Abstract
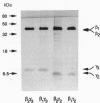
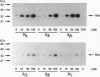
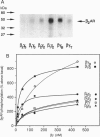
The beta and gamma subunits of heterotrimeric guanine nucleotide-binding proteins (G proteins) have recently been shown to play an active role in signal transduction. Among other effects they enable translocation of the beta-adrenergic receptor kinase (beta ARK) from the cytosol to the plasma membrane and thus permit phosphorylation and ultimately desensitization of beta-adrenergic receptors and other G-protein-coupled receptors. To investigate the specificity of this effect, we have purified various combinations of recombinant beta and gamma subunits expressed in Sf9 cells and measured their effects on beta ARK-catalyzed phosphorylation of beta 2-adrenergic receptors and of rhodopsin. The combinations tested were beta 1 gamma 2, beta 1 gamma 3, beta 2 gamma 2, beta 2 gamma 3, and transducin beta gamma (beta 1 gamma 1). There were clear differences in enhancement of rhodopsin phosphorylation, with an order of efficacy beta 2 gamma 2 > beta 1 gamma 2 >> beta 2 gamma 3 approximately beta 1 gamma 3 approximately beta 1 gamma 1. The first two combinations had larger effects than a mixed beta gamma preparation from bovine brain. In enhancing phosphorylation of beta 2-adrenergic receptors, beta 1 gamma 2 was more efficient and potent than all other combinations. These data suggest a twofold specificity of beta gamma complexes in enhancing beta ARK-catalyzed receptor phosphorylation: the gamma subunits may be important in interacting with beta ARK, with gamma 2 being more potent than gamma 3, whereas the beta subunits may determine coupling to the receptors, with beta 2 being more effective than beta 1 for rhodopsin and beta 1 being more effective than beta 2 for beta 2-adrenergic receptors.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bauer P. H., Müller S., Puzicha M., Pippig S., Obermaier B., Helmreich E. J., Lohse M. J. Phosducin is a protein kinase A-regulated G-protein regulator. Nature. 1992 Jul 2;358(6381):73–76. doi: 10.1038/358073a0. [DOI] [PubMed] [Google Scholar]
- Benovic J. L., DeBlasi A., Stone W. C., Caron M. G., Lefkowitz R. J. Beta-adrenergic receptor kinase: primary structure delineates a multigene family. Science. 1989 Oct 13;246(4927):235–240. doi: 10.1126/science.2552582. [DOI] [PubMed] [Google Scholar]
- Birnbaumer L. Receptor-to-effector signaling through G proteins: roles for beta gamma dimers as well as alpha subunits. Cell. 1992 Dec 24;71(7):1069–1072. doi: 10.1016/s0092-8674(05)80056-x. [DOI] [PubMed] [Google Scholar]
- Bourne H. R., Sanders D. A., McCormick F. The GTPase superfamily: a conserved switch for diverse cell functions. Nature. 1990 Nov 8;348(6297):125–132. doi: 10.1038/348125a0. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cali J. J., Balcueva E. A., Rybalkin I., Robishaw J. D. Selective tissue distribution of G protein gamma subunits, including a new form of the gamma subunits identified by cDNA cloning. J Biol Chem. 1992 Nov 25;267(33):24023–24027. [PubMed] [Google Scholar]
- Camps M., Carozzi A., Schnabel P., Scheer A., Parker P. J., Gierschik P. Isozyme-selective stimulation of phospholipase C-beta 2 by G protein beta gamma-subunits. Nature. 1992 Dec 17;360(6405):684–686. doi: 10.1038/360684a0. [DOI] [PubMed] [Google Scholar]
- Cerione R. A., Codina J., Benovic J. L., Lefkowitz R. J., Birnbaumer L., Caron M. G. The mammalian beta 2-adrenergic receptor: reconstitution of functional interactions between pure receptor and pure stimulatory nucleotide binding protein of the adenylate cyclase system. Biochemistry. 1984 Sep 25;23(20):4519–4525. doi: 10.1021/bi00315a003. [DOI] [PubMed] [Google Scholar]
- Codina J., Stengel D., Woo S. L., Birnbaumer L. Beta-subunits of the human liver Gs/Gi signal-transducing proteins and those of bovine retinal rod cell transducin are identical. FEBS Lett. 1986 Oct 27;207(2):187–192. doi: 10.1016/0014-5793(86)81486-7. [DOI] [PubMed] [Google Scholar]
- Fawzi A. B., Fay D. S., Murphy E. A., Tamir H., Erdos J. J., Northup J. K. Rhodopsin and the retinal G-protein distinguish among G-protein beta gamma subunit forms. J Biol Chem. 1991 Jul 5;266(19):12194–12200. [PubMed] [Google Scholar]
