Abstract
Purpose: Testicular cancer is the most common cancer among adolescent and young adult (AYA) men 15–39 years of age. This study aims to determine whether race/ethnicity and/or neighborhood socioeconomic status (SES) contribute independently to survival of AYAs with testicular cancer.
Methods: Data on 14,249 eligible AYAs with testicular cancer diagnosed in California between 1988 and 2010 were obtained from the population-based California Cancer Registry. Multivariable Cox proportional hazards regression was used to examine overall and testicular cancer-specific survival and survival for the seminoma and nonseminoma histologic subtypes according to race/ethnicity, census-tract level neighborhood SES, and other patient and clinical characteristics.
Results: Compared with White AYAs, Hispanic AYAs had worse overall and testicular cancer-specific survival (hazard ratio [HR], 1.21; 95% confidence interval [CI], 1.07–1.37) and Black AYAs had worse overall survival (HR, 1.41; 95% CI, 1.01–1.97), independent of neighborhood SES and other demographic and clinical factors. Racial/ethnic disparities in survival were more pronounced for nonseminoma than for seminoma. AYAs residing in middle and low SES neighborhoods experienced worse survival across both histologic subtypes independent of race/ethnicity and other factors, while improvements in survival over time were more pronounced for seminoma. Longer time to treatment was also associated with worse survival, particularly for AYAs with nonseminoma.
Conclusion: Among AYAs, race/ethnicity, and neighborhood SES are independently associated with survival after testicular cancer. Variation in disparities by histologic type according to demographic factors, year of diagnosis, and time to treatment may reflect differences in prognosis and extent of treatment for the two histologies.
Keywords: : testicular cancer, survival, disparities, race/ethnicity, neighborhood SES, epidemiology
Testicular cancer is the most common cancer among adolescent and young adult (AYA) men 15–39 years of age1 and peaks in incidence at 30–34 years of age.2 In addition, AYAs with cancer are a particularly vulnerable group:3,4 compared with older adults and children, AYAs have different survival patterns,5–7 are least likely to participate in clinical trials, are more likely to experience delays in diagnosis or treatment,8 have greater difficulty maintaining education and employment positions,9 and are more likely to suffer psychosocial problems.10–12 As a result, in 2006 The National Cancer Institute and the LIVESTRONG Young Adult Alliance called for research to determine factors that may affect cancer outcomes among AYAs.13 Evaluating the role of sociodemographic factors in testicular cancer survival among AYAs is important to achieving this goal.
Although survival after testicular cancer is high, with 5-year relative survival over 90%,14 several large population-based studies of testicular cancer survival in men of all ages have reported disparities in survival by race/ethnicity.15–18 These reports, based on data from the Surveillance, Epidemiology and End Results (SEER) national cancer registry database, have established that testicular cancer–specific survival is lower for Hispanic Whites,15,18 African Americans,16,18 and non-Whites compared with non-Hispanic Whites17 and that overall and testicular cancer–specific survival is lower for men living in counties with lower socioeconomic status (SES) compared with higher SES counties, as determined by a limited number of county-level SES indicators.15–17
Testicular cancer includes two main histologic subtypes, seminoma and nonseminoma, which each comprise approximately half of all testicular cancers.14 Pure seminoma has a favorable prognosis and infrequently metastasizes; it is usually curable with surgery alone.14 Nonseminoma, however, comprises several distinct subtypes,19 which frequently present with metastatic disease at the time of diagnosis;19 involve more complicated treatment decisions;20 and have a higher rate of relapse.19 Previous studies among men of all ages have reported worse survival among African Americans or non-Whites compared with Whites for the nonseminoma type, but not for the seminoma histologic type.16,17
To our knowledge, no study has considered the associations between patient sociodemographic characteristics, including race/ethnicity and neighborhood SES, with survival among AYAs with testicular cancer. Documenting disparities in survival by race/ethnicity or neighborhood SES and determining whether those disparities are independently associated with survival is important to identifying modifiable factors that contribute to survival among AYAs. Accordingly, this study will examine whether survival of AYAs with testicular cancer overall and by histology differs by race/ethnicity and neighborhood SES using the population-based California Cancer Registry (CCR).
Methods
Cancer cases
California law mandates that cancer cases be reported to the population-based CCR, which participates in the National Cancer Institute's SEER program. We obtained information about California residents 15–39 years of age diagnosed with first-primary, invasive testicular cancer (International Classification of Disease for Oncology, third edition [ICD-O-3] site codes C620–C621, C629) from January 1, 1988, through December 31, 2010. For each case, we obtained cancer registry information routinely abstracted from the medical record (Table 1)—race/ethnicity, age at diagnosis, marital status, year of diagnosis, histology, stage at diagnosis, and first course treatment—as well as vital status as of December 31, 2012, and cause of death. Race/ethnicity was collapsed into the categories non-Hispanic White, non-Hispanic Black, non-Hispanic Asian/Pacific Islander, Hispanic, and other/unknown; hereafter referred to as White, Black, Asian/Pacific Islander (PI), Hispanic, and other. Vital status is routinely determined by the CCR through hospital follow-up and database linkages. Cause of death was categorized as testicular cancer (cause of death codes 1860, 1869, or C629), other cancer (cause of death codes 1400-2399 or C000-D480, excluding testicular cancer), unknown (cause of death codes 7777 or 7797), and non-cancer (all other cause of death codes).
Table 1.
