Abstract
Significance: Fibroblasts play a critical role in normal wound healing. Various extracellular matrix (ECM) components, including collagens, fibrin, fibronectin, proteoglycans, glycosaminoglycans, and matricellular proteins, can be considered potent protagonists of fibroblast survival, migration, and metabolism.
Recent Advances: Advances in tissue culture, tissue engineering, and ex vivo models have made the examination and precise measurements of ECM components in wound healing possible. Likewise, the development of specific transgenic animal models has created the opportunity to characterize the role of various ECM molecules in healing wounds. In addition, the recent characterization of new ECM molecules, including matricellular proteins, dermatopontin, and FACIT collagens (Fibril-Associated Collagens with Interrupted Triple helices), further demonstrates our cursory knowledge of the ECM in coordinated wound healing.
Critical Issues: The manipulation and augmentation of ECM components in the healing wound is emerging in patient care, as demonstrated by the use of acellular dermal matrices, tissue scaffolds, and wound dressings or topical products bearing ECM proteins such as collagen, hyaluronan (HA), or elastin. Once thought of as neutral structural proteins, these molecules are now known to directly influence many aspects of cellular wound healing.
Future Directions: The role that ECM molecules, such as CCN2, osteopontin, and secreted protein, acidic and rich in cysteine, play in signaling homing of fibroblast progenitor cells to sites of injury invites future research as we continue investigating the heterotopic origin of certain populations of fibroblasts in a healing wound. Likewise, research into differently sized fragments of the same polymeric ECM molecule is warranted as we learn that fragments of molecules such as HA and tenascin-C can have opposing effects on dermal fibroblasts.

E. J. Caterson, MD, PhD
Scope and Significance
This review highlights translational research regarding the effect of the local extracellular matrix (ECM) on dermal fibroblasts. The modulation of ECM components in the healing wound is discussed, with some examples of current research related to the topic.
Clinical Relevance
Historically, the ECM has been misconstrued as relatively inert scaffolding, existing only to support cells. An initial insight into the role of ECM was expanded with the revelation that growth of most human cells, including skin cells, depends on cellular adhesion to the ECM through a mechanism called anchorage dependence.1 This gave greater credibility to the idea that the ECM is a vital participant in tissue activity and wound healing. Beyond simply anchoring cells, it is now known that the ECM is an active and complex tissue component which is capable of influencing cell survival, proliferation, and function.2 Due to their ability to modify cellular properties in a healing wound, ECM components are attractive targets for the emerging clinical use of bioactive wound dressings, engineered tissues, and topical wound treatments (Table 1).
Table 1.
Selected list of clinically available extracellular matrix components for modulation of wound healing or scar treatment
| Extracellular Matrix Component | Wound Dressings | Dermal Substitutes/Tissue Scaffolds | Soft Tissue Fillers | |||
|---|---|---|---|---|---|---|
| Collagen I | Biobrane (p) Biostep (p) Biopad (e) Catrix (b) CellerateRX (b) Colactive AG (p) Collasorb (b) Collieva (p) DermaCol (p) DermADAPT Dermagen |
DermaSIL Fibracol (b) FortaFlex (p) FortaDerm Medifil II (b) Prisma Matrix Promogran Matrix (p) Puracol (b) Skintemp (b) Stimulen |
Alloderm (h) Allomax (h) Apligraf (h,b) Biodesign (p) Collamend (p) Dermagraft (h) DermaMatrix (h) Endoform (o) EZ-Derm (p) FlexHD (h) Fortaflex (p) GammaGraft (h) |
Glyaderm (h) GraftJacket (h) ICX-SKN (h) Integra (b) Karoderm (h) Matriderm (b) MatriStem (p) NeoForm (h) Oasis (p) OrCel (h,b) Permacol (p) PermaDerm (b) |
Primatrix (b) Repliform (h) SIS (p) Strattice (p) Surederm (h) Strattice (p) Surgimend (b) TheraSkin (h) TransCyte (h,p) Unite (e) Veritas (b) XenMatrix (p) |
Artefill (b) Cosmoderm (b) Cosmoplast (b) Cymetra (h) Dermalogen (h) Fascian (h) Zyderm (b) Zyplast (b) |
| Collagen II | BioCell collagen | None | None | |||
| Collagen III | None | Biodesign (p) DermaMatrix (h) Dermagraft (h) Endoform (o) |
GraftJacket (h) ICX-SKN (h) Matriderm (b) Oasis (p) |
Primatrix (b) Surgimend (b) TheraSkin (h) TransCyte (h,p) |
None | |
| Collagen IV | None | Endoform (o) Dermagraft (h) |
GraftJacket (h) MatriStem (p) |
Oasis (p) TheraSkin (h) |
None | |
| Collagen V | None | Matriderm (b) | TheraSkin (h) | TransCyte (h,p) | None | |
| Collagen VI | None | Biodesign (p) | Oasis (p) | TheraSkin (h) | None | |
| Collagen VII | None | GraftJacket (h) | MatriStem (p) | None | ||
| Collagen XIV | None | TheraSkin (h) | None | |||
| Fibrin | Crosseal (h) Evarrest (h) |
Evicel (h) Tisseel (h) |
BioSeed-S | ICX-SKN (h) | None | |
| Fibronectin | None | Biodesign (p) Endoform (o) |
Oasis (p) | TransCyte (h,p) | None | |
| Vitronectin | None | DermaMatrix (h) | None | |||
| Elastin | Elastatropin TropolActive-F |
Prolastil E-50 | Alloderm (h) Allomax (h) Dermagraft (h) |
DermaMatrix (h) Endoform (o) Glyaderm (h) |
GraftJacket (h) Matriderm (b) Oasis (p) |
None |
| Hyaluronan | BioCell collagen HealSmart Hyalofill Hyalomatrix |
Hyiodine Hylase IPM Wound Gel RadiaPlexRx |
Biodesign (p) DermaMatrix (h) Endoform (o) |
Hyalograft Hyalomatrix GraftJacket (h) |
Laserskin Oasis (p) |
Belotero (es) Captique (es) Hylaform (r) Hydrelle (es) Juvederm (es) Restylane (es) Perlane (es) |
| Chondroitin sulfate | BioCell collagen | Dermagen | Biodesign (p) GraftJacket (h) |
Integra (b) | Oasis (p) | None |
| Decorin | None | Dermagraft (h) | Oasis (p) | TransCyte (h,p) | None | |
| Versican | None | TransCyte (h,p) | None | |||
| SPARC | None | TransCyte (h,p) | None | |||
| Tenascin | None | GraftJacket (h) | TransCyte (h,p) | None | ||
More than 30 dermal substitutes containing multiple ECM components are available clinically today. Numerous unlisted topical gels and powders featuring ECM components also exist.
b, bovine; e, equine; es, equine streptococci; h, human; o, ovine; p, porcine; r, rooster comb.
Translational Relevance
Since dermal fibroblasts are so intimately linked to the ECM, as both an originator and a resident, the modulatory properties of the local ECM are perhaps most apparent in the functioning of dermal fibroblasts. The ECM is capable of influencing the migration, senescence, and gene expression of the fibroblast, and it is even capable of differentiating the fibroblast into entirely different cell types.3 Since it is so poorly characterized on its own terms, in some sense the fibroblast is actually defined by its local ECM. Since the ECM sequesters and regulates exposure of the fibroblast to soluble cytokines, it is not possible to completely dissociate the role of these signaling molecules from that of the fibroblast's proteinacious matrix microenvironment.4 However, this review focuses on the classic, macromolecular, insoluble components of the skin ECM, and the effect these structures have on the fibroblast. This review discusses the impact of various ECM components on wound-healing functions of dermal fibroblasts, including migration, proliferation, gene expression, and protein metabolism. As is the case with many aspects of wound healing, a balance should be struck between inadequate fibroblast activity, which may lead to chronic wound formation, and overly exuberant fibroblast activity, which can lead to fibrotic healing. Many components of the ECM are involved in regulating this equilibrium, and can potentially be manipulated to swing the balance one way or the other to improve overall wound healing.
The Fibroblast and the ECM
Defining the fibroblast
Although it is the most prevalent cell in human dermis, and one of the most important architects of cutaneous wound healing, the fibroblast as a cell type remains relatively ill defined. The word “fibroblast” encompasses any stromal cell that does not express markers for a more specific mesenchymal lineage. This imprecise definition, and our impaired ability to track cellular changes due to a lack of distinguishing cell markers, lends to the misconception of the fibroblast as a homogenous and static cell. However, fibroblasts are an impressively heterogeneous and dynamic cell lineage. Fibroblast populations differ between human tissues, with significant alterations even apparent within a given tissue type. In skin dermis, for example, different dermal layers host diverse subpopulations of fibroblasts with both unique morphologies and physiologic functions.5–8 Depending on their resident depth of dermis, fibroblasts express different quantities of collagen and ratios of collagen type I and III mRNA. Further, fibroblasts within the deeper dermis produce less collagenase mRNA than do fibroblasts in more superficial layers.9
Fibroblasts are also remarkably plastic in nature. Cells that share fibroblasts' mesenchymal origin, including adipocytes and pericytes, are able to de-differentiate into fibroblasts with an appropriate stimulus.10–14 Even cells of endothelial and epithelial origin are capable of taking on the phenotype into fibroblasts, a fact that has emerged as an important pathologic etiology for fibrotic disease and likely tumor metastasis.15–18 Likewise, fibroblasts themselves are capable of altering their cellular profile, with the most common transition being that into contractile myofibroblasts. The dermal fibroblast also has the unique title of being the first human somatic cell to be induced into a pluripotent stem cell line.19–21
The fibroblast is a malleable cell, capable of altering its function and physiology or even transforming into a new cell type, based on its location within the body. The mechanisms driving these alterations are still poorly understood; however, it is thought that both soluble signaling molecules and the resident molecular framework of the fibroblast's extracellular microenvironment can act to modify the activity of the fibroblast. The role of growth factors and cytokines in the fibroblast during wound healing has been discussed in other excellent reviews.22–25 Since its manipulation and augmentation is emerging in clinical treatment (including use of acellular dermal matrices and topical wound dressings), the influence of the ECM on the fibroblast, both mechanically and through modulation of molecular pathways, warrants further discussion.
Defining the ECM
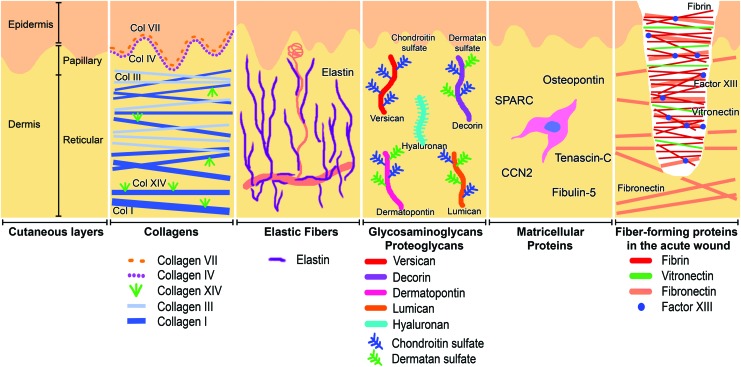
The extracellular components of the skin can be divided into fiber-forming structural molecules, nonfiber-forming structural molecules, and “matricellular proteins” that do not function structurally but modify cell–matrix interactions (Fig. 1).26 Fiber-forming molecules provide a structure to the ECM by creating a complex three-dimensional framework of rigid proteins. The nonfiber-forming molecules, mostly proteoglycans and glycosaminoglycans (GAGs), function to create a charged, dynamic, and osmotically active space. In the skin, the most prevalent fiber-forming protein, by far, is collagen, which comprises 77% of the fat-free dry weight of human skin.27 Other fiber-forming proteins in the skin include fibrin, fibronectin, vitronectin, elastin, and fibrillin.28 Glycoproteins such as laminins, secreted molecules that comprise the basement membrane, and integrins, which act as cell membrane-bound receptors, are also structural proteins that facilitate skin cell adhesion and migration. While these fibrous proteins define the rigidity and elasticity of a tissue, it is the nonfiber-forming proteoglycans and GAGs that fill the majority of the tissue's interstitial space.26 Their negatively charged and hydrophilic nature enable proteoglycans and GAGs to function in hydration, buffering, and force dispersion within tissues. The most abundant proteoglycans in the skin include hyaluronan (HA), decorin, versican, and dermatopontin.29,30 Also present in the ECM are the matricellular proteins, a recently described group of secreted local proteins that interact in autocrine or paracrine cell–matrix signaling while not contributing significantly to ECM mechanical structure. Included among the matricellular proteins are osteopontin, secreted protein, acidic and rich in cysteine (SPARC) (also known as osteonectin), tenascin-C, fibulins, and the CCN family.31 Unlike most ECM components that are constantly present in normal skin and repopulate wounded skin after predictable intervals, matricellular proteins can be absent in healthy skin and expressed temporarily only after skin wounding (Table 2). In the healing wound, fibroblasts produce the majority of these ECM components while these same molecules simultaneously act to modify the function of the fibroblast.24 In this sense, the interaction of the fibroblast with the ECM can be thought of as a form of autocrine regulation that is crucial in the process of wound healing. The fibroblast should continually create new matrix proteins to properly heal a cutaneous wound, while concurrently being regulated by proteins of its own creation.
