Abstract
Stem cell-based tissue-engineered tracheas are at an early stage in their product development cycle. Tens of patients have been treated worldwide in predominantly compassionate use settings, demonstrating significant promise. This potentially life-saving treatment is complex, and the cost and its implications for such treatments are yet to be fully understood. The costs are compounded by varying strategies for graft preparation and transplant, resulting in differing clinical and laboratory costs from different research groups. In this study, we present a detailed breakdown of the clinical and manufacturing costs for three of the United Kingdom (UK) patients treated with such transplants. All three patients were treated under Compassionate Use legislation, within the UK National Health Service (NHS) hospital setting. The total costs for the three UK patients treated ranged from $174,420 to $740,500. All three patients were in a state of poor health at time of treatment and had a number of complexities in addition to the restricted airway. This is the first time a cost analysis has been made for a tissue-engineered organ and provides a benchmark for future studies, as well as comparative data for use in reimbursement considerations.
Introduction
To date, 10's of patients worldwide have received a stem cell-based tissue-engineered airway transplant. These airway transplants are distinctly different from conventional organ transplants in that they need to be fabricated de novo. They generally constitute cells obtained from the patient (autologous) seeded on a nonimmunogenic scaffold, to create a bespoke, personalized treatment that avoids the need for immunosuppression. A 5-year follow-up of the first adult patient treated with such an airway was published recently, demonstrating that the tissue-engineered trachea had become well vascularized and completely recellularized, and the recipient was leading a normal life.1,2
There are several steps involved in generating a stem cell-engineered airway construct ready for surgical implantation. First, a suitable donor human trachea is decellularized, leaving a porous scaffold of largely connective tissue with little or no measurable allogenicity, thus removing the need for posttransplantation immunosuppressive therapy. More recently, customized synthetic scaffolds have also been used clinically as an alternative; however, both scaffold types have advantages and disadvantages.3 The chosen scaffold is then recellularized, using autologous epithelial and mesenchymal stem cells (MSCs), either undifferentiated or differentiated into one or more daughter populations (e.g., chondrocyte). The engineered trachea is then maintained in appropriate conditions until it is ready for surgical transplantation into the patient.
Since 2009, three United Kingdom (UK) patients have received stem cell-engineered airway transplants, including the first transplant of this kind in a child. Two of these patients were treated with the aim of establishing a permanent airway, in order to both significantly extend life as well as improve quality of life. The third patient had significantly more advanced disease progression, but was implanted with a tissue-engineered trachea as a palliative measure in order for the patient to leave hospital with symptoms optimized for their remaining short months of life, in line with their goals. For the first transplant in a child, at the 2-year follow-up stage, the child had a functional airway that demonstrated appropriate biomechanical strength and a restored epithelium.4 At the 4-year follow-up stage, the child's clinical progress has been good, and evidence of the viability of the technique is clear,5 although the graft now has required the support of a stent.
While the safety, efficacy and viability of the novel treatment is of primary concern, affordability and an ability to obtain reimbursement are vital if this therapy is ever to be incorporated into routine healthcare in the public and/or private clinical setting. This article contains the breakdown costs for both the clinical and good manufacturing practice (GMP) production components for the treatment of this child, together with the two other UK patients who have been implanted with this technology. All three cases had full local ethics approvals and were treated entirely according to UK Compassionate Use legislation, using products prepared in a GMP laboratory with the necessary Specials License. A significant extension to life has been achieved in one of the three patients; hence this patient's costing data are more comprehensive for the longer-term viability and the potential financial value of such a treatment. Data from the other two patients are included to enable a broadening of the data points and to reflect the breadth of patients and their medical conditions that are encountered early in the development of a novel therapy, before successfully completing clinical trials and entering routine clinical practice. The aim of this article is to provide the starting point for considering how to best deliver these therapies into a cost-constrained healthcare market by providing accurate and detailed data to enable future discussion.
Materials and Methods
Cost data were collected for each patient relating to the clinical care and laboratory-based work involved in the generation of their airway transplants. Two of the patients were children treated at Great Ormond Street Hospital for Children (GOSH) in London (United Kingdom), and the remaining patient was an adult treated initially in Italy and then later at University College Hospital (UCLH) in London (United Kingdom). Clinical care included both inpatient and outpatient-related use of UK National Health Service (NHS) medical services, including all investigations, operations, and procedures performed while admitted to hospital and until the point of discharge. For Patient 1, clinical procedures performed upon subsequent admissions to GOSH were also taken into account. Unit cost data were obtained from a combination of NHS reference costs within the National Schedule of Reference Costs (2010–2011 period, when the bulk of procedures was incurred) and typical costs for complex tracheal patients provided by GOSH. The information for each patient's service usage was deduced from the individual patient's notes, computerized records, and clinical codings documenting their care.