Frequency Distribution of Sociodemographic and Clinical Characteristics According to Tumor Histologic Type for Adolescent and Young Adult Men 15–39 Years of Age with Testicular Cancer, 1988–2010 California
| All histologiesa | Seminoma | Nonseminoma | ||||
|---|---|---|---|---|---|---|
| N = 14,249 | N = 7071 | N = 7045 | ||||
| n | (%) | n | (%) | n | (%) | |
| Race/ethnicity | ||||||
| White | 8672 | (60.9%) | 4471 | (63.2%) | 4137 | (58.7%) |
| Black | 255 | (1.8%) | 141 | (2.0%) | 101 | (1.4%) |
| Asian/PI | 539 | (3.8%) | 294 | (4.2%) | 238 | (3.4%) |
| Hispanic | 4535 | (31.8%) | 2042 | (28.9%) | 2446 | (34.7%) |
| Other | 248 | (1.7%) | 123 | (1.7%) | 123 | (1.7%) |
| Neighborhood SES | ||||||
| High | 2803 | (19.7%) | 1430 | (20.2%) | 1352 | (19.2%) |
| Middle | 6291 | (44.2%) | 3216 | (45.5%) | 3031 | (43.0%) |
| Low | 5155 | (36.2%) | 2425 | (34.3%) | 2662 | (37.8%) |
| Age at diagnosis | ||||||
| 15–24 | 3555 | (24.9%) | 794 | (11.2%) | 2696 | (38.3%) |
| 25–39 | 10694 | (75.1%) | 6277 | (88.8%) | 4349 | (61.7%) |
| Marital status | ||||||
| Married | 5568 | (39.1%) | 3332 | (47.1%) | 2205 | (31.3%) |
| Not married | 8308 | (58.3%) | 3555 | (50.3%) | 4657 | (66.1%) |
| Unknown | 373 | (2.6%) | 184 | (2.6%) | 183 | (2.6%) |
| Year of diagnosis | ||||||
| 1988–1995 | 4657 | (32.7%) | 2452 | (34.7%) | 2173 | (30.8%) |
| 1996–2003 | 4801 | (33.7%) | 2447 | (34.6%) | 2306 | (32.7%) |
| 2004–2010 | 4791 | (33.6%) | 2172 | (30.7%) | 2566 | (36.4%) |
| Stage at diagnosis | ||||||
| Local | 9346 | (65.6%) | 5517 | (78.0%) | 3773 | (53.6%) |
| Regional | 2690 | (18.9%) | 1043 | (14.8%) | 1623 | (23.0%) |
| Metastatic | 1997 | (14.0%) | 418 | (5.9%) | 1547 | (22.0%) |
| Unknown | 216 | (1.5%) | 93 | (1.3%) | 102 | (1.4%) |
| Surgery | ||||||
| No | 335 | (2.4%) | 135 | (1.9%) | 189 | (2.7%) |
| Yes | 13914 | (97.6%) | 6936 | (98.1%) | 6856 | (97.3%) |
| Radiotherapy | ||||||
| No | 9958 | (69.9%) | 2959 | (41.8%) | 6886 | (97.7%) |
| Yes | 4291 | (30.1%) | 4112 | (58.2%) | 159 | (2.3%) |
| Chemotherapy | ||||||
| No | 9378 | (65.8%) | 5924 | (83.8%) | 3382 | (48.0%) |
| Yes | 4684 | (32.9%) | 1081 | (15.3%) | 3546 | (50.3%) |
| Unknown | 187 | (1.3%) | 66 | (0.9%) | 117 | (1.7%) |
| Time to treatment (days) | ||||||
| 0 | 10562 | (74.1%) | 5401 | (76.4%) | 5073 | (72.0%) |
| 1–14 | 2792 | (19.6%) | 1228 | (17.4%) | 1537 | (21.8%) |
| 15–30 | 528 | (3.7%) | 273 | (3.9%) | 250 | (3.5%) |
| 31–60 | 185 | (1.3%) | 83 | (1.2%) | 100 | (1.4%) |
| 60+ | 182 | (1.3%) | 86 | (1.2%) | 85 | (1.2%) |
| Cause of death | ||||||
| Alive | 12891 | (90.5%) | 6588 | (93.2%) | 6205 | (88.1%) |
| Deceased | ||||||
| Testicular cancer | 627 | (4.4%) | 132 | (1.9%) | 473 | (6.7%) |
| Other cancer | 201 | (1.4%) | 80 | (1.1%) | 113 | (1.6%) |
| Non-cancer | 436 | (3.1%) | 230 | (3.3%) | 202 | (2.9%) |
| Unknown | 94 | (0.7%) | 41 | (0.6%) | 52 | (0.7%) |
“All histologies” includes seminoma, nonseminoma, and other histologies.
PI, Pacific Islander; SES, socioeconomic status.
Of the 14,605 AYAs diagnosed with first primary invasive testicular cancer, we excluded those diagnosed by autopsy/death certificate only or with no survival time (n = 56) and those without histologic confirmation of diagnosis (n = 59).
Clinical variables
Testicular cancer histology was grouped into three categories defined by ICD-O3 codes. Groups consisted of seminoma tumors (ICD-03 codes 9060–9064), nonseminoma tumors (ICD-O3 codes 9065, 9070–9102), and other tumors (9500, 9735, 8000–8991), including non-germ cell tumors and lymphomas (<1% of AYAs). The more detailed American Joint Committee on Cancer staging criteria were not available in the CCR for testicular cancers diagnosed before 2004, so SEER summary stage categories were used: local, regional, metastatic (remote), and unknown/unspecified (not abstracted, unknown, or unspecified).
Information on first-course treatment modality from the cancer registry was included in analyses as surgery (yes, no, unknown), radiation (yes, no, unknown), and chemotherapy (yes, no, unknown). A single patient with unknown surgery was included with the “yes” category of surgery, because this was the predominant occurrence.
Time to treatment was calculated as the number of days between the date of diagnosis and the date of first surgery or the earliest date of administration of non-surgical therapy, whichever occurred first. Time to treatment was defined by five intervals; 0, 1–14, 15–30, 31–60, or greater than 60 days (60+), which have been significantly associated with age and cancer stage among AYAs.21
Neighborhood socioeconomic status
As information on patient education or other individual-level measures of SES are not collected by the CCR, we assigned a multicomponent index of neighborhood SES based on patients' residential census-tract group at diagnosis using a previously described index that incorporates 2000 United States Census (for cases diagnosed through 2005)22 and 2006–2010 American Community Survey data (for cases diagnosed in 2006 forward)23 on education, occupation, unemployment, household income, poverty, rent, and house values. Index scores are grouped into quintiles from highest to lowest SES index value based on the distribution of scores across census tracts in California.22,23 In order to assure sufficient numbers of patients and deaths in each neighborhood SES category, the SES quintile scores have been collapsed into three SES categories: high (highest quintile), middle (higher-middle and middle quintiles), and low (lower-middle and lowest quintile).
