Abstract
Objective: We present the rationale and design of a randomized controlled trial of cognitive-behavioral therapy (CBT) for aggression in children and adolescents, which is conducted in response to the National Institute of Mental Health (NIMH) Research Domain Criteria (RDoC) approach initiative. Specifically, the study is focused on the brain-behavior associations within the RDoC construct of frustrative non-reward. On the behavioral level, this construct is defined by reactions elicited in response to withdrawal or prevention of reward, most notably reactive aggression. This study is designed to test the functional magnetic resonance (fMRI) and electrophysiological (EEG) correlates of aggression and its reduction after CBT.
Methods: Eighty children and adolescents with high levels of aggression across multiple traditional diagnostic categories, ages 8–16, will be randomly assigned to receive 12 sessions of CBT or 12 sessions of supportive psychotherapy. Clinical outcomes will be measured by the ratings of aggressive behavior collected at baseline, midpoint, and endpoint evaluations, and by the Improvement Score of the Clinical Global Impressions Scale assigned by an independent evaluator (blinded rater). Subjects will also perform a frustration-induction Go-NoGo task and a task of emotional face perception during fMRI scanning and EEG recording at baseline and endpoint.
Results: Consistent with the NIMH strategic research priorities, if functional neuroimaging and EEG variables can identify subjects who respond to CBT for aggression, this can provide a neuroscience-based classification scheme that will improve treatment outcomes for children and adolescents with aggressive behavior.
Conclusions: Demonstrating that a change in the key nodes of the emotion regulation circuitry is associated with a reduction of reactive aggression will provide evidence to support the validity of the frustrative non-reward construct.
Introduction
This article describes the rationale for and design of a randomized controlled study of cognitive-behavioral therapy (CBT) in children and adolescents with aggressive behavior across diagnostic categories. The study was developed in response to the National Institute of Mental Health (NIMH) Research Domain Criteria (RDoC) initiative that calls for explicating the core dimensions of psychopathology along multiple levels of analysis ranging from behavior to neural circuits and to molecules and genes. Specifically, we were interested in aggressive behavior and its neural correlates, the variables that fall within Frustrative Non-Reward, one of the five constructs of the Negative Valence Domain outlined by the RDoC project (National Institute of Mental Health Research Domain Criteria Project 2011). Frustrative non-reward is defined by reactions elicited in response to withdrawal or prevention of reward, most notably aggressive behavior. The aims of the study are to examine the association of aggression with the neural correlates of emotion regulation, social perception, and reward processing in the context of a randomized controlled trial. In clinically referred samples, virtually any childhood psychiatric disorder confers elevated risk for aggressive behavior (Jensen et al. 2007), underscoring the relevance of a dimensional approach to aggression across diagnostic categories as advocated by the RDoC project. Therefore, we designed this study to examine whether reduction of aggressive behavior after treatment with CBT is paralleled by the changes in the brain circuitry of aggression. Because randomized controlled trials are experiments that enable interpretation of directionality of change in neural activity relative to a predicted change in behavior, this study also leverages the explanatory power of a randomized design to examine neural mechanisms of the RDoC frustrative non-reward construct.
Characteristics and Subtypes of Childhood Aggression
Aggression encompasses a wide range of behaviors that can result in harm to self or others. Subtypes of aggression have been distinguished based on function (i.e., why the behavior is performed) and form of manifestation (what does the behavior look like). The frustration-aggression model posits that aggression is an angry response to frustration (Dollard et al. 1939; Berkowitz 1963). In contrast, social learning theory suggests that aggression is a goal-oriented instrumental behavior (Bandura 1973). Consistent with these theoretical formulations, reactive aggression has been distinguished from proactive aggression (Vitiello and Stoff 1997). Reactive aggression (also referred to as hostile or affective aggression) is viewed as an affectively fueled response to frustration or provocation that includes overt behaviors inappropriate to social context such as yelling and hitting. Proactive or instrumental aggression is viewed as purposeful behavior to gain resources (e.g. mugging) or social status (e.g. bullying). Another related and well-known classification distinguishes between overt or confrontational aggression such as arguing and fighting and covert or concealed antisocial acts such as lying, stealing, and breaking rules (Achenbach et al. 1989; Frick et al. 1993). Factor analyses of childhood disruptive behavior symptoms commonly reveal dimensions of overt aggression versus covert antisocial behavior (Frick et al.1993). Although different types of aggressive behavior tend to co-occur, reactive and proactive aggression have emerged as two subtypes with distinct profiles of psychosocial correlates and neural underpinnings. Reactive aggression in children is associated with impaired social-information processing and emotion dysregulation (Raine et al. 2006; Arsenio et al. 2009). Proactive aggression and covert forms of antisocial behavior are associated with callous-unemotional (CU) traits and abnormalities in reward processing (Frick and White 2008; Blair 2010a). One of the challenges in designing a treatment study for aggressive behavior is selection of appropriate inclusion criteria for the type and severity of aggressive behavior and selection of the primary outcomes measures that would be sensitive in detecting clinically meaningful change with treatment. This article describes the rationale for our choices of measures of aggressive behavior for study inclusion criteria, and the primary and exploratory outcome measures.
Neural Mechanisms of Reactive Aggression
The first aim of the study is to examine the effects of CBT on the neural indices of emotion regulation in children and adolescents with high levels of aggression by collecting functional magnetic resonance imaging (fMRI) and electroencephalography (EEG) during the frustration-induction Go-NoGo task before and after treatment with CBT relative to a supportive psychotherapy (SPT) control condition.
Abnormal reactivity in the neural circuitry of experience and regulation of emotions in response to frustration has been implicated in aggressive behavior (Davidson et al. 2000). Cross-sectional fMRI studies comparing aggressive individuals to nonaggressive counterparts provide support to the emotion dysregulation model of aggression. Thus, adults with intermittent explosive disorder (Coccaro et al. 2007) and domestic violence offenders (Lee et al. 2008, 2009) exhibit increased amygdala and reduced dorsal anterior cingulate cortex (dACC) response during emotional processing. Similarly, an fMRI study of 22 adolescents with conduct disorder, ages 12–17, versus 22 healthy controls, revealed increased amygdala activation when viewing negative affective pictures (Herpertz et al. 2008). Another study of eight subjects with conduct disorder, ages 16–18, versus eight healthy controls, reported reduced amygdala/prefrontal coupling when watching animations of people experiencing pain (Decety et al. 2009). Lastly, an fMRI study with negative affective picture viewing that compared 13 children and adolescents with conduct disorder, ages 9–15, to 14 healthy controls, revealed differences in the right dACC and the left amygdala (Sterzer et al. 2005). Because the dACC has been implicated in cognitive processes such as monitoring of response conflicts and decision making (Bush et al. 2000), the abnormal deactivation in this region during negative affect in subjects with conduct disorder might explain the failure of cognitive control of emotional behavior. Because CBT for aggression teaches emotion regulation strategies for reducing frustration, we hypothesize that reduction of aggression after CBT will engage the neural circuitry of emotion regulation.
The emotion dysregulation model of aggressive behavior has guided our selection of the fMRI task that elicits frustration and requires emotion regulation in the context of the Go-NoGo paradigm (see subsequent fMRI tasks section). The study hypotheses are formulated about a subset of regions – amygdala, ventromedial prefrontal cortex (vmPFC), and dACC – implicated in dysfunctional processing and regulation of emotions in individuals with aggressive behavior. Positive response to CBT in children and adolescents with aggressive behavior was associated with more efficient recruitment of the vmPFC in studies using source localization analysis of the EEG during an emotion-induction Go-NoGo task (Lewis et al. 2008). Using a version of the same task during fMRI, we showed that increased activation of the dACC was positively associated with emotion regulation in healthy subjects (Perlman and Pelphrey 2010). Based on this work, we predict that reduction of aggression after CBT will be associated with increased activation of the dACC and vmPFC, decreased activation in the amygdala, and increased functional connectivity among these regions. In turn, demonstrating that a change in the key nodes of the aggression neurocircuitry is associated with a reduction of aggressive behavior will contribute new information toward developing and validating the construct of frustrative non-reward within the dimensional RDoC framework.
