Abstract
The vast majority (85%) of pancreatic ductal adenocarcinomas (PDACs) are discovered at too of a late stage to allow curative surgery. In addition, PDAC is highly resistant to conventional methods of chemotherapy and radiotherapy, which only offer a marginal clinical benefit. Consequently, the prognosis of this cancer is devastating, with a 5-year survival rate of less than 5%. In this dismal context, we recently demonstrated that PDAC gene therapy using nonviral vectors is safe and feasible, with early signs of efficacy in selected patients. Our next step is to transfer to the clinic HIV-1-based lentiviral vectors (LVs) that outshine other therapeutic vectors to treat experimental models of PDAC. However, a primary safety issue presented by LVs that may delay their use in patients is the risk of oncogenesis after vector integration in the host's cell DNA. Thus, we developed a novel anticancerous approach based on integrase-defective lentiviral vectors (IDLVs) and demonstrated that IDLVs can be successfully engineered to transiently deliver therapeutic genes to inhibit pancreatic cancer cells proliferation. This work stems for the use of therapeutic IDLVs for the management of PDAC, in forthcoming early phase gene therapy clinical trial for this disease with no cure.
Introduction
Despite the continued efforts of scientists and clinicians, pancreatic ductal adenocarcinoma (PDAC) is associated with a very high death rate. Two-thirds of patients with PDAC die within one year of diagnosis, as the median survival hardly reaches six months for patients with advanced metastatic disease.1 Pancreatic cancer cells are resistant to endogenous antiproliferative signals, evade apoptosis, have limitless replicative potential, and undergo tissue invasion and metastasis.2,3 Consequently, PDAC is usually resistant to conventional therapeutic approaches (chemotherapy and radiotherapy) and targeted biotherapies. Without active treatment, metastatic pancreatic cancer has a median survival of 3–5 months. Therefore, there is an urgent need to develop new therapeutic strategies such as gene therapy to improve pancreatic cancer managing.
PDAC has been actively targeted by gene therapy approaches, and gene therapy products are currently in late clinical trials, alone or in combination with chemotherapeutic agents. We performed the first-in-human clinical trial, based on the use of nonviral vectors to transfer anticancer genes that sensitize PDAC cells to gemcitabine chemotherapy.4 This early phase clinical trial demonstrated that intratumoral gene delivery is safe and feasible in patients with PDAC who cannot undergo surgery. In addition, a population of patients with locally advanced tumors benefited from this treatment, with two patients surviving for two years after gene therapy.4
From our experience, the success of gene therapy protocols strongly relies on the identification of gene delivery vectors with significant delivery efficacy, as we have found that PDAC-derived cells are very resistant to gene transfer. Indeed, we have experimented synthetic (polyethylenimine, PEI) and viral-based (adenovirus, SV40) vectors, which demonstrated gene delivery to PDAC cells and evidence of therapeutic efficacy, both in vitro and in vivo.5–7 However, the low efficacy of gene transfer using PEI,5,7 the inherent immunogenicity of adenovirus,8 and the manufacturing hurdles of SV40 vectors9 challenge the use of these delivery vehicles in clinical trials. On the other hand, we recently found that lentiviral vectors (LVs) demonstrated the highest efficacy and reliability to inhibit PDAC cell proliferation both in vitro and in vivo,10–13 and elected LVs as promising gene delivery vectors for PDAC gene therapy. However, despite successful lentiviral-based gene therapy applications in patients after infusion of corrected cells,14–17 and more recently, after direct intracranial gene transfer,18 the risk of insertional mutagenesis and subsequent malignant transformation of transduced cells,19 historically demonstrated in patients during the X-SCID trial using gamma-retroviral vectors, continue to hinder the development of other integrating vectors such as LVs for in situ and in vivo cancer gene therapy, including PDAC.
HIV-1 integration is driven by the ability of the lentiviral preintegration complex to enter the cellular nucleus. The main protein components of this complex are HIV reverse transcriptase, matrix protein, accessory protein vpr, and viral integrase (IN) that mediates both the nuclear import and the integration of the lentiviral genome within the host DNA. Because of its multiplicity of function, HIV IN cannot be deleted entirely, but a different class of mutations has been engineered to prevent integration.20 Although integration was thought to be essential for viral stability and expression, recent studies by several groups reported efficient gene expression in vitro and in vivo using integrase-defective lentiviral vectors (IDLVs).21 In greater details, “class I” mutations of D64, D116, and E152 residues within HIV IN specifically inhibit the integration of viral DNA into the host genome, without reducing DNA synthesis or disturbing Gag-Pol functions.21
In this work, we evaluated the efficacy of IDLVs for gene transfer in human-derived PDAC cells. We found that IDLVs can be produced to high levels following routine protocols and can transduce PDAC cell lines with high efficacy to ensure transient gene expression without residual integration. Last, therapeutic NILVs showed preliminary evidence of efficacy by inhibiting PDAC cells' proliferation when combined with chemotherapy. This study stems for the development of IDLV for the gene therapy of PDAC.
Materials and Methods
Cells
Capan-2 and Capan-1 cells were grown in RPMI medium supplemented with 10% fetal calf serum, L-glutamine, antibiotic and antimycotic cocktail (Invitrogen), and Plasmocin (InvivoGen). 293 FT, Mia PACA-2, and Panc-1 cells were grown in DMEM containing 4.5 g/liter glucose (Invitrogen), 10% fetal calf serum, L-glutamine, antibiotics, Fungizone, and Plasmocin (InvivoGen). Cell lines were grown in a humidified incubator at 37°C in 5% CO2.