We excluded patients with missing neighborhood SES (n = 241). The final study population thus included 14,249 cases and 1358 deaths due to any cause.
Survival analyses
All analyses were conducted using SAS software version 9.3 (SAS Institute Inc., Cary, North Carolina). Frequencies and column percents of all patients and by seminoma and nonseminoma were determined according to covariates, vital status, and cause of death. Survival time was calculated in days from day of diagnosis to date of death, date of last follow-up, or study end date (December 31, 2012). For models of testicular cancer–specific survival, individuals who died of other or unknown causes were censored at the time of death. The average follow-up for censored patients was 11.3 years (standard deviation, 7.1 years). Ninety-six percent (95.6%) of Whites, 92.5% of Blacks, 92.9% of Asian/PIs, and 87.2% of Hispanics alive at the study end date had a follow-up date within the prior two years. Ninety-four percent (94.2%) of AYAs living in high SES neighborhoods, 94.5% of AYAs living in middle SES neighborhoods, and 89.4% of AYAs living in low SES neighborhoods alive at the study end date had a follow-up date within the prior two years. Sensitivity analyses confirmed that differential loss to follow-up by race/ethnicity and neighborhood SES did not affect survival estimates (data not shown).
Unadjusted Kaplan-Meier survival estimates were generated for categories of race/ethnicity and neighborhood SES for seminoma and nonseminoma. Stratified Cox proportional hazards regression models were used to estimate hazard ratios (HR) and 95% confidence intervals (CIs) for testicular cancer overall and by histologic type (seminoma, nonseminoma). We assessed the proportional hazards assumption by statistical testing of the correlation between weighted Schoenfeld residuals and logarithmically transformed survival time. Covariates violating the proportional hazards assumption (stage at diagnosis, histology, surgery, and chemotherapy) were included in final models as stratifying variables to account for varying baseline hazards. Receipt of radiation interacted with model strata, so was subsequently also included as a stratifying variable. We are unable to report HRs for stratifying variables. In the final models, there were no interactions of strata with covariates or violations of the proportional hazards assumption. Possible interactions among covariates were examined and none were significant at p < 0.05.
Results
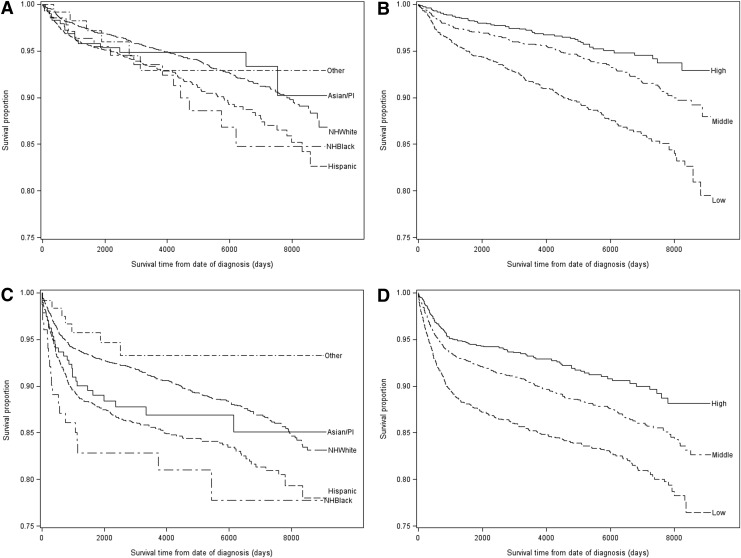
The largest proportion of AYAs were of White race/ethnicity (60.9%), resided in middle SES neighborhoods (44.2%), were 25–39 years of age (75.1%) and were unmarried (58.3%) (Table 1). Similar proportions of patients were diagnosed with seminomas (49.6%) as with nonseminomas (49.4%) and most had localized disease at the time of diagnosis (65.6%). Most patients underwent surgery (97.6%), and 30.1% received radiation, primarily for seminoma (95.8%). On the other hand, the 32.9% of AYAs receiving chemotherapy predominantly received it for nonseminoma (75.7%). Nearly two-thirds (74.1%) of patients started treatment on the day of diagnosis. Less than ten percent of the study population died by the study end date, the largest proportion (46.2%) from testicular cancer. Of AYAs that died of testicular cancer, 75.4% had nonseminoma. Unadjusted Kaplan-Meier survival curves suggest large disparities in survival according to neighborhood SES and race/ethnicity that are more pronounced for nonseminoma than seminoma (Fig 1).
FIG. 1.
Unadjusted Kaplan-Meyer curves of overall survival of adolescent and young adult men 15–39 years of age with testicular cancer according to neighborhood socioeconomic status (SES) or race/ethnicity, in California for the years 1988–2010. (A, B) Overall survival for seminoma by survival time from date of diagnosis to date of death or censoring in days according to (A) race/ethnicity and (B) neighborhood SES. (C, D) Overall survival for nonseminoma by survival time from date of diagnosis to date of death from testicular cancer or censoring in days according to (C) race/ethnicity and (D) neighborhood SES. PI, Pacific Islander.
Disparities in overall and testicular cancer–specific survival
Black and Hispanic AYAs had worse overall survival after adjustment for neighborhood SES (in addition to other covariates listed in Table 2), compared with Whites (HR, 1.41; 95% CI, 1.01–1.97 and HR, 1.21, 95% CI 1.07–1.37 respectively). Lower survival for Black and Hispanic AYAs compared with White AYAs was also present for testicular cancer–specific survival, although small numbers of Black AYAs resulted in wide confidence intervals (HR, 1.44; 95% CI, 0.87–2.37 and HR, 1.23; 95% CI, 1.02–1.47 respectively). Testicular cancer–specific survival was also lower for Asian/PI AYAs compared with White AYAs, but confidence intervals are wide (HR, 1.26; 95% CI, 0.83–1.91).