The clinical costs included charges associated to inpatient bed days on either a regular ward or within the intensive care unit, imaging (e.g., computerized tomography [CT] scans, and videofluoroscopy), and meetings with specialists and consultants. Charges relating to other clinical items more specifically related to this type of airway treatment were also taken into account, such as procedures to investigate and maintain the airway itself (e.g., microlaryngoscopy, bronchoscopy and bronchography, balloon dilatation, and airway stent procedures), bone marrow aspiration to obtain autologous MSCs, and the numerous surgical procedures included in the graft transplant itself. Charges for blood tests and prescribed medicines were not included in the final calculation. An estimated clinical cost for a patient treated within a formal clinical trial setting was also calculated. This was estimated by costing the relevant items in a schedule of activities and timeline drafted for a phase I clinical study of stem cell-engineered airway transplants in pediatric patients with advanced airway disorders unresponsive to conventional treatments. The schedule comprises a transplant operation and 6 months of follow-up and does not include any subsequent surgical interventions or complications related to the airway transplant.
The laboratory-based work to generate the stem cell-engineered airway transplants was costed separately using information in batch manufacturing records collected from the UCL Biobank. Where possible, we calculated the costs from procurement of the donor trachea, its decellularization, and subsequent recellularization, including laboratory equipment, consumables, staff, and laboratory overheads. Laboratory equipment costs and running costs were calculated by taking into account the required staffing, the initial costs for the equipment, and the estimated number of products able to be produced per year.
Patient Summaries
Patient 1
This patient was an 11-year-old boy at the time of tracheal transplant, with congenital tracheal stenosis (previously repaired by patch tracheoplasty as a neonate and then again at 3 years using a large, preserved homograft patch), who received airway transplant surgery on compassionate grounds due to erosion of a metal tracheal stent into his aorta. Due to the urgent clinical need to intervene surgically because of slow, but active bleeding from the aorta into his airway, a decellularized donor trachea was recellularized intraoperatively with bone marrow-derived MSCs obtained at the same time as the transplant surgery itself. The donor trachea had been sourced from Italy and tissue decellularization performed there as part of an ongoing collaboration. The tracheal scaffold was then transported to GOSH for surgery, where all the postoperative care also took place. Today, the patient is well and back at school, with a patent, but stented airway. The airway has required a single, radiologically guided balloon dilatation in three out of the 5 years since implantation.
Patient 2
This patient was a 19-year-old woman with adenoid cystic carcinoma of the trachea, who had two separate transplants. The first transplant was a decellularized donor trachea, recellularized with autologous stem cells, and transplanted into the patient in Italy. The patient then travelled to UCLH for postsurgery care. Infection, likely exacerbated by the side effects of radiotherapy, caused a breakdown of the first transplanted trachea. A second transplant was performed at UCLH 15 months after the first, with a synthetic trachea seeded with autologous stem cells at the UCL Biobank. Surgery was performed using the existing UK compassionate use legislation and with fully informed written consent. Sadly, the patient died 6 months after the second transplant due to mediastinitis, secondary to an esophageal fistula that is believed to have been unrelated to the transplant. However, in the interim, she had 3 months at home with a good quality of life before her death. This is probably the first reported case of a complex tissue-engineered construct being deployed from the outset as a purely palliative surgical intervention.
Patient 3
This patient was a 15-year-old girl who, along with several other congenital conditions, had long-segment congenital tracheal stenosis with a single left lung. She had undergone multiple surgical procedures in another institution, despite which her stenosis had become critical over time and not amenable to conventional therapy such as resection, stenting, or balloon dilatation. A tracheal transplant was performed at GOSH using a decellularized donor trachea obtained by permission from NHS Blood and Transplant. It was recellularized with autologous stem cells at the UCL Biobank, in a manner similar to the first adult treated in Spain,2 but with processes adapted to become fully GMP compliant. She had immediate resolution of airway problems and was discharged well at 2 weeks. However, at 1 month after surgery, she suffered an acute deterioration at her base hospital and died. A postmortem was not performed.