Ethics statement and experimental protocol
All animal experiments were conducted according to the national ethics guidelines for experimental research and were performed in accordance with the Guide for the Care and Use of Laboratory Animals (U.S. National Institutes of Health); Protocol No. 05/1037/12/13 was approved by the regional Midi-Pyrenees' ethics committee for animal experimentation. Human PDAC-derived Mia PACA-2 were implanted subcutaneously in athymic mice as previously described.5 IDLV D64V vectors encoding for copGFP were injected in level 2 animal safety facility in exponentially growing tumors at 15 days after tumor induction.22 Control animals received phosphate buffered saline. At the time of injection, tumor size was 125 ± 19 mm3. In selected experimental groups, tumors received a second injection four days later. At seven days post-IDLV injection, animals were killed and tumors were dissociated as previously described.13 GFP-positive cells were detected by FACS. Part of tumors were frozen in liquid nitrogen and stored at −80°C before RNA extraction using Trizol (Thermo) and RT-PCR for copGFP expression using Revertaid enzyme (Thermo), Phuions Taq polymerase (New England Bioloabs), and forward (5′-CTTCTACCACTTCG GGACCT-3′) and reverse (5′-TCTTGAAGTGCATG TGGCTG-3′) primers for CopGFP designed with Perlprimer software.23
Plasmids, vector cloning, production, and titration
Lentiviral plasmids derived from pCMVΔ8.91 with WT HIV-1 IN, D64V-mutated HIV-1 IN, and D116N-mutated HIV-1 IN or encoding for Gaussia luciferase were a kind gift from Dr. E. Ravet (Invivogen). The DNA vector TRIP-ΔU3-EF1a-EGFP, encoding for EGFP, has been described elsewhere.10 CopGFP and deoxycytidine kinase (DCK) cDNA were PCR-amplified from pMIRZIP-has-miR-2113 and normal pancreatic cells, respectively, and cloned into pPS-EF1-LCS-T2A (System Biosciences) following the manufacturer's recommendations. Successful cloning was verified by sequencing. Replication-defective, self-inactivating LVs were produced, concentrated, and titrated as described elsewhere.13 Briefly, lentiviral particles were produced in a BSL-3 facility (INSERM U1037) using Lenti-SmartINT and Lenti-SmartNIL kits (Invivogen) for production of parental LV and IDLV, respectively, using 293FT cells (Invitrogen), following the manufacturer's recommendations. Lentiviral particles were concentrated using Vivaspin filter devices (Vivaspin) or by ultracentrifugation and stored in phosphate buffered saline at −80°C. The viral titers were determined on HT1080 cells and expressed in transduction unit/ml (TU/ml) as described elsewhere.10 Vector concentrations were quantified by p24 ELISA (Ingen). All batches were checked whether they were replicative virus-free after transduction of 293FT cells and analysis of cellular extracts and culture supernatant for p24 presence, up to 3 passages (10 days) in culture.
Cell transduction
An amount of 5 × 104 PDAC-derived cells were plated in 48-well clusters and transduced overnight with LVs at the multiplicity of infection (MOI) of 5, or with the indicated ng of p24, in 250 μl of transduction medium (complete culture medium +4 μg/ml Protamine Choay; Sanofi Aventis France). Cell medium was replaced the next day. For integration studies, 1 × 105 cells were seeded in 6-well clusters, transduced overnight, and selected using 5 μg/ml puromycin (Invivogen). GFP-positive cells were quantified by flow cytometry analysis (FACS) on a FACScalibur (Beckton Dickinson). Gaussia production was measured in 5 μl of culture medium using coelenterazine (0.5 μM; Promega) as a substrate, as previously described.13 Crystal violet (Sigma) staining was performed as per manufacturer's recommendations.
Western blotting
Proteins were extracted from transduced cells, resolved on SDS-polyacrylamide gels, and transferred to nitrocellulose membrane. After room-temperature blocking for 1 hr, blots were incubated overnight at 4°C with antibodies against HA Tag (Sigma) and actin (Santa Cruz Biotechnology) diluted according to the manufacturer's recommendations. Secondary HRP-conjugated antibodies (dilution 1:10,000; Perbio Science) were added, and blots were incubated for 1 hr at room temperature. Immunoreactive proteins were visualized using Clarity ECL (Biorad) and imaged with ChemiDoc XRS+ (Biorad).
Cell proliferation
Cell proliferation assays were performed in 6-well clusters. An amount of 105 Mia PaCa-2 cells were cultured in complete medium for 24 hr (2 ml/dish). The next day, cells were transduced with 500 ng p24/ml of LVs expressing DCK. Control cells were transduced with LV(GFP). Two days later, cells were treated or not with 10 μM gemcitabine (Lilly). Cell growth was measured at day 3 after gemcitabine treatment (day 5 after transduction) by cell counting using a Coulter counter model ZM (Beckman Coulter). All experiments were conducted with different batches of LVs. Transduced cells were not selected in this study.
Statistical analysis
Results are expressed as mean ± standard error (SE). Data were compared using unpaired t tests using GraphPad Instat software (GraphPad Software) (*p < 0.05, **p < 0.01, ***p < 0.001). p < 0.05 was considered significant.