Biomarkers of CU Traits as Predictors of Response to CBT for Aggression
The second aim of the study is to examine the moderating effects of the fMRI and EEG correlates of CU traits on the response to CBT for aggression. The rationale for this aim stems from studies on neural correlates of psychopathy or CU traits in children and adolescents with conduct disorder. CU traits (lack of guilt and empathy) are present in ∼25% of children and adolescents with conduct disorder. These traits have been associated with persistent antisocial behavior (Frick and White 2008) and distinct neurocognitive characteristics, including difficulty in processing facial expressions (Blair 2010a). Individuals with CU traits are also prone to reactive aggression (Blair 2010b) which makes it important to investigate whether the neural correlates of CU traits should be considered within the frustrative non-reward RDoC construct. Specifically, adolescents with conduct disorder and a high level of CU traits showed reduced amygdala activation in response to fearful faces (Marsh et al. 2008; Jones et al. 2009). Further, the severity of CU traits was inversely correlated with the connectivity of the amygdala with the vmPFC (Marsh et al. 2008) These findings are consistent with the critical role of the amygdala as an interface between the perception of social stimuli and the triggering of emotional reactions (Adolphs 2010). Hypoactivation of the amygdala during encoding of fearful faces in youth with CU traits could reflect an impairment of social perception; specifically, reduced capacity to recognize salient distress cues, a factor that decreases aggression in healthy populations (Marsh and Blair 2008; Carlson et al. 2010). This interpretation is consistent with the findings of impaired social perception and problem-solving in aggressive children (Lochman and Dodge 1994). Despite the growing recognition of CU traits in children and adolescents with conduct disorder, little is known about their effect on treatment for aggression (Hawes and Dadds 2005; Haas et al. 2011). We will evaluate whether the decreased amygdala response to fearful faces, as a biomarker of CU traits, predicts poorer response to CBT.
Rationale for Using both fMRI and EEG
The rationale for using both fMRI and EEG to measure the effects of CBT in this randomized controlled study is twofold. First, these two neuroimaging methods collect different, but complementary, information about neural processes. By collecting both fMRI and EEG data, we will obtain unique information about regional activation and temporal dynamics, both of which are relevant for examining the RDoC dimensions of psychopathology, including frustrative non-reward. Second, because this study will investigate neural mechanisms of response to CBT in children with aggression, collection of both EEG and fMRI will allow us to cross-validate the EEG and fMRI variables as biomarkers of response to treatment. Then, future studies can rely on less-expensive EEG biomarkers to optimize treatments for aggression in subjects with specific neurobiological profiles. We formulated specific hypotheses regarding evoked related potentials to the frustration-induction Go-NoGo and face perception tasks that were modified for EEG.
When the EEG is time-locked to specific events (i.e., the presentation of a stimulus or the execution of a response), the averaged positive and negative voltage changes over time are referred to as event-related potentials (ERPs). ERPs reflect the phase-locked activity of large populations of neurons, in particular, summated postsynaptic potentials (Hajcak et al. 2010). Lewis and colleagues have conducted a series of ERP studies using the emotion-induction Go-NoGo task. In the first study, 56 healthy children and adolescents ages 5–16 years performed the emotion-induction Go-NoGo task while earning points for correct responses (Lewis et al. 2006). The N2 and P3 ERPs to the Go versus NoGo trials during neutral and frustration portions of the task were assessed to investigate the neural mechanisms subserving cognitive control processes engaged by the response inhibition condition of the task. Frustration induction resulted in increased amplitudes of the NoGo N2 and P3 ERPs, suggesting an effect of emotion induction on response inhibition. Because N2 is believed to reflect the cognitive effort involved in the monitoring of conflicting information, and P3 is associated with the inhibition of movement (Johnstone et al. 2005), enhanced N2 amplitude has been interpreted as an increase of cognitive effort required for the performance of the task when frustrated. In the second study (Lewis et al. 2008), 27 8–12-year-old children received CBT for disruptive behavior and were evaluated with EEG while performing the same emotion-induction Go-NoGo task before and after treatment. Fifteen of the 27 children revealed significant reduction in disruptive behavior that was paralleled by decreased amplitude of the N2 ERP in the emotion regulation condition of the task, suggesting changes in cognitive regulatory processes. These findings were replicated in an open-study of 71 children who received CBT for aggressive behavior and completed the emotion-induction Go-NoGo before and after treatment (Woltering et al. 2011). In this larger sample, reduction of aggression was also associated with the reduction of the N2 ERP amplitude. Our randomized controlled study will test whether changes in N2 and P3 evoked potentials during frustration-induction Go-NoGo are specific to CBT rather than nonspecific effects of the passage of time or interaction with a therapist.
ERP studies of reward processing identified two feedback-locked components associated with positive and negative outcomes (Walsh and Anderson 2012). First, the P3 component is observed in response to positive feedback for correct performance, and it is believed to reflect cognitive processing of reward value (Yeung and Sanfey 2004). The amplitude of the P3 component was proportionate to the magnitude of reward in gambling tasks, and the P3 component to the correct Go trials in the Go-NoGo task was correlated with behavioral ratings of reward sensitivity (De Pascalis et al. 2010). The second component, feedback-related negativity (FRN), occurs ∼250 ms after receiving performance feedback. The amplitude of the FRN is greater in response to negative feedback, and it is presumed to reflect the evaluation of outcome valence along a good–bad dimension (Lange et al. 2012). Reduced FRN amplitudes were associated with externalizing psychopathology (Nelson et al. 2011), as well as with risk-taking in adolescents (Crowley et al. 2009). We will explore whether the amplitude of the P3 to the correct, and of FRN to the incorrect, Go trials of the Go-NoGo task in the proposed study can be associated with response to CBT for aggression.
ERPs have been used extensively to investigate social processing using face perception tasks (Eimer 2000). The N170 is a negative component peaking ∼170 ms after viewing a face (Bentin et al. 1996). It is presumed to reflect structural decoding of the face and tends to be larger in amplitude and shorter in latency relative to ERPs elicited by other visual stimuli. Recent studies also show that the amplitude of N170 was greater when visual attention was drawn to the eyes, suggesting its role in orienting to the most socially salient features of the stimuli (Hinojosa et al. 2015). The N250 component reflects processing of face emotional valence (Hinojosa et al. 2009). Both N170 and N250 were shown to be abnormal in children with social disabilities (McPartland et al. 2004; Webb et al. 2010) and anxiety (Holmes et al. 2009), but electrophysiological indices of face perception have not been studied in children with aggression. We will explore whether reduced amplitudes and latencies of the N170 and N250 ERPs to fearful faces (as indices of impaired socioemotional processing), predict poorer response to CBT.
Study Design
This is a randomized controlled study of CBT in 80 children and adolescents with aggressive behavior. Subjects are randomly assigned to receive 12 sessions of CBT or 12 sessions of SPT. Primary clinical outcome measures include the Modified Overt Aggression Scale (MOAS) collected at baseline (week 0), midpoint (week 6), and endpoint (week 12) evaluations and the Clinical Global Impressions-Improvement (CGI-I) score assigned by an independent evaluator (IE) at midpoint and endpoint. Participants also perform neurocognitive tasks of emotion regulation and face perception during fMRI scanning and EEG recording at baseline and endpoint. The main contrasts of interest for the blood-oxygen-level dependent (BOLD) signal will be the difference between: 1) Winning versus frustration blocks of the Go-NoGo task, 2) neutral versus emotional faces, and 3) correct versus incorrect Go trials of the Go-NoGo. The main variables of interest for the ERP data will be: 1) The N2 component to the NoGo trials, 2) P3 to the correct and FRN to the incorrect Go trials, and 3) N170 and N250 ERPs to the face perception task. Figure 1 shows the flow chart of the study design.