Figure 1.
The extracellular matrix (ECM) of normal skin with selected components depicted. Fiber-forming proteins and collagens define the rigid mechanical structure of skin, while elastic fibers enable stretching. Proteoglycans and glycoproteins create an osmotically active hydrated interstitial space. Matricellular proteins do not contribute to the mechanical structure of the ECM but instead act as paracrine signaling molecules. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Table 2.
Chronology of selected extracellular matrix component expression in healing human wounds
| Earliest Appearance | Regular Appearance | References | |
|---|---|---|---|
| Fiber-forming collagens | |||
| Collagen I | 4 days | 7 days | 160, 161, 164 |
| Collagen III | 2 days | 6 days | 163 |
| Collagen V | 3 days | 7 days | 161, 185 |
| Nonfiber-forming collagens | |||
| Collagen IV | 4 days | 7 days | 72, 161, 185 |
| Collagen VI | 3 days | 7 days | 161, 165 |
| Collagen VII | 4 days | 12 days | 161, 166, 185 |
| Collagen XIV | Unknown | Unknown | N/A |
| Other fiber-forming proteins | |||
| Fibrin | Seconds | 30 s | 167 |
| Fibronectin | 10 min | 4 h | 72, 164 |
| Vitronectin | Unknown | 3 days | 168 |
| Elastin | 3 days | 14 days | 170 |
| Proteoglycans/glycosaminoglycans | |||
| Hyaluronic acid | 1 day | >1.5 days | 171, 172 |
| Chondroitin sulfate | Unknown | 3 days | 169, 173, 174 |
| Dermatan sulfate | Unknown | 3 days | 169, 173, 174 |
| Decorin | 7 days | 12 days | 171, 173 |
| Lumican | 3 days | 7 days | 175 |
| Versican | Unknown | 14 days | 176, 177 |
| Dermatopontin | 2 days | 14 days | 30 |
| Basement membrane | |||
| Complete intact basement membrane | 8 days | 21 days | 161, 178, 185 |
| Matricellular proteins | |||
| Osteopontin | 4 days | 14 days | 121, 179 |
| SPARC | 2 days | 7 days | 180, 181 |
| CCN2 | 1 day | 5 days | 161, 182 |
| Tenascin-C | 1 day | 5 days | 161, 182 |
| Fibulin-5 | Unknown | 14 days | 147 |
| Cell types | |||
| Fibrocytes | Unknown | >4 days | 183 |
| Myofibroblasts | Unknown | 5 days | 72, 161, 162 |
Earliest demonstrable histologic appearance and regular histologic appearance of ECM components in human wounds is indicated.
Collagen
The tensile strength and compressibility of fiber-forming collagen make it a ubiquitous protein in the human body. In fact, collagen is thought to be the single most abundant protein in the animal kingdom.32 Although 28 types of collagen have been identified, collagens I and III, respectively compose approximately 90% and 10% of the total collagen in the skin, with collagen V representing about 2%.33 However, less prevalent collagen types are also integral to normal skin functioning as indicated by the pathologic skin conditions associated with mutations in collagens IV, VII, and XVII (Table 3).
Table 3.
Genetic disorders involving extracellular matrix proteins, and the characteristic effect these gene mutations have on wound healing
| Extracellular Matrix Component | Encoding Gene | Disease Associated with Gene Defect | Characteristic Wound-Healing Pathology |
|---|---|---|---|
| Collagen I | COL1A1 | Arthochalasia EDS Caffey disease Osteogenesis imperfecta |
Tissue fragility Atrophic scar Hyperextensible skin Delayed wound healing |
| Collagen III | COL3A1 | Vascular EDS | Tissue fragility Delayed wound healing |
| Collagen IV | COL4A4 | Familial porencephaly Hereditary angiopathy Alport syndrome Leiomyomatosis |
Unknown |
| Collagen V | COL5A2 | Classic EDS | Impaired wound healing Atrophic “cigarette paper” scars Hyperextensible skin |
| Collagen VI | COL6A1 | Bethlem myopathy Ulrich muscular dystrophy |
Wound dehiscence Atrophic scars |
| Collagen VII | COL7A1 | Dystrophic epidermolysis bullosa | Extreme skin fragility Frequent wounding from minor trauma Delayed wound healing |
| Collagen XVII | COL17A1 | Epidermolysis bullosa junctionalis | Extreme skin fragility Frequent wounding from minor trauma Delayed wound healing |
| Decorin | DCN | Congenital stromal corneal dystrophy | Unknown |
| Elastin | ELN | Cutis laxa Williams syndrome |
Loose skin Normal wound healing |
| Fibrillin 1 | FBN1 | Marfan's syndrome Weill-Marchensani syndrome Congenital scleroderma |
Impaired wound healing Atrophic or distended scar Thick skin covering most of body (in congenital scleroderma only) |
| Fibrillin 2 | FBN2 | Contractural arachnodactyly | Unknown |
| Fibulin 4 | EFEMP2 | Cutis laxa | Loose skin Normal wound healing |
| Fibulin 5 | FBLN5 | Cutis laxa | Loose skin Normal wound healing |
| Laminin-332 | LAMA3, LAMB3, LAMC2 | Epidermolysis bullosa juncionalis | Extreme skin fragility Frequent wounding from minor trauma Delayed wound healing |
| Tenascin-X | TNXB | Hypermobility EDS | Hyperextensible skin Normal wound healing |
Genetic mutations affecting ECM proteins have varying characteristic effects on skin and wound healing.
EDS, Ehlers Danlos syndrome.
Collagen-mediated mechanical tension
Interwoven collagen fibrils form the structural scaffold of the healing wound and enable cell adhesion, chemotaxis, and migration. Since collagen fibers align in different orientations, they are able to produce mechanical tension vectors within the skin. Surgeons have long noted this property in the existence of Langer's lines, or skin tension lines, which are believed to correlate histologically with the orientation of collagen fibrils in the dermis.34–38 Although the tensile strength of collagen is integral to appropriate acute wound healing, too much tautness of collagen bundles in the early wound, among other variables, is thought to contribute to later formation of hypertrophic scars.39,40 Conversely, the chronically diminished mechanical stimulation imposed by the ECM on dermal fibroblasts has been linked to the reduced production of collagen type I and III and associated dermal laxity characteristic of aging skin.41 While fibroblasts are responsible for the production and early tensing of the ECM collagen, they are also considerably affected by it. Since the myofibroblast was first described in 1971, molecular mechanical stress (via tension in the ECM) has been implicated in fibroblast differentiation to this cell type.3 More recent work has shown that fibroblast gene expression, including apoptosis signaling, matrix metalloproteinase (MMP) activity, and differentiation into myofibroblasts, are strongly linked to the rigidity or laxity of the ECM, even independent of transforming growth factor (TGF)-β signaling.42–48 Even a 15% increase in cell stretch is capable of altering a fibroblast's orientation in relation to the ECM, inducing alterations in focal adhesion kinase signaling, or up-regulating expression of alpha smooth muscle actin (αSMA), consistent with differentiation into the contractile myofibroblast.49,50 Some clinicians have theorized that reducing the tension on wound fibroblasts by paralyzing local muscle fibers may reduce myofibroblast proliferation and collagen production, thereby decreasing scarring. Botulinum A toxin is an injectable neurotoxin that causes flaccid paralysis of muscles, and if injected around a wound would thus lessen the mechanical stretch on nearby fibroblasts. Attempts at reducing hypertrophic scar formation through the use of botulinum A have thus far yielded conflicting results.51,52
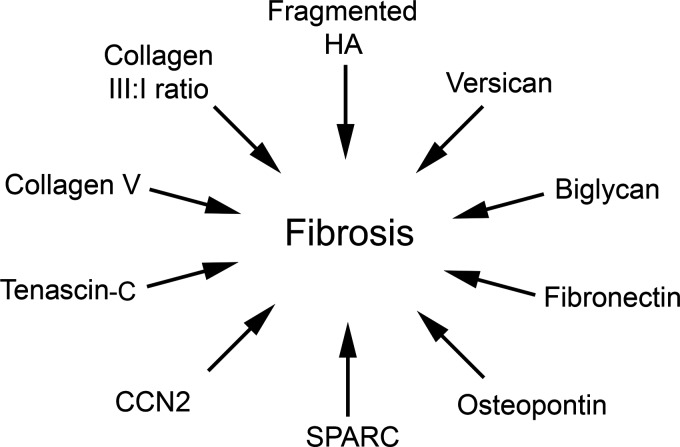
Similarly, fibroblasts grown on type I collagen substrates with greater stiffness show increased expression of αvβ3 integrin, which binds to noncollagen ECM proteins including vitronectin and fibronectin. The same fibroblasts also show simultaneous decreased expression of α2β1 integrin, which is recognized as the fibroblast-collagen binding integrin and is required for fibroblast migration.50,53 Conceptually, this correlates with the idea that as an open wound contracts and builds up tension through tightening of collagen fibrils, myofibroblasts transition away from α2β1 integrin-mediated organization of collagen fibers, and toward αvβ3 integrin-mediated migration along the noncollagen proteins present in acute wounds. This wound contraction and subsequent re-organization of collagen fibers can be seen histologically (Fig. 2). The pathway by which mechanical strain induces fibroblast differentiation or modifies myofibroblast behavior is still unclear, although pathways involving src kinase substrate p130cas, FAK-ERK pathway, PI3K-Akt anti-apoptotic signaling, or specific calcium channel signals are thought to be involved.54–60
Figure 2.
ECM components implicated in fibrosis or hypertrophic scarring. Overexpression or modification of certain ECM components is associated with myofibroblast differentiation and fibrotic wound healing.
Fibrillar collagens
Collagen can broadly be divided into fibrillar and nonfibrillar families. Fibrillar collagens include types I-III, and V. The presence, absence, or ratio of specific collagen types can affect fibroblast function apart from the mechanical stress imposed by collagen fibrils. For example, mice with a genetic deficiency in collagen III exhibit greater myofibroblast differentiation and more pronounced wound contracture.61 These same mice also demonstrate abnormal collagen I fibrillogensis in the skin and other organs, indicating that collagen III may play a regulatory role in collagen I synthesis.62 This correlates with the observation that scarless fetal wound healing is associated with a higher ratio of collagen III to collagen I in multiple small and large mammal models.63–66 Likewise, collagen type V has been shown to regulate collagen I fiber assembly, with mice deficient in collagen V demonstrating large structurally abnormal collagen fibrils.67,68
However its presence has been associated with decreased dermal fibroblast apoptosis, increased skin thickness (as observed in systemic sclerosis), and, in fibroblasts isolated from patients with Ehlers–Danlos Syndrome, rescue of fibronectin fibrillogenesis and improved α2β1-mediated fibroblast migration.69–71
Experience in reconstructive surgery and wound care show that fibroblasts are able to successfully migrate and infiltrate acellular dermal matrices that are rich in collagen I; however, the effect of isolated zoonotic collagen I on fibroblasts in human wounds remains uncertain. Topical collagen dressings are effective in reducing wound size of diabetic foot ulcers; however, they showed no effect on healing of facial wounds after excisional Mohs surgery.72,73 Further studies on stage II and III pressure ulcers showed good healing after collagen application, but the same effect was also achieved with neutral hydrocolloid control.74
Nonfibrillar collagens
Nonfibrillar collagens, including collagen IV, VI-VIII, and XIV, do not form classic fibrils but instead compose reticular nets, connect cells to the basement membrane, or in the case of FACIT collagens (Fibril-Associated Collagens with Interrupted Triple helices) aid in the organization of other collagens fibers. Despite forming the basal lamina and enabling keratinocyte and fibroblast migration and adhesion to the basement membrane, the role of collagen IV in wound healing has remained relatively unstudied.