- Federman A. D., Conklin B. R., Schrader K. A., Reed R. R., Bourne H. R. Hormonal stimulation of adenylyl cyclase through Gi-protein beta gamma subunits. Nature. 1992 Mar 12;356(6365):159–161. doi: 10.1038/356159a0. [DOI] [PubMed] [Google Scholar]
- Fisher K. J., Aronson N. N., Jr Characterization of the cDNA and genomic sequence of a G protein gamma subunit (gamma 5). Mol Cell Biol. 1992 Apr;12(4):1585–1591. doi: 10.1128/mcb.12.4.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong H. K., Amatruda T. T., 3rd, Birren B. W., Simon M. I. Distinct forms of the beta subunit of GTP-binding regulatory proteins identified by molecular cloning. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3792–3796. doi: 10.1073/pnas.84.11.3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautam N., Baetscher M., Aebersold R., Simon M. I. A G protein gamma subunit shares homology with ras proteins. Science. 1989 May 26;244(4907):971–974. doi: 10.1126/science.2499046. [DOI] [PubMed] [Google Scholar]
- Gautam N., Northup J., Tamir H., Simon M. I. G protein diversity is increased by associations with a variety of gamma subunits. Proc Natl Acad Sci U S A. 1990 Oct;87(20):7973–7977. doi: 10.1073/pnas.87.20.7973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gierschik P., Simons C., Woodard C., Somers R., Spiegel A. Antibodies against a retinal guanine nucleotide-binding protein cross-react with a single plasma membrane protein in non-retinal tissues. FEBS Lett. 1984 Jul 9;172(2):321–325. doi: 10.1016/0014-5793(84)81149-7. [DOI] [PubMed] [Google Scholar]
- Haga K., Haga T. Activation by G protein beta gamma subunits of agonist- or light-dependent phosphorylation of muscarinic acetylcholine receptors and rhodopsin. J Biol Chem. 1992 Feb 5;267(4):2222–2227. [PubMed] [Google Scholar]
- Haga K., Haga T. Dual regulation by G proteins of agonist-dependent phosphorylation of muscarinic acetylcholine receptors. FEBS Lett. 1990 Jul 30;268(1):43–47. doi: 10.1016/0014-5793(90)80968-o. [DOI] [PubMed] [Google Scholar]
- Hausdorff W. P., Caron M. G., Lefkowitz R. J. Turning off the signal: desensitization of beta-adrenergic receptor function. FASEB J. 1990 Aug;4(11):2881–2889. [PubMed] [Google Scholar]
- Heithier H., Fröhlich M., Dees C., Baumann M., Häring M., Gierschik P., Schiltz E., Vaz W. L., Hekman M., Helmreich E. J. Subunit interactions of GTP-binding proteins. Eur J Biochem. 1992 Mar 15;204(3):1169–1181. doi: 10.1111/j.1432-1033.1992.tb16744.x. [DOI] [PubMed] [Google Scholar]
- Hekman M., Holzhöfer A., Gierschik P., Im M. J., Jakobs K. H., Pfeuffer T., Helmreich E. J. Regulation of signal transfer from beta 1-adrenoceptor to adenylate cyclase by beta gamma subunits in a reconstituted system. Eur J Biochem. 1987 Dec 1;169(2):431–439. doi: 10.1111/j.1432-1033.1987.tb13630.x. [DOI] [PubMed] [Google Scholar]
- Hepler J. R., Gilman A. G. G proteins. Trends Biochem Sci. 1992 Oct;17(10):383–387. doi: 10.1016/0968-0004(92)90005-t. [DOI] [PubMed] [Google Scholar]
- Hurley J. B., Fong H. K., Teplow D. B., Dreyer W. J., Simon M. I. Isolation and characterization of a cDNA clone for the gamma subunit of bovine retinal transducin. Proc Natl Acad Sci U S A. 1984 Nov;81(22):6948–6952. doi: 10.1073/pnas.81.22.6948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im M. J., Holzhöfer A., Böttinger H., Pfeuffer T., Helmreich E. J. Interactions of pure beta gamma-subunits of G-proteins with purified beta 1-adrenoceptor. FEBS Lett. 1988 Jan 25;227(2):225–229. doi: 10.1016/0014-5793(88)80903-7. [DOI] [PubMed] [Google Scholar]
- Iñiguez-Lluhi J. A., Simon M. I., Robishaw J. D., Gilman A. G. G protein beta gamma subunits synthesized in Sf9 cells. Functional characterization and the significance of prenylation of gamma. J Biol Chem. 1992 Nov 15;267(32):23409–23417. [PubMed] [Google Scholar]
- Katz A., Wu D., Simon M. I. Subunits beta gamma of heterotrimeric G protein activate beta 2 isoform of phospholipase C. Nature. 1992 Dec 17;360(6405):686–689. doi: 10.1038/360686a0. [DOI] [PubMed] [Google Scholar]
- Kleuss C., Scherübl H., Hescheler J., Schultz G., Wittig B. Different beta-subunits determine G-protein interaction with transmembrane receptors. Nature. 1992 Jul 30;358(6385):424–426. doi: 10.1038/358424a0. [DOI] [PubMed] [Google Scholar]