FIG. 1.
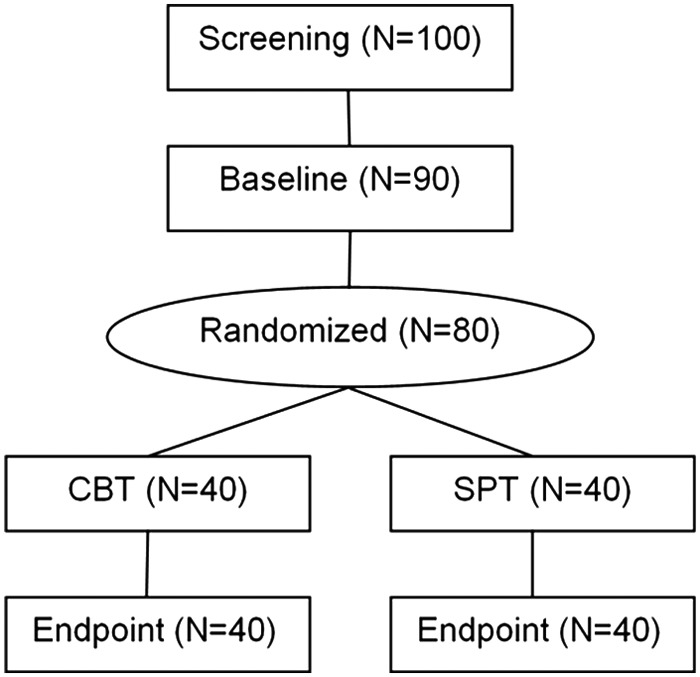
Flow chart of study design.
Study Inclusion and Exclusion Criteria
1. Boys and girls in the age range from 8 to 16 years are eligible for the study. Our rationale for selecting this age range for this study was twofold. First, CBT has been well studied across this age range and we reasoned it would be clinically relevant in this sample. Second, we wanted to enable investigation of the age effects on the neural circuity of aggressive behavior.
2. T Score ≥65 on the parent-rated Aggressive Behavior Scale of the Child Behavior Checklist (CBCL) (Achenbach and Rescorla 2001) is required for inclusion. We selected the CBCL aggression scale to measure severity of aggression as inclusion criterion because CBCL is one of the best-standardized dimensional measures of psychopathology in children. This score represents a cutoff for a clinically significant level of aggression and it is 1.5 standard deviation units above the mean in the standardization sample.
3. Subjects must be unmedicated or on stable medication for aggression, attention-deficit/hyperactivity disorder (ADHD), anxiety, or depression for at least 6 weeks, with no planned changes for the duration of the study. Our rationale for allowing children receiving psychiatric medication in the study is that children with high levels of aggression may be receiving medication for either aggression or other forms of co-occurring psychopathology. Therefore, including subjects on stable medication is relevant to the generalizability of results of this study to children with a wider range of severity of aggressive behavior.
4. Full-scale intelligence quotient (IQ) ≥85. We opted to set a cutoff for a full-scale IQ at 85 based on the rationale that CBT for aggression has been best studied in children without cognitive impairments. However, there is emerging evidence that CBT for aggression may be helpful for adults with intellectual disabilities (Novaco and Taylor 2015) and studies of CBT for anxiety have shown positive effects in in children with verbal IQ as low as 70 (Sukhodolsky et al. 2013).
5. Children across various Diagnostic and Statistical Manual of Mental Disorders (DSM) diagnoses will be eligible for participation. However, significant levels of psychopathology that require immediate clinical attention such as severe depression or psychosis will be exclusionary because they would require alternative, immediate treatments (to which excluded participants will be referred). This study was developed to examine one dimension of psychopathology, aggression, in children and adolescents across diagnostic categories. However, participation in a randomized trial in which subjects are assigned to active or control interventions and are asked not to initiate any new treatments for the duration of the active phase of the study requires comprehensive evaluation of co-occurring psychopathology to assure that psychiatric disorders requiring immediate treatment are not overlooked. Therefore, participants in this study receive a comprehensive diagnostic evaluation to determine eligibility, which includes absence of psychiatric psychopathology necessitating immediate treatment. In addition, all past and current psychopharmacological and psychosocial treatments are carefully documented, and will be reported in the participant characterization sections in articles describing the results of this study.
6. Absence of significant medical condition such as heart disease or seizure disorder based on medical history. Our rationale for this criterion is that subjects with significant medical conditions have more pressing treatment needs and may be unable to participate in a clinical trial that requires fMRI and EEG assessments and weekly visits over the course of several months.
7. Concurrent psychotherapy can continue, but an adequate dose of CBT for aggressive behavior is exclusionary. Participants are asked not to initiate any new psychotherapy during the study, because initiation of new interventions could interfere with the interpretation of results.
8. Because fMRI is one of the primary outcome measures in this study, participants have to meet fMRI safety requirements such as absence of metal medical implants and claustrophobia.
9. Participants also have to meet fMRI data quality requirements at baseline to enable pre- to posttreatment comparisons. This criterion includes confirming that participants pass the fMRI motion quality and behavioral performance parameters for the frustration-induction Go-NoGo, the primary fMRI outcome measure.
Clinical Assessment Procedures
Screening and characterization assessments are conducted to confirm eligibility and establish the baseline for outcome measures. The Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL) (Kaufman et al. 1997), a structured interview with excellent reliability, will be conducted with parent and child by an experienced clinician, to establish Diagnostic and Statistical Manual of Mental Disorders, 5th ed. (DSM-5) diagnoses (American Psychiatric Association 2013). Full scale IQ is evaluated by the Wechsler Abbreviated Scale of Intelligence (WASI) conducted by an experienced research assistant (Wechsler 1997). Parents fill out demographic, medical history, and treatment history forms that were used in our previous clinical trials (Aman et al. 2009; Sukhodolsky et al. 2009; Piacentini et al. 2010) as well as ratings of disruptive behavior, adaptive functioning, and associated symptoms. The Pubertal Development Scale (PDS)(Petersen et al. 1988) is completed by parent and children to measure pubertal development status. Primary and exploratory outcome measures are collected at baseline (week 0), midpoint (6 weeks), endpoint (12 weeks), and 3 month follow up.
Primary Outcome Measures
The MOAS (Yudofsky et al. 1986; Silver and Yudofsky 1991) is a 16 item scale that reflects the frequency and severity of incidents of aggressive behavior. We selected the MOAS as a primary dimensional outcome measure because it reflects the construct of reactive aggression (Jensen et al 2007; Knapp et al. 2012) and it has been sensitive to change in clinical studies for children with aggressive behavior (Malone et al. 2000; Saxena et al. 2006; Blader et al. 2009). The scale items are grouped in four categories: 1) Verbal aggression, 2) aggression against objects, 3) self-directed aggression, and 4) aggression against others. Each item is rated on a four point Likert scale reflecting frequency of behavior during the past week, and assigned a weighted score reflecting the harmfulness of each behavior. The MOAS has excellent reliability, including internal consistency of 0.78 and interrater reliability of 0.87 (Sukhodolsky et al. 2005).
The CGI-I score (Guy 1976) assigned by an independent evaluator (IE) blind to treatment assignment is the categorical primary outcome measure. The CGI-I reflects the IE's assessment of overall change from baseline rated on a scale from very much improved (score of 1) through no change (score of 4) to very much worse (score of 7). Ratings of very much improved (1) or much improved (2) define positive response; all other scores are classified as a negative response. To rate the CGI-I, the IE uses all available information, including the parent-nominated target symptoms (i.e., two most pressing behavioral problems recorded at baseline) (Arnold et al. 2003). These target symptoms are documented according to frequency (episodes per day or per week), intensity (duration and appearance of the behavior), and impact (degree of disruption at home and school); for example, “anger outbursts, three to five times per day, lasting 10–30 minutes and accompanied by yelling, slamming doors, verbal threats, occasional physical aggression or property destruction.” The interrater reliability of the parent-nominated target symptom is 0.9 (Arnold et al. 2003).
