Abstract
Background. Liver disease is common during human immunodeficiency virus (HIV) infection, but valid serum fibrosis markers are lacking. We hypothesize that HIV monoinfection and HIV/hepatitis C virus (HCV) coinfection is associated with an enhanced liver fibrosis (ELF) score higher than that for uninfected controls and examine whether this association is affected by factors other than liver injury.
Methods. The association of HIV and HIV/HCV coinfection with the ELF score was evaluated using multivariable regression after controlling for transient elastography–measured liver stiffness and traditional and HIV-related factors in a cross-sectional analysis of 297 women.
Results. HIV/HCV-coinfected and HIV-monoinfected women had higher median ELF scores than controls (9.6, 8.5, and 8.2, respectively). After adjustment for demographic, behavioral, and metabolic factors and for inflammatory markers, HIV/HCV coinfection remained associated with a 9% higher ELF score (95% confidence interval [CI], 5%–13%), while the association of HIV monoinfection was substantially attenuated (1% higher ELF score; 95% CI, −2% to 4%). After further adjustment for liver stiffness, HIV/HCV coinfection remained associated with 6% higher levels (95% CI, 3%–10%). In HIV/HCV-coinfected and HIV-monoinfected women, higher liver stiffness values were associated with higher ELF scores, as were older age and a nadir CD4+ T-cell count of <200 cells/mm3.
Conclusions. Our findings suggest that the ELF score can be used to assess liver fibrosis severity in HIV-infected women. However, higher ELF scores may reflect extrahepatic fibrosis in HIV-infected patients with a history of severe immunosuppression or advanced age.
Keywords: HIV, HCV, transient elastography, enhanced liver fibrosis score, women
Liver disease is highly prevalent among human immunodeficiency virus (HIV)–infected adults, and liver-related deaths are a leading cause of non–AIDS-related mortality [1, 2]. While this is often attributed to hepatitis virus coinfection, HIV has been associated with liver fibrosis even in the absence of viral hepatitis [3, 4]. Specific antiretroviral and other medications, alcohol use, fatty liver disease, obesity, and immunosuppression are thought to be contributing factors. HIV infection may also lead directly to liver disease by infecting hepatocytes, activating hepatic stellate cells, and stimulating collagen and proinflammatory cytokines [5–8].
The evaluation of liver fibrosis in the setting of HIV infection poses a clinical dilemma. While liver biopsy is considered the clinical gold standard, it is invasive and HIV-infected persons may have greater contraindications to biopsy than those without HIV infection. Previous studies have used indirect serum markers such as the aspartate aminotransferase-to-platelet ratio index [4] and FIB-4 index [3] and noninvasive imaging techniques such as ultrasonography-based transient elastography (TE) [9] to compare the effect of HIV monoinfection to that of no infection on liver fibrosis. However, there are limitations to these noninvasive markers in subpopulations of HIV-infected patients. Indirect markers of fibrosis are calculated from standard clinical laboratory findings (such as transaminase levels and platelet counts) that may be affected by HIV infection itself rather than by liver injury. While TE provides direct imaging of the liver, increased abdominal subcutaneous fat can limit the ability of TE to capture valid images [10].
Few if any studies have examined the association of HIV monoinfection with the enhanced liver fibrosis (ELF) score in HIV-infected persons. The ELF score is derived from 3 direct serum fibrosis markers: hyaluronic acid (HA), amino terminal propeptide of type III collagen (PIIINP), and tissue inhibitor of matrix metalloproteinase 1 (TIMP-1) [11]. These 3 markers are reflective of the extracellular matrix modeling process, which is important in fibrin degradation and synthesis but not necessarily specific to the liver. In patients with systemic sclerosis in the absence of liver fibrosis, the ELF score has been correlated with fibrosis of the skin and interstitial lung disease [12]. Examination of whether the ELF score might also reflect extrahepatic fibrosis is needed as HIV infection has been associated with lymphoid tissue fibrosis [13, 14].
We evaluated the associations of HIV monoinfection, HIV/hepatitis C virus (HCV) coinfection, and TE-measured liver stiffness with ELF scores in a geographically and ethnically diverse cohort of US women with or at risk for HIV infection, from the Women's Interagency HIV Study (WIHS). We hypothesized that HIV/HCV coinfection and HIV monoinfection would be associated with a greater ELF score. We also sought to determine whether extrahepatic factors were independently associated with ELF scores after adjustment for liver fibrosis severity, based on TE-measured liver stiffness.
METHODS
Study Population
The WIHS is a multicenter prospective cohort study that was established in 1994 to investigate the impact of HIV on women with and at risk for HIV infection. A total of 4137 women (3067 with and 1070 without HIV infection) were enrolled between 1994 and 2012 from 5 US cities (New York [Bronx and Brooklyn], Chicago, Los Angeles, San Francisco, and Washington, DC). Baseline sociodemographic characteristics and HIV risk factors were similar between HIV-infected and uninfected women [15, 16]. An institutional review board approved study protocols and consent forms, and each study participant gave written informed consent.
Every 6 months, participants complete a comprehensive physical examination, provide biological specimens for CD4+ T-cell count and HIV RNA load determination, and complete an interviewer-administered questionnaire, which collects information on sociodemographic characteristics, disease characteristics, and specific antiretroviral therapy (ART) use.