Results
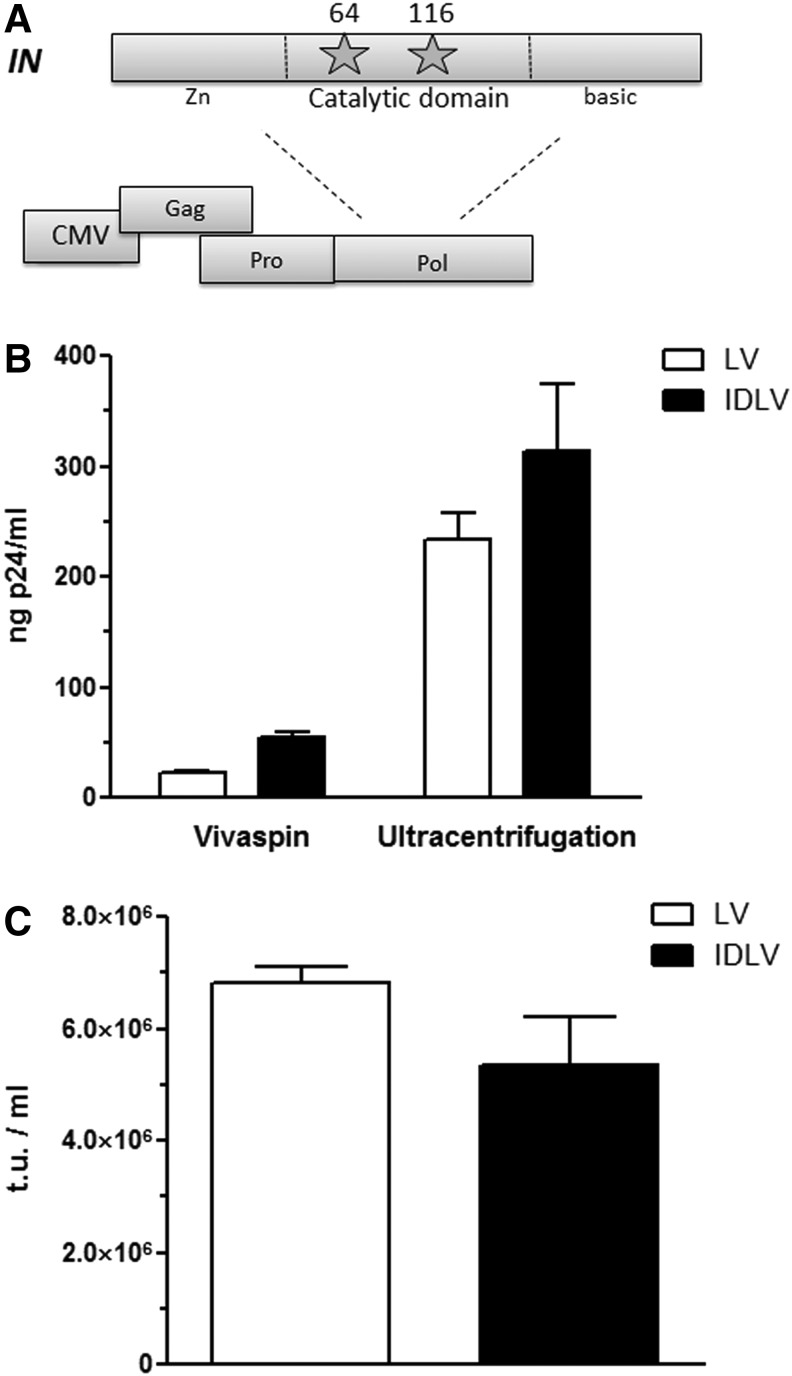
In this work, we used two different packaging vectors containing class I mutation of HIV-1 IN, in residue No. 64 (D64V) or No. 116 (D116N, located in the catalytic domain of the enzyme (Fig. 1A). These two residues are conserved across all reverse-transcribed elements, and are known to have the greatest effect on integration with no apparent effect on other viral processes.24 Second-generation IDLVs encoding for GFP were produced as described in Materials and Methods. As control, 293FT cells were transfected using packaging vectors containing wild-type HIV-1 IN. As shown in Fig. 1B, 293FT cells transfected with wild-type and mutated (D116N) HIV-1 IN demonstrated evidence of cytopathic effect and syncitia formation, two characteristics of LV production. We next assayed p24 antigen concentration (pg p24/ml) by ELISA or the number of transducing units (TU/ml) by FACS analysis after limiting dilution in cell culture. As shown in Fig. 1C, both LV and IDLV preparations contain equivalent functional and nonfunctional vector particle numbers. When assaying for functional vector particles only, we found that titration using HT-1080 cells gives equivalent levels of transducing units for both vector batches. Accordingly, we used p24 and functional titration of lentiviral batches for the remainder of the study.
Figure 1.
Production and titration of integrase-defective lentiviral vectors. (A) Schematic representation of the HIV-1 IN mutants used in this study. (B) Quantification of p24 content of vector batches. Results are means ± SD of three different batches of vectors with three experimental replicates. (C) Functional titration using HT-1080 cells of vector batches. Results are means ± SD of three different batches of vectors with three experimental replicates.