Table 2.
Hazard Ratios and 95% Confidence Intervals from Stratified Cox Proportional Hazards Models of Overall and Testicular Cancer-Specific Survival Among Adolescent and Young Adult Men 15–39 Years of Age with Testicular Cancer According to Sociodemographic Characteristics and Year of Diagnosis, 1988–2010 California
| Overall survivala | Testicular cancer-specific survivala | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adjusted for race/ethnicity | Adjusted for neighborhood SES | Adjusted for race/ethnicity and neighborhood SES | Adjusted for race/ethnicity | Adjusted for neighborhood SES | Adjusted for race/ethnicity and neighborhood SES | |||||||||
| Deaths | HR | 95% CI | HR | 95% CI | HR | 95% CI | Deaths | HR | 95% CI | HR | 95% CI | HR | 95% CI | |
| Race/ethnicity | ||||||||||||||
| White | 753 | 1.00 | Reference | 1.00 | Reference | 311 | 1.00 | Reference | 1.00 | Reference | ||||
| Black | 41 | 1.58 | (1.14–2.20) | 1.41 | (1.01–1.97) | 19 | 1.59 | (0.97–2.61) | 1.44 | (0.87–2.37) | ||||
| Asian/PI | 46 | 1.09 | (0.80–1.47) | 1.11 | (0.82–1.50) | 25 | 1.24 | (0.81–1.88) | 1.26 | (0.83–1.91) | ||||
| Hispanic | 504 | 1.39 | (1.23–1.56) | 1.21 | (1.07–1.37) | 267 | 1.38 | (1.16–1.64) | 1.23 | (1.02–1.47) | ||||
| Other | 14 | 0.78 | (0.45–1.35) | 0.78 | (0.45–1.35) | 5 | 0.75 | (0.31–1.82) | 0.52 | (0.31–1.81) | ||||
| Neighborhood SES | ||||||||||||||
| High | 165 | 1.00 | Reference | 1.00 | Reference | 67 | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | ||
| Middle | 528 | 1.37 | (1.14–1.63) | 1.34 | (1.12–1.60) | 241 | 1.44 | (1.09–1.89) | 1.40 | (1.06–1.84) | ||||
| Low | 665 | 1.93 | (1.62–2.29) | 1.79 | (1.50–2.14) | 319 | 1.85 | (1.42–2.41) | 1.71 | (1.29–2.25) | ||||
| Age at diagnosis (years) | ||||||||||||||
| 15–24 | 348 | 0.71 | (0.62–0.81) | 0.73 | (0.63–0.83) | 0.70 | (0.61–0.80) | 222 | 0.92 | (0.77–1.10) | 0.96 | (0.80–1.14) | 0.92 | (0.77–1.11) |
| 25–39 | 1010 | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 405 | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference |
| Marital status | ||||||||||||||
| Married | 394 | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 159 | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference |
| Not married | 942 | 1.57 | (1.38–1.77) | 1.57 | (1.38–1.77) | 1.57 | (1.39–1.78) | 463 | 1.45 | (1.20–1.76) | 1.45 | (1.20–1.75) | 1.46 | (1.20–1.76) |
| Unknown | 22 | 1.22 | (0.78–1.91) | 1.23 | (0.78–1.92) | 1.23 | (0.79–1.93) | 5 | 0.52 | (0.19–1.43) | 0.52 | (0.19–1.42) | 0.52 | (0.19–1.43) |
| Year of diagnosis | ||||||||||||||
| 1988–1995 | 677 | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 248 | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference |
| 1996–2003 | 397 | 0.76 | (0.67–0.87) | 0.78 | (0.68–0.89) | 0.77 | (0.67–0.88) | 192 | 0.77 | (0.63–0.94) | 0.79 | (0.65–0.96) | 0.77 | (0.64–0.94) |
| 2004–2010 | 284 | 0.74 | (0.63–0.86) | 0.77 | (0.66–0.90) | 0.75 | (0.64–0.87) | 187 | 0.82 | (0.67–1.01) | 0.86 | (0.71–1.06) | 0.83 | (0.68–1.02) |
| Time to treatment (days) | ||||||||||||||
| 0 | 780 | Reference | 1.00 | Reference | 1.00 | Reference | 297 | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | |
| 1–14 | 418 | 1.24 | (1.08–1.41) | 1.26 | (1.11–1.44) | 1.25 | (1.09–1.42) | 248 | 1.38 | (1.15–1.66) | 1.41 | (1.18–1.69) | 1.39 | (1.16–1.67) |
| 15–30 | 88 | 1.39 | (1.09–1.76) | 1.42 | (1.12–1.80) | 1.40 | (1.10–1.78) | 38 | 1.09 | (0.76–1.56) | 1.12 | (0.78–1.60) | 1.10 | (0.77–1.57) |
| 31–60 | 35 | 1.58 | (1.10–2.26) | 1.57 | (1.10–2.25) | 1.56 | (1.09–2.23) | 21 | 1.75 | (1.09–2.80) | 1.72 | (1.07–2.75) | 1.72 | (1.07–2.75) |
| 60+ | 37 | 1.31 | (0.89–1.91) | 1.35 | (0.92–1.97) | 1.32 | (0.90–1.93) | 23 | 1.37 | (0.83–2.24) | 1.41 | (0.86–2.32) | 1.39 | (0.84–2.27) |
All models include stage at diagnosis, tumor histology, surgery, radiation, and chemotherapy as stratifying variables and are adjusted for age at diagnosis, marital status, year of diagnosis, and time to treatment. Adjustments for race/ethnicity and neighborhood SES are indicated in table.
Bold type indicates statistical significance.
HR, hazard ratio; CI, confidence interval.