From November 2010 through October 2012, WIHS participants from the San Francisco, Chicago, and Washington, DC, sites between the ages of 25 and 65 years who were hepatitis B virus surface antigen negative, not pregnant, and not receiving interferon-based therapy were approached at their WIHS semiannual visit to participate in the TE substudy. The TE substudy was designed as a nested cohort study to investigate the determinants of liver disease in HIV-monoinfected and HIV/HCV-coinfected women. In addition, a comparison group of WIHS women with neither HIV infection nor HCV infection were included. Women who had a body mass index (calculated as the weight in kilograms divided by the height in meters squared) of > 35 or evidence of decompensated cirrhosis (ascites, hepatic encephalopathy, and/or esophageal varices), acute hepatitis, hepatitis flare, or cholestasis were also excluded from the study, owing to reported interference with the TE measurement [17]. Of the 381 women who agreed to participate in the TE substudy, we excluded 48 who did not have a valid TE measurement [9] and 26 who did not have sera available to enable ELF marker testing. We also excluded 10 HCV-monoinfected women because the sample size of this group was small. This cross-sectional analysis therefore included 297 women (165 HIV-monoinfected women, 73 HIV/HCV-coinfected women, and 59 uninfected women).
Ascertainment of ELF Outcome
Components of the ELF score were quantitatively measured from frozen sera that were stored at −70°C. An automated IMMUNO 1 immunoanalyzer (Siemens Medical Solutions Diagnostics, Tarrytown, New York) determined levels of TIMP-1, PIIINP, and hyaluronic acid (HA) via magnetic particle separation immunoassays as per the European Liver Fibrosis Study [11]. The TIMP-1 and PIIINP assays each use 2 monoclonal antibodies that bind to independent binding sites on their respective antigens. The HA assay uses HA binding protein, which is isolated from bovine nasal septum, in place of monoclonal antibodies. Tests were performed according to the manufacturer's instruction at iQur Limited (London, United Kingdom), and the levels of the individual markers and the ELF composite score were provided.
Covariates
Our primary predictors included HIV infection status (defined by prior documentation of an HIV-positive enzyme immunoassay [EIA] confirmed by Western blot) and chronic HCV infection (defined by documentation of an HCV-positive EIA confirmed by detection of HCV RNA). Candidate covariates included sociodemographic factors (age and ethnicity), menopausal status, lifestyle factors (history of injection drug use, alcohol use [none; light drinking, defined as consumption of 1–15 g of alcohol/day, moderate drinking, defined as consumption of 15–30 g/day; or heavy drinking, defined as consumption of >30 g/day], marijuana use [none, occasional, or daily], smoking [none, current, or past]), anthropometric characteristics (waist circumference, waist-to-hip ratio, and body mass index), and metabolic factors, including diabetes mellitus (defined by a confirmed elevated fasting glucose level, elevated hemoglobin A1C level, or self-report of antidiabetes medications), insulin resistance estimated using the homeostasis model assessment (HOMA), use of antidiabetes medications, fasting lipid levels (high-density lipoprotein [HDL] cholesterol, low-density lipoprotein [LDL] cholesterol, triglyceride level, and total cholesterol level), use of lipid-level-lowering therapy, and inflammatory markers (interleukin 6 [IL-6] and C-reactive protein [CRP] levels). In separate models, we also tested liver stiffness and liver enzyme (alanine aminotransferase [ALT] and aspartate aminotransferase [AST]) levels. HIV-related factors included current CD4+ T-cell count, nadir CD4+ T-cell count, current HIV RNA load, history of clinical AIDS, and current use of highly active antiretroviral therapy (HAART) and ART, by class. HCV-related factors included HCV RNA level and HCV genotype. Multiple imputation using the Markov chain Monte Carlo method was used to impute missing covariates [18].
Statistical Analysis
We compared sociodemographic and clinical characteristics among 3 groups: HIV-monoinfected women, HIV/HCV-coinfected women, and those with neither infection, using the Kruskal–Wallis test for continuous variables and the Fisher exact test for categorical variables.
The ELF score was found to be right skewed and showed evidence of heteroscedasticity, even after log transformation. Therefore, we used generalized linear regression with a log link function and pseudo-maximum likelihood estimator, using a robust variance estimator to adjust for potential overdispersion in the models [19–21]. The relative percentage difference was then estimated to evaluate the association of HIV/HCV coinfection, HIV monoinfection, and other factors with the ELF score.
To determine whether HIV monoinfection and HCV infection were independently associated with the ELF score, multivariable models were sequentially adjusted for (1) sociodemographic characteristics, (2) lifestyle factors, (3) metabolic and inflammation parameters, and (4) liver fibrosis severity. Age and race/ethnicity were retained in all models. We then constructed separate models in participants with HIV/HCV coinfection and HIV infection alone to identify correlates of the ELF score within each group. Additional adjustment for liver fibrosis severity, using transient elastography and markers of liver inflammation (ie, AST and ALT levels), was performed to determine whether the HIV and HCV effects on the ELF score reflected extrahepatic fibrosis and liver inflammation. Stepwise regression was performed, with a P value of ≤.10 used for entry and retention; the candidate variables were selected on the basis of their hypothesized associations with liver fibrosis.