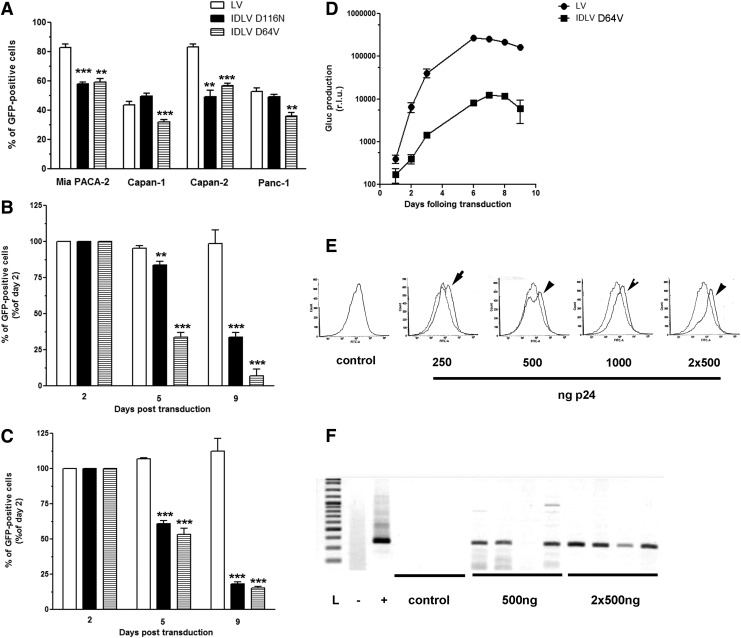
Next, human PDAC-derived cell lines were transduced with equivalent MOI of LV and IDLV encoding for eGFP. As shown in Fig. 2A, both LV and IDLV transduce PDAC-derived cell lines with high efficacy; however, IDLVs give rise to a lower percentage of GFP-positive cells as compared with the parental vectors. We next assessed the duration of gene transfer in human PDAC-derived cell lines using LV and IDLV. Mia PACA-2 and Capan-1 cells were transduced with MOI = 5 of LV(GFP) and IDLV(GFP) and cells were collected up to 9 days after gene transfer. Figures 2B and 2C demonstrate stable gene expression in PDAC-derived Mia PACA-2 and Capan-1 cell lines, respectively, after LV transduction; as expected, transduction with IDLVs results in transient gene expression with minimal numbers of GFP-positive cells detected after nine days in culture. We next constructed lentiviral backbones expressing secreted Gaussia luciferase (Gluc), to facilitate the noninvasive monitoring of gene expression after transduction. Results shown in Fig. 2D demonstrate that parental vectors are 25 ± 5-fold more effective than IDLVs to drive protein expression in PDAC-derived cell lines throughout the course of the experiment, whereas there is no significant difference between the D64V and D116N IDLV mutants.
Figure 2.
Transduction of PDAC cells with parental and IDLV HIV-1 vectors. (A) Mia PACA-2, Capan-1, Capan-2, and Panc-1 pancreatic cancer-derived human cell lines were transduced with MOI = 5 of LV(GFP), IDLV D116N(GFP), and IDLV D64V(GFP), respectively. Forty-eight hours later, GFP-positive cells were quantified by FACS analysis. Results are means ± SD of three different batches of vectors with three experimental replicates. Mia PACA-2 (B) and Capan-1 (C) pancreatic cancer-derived human cell lines were transduced with MOI = 5 of LV(GFP), IDLV D116N(GFP), and IDLV D64V(GFP), respectively. GFP-positive cells quantified by FACS analysis at the time indicated. Results are means ± SD of three different batches of vectors with three experimental replicates. (D) MIA Paca-2 cells were transduced with 500 ng p24/ml of LV(Gluc) and IDLV D64V(Gluc), respectively. Secreted Gluc was sampled from the culture medium at the time indicated and quantified as described in Materials and Methods. Results are means ± SD of three different batches of vectors with three experimental replicates. **p < 0.01, ***p < 0.001. Mia PACA-2 cells were engrafted subcutaneously in athymic mice. Fifteen days later, increasing amounts of IDLV D64V encoding for copepod Pontellina plumata GFP (CopGFP) were injected in exponentially growing tumors (n = 4 per group). In selected experimental groups, tumors received a second injection four days later. Seven days after the first intratumoral injection, mice were killed and tumors were anmysed for GFP using FACS analysis (E) or RT-PCR (F). Arrows indicate IDLV-transduced tumors. Dotted line, control GFP; IDLV, integrase-defective lentiviral vector; L, ladder; LV, lentiviral vector; MOI, multiplicity of infection; PDAC, pancreatic ductal adenocarcinoma.
We next examined the gene delivery efficacy of IDLVs in vivo in experimental PDACs. Mia PACA-2 cells were engrafted subcutaneously in athymic mice as described before.5 Fifteen days later, increasing amounts of IDLV D64V encoding for copepod Pontellina plumata GFP (CopGFP) were injected in exponentially growing tumors. In selected experimental groups, tumors received a second injection four days later. Seven days after the first intratumoral injection, mice were killed and tumors were dissociated and snap-frozen. Using FACS analysis, we found that IDLV achieved detectable gene expression in PDACs, in a dose-dependent manner, with the highest level of gene transfer obtained after repeated injection of the vector (Fig. 2E). We further confirmed successful in vivo gene expression using IDLV by RT-PCR (Fig. 2F). Taken together, we provide evidence herein for the first time that IDLVs are suitable for in vivo gene delivery into exponentially growing PDACs.