Results
Table 1 shows the laboratory costs involved for each patient. The maximum laboratory cost for manufacturing the transplant was $27,490, which included the purchase of a donor trachea, the decellularization process, and recellularization with autologous epithelial cells and MSCs. There were no costs involved for recellularization of patient 1's trachea due to the cells purely being seeded intraoperatively, and the costing of the trachea is, therefore, an estimate based on the decellularized donor tracheas used for both patient 3 and the second transplant for patient 2. In addition, since patient 2's second trachea replacement was performed using a synthetic scaffold, there were no costs involved for a decellularization process for this. The cost of the synthetic trachea was estimated at $1000 taking into account materials, equipment, and laboratory running costs.
Table 1.
The Laboratory Costs for the Three United Kingdom Patients Treated with Stem Cell-Engineered Airways in USD (Converted from GBP to USD Using Thompson-Reuters ForEx Rate on 25 March 2015), Rounded to Nearest $10
| Patient 1 | Patient 2 (first) | Patient 2 (second) | Patient 3 | |
|---|---|---|---|---|
| Trachea | $3730 | $3730 | $1000 | $3730 |
| Decellularization | ||||
| Consumables | $870 | $870 | $870 | |
| Equipmenta | $1200 | $1200 | n/a | $1200 |
| Laboratory running cost | $6890 | $6890 | $6890 | |
| Recellularization | ||||
| MSCs | $1810 | $1810 | $1810 | |
| Epithelial cells | $1040 | $1040 | $1040 | |
| Reagants | n/a | $2380 | $2380 | $2380 |
| Laboratory running cost | $7580 | $7580 | $7580 | |
| Equipmenta | $1990 | $1990 | $1990 | |
| Total | $12,690 | $27,490 | $15,800 | $27,490 |
Equipment costs calculated by assuming three products produced per year, over a 3-year period.
MSCs, mesenchymal stem cells.
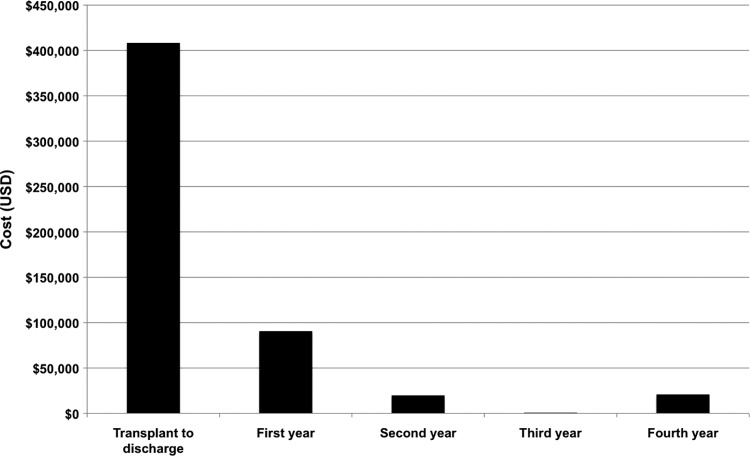
Table 2 shows a summary of the clinical costs for each patient. Figure 1 shows the decreasing clinical cost calculated for patient 1 (data recently published by Hamilton et al.5). Table 3 shows the total cost per patient, after combining the laboratory and clinical costs. The total costs for patient 1 and the first tracheal replacement for patient 2 are comparable, at $553,140 and $506,130 respectively. The total cost for patient 1 takes into account the graft, the operation, and 4 years of follow-up care. For patient 2, the costs are calculated from the graft and 15 months of follow-up care only. The clinical costs relating to the surgical implantation of the first trachea for patient 2 were not costed as hospital admission costs from Italy were unobtainable, hence the total clinical costing for patient 2 is likely to be an underestimate. The total cost for patient 3 of $174,420 is around a third of that of patient 1, understandably so since patient 3 was an inpatient for only 21 days. The cost of patient 3's care at her local hospital after discharge from GOSH was not included. Patient 1 is enjoying long-term survival coupled with good quality of life following the transplant with the tissue-engineered trachea, hence long-term follow-up data and clinical costs are available to be collected (Fig. 1). These data demonstrate decreasing clinical costs in the subsequent years after treatment. Due to their short posttransplant survival periods, patients 2 and 3 had significantly different degrees of follow-up procedures and interventions. Thus, directly comparing the total costs associated with treating the three patients is difficult, however, the three patients' data combined reflect the breadth of medical indications and the health of the patients that are likely to be candidates for future clinical trials and treatment. Therefore, this 3-patient series needs to be collectively considered a starting point for a future formal case study series or clinical trial.