All analyses were performed using the SAS system, version 9.3 (SAS Institute, Cary, North Carolina).
RESULTS
Population Characteristics
The sociodemographic, behavioral, metabolic, and clinical characteristics of the 297 women included in the analysis are shown in Table 1, stratified by HIV and HCV status. Over half of all women were African American. HIV/HCV-coinfected women were older, more often postmenopausal, and more often smokers, compared with HIV-monoinfected and uninfected women. HIV/HCV-coinfected women were also more likely to have diabetes and insulin resistance but lower LDL and total cholesterol levels. HIV/HCV-coinfected women also had higher IL-6 levels but lower CRP levels, consistent with previous reports [22, 23]. About half of HIV/HCV-coinfected women had significant liver fibrosis (defined by a TE-measured liver stiffness of > 7.1 kPa [24]), compared with 10% of controls and 11% of HIV-monoinfected women. HIV/HCV-coinfected women were also more likely than HIV-monoinfected women to have a history of AIDS.
Table 1.
Demographic and Clinical Characteristics Among 73 Women With Human Immunodeficiency Virus (HIV)/Hepatitis C Virus (HCV) Coinfection, 165 Women With HIV Monoinfection, and 59 Uninfected Control Women
| Characteristic | HIV/HCV Coinfection | HIV Monoinfection | Control | P Value |
|---|---|---|---|---|
| Demographic parameters | ||||
| Age, y | 53 (50–57) | 46 (40–52) | 42 (35–49) | <.0001 |
| Race/ethnicity | .24 | |||
| White | 10 | 21 | 14 | |
| African American | 77 | 61 | 66 | |
| Hispanic | 11 | 12 | 12 | |
| Other | 3 | 6 | 9 | |
| Menopause | 77 | 38 | 31 | <.0001 |
| Behavioral parameters | ||||
| Current smoker | 67 | 35 | 48 | <.0001 |
| Heavy alcohol use | 7 | 5 | 7 | .89 |
| Marijuana user | 30 | 22 | 31 | .31 |
| Body-composition parameters | ||||
| BMIa | 26 (23–29) | 25 (22–29) | 27 (23–29) | .27 |
| Waist circumference, cm | 92 (81–101) | 87 (79–96) | 86 (81–97) | .12 |
| Lipid profile | ||||
| LDL level, mg/dL | 75 (59–105) | 99 (81–121) | 97 (81–129) | .02 |
| HDL level, mg/dL | 53 (40–70) | 55 (44–68) | 62 (47–78) | .17 |
| Total cholesterol level, mg/dL | 160 (132–184) | 178 (155–204) | 182 (169–209) | .0002 |
| Triglyceride level, mg/dL | 97 (83–132) | 96 (69–136) | 74 (51–122) | .008 |
| DM | 22 | 9 | 15 | .03 |
| HOMA-IR | 1.8 (0.8–3.4) | 0.9 (0.4–1.8) | 0.6 (0.4–1.7) | .002 |
| Inflammatory markers | ||||
| IL-6 level, pg/mL | 1.5 (0.9–2.5) | 0.9 (0.6–1.4) | 0.9 (0.5–1.5) | <.0001 |
| CRP level, mg/L | 0.7 (0.2–2.4) | 1.2 (0.5–2.4) | 1.4 (0.6–4.4) | .03 |
| Liver-related parameters | ||||
| HCV RNA level, ×1000 IU/mL | 2315 (834–3930) | … | … | <.0001 |
| Liver stiffness, kPa | 7.1 (5.5–10.4) | 4.3 (3.6–5.4) | 4.4 (3.7–5.3) | <.0001 |
| AST level, U/L | 40 (30–71) | 21 (18–27) | 17 (15–21) | <.0001 |
| ALT level, U/L | 29 (21–44) | 17 (12–24) | 13 (10–19) | <.0001 |
| HIV-related parameters | ||||
| HIV RNA load, copies/mL | 66 (20–470) | 32 (20–442) | … | .36 |
| CD4+ T-cell count, cells/mm3 | ||||
| Current | 517 (326–746) | 582 (360–745) | … | .35 |
| Nadir | 190 (92–288) | 227 (123–331) | … | .08 |
| History of AIDS | 49 | 33 | … | .02 |
| HAART use | 81 | 84 | … | .52 |
Data are % of women or median value (interquartile range).
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; CRP, C-reactive protein; DM, diabetes mellitus; HAART, highly active antiretroviral therapy; HDL, high-density lipoprotein cholesterol; HOMA-IR, homeostasis model assessment of insulin resistance; IL-6, interleukin 6; LDL, low-density lipoprotein cholesterol.
a Body mass index (BMI) is calculated as the weight in kilograms divided by the height in meters squared.