AYAs from middle and low SES neighborhoods had much lower overall (HR, 1.34; 95% CI, 1.12–1.60 and HR, 1.79; 95% CI, 1.50–2.14 respectively) and testicular cancer–specific (HR, 1.40; 95% CI, 1.06–1.84 and HR, 1.71; 95% CI, 1.29–2.25 respectively) survival than AYAs from high SES neighborhoods, even after controlling for race/ethnicity.
Patients 15–24 years of age had greater overall, but not testicular cancer–specific, survival compared with patients 25–39 years of age (HR, 0.70; 95% CI, 0.61–0.80 and HR, 0.92; 95% CI, 0.77–1.11 respectively). Unmarried AYAs had worse overall and testicular cancer–specific survival compared with married AYAs (HR, 1.57; 95% CI, 1.39–1.78 and HR, 1.46; 95% CI, 1.20–1.76 respectively). In addition, more recent diagnosis and greater time to treatment were generally associated with lower overall survival and testicular cancer–specific survival.
Disparities in survival vary by histology
There were no racial/ethnic differences in overall survival among AYAs with seminoma (Table 3). For nonseminoma, however, Hispanics had lower overall survival, even after controlling for neighborhood SES (HR, 1.25; 95% CI, 1.07–1.47). Blacks and Asian/PIs also had a suggestively lower survival after nonseminoma testicular cancer (HR, 1.46; 95% CI, 0.91–2.33 and HR, 1.30; 95% CI, 0.88–1.90 respectively). For both seminoma and nonseminoma testicular cancer, AYAs living in middle and low SES neighborhoods had worse overall survival compared with AYAs living in high SES neighborhoods.
Table 3.
Hazard Ratios and 95% CIs from Stratified Cox Proportional Hazards Models1 of Overall Survival Among Adolescent and Young Adult Men 15–39 Years of Age with Seminoma or Nonseminoma Testicular Cancer According to SES Characteristics and Year of Diagnosis, 1988–2010 California
| Seminomaa | Nonseminomaa | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adjusted for race/ethnicity | Adjusted for neighborhood SES | Adjusted for race/ethnicity and neighborhood SES | Adjusted for race/ethnicity | Adjusted for neighborhood SES | Adjusted for race/ethnicity and neighborhood SES | |||||||||
| Deaths | HR | 95% CI | HR | 95% CI | HR | 95% CI | Deaths | HR | 95% CI | HR | 95% CI | HR | 95% CI | |
| Race/ethnicity | ||||||||||||||
| White | 295 | 1.00 | Reference | 1.00 | Reference | 446 | 1.00 | Reference | 1.00 | Reference | ||||
| Black | 14 | 1.33 | (0.77–2.28) | 1.17 | (0.68, 2.01) | 19 | 1.60 | (1.00–2.55) | 1.46 | (0.91–2.33) | ||||
| Asian/PI | 16 | 0.81 | (0.49–1.35) | 0.81 | (0.49, 1.35) | 29 | 1.27 | (0.87–1.87) | 1.30 | (0.88–1.90) | ||||
| Hispanic | 152 | 1.32 | (1.08–1.62) | 1.12 | (0.90, 1.38) | 339 | 1.40 | (1.21–1.63) | 1.25 | (1.07–1.47) | ||||
| Other | 6 | 1.04 | (0.46–2.35) | 1.00 | (0.44, 2.27) | 7 | 0.64 | (0.30–1.37) | 0.65 | (0.31–1.38) | ||||
| Neighborhood SES | ||||||||||||||
| High | 55 | 1.00 | Reference | Reference | 108 | 1.00 | Reference | 1.00 | Reference | |||||
| Middle | 188 | 1.50 | (1.11–2.04) | 1.49 | (1.09, 2.02) | 330 | 1.27 | (1.02–1.58) | 1.24 | (0.99–1.54) | ||||
| Low | 240 | 2.30 | (1.70–3.11) | 2.21 | (1.62, 3.01) | 402 | 1.71 | (1.38–2.12) | 1.57 | (1.26–1.97) | ||||
| Age at diagnosis | ||||||||||||||
| 15–24 | 56 | 0.89 | (0.66–1.18) | 0.87 | (0.65–1.15) | 0.85 | (0.64, 1.14) | 278 | 0.69 | (0.59–0.81) | 0.72 | (0.62–0.84) | 0.69 | (0.59–0.81) |
| 25–39 | 427 | 1.00 | Reference | 1.00 | Reference | Reference | 562 | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | |
| Marital status | ||||||||||||||
| Married | 156 | 1.00 | Reference | 1.00 | Reference | Reference | 228 | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | |
| Not married | 317 | 1.91 | (1.57–2.33) | 1.91 | (1.57–2.33) | 1.92 | (1.58, 2.34) | 600 | 1.39 | (1.18–1.63) | 1.39 | (1.18–1.63) | 1.39 | (1.18–1.64) |
| Unknown | 10 | 1.63 | (0.82–3.21) | 1.63 | (0.82–3.21) | 1.63 | (0.82, 3.21) | 12 | 1.09 | (0.60–1.98) | 1.09 | (0.60–1.98) | 1.10 | (0.60–2.00) |
| Year of diagnosis | ||||||||||||||
| 1988–1995 | 292 | 1.00 | Reference | 1.00 | Reference | Reference | 374 | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | |
| 1996–2003 | 137 | 0.70 | (0.56–0.87) | 0.71 | (0.57–0.88) | 0.70 | (0.56–0.88) | 247 | 0.81 | (0.68–0.96) | 0.83 | (0.70–0.98) | 0.81 | (0.68–0.96) |
| 2004–2010 | 54 | 0.46 | (0.33–0.64) | 0.48 | (0.35–0.67) | 0.47 | (0.34–0.66) | 219 | 0.85 | (0.70–1.02) | 0.89 | (0.74–1.07) | 0.85 | (0.70–1.02) |
| Time to treatment (days) | ||||||||||||||
| 0 | 308 | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 453 | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference |
| 1–14 | 118 | 1.16 | (0.91–1.46) | 1.17 | (0.93–1.48) | 1.17 | (0.93–1.48) | 291 | 1.27 | (1.08–1.49) | 1.30 | (1.11–1.53) | 1.28 | (1.09–1.50) |
| 15–30 | 33 | 1.32 | (0.89–1.95) | 1.30 | (0.87–1.92) | 1.29 | (0.87–1.91) | 54 | 1.44 | (1.06–1.94) | 1.51 | (1.12–2.03) | 1.47 | (1.08–1.98) |
| 31–60 | 12 | 1.50 | (0.81–2.80) | 1.49 | (0.80–2.77) | 1.48 | (0.79–2.74) | 23 | 1.65 | (1.06–2.56) | 1.70 | (1.10–2.64) | 1.65 | (1.06–2.57) |
| 60+ | 12 | 1.08 | (0.54–2.17) | 1.10 | (0.55–2.20) | 1.08 | (0.54–2.16) | 19 | 1.35 | (0.83–2.18) | 1.40 | (0.87–2.26) | 1.37 | (0.84–2.22) |
All models include stage at diagnosis, surgery, radiation, and chemotherapy as stratifying variables and are adjusted for age at diagnosis, marital status, year of diagnosis, and time to treatment. Adjustments for race/ethnicity and neighborhood SES indicated in table.