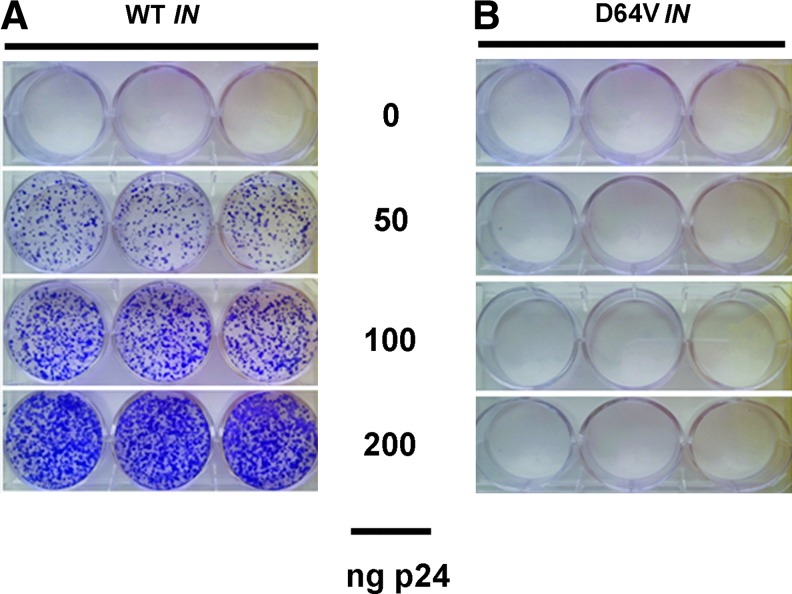
To assess whether the integration process in PDAC-derived cells is attenuated using IDLV, Mia PACA-2 cells were transduced with parental and D64V IDLV encoding for CopGFP and Puro at the dose indicated in Fig. 3. FACS analysis performed two days later revealed equivalent transduction rate for parental and D64V LVs (data not shown). Twenty-four hours later, puromycin was added to the culture medium. Cells were grown for an additional seven days, fixed, and labeled with crystal violet as described in Materials and Methods. Results shown in Fig. 3A indicate the presence of numerous clones when cells are transduced with increasing amounts of parental LVs; on the other hand, transduction of human PDAC-derived cell lines with D64V IDLVs yields no detectable clone, strongly suggesting the lack of effective integrase activity in these vectors.
Figure 3.
Residual integration with IDLV. Mia PACA-2 cells were transduced with LV(CopGFP-Puro) (A) or D64V(CopGFP-Puro) (B) at the dose indicated. Two days later, puromycin (5 μg/ml) was added to the culture medium. Cells were grown for an additional seven days, fixed, and labeled with crystal violet as described in Materials and Methods. Results are representative of three experiments performed with three different batches of vectors with three experimental replicates. Color images available online at www.liebertpub.com/hum
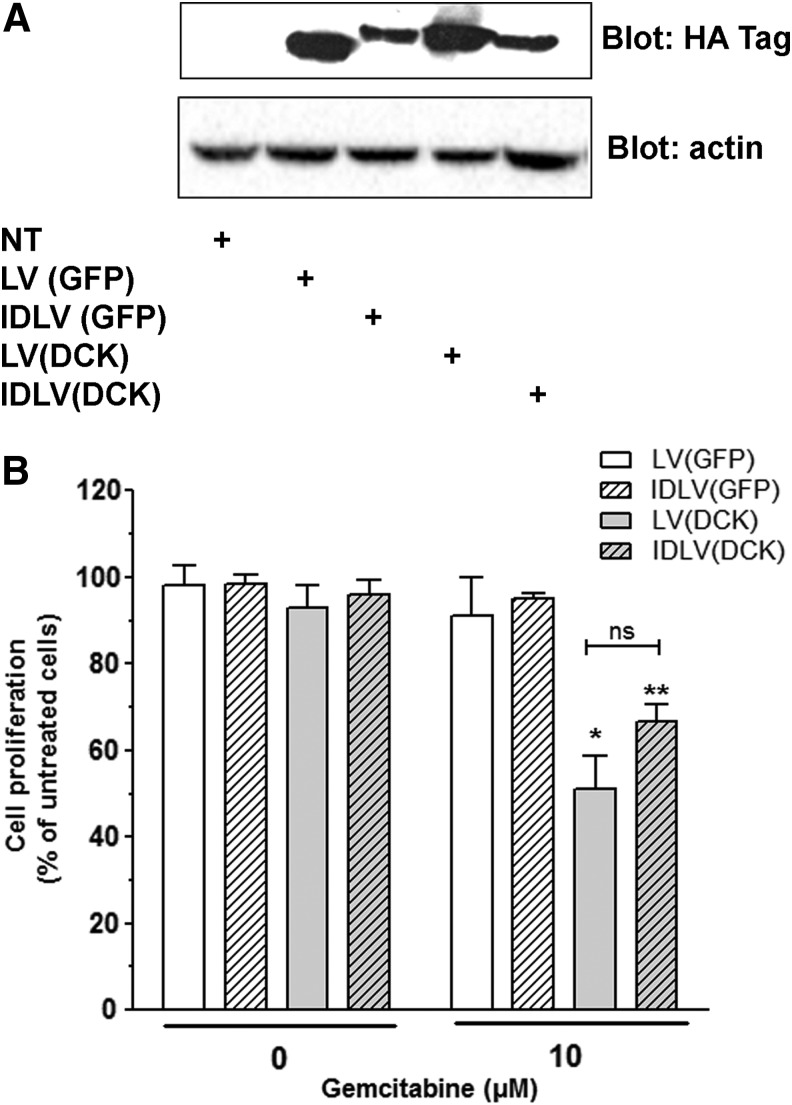
We next generated therapeutic LVs encoding for DCK, which phosphorylates gemcitabine (dFdC) into toxic metabolites for cancer cells. We and others have demonstrated that (1) DCK is underexpressed in samples from patients with PDAC resisting to chemotherapy25 and that (2) the enforced expression of DCK into PDAC-derived cell lines using nonviral gene therapy sensitizes cells to gemcitabine chemotherapy.26 Mia PACA-2 cells were transduced with parental and D64V IDLVs encoding for DCK. As control, cells were transduced with vectors encoding for CopGFP. Figure 4A demonstrates that both LV and IDLV drive successful expression of DCK in cancer cells, two days after transduction. Cells were subsequently treated for 72 hr with 10 μM gemcitabine. Control cells were treated with placebo (ddH2O). Cell proliferation was measured by cell counting. Figure 4B indicates that neither gemcitabine treatment at this dose nor transduction of PDAC-derived cell lines with vectors encoding GFP or DCK inhibits cell proliferation. However, delivering DCK strongly sensitizes cells to gemcitabine, as cell proliferation is inhibited by 49% ± 19% and 34% ± 6% when transduced by LV(DCK) and IDLV(DCK), respectively. Interestingly, the level of chemosensitization mediated by the HIV-1 IDLVs was comparable to the parental HIV-1 vector.