Exploratory Measures of Aggression and CU Traits
Exploratory measures of aggression, CU traits, and anger/irritability are included to enable a more fine-grained characterization of disruptive behavior in our sample and to explore the utility of these measures to detect brain-behavior associations. The CBCL, one of the best-researched and most widely used parent ratings of child psychopathology, has two factor-analytically derived scales of disruptive behavior (Achenbach 1991; Achenbach and Rescorla 2001) The 16 item Aggressive Behavior scale includes items reflecting inappropriate anger outbursts as well as verbal and physical aggression. The 11 item Rule-Breaking Behavior Scale (called delinquent behavior scale in the first edition) includes antisocial behaviors such as lying and stealing. We will use the Aggressive and Rule-Breaking Behavior scales as secondary outcome measures. The Inventory of Callous-Unemotional Traits (ICU) (Frick 2003) is a 24 item questionnaire that was developed based on the six item Callous-Unemotional Subscale of the earlier Antisocial Process Screening Device (APSD) (Frick and Hare 2001). Both parent-rated and child self-report forms of the ICU are used in the study. The Home Situations Questionnaire (HSQ) (Barkley 1997) is a parent-rated measure of noncompliance across 20 everyday situations. Other parent-rated measures of aggression and disruptive behavior include: The Reactive-Proactive Aggression Questionnaire (RPQ) (Raine et al. 2006), the Children's Scale of Hostility and Aggression: Reactive/Proactive (C-SHARP) (Farmer and Aman 2009), the Disruptive Behavior Rating Scale (DBRS) (Barkley 1997), the Antisocial Process Screening Device (APSD) (Frick and Hare 2001), and the Affective Reactivity Index (ARI) (Stringaris et al. 2012).
The child self-reports of anger and aggression include: The Children's Inventory of Anger (ChIA) (Nelson and Finch 2000), a 39 item measure normed for 6–16-year-old children and adolescents; The Social Problem Solving Measure (SPSM) (Lochman and Dodge 1994), an 8 item measure of aggressive and nonaggressive problem-solving strategies in hypothetical situations; the ARI (Stringaris et al. 2012), an 8 item measure of anger and irritability; the Aggression Questionnaire (AQ) (Buss and Warren 2000), a 34 item self-report of aggressive behavior; the Anger Rumination Scale (ARS) (Sukhodolsky et al. 2001) a 19 item rating of a tendency to think about anger experiences; and the State-Trait Anger Expression Inventory (STAXI) (Spielberger 1988), a 44 item self-report of experience and control of anger.
Exploratory Measures of Associated Psychopathology and Functioning
We are also collecting measures of other forms of psychopathology that commonly co-occur with aggressive behavior including ADHD, anxiety, and depression and measures of the child's social functioning as well as family stress, as these areas of functioning can be affected by the child's aggressive behavior. The Swanson, Nolan, and Pelham, Version IV (SNAP-IV) ADHD scale (MTA cooperative group 1999) is completed by parents to evaluate severity of ADHD symptoms. The Children's Depression Inventory (CDI-2) is a 27 item measure of depressive symptoms over the preceding 2 weeks (Kovacs 2003); and The Multidimensional Anxiety Scale for Children-2nd edition (MASC-2) (March 2013) is a 50 item scale of child anxiety. Both parent-rated and child self-report versions of the CDI-2 and MASC-2 are collected in this study. The Social Responsiveness Scale – Second Edition (SRS-2) (Constantino and Gruber 2005) is a 65 item, parent-report scale that measures social functioning. The Child Mania Rating Scale (CMRS) (Pavuluri 2006) is a 21 item parent-report screening instrument for mania. The Family Assessment Measure-III, Short form (Brief FAM) (Skinner et al. 1995) is a 14 item scale that provides a global index of family dysfunction. Perceived Stress Scale (PSS) (Cohen et al. 1983) is a 10 item parent rating of stress that has been commonly used in clinical trials.
Treatment Procedures
CBT
CBT consists of 12 weekly, individually administered sessions delivered according to a structured manual (Sukhodolsky and Scahill 2012) (see our companion article for details on behavioral intervention for anger and aggression (Sukhodolsky et al., this issue). Briefly, the first three sessions include education about anger triggers, prevention strategies, and emotion regulation skills such as cognitive reappraisal and relaxation. Sessions 4–6 include practicing problem-solving skills such as generation of multiple solutions and considering consequences. Sessions 7–9 focus on practicing skills for preventing or resolving potentially anger-provoking situations with friends, siblings, parents, and teachers. For example, participants are asked to recall a situation in which they acted aggressively and to role-play behaviors that would have prevented aggression. All sessions are organized by the child's target problems and connected by a behavioral support plan providing for a consistent acquisition of skills. Each session consists of a menu of therapeutic techniques and activities that can be used in a flexible yet reliable manner to achieve session goals. There are also parent check-ins during each visit and several sessions conducted with parents only, in which parents learn how to reward their child's nonaggressive behaviors with praise, attention, and privileges.
SPT control condition
We decided to use SPT as a control condition because many children and adolescents with aggressive behavior experience difficulties in peer relationship and family functioning and may benefit from psychological support. SPT is less specific than CBT and does not teach emotion regulation and problem-solving skills. SPT consists of 12 1-hour weekly sessions. The clinician, through the use of supportive, empathic, and nondirective actions, provides participants with a “sounding-board” so that they can voice their concerns regarding problems that may require discussion and assistance. Each SPT session starts with a review of events of the past week and includes queries on topics such as school, interests, hobbies, and family, with an overarching goal of enhancing subjective well-being. A major objective is to provide a clinical contact that enables children to discuss their concerns with a sympathetic adult. Subjects randomized to SPT are offered CBT after endpoint evaluations.
Neurocognitive Tasks of Emotion Regulation and Social Perception
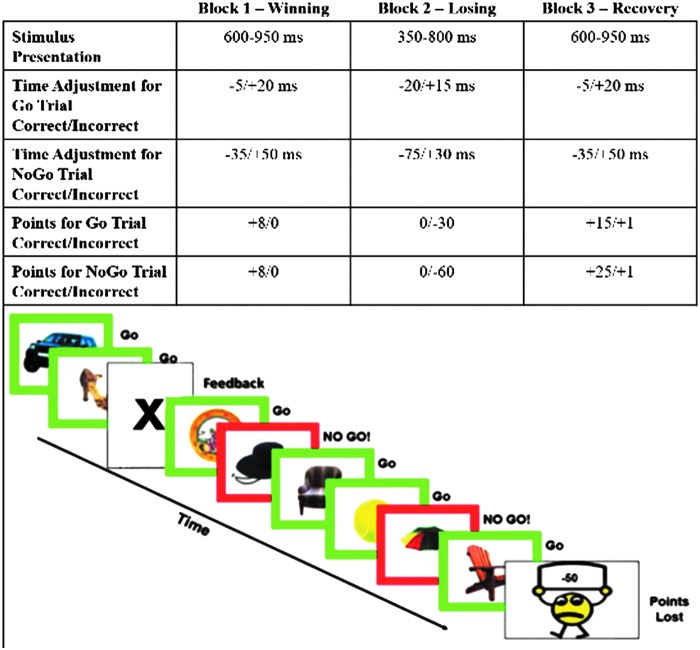
The frustration-induction Go-NoGo task is a mixed blocked/event-related design (see Fig. 2). Subjects are instructed to view a steady stream of common objects (balls, hats, chairs) and to press a button every time an object is presented in a green frame but to inhibit their response when an object appears in a red frame (∼ 33% of trials).
FIG. 2.
Frustration-induction Go-NoGo task. Upper panel shows latency windows for stimulus presentation and correct responses by three task blocks. Lower panel shows a sample of the task stimuli. A color version of this figure is available in the online article at www.liebertpub.com/cap.