Bold type indicates statistical significance.
For nonseminoma, younger AYAs 15–24 years of age had better survival than older AYAs (HR, 0.69; 95% CI, 0.59–0.81). Unmarried AYAs had worse survival compared with married AYAs for both seminoma and nonseminoma. The improvement in survival for more recent diagnoses was more marked among AYAs with seminoma, as the better survival of patients diagnosed in 2004–2010 with nonseminoma was of only borderline statistical significance compared with patients diagnosed in 1988–1995. Lower survival with greater time to treatment was more pronounced for nonseminoma than for seminoma.
Discussion
In this large, population-based study of testicular cancer among AYAs, Hispanic AYAs had worse survival after testicular cancer than White AYAs, with these survival disparities more pronounced for Hispanics with the nonseminoma histologic subtype. Although testicular cancer is rare among Black AYAs (<2% of testicular cancer cases were Black AYAs), they experienced worse overall survival after diagnosis of testicular cancer than Whites. AYAs residing in lower SES neighborhoods experienced worse survival across both histologic subtypes, while improvements over time were most notable for seminoma. Longer time to treatment was also associated with worse survival, especially for AYAs with nonseminoma.
One study of men of all ages reported worse survival for non-Whites (including Hispanics) compared with non-Hispanic Whites only for nonseminoma cancer.17 Our analyses have further shown that Hispanic AYAs have lower survival compared with White AYAs for nonseminoma, and suggest that Black and Asian/PI AYAs also may have lower survival from nonseminoma. Further, we only observed better survival for patients diagnosed in more recent years for the seminoma histologic type and the association of time to treatment with survival is more marked for nonseminoma; these observed survival differences may be a reflection of differences in prognosis and treatment availability by subtype. Nonseminoma testicular cancer comprises several distinct subtypes19 and is less sensitive to radiation,19 more prone to relapse,19 and can involve more complicated treatment decisions20 compared with seminoma. As a result, chemotherapy and retroperitoneal lymph node dissection (RPLND), a surgery to remove abdominal lymph nodes that can harbor cancer cells even in early stage nonseminoma,19 are used more often to treat nonseminoma.19 Both chemotherapy and RPLND, however, can be accompanied by significant complications.24 Moreover, RPLND is performed in specialized centers of excellence, which may contribute to delays in delivery of continuing care. As a result, difficulties experienced by Hispanic, Black, or Asian/PI AYAs or AYAs residing in lower SES neighborhoods25–31 may exaggerate survival disparities for nonseminoma. In addition, the aggressive nature of nonseminoma may contribute to the more marked association of time to treatment with this histology.19
Our study extends the findings in prior studies of men of all ages15,16,18 by additionally considering neighborhood SES and relevant treatment variables and showing that worse overall survival of Hispanic and Black AYAs after testicular cancer, compared with White AYAs, is independent of treatment and neighborhood SES. We did not observe racial/ethnic differences in the distribution of cancer histology in our study and no prior studies have reported differences in the biology of testicular cancer by race/ethnicity, so these factors are unlikely to explain the poorer survival we observed for Hispanic, Black, and Asian/PI AYAs. Other potential mediators of the effects of race/ethnicity on testicular cancer are chronic stress due to racial/ethnic discrimination25 or differences in treatment compliance (after treatment initiation) between racial/ethnic groups,29 factors we could not measure in this study.