In contrast to this, collagen VI has been shown to drive in vitro serum-starved fibroblasts through the S phase, and prevent apoptosis via downregulation of Bax compared with controls. This effect was more pronounced with soluble collagen VI than immobilized collagen VI, and was partially independent from β1 integrin signaling and collagen VI's ability to anchor cells.75 Collagen VI persists in wound histology from 3 days till many months postwounding; this perhaps suggests that the ability of collagen VI to inhibit fibroblast apoptosis could be relevant during this critical time in wound healing (Fig. 3).76 The gene encoding collagen VI is located on chromosome 21. Interestingly, dermal fibroblasts isolated from patients with trisomy 21 show increased expression of collagen VI, greater adherence to collagen VI, and also increased hyaluronan (HA) synthase correlated to the overexpression of collagen VI.77,78 These facts, coupled with the progeroid skin aging and sometimes impaired healing of surgical wounds characteristic of trisomy 21, could provide future avenues of study.79
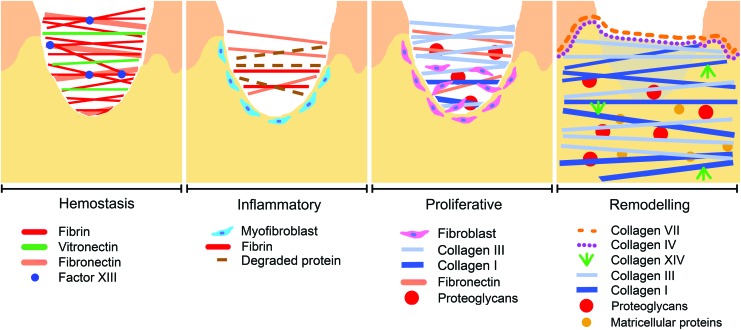
Figure 3.
The appearance of ECM proteins correlates with phases of wound healing. The ECM composition of a wound changes as it progresses through the hemostatic, inflammatory, proliferative, and remodeling phases of healing. Fibrin, fibronectin, and other clotting proteins dominate in the hemostatic acute wound, but as fibroblasts migrate into the wound they produce proteoglycans and collagen that are associated with mature healing wounds. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Collagen VII has been shown to support dermal fibroblast migration and, in addition, regulates cytokine expression in wound-infiltrating macrophages.80 Topically applied human recombinant collagen VII was incorporated into skin wounds on mice and decreased expression of fibrogenic TGF-β2 and αSMA while increasing the expression of anti-fibrogenic TGF-β3. This resulted in less collagen deposition in the healed wound, and may be of interest in controlling fibrosis in normal healing wounds as well as in treating patients with recessive dystrophic epidermolysis bullosa (RDEB), a disease characterized by absence of collagen VII.81 Fibroblasts from RDEB patients who underwent restorative lentiviral transfection with the gene encoding collagen VII demonstrated homing, incorporation, and production of functional collagen VII in mice after an intravenous injection of these cells.82
Collagen XIV is a member of the increasingly studied FACIT collagen family. It has been shown to decrease dermal fibroblast differentiation and reduce fibroblast DNA synthesis by 75% while maintaining normal viability and stable cell numbers.83 In mechanical studies, skin isolated from collagen XIV null mice exhibited significantly decreased stretch compared with normal skin.84 Further research into collagen XIV is needed to assess whether its role in decreasing fibroblast differentiation could perhaps reduce scar formation.
Other Fiber-Forming Proteins
Other fiber-forming proteins include fibrin, fibronectin, vitronectin, and elastin. Elastin helps define the rigidity and elasticity of the normal skin, while fibrin, fibronectin, and vitronectin are key mediators in hemostasis and cell migration in the healing wound.
Fibrin
In the earliest hemostatic stages of tissue repair, a provisional clot matrix is formed with fibrin, a fibrillar protein derived from soluble plasma fibrinogen.85 Fibroblasts quickly contract the loose fibrin matrix, and then utilize the contracted matrix as a surface for migration and tissue remodeling. As the wound progresses into the proliferative phase of healing, fibroblasts are then able to synthesize collagen and other ECM proteins to replace the contracted fibrin matrix and progress toward a granulation tissue-like matrix (Fig. 3).86
It has been shown that the contraction of the fibrin matrix can influence fibroblast responsiveness to TGF-β, which, in turn, activates collagen synthesis. Fibroblasts placed within noncontracted fibrin matrices in vitro had increased collagen and total protein synthesis when stimulated by various concentrations of TGF-β compared with fibroblasts embedded in contracted fibrin matrices with identical exposures.87 In addition, manipulation of the fibrin fiber alignment and overall fibrin matrix geometry has also been implicated in influencing fibroblast matrix remodeling.88 For example, an in vitro study utilizing cruciform fibroblast-populated fibrin gels that varied in arm length and fiber alignment demonstrated that collagen synthesis, and to a lesser extent elastin synthesis, was increased in specific regions of gels of certain geometries. Each of the cruciform fibrin gels were contracted and remodeled by fibroblasts without manipulation; however, the areas with the greatest collagen synthesis per cell correlated to the areas in which the greatest amount of tensile stress was imposed by the fibrin fiber alignment, as was predicted by anisotropic biphasic theory simulations.88 These data imply that fibroblast remodeling is influenced by both regional mechanical stress and the vector geometry of the fibrin matrix.
Fibronectin
Fibronectin is a complex component of the ECM, and the target of a growing body of research analyzing its ability to stimulate fibroblast growth, spreading, migration, and contractility.89 It is a dimeric glycoprotein that exists in a modified soluble form primarily found in plasma and an insoluble cellular form found in the ECM. Soluble plasma fibronectin assembles into insoluble ECM fibrils in the acute wound through a complex, cell-dependent process involving conformational changes that expose specific matricryptic sites.85,89 One of these sites is an RGD (Arg-Gly-Asp) sequence known as the “cell-binding domain,” or “growth promoting site” through which fibronectin binds to the αvβ3 integrin expressed by fibroblasts, stimulating their migration into the wound.85 After tissue injury and deposition of a fibrin clot, fibronectin, in addition, has a unique site to bind and stabilize fibrin. This covalent bond is further stabilized by factor XIII, and is an absolute requirement for migration of fibroblasts into plasma clots, at least in vitro.90 Due to its many binding domains, ECM fibronectin fibrils have the ability to interact with cells, other fibronectin fibrils, and multiple other proteins within the ECM, making it a unique responder in tissue injury.
In wound healing, fibronectin plays a critical role in ECM organization and stability. In vitro studies revealed that fibronectin is actually required for deposition of collagen I and other structural proteins into the ECM.91 Further, maintenance of the fibrillar organization of both collagen and fibronectin in vitro requires the active polymerization of fibronectin. Fibrils were lost from the ECM when fibronectin polymerization was inhibited.91 Fibronectin has also been shown to regulate the proteolytic activation of lysyl oxidase (LOX), the enzyme responsible for covalent cross-linking of collagen fibrils into mature collagen fibers. In the absence of fibronectin, the catalytic activity of LOX is dramatically decreased, resulting in less mature collagen matrices.92 Furthermore, fibronectin has been shown to play a role in fibroblast-mediated collagen gel contraction in vivo. Inhibition of a specific 122-amino-acid sequence within the III4-III5 repeats of fibronectin leads to inhibition of the initial phase of fibroblast-mediated collagen gel contraction.93
Fibronectin and fibroblasts share a dynamic, reciprocal relationship. While the intracellular cytoskeleton of fibroblasts can influence the orientation of fibronectin fibrils, the fibronectin matrix can also influence intracellular events. For example, fibroblasts grown on a fibronectin matrix orient their intracellular actin filament bundles in alignment with the matrix.2 In addition, stimulation of fibroblast proliferation by TGF-β has been shown to be dependent on preassembly of a fibronectin matrix. In vivo studies in a rat model have shown that topical application of fibronectin enhances wound-healing parameters, including migration of a greater numbers of fibroblasts expressing TGF-β after wounding.94
Vitronectin
Vitronectin is important for early contraction of wounds.95 In an in vitro model of wound healing, fibroblasts were shown to generate tension in the ECM by binding proteins in a chronological manner. In order to generate tension, fibroblasts first attach to fibronectin, followed by attachment to vitronectin, and finally attachment to collagen.92 These processes were shown to be interdependent, with fibroblasts only generating full force through normal collagen attachment after having previously bound fibronectin and vitronectin.92 Thus, vitronectin serves as a facilitating step toward maximal wound contraction.
Vitronectin has also been shown to influence fibroblast proliferation as mediated by fibronectin. In an in vitro study, fibroblasts bound to vitronectin produced fibronectin fibrils that were significantly less effective at stimulating fibroblast proliferation.89 Vitronectin is thought to induce this effect by guiding formation of fibronectin fibrils with less exposed “growth promoting sites,” thereby preventing effective binding between their RGD sequences and the αvβ3 integrins expressed by fibroblasts.89 In this sense, vitronectin acts to modulate or balance the migratory and proliferative fibroblast responses induced by fibronectin. By reducing the “growth promoting sites” exposed on fibronectin, vitronectin when introduced early into a healing wound may temper the pro-fibrotic effect of fibronectin.
Elastin
Rubber-like elastin fibers contribute to resilience and elasticity in tissues. Long cross-linked elastin fibers are intertwined with the relatively rigid collagen fibers in the ECM.96 Although elastin is conventionally known for its structural role, elastin-derived peptides have shown beneficial effects on wound repair and regeneration. Within skin wounds, various proteases such as MMPs act on elastin to liberate these fiber-forming proteins. This leads to accelerated fibroblast proliferation, increased collagen type I, and increased tropoelastin.97 Tropoelastin has been shown to promote attachment, spreading, and proliferation of fibroblasts through binding integrin αvβ3 and elastin binding protein (EBP) in an in vitro model. Although the mechanism of tropoelastin's effects are not completely understood, signaling involving EBP has been found to be mediated by a protein kinase A circuit.98
Proteoglycans and Gags
Proteoglycans consist of a core protein that is bound to one or more GAG side chains. In turn, GAGs are linear polysaccharides that can exist independently or bound to a proteoglycan core. Both types of these polymeric molecules have extreme variability in size.
Hyaluronan
HA, also known as hyaluronic acid, is one of the most common GAGs in the skin and has been a topic of growing interest over the last two decades. In addition to its role in maintaining tissue hydration and osmotic balance, HA plays key roles in both fibrotic and regenerative wound healing. Increased expression of HA and its receptor CD44 have classically been associated with fibrotic disease in a variety of tissues. This concept is recapitulated by the observation that human oral fibroblasts, which are associated with rapid and scarless wound healing, are unable to express HA synthase and have decreased pericellular assembly of HA.99 Divergently, research in fetal scarless wound healing has demonstrated much greater expression of HA in fetal rabbit wounds, and treatment of in vitro human adult wounds with HA-rich amniotic fluid results in improved re-epithelialization.100,101 Likewise, human keloid scars contain less HA and fibroblasts isolated from keloid scars produce less HA than their nonfibrotic counterparts.102
These conflicting actions of HA were recently resolved with the discovery that changing the size of the HA molecule modifies its effect. Native high-molecular-weight HA (>500 kDa) is associated with decreased inflammation, increased expression of collagen III, and increased activity of anti-fibrotic TGF-β3. Conversely, fragmented HA (<400 kDa) has been linked with increased inflammation, greater collagen I expression, increased proliferation of fibroblasts, and increased myofibroblast differentiation.103,104 Adding interest, if not a little confusion, it has further been shown that specific very small-sized hexameric HA fragments (∼1 kDa) improve fibroblast migration and promote wound closure, while decreasing myofibroblast differentiation and fibrosis.105 These data suggest that hexameric HA might be of interest in the healing of chronic wounds.
Although HA likely exerts its effect on the fibroblast via its CD44 and RHAMM (Receptor for HA Mediated Motility) receptors, the large branching pericellular molecule is also known to trap TGF-β1 molecules close to the fibroblast. This creates a positive feedback autocrine loop that contributes to the fibroblast's differentiation into the myofibroblast.106,107 Fibroblasts taken from aging patients demonstrate decreased pericellular HA, and accordingly have reduced TGF-β1-mediated CD44- EGF-R-ERK interaction and, thus, resist differentiation into myofibroblasts.108 This perhaps contributes, in part, to the impaired healing observed in some elderly patients.
Unlike most other ECM GAGs, HA does not exist naturally in a sulfated form. However, artificially sulfated HA has dramatic and complex effects on the dermal fibroblast that are of interest in the engineering of artificial tissues. A quantitative proteomics study revealed that sulfated HA upregulates 84 proteins, and downregulates various MMPs and collagen I.109 These effects are likely related, in part, to the ability of sulfated HA to bind even more avidly to TGF-β1 than does native HA.110 Investigation of artificial tissue scaffolds containing sulfated HA is an ongoing topic of research.