Figure 4.
Transduction of DCK with IDLV sensitizes pancreatic cancer cells to chemotherapy. MIA Paca-2 cells were transduced with 500 ng p24/ml of LV(DCK-Puro) and IDLV D64V(DCK-Puro), respectively. Control cells were left untransduced, or transduced with LV(CopGFP-Puro) and IDLV D64V(CopGFP-Puro), respectively. DCK gene expression was verified by Western blotting two days after gene transfer (A). Cells were treated with 10 μM gemcitabine, and cell proliferation was quantified 72 hr later as described in Materials and Methods (B). Results are expressed as mean ± SD compared with untreated cells, of three different experiments done in experimental triplicates with three different batches of vectors. *p < 0.05, **p < 0.001.
Discussion
PDAC is the fourth leading cause of cancer death in Western countries, with the lowest 5-year relative and 1-year survival rates among commonly diagnosed cancers.1 Its incidence is increasing for the last 40 years. Curative surgery for PDAC management is possible in only a fraction of patients because a vast majority (85%) of patients are diagnosed with advanced tumors. Consequently, PDAC is projected to become the third leading cause of cancer-related death by 2030.27 Since 1997, gemcitabine is the only approved first-line treatment for these patients, with limited clinical benefit.28 Recently, phase II and III trials exploring gemcitabine-based combinations with erlotinib,29 FOLFIRINOX,30 or nab-Paclitaxel31 were found to improve overall survival of patients. The armamentarium developed so far for PDAC patients offers at best a marginal survival benefit32; the prognosis of PDAC is still very poor, and developing new treatments that may profoundly change the therapeutic landscape is urgently needed.
We are among the first to provide evidence that cancer gene therapy may help alleviate the dismal prognosis of PDAC, a disease currently with no cure and fatal in a short time span. During the Thergap clinical trial, we demonstrated that the intratumoral injection of gene therapy is well tolerated, safe, and feasible, and that patients may benefit from the therapy, as 7 out of 9 patients survived more than 1 year after treatment, with 2 long survivors (>2 years).4 While this clinical trial is important, our next goal is to improve the delivery vehicles used in patients, that is, PEI nonviral vector, to elevate the therapeutic index of gene therapy, to serve in future early phase clinical trials. Our group extensively demonstrated the efficacy of lentiviral-based vectors to transduce and kill PDAC cells both in vitro and in vivo.10,12,13,33 However, despite recent advances in lentiviral-based ex vivo therapeutic gene delivery in patients suffering from monogenic diseases,14–18 the use of HIV as the origin of a vector system for in situ and in vivo cancer gene therapy is still surprisingly controversial. This is probably because of the pathogenic nature of the wild-type virus itself, but also to the inherent property of LVs to affect the host genome. Importantly, for ex vivo approaches, such as CAR-T cells engineering, integration and long-term expression is mandatory, and was proven to be safe and efficient in several clinical trials.34 Accordingly, IDLVs have recently gained increasing interest for in situ gene delivery strategies as they significantly reduce the potential to generate replication-competent lentivirus and the risk of insertional mutagenesis, while maintaining high level of transduction and gene expression in target cells.21 However, most if not all of the data were obtained in non- or slow-dividing cell lines; evidence for considering the use of IDLV in highly proliferating cancer cells is currently lacking.
In this study, we provide early evidence of using IDLVs as gene transfer vehicles in PDAC experimental models. We selected two class I mutations of HIV-1 IN, namely, D64V and D116N, because they have been previously demonstrated to have the greatest effect on integration with no apparent effect on other viral processes.24 We found that IDLVs can be produced at high levels following routine protocols, and that IDLV titers were equivalent to those of parental vectors. Using GFP-encoding vectors, we demonstrate that both D64V and D116N IDLVs transduce PDAC-derived cells with high efficacy at an MOI of 5 (33–65%, mean 46% ± 13%, and 41–60%, mean 51% ± 5%, respectively). Although mutant IDLVs give rise to a relatively lower percentage of GFP-positive cells (i.e., transduction efficiency) compared with the parental vectors, they outshine nonviral vectors, such as PEI, for gene transfer in PDAC-derived cells. Transduction of Mia PACA-2 cells with IDLVs resulted in a 20-time fold decrease in gene expression compared with parental vectors. This is a common observation, as other reports suggest that IDLVs do not support levels of gene expression equivalent to integrating LVs.21 In vivo, IDLV gene delivery into subcutaneous tumors was not measurable using FACS, as opposed to parental LVs. However, using RT-PCR amplification, we found that ILDVs successfully drive gene expression in vivo, after a route of administration validated in patients, as in vivo gene delivery of unmodified LVs is prone to inactivation by serum.35 Although LVs and IDLVs have the propensity of transducing the same cell types, endogenous restriction factors may further influence transgene expression from unintegrated episomes. Interestingly, DNA methylation is also considered as a potential cause of transcriptional repression after plasmid-mediated gene expression.36 IDLVs could be more susceptible to provirus methylation, a phenomenon largely described for retroviral vectors.37 Studies are undergoing to find whether epigenetic drugs (5-Aza, trichostatin A, etc.) may enhance IDLV-based gene expression in PDAC cells.