Association of HIV/HCV Coinfection and HIV Monoinfection With ELF Score
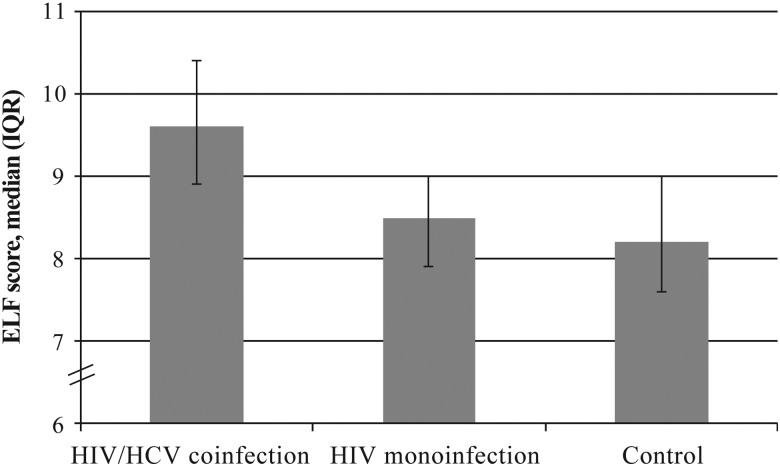
ELF scores were highest in HIV/HCV-coinfected women (median, 9.6; interquartile range [IQR], 8.9–10.4; P < .05 vs controls), intermediate in HIV-monoinfected women (median, 8.5; IQR, 7.9–9.0; P < .05 vs controls), and lowest in control women (median, 8.2; IQR, 7.6–9.0; Figure 1). In unadjusted analysis, ELF scores were 18% higher in HIV/HCV-coinfected women (95% CI, 14%–23%), relative to controls, while HIV-monoinfected women had a 4% higher (95% CI, 1%–7%) ELF score.
Figure 1.
Comparison of enhanced liver fibrosis (ELF) scores among 73 women with human immunodeficiency virus (HIV)/hepatitis C virus (HCV) coinfection, 165 women with HIV monoinfection, and 59 uninfected control women. The unadjusted percentage differences in ELF scores were 18% (95% confidence interval [CI], 14%–23%) for HIV/HICV-coinfected women and 4% (95% CI, 1%–7%) for HIV-monoinfected women, compared with score for control women. Abbreviation: IQR, interquartile range.
After adjustment for demographic, behavioral, and metabolic factors and for markers of inflammation (Table 2), HIV/HCV coinfection remained associated with a 9% higher ELF score (95% CI, 5%–13%), while the effect of HIV monoinfection was substantially attenuated and no longer statistically significant (relative difference, 1%; 95% CI, −2% to 4%).
Table 2.
Estimated Percentage Differences in Enhanced Liver Fibrosis Scores for 73 Women With Human Immunodeficiency Virus (HIV)/Hepatitis C Virus (HCV) Coinfection and 165 Women With HIV Monoinfection, Compared With Scores for 59 Control Women
| Model | Percentage Difference (95% CI) |
|
|---|---|---|
| HIV/HCV Coinfection | HIV Monoinfection | |
| Demographic adjusted | 12 (8–16) | 2 (−1 to 4) |
| Fully adjusteda | 9 (5–13) | 1 (−2 to 4) |
| Fully adjusteda plus TE-measured liver stiffness | 6 (3–10) | 2 (−1 to 4) |
Data are from generalized linear regression analyses.
Abbreviations: CI, confidence interval; TE, ultrasonography-based transient elastography.
a Includes age, race/ethnicity, homeostasis model assessment of insulin resistance score, interleukin 6 level, and total cholesterol level.
After additional adjustment for liver stiffness, HIV/HCV coinfection remained associated with a 6% higher (95% CI, 3%–10%) ELF score, relative to controls (Table 2). There was little change in the association of HIV monoinfection with ELF score after further adjustment for liver stiffness (2%; 95% CI, −1% to 4%).
When we further adjusted for markers of liver inflammation, ALT level, and AST level, the association of HIV/HCV coinfection with a higher ELF score was attenuated but remained statistically significant (relative difference, 4%; 95% CI, 1%–8%). By contrast, the effect of HIV monoinfection on the ELF score remained small and not statistically significant (relative difference, 1%; 95% CI, −2% to 4%).
Association of HCV Infection With ELF Score in HIV-Infected Women
We also compared ELF scores and determinants for HIV/HCV-coinfected women to those for HIV-monoinfected women, to determine whether differences were related to HIV replication and immunosuppression (Table 3). In models that controlled for sociodemographic, behavioral, metabolic, and inflammatory factors, ELF scores were 9% higher (95% CI, 5%–12%) in HIV/HCV-coinfected women, compared with HIV-monoinfected women. We then adjusted for HIV RNA levels and CD4+ T-cell counts. After additional adjustment for these HIV-related factors, HIV/HCV coinfection remained associated with 8% higher (95% CI, 5%–11%) ELF scores, relative to women with HIV monoinfection. We saw substantial attenuation when we controlled for liver stiffness, although HIV/HCV coinfection remained associated with 5% higher levels. When both HIV-related factors and liver stiffness were included in the model, ELF scores were 4% higher (95% CI, 2%–7%) on average in HIV/HCV-coinfected women, relative to HIV-monoinfected women (Table 3).
Table 3.
Estimated Percentage Differences in Enhanced Liver Fibrosis Scores for 73 Women With Human Immunodeficiency Virus (HIV)/Hepatitis C Virus (HCV) Coinfection, Compared With Scores for 165 Women With HIV Monoinfection
| Model | Percentage Difference (95% CI) |
|---|---|
| Demographic adjusted | 11 (7–14) |
| Fully adjusteda | 9 (5–12) |
| Fully adjusted plus HIV-related factorsb | 8 (5–11) |
| Fully adjusted plus TE-measured liver stiffness | 5 (2–8) |
| Fully adjusted plus HIV-related factors and TE-measured liver stiffness | 4 (2–7) |
Data are from generalized linear regression analyses.