SEER studies of men of all ages utilized a limited number of county-level SES indicators to study the relationship of SES with testicular cancer.15–18 Our results with census-tract level neighborhood SES support lower overall and testicular cancer–specific survival for AYAs residing in low SES neighborhoods for both seminoma and nonseminoma. Lower neighborhood SES has frequently been shown to correlate with worse cancer outcomes at all ages of diagnosis and for many different types of cancer.6,7,30,32,33 In this context, neighborhood SES is conceptualized as an independent risk factor for survival, not a proxy for individual SES, and mediates poorer individual survival outcomes through neighborhood-level factors different from individual SES indicators.26,28 These mediators may include reduced mental health,26–28 chronic stress,26,27 aspects of the neighborhood social environment,34–37 and reduced quality or availability of healthcare and support services in lower SES neighborhoods.26,28,30,31
Previous studies have reported greater survival among AYAs compared with survival among older men.16,17 We have shown that younger AYAs (15–24 years of age) have greater overall survival than older AYAs (25–39 years of age) from nonseminoma, which could be due to younger AYAs having fewer comorbidities, differences in treatment in the pediatric setting versus the adult setting, or the ability of younger patients to withstand more intense treatment regimens.14 Worse survival among unmarried compared with married patients has been reported previously for men of all ages with testicular cancer15,17 and for many cancer types.38 The reason for this disparity is unknown, but may relate to less social support for unmarried persons following a cancer diagnosis.39 Improved survival for patients more recently diagnosed with seminoma may be due to incremental improvements in approaches to radiotherapy and chemotherapy that better manage toxicity and secondary effects.19
This study of survival of AYAs with testicular cancer is unique among other studies of testicular cancer survival in that it examines survival specifically among young men. These young men bear the majority of the burden from testicular cancer 2 and AYAs as a group can be especially vulnerable to factors that affect cancer treatment and outcome.40 Our study does have limitations. Data on comorbid conditions were not available in the registry and information on health insurance was not available until 2001. In addition, detailed American Joint Committee on Cancer staging information was not available for testicular cancer before 2004, so SEER summary staging was used. SEER summary stage does not account for factors within its categories that may affect survival, such as degree of extension or the size of the primary tumor. We were also not able to include information on serum markers of testicular cancer or receipt of RPLND. In addition, the CCR does not provide information on individual level SES. In the future, it will be important to consider differences in survival according to individual level SES and whether individual and neighborhood SES independently contribute to survival. Finally, as with all registry studies, we must consider differential misclassification of race/ethnicity. However, it has previously been determined that the level of agreement between CCR data and self-reported race/ethnicity is excellent for Whites and Blacks and intermediate for Hispanics and Asians.41,42
Conclusion
Race/ethnicity and neighborhood SES were independently associated with overall and testicular cancer specific survival among AYAs. Hispanic AYAs experienced significantly worse, and Black and Asian/PI AYAs experienced suggestively worse, overall survival after nonseminoma testicular cancer. Improvements in survival over time were more pronounced for seminoma testicular cancer, while the better survival observed among younger AYAs was limited to nonseminoma testicular cancer. The survival differences between race/ethnicity, age group, and year of diagnosis by histologic type may reflect differences in prognosis and extent of testicular cancer treatment. Further research should address factors that mediate the observed associations between race/ethnicity and neighborhood SES with testicular cancer survival among AYAs.
Acknowledgments
This work was supported by the California Department of Public Health as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885 and the SEER program of the National Cancer Institute at the National Institutes of Health under contract HHSN2612010000140C awarded to the Cancer Prevention Institute of California (M.C.D., T.H.M.K.) and the Stanford Cancer Institute (T.H.M.K.). M.M. and S.S. did not receive financial support for this project.
The collection of cancer incidence data used in this study was supported by the California Department of Public Health as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885; the National Cancer Institute's SEER program under contract HHSN2612010000140C awarded to the Cancer Prevention Institute of California, contract HHSN261201000035C awarded to the University of Southern California, and contract HHSN261201000034C awarded to the Public Health Institute; and the Centers for Disease Control and Prevention's National Program of Cancer Registries, under agreement No. 1U58 DP000807-01 awarded to the Public Health Institute.
The ideas and opinions expressed herein are those of the authors and endorsement by the State of California, Department of Health Services, the National Cancer Institute, and the Centers for Disease Control and Prevention or their contractors and subcontractors is not intended, nor should it be inferred.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Bleyer A, Barr R, Hayes-Lattin B, et al. . The distinctive biology of cancer in adolescents and young adults. Nat Rev Cancer. 2008;8(4):288–98 [DOI] [PubMed] [Google Scholar]
- 2.Townsend JS, Richardson LC, German RR. Incidence of testicular cancer in the United States, 1999–2004. Am J Mens Health. 2010;4(4):353–60 [DOI] [PubMed] [Google Scholar]
- 3.Bleyer A, Budd T, Montello M. Adolescents and young adults with cancer: the scope of the problem and criticality of clinical trials. Cancer. 2006;107(7 Suppl):1645–55 [DOI] [PubMed] [Google Scholar]
- 4.Bleyer WA. Cancer in older adolescents and young adults: epidemiology, diagnosis, treatment, survival, and importance of clinical trials. Med Pediatr Oncol. 2002;38(1):1–10 [DOI] [PubMed] [Google Scholar]
- 5.Keegan TH, Press DJ, Tao L, et al. . Impact of breast cancer subtypes on 3-year survival among adolescent and young adult women. Breast Cancer Res. 2013;15(5):R95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Derouen MC, Gomez SL, Press DJ, et al. . A population-based observational study of first-course treatment and survival for adolescent and young adult females with breast cancer. J Adolesc Young Adult Oncol. 2013;2(3):95–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kent EE, Morris RA, Largent JA, et al. . Socioeconomic impacts on survival differ by race/ethnicity among adolescents and young adults with non-Hodgkin's lymphoma. J Cancer Epidemiol. 2010;2010:824691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parsons HM, Harlan LC, Seibel NL, et al. . Clinical trial participation and time to treatment among adolescents and young adults with cancer: does age at diagnosis or insurance make a difference? J Clin Oncol. 2011;29(30):4045–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parsons HM, Harlan LC, Lynch CF, et al. . Impact of cancer on work and education among adolescent and young adult cancer survivors. J Clin Oncol. 2012;30(19):2393–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bellizzi KM, Smith A, Schmidt S, et al. . Positive and negative psychosocial impact of being diagnosed with cancer as an adolescent or young adult. Cancer. 2012;118(20):5155–62 [DOI] [PubMed] [Google Scholar]