- Kleuss C., Scherübl H., Hescheler J., Schultz G., Wittig B. Selectivity in signal transduction determined by gamma subunits of heterotrimeric G proteins. Science. 1993 Feb 5;259(5096):832–834. doi: 10.1126/science.8094261. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levine M. A., Smallwood P. M., Moen P. T., Jr, Helman L. J., Ahn T. G. Molecular cloning of beta 3 subunit, a third form of the G protein beta-subunit polypeptide. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2329–2333. doi: 10.1073/pnas.87.6.2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohse M. J., Andexinger S., Pitcher J., Trukawinski S., Codina J., Faure J. P., Caron M. G., Lefkowitz R. J. Receptor-specific desensitization with purified proteins. Kinase dependence and receptor specificity of beta-arrestin and arrestin in the beta 2-adrenergic receptor and rhodopsin systems. J Biol Chem. 1992 Apr 25;267(12):8558–8564. [PubMed] [Google Scholar]
- Lohse M. J., Benovic J. L., Codina J., Caron M. G., Lefkowitz R. J. beta-Arrestin: a protein that regulates beta-adrenergic receptor function. Science. 1990 Jun 22;248(4962):1547–1550. doi: 10.1126/science.2163110. [DOI] [PubMed] [Google Scholar]
- Lohse M. J., Klotz K. N., Schwabe U. Agonist photoaffinity labeling of A1 adenosine receptors: persistent activation reveals spare receptors. Mol Pharmacol. 1986 Oct;30(4):403–409. [PubMed] [Google Scholar]
- Lohse M. J., Lefkowitz R. J., Caron M. G., Benovic J. L. Inhibition of beta-adrenergic receptor kinase prevents rapid homologous desensitization of beta 2-adrenergic receptors. Proc Natl Acad Sci U S A. 1989 May;86(9):3011–3015. doi: 10.1073/pnas.86.9.3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park D., Jhon D. Y., Lee C. W., Lee K. H., Rhee S. G. Activation of phospholipase C isozymes by G protein beta gamma subunits. J Biol Chem. 1993 Mar 5;268(7):4573–4576. [PubMed] [Google Scholar]
- Pitcher J. A., Inglese J., Higgins J. B., Arriza J. L., Casey P. J., Kim C., Benovic J. L., Kwatra M. M., Caron M. G., Lefkowitz R. J. Role of beta gamma subunits of G proteins in targeting the beta-adrenergic receptor kinase to membrane-bound receptors. Science. 1992 Aug 28;257(5074):1264–1267. doi: 10.1126/science.1325672. [DOI] [PubMed] [Google Scholar]
- Schägger H., von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987 Nov 1;166(2):368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- Simon M. I., Strathmann M. P., Gautam N. Diversity of G proteins in signal transduction. Science. 1991 May 10;252(5007):802–808. doi: 10.1126/science.1902986. [DOI] [PubMed] [Google Scholar]
- Sternweis P. C., Robishaw J. D. Isolation of two proteins with high affinity for guanine nucleotides from membranes of bovine brain. J Biol Chem. 1984 Nov 25;259(22):13806–13813. [PubMed] [Google Scholar]
- Sternweis P. C. The purified alpha subunits of Go and Gi from bovine brain require beta gamma for association with phospholipid vesicles. J Biol Chem. 1986 Jan 15;261(2):631–637. [PubMed] [Google Scholar]
- Sugimoto K., Nukada T., Tanabe T., Takahashi H., Noda M., Minamino N., Kangawa K., Matsuo H., Hirose T., Inayama S. Primary structure of the beta-subunit of bovine transducin deduced from the cDNA sequence. FEBS Lett. 1985 Oct 28;191(2):235–240. doi: 10.1016/0014-5793(85)80015-6. [DOI] [PubMed] [Google Scholar]
- Söhlemann P., Hekman M., Buchen C., Elce J. S., Lohse M. J. Purification and functional characterization of beta-adrenergic receptor kinase expressed in insect cells. FEBS Lett. 1993 Jun 7;324(1):59–62. doi: 10.1016/0014-5793(93)81532-5. [DOI] [PubMed] [Google Scholar]
- Tang W. J., Gilman A. G. Type-specific regulation of adenylyl cyclase by G protein beta gamma subunits. Science. 1991 Dec 6;254(5037):1500–1503. doi: 10.1126/science.1962211. [DOI] [PubMed] [Google Scholar]
- Taussig R., Quarmby L. M., Gilman A. G. Regulation of purified type I and type II adenylylcyclases by G protein beta gamma subunits. J Biol Chem. 1993 Jan 5;268(1):9–12. [PubMed] [Google Scholar]
- Wu D., Katz A., Simon M. I. Activation of phospholipase C beta 2 by the alpha and beta gamma subunits of trimeric GTP-binding protein. Proc Natl Acad Sci U S A. 1993 Jun 1;90(11):5297–5301. doi: 10.1073/pnas.90.11.5297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Weizsäcker E., Strathmann M. P., Simon M. I. Diversity among the beta subunits of heterotrimeric GTP-binding proteins: characterization of a novel beta-subunit cDNA. Biochem Biophys Res Commun. 1992 Feb 28;183(1):350–356. doi: 10.1016/0006-291x(92)91650-f. [DOI] [PubMed] [Google Scholar]