Subjects are also told that they will earn points for correct responses that can be exchanged for a prize. Unbeknownst to the subject, the task contains three “blocks” designed to induce frustration and require emotion regulation. In the first block (winning condition), participants see their points steadily increase. However, changes in the point-adjustment algorithm cause the task to become more difficult by the end of the second block (losing or frustration induction), thereby leading to a loss of all of the accumulated points, and an induction of frustration at the possible loss of a prize. With a return to the more generous algorithm, subjects regain their points in the third block (recovery from frustration or emotion-regulation condition) and, ultimately, win their desired prize. The duration of the winning and losing blocks is 1 minute and the duration of the recovery block is 1.5 minutes. This sequence of winning – losing – recovery blocks is repeated four times throughout the task. An earlier version of this task has been shown to robustly activate emotion regulation circuitry in children (Perlman and Pelphrey 2010; Perlman and Pelphrey 2011). Differences in activation 1) in neutral versus recovery from frustration conditions and 2) in correct versus incorrect Go trials will measure brain processes involved in emotion regulation and reward sensitivity. The experimental manipulation used to induce frustration during this task maps well on the definition of frustrative non-reward in the RDoC workshop as “the inability to obtain positive rewards following repeated or sustained efforts”(National Institute of Mental Health Research Domain Criteria Project 2011).
The emotional face perception task consists of images taken from the NimStim collection (Tottenham et al. 2009) and utilizes an equal number of male and female faces. Each of the 14 individuals selected from the set had two images, a “calm” facial expression, and a “fearful” facial expression, yielding 28 individual face-expression pair images.
Figure 3 illustrates the task stimuli. The task utilizes a pseudorandomized block design with two conditions: One in which only fearful faces are shown, and the other in which calm faces are shown. Each of the twelve blocks contain two randomly selected faces exhibiting the same facial expression; the calm condition comprises 6 of the 12 blocks, whereas the other 6 are of the fearful condition. The face-expression pair images are randomly selected throughout the blocks and no individual face-expression image is displayed more than once throughout the paradigm. The participants see some individuals twice, but never the same individual with a previously seen expression. The duration of each block is 12 seconds, composed of two faces displayed for 5.5 seconds each, separated by a 1 second intertrial fixation cross. Blocks are separated by a jittered interblock fixation, the duration of which is either 8, 10, or 12 seconds. These interblock intervals (IBIs) were pseudorandomly chosen such that the mean of all IBIs equals 10 seconds. The first block is preceded by a 10 second initial fixation cross and the final block is succeeded by an identical 10 second fixation. The total duration of the paradigm is 284 seconds (4 minutes 44 seconds). Throughout the paradigm, participants perform a gender discrimination task by pressing a button in their left hand to indicate that the face displayed is a male, or a button in their right hand to indicate the face is female. Participants are not given feedback of their gender discrimination accuracy, which serves as a behavioral measure of attention orthogonal to the primary hypothesis about the main effect of facial expression. Differences in amygdala activation to fearful versus neutral faces will represent the main variable of interest to examine the role of CU traits in response to treatment with CBT for aggression, as fearful faces are shown to strongly activate the amygdala (Morris et al. 1996).
FIG. 3.

Example of stimuli in the emotional face perception task. A color version of this figure is available in the online article at www.liebertpub.com/cap.
Both the frustration-induction Go-NoGo and emotional face perception tasks are modified for EEG by adjusting the duration of stimuli presentation to reflect a faster time course of the ERPs. Exploratory analysis will also include a brief task of matching emotional faces to geometric shapes (Hariri et al. 2000) and resting-state fMRI and EEG data.
Case Illustration
To illustrate the utility of using fMRI with the frustration-induction Go-NoGo task in the context of a clinical trial, we show the change in brain activation of one subject before and after CBT for aggression. The subject, a 12-year-old boy, received a T score of 73 on the parent-rated CBCL aggression scale at baseline and a K-SADS diagnosis of oppositional defiant disorder. The MOAS revealed daily anger outbursts lasting ≥5 minutes and daily physical fights (without injury) with his 9-year-old sister resulting in a total MOAS score of 38. After 12 sessions of CBT, there was a clinically meaningful reduction in aggressive behavior evidenced by the CBCL aggression T score of 52 and MOAS score of 10. This reduction in aggressive behavior was paralleled by changes in the brain activation during the frustration-induction Go-NoGo from before to after treatment with CBT. The fMRI data collection, preprocessing, and motion correction were conducted using procedures described in our previous articles (Perlman and Pelphrey 2010; Ahmed and Vander Wyk, 2013; Vander Wyk et al. 2014; Ventola et al. 2015). The contrasts between the recovery and winning conditions were compared across pre- and post-CBT scans: (Recovery - Winning)Post-CBT ≠ (Recovery - Winning)Pre-CBT. Statistical thresholds were set at a false discovery rate of q < 0.05, to control for multiple comparisons (Genovese et al. 2002). Several regions showed significant changes from before to after treatment, including the vmPFC and the dACC (see Fig. 4). Mean activation levels were extracted from the region and also depicted in Figure 4. This case illustration serves to demonstrate the type of changes in aggressive behavior and brain activity that are tested in our randomized controlled study of CBT versus SPT in a sample of 80 subjects.
FIG. 4.
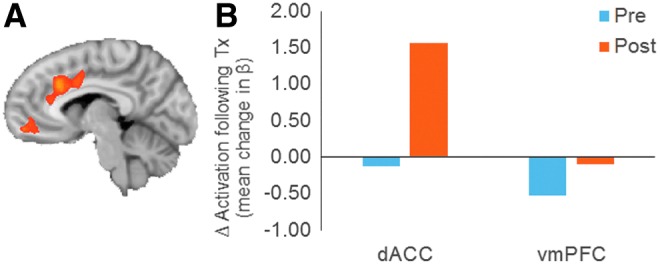
(A) Voxels found to show significant treatment-related change in differential activation during recovery versus winning conditions in a single exemplar participant. (B) Extracted beta values for the recovery minus winning contrast reflecting change in emotion regulation activation following treatment in the dorsal anterior cingulate (dACC) and ventral medial prefrontal cortex (vmPFC). A color version of this figure is available in the online article at www.liebertpub.com/cap.
Additional Study Design Considerations and Potential Limitations
Combining the research design requirements of randomized clinical trials with those of developmental cognitive neuroscience presents unique challenges and opportunities. For example, randomized designs are the gold standard in clinical research aimed at showing effects of treatment on clinical outcomes. In contrast, the majority of studies testing effects of intervention on the neural targets have utilized open designs that cannot rule out effects of time and other factors that may influence pre- to posttreatment change. Another important consideration in clinical trials is careful documentation of recruitment efforts and providing explanation for subjects who do not meet eligibility criteria. This improves understanding of the generalizability of results from the study sample to broader populations. Therefore, we provide a detailed description of our study design and inclusion criteria in this article. Such a detailed description, including prespecification of hypotheses, premeditated data analytic strategies, and open access to our (deidentified) raw data is wholly consistent with an “open science” perspective.
Effects of Concomitant Medication
Randomized controlled trials are experiments that enable testing hypotheses about the effects of independent variables (e.g., treatment with CBT or SPT) on dependent variables (e.g., aggressive behavior, BOLD signal) while keeping other variables equally distributed between treatment groups by virtue of random assignment. The effects of extraneous variables that may be associated with primary outcome variables can be further controlled by stratification as part of the randomization process, or by analysis of covariance at the stage of data analyses. For example, the possible effects of concomitant psychiatric medication on behavioral and neuroimaging outcomes require careful consideration. Limiting the study to children not taking medication would constrain generalizability; therefore, stable psychotropic medication is allowed in this study. Common drug treatments for aggressive behavior in children include novel antipsychotic agents such as risperidone (RUPP Autism Network 2002), mood stabilizers such as divalproex (Donovan et al. 2000) and lithium (Malone et al. 2000), and stimulant medications such as methylphenidate (MTA cooperative group 1999). We anticipate that approximately half of our sample will be receiving medication, and we stratify our sample by medication status as receiving or not receiving psychiatric medication and will include this mediation status variable as a covariate in the analysis. In addition to general medication status, we will also code current medication by its class (i.e., selective serotonin reuptake inhibitors [SSRIs], anxiolytics, novel antipsychotics, stimulants, mood stabilizers) and conduct additional exploratory analyses by entering each medication class variable as a covariate in data analyses.