Dermatan sulfate, chondroitin sulfate
Dermatan sulfate (DS) and chondroitin sulfate (CS) are the GAGs most commonly bound to proteoglycans, along with keratan sulfate and herparan sulfate. When they are not bound to proteoglycans, they can also exist, similar to HA, in a free unbound state. DS and CS are very similar in structure, with DS distinguishing itself only by the presence of iduronic acid, a modified sugar. In vitro fibroblasts have been observed to express greater quantities of free DS and CS while in their subconfluent growing phase. The presence of these loosely bound GAGs may be a useful substrate for early fibroblast migration.111 DS is the most prevalent GAG in the skin, and has been shown to be the most common soluble GAG in acute human wounds.112 It has been demonstrated that DS is required to modify CD44 and enable fibroblast migration in fibronectin/fibrin gel during the earliest phases of wound healing. In addition, fibroblast growth factor-2 (FGF-2) and FGF-10 are known to bind DS; this affinity is increased even further for DS containing more iduronic acid, indicating that the iduronic acid component of DS is responsible for mediating FGF responsiveness in the fibroblast.112,113 CS and DS may also prove to be players in scarless fetal wound healing, as proteoglycans isolated from human fetal skin have been shown to contain much longer GAG chains compared with those of the adult human skin.29
Decorin
Decorin is a proteoglycan of the small leucine-rich proteoglycan (SLRP) family that is bound to a variable number of CS or DS GAG chains. Decorin is the most prevalent proteoglycan in adult human skin and demonstrates a binding affinity for TGF-β1 that is even further increased by removing its chondroitin and dermatan side chains.114 In this way, decorin can act as a natural inhibitor of TGF-β1 and is associated with decreased fibrosis and formation of normal scars.115 In addition to TGF-β1, decorin also effectively binds collagen at β1-integrin binding sites and is required for normal collagen fibrillogenesis. The dramatically reduced expression of decorin (25% that of normal) in keloid tissue and hypertrophic burn scars may explain why collagen fibrils within these fibrotic scars are thicker and less organized than in normally healed skin.116,117 Likewise, delayed expression of decorin has been observed in the healing of hypertrophic burns.118 Experiments in a knock-out mouse model indicate that the absence of decorin results in increased fibroblast proliferation, increased fibroblast adhesion to collagen or fibronectin, increased fibroblast migration in vitro, and paradoxically significantly delayed wound healing and cutis laxa in vivo.119,120 These experiments are consistent with a role of decorin in reduced fibroblast migration and decreased fibrosis in healing wounds. Interestingly, exogenous decorin added to cultured keloid fibroblasts resulted in a reduction of collagen I, MMP1, MMP9, and MMP13.121
Versican
Hypertrophic burn scars in humans show a six-fold higher expression of versican (and biglycan) compared with normal skin.117 Further evidence that versican is associated with fibrotic healing is the observation that deep dermal fibroblasts, which have a proven role in hypertrophic scars after deep dermal injury, produce significantly more versican than do superficial fibroblasts.122,123 Fibrotic or hypertrophic scars have multivariate pathologies, with many contributing factors. In addition to genetics and initial wound tension, many extracellular molecules (including versican) have been associated with fibrotic healing (Fig. 4). Interestingly, targeted RNAi against versican resulted in less aggregative behavior in dermal papilla fibroblasts associated with hair follicles.124 Although these data are of unclear importance for wound healing, they point toward a further investigation into the effect of versican on fibrosis.
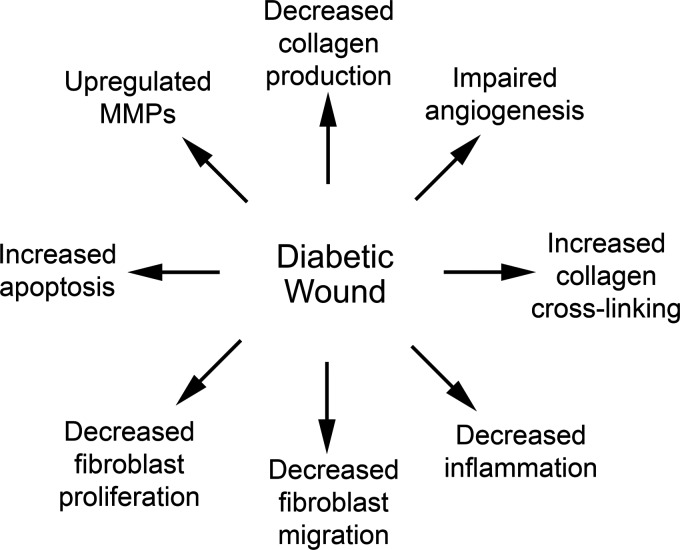
Figure 4.
Glycosylated matrix proteins impair and delay healing of diabetic wounds. Many factors, including reduced vasculogenesis, impair the healing of diabetic wounds. The presence of advanced glycosylation end products in diabetic wounds is also associated with decreased collagen production, migration, and proliferation of fibroblasts.
Lumican
Lumican is so named, because it is a translucent proteoglycan that is abundant in the cornea, and also present in the skin. Lumican promotes myofibroblast differentiation and contraction via an α2 integrin-mediated pathway.125 Mice that are deficient in lumican develop abnormally thick collagen fibrils and fragile skin, which are associated with increased proliferation, decreased apoptosis, and downregulation of p53 in fibroblasts in vitro.126 These same fibroblasts exhibited a decreased response to apoptotic Fas-Fas ligand signaling. These data suggest that lumican plays a significant role in pro-apoptotic signaling in fibroblasts, and may be important in fibrotic healing.
Dermatopontin
Dermatopontin is a recently described SLRP proteoglycan that increases skin elasticity, tensile strength, and collagen fibrillogenesis.127 Reduced expression of dermatopontin is associated with hypertrophic scar and systemic sclerosis.128 Dermatopontin has been shown to increase fibroblast adhesion to fibrin matrices, and promote fibronectin fibril formation in a dose-dependent manner.129 There is some indication that pro-migratory dermatopontin and anti-migratory decorin act to balance and modify each other's activity in the healing wound.30 Since it was only fully described less than 25 years ago, the full physiology of dermatopontin in wound healing awaits further investigation.
Biglycan, aggrecan
Biglycan is a proteoglycan commonly present in the skin, while aggrecan is only minimally expressed in the dermis. Although biglycan has been found to be upregulated in fibrotic skin diseases, little else is known about the effect of these proteoglycans on fibroblasts in wound healing.130
Matricellular Proteins
Matricellular proteins are secreted proteins that are incorporated into the ECM while not significantly defining its structure. These proteins are often completely absent or present only in low levels in uninjured skin. However, they are upregulated after tissue injury. While they do not contribute to the mechanical organization of the ECM, they act as temporally dynamic signaling molecules.
Osteopontin
Osteopontin is a relatively recently described glycoprotein that was first characterized in the bone. In addition to its role in biomineralization, osteopontin has also been implicated in dermal fibrosis.131 Osteopontin is required for the differentiation of myofibroblasts in response to TGF-β signaling, and increases fibroblast migration and proliferation.132,133 By decreasing osteopontin concentration via delivery of osteopontin antisense oligodeoxynucleotides to a mouse model wound bed, the wound healed significantly faster with less scarring and granulation tissue formation.134
Secreted protein, acidic and rich in cysteine
SPARC, also known as osteonectin, is another glycoprotein most commonly found in the bone. Similar to osteopontin, increased presence of SPARC is also linked to fibrotic disease in the skin and other tissues. In vitro experiments on dermal fibroblasts show SPARC increased gene expression, protein assembly, and ECM incorporation of collagen I.135,136 Silencing SPARC using siRNA reduced these effects, even in fibroblasts stimulated with TGF-β and fibroblasts from patients with systemic sclerosis.135,137 The in vivo data for SPARC are less clear. SPARC-null mice demonstrated accelerated wound closure with decreased collagen production when subjected to 5 mm wounds, but showed significantly delayed wound healing with impaired ECM production in larger 25 mm wounds.138 The effect of SPARC inhibition in the prevention of fibrosis in human wounds remains unknown.
CCN2
CCN2, previously known as CTGF, is a matricellular peptide that is not usually expressed in the skin, but is upregulated during tissue injury. CCN2 increases fibroblast expression of collagen I, collagen III, tissue inhibitors of MMP and basic FGF while not affecting expression of proteoglycans.139 Although CCN2 does not appear to affect local myofibroblast differentiation, it is believed to stimulate mesenchymal stem cell recruitment to the wound site and subsequent differentiation into dermal fibroblasts.140 Although this implies that CCN2 contributes to the anabolic environment of dermal wound healing, it has also been shown that CCN2 is not a requirement for dermal healing, as fibroblast CCN1/CCN2 knock-out mice are capable of unimpaired wound healing.141 In fact, CCN2 expression in skin is associated with hypertrophic scar and fibrosis.142,143 Conversely, CCN2 seems to be involved in promoting keratinocyte migration during wound healing via integrin α5β1.144 Interestingly, collagen I production and scar were reduced by inhibiting the ERK and JNK signaling downstream of CCN2 in a rabbit model of hypertrophic scarring.145
Tenascin-C
Tenascin-C is a glycoprotein that, while sparsely distributed in the skin, is upregulated in areas of tissue injury, and especially localized to wound edges. Tenascin-C is persistently upregulated in fibrotic disease, including keloids and photo-damaged skin.146,147 Native full-length tenascin-C arrests fibroblast cell-cycle progression in G1 phase and promotes fibroblast migration along the fibrin-fibronectin matrices characteristic of early wounds.148 Conversely, fragmented tenascin-C was shown to almost completely inhibit fibroblast migration. This behavior pattern of tenascin-C complements the physiology of wound healing. Initially, tenascin-C is expressed at the wound edge and encourages fibroblast migration. As the wound matures, proteinases are expressed that generate fragmented tenascin-C, which, in turn, inhibits further fibroblast migration and limits hypertrophic scar growth. Perhaps the persistence of full-length tenascin-C in fibrotic disease contributes to this pathologic healing.149
Fibulin-5
Fibulin-5 is a secreted glycoprotein that binds to and mediates development of elastin fibers in the ECM. Overexpression of fibulin-5 by retroviral transfection in rabbits was shown to induce formation of granulation tissue and instigate remodeling of the ECM.150 The fact that fibulin-5 regulates remodeling of granulation tissue correlates with the fact that under normal conditions fibulin-5 does not appear until 14 days after wounding. Despite this promising result of overexpression, it was found that fibulin-5 null mice had normal wound healing, with the exception of a complete absence of elastin in their skin. Experiments performed on fibroblasts isolated from these mice indicate that fibulin-5 does not affect fibroblast migration or proliferation.151
Nonenzymatically Glycosylated Matrix Proteins
Nonenzymatic glycosylation is a process in which reducing sugars irreversibly bind to the amino groups of proteins, producing Advanced Glycosylation Endproducts (AGEs). It is commonly observed in ECM proteins of patients with diabetes mellitus (DM) and increased age.152 AGEs contribute to a number of the pathologic processes caused by hyperglycemia, affecting everything from entire organ systems (as in diabetic renal nephropathy) to the individual fibroblast in a healing wound. Some of these pathologic processes are thought to be related to the inflammatory function of an immunoglobulin receptor that targets AGEs, Receptor for Advanced Glycation Endproducts (RAGE).
Advanced glycation endproducts
Since dermal macromolecular ECM has a slow turnover rate, the presence of glycosylated ECM can be especially problematic in the skin, with approximately 30% of diabetic patients experiencing some cutaneous involvement during their lifetime (Fig. 5).153 Accumulation of AGEs on collagen in the ECM of skin has been widely studied.152,154,155 Glycosylation of collagen leads to increased cross-linking between collagen fibers, increased stiffness, decreased solubility, and decreased susceptibility to proteolysis.152,155,156 Some authors highlight differences between the collagen cross-linking due to DM compared with aging, stressing that diabetic cross-linking is more extensive and causes a further decrease in solubility. AGEs have also been found to accumulate on elastin fibers within skin that has undergone solar elastosis.154 AGEs generate active oxygen-free radical species during UVA irradiation, thereby increasing damage to and photoaging of the skin.154
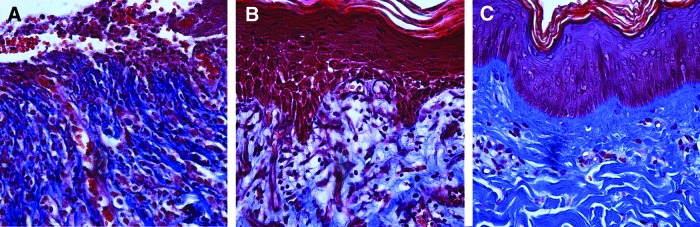
Figure 5.