Abbreviations: CI, confidence interval; TE, ultrasonography-based transient elastography.
a Includes age, race/ethnicity, homeostasis model assessment of insulin resistance score, interleukin 6 level, and total cholesterol level.
b HIV-related factors include HIV RNA load and CD4+ T-cell count.
Factors Associated With ELF Score in HIV/HCV-Coinfected and HIV-Monoinfected Women
In unadjusted analyses, factors associated with higher ELF scores in HIV/HCV-coinfected women included older age; Hispanic ethnicity; greater insulin resistance; higher IL-6 level, liver stiffness value, and AST level; nadir CD4+ T-cell count of <200 cells/mm3; and a lower HDL cholesterol level (Table 4). After multivariable adjustment, older age, higher liver stiffness value, and a nadir CD4+ T-cell count of <200 cells/mm3 remained independently associated with a higher ELF score, while a higher HDL cholesterol level remained associated with a lower ELF score.
Table 4.
Factors Associated With Enhanced Liver Fibrosis (ELF) Scores for 73 Women With Human Immunodeficiency Virus (HIV)/Hepatitis C Virus (HCV) Coinfection and 165 Women With HIV Monoinfection
| Factor | HIV/HCV Coinfection, Percentage Difference (95% CI) |
HIV Monoinfection, Percentage Difference (95% CI) |
||
|---|---|---|---|---|
| Unadjusted | Adjusted | Unadjusted | Adjusted | |
| Demographic factors | ||||
| Age (per decade) | 5 (1–8) | 4 (1–7) | 4 (2–7) | 4 (2–6) |
| Race/ethnicity (reference, white) | ||||
| Black | 2 (−4 to 8) | −2 (−7 to 2) | −1 (−5 to 3) | −2 (−6 to 2) |
| Hispanic | 10 (0–21) | 3 (−3 to 9) | 1 (−4 to 7) | 0 (−5 to 5) |
| Other | −8 (−13 to −3) | −3 (−11 to 5) | 3 (−4 to 9) | 5 (−1 to 12) |
| HOMA-IR score (per doubling) | 2 (1–4) | … | 1 (0–3) | … |
| IL-6 level (per doubling) | 3 (0–5) | … | 2 (1–10) | 1 (0–3) |
| HDL level (per doubling) | −8 (−12 to −4) | −4 (−7 to 0) | 0 (−3 to 4) | 0 (−3 to 3) |
| HIV-related factors | ||||
| HIV RNA level (> 50 vs ≤50 copies/mL) | 0 (−5 to 5) | … | 2 (−1 to 6) | … |
| Nadir CD4+ T-cell count (<200 vs >200 cells/mm3) | 5 (0–11) | 4 (0–7) | 5 (2–9) | 3 (0,–6) |
| Liver-related factors | ||||
| HCV RNA load (per doubling) | 0 (−1 to 1) | … | … | … |
| ALT level (per doubling) | 2 (−1 to 5) | … | 1 (−2 to 4) | … |
| AST level (per doubling) | 3 (0–6) | … | 4 (0–8) | … |
| Liver stiffness (per doubling) | 8 (6–10) | 7 (5–9) | 6 (2–10) | 5 (1–8) |
Data are from generalized linear regression analyses.
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; CI, confidence interval; HDL, high-density lipoprotein cholesterol; HOMA-IR, homeostasis model assessment of insulin resistance; IL-6, interleukin 6.
Among HIV-monoinfected women, older age, greater insulin resistance, higher IL-6 level, higher liver stiffness value, higher AST level, and a nadir CD4+ T-cell count of <200 cells/mm3 were associated with higher ELF score in unadjusted analysis (Table 4). After multivariate adjustment, older age, higher IL-6 and liver stiffness values, as well as a nadir CD4+ T-cell count of <200 cells/mm3 remained independently associated with higher ELF scores.
When we examined the individual components of the ELF score, we found that hyaluronic acid had a very strong correlation with the total ELF score (Spearman r = 0.96, P < .0001), while TIMP1 (r = 0.65, P < .0001) and PIIINP (r = 0.52, P < .0001) were moderately correlated.
DISCUSSION
With the increasing burden of liver disease during HIV infection, there is an urgent need for reliable, noninvasive methods to measure liver fibrosis severity and monitor its progression. In this contemporary cohort of women with HIV/HCV coinfection, HIV monoinfection, and neither infection, we found that HIV/HCV-coinfected women had higher ELF scores than women with neither infection, as expected, while elevations in HIV-monoinfected women were much more modest. The association of HIV/HCV coinfection with higher ELF scores remained independent of sociodemographic, metabolic, and behavioral factors and markers of inflammation, whereas HIV monoinfection was no longer associated after adjustment for these factors. A notable result of our study was that HIV/HCV coinfection remained associated with a higher ELF score, even after adjustment for TE-measured liver stiffness, suggesting that the ELF score may reflect liver fibrosis that is not detected by TE or that factors beyond the liver may lead to elevations in the ELF score.