Head Motion
Controlling for head movement is a critical issue for fMRI studies with pediatric populations, especially for studies of resting state functional connectivity, as we have previously discussed (Deen and Pelphrey 2012). We measure head motion in each participant over the course of a scan by computing the displacement of the head between consecutive time points, and average this over time. We will then check that the groups (CBT and PST) are well matched on this measure, and include this value as a nuisance variable in regression analyses (Power et al. 2012) Also, any volume that exceeds 3 mm or 3 degrees of motion relative to the first undiscarded volume will be excised. Subjects for whom >25% of the data have to be removed from one experimental condition of the primary task are asked to repeat the fMRI. If the baseline fMRI is failed twice, subjects are not randomized, and are referred for appropriate clinical services.
Conclusion and Clinical Significance
In addition to testing clinical efficacy of CBT for aggression in children and adolescents across diagnostic categories, this study will investigate the neural mechanisms of CBT by evaluating functional fMRI and EEG markers of socioemotional functioning before and after treatment. Investigating neural mechanisms of psychosocial interventions, including the CBT for aggression used in this study, is important for two main reasons. First, as with any treatment aimed at ameliorating a clinical condition, CBT needs to be based on a sound understanding of the biological processes involved. We will test whether the neural circuitry involved in emotion regulation and social perception is associated with response to CBT for aggression. Second, a better understanding of these biological mechanisms could guide the refinement of existing treatments, lead to the development of algorithms that predict which treatment is likely to benefit a given patient, and inform the development of novel interventions that may be based on altering plasticity or retuning circuitry rather than neurotransmitter pharmacology. Consistent with the NIMH strategic research priorities, if functional neuroimaging and electrophysiological variables can identify subjects who respond to CBT for aggression, this can provide a neuroscience-based classification scheme that will improve treatment outcomes for children and adolescents with aggressive behavior.
Disclosures
Dr. Sukhodolsky receives royalties from Guilford Press for a treatment manual on CBT for anger and aggression in children. Dr. Pelphrey, Dr. Vander Wyk, Dr. Crowley, Mr. Eilbott, Ms. McCauley, and Mr. Ibrahim report no conflicts of interest.
References
- Achenbach TM: Manual for the Child Behavior Checklist/4-18 and 1991 Profile. Burlington, VT: University of Vermont Press; 1991 [Google Scholar]
- Achenbach TM, Conners CK, Quay HC, Verhulst FC, Howell CT: Replication of empirically derived syndromes as a basis for taxonomy of child/adolescent psychopathology. J Abnorm Child Psychol 17:299–323, 1989 [DOI] [PubMed] [Google Scholar]
- Achenbach TM, Rescorla LA: Manual for the ASEBA School-Age Forms & Profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth, and Families; 2001 [Google Scholar]
- Adolphs R: What does the amygdala contribute to social cognition? Ann N Y Acad Sci 1191:42–61, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed A, Vander Wyk BC: Neural processing of intentional biological motion in unaffected siblings of children with autism spectrum disorder: An fMRI study. Brain Cogn 83:297–306, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aman MG, McDougle CJ, Scahill L, Handen B, Arnold LE, Johnson C, Stigler KA, Bearss K, Butter E, Swiezy NB, Sukhodolsky DD, Ramadan Y, Pozdol SL, Nikolov R, Lecavalier L, Kohn AE, Koenig K, Hollway JA, Korzekwa P, Gavaletz A, Mulick JA, Hall KL, Dziura J, Ritz L, Trollinger S, Yu S, Vitiello B, Wagner A: Medication and parent training in children with pervasive developmental disorders and serious behavior problems: Results from a randomized clinical Trial. J Am Acad Child Adolesc Psychiatry 48:1143–1154, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013 [Google Scholar]
- Arnold LE, Vitiello B, McDougle C, Scahill L, Shah B, Gonzalez NM, Chuang S, Davies M, Hollway J, Aman MG, Cronin P, Koenig K, Kohn AE, McMahon DJ, Tierney E: Parent-defined target symptoms respond to risperidone in RUPP Autism study: Customer approach to clinical trials. J Am Acad Child Adolesc Psychiatry 42:1443–1450, 2003 [DOI] [PubMed] [Google Scholar]
- Arsenio WF, Adams E, Gold J: Social Information processing, moral reasoning, and emotion attributions: Relations with adolescents' reactive and proactive aggression. Child Dev 80:1739–1755, 2009 [DOI] [PubMed] [Google Scholar]
- Bandura A: Aggression: A Social Learning Analysis. Oxford: Prentice-Hall; 1973 [Google Scholar]
- Barkley RA: Defiant Children: A Clinician's Manual for Assessment and Parent Training, 2nd ed. New York: Guilford Press; 1997 [Google Scholar]
- Bentin S, Allison T, Puce A, Perez E, McCarthy G: Electrophysiological studies of face perception in humans. J Cogn Neurosci 8:551–565, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkowitz L: Aggression: A social psychological analysis. New York: McGraw-Hill; 1963 [Google Scholar]
- Blader JC, Schooler NR, Jensen PS, Pliszka SR, Kafantaris V: Adjunctive divalproex versus placebo for children with ADHD and aggression refractory to stimulant monotherapy. Am J Psychiatry 166:1392–1401, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair RJ: Neuroimaging of psychopathy and antisocial behavior: a targeted review. Curr Psychiatry Rep 12:76–82, 2010a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair RJR: Psychopathy, frustration, and reactive aggression: The role of ventromedial prefrontal cortex. Br J Psychol 101:383–399, 2010b [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI: Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci 4:222, 2000 [DOI] [PubMed] [Google Scholar]
- Buss AH, Warren WL: The Aggression Questionnaire: Manual. Los Angeles: Western Psychological Services; 2000 [Google Scholar]
- Carlson JM, Greenberg T, Mujica–Parodi LR: Blind rage? Heightened anger is associated with altered amygdala responses to masked and unmasked fearful faces. Psychiatry Res 182:281–283, 2010 [DOI] [PubMed] [Google Scholar]
- Coccaro EF, McCloskey MS, Fitzgerald DA, Phan KL: Amygdala and orbitofrontal reactivity to social threat in individuals with impulsive aggression. Biol Psychiatry 62:168–178, 2007 [DOI] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav 24:385–396, 1983 [PubMed] [Google Scholar]
- Constantino JN, Gruber CP: Social Responsiveness Scale (SRS). Los Angeles: Western Psychological Services; 2005 [Google Scholar]
- Crowley MJ, Wu J, Crutcher C, Bailey CA, Lejuez CW, Mayes LC: Risk-taking and the feedback negativity response to loss among at-risk adolescents. Dev Neurosci 31:137–148, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson RJ, Putnam KM, Larson CL: Dysfunction in the neural circuitry of emotion regulation – A possible prelude to violence. Science 289:591–594, 2000 [DOI] [PubMed] [Google Scholar]
- De Pascalis V, Varriale V, D'Antuono L: Event-related components of the punishment and reward sensitivity. Clin Neurophysiol 121:60–76, 2010 [DOI] [PubMed] [Google Scholar]