Histological evaluation of wound contraction and collagen fiber orientation in a healing full-thickness excisional porcine wound stained with Masson Trichrome (collagen appears blue). (A) After 7 days of healing, the wound is contracted and loosely organized collagen is present in granulation tissue. (B) 14 days after wounding, the wound is re-epithelialized with reduced contraction and gradual re-modeling of collagen fibers. (C) Intact porcine skin demonstrates highly organized collagen bundles and fibers in dermis. All images are taken with a 20×objective. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
With regard to wound healing, nonenzymatic glycosylation of collagen leads to impaired fibroblast-induced contraction.152 During ECM remodeling in the normal healing process, contraction of collagen matrices is associated with downregulation of type I collagen and upregulation of MMPs by fibroblasts. In vitro analysis revealed that collagen glycosylation inhibited production and activation of MMP-1 and MMP-2 by fibroblasts, suggesting a mechanism for the decreased lattice contraction.152 Other studies which support the finding of decreased MMP activity found that fibroblast synthesis of HA was also decreased by AGE accumulation within skin ECM. These changes elicited by AGEs are thought to be due to interactions with fibroblast cell membranes, as both HA and MMPs are located within fibroblast cell membranes.154
AGE accumulation-induced cross-linking of collagen within the ECM has been implicated in impaired neovascularization of chronic diabetic wounds.156 In addition to release of angiogenic factors, ECM proteins are necessary for mechanical support of capillary buds during normal angiogenesis. AGEs affect the ability of the ECM to support this regulated vasculogenesis. Endothelial cells studied in glycated collagen lattices were shown to be significantly impaired, with inhibited invasion, decreased proliferation, and significantly reduced capillary formation.156 While outside the scope of this review, AGEs can greatly impair vasculogenesis in diabetic wounds, thereby significantly impairing healing in addition to their effect on dermal fibroblasts.
A study of human diabetic skin specimens revealed more apoptotic cells and increased expression of AGE and RAGE compared with nondiabetic skin.153 It was also shown that fibroblasts within the glycosylated ECM of these skin samples exhibited cell-cycle arrest between G2/M phases, leading to apoptosis. This arrest was reversed by application of RAGE-blocking antibodies, demonstrating causality.153 In addition, it was shown that the fibroblasts within these glycosylated ECMs had modified actin cytoskeletons and increased release of cytoplasmic lactate dehydrogenase, indicating damage to their cell membranes.152,154
In vivo studies involving genetically diabetic mice demonstrated that delayed healing of diabetic wounds is accompanied by a delayed localization of inflammatory cells to the wound, diminished generation of growth factors by inflammatory cells, exaggerated generation of pro-inflammatory factors (e.g., cytokines and MMPs), and delayed egress of inflammatory cells.157 By inducing a RAGE blockade in the diabetic mice, beneficial inflammatory responses were restored, including re-established migration of inflammatory cells both into and out of wounds and their appropriately limited activation. RAGE-bearing cells responding to AGE accumulation within the ECM of skin have thus been implicated as having a global effect on diabetic wound healing in this in vivo study.157
The ability of AGEs in the ECM to negatively impact almost every component of wound healing, including migration and proliferation of the fibroblast, matrix remodeling, angiogenesis, and inflammatory signaling, is profound. Methods of preventing AGE formation or modulating their effect through wound interventions are an important topic of current research.
Take-Home Messages.
• The ECM plays important signaling roles during dermal wound healing, independent from its ability to sequester growth factors.
• Individual ECM components have varied effects on fibroblast proliferation, migration, collagen production, and differentiation into myofibroblasts (Table 4).
• Different fragment sizes of ECM molecules, such as HA and tenascin-C, can induce opposing effects on dermal fibroblasts.
• Manipulation of certain ECM components may reduce fibrosis in healing wounds.
• Glycosylated matrix proteins are major factors in compromised healing of diabetic wounds, due to both weakened vasculogenesis and impaired fibroblast function caused by AGEs.
Table 4.
Effect of selected extracellular matrix components on dermal fibroblast functions
| Extracellular Matrix Component | Fibroblast Proliferation | Fibroblast Migration | Collagen Production/Assembly | Myofibroblast Differentiation/Contraction | References |
|---|---|---|---|---|---|
| Collagen I | ↓↑ | ↑ | ↑ | ↑ | 39, 40, 49, 50, 53 |
| Collagen III | – | – | ↑ | ↓ | 61, 62 |
| Collagen IV | – | – | – | – | NA |
| Collagen V | ↑ | ↑ | ↑ | – | 63, 64, 66, |
| Collagen VI | ↑ | ↓↑ | ↑ | – | 154 |
| Collagen VII | – | ↑ | ↓ | ↓ | 75, 77 |
| Collagen XIV | ↓ | – | ↑ | ↓ | 78, 79 |
| Fibrin | ↑ | ↑ | ↑ | ↑ | 83, 85 |
| Fibronectin | ↑ | ↑ | ↑ | ↑ | 158 |
| Vitronectin | ↓ | ↑ | – | ↑ | 84, 88, 90 |
| Elastin | ↑ | ↑ | ↑ | ↓ | 91, 92, 93 |
| Hyaluronan | ↓↑ | ↑ | ↓↑ | ↓↑ | 158, 186 |
| Dermatan sulfate | ↑ | ↑ | – | – | 187 |
| Chondroitin sulfate | ↑ | ↑ | – | – | 188 |
| Decorin | ↓ | ↓ | ↓ | ↓ | 115, 158 |
| Versican | – | ↓↑ | ↑ | ↑ | 184 |
| Lumican | ↓ | – | ↑ | ↑ | 121 |
| Dermatopontin | – | ↑ | ↑ | ↓ | 158 |
| Osteopontin | ↑ | ↑ | ↑ | ↑ | 127 |
| SPARC | – | ↓ | ↑ | – | 130 |
| CCN2 | ↑ | ↑ | ↑ | ↓↑ | 134, 137, 140 |
| Tenascin-C | ↓ | ↓↑ | ↑ | ↑ | 144, 158, 159 |
| Fibulin-5 | ↓↑ | ↓↑ | ↓↑ | ↑ | 145, 146 |
Up-arrows (↑) show an increase or improvement, down-arrows (↓) show a decrease or impairment, bidirectional arrows (↓↑) show no apparent effect, and dashes (–) indicate an unknown or unstudied effect.
(↑) Increased; (↓) Decreased; (↓↑) No effect; (–) Unknown effect.
Abbreviations and Acronyms
- αSMA
alpha smooth muscle actin
- AGE
advanced glycosylation end product
- CS
chondroitin sulfate
- CCN2
Cyr61-Ctgf-Nov-2
- DM
diabetes mellitus
- DS
dermatan sulfate
- EBP
elastin binding protein
- ECM
extracellular matrix
- FACIT collagens
fibril-associated collagens with interrupted triple helices
- FGF-2
fibroblast growth factor-2
- GAG
glycosaminoglycan
- HA
hyaluronan
- LOX
lysyl oxidase
- MMP
matrix metalloproteinase
- RAGE
receptor for advanced glycation endproducts
- RDEB
recessive dystrophic epidermolysis bullosa
- RHAMM
receptor for hyaluronan-mediated motility
- SPARC
secreted protein, acidic and rich in cysteine
- SLRP
small leucine-rich proteoglycan
- TGF-β
transforming growth factor beta
Acknowledgments and Funding Sources
We have no funding sources to report.
Author Disclosure and Ghostwriting
No competing financial interests exist. The content of this article was expressly written by the authors listed. No ghostwriters were used to write this article.
About the Authors
Lauren Tracy, BA, is a research fellow in the Wound Healing and Tissue Engineering Laboratory at Brigham and Women's Hospital, and a medical student at the University of California - Irvine. Raquel Minasian, BA, is a research fellow in the Wound Healing and Tissue Engineering Laboratory at Brigham and Women's Hospital, and a medical student at the Tufts University School of Medicine. E.J. Caterson, MD, PhD, is a plastic surgeon at Brigham and Women's Hospital and an Instructor of Surgery at Harvard Medical School. He is involved in clinical and experimental efforts to modulate wound healing and tissue engineer skin.
References
- 1.Stoker M, O'Neill C, Berryman S, Waxman V. Anchorage and growth regulation in normal and virus-transformed cells. Int J Cancer 1968;3:683–693 [DOI] [PubMed] [Google Scholar]
- 2.Alberts J, Johnson B, Lewis A. The Extracellular Matrix of Animals. New York: Garland Science, 2002 [Google Scholar]
- 3.Majno G, Gabbiani G, Hirschel BJ, Ryan GB, Statkov PR. Contraction of granulation tissue in vitro: similarity to smooth muscle. Science 1971;173:548–550 [DOI] [PubMed] [Google Scholar]
- 4.Schultz GS, Wysocki A. Interactions between extracellular matrix and growth factors in wound healing. Wound Repair Regen 2009;17:153–162 [DOI] [PubMed] [Google Scholar]
- 5.Sorrell JM, Baber MA, Caplan AI. Clonal characterization of fibroblasts in the superficial layer of the adult human dermis. Cell Tissue Res 2007;327:499–510 [DOI] [PubMed] [Google Scholar]
- 6.Sorrell JM, Baber MA, Caplan AI. Site-matched papillary and reticular human dermal fibroblasts differ in their release of specific growth factors/cytokines and in their interaction with keratinocytes. J Cell Physiol 2004;200:134–145 [DOI] [PubMed] [Google Scholar]
- 7.Nolte SV, Xu W, Rennekampff H-O, Rodemann HP. Diversity of fibroblasts—a review on implications for skin tissue engineering. Cells Tissues Organs 2008;187:165–176 [DOI] [PubMed] [Google Scholar]
- 8.Sorrell JM, Caplan AI. Fibroblast heterogeneity: more than skin deep. J Cell Sci 2004;117:667–675 [DOI] [PubMed] [Google Scholar]
- 9.Ali-Bahar M, Bauer B, Tredget EE, Ghahary A. Dermal fibroblasts from different layers of human skin are heterogeneous in expression of collagenase and types I and III procollagen mRNA. Wound Repair Regen 2004;12:175–182 [DOI] [PubMed] [Google Scholar]
- 10.Andrade ZA, de-Oliveira-Filho J, Fernandes AL. Interrelationship between adipocytes and fibroblasts during acute damage to the subcutaneous adipose tissue of rats: an ultrastructural study. Braz J Med Biol Res 1998;31:659–664 [DOI] [PubMed] [Google Scholar]
- 11.Matsumoto T, et al. Mature adipocyte-derived dedifferentiated fat cells exhibit multilineage potential. J Cell Physiol 2008;215:210–222 [DOI] [PubMed] [Google Scholar]
- 12.Tholpady SS, et al. The cellular plasticity of human adipocytes. Ann Plast Surg 2005;54:651–656 [DOI] [PubMed] [Google Scholar]
- 13.Schrimpf C, Duffield JS. Mechanisms of fibrosis: the role of the pericyte. Curr Opin Nephrol Hypertens 2011;20:297–305 [DOI] [PubMed] [Google Scholar]
- 14.Karén J, et al. Effects of the histone deacetylase inhibitor valproic acid on human pericytes in vitro. PLoS One 2011;6:e24954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Piera-Velazquez S, Li Z, Jimenez SA. Role of endothelial-mesenchymal transition (EndoMT) in the pathogenesis of fibrotic disorders. Am J Pathol 2011;179:1074–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee JG, Ko MK, Kay EP. Endothelial mesenchymal transformation mediated by IL-1β-induced FGF-2 in corneal endothelial cells. Exp Eye Res 2012;95:35–39 [DOI] [PubMed] [Google Scholar]
- 17.Lin F, Wang N, Zhang T-C. The role of endothelial-mesenchymal transition in development and pathological process. IUBMB Life 2012;64:717–723 [DOI] [PubMed] [Google Scholar]
- 18.Slukvin II, Vodyanik M. Endothelial origin of mesenchymal stem cells. Cell Cycle 2011;10:1370–1373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006;126:663–676 [DOI] [PubMed] [Google Scholar]
- 20.Takahashi K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007;131:861–872 [DOI] [PubMed] [Google Scholar]