Our finding that HIV/HCV coinfection is independently associated with higher ELF scores and, in HIV/HCV-coinfected and HIV-monoinfected women, that TE-measured liver stiffness is strongly associated with higher ELF scores expands the clinical population in which the ELF score can be used to assess liver fibrosis. To date, no study has assessed the ELF score in HIV/HCV-coinfected and HIV-monoinfected persons. The diagnostic accuracy of the ELF score to assess liver disease has been validated in populations with significant liver fibrosis [11, 25, 26] by comparing scores to liver histology findings, including those for persons with viral hepatitis [27], nonalcoholic fatty liver disease [28, 29], and primary biliary cirrhosis [30]. Those studies report diagnostic cutoffs (relative to stages of histologic fibrosis) ranging from 8.5 to 10.2 for significant liver fibrosis (ie, moderate fibrosis or histologic stage 2 fibrosis) [26, 28, 29, 31–33], 9.3–10.5 for severe liver fibrosis (histologic stage 3) [29, 32, 34], and 9.5–11.3 for cirrhosis (histologic stage 4) [26, 27, 29, 31, 32, 34]. The median ELF scores of 9.6, 8.5, and 8.2 in our HIV/HCV-coinfected women, HIV-monoinfected women, and control women, respectively, are within the expected range of liver fibrosis severity and lend further support to the use of ELF in HIV-infected women with little or no disease and significant HCV-associated liver disease.
Contrary to prior studies that showed an association of HIV monoinfection with serum markers of liver fibrosis [3, 4], we found little association of HIV monoinfection with ELF scores after adjustment for sociodemographic, metabolic, and behavioral factors and markers of inflammation. A possible reason for these disparate findings is that previous studies assessed fibrosis by using indirect serum markers that may be determined in part by factors other than fibrosis, such as hepatotoxicity and HIV-associated thrombocytopenia. In our study, women with neither infection also had similar risk behaviors as the HIV-infected women, enabling us to determine the independent effect of HIV infection on liver fibrosis. Our findings are consistent with previous analysis in this same cohort, which investigated factors associated with TE-measured liver stiffness (which directly visualizes the liver) [9]. The consistent associations between HIV and liver fibrosis measured by TE [9] and ELF, as well as our finding that TE-measured liver stiffness was strongly associated with ELF scores in both HIV/HCV-coinfected and HIV-monoinfected women, validates its use in HIV-infected women.
We also showed that older age and a low nadir CD4+ T-cell count were associated with higher ELF scores, independent of liver fibrosis, in both HIV/HCV-coinfected and HIV-monoinfected women. Older age and HIV infection have previously been associated with extrahepatic fibrosis [13, 14]. One small study of primarily treated HIV-infected patients showed that older age was associated with tonsillar lymphoid tissue fibrosis [13]. HIV infection has also been associated with both tonsillar [13] and rectal lymphoid tissue fibrosis [14]. The association of a low nadir CD4+ T-cell count observed in our study could represent a marker of the degree of lymphoid destruction and, hence, fibrosis. In non–HIV-infected patients with systemic sclerosis, ELF scores correlated with fibrosis of the skin and interstitial lung disease [12]. The constellation of these findings suggests that the ELF score is likely also a marker of extrahepatic fibrosis during HIV infection.
We also showed that, in HIV-monoinfected women, a higher level of the proinflammatory cytokine IL-6 was associated with a higher ELF score and that, in the HIV/HCV-coinfected women, a lower HDL cholesterol level was associated with a higher ELF score. Studies suggest that the lower HDL cholesterol level in HIV-infected persons, relative to HIV-uninfected persons, may be a marker of systemic inflammation [35]. Another recent study of HIV/HCV-coinfected persons found that higher IL-6 levels were associated with higher hyaluronic acid levels (a component of the ELF score) [36]. That study was not able to distinguish whether the higher IL-6 levels were a result of HIV infection or HCV-associated liver fibrosis. We found that the association of an enhanced inflammatory state with the ELF score was independent of liver fibrosis and markers of liver inflammation (eg, AST). Another study also showed that liver inflammation did not influence the ELF score [11], although a smaller study comparing TE findings and ELF scores to stages of histologic fibrosis in patients with chronic liver diseases concluded that ELF scores appeared more strongly influenced by inflammatory liver injury than TE findings [37]. Controlling for liver fibrosis using TE provides support to our findings that, in addition to being a marker of extrahepatic fibrosis, the ELF score is likely also a marker of inflammation beyond the liver in people with HIV infection.
Our study has some important limitations. First, we were unable to examine causal relationships owing to the cross-sectional design of our study. Second, we were unable to identify determinants of the ELF score in HCV-monoinfected women because of the small sample size of this group in the WIHS. Third, we were unable to compare histologic fibrosis (which is considered the clinical gold standard) with ELF scores. Although we found that TE-measured liver stiffness was strongly associated with a higher ELF score, further study is needed to determine the accuracy of ELF scores in predicting liver biopsy results in the HIV-infected individuals.
We conclude that ELF scores are elevated in HIV/HCV-coinfected women and that the ELF score can be used to assess liver fibrosis severity in people infected with HIV and HCV. However, use of the ELF score as a marker of liver fibrosis should be done with caution, especially among older patients and in those with a history of severe immunosuppression, for whom greater ELF scores may reflect extrahepatic fibrosis and systemic inflammation during HIV infection. In these settings, further imaging of the liver, using TE, may be warranted to distinguish hepatic fibrosis from extrahepatic fibrosis. Future studies need to investigate whether ELF scores can be used to monitor systemic fibrosis and predict the risk of non–AIDS-related clinical outcomes that are hepatic and extrahepatic in individuals with HIV infection.