As expected, the number of GFP-positive cells was rapidly reduced as the nonintegrated genome should be lost by dilution in highly proliferating PDAC-derived cells. We further confirm the decrease of gene expression with time using noninvasive monitoring. These results strongly suggest the lack of effective integrase activity in IDLVs. Nevertheless, IDLVs with a single mutation in D64, as we used in this study, still possess a low level of integration activity (<1%), probably because of integrase-independent cellular processes, including DNA repair.38 During this study, we failed to identify residual integrants after transduction of PDAC-derived cell lines with D64V IDLVs, when compared with parental vectors. Although residual integration quantitation by this method may be an underestimate of the true integration rate, our results strongly suggest that IDLVs integrate with low frequency in PDAC cells' genome, thus limiting the risk of insertional mutagenesis.
As a proof-of-concept, we generated HIV-1 IDLVs with therapeutic activity. These vectors encode for DCK, a protein (1) we previously demonstrated to sensitize PDAC-derived cells to chemotherapy and to elicit a strong antitumoral bystander effect,26 and (2) that was recently transferred in patients using nonviral gene therapy during a phase I clinical trial for PDAC.4 We found that both IDLV and parental vectors drive detectable expression of DCK in PDAC-derived cell lines. When combined with gemcitabine, both vectors inhibited cancer cell proliferation to a similar extent. These results demonstrate that IDLVs are suitable delivery vehicles for PDAC cells for the transient expression of potent tumor suppressor genes. Studies are ongoing to find whether these vectors may drive successful, targeted gene expression using tumor-specific promoters in experimental animal models of PDAC to alter tumor progression, and spare the normal pancreatic parenchyma, respectively.
In summary, we demonstrate for the first time that IDLVs show promise for achieving gene expression without integration in pancreatic cancer-derived cells, preserving some benefits of LVs, while reducing the risk of insertional mutagenesis. Using IDLVs to deliver anticancerous gene successfully altered cancer cell proliferation. Although further testing in experimental tumors is obviously required, our results strongly suggest that IDLVs represent a promising class of novel viral vectors for the intratumoral gene therapy of pancreatic cancer.
Acknowledgments
The authors would like to thank the Région Midi Pyrénées (Grant No. 10051310) and the Fondation de l'Avenir (Grant No. ET2-656) for their financial support. M.G. was supported by a PhD grant from Université Paul Sabatier Toulouse III. A.P. was supported by a grant from the CHU of Toulouse.
Author Disclosure
No competing financial interests exist.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin 2013;63:11–30 [DOI] [PubMed] [Google Scholar]
- 2.Yachida S, Jones S, Bozic I, et al. Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature 2010;467:U114–U126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jones S, Zhang XS, Parsons DW, et al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science 2008;321:1801–1806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buscail L, Bournet B, Vernejoul F, et al. First-in-man phase I clinical trial of gene therapy for advanced pancreatic cancer: Safety, biodistribution and preliminary clinical findings. Mol Ther 2015;23:779–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carrere N, Vernejoul F, Souque A, et al. Characterization of the bystander effect of somatostatin receptor sst2 after in vivo gene transfer into human pancreatic cancer cells. Hum Gene Ther 2005;16:1175–1193 [DOI] [PubMed] [Google Scholar]
- 6.Cordelier P, Bienvenu C, Lulka H, et al. Replication-deficient rSV40 mediate pancreatic gene transfer and long-term inhibition of tumor growth. Cancer Gene Ther 2007;14:19–29 [DOI] [PubMed] [Google Scholar]
- 7.Vernejoul F, Faure P, Benali N, et al. Antitumor effect of in vivo somatostatin receptor subtype 2 gene transfer in primary and metastatic pancreatic cancer models. Cancer Res 2002;62:6124–6131 [PubMed] [Google Scholar]
- 8.Alonso-Padilla J, Papp T, Kaján GL, et al. Development of novel adenoviral vectors to overcome challenges observed with HAdV-5 based constructs. Mol Ther 2015. DOI: 10.1038/mt.2015.194. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buscail L, Cordelier P. Potential of recombinant SV40-based vectors for gene therapy. Recent Pat DNA Gene Seq 2007;1:93–99 [DOI] [PubMed] [Google Scholar]
- 10.Ravet E, Lulka H, Gross F, et al. Using lentiviral vectors for efficient pancreatic cancer gene therapy. Cancer Gene Ther 2010;17:315–324 [DOI] [PubMed] [Google Scholar]
- 11.Delpu Y, Lulka H, Sicard F, et al. The rescue of miR-148a expression in pancreatic cancer: An inappropriate therapeutic tool. PLoS ONE 2013;8:e55513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Torrisani J, Bournet B, du Rieu MC, et al. let-7 MicroRNA transfer in pancreatic cancer-derived cells inhibits in vitro cell proliferation but fails to alter tumor progression. Hum Gene Ther 2009;20:831–844 [DOI] [PubMed] [Google Scholar]