Table 2.
A Table Showing the Clinical Costs for the Three United Kingdom Patients Treated with Stem Cell-Engineered Airways, Using National Health Service Reference Costs Applied to All Clinical Activity Performed While the Patients were Admitted to the United Kingdom Hospital, up Until the Point of Discharge
| Patient 1 | Patient 2 | Patient 3 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Clinical items | Transplant to discharge | First year | Second year | Third year | Fourth year | Total costs | First transplant | Second transplant | Total costs | Total costs |
| Standard ward staysa | $45,360 | $17,960 | $6620 | $470 | $7560 | $77,970 | $94,970 | $28,350 | $123,320 | $3030 |
| ITU/CITU stays | $104,570 | $37,820 | $4450 | $0 | $0 | $146,840 | $47,300 | $63,660 | $110,960 | $33,380 |
| Imaging (e.g., CXR, CT, and ECHO) | $320 | $1160 | $370 | $0 | $320 | $2170 | $10,890 | $3330 | $14,220 | $1020 |
| Broncoscopies (e.g., B&B) and specialist meetings (e.g., dietetic assessment) | $1850 | $3690 | $2220 | $370 | $740 | $8870 | $2830 | $2850 | $5680 | $4080 |
| Surgery (e.g., MLB, stent insertions and removals, graft transplant) | $256,250 | $29,960 | $6130 | $0 | $12,260 | $304,600 | $322,650 | $120,380 | $443,030 | $105,420 |
| Total | $408,350 | $90,590 | $19,790 | $840 | $20,880 | $540,450 | $478,640 | $218,570 | $697,210 | $146,930 |
Costs shown in USD (calculated using Thompson-Reuters ForEx rate on 25 March 2015), rounded to nearest $10.
Includes two nights of overnight patient hotel costs for Patient 3.
B&B, bronchoscopy and bronchogram; CT, computerized tomography; CXR, chest X-ray; ECHO, echocardiogram; MLB, microlaryngoscopy.
FIG. 1.
A chart showing the decreasing clinical costs calculated for Patient 1, across the different periods from the operation up until the most recent follow-up (reproduced with permission granted from Am. J. Transplant. Copyright © American Society of Transplantation and the American Society of Transplant Surgeons)5 Costs shown in USD (converted from GBP to USD using Thompson-Reuters ForEx rate on 25 March 2015), rounded to nearest $10.
Table 3.
The Total Patient Costs for the Three United Kingdom Patients Treated with Stem Cell-Engineered Tracheas in USD (converted from GBP to USD Using Thompson-Reuters ForEx Rate on 25 March 2015), Rounded to Nearest $10
| Patient 1 | Patient 2 (first) | Patient 2 (second) | Patient 2 (total) | Patient 3 | |
|---|---|---|---|---|---|
| Laboratory | $12,690 | $27,490 | $15,800 | $43,290 | $27,490 |
| Clinical | $540,450 | $478,640 | $218,570 | $697,210 | $146,930 |
| Total | $553,140 | $506,130 | $234,370 | $740,500 | $174,420 |
Estimated clinical costs for a future patient treated within a formal early-stage clinical trial setting are in the region of $105,650–$116,690 (data not shown). This cost is based on a 5–7 day stay in the pediatric intensive care unit followed by a 2–4 week stay in an open pediatric ward (both at GOSH), an identical implantation procedure to those used for both pediatric patients in this case series, CT scans, bronchoscopies and a bone marrow aspiration, and an airway biopsy ahead of transplant to obtain the autologous cells. With the same laboratory costs as for patient 2 (second transplant) and patient 3, the total cost for a future patient within a formal clinical trial setting can be estimated to be in the range $133,140–$144,180. It is important to note that the calculated clinical costs shown here are based on UK NHS reference costs; clinical costs in other regions will undoubtedly differ due to variations in cost structures and healthcare delivery.