- 21.Yu J., et al. Induced pluripotent stem cell lines derived from human somatic cells. Science 2007;318:1917–1920 [DOI] [PubMed] [Google Scholar]
- 22.Kiwanuka E, Junker J, Eriksson E. Harnessing growth factors to influence wound healing. Clin Plast Surg 2012;39:239–248 [DOI] [PubMed] [Google Scholar]
- 23.Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M. Growth factors and cytokines in wound healing. Wound Repair Regen 2008;16:585–601 [DOI] [PubMed] [Google Scholar]
- 24.Bainbridge P. Wound healing and the role of fibroblasts. J Wound Care 2013;22:407–408, 410–412 [DOI] [PubMed] [Google Scholar]
- 25.Donovan J, Abraham D, Norman J. Platelet-derived growth factor signaling in mesenchymal cells. Front Biosci (Landmark Ed) 2013;18:106–119 [DOI] [PubMed] [Google Scholar]
- 26.Järveläinen H, Sainio A, Koulu M, Wight TN, Penttinen R. Extracellular matrix molecules: potential targets in pharmacotherapy. Pharmacol Rev 2009;61:198–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weinstein G, Boucek R. Collagen and elastin of human dermis. J Invest Dermatol 1960;35:227–229 [PubMed] [Google Scholar]
- 28.Frantz C, Stewart KM, Weaver VM. The extracellular matrix at a glance. J Cell Sci 2010;123:4195–4200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carrino DA, Sorrell JM, Caplan AI. Age-related changes in the proteoglycans of human skin. Arch Biochem Biophys 2000;373:91–101 [DOI] [PubMed] [Google Scholar]
- 30.Okamoto O, Fujiwara S. Dermatopontin, a novel player in the biology of the extracellular matrix. Connect Tissue Res 2006;47:177–189 [DOI] [PubMed] [Google Scholar]
- 31.Bornstein P, Sage EH. Matricellular proteins: extracellular modulators of cell function. Curr Opin Cell Biol 2002;14:608–616 [DOI] [PubMed] [Google Scholar]
- 32.Eyre DR. Collagen: molecular diversity in the body's protein scaffold. Science 1980;207:1315–1322 [DOI] [PubMed] [Google Scholar]
- 33.Smith LT, Holbrook KA, Madri JA. Collagen types I, III, and V in human embryonic and fetal skin. Am J Anat 1986;175:507–521 [DOI] [PubMed] [Google Scholar]
- 34.Cox HT. The cleavage lines of the skin. Br J Surg 1941;29:234–240 [Google Scholar]
- 35.KRAISSL CJ. The selection of appropriate lines for elective surgical incisions. Plast Reconstr Surg (1946) 1951;8:1–28 [DOI] [PubMed] [Google Scholar]
- 36.Piérard GE, Lapière CM. Microanatomy of the dermis in relation to relaxed skin tension lines and Langer's lines. Am J Dermatopathol 1987;9:219–224 [DOI] [PubMed] [Google Scholar]
- 37.Ridge MD, Wright V. The directional effects of skin. A bio-engineering study of skin with particular reference to Langer's lines. J Invest Dermatol 1966;46:341–346 [PubMed] [Google Scholar]
- 38.Sakai S, Yamanari M, Lim Y, Nakagawa N, Yasuno Y. In vivo evaluation of human skin anisotropy by polarization-sensitive optical coherence tomography. Biomed Opt Express 2011;2:2623–2631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baur PS, Larson DL, Stacey TR. The observation of myofibroblasts in hypertrophic scars. Surg Gynecol Obstet 1975;141:22–26 [PubMed] [Google Scholar]
- 40.Junker JPE, Kratz C, Tollbäck A, Kratz G. Mechanical tension stimulates the transdifferentiation of fibroblasts into myofibroblasts in human burn scars. Burns 2008;34:942–946 [DOI] [PubMed] [Google Scholar]
- 41.Varani J, et al. Decreased collagen production in chronologically aged skin: roles of age-dependent alteration in fibroblast function and defective mechanical stimulation. Am J Pathol 2006;168:1861–1868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu F, et al. Feedback amplification of fibrosis through matrix stiffening and COX-2 suppression. J Cell Biol 2010;190:693–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Prager-Khoutorsky M., et al. Fibroblast polarization is a matrix-rigidity-dependent process controlled by focal adhesion mechanosensing. Nat Cell Biol 2011;13:1457–1465 [DOI] [PubMed] [Google Scholar]
- 44.Huang X, et al. Matrix stiffness-induced myofibroblast differentiation is mediated by intrinsic mechanotransduction. Am J Respir Cell Mol Biol 2012;47:340–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marinković A, Mih JD, Park J-A, Liu F, Tschumperlin DJ. Improved throughput traction microscopy reveals pivotal role for matrix stiffness in fibroblast contractility and TGF-β responsiveness. Am J Physiol Lung Cell Mol Physiol 2012;303:L169–L180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Leight JL, Wozniak MA, Chen S, Lynch ML, Chen CS. Matrix rigidity regulates a switch between TGF-β1-induced apoptosis and epithelial-mesenchymal transition 2012; Mol Biol Cell 23:781–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Karamichos D, Brown RA, Mudera V. Collagen stiffness regulates cellular contraction and matrix remodeling gene expression. J Biomed Mater Res 2007;A 83:887–894 [DOI] [PubMed] [Google Scholar]
- 48.Derderian CA, et al. Mechanical strain alters gene expression in an in vitro model of hypertrophic scarring. Ann Plast Surg 2005;55:69–75; discussion 75 [DOI] [PubMed] [Google Scholar]
- 49.Wen H, Blume PA, Sumpio BE. Role of integrins and focal adhesion kinase in the orientation of dermal fibroblasts exposed to cyclic strain. Int Wound J 2009;6:149–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jones C, Ehrlich HP. Fibroblast expression of α-smooth muscle actin, α2β1 integrin and αvβ3 integrin: influence of surface rigidity. Exp Mol Pathol 2011;91:394–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ziade M, et al. Use of botulinum toxin type A to improve treatment of facial wounds: a prospective randomised study. J Plast Reconstr Aesthet Surg 2013;66:209–214 [DOI] [PubMed] [Google Scholar]
- 52.Gauglitz GG, et al. Botulinum toxin A for the treatment of keloids. Skin Pharmacol Physiol 2012;25:313–318 [DOI] [PubMed] [Google Scholar]
- 53.Lygoe KA, Wall I, Stephens P, Lewis MP. Role of vitronectin and fibronectin receptors in oral mucosal and dermal myofibroblast differentiation. Biol Cell 2007;99:601–614 [DOI] [PubMed] [Google Scholar]
- 54.Nakamoto T, et al. Analysis of gene expression profile in p130(Cas)-deficient fibroblasts. Biochem Biophys Res Commun 2002;294:635–641 [DOI] [PubMed] [Google Scholar]
- 55.Zouq NK, et al. FAK engages multiple pathways to maintain survival of fibroblasts and epithelia: differential roles for paxillin and p130Cas. J Cell Sci 2009;122:357–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wong VW, Longaker MT, Gurtner GC. Soft tissue mechanotransduction in wound healing and fibrosis. Semin Cell Dev Biol 2012;23:981–986 [DOI] [PubMed] [Google Scholar]
- 57.Wong VW, et al. Focal adhesion kinase links mechanical force to skin fibrosis via inflammatory signaling. Nat Med 2012;18:148–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Paterno J, et al. Akt-mediated mechanotransduction in murine fibroblasts during hypertrophic scar formation. Wound Repair Regen 2011;19:49–58 [DOI] [PubMed] [Google Scholar]
- 59.Xia H, Nho RS, Kahm J, Kleidon J, Henke CA. Focal adhesion kinase is upstream of phosphatidylinositol 3-kinase/Akt in regulating fibroblast survival in response to contraction of type I collagen matrices via a beta 1 integrin viability signaling pathway. J Biol Chem 2004;279:33024–33034 [DOI] [PubMed] [Google Scholar]
- 60.Davis J, Burr AR, Davis GF, Birnbaumer L, Molkentin JD. A TRPC6-dependent pathway for myofibroblast transdifferentiation and wound healing in vivo. Dev Cell 2012;23:705–715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Volk SW, Wang Y, Mauldin EA, Liechty KW, Adams SL. Diminished type III collagen promotes myofibroblast differentiation and increases scar deposition in cutaneous wound healing. Cells Tissues Organs 2011;194:25–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu X, Wu H, Byrne M, Krane S, Jaenisch R. Type III collagen is crucial for collagen I fibrillogenesis and for normal cardiovascular development. Proc Natl Acad Sci U S A 1997;94:1852–1856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Merkel JR, DiPaolo BR, Hallock GG, Rice DC. Type I and type III collagen content of healing wounds in fetal and adult rats. Proc Soc Exp Biol Med 1988;187:493–497 [DOI] [PubMed] [Google Scholar]
- 64.Carter R, Jain K, Sykes V, Lanning D. Differential expression of procollagen genes between mid- and late-gestational fetal fibroblasts. J Surg Res 2009;156:90–94 [DOI] [PubMed] [Google Scholar]
- 65.Cuttle L, et al. Collagen in the scarless fetal skin wound: detection with picrosirius-polarization. Wound Repair Regen 2005;13:198–204 [DOI] [PubMed] [Google Scholar]
- 66.Burd DA, Longaker MT, Adzick NS, Harrison MR, Ehrlich HP. Foetal wound healing in a large animal model: the deposition of collagen is confirmed. Br J Plast Surg 1990;43:571–577 [DOI] [PubMed] [Google Scholar]
- 67.Wenstrup RJ, et al. Type V collagen controls the initiation of collagen fibril assembly. J Biol Chem 2004;279:53331–53337 [DOI] [PubMed] [Google Scholar]
- 68.Zoppi N, Gardella R, De Paepe A, Barlati S, Colombi M. Human fibroblasts with mutations in COL5A1 and COL3A1 genes do not organize collagens and fibronectin in the extracellular matrix, down-regulate alpha2beta1 integrin, and recruit alphavbeta3 Instead of alpha5beta1 integrin. J Biol Chem 2004;279:18157–18168 [DOI] [PubMed] [Google Scholar]
- 69.Chanut-Delalande H, et al. Development of a functional skin matrix requires deposition of collagen V heterotrimers. Mol Cell Biol 2004;24:6049–6057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Martin P, et al. Abnormal collagen V deposition in dermis correlates with skin thickening and disease activity in systemic sclerosis. Autoimmun Rev 2012;11:827–835 [DOI] [PubMed] [Google Scholar]
- 71.Viglio S, et al. Rescue of migratory defects of Ehlers-Danlos syndrome fibroblasts in vitro by type V collagen but not insulin-like binding protein-1. J Invest Dermatol 2008;128:1915–1919 [DOI] [PubMed] [Google Scholar]
- 72.Donaghue VM, et al. Evaluation of a collagen-alginate wound dressing in the management of diabetic foot ulcers. Adv Wound Care 11:114–119 [PubMed] [Google Scholar]
- 73.Becker GD, Adams LA, Hackett J. Collagen-assisted healing of facial wounds after Mohs surgery. Laryngoscope 1994;104:1267–1270 [DOI] [PubMed] [Google Scholar]
- 74.Graumlich JF, et al. Healing pressure ulcers with collagen or hydrocolloid: a randomized, controlled trial. J Am Geriatr Soc 2003;51:147–154 [DOI] [PubMed] [Google Scholar]
- 75.Rühl M, et al. Soluble collagen VI drives serum-starved fibroblasts through S phase and prevents apoptosis via down-regulation of Bax. J Biol Chem 1999;274:34361–34368 [DOI] [PubMed] [Google Scholar]
- 76.Betz P, et al. Time-dependent pericellular expression of collagen type IV, laminin, and heparan sulfate proteoglycan in myofibroblasts. Int J Legal Med 1992;105:169–172 [DOI] [PubMed] [Google Scholar]
- 77.Jongewaard IN, Lauer RM, Behrendt DA, Patil S, Klewer SE. Beta 1 integrin activation mediates adhesive differences between trisomy 21 and non-trisomic fibroblasts on type VI collagen. Am J Med Genet 2002;109:298–305 [DOI] [PubMed] [Google Scholar]
- 78.Karousou E, et al. New insights into the pathobiology of Down syndrome—hyaluronan synthase-2 overexpression is regulated by collagen VI α2 chain. FEBS J 2013;280:2418–2430 [DOI] [PubMed] [Google Scholar]