Notes
Acknowledgments. Data in this manuscript were collected by the Women's Interagency HIV Study (WIHS).
Disclaimer. The contents of this publication are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health (NIH).
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases (U01-AI-103401, U01-AI-103408, UO1-AI-35004, UO1-AI-31834, UO1-AI-34994, U01-AI-103397, U01-AI-103390, UO1-AI-34989, and UO1-AI-42590 [all to the WIHS] and K24 AI 108516 [to P. C. T.] and R01 AI 087176 [to P. C. T. and administered by the Northern California Institute for Research and Education and with resources of the Veterans Affairs Medical Center, San Francisco, CA]), the Eunice Kennedy Shriver National Institute of Child Health and Human Development (to the WIHS), the National Cancer Institute (to the WIHS), the National Institute on Drug Abuse (to the WIHS), and the National Institute on Mental Health (to the WIHS), the National Institute of Dental and Craniofacial Research (to the WIHS), the National Institute on Alcohol Abuse and Alcoholism (to the WIHS), the National Institute on Deafness and other Communication Disorders (to the WIHS), the NIH Office of Research on Women's Health (to the WIHS), the NIH (UL1-TR000004 [to the UCSF CTSA] and UL1-TR000454 [to the Atlanta CTSA]), the National Institute of Diabetes, Digestive and Kidney Diseases-funded UCSF Liver Center (P30 DK026743 to the WIHS), and the NIH Research (to W. M. R.).
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Weber R, Sabin CA, Friis-Moller N et al. Liver-related deaths in persons infected with the human immunodeficiency virus: the D:A:D study. Arch Intern Med 2006; 166:1632–41. [DOI] [PubMed] [Google Scholar]
- 2.DallaPiazza M, Amorosa VK, Localio R, Kostman JR, Lo Re V III. Prevalence and risk factors for significant liver fibrosis among HIV-monoinfected patients. BMC Infect Dis 2010; 10:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blackard JT, Welge JA, Taylor LE et al. HIV mono-infection is associated with FIB-4 - A noninvasive index of liver fibrosis - in women. Clin Infect Dis 2011; 52:674–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Price JC, Seaberg EC, Badri S, Witt MD, D'Acunto K, Thio CL. HIV monoinfection is associated with increased aspartate aminotransferase-to-platelet ratio index, a surrogate marker for hepatic fibrosis. J Infect Dis 2012; 205:1005–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blackard JT, Sherman KE. HCV/ HIV co-infection: time to re-evaluate the role of HIV in the liver? J Viral Hepat 2008; 15:323–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tuyama AC, Hong F, Saiman Y et al. Human immunodeficiency virus (HIV)-1 infects human hepatic stellate cells and promotes collagen I and monocyte chemoattractant protein-1 expression: implications for the pathogenesis of HIV/hepatitis C virus-induced liver fibrosis. Hepatology 2010; 52:612–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vlahakis SR, Villasis-Keever A, Gomez TS, Bren GD, Paya CV. Human immunodeficiency virus-induced apoptosis of human hepatocytes via CXCR4. J Infect Dis 2003; 188:1455–60. [DOI] [PubMed] [Google Scholar]
- 8.Wheeler AL, Scherzer R, Lee D et al. HIV/hepatitis C virus coinfection ameliorates the atherogenic lipoprotein abnormalities of HIV infection. AIDS 2014; 28:49–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bailony MR, Scherzer R, Huhn G, Plankey MW, Peters MG, Tien PC. Association of HIV infection, hepatitis C virus infection, and metabolic factors with liver stiffness measured by transient elastography. J Infect Dis 2013; 208:1776–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Castera L, Foucher J, Bernard PH et al. Pitfalls of liver stiffness measurement: a 5-year prospective study of 13,369 examinations. Hepatology 2009; 51:828–35. [DOI] [PubMed] [Google Scholar]
- 11.Rosenberg WM, Voelker M, Thiel R et al. Serum markers detect the presence of liver fibrosis: a cohort study. Gastroenterology 2004; 127:1704–13. [DOI] [PubMed] [Google Scholar]
- 12.Abignano G, Cuomo G, Buch MH et al. The enhanced liver fibrosis test: a clinical grade, validated serum test, biomarker of overall fibrosis in systemic sclerosis. Ann Rheum Dis 2014; 73:420–7. [DOI] [PubMed] [Google Scholar]
- 13.Diaz A, Alos L, Leon A et al. Factors associated with collagen deposition in lymphoid tissue in long-term treated HIV-infected patients. AIDS 2010; 24:2029–39. [DOI] [PubMed] [Google Scholar]
- 14.Sanchez JL, Hunt PW, Reilly CS et al. Lymphoid fibrosis occurs in long-term nonprogressors and persists with antiretroviral therapy but may be reversible with curative interventions. J Infect Dis 2015; 211:1068–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bacon MC, Von Wyl V, Alden C et al. The Women's Interagency HIV Study: an observational cohort brings clinical sciences to the bench. Clin Diagn Lab Immunol 2005; 12:1013–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barkan SE, Melnick SL, Preston-Martin S et al. The women's interagency HIV study. Epidemiology 1998; 9:117–25. [PubMed] [Google Scholar]
