1159/000320379. [DOI] [PubMed] [Google Scholar]
- 38.Witjes CD, van Aalten SM, Steyerberg EW, Borsboom GJ, de Man RA, Verhoef C, et al. Recently introduced biomarkers for screening of hepatocellular carcinoma: a systematic review and meta-analysis. Hepatol Int. 2013;7(1):59–64. doi: 10.1007/s12072-012-9374-3. [DOI] [PMC free article] [PubMed] [Google Scholar]