- 13.Sicard F, Gayral M, Lulka H, et al. Targeting miR-21 for the therapy of pancreatic cancer. Mol Ther. 2013;21:986–994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aiuti A, Biasco L, Scaramuzza S, et al. Lentiviral hematopoietic stem cell gene therapy in patients with Wiskott-Aldrich syndrome. Science 2013;341:1233151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Biffi A, Montini E, Lorioli L, et al. Lentiviral hematopoietic stem cell gene therapy benefits metachromatic leukodystrophy. Science 2013;341:1233158. [DOI] [PubMed] [Google Scholar]
- 16.Cavazzana-Calvo M, Payen E, Negre O, et al. Transfusion independence and HMGA2 activation after gene therapy of human β-thalassaemia. Nature 2010;467:318–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cartier N, Hacein-Bey-Abina S, Bartholomae CC, et al. Hematopoietic stem cell gene therapy with a lentiviral vector in X-linked adrenoleukodystrophy. Science 2009;326:818–823 [DOI] [PubMed] [Google Scholar]
- 18.Palfi S, Gurruchaga JM, Ralph GS, et al. Long-term safety and tolerability of ProSavin, a lentiviral vector-based gene therapy for Parkinson's disease: A dose escalation, open-label, phase 1/2 trial. Lancet 2014;383:1138–1146 [DOI] [PubMed] [Google Scholar]
- 19.Fischer A, Hacein-Bey Abina S, Touzot F, et al. Gene therapy for primary immunodeficiencies. Clin Genet 2015;88:507–515 [DOI] [PubMed] [Google Scholar]
- 20.Staunstrup NH, Mikkelsen JG. Integrase-defective lentiviral vectors—a stage for nonviral integration machineries. Curr Gene Ther 2011;11:350–362 [DOI] [PubMed] [Google Scholar]
- 21.Banasik MB, McCray PB. Integrase-defective lentiviral vectors: Progress and applications. Gene Ther 2010;17:150–157 [DOI] [PubMed] [Google Scholar]
- 22.Benali N, Cordelier P, Calise D, et al. Inhibition of growth and metastatic progression of pancreatic carcinoma in hamster after somatostatin receptor subtype 2 (sst2) gene expression and administration of cytotoxic somatostatin analog AN-238. Proc Natl Acad Sci U S A 2000;97:9180–9185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marshall OJ. PerlPrimer: Cross-platform, graphical primer design for standard, bisulphite and real-time PCR. Bioinforma Oxf Engl 2004;20:2471–2472 [DOI] [PubMed] [Google Scholar]
- 24.Leavitt AD, Robles G, Alesandro N, et al. Human immunodeficiency virus type 1 integrase mutants retain in vitro integrase activity yet fail to integrate viral DNA efficiently during infection. J Virol 1996;70:721–728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maréchal R, Bachet J-B, Mackey JR, et al. Levels of gemcitabine transport and metabolism proteins predict survival times of patients treated with gemcitabine for pancreatic adenocarcinoma. Gastroenterology 2012;143:664–674.e1–6. [DOI] [PubMed] [Google Scholar]
- 26.Vernejoul F, Ghénassia L, Souque A, et al. Gene therapy based on gemcitabine chemosensitization suppresses pancreatic tumor growth. Mol Ther J Am Soc Gene Ther 2006;14:758–767 [DOI] [PubMed] [Google Scholar]
- 27.Rahib L, Smith BD, Aizenberg R, et al. Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res 2014;74:2913–2921 [DOI] [PubMed] [Google Scholar]
- 28.Burris HA, 3rd, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: A randomized trial. J Clin Oncol Off J Am Soc Clin Oncol 1997;15:2403–2413 [DOI] [PubMed] [Google Scholar]
- 29.Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: A phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol Off J Am Soc Clin Oncol 2007;25:1960–1966 [DOI] [PubMed] [Google Scholar]
- 30.Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011;364:1817–1825 [DOI] [PubMed] [Google Scholar]
- 31.Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 2013;369:1691–1703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Costello E, Greenhalf W, Neoptolemos JP. New biomarkers and targets in pancreatic cancer and their application to treatment. Nat Rev Gastroenterol Hepatol 2012;9:435–444 [DOI] [PubMed] [Google Scholar]
- 33.Delpu Y, Lulka H, Sicard F, et al. The rescue of miR-148a expression in pancreatic cancer: An inappropriate therapeutic tool. PLoS ONE 2013;8:e55513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brenner MK, Gottschalk S, Leen AM, et al. Is cancer gene therapy an empty suit? Lancet Oncol 2013;14:e447–e456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.DePolo NJ, Reed JD, Sheridan PL, et al. VSV-G pseudotyped lentiviral vector particles produced in human cells are inactivated by human serum. Mol Ther J Am Soc Gene Ther 2000;2:218–222 [DOI] [PubMed] [Google Scholar]
- 36.Hyde SC, Pringle IA, Abdullah S, et al. CpG-free plasmids confer reduced inflammation and sustained pulmonary gene expression. Nat Biotechnol 2008;26:549–551 [DOI] [PubMed] [Google Scholar]
- 37.Antoniou MN, Skipper KA, Anakok O. Optimizing retroviral gene expression for effective therapies. Hum Gene Ther 2013;24:363–374 [DOI] [PubMed] [Google Scholar]
- 38.Nightingale SJ, Hollis RP, Pepper KA, et al. Transient gene expression by nonintegrating lentiviral vectors. Mol Ther J Am Soc Gene Ther 2006;13:1121–1132 [DOI] [PubMed] [Google Scholar]