- 79.Mik G, Gholve PA, Scher DM, Widmann RF, Green DW. Down syndrome: orthopedic issues. Curr Opin Pediatr 2008;20:30–36 [DOI] [PubMed] [Google Scholar]
- 80.Nyström A, et al. Collagen VII plays a dual role in wound healing. J Clin Invest 2013;123:3498–3509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang X, et al. Topical application of recombinant type VII collagen incorporates into the dermal-epidermal junction and promotes wound closure. Mol Ther 2013;21:1335–1344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Woodley DT, et al. Intravenously injected human fibroblasts home to skin wounds, deliver type VII collagen, and promote wound healing. Mol Ther 2007;15:628–635 [DOI] [PubMed] [Google Scholar]
- 83.Ruehl M, et al. The elongated first fibronectin type III domain of collagen XIV is an inducer of quiescence and differentiation in fibroblasts and preadipocytes. J Biol Chem 2005;280:38537–38543 [DOI] [PubMed] [Google Scholar]
- 84.Ansorge HL, et al. Type XIV Collagen Regulates Fibrillogenesis: PREMATURE COLLAGEN FIBRIL GROWTH AND TISSUE DYSFUNCTION IN NULL MICE. J Biol Chem 2009;284:8427–8438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pereira M, et al. The incorporation of fibrinogen into extracellular matrix is dependent on active assembly of a fibronectin matrix. J Cell Sci 2002;115:609–617 [DOI] [PubMed] [Google Scholar]
- 86.Tuan TL, Song A, Chang S, Younai S, Nimni ME. In vitro fibroplasia: matrix contraction, cell growth, and collagen production of fibroblasts cultured in fibrin gels. Exp Cell Res 1996;223:127–134 [DOI] [PubMed] [Google Scholar]
- 87.Coustry F, Gillery P, Maquart FX, Borel JP. Effect of transforming growth factor beta on fibroblasts in three-dimensional lattice cultures. FEBS Lett 1990;262:339–341 [DOI] [PubMed] [Google Scholar]
- 88.Sander EA, Barocas VH, Tranquillo RT. Initial fiber alignment pattern alters extracellular matrix synthesis in fibroblast-populated fibrin gel cruciforms and correlates with predicted tension. Ann Biomed Eng 2011;39:714–729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gildner CD, Roy DC, Farrar CS, Hocking DC. Opposing effects of collagen I and vitronectin on fibronectin fibril structure and function. Matrix Biol 2014;34:33–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Corbett SA, Lee L, Wilson CL, Schwarzbauer JE. Covalent cross-linking of fibronectin to fibrin is required for maximal cell adhesion to a fibronectin-fibrin matrix. J Biol Chem 1997;272:24999–25005 [DOI] [PubMed] [Google Scholar]
- 91.Sottile J, Hocking DC. Fibronectin polymerization regulates the composition and stability of extracellular matrix fibrils and cell-matrix adhesions. Mol Biol Cell 2002;13:3546–3559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sethi KK, et al. Evidence for sequential utilization of fibronectin, vitronectin, and collagen during fibroblast-mediated collagen contraction. Wound Repair Regen 2002;10:397–408 [DOI] [PubMed] [Google Scholar]
- 93.Obara M, Yoshizato K. A novel domain of fibronectin revealed by epitope mapping of a monoclonal antibody which inhibits fibroblasts-mediated collagen gel contraction. FEBS Lett 1997;412:48–52 [DOI] [PubMed] [Google Scholar]
- 94.Kwon A-H, Qiu Z, Hirao Y. Topical application of plasma fibronectin in full-thickness skin wound healing in rats. Exp Biol Med (Maywood) 2007;232:935–941 [PubMed] [Google Scholar]
- 95.Schvartz I, Seger D, Shaltiel S. Vitronectin. Int J Biochem Cell Biol 1999;31:539–544 [DOI] [PubMed] [Google Scholar]
- 96.Nissen NN, Shankar R, Gamelli RL, Singh A, DiPietro LA. Heparin and heparan sulphate protect basic fibroblast growth factor from non-enzymic glycosylation. Biochem J 1999;338:637–642 [PMC free article] [PubMed] [Google Scholar]
- 97.Antonicelli F, Bellon G, Lorimier S, Hornebeck W. Role of the elastin receptor complex (S-Gal/Cath-A/Neu-1) in skin repair and regeneration. Wound Repair Regen 2009;17:631–638 [DOI] [PubMed] [Google Scholar]
- 98.Almine JF, et al. Elastin sequences trigger transient proinflammatory responses by human dermal fibroblasts. FASEB J 2013;27:3455–3465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Meran S, et al. Involvement of hyaluronan in regulation of fibroblast phenotype. J Biol Chem 2007;282:25687–25697 [DOI] [PubMed] [Google Scholar]
- 100.DePalma RL, et al. Characterization and quantitation of wound matrix in the fetal rabbit. Matrix 1989;9:224–231 [DOI] [PubMed] [Google Scholar]
- 101.Nyman E, Huss F, Nyman T, Junker J, Kratz G. Hyaluronic acid, an important factor in the wound healing properties of amniotic fluid: in vitro studies of re-epithelialisation in human skin wounds. J Plast Surg Hand Surg 2013;47:89–92 [DOI] [PubMed] [Google Scholar]
- 102.Sidgwick GP, Iqbal SA, Bayat A. Altered expression of hyaluronan synthase and hyaluronidase mRNA may affect hyaluronic acid distribution in keloid disease compared with normal skin. Exp Dermatol 2013;22:377–379 [DOI] [PubMed] [Google Scholar]
- 103.Tolg C, et al. A RHAMM mimetic peptide blocks hyaluronan signaling and reduces inflammation and fibrogenesis in excisional skin wounds. Am J Pathol 2012;181:1250–1270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.David-Raoudi M, et al. Differential effects of hyaluronan and its fragments on fibroblasts: relation to wound healing. Wound Repair Regen 2008;16:274–287 [DOI] [PubMed] [Google Scholar]
- 105.Tolg C, Telmer P, Turley E. Specific sizes of hyaluronan oligosaccharides stimulate fibroblast migration and excisional wound repair. PLoS One 2014;9:e88479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Webber J, Jenkins RH, Meran S, Phillips A, Steadman R. Modulation of TGFbeta1-dependent myofibroblast differentiation by hyaluronan. Am J Pathol 2009;175:148–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Webber J, Meran S, Steadman R, Phillips A. Hyaluronan orchestrates transforming growth factor-beta1-dependent maintenance of myofibroblast phenotype. J Biol Chem 2009;284:9083–9092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Simpson RML, et al. Aging fibroblasts resist phenotypic maturation because of impaired hyaluronan-dependent CD44/epidermal growth factor receptor signaling. Am J Pathol 2010;176:1215–1228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Müller SA, et al. Quantitative proteomics reveals altered expression of extracellular matrix related proteins of human primary dermal fibroblasts in response to sulfated hyaluronan and collagen applied as artificial extracellular matrix. J Mater Sci Mater Med 2012;23:3053–3065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hintze V, et al. Sulfated hyaluronan and chondroitin sulfate derivatives interact differently with human transforming growth factor-β1 (TGF-β1). Acta Biomater 2012;8:2144–2152 [DOI] [PubMed] [Google Scholar]
- 111.Kosir MA, Quinn CC, Wang W, Tromp G. Matrix glycosaminoglycans in the growth phase of fibroblasts: more of the story in wound healing. J Surg Res 2000;92:45–52 [DOI] [PubMed] [Google Scholar]
- 112.Penc SF, et al. Dermatan sulfate released after injury is a potent promoter of fibroblast growth factor-2 function. J Biol Chem 1998;273:28116–28121 [DOI] [PubMed] [Google Scholar]
- 113.Radek KA, Taylor KR, Gallo RL. FGF-10 and specific structural elements of dermatan sulfate size and sulfation promote maximal keratinocyte migration and cellular proliferation. Wound Repair Regen 2009;17:118–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hildebrand A, et al. Interaction of the small interstitial proteoglycans biglycan, decorin and fibromodulin with transforming growth factor beta. Biochem J 1994;302:527–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mohan RR, Gupta R, Mehan MK, Cowden JW, Sinha S. Decorin transfection suppresses profibrogenic genes and myofibroblast formation in human corneal fibroblasts. Exp Eye Res 2010;91:238–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Meenakshi J, et al. Low decorin expression along with inherent activation of ERK1,2 in ear lobe keloids. Burns 2009;35:519–526 [DOI] [PubMed] [Google Scholar]
- 117.Scott PG, Dodd CM, Tredget EE, Ghahary A, Rahemtulla F. Immunohistochemical localization of the proteoglycans decorin, biglycan and versican and transforming growth factor-beta in human post-burn hypertrophic and mature scars. Histopathology 1995;26:423–431 [DOI] [PubMed] [Google Scholar]
- 118.Sayani K, et al. Delayed appearance of decorin in healing burn scars. Histopathology 2000;36:262–272 [DOI] [PubMed] [Google Scholar]
- 119.Järveläinen H, et al. A role for decorin in cutaneous wound healing and angiogenesis. Wound Repair Regen 2006;14:443–452 [DOI] [PubMed] [Google Scholar]
- 120.Ferdous Z, et al. A role for decorin in controlling proliferation, adhesion, and migration of murine embryonic fibroblasts. J Biomed Mater Res A 2010;93:419–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Li H, Nahas Z, Feng F, Elisseeff JH, Boahene K. Tissue engineering for in vitro analysis of matrix metalloproteinases in the pathogenesis of keloid lesions. JAMA Facial Plast Surg 15:448–456 [DOI] [PubMed] [Google Scholar]
- 122.Wang J, Dodd C, Shankowsky HA, Scott PG, Tredget EE. Deep dermal fibroblasts contribute to hypertrophic scarring. Lab Invest 2008;88:1278–1290 [DOI] [PubMed] [Google Scholar]
- 123.Varkey M, Ding J, Tredget EE. Fibrotic remodeling of tissue-engineered skin with deep dermal fibroblasts is reduced by keratinocytes. Tissue Eng Part A 2014;20:716–727 [DOI] [PubMed] [Google Scholar]
- 124.Feng M, Yang G, Wu J. Versican targeting by RNA interference suppresses aggregative growth of dermal papilla cells. Clin Exp Dermatol 2011;36:77–84 [DOI] [PubMed] [Google Scholar]
- 125.Liu X-J, et al. Lumican accelerates wound healing by enhancing α2β1 integrin-mediated fibroblast contractility. PLoS One 2013;8:e67124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Vij N, Roberts L, Joyce S, Chakravarti S. Lumican suppresses cell proliferation and aids Fas-Fas ligand mediated apoptosis: implications in the cornea. Exp Eye Res 2004;78:957–971 [DOI] [PubMed] [Google Scholar]
- 127.Takeda U, et al. Targeted disruption of dermatopontin causes abnormal collagen fibrillogenesis. J Invest Dermatol 2002;119:678–683 [DOI] [PubMed] [Google Scholar]
- 128.Kuroda K, Okamoto O, Shinkai H. Dermatopontin expression is decreased in hypertrophic scar and systemic sclerosis skin fibroblasts and is regulated by transforming growth factor-beta1, interleukin-4, and matrix collagen. J Invest Dermatol 1999;112:706–710 [DOI] [PubMed] [Google Scholar]
- 129.Kato A, et al. Dermatopontin interacts with fibronectin, promotes fibronectin fibril formation, and enhances cell adhesion. J Biol Chem 2011;286:14861–14869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hunzelmann N, Anders S, Sollberg S, Schönherr E, Krieg T. Co-ordinate induction of collagen type I and biglycan expression in keloids. Br J Dermatol 1996;135:394–399 [PubMed] [Google Scholar]
- 131.Wu M, et al. Osteopontin in systemic sclerosis and its role in dermal fibrosis. J Invest Dermatol 2012;132:1605–1614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hunter C, Bond J, Kuo PC, Selim MA, Levinson H. The role of osteopontin and osteopontin aptamer (OPN-R3) in fibroblast activity. J Surg Res 2012;176:348–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lenga Y, et al. Osteopontin expression is required for myofibroblast differentiation. Circ Res 2008;102:319–327 [DOI] [PubMed] [Google Scholar]