Discussion
At present, the clinical costs in treating these patients far outweigh the laboratory-based product costs associated with preparing the novel engineered tracheal graft. This is due to the fact that the patients being treated with the technique under Compassionate Use legislation were severely ill and had significant chronic comorbidities with associated high-care costs. In addition, two of the patients had complications affecting their airway and esophagus, making it much harder for the transplant to efficiently engraft, and thus requiring a more invasive and costly treatment. If formal early-phase clinical trials demonstrate safety and potential efficacy for this technology, it is hoped that future patients with less severe comorbidities and performance status would also become candidates for such treatment, to minimize confounding factors. In addition to potentially reducing total care costs due to less treatment required for comorbidities, earlier intervention might also greatly improve quality of life sooner for the patient. To validate such claims, a formal clinical trial with strict inclusion and exclusion criteria will be required to narrow the high rate of patient and disease variability that the clinical team faced with these first three patients. The data presented in this article should act as a base line to enable researchers to better design future clinical trials for tissue-engineered airways.
The high cost of these transplants is comparable to the closest surgical option available for such severely diseased airways: slide tracheoplasty. In 2004, Kocyildirim et al.6 calculated the total cost of hospital stays for patients undergoing slide tracheoplasty to have a median of $27,380 in a case series of surgeries performed at GOSH. They also reported that before the introduction of the multidisciplinary team in 2001, the costs were substantially higher, with a median of $83,820. Today, slide tracheoplasty for a very complex patient at GOSH costs in the region of $52,080 for the procedure alone. The costs are also comparable to other, more common transplant operations such as renal transplantation. In 2012, Rocha et al. reported7 this to cost $67,580 to cover initial admission and first year follow-up costs (including office visits and immunosuppression) within a European public national health system hospital. Another group in Sao Paulo (Brazil) have recently further analyzed the hospital costs of pancreas-kidney transplantations in a Brazilian hospital, to determine financial feasibility depending on the particular source of payment.8 Regarding islet transplantation, an EU group has calculated the cost of the procedure and first year follow-up for type 1 diabetic patients to be $85,220, with the two main cost components deduced as islet preparation and adverse events, at 30% and 24% of the total cost, respectively.9
Critically, a substantial reduction in recurrent healthcare costs is feasible as shown by patient 1's follow-up data (Fig. 1). This is an important demonstration of how high-cost cell-based therapeutic interventions may ultimately save costs for healthcare systems and society in the medium and longer term. If clinical trials are successful, and these interventions become routine, then cost efficiencies will likely follow the increased demand; the more airways manufactured per year, the lower the cost of the goods. These reductions will principally come from the economies of scale related to higher throughput at the GMP facility and its equipment (fixed costs).
Indirect costs for these patients have not been captured herein due to the small number of patients and retrospective nature of this study. One quality of life assessment was performed for patient 1, but since it was only assessed at this single time point, it was not possible to estimate quality-adjusted life years (QALYs), without making substantial assumptions of the improvement in the child's health over the 4 years since implantation.5 Some patients (and/or their parents) treated within the tracheal program at GOSH complete a generic quality of life questionnaire, but at present these do not constitute sufficient data to inform a QALY calculation. In the United Kingdom, QALY data are used by the National Institute for Health and Care Excellence (NICE) to measure the cost-effectiveness of a treatment, a key consideration in decisions on whether or not the treatment should be recommended for NHS patients. NICE determines a treatment as cost-effective if the cost per QALY is under a threshold of $29,760–$44,640. In circumstances where NICE's “end of life criteria” apply, a threshold of up to $74,410 per QALY may be considered cost-effective.10 In the case of the stem cell-engineered tracheal transplants, the total patient cost (i.e., manufacturing and clinical care) of around $140,000 per treatment might be deemed cost-effective by NICE for future UK patients in circumstances where substantial improvements in the length and/or quality of life can be achieved. For example, if 5 years of life at full health could be restored with a treatment costing $140,000 (comprising full care pathway costs), the $28,000 cost per QALY would certainly fall within NICE's threshold to be considered affordable. However, in patient groups with complex comorbidities, where replacing the trachea would give modest improvements to the length and quality of life, the cost per QALY could be very high and thus outside of where NICE would typically consider a treatment cost-effective. Therefore, for patients suffering from congenital abnormalities, where there is a high degree of complexity and concurrent comorbidities, the health gains upon restoration of the airway with the engineered trachea may be insufficient for the treatment to be considered cost-effective. Whereas, for an oncology patient, where long-term remission and hence restoration to full health may be achievable, for a UK NHS cost consideration, the treatment may fall under the threshold and become feasible. Affordability for new treatments such as these tracheal replacement grafts, where the same technology is applicable in treating a number of indications, may well thus depend on that indication treated.