- Decety J, Michalska KJ, Akitsuki Y, Lahey BB: Atypical empathic responses in adolescents with aggressive conduct disorder: A functional MRI investigation. Biol Psychol 80:203–211, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deen B, Pelphrey K: Perspective: Brain scans need a rethink. Nature 491:S20, 2012 [DOI] [PubMed] [Google Scholar]
- Dollard J, Dood L, Miller N, Mowrer O, Sears R: Frustration and Aggression. New Haven: Yale University Press; 1939 [Google Scholar]
- Donovan SJ, Stewart JW, Nunes EV, Quitkin FM, Parides M, Daniel W, Susser E, Klein DF: Divalproex treatment for youth with explosive temper and mood lability: A double-blind, placebo-controlled crossover design. Am J Psychiatry 157:818–820, 2000 [DOI] [PubMed] [Google Scholar]
- Eimer M: Event-related brain potentials distinguish processing stages involved in face perception and recognition. Clin Neurophysiol 111:694–705, 2000 [DOI] [PubMed] [Google Scholar]
- Farmer CA, Aman MG: Development of the Children's Scale of Hostility and Aggression: Reactive/Proactive (C-SHARP). Res Dev Disabil 30:1155–1167, 2009 [DOI] [PubMed] [Google Scholar]
- Frick PJ: The Inventory of Callous-Unemotional Traits. New Orleans: University of New Orleans; 2003 [Google Scholar]
- Frick PJ, Hare RD: Antiosocial Process Screening Device. Toronto: Multi Health Systems; 2001 [Google Scholar]
- Frick PJ, Lahey BB, Loeber R, Tannenbaum L, Van Horn Y, Christ MAG, Hart EA, Hanson K: Oppositional defiant disorder and conduct disorder: A meta-analytic review of factor analyses and cross-validation in a clinic sample. Clin Psychol Rev 13:319–340, 1993 [Google Scholar]
- Frick PJ, White SF: Research review: The importance of callous-unemotional traits for developmental models of aggressive and antisocial behavior. J Child Psychol Psychiatry 49:359–375, 2008 [DOI] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T: Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage 15:870–878, 2002 [DOI] [PubMed] [Google Scholar]
- Guy W: Clinical Global Impressions Scales (CGI). ECDEU Assessment Manual for Psychopharmacology (Publication 76-338). Washington, DC: Department of Health, Education, and Welfare; 1976 [Google Scholar]
- Haas SM, Waschbusch DA, Pelham Jr WE, King S, Andrade BF, Carrey NJ: Treatment response in CP/ADHD children with callous/unemotional traits. J Abnorm Child Psychol 39:541–552, 2011 [DOI] [PubMed] [Google Scholar]
- Hajcak G, Macnamara A, Olvet DM: Event-related potentials, emotion, and emotion regulation: An integrative review. Dev Neuropsychol 35:129–155, 2010 [DOI] [PubMed] [Google Scholar]
- Hariri AR, Bookheimer SY, Mazziotta JC: Modulating emotional responses: Effects of a neocortical network on the limbic system. Neuroreport 11:43–48, 2000 [DOI] [PubMed] [Google Scholar]
- Hawes DJ, Dadds MR: The treatment of conduct problems in children with callous-unemotional traits. J Consult Clin Psychol 73:737–741, 2005 [DOI] [PubMed] [Google Scholar]
- Herpertz SC, Huebner T, Marx I, Vloet TD, Fink GR, Stoecker T, Shah NJ, Konrad K, Herpertz–Dahlmann B: Emotional processing in male adolescents with childhood-onset conduct disorder. J Child Psychol Psychiatry 49:781–791, 2008 [DOI] [PubMed] [Google Scholar]
- Hinojosa JA, Carretie L, Valcarcel MA, Mendez–Bertolo C, Pozo MA: Electrophysiological differences in the processing of affective information in words and pictures. Cogn Affect Behav Neurosci 9:173–189, 2009 [DOI] [PubMed] [Google Scholar]
- Hinojosa JA, Mercado F, Carretié L: N170 sensitivity to facial expression: A meta-analysis. Neurosci Biobehav Rev 55:498–509, 2015 [DOI] [PubMed] [Google Scholar]
- Holmes A, Nielsen MK, Tipper S, Green S. An electrophysiological investigation into the automaticity of emotional face processing in high versus low trait anxious individuals. Cogn Affect Behav Neurosci 9:323–334, 2009 [DOI] [PubMed] [Google Scholar]
- Jensen PS, Youngstrom EA, Steiner H, Findling RL, Meyer RE, Malone RP, Carlson GA, Coccaro EF, Aman MG, Blair J, Dougherty D, Ferris C, Flynn L, Green E, Hoagwood K, Hutchinson J, Laughren T, Leve LD, Novins DK, Vitiello B: Consensus report on impulsive aggression as a symptom across diagnostic categories in child psychiatry: Implications for medication studies. J Am Acad Child Adolesc Psychiatry 46:309–322, 2007 [DOI] [PubMed] [Google Scholar]
- Johnstone SJ, Pleffer CB, Barry RJ, Clarke AR, Smith JL: Development of inhibitory processing during the Go/NoGo task: A behavioral and event-related potential study of children and adults. J Psychophysiol 19:11–23, 2005 [Google Scholar]
- Jones AP, Laurens KR, Herba CM, Barker GJ, Viding E: Amygdala hypoactivity to fearful faces in boys with conduct problems and callous-unemotional traits. Am J Psychiatry 166:95–102, 2009 [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N: Schedule for affective disorders and schizophrenia for school-age children–present and lifetime version (K-SADS-PL): Initial reliability and validity data. J Am Acad Child Adolesc Psychiatry 36:980–988, 1997 [DOI] [PubMed] [Google Scholar]
- Knapp P, Chait A, Pappadopulos E, Crystal S, Jensen PS: Treatment of maladaptive aggression in youth: CERT guidelines I. Engagement, assessment, and management. Pediatrics 129:e1562–e1576, 2012 [DOI] [PubMed] [Google Scholar]
- Kovacs M. Children's Depression Inventory. North Tonawanda, NY: Multi-Health Systems; 2003 [Google Scholar]
- Lange S, Leue A, Beauducel A: Behavioral approach and reward processing: Results on feedback-related negativity and P3 component. Biol Psychol 89:416–425, 2012 [DOI] [PubMed] [Google Scholar]
- Lee TMC, Chan SC, Raine A: Hyperresponsivity to threat stimuli in domestic violence offenders: A functional magnetic resonance imaging study. J Clin Psychiatry 70:36–45, 2009 [DOI] [PubMed] [Google Scholar]
- Lee TMC, Chan SC, Raine A: Strong limbic and weak frontal activation to aggressive stimuli in spouse abusers. Mol Psychiatry 13:655–656, 2008 [DOI] [PubMed] [Google Scholar]
- Lewis MD, Granic I, Lamm C, Zelazo PD, Stieben J, Todd RM, Moadab I, Pepler D: Changes in the neural bases of emotion regulation associated with clinical improvement in children with behavior problems. Dev Psychopathol 20:913–939, 2008 [DOI] [PubMed] [Google Scholar]
- Lewis MD, Lamm C, Segalowitz SJ, Stieben J, Zelazo PD: Neurophysiological correlates of emotion regulation in children and adolescents. J Cogn Neurosci 18:430–443, 2006 [DOI] [PubMed] [Google Scholar]
- Lochman JE, Dodge KA: Social-cognitive processes of severly violent, moderately aggressive, and nonaggressive boys. J Consult Clin Psychol 62:366–374, 1994 [DOI] [PubMed] [Google Scholar]
- Malone RP, Delaney MA, Luebbert JF, Cater J, Campbell M: A double-blind placebo-controlled study of lithium in hospitalized aggressive children and adolescents with conduct disorder. Arch Gen Psychiatry 57:649–654, 2000 [DOI] [PubMed] [Google Scholar]
- March JS. Multidimensional Anxiety Scale for Children 2nd Edition (MASC 2). Toronto, Canada: Multi-Health Systems, 2013 [Google Scholar]
- Marsh AA, Blair RJ: Deficits in facial affect recognition among antisocial populations: A meta-analysis. Neurosci Biobehav Rev 32:454–465, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh AA, Finger EC, Mitchell DGV, Reid ME, Sims C, Kosson DS, Towbin KE, Leibenluft E, Pine DS, Blair RJR: Reduced amygdala response to fearful expressions in children and adolescents with callous-unemotional traits and disruptive behavior disorders. Am J Psychiatry 165:712–720, 2008 [DOI] [PubMed] [Google Scholar]