- 11.Smith A, Bellizzi KM, Keegan TH, et al. . Health-related quality of life of adolescent and young adult cancer patients in the United States: the AYA HOPE study. J Clin Oncol. 2013;31(17):2136–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith A, Parsons HM, Kent EE, et al. . Unmet support service needs and health-related quality of life among adolescent and young adults with cancer: the AYA HOPE study. Front Pediatr Oncol. 2013;3:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schmidt C. Lack of progress in teen and young adult cancers concerns researchers, prompts study. J Natl Cancer Inst. 2006;98(24):1760–3 [DOI] [PubMed] [Google Scholar]
- 14.Hayes-Lattin B, Nichols CR, editors. Testicular cancer: a prototypic tumor of young adults. Semin Oncol. 2009;36(5):432–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson TV, Hsiao W, Jani A, Master VA. Increased mortality among Hispanic testis cancer patients independent of neighborhood socioeconomic status: A SEER study. J Immigr Minor Health. 2011;13(5):818–24 [DOI] [PubMed] [Google Scholar]
- 16.Sun M, Abdollah F, Liberman D, et al. . Racial disparities and socioeconomic status in men diagnosed with testicular germ cell tumors. Cancer. 2011;117(18):4277–85 [DOI] [PubMed] [Google Scholar]
- 17.Fosså SD, Cvancarova M, Chen L, et al. . Adverse prognostic factors for testicular cancer–specific survival: a population-based study of 27,948 patients. J Clin Oncol. 2011;29(8):963–70 [DOI] [PubMed] [Google Scholar]
- 18.Biggs ML, Schwartz SM. Differences in testis cancer survival by race and ethnicity: a population-based study, 1973–1999 (United States). Cancer Causes Control. 2004;15(5):437–44 [DOI] [PubMed] [Google Scholar]
- 19.Feldman DR, Bosl GJ, Sheinfeld J, Motzer RJ. Medical treatment of advanced testicular cancer. JAMA. 2008;299(6):672–84 [DOI] [PubMed] [Google Scholar]
- 20.Foster RS. Early-stage testis cancer. Curr Treat Options Oncol. 2001;2(5):413–9 [DOI] [PubMed] [Google Scholar]
- 21.Parsons HM, Harlan LC, Seibel NL, et al. . Clinical trial participation and time to treatment among adolescents and young adults with cancer: does age at diagnosis or insurance make a difference? J Clin Oncol. 2011;29(30):4045–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yost K, Perkins C, Cohen R, et al. . Socioeconomic status and breast cancer incidence in California for different race/ethnic groups. Cancer Causes Control. 2001;12(8):703–11 [DOI] [PubMed] [Google Scholar]
- 23.Yang J SC, Harrati A, Clarke C, Keegan THM, Gomez SL. Developing an area-based socioeconomic measure from American Community Survey data. Fremont, CA: Cancer Prevention Institute of California; 2014 [Google Scholar]
- 24.Kaufman MR, Chang SS. Short- and long-term complications of therapy for testicular cancer. Urol Clin North Am. 2007;34(2):259–68 [DOI] [PubMed] [Google Scholar]
- 25.Krieger N. Embodying inequality: a review of concepts, measures, and methods for studying health consequences of discrimination. Int J Health Serv. 1999;29(2):295–352 [DOI] [PubMed] [Google Scholar]
- 26.Ellen IG, Mijanovich T, Dillman KN. Neighborhood effects on health: exploring the links and assessing the evidence. J Urban Aff. 2001;23(3–4):391–408 [Google Scholar]
- 27.Chida Y, Hamer M, Wardle J, Steptoe A. Do stress-related psychosocial factors contribute to cancer incidence and survival? Nat Clin Pract Oncol. 2008;5(8):466–75 [DOI] [PubMed] [Google Scholar]
- 28.Galster GC. The mechanism(s) of neighbourhood effects: theory, evidence, and policy implications. In: van Ham M, Manley D, Bailey N, et al. (Eds.) Neighbourhood effects research: new perspectives. Netherlands: Springer; 2012; pp. 23–56 [Google Scholar]
- 29.Hershman DL, Shao T, Kushi LH, et al. . Early discontinuation and non-adherence to adjuvant hormonal therapy are associated with increased mortality in women with breast cancer. Breast Cancer Res Treat. 2011;126(2):529–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Freeman VL, Ricardo AC, Campbell RT, et al. . Association of census tract-level socioeconomic status with disparities in prostate cancer-specific survival. Cancer Epidemiol Biomarkers Prev. 2011;20(10):2150–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Falagas ME, Zarkadoulia EA, Ioannidou EN, et al. . The effect of psychosocial factors on breast cancer outcome: a systematic review. Breast Cancer Res. 2007;9(4):R44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Byers TE, Wolf HJ, Bauer KR, et al. . The impact of socioeconomic status on survival after cancer in the United States: findings from the National Program of Cancer Registries Patterns of Care study. Cancer. 2008. 1;113(3):582–91 [DOI] [PubMed] [Google Scholar]
- 33.Keegan TH, Quach T, Shema S, et al. . The influence of nativity and neighborhoods on breast cancer stage at diagnosis and survival among California Hispanic women. BMC Cancer. 2010;10:603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eschbach K, Ostir GV, Patel KV, et al. . Neighborhood context and mortality among older Mexican Americans: is there a barrio advantage? Am J Public Health. 2004;94(10):1807–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Warner ET, Gomez SL. Impact of neighborhood racial composition and metropolitan residential segregation on disparities in breast cancer stage at diagnosis and survival between black and white women in California. J Community Health. 2010;35(4):398–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lim JW, Ashing-Giwa KT. Examining the effect of minority status and neighborhood characteristics on cervical cancer survival outcomes. Gynecol Oncol. 2011;121(1):87–93 [DOI] [PubMed] [Google Scholar]
- 37.Russell E, Kramer MR, Cooper HL, et al. . Residential racial composition, spatial access to care, and breast cancer mortality among women in Georgia. J Urban Health. 2011;88(6):1117–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kravdal O. The impact of marital status on cancer survival. Soc Sci Med. 2001;52(3):357–68 [DOI] [PubMed] [Google Scholar]
- 39.Coombs RH. Marital status and personal well-being: A literature review. Fam Relat. 1991;40(1):97–102 [Google Scholar]
- 40.Bleyer A. Young adult oncology: the patients and their survival challenges. CA Cancer J Clin. 2007;57(4):242–55 [DOI] [PubMed] [Google Scholar]
- 41.Gomez SL, Glaser SL. Misclassification of race/ethnicity in a population-based cancer registry (United States). Cancer Causes Control. 2006;17(6):771–81 [DOI] [PubMed] [Google Scholar]
- 42.Clegg LX, Reichman ME, Hankey BF, et al. . Quality of race, Hispanic ethnicity, and immigrant status in population-based cancer registry data: implications for health disparity studies. Cancer Causes Control. 2007;18(2):177–87 [DOI] [PubMed] [Google Scholar]