Conclusion
We describe the laboratory and clinical costings for the first three UK patients treated with stem cell-based tissue-engineered tracheas, transplanted according to UK Compassionate Use legislation in the NHS setting. Our data suggest that this technology has evidence of efficacy, at least in these limited indications, and that it is not excessively expensive, in terms of cost of goods and clinical application, as well as in comparison to the closest surgical option available. These observations have implications for the development, clinical testing, and commercialization of a range of proposed tissue-engineered and stem cell-based technologies.
Patients 2 and 3 had significant comorbidities at the outset, and while both unfortunately died, the causes appeared to be unrelated to the grafts. Before death, both patients had a high quality of life, although for short periods of time, and both would almost certainly have died much earlier if untreated. The costs of the novel treatment in these cases, especially when the periods of wellness are included, are thus hypothesized to be less than those that would otherwise have been incurred, at the very least from prolonged intensive care stays. Patient 1's health continues to improve, although he continues to be closely monitored and receives procedures to maintain his airway, as and when required.
Looking ahead, it will be important for these novel treatments to be examined by formal clinical trials and such trials should include a detailed assessment of costs from a number of perspectives (patient, carers, healthcare providers, and society in general), as well as robust quality of life outcome measures. If efficacy and safety in such groups of patients are confirmed, then moving the technology into routine practice will drive down product costs. For such a transition to occur, effective commercialization strategies with appropriate reimbursement considerations will be essential.
Acknowledgments
Sincere thanks to the Finance Department at GOSH, including M. Isaac, J. Atmore, A. Skowronska, and B. Ashdown, for their kind assistance in collating clinical costs for the patients treated. M.A.B, C.M. and M.L. are Principal Investigators, and E.C.-S. and C. Carvalho are investigators on the RegenVOX Phase I/II clinical trial funded by the Medical Research Council (MRC) (https://clinicaltrials.gov/ct2/show/NCT01977911).
Author Contributions and Ethics Approval
M.J.E. and M.A.B. were the clinical leads for the three patients. M.L. and C.M. contributed to planning and conduct. K.M., E.C.-S., L.V.-T., C.C., L.P., C.C., N.H., E.T., C.B., and M.L. contributed to data collection and reporting. K.M, E.C.-S., L.V.-T., M.J.E., M.A.B., and C.M. contributed to the drafting of the article, figures, and tables. Local ethics board approval was in place for both the treatment and subsequent data collection, and all three patients were treated under UK Compassionate Use legislation. Foreign exchange rates for GBP (or EUR, for costs referenced from7 and9) to USD were calculated using Thompson-Reuters on 25 March 2015. Costs displayed have been rounded to the nearest $10.
Disclosure Statement
No competing financial interests exist.
References
- 1.Gonfiotti A., et al. The first tissue-engineered airway transplantation: 5-year follow-up results. Lancet 383, 238, 2014 [DOI] [PubMed] [Google Scholar]
- 2.Macchiarini P., et al. Clinical transplantation of a tissue-engineered airway. Lancet 372, 2023, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Weiss D.J., Elliott M., Jang Q., Poole B., and Birchall M. Tracheal bioengineering: the next steps. Proceeds of an international society of cell therapy pulmonary cellular therapy signature series workshop, Paris, France, April 22, 2014. Cytotherapy 16, 1601, 2014 [DOI] [PubMed] [Google Scholar]
- 4.Elliott M.J., et al. Stem-cell-based, tissue engineered tracheal replacement in a child: a 2-year follow-up study. Lancet 380, 994, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hamilton N.J., et al. Tissue-Engineered Tracheal Replacement in a Child: A 4-Year Follow-Up Study. Am J Transplant 15, 10, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kocyildirim E., et al. Long-segment tracheal stenosis: slide tracheoplasty and a multidisciplinary approach improve outcomes and reduce costs. J Thorac Cardiovasc Surg 128, 876, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Rocha M.J., et al. Cost analysis of renal replacement therapy by transplant in a system of bundled payment of dialysis. Clin Transplant 26, 529, 2012 [DOI] [PubMed] [Google Scholar]
- 8.Miziara Gonzalez A., et al. Component analysis of hospital cost of pancreas-kidney transplant and correlations with different variables in a Brazilian hospital. Transplant Proc 46, 1836, 2014 [DOI] [PubMed] [Google Scholar]
- 9.Guignard A.P., et al. Cost analysis of human islet transplantation for the treatment of type 1 diabetes in the Swiss-French Consortium GRAGIL. Diabetes Care 27, 895, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Guide to the methods of technology appraisal 2013. NICE article [PMG9]. Published April 2013 [PubMed]