- 17.Cohen EB, Afdhal NH. Ultrasound-based hepatic elastography: origins, limitations, and applications. J Clin Gastroenterol 2010; 44:637–45. [DOI] [PubMed] [Google Scholar]
- 18.Gilks WR, Richardson S, Spiegelhalter DJ. Markov chain Monte Carlo in practice. London: Chapman & Hall, 1996. [Google Scholar]
- 19.Manning WG, Mullahy J. Estimating log models: to transform or not to transform? J Health Econ 2001; 20:461–94. [DOI] [PubMed] [Google Scholar]
- 20.Santos Silva J, Tenreyro S. The log of gravity. Rev Econ Stat 2006; 88:641–58. [Google Scholar]
- 21.Santos Silva J, Tenreyro S. On the existence of the maximum likelihood estimates in poisson regression. Econ Lett 2010; 107:310–2. [Google Scholar]
- 22.Reingold J, Wanke C, Kotler D et al. Association of HIV infection and HIV/HCV coinfection with C-reactive protein levels: the fat redistribution and metabolic change in HIV infection (FRAM) study. J Acquir Immune Defic Syndr 2008; 48:142–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shah S, Ma Y, Scherzer R et al. Association of HIV, hepatitis C virus and liver fibrosis severity with intreleukin-6 and C-reactive protein levels. AIDS 2015; 29:1325–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vergara S, Macias J, Rivero A et al. The use of transient elastometry for assessing liver fibrosis in patients with HIV and hepatitis C virus coinfection. Clin Infect Dis 2007; 45:969–74. [DOI] [PubMed] [Google Scholar]
- 25.Xie Q, Zhou X, Huang P, Wei J, Wang W, Zheng S. The performance of enhanced liver fibrosis (ELF) test for the staging of liver fibrosis: a meta-analysis. PLoS One 2014; 9:e92772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Friedrich-Rust M, Rosenberg W, Parkes J, Herrmann E, Zeuzem S, Sarrazin C. Comparison of ELF, FibroTest and FibroScan for the non-invasive assessment of liver fibrosis. BMC Gastroenterol 2010; 10:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parkes J, Guha IN, Roderick P et al. Enhanced Liver Fibrosis (ELF) test accurately identifies liver fibrosis in patients with chronic hepatitis C. J Viral Hepat 2011; 18:23–31. [DOI] [PubMed] [Google Scholar]
- 28.Guha IN, Parkes J, Roderick P et al. Noninvasive markers of fibrosis in nonalcoholic fatty liver disease: validating the european liver fibrosis panel and exploring simple markers. Hepatology 2008; 47:455–60. [DOI] [PubMed] [Google Scholar]
- 29.Nobili V, Parkes J, Bottazzo G et al. Performance of ELF serum markers in predicting fibrosis stage in pediatric non-alcoholic fatty liver disease. Gastroenterology 2009; 136:160–7. [DOI] [PubMed] [Google Scholar]
- 30.Mayo MJ, Parkes J, Adams-Huet B et al. Prediction of clinical outcomes in primary biliary cirrhosis by serum enhanced liver fibrosis assay. Hepatology 2008; 48:1549–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guechot J, Trocme C, Renversez JC, Sturm N, Zarski JP, Group AHEFS. Independent validation of the Enhanced Liver Fibrosis (ELF) score in the ANRS HC EP 23 Fibrostar cohort of patients with chronic hepatitis C. Clin Chem Lab Med 2012; 50:693–9. [DOI] [PubMed] [Google Scholar]
- 32.Kim BK, Kim HS, Park JY et al. Prospective validation of ELF test in comparison with Fibroscan and FibroTest to predict liver fibrosis in Asian subjects with chronic hepatitis B. PLoS One 2012; 7:e41964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lichtinghagen R, Pietsch D, Bantel H, Manns MP, Brand K, Bahr MJ. The Enhanced Liver Fibrosis (ELF) score: normal values, influence factors and proposed cut-off values. J Hepatol 2013; 59:236–42. [DOI] [PubMed] [Google Scholar]
- 34.Wong GL, Chan HL, Choi PC et al. Non-invasive algorithm of enhanced liver fibrosis and liver stiffness measurement with transient elastography for advanced liver fibrosis in chronic hepatitis B. Aliment Pharmacol Ther 2014; 39:197–208. [DOI] [PubMed] [Google Scholar]
- 35.Baker J, Ayenew W, Quick H et al. High-density lipoprotein particles and markers of inflammation and thrombotic activity in patients with untreated HIV infection. J Infect Dis 2010; 201:285–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peters L, Neuhaus J, Duprez D et al. Biomarkers of inflammation, coagulation and microbial translocation in HIV/HCV co-infected patients in the SMART study. J Clin Virol 2014; 60:295–300. [DOI] [PubMed] [Google Scholar]
- 37.Wahl K, Rosenberg W, Vaske B et al. Biopsy-controlled liver fibrosis staging using the enhanced liver fibrosis (ELF) score compared to transient elastography. PLoS One 2012; 7:e51906. [DOI] [PMC free article] [PubMed] [Google Scholar]