- McPartland J, Dawson G, Webb SJ, Panagiotides H, Carver LJ: Event-related brain potentials reveal anomalies in temporal processing of faces in autism spectrum disorder. J Child Psychol Psychiatry 45:1235–1245, 2004 [DOI] [PubMed] [Google Scholar]
- Morris JS, Frith CD, Perrett DI, Rowland D, Young AW, Calder AJ, Dolan RJ: A differential neural response in the human amygdala to fearful and happy facial expressions. Nature 383:812–815, 1996 [DOI] [PubMed] [Google Scholar]
- MTA Cooperative Group: A 14-month randomized clinical trial of treatment strategies for attention-deficit/hyperactivity disorder. The MTA Cooperative Group. Multimodal Treatment Study of Children with ADHD. Arch Gen Psychiatry 56:1073–1086, 1999 [DOI] [PubMed] [Google Scholar]
- National Institute of Mental Health Research Domain Criteria (RDOC) Project: Negative Valence Systems. Workshop Proceedings, Rockville, MO, 2011 [Google Scholar]
- Nelson LD, Patrick CJ, Bernat EM: Operationalizing proneness to externalizing psychopathology as a multivariate psychophysiological phenotype. Psychophysiol 48:64–73, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson WM, Finch AJ: Children's Inventory of Anger. Los Angeles: Western Psychological Services; 2000 [Google Scholar]
- Novaco RW, Taylor JL: Reduction of assaultive behavior following anger treatment of forensic hospital patients with intellectual disabilities. Behav Res Ther 65:52–59, 2015 [DOI] [PubMed] [Google Scholar]
- Perlman SB, Pelphrey KA: Regulatory brain development: Balancing emotion and cognition. Soc Neurosci 5:533–542, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman SB, Pelphrey KA: Developing connections for affective regulation: Age-related changes in emotional brain connectivity. J Exp Child Psychol 108:607–620, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen AC, Crockett L, Richards M, Boxer A: A self-report measure of pubertal status: Reliability, validity, and initial norms. J Youth Adolesc 17:117–133, 1988 [DOI] [PubMed] [Google Scholar]
- Piacentini J, Woods DW, Scahill L, Wilhelm S, Peterson AL, Chang S, Ginsburg GS, Deckersbach T, Dziura J, Levi–Pearl S, Walkup JT: Behavior therapy for children with Tourette disorder: A randomized controlled trial. JAMA 303:1929–1937, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE: Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage 59:2142–2154, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raine A, Dodge K, Loeber R, Gatzke–Kopp L, Lynam D, Reynolds C, Stouthamer–Loeber M, Liu J: The reactive-proactive aggression questionnaire: Differential correlates of reactive and proactive aggression in adolescent boys. Aggress Behav 32:159–171, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUPP Autism Network: Risperidone in children with autism and serious behavioral problems. N Engl J Med 347:314–321, 2002 [DOI] [PubMed] [Google Scholar]
- Saxena K, Howe M, Simeonova D, Steiner H, Chang K: Divalproex sodium reduces overall aggression in youth at high risk for bipolar disorder. J Child Adolesc Psychopharmacol 16:252–259, 2006 [DOI] [PubMed] [Google Scholar]
- Silver JM, Yudofsky SC: The overt aggression scale: Overview and guiding principles. J Neuropsychiatry Clin Neurosci 3:S22–29, 1991 [PubMed] [Google Scholar]
- Skinner H, Steinhauer P, Santa–Barbara J: Family Assessment Measure-III (FAM-III). North Tonawanda, NY: Multi-Health Systems; 1995 [Google Scholar]
- Spielberger CD: Manual for the State-Trait Anger Expression Inventory (STAXI). Odessa, FL: Psychological Assessment Resources; 1988 [Google Scholar]
- Sterzer P, Stadler C, Krebs A, Kleinschmidt A, Poustka F: Abnormal neural responses to emotional visual stimuli in adolescents with conduct disorder. Biol Psychiatry 57:7–15, 2005 [DOI] [PubMed] [Google Scholar]
- Stringaris A, Goodman R, Ferdinando S, Razdan V, Muhrer E, Leibenluft E, Brotman MA: The Affective Reactivity Index: A concise irritability scale for clinical and research settings. J Child Psychol Psychiatry 53:1109–1117, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukhodolsky DG, Bloch MH, Panza KE, Reichow B: Cognitive-behavioral therapy for anxiety in children with high-functioning autism: A meta-analysis. Pediatrics 132:e1341–e1350, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukhodolsky DG, Cardona L, Martin A: Characterizing aggressive and noncompliant behaviors in a children's psychiatric inpatient setting. Child Psychiatry Hum Dev 36:177–193, 2005 [DOI] [PubMed] [Google Scholar]
- Sukhodolsky DG, Golub A, Cromwell EN: Development and validation of the Anger Rumination Scale. Pers Individ Diff 31:689–700, 2001 [Google Scholar]
- Sukhodolsky DG, Scahill L: Cognitive-Behavioral Therapy for Anger and Aggression in Children. New York: Guilford Press; 2012 [Google Scholar]
- Sukhodolsky DG, Smith SD, McCauley SA, Ibrahim K, Piasecka JB: Behavioral interventions for anger, irritability and aggression in children and adolescents. J Child Adolesc Psychopharmacol, this issue [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukhodolsky DG, Vitulano LA, Carroll DH, McGuire J, Leckman JF, Scahill L: Randomized trial of anger control training for adolescents with Tourette's Syndrome and disruptive behavior. J Am Acad Child Adolesc Psychiatry 48:413–421, 2009 [DOI] [PubMed] [Google Scholar]
- Tottenham N, Tanaka JW, Leon AC, McCarry T, Nurse M, Hare TA, Marcus DJ, Westerlund A, Casey BJ, Nelson C: The NimStim set of facial expressions: Judgments from untrained research participants. Psychiatry Res 168:242–249, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vander Wyk BC, Hoffman F. and Pelphrey KA: Equivalent neural responses in children and adolescents with and without autism during judgments of affect. Dev Cogn Neurosci 8:121–30, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventola P, Yang DYJ, Friedman HE, Oosting D, Wolf J, Sukhodolsky DG, Pelphrey KA: Heterogeneity of neural mechanisms of response to pivotal response treatment. Brain Imaging Behav 9:74–88, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitiello B, Stoff DM: Subtypes of aggression and their relevance to child psychiatry. J Am Acad Child Adolesc Psychiatry 36:307–315, 1997 [DOI] [PubMed] [Google Scholar]
- Walsh MM, Anderson JR: Learning from experience: Event-related potential correlates of reward processing, neural adaptation, and behavioral choice. Neurosci Biobehav Rev 36:1870–1884, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb SJ, Jones EJH, Merkle K, Murias M, Greenson J, Richards T, Aylward E, Dawson G: Response to familiar faces, newly familiar faces, and novel faces as assessed by ERPs is intact in adults with autism spectrum disorders. Int J Psychophysiol 77:106–117, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D: WAIS-III Administration and Scoring Manual. San Antonio, TX: The Psychological Corporation; 1997 [Google Scholar]
- Woltering S, Granic I, Lamm C, Lewis MD: Neural changes associated with treatment outcome in children with externalizing problems. Biol Psychiatry 70:873–879, 2011 [DOI] [PubMed] [Google Scholar]
- Yeung N, Sanfey AG: Independent coding of reward magnitude and valence in the human brain. J Neurosci 24:6258–6264, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yudofsky SC, Silver JM, Jackson W, Endicott J, Williams D: The overt aggression scale for the objective rating of verbal and physical aggression. Am J Psychiatry 143:35–39, 1986 [DOI] [PubMed] [Google Scholar]