- 134.Mori R, Shaw TJ, Martin P. Molecular mechanisms linking wound inflammation and fibrosis: knockdown of osteopontin leads to rapid repair and reduced scarring. J Exp Med 2008;205:43–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wang J-C, et al. Attenuation of fibrosis in vitro and in vivo with SPARC siRNA. Arthritis Res Ther 2010;12:R60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Rentz TJ, Poobalarahi F, Bornstein P, Sage EH, Bradshaw AD. SPARC regulates processing of procollagen I and collagen fibrillogenesis in dermal fibroblasts. J Biol Chem 2007;282:22062–22071 [DOI] [PubMed] [Google Scholar]
- 137.Zhou X, Tan FK, Guo X, Arnett FC. Attenuation of collagen production with small interfering RNA of SPARC in cultured fibroblasts from the skin of patients with scleroderma. Arthritis Rheum 2006;54:2626–2631 [DOI] [PubMed] [Google Scholar]
- 138.Bradshaw AD, Reed MJ, Sage EH. SPARC-null mice exhibit accelerated cutaneous wound closure. J Histochem Cytochem 2002;50:1–10 [DOI] [PubMed] [Google Scholar]
- 139.Wang JF, Olson ME, Ball DK, Brigstock DR, Hart DA. Recombinant connective tissue growth factor modulates porcine skin fibroblast gene expression. Wound Repair Regen 2003;11:220–229 [DOI] [PubMed] [Google Scholar]
- 140.Lee CH, Shah B, Moioli EK, Mao JJ. CTGF directs fibroblast differentiation from human mesenchymal stem/stromal cells and defines connective tissue healing in a rodent injury model. J Clin Invest 2010;120:3340–3349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Liu S, Thompson K, Leask A. CCN2 expression by fibroblasts is not required for cutaneous tissue repair. Wound Repair Regen 2014;22:119–124 [DOI] [PubMed] [Google Scholar]
- 142.Leask A. Transcriptional profiling of the scleroderma fibroblast reveals a potential role for connective tissue growth factor (CTGF) in pathological fibrosis. Keio J Med 2004;53:74–77 [DOI] [PubMed] [Google Scholar]
- 143.Xia W, et al. Increased CCN2 transcription in keloid fibroblasts requires cooperativity between AP-1 and SMAD binding sites. Ann Surg 2007;246:886–895 [DOI] [PubMed] [Google Scholar]
- 144.Kiwanuka E, et al. CCN2 promotes keratinocyte adhesion and migration via integrin α5β1. Exp Cell Res 2013;319:2938–2946 [DOI] [PubMed] [Google Scholar]
- 145.Hu X, et al. The role of ERK and JNK signaling in connective tissue growth factor induced extracellular matrix protein production and scar formation. Arch Dermatol Res 2013;305:433–445 [DOI] [PubMed] [Google Scholar]
- 146.Dalkowski A, Schuppan D, Orfanos CE, Zouboulis CC. Increased expression of tenascin C by keloids in vivo and in vitro. Br J Dermatol 1999;141:50–56 [DOI] [PubMed] [Google Scholar]
- 147.Filsell W, Rudman S, Jenkins G, Green MR. Coordinate upregulation of tenascin C expression with degree of photodamage in human skin. Br J Dermatol 1999;140:592–599 [DOI] [PubMed] [Google Scholar]
- 148.Orend G, Huang W, Olayioye MA, Hynes NE, Chiquet-Ehrismann R. Tenascin-C blocks cell-cycle progression of anchorage-dependent fibroblasts on fibronectin through inhibition of syndecan-4. Oncogene 2003;22:3917–3926 [DOI] [PubMed] [Google Scholar]
- 149.Trebaul A, Chan EK, Midwood KS. Regulation of fibroblast migration by tenascin-C. Biochem Soc Trans 2007;35:695–697 [DOI] [PubMed] [Google Scholar]
- 150.Lee MJ, Roy NK, Mogford JE, Schiemann WP, Mustoe TA. Fibulin-5 promotes wound healing in vivo. J Am Coll Surg 2004;199:403–410 [DOI] [PubMed] [Google Scholar]
- 151.Zheng Q, et al. Normal wound healing in mice deficient for fibulin-5, an elastin binding protein essential for dermal elastic fiber assembly. J Invest Dermatol 2006;126:2707–2714 [DOI] [PubMed] [Google Scholar]
- 152.Rittié L, Berton A, Monboisse JC, Hornebeck W, Gillery P. Decreased contraction of glycated collagen lattices coincides with impaired matrix metalloproteinase production. Biochem Biophys Res Commun 1999;264:488–492 [DOI] [PubMed] [Google Scholar]
- 153.Niu Y, Xie T, Ge K, Lin Y, Lu S. Effects of extracellular matrix glycosylation on proliferation and apoptosis of human dermal fibroblasts via the receptor for advanced glycosylated end products. Am J Dermatopathol 2008;30:344–351 [DOI] [PubMed] [Google Scholar]
- 154.Okano Y, Masaki H, Sakurai H. Dysfunction of dermal fibroblasts induced by advanced glycation end-products (AGEs) and the contribution of a nonspecific interaction with cell membrane and AGEs. J Dermatol Sci 2002;29:171–180 [DOI] [PubMed] [Google Scholar]
- 155.Reihsner R, Melling M, Pfeiler W, Menzel EJ. Alterations of biochemical and two-dimensional biomechanical properties of human skin in diabetes mellitus as compared to effects of in vitro non-enzymatic glycation. Clin Biomech (Bristol, Avon) 2000;15:379–386 [DOI] [PubMed] [Google Scholar]
- 156.Kuzuya M, et al. Inhibition of angiogenesis on glycated collagen lattices. Diabetologia 1998;41:491–499 [DOI] [PubMed] [Google Scholar]
- 157.Goova MT, et al. Blockade of receptor for advanced glycation end-products restores effective wound healing in diabetic mice. Am J Pathol 2001;159:513–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Sidgwick GP, Bayat A. Extracellular matrix molecules implicated in hypertrophic and keloid scarring. J Eur Acad Dermatol Venereol 2012;26:141–152 [DOI] [PubMed] [Google Scholar]
- 159.End P, Panayotou G, Entwistle A, Waterfield MD, Chiquet M. Tenascin: a modulator of cell growth. Eur J Biochem 1992;209:1041–1051 [DOI] [PubMed] [Google Scholar]
- 160.Kubo H, et al. Temporal expression of wound healing-related genes in skin burn injury. Leg Med (Tokyo) 2014;16:8–13 [DOI] [PubMed] [Google Scholar]
- 161.Betz P. Immunohistochemical parameters for the age estimation of human skin wounds. A review. Am J Forensic Med Pathol 1995;16:203–209 [DOI] [PubMed] [Google Scholar]
- 162.Betz P, et al. Time-dependent appearance of myofibroblasts in granulation tissue of human skin wounds. Int J Legal Med 1992;105:99–103 [DOI] [PubMed] [Google Scholar]
- 163.Betz P, et al. Comparison of the solophenyl-red polarization method and the immunohistochemical analysis for collagen type III. Int J Legal Med 1992;105:27–29 [DOI] [PubMed] [Google Scholar]
- 164.Betz P, et al. Immunohistochemical localization of fibronectin as a tool for the age determination of human skin wounds. Int J Legal Med 1992;105:21–26 [DOI] [PubMed] [Google Scholar]
- 165.Oono T, et al. Expression of type VI collagen mRNA during wound healing. J Invest Dermatol 1993;100:329–334 [DOI] [PubMed] [Google Scholar]
- 166.McGrath JA, Leigh IM, Eady RA. Intracellular expression of type VII collagen during wound healing in severe recessive dystrophic epidermolysis bullosa and normal human skin. Br J Dermatol 1992;127:312–317 [DOI] [PubMed] [Google Scholar]
- 167.Wester J, Sixma JJ, Geuze JJ, van der Veen J. Morphology of the early hemostasis in human skin wounds: influence of acetylsalicylic acid. Lab Invest 1978;39:298–311 [PubMed] [Google Scholar]
- 168.Olczyk P, et al. Propolis modulates vitronectin, laminin, and heparan sulfate/heparin expression during experimental burn healing. J Zhejiang Univ Sci B 2012;13:932–941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Olczyk P, et al. Propolis induces chondroitin/dermatan sulphate and hyaluronic Acid accumulation in the skin of burned wound. Evid Based Complement Alternat Med 2013;2013:290675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Ashcroft GS, Kielty CM, Horan MA, Ferguson MW. Age-related changes in the temporal and spatial distributions of fibrillin and elastin mRNAs and proteins in acute cutaneous wounds of healthy humans. J Pathol 1997;183:80–89 [DOI] [PubMed] [Google Scholar]
- 171.Oksala O, et al. Expression of proteoglycans and hyaluronan during wound healing. J Histochem Cytochem 1995;43:125–135 [DOI] [PubMed] [Google Scholar]
- 172.Tammi R, Pasonen-Seppänen S, Kolehmainen E, Tammi M. Hyaluronan synthase induction and hyaluronan accumulation in mouse epidermis following skin injury. J Invest Dermatol 2005;124:898–905 [DOI] [PubMed] [Google Scholar]
- 173.Siméon A, Wegrowski Y, Bontemps Y, Maquart FX. Expression of glycosaminoglycans and small proteoglycans in wounds: modulation by the tripeptide-copper complex glycyl-L-histidyl-L-lysine-Cu(2+). J Invest Dermatol 2000;115:962–968 [DOI] [PubMed] [Google Scholar]
- 174.Yeo TK, Brown L, Dvorak HF. Alterations in proteoglycan synthesis common to healing wounds and tumors. Am J Pathol 1991;138:1437–1450 [PMC free article] [PubMed] [Google Scholar]
- 175.Honardoust D, Eslami A, Larjava H, Häkkinen L. Localization of small leucine-rich proteoglycans and transforming growth factor-beta in human oral mucosal wound healing. Wound Repair Regen 2008;16:814–823 [DOI] [PubMed] [Google Scholar]
- 176.Geary RL, et al. Wound healing: a paradigm for lumen narrowing after arterial reconstruction. J Vasc Surg 1998;27:96–106; discussion 106–108 [DOI] [PubMed] [Google Scholar]
- 177.Zimmermann DR, Dours-Zimmermann MT, Schubert M, Bruckner-Tuderman L. Versican is expressed in the proliferating zone in the epidermis and in association with the elastic network of the dermis. J Cell Biol 1994;124:817–825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Wick G, Glanville RW, Timpl R. Characterization of antibodies to basement membrane (type IV) collagen in immunohistological studies. Immunobiology 1980;156:372–381 [DOI] [PubMed] [Google Scholar]
- 179.Sharma A, Singh AK, Warren J, Thangapazham RL, Maheshwari RK. Differential regulation of angiogenic genes in diabetic wound healing. J Invest Dermatol 2006;126:2323–2331 [DOI] [PubMed] [Google Scholar]
- 180.Reed MJ, et al. Differential expression of SPARC and thrombospondin 1 in wound repair: immunolocalization and in situ hybridization. J Histochem Cytochem 1993;41:1467–1477 [DOI] [PubMed] [Google Scholar]
- 181.Wu RX, et al. Fibroblast migration after myocardial infarction is regulated by transient SPARC expression. J Mol Med (Berl) 2006;84:241–252 [DOI] [PubMed] [Google Scholar]
- 182.Mackie EJ, Halfter W, Liverani D. Induction of tenascin in healing wounds. J Cell Biol 1988;107:2757–2767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Ishida Y, Kimura A, Takayasu T, Eisenmenger W, Kondo T. Detection of fibrocytes in human skin wounds and its application for wound age determination. Int J Legal Med 2009;123:299–304 [DOI] [PubMed] [Google Scholar]
- 184.Wu YJ, La Pierre DP, Wu J, Yee AJ, Yang BB. The interaction of versican with its binding partners. Cell Res 2005;15:483–494 [DOI] [PubMed] [Google Scholar]
- 185.Betz P, et al. The time-dependent rearrangement of the epithelial basement membrane in human skin wounds—immunohistochemical localization of collagen IV and VII. Int J Legal Med 1992;105:93–97 [DOI] [PubMed] [Google Scholar]
- 186.Huang-Lee LL, Wu JH, Nimni ME. Effects of hyaluronan on collagen fibrillar matrix contraction by fibroblasts. J Biomed Mater Res 1994;28:123–132 [DOI] [PubMed] [Google Scholar]
- 187.Clark RAF, Lin F, Greiling D, An J, Couchman JR. Fibroblast invasive migration into fibronectin/fibrin gels requires a previously uncharacterized dermatan sulfate-CD44 proteoglycan. J Invest Dermatol 2004;122:266–277 [DOI] [PubMed] [Google Scholar]
- 188.Zou XH, et al. Chondroitin sulfate in palatal wound healing. J Dent Res 2004;83:880–885 [DOI] [PubMed] [Google Scholar]