Abstract
Background. Anemia has been linked to adverse human immunodeficiency virus (HIV) outcomes, including dementia, in the era before highly active antiretroviral therapy (HAART). Milder forms of HIV-associated neurocognitive disorder (HAND) remain common in HIV-infected persons, despite HAART, but whether anemia predicts HAND in the HAART era is unknown.
Methods. We evaluated time-dependent associations of anemia and cross-sectional associations of red blood cell indices with neurocognitive impairment in a multicenter, HAART-era HIV cohort study (N = 1261), adjusting for potential confounders, including age, nadir CD4+ T-cell count, zidovudine use, and comorbid conditions. Subjects underwent comprehensive neuropsychiatric and neuromedical assessments.
Results. HAND, defined according to standardized criteria, occurred in 595 subjects (47%) at entry. Mean corpuscular volume and mean corpuscular hemoglobin were positively associated with the global deficit score, a continuous measure of neurocognitive impairment (both P < .01), as well as with all HAND, milder forms of HAND, and HIV-associated dementia in multivariable analyses (all P < .05). Anemia independently predicted development of HAND during a median follow-up of 72 months (adjusted hazard ratio, 1.55; P < .01).
Conclusions. Anemia and red blood cell indices predict HAND in the HAART era and may contribute to risk assessment. Future studies should address whether treating anemia may help to prevent HAND or improve cognitive function in HIV-infected persons.
Keywords: human immunodeficiency virus (HIV), anemia, red blood cell indices, mitochondrial dysfunction, HIV-associated neurocognitive disorder, neurocognitive impairment, iron metabolism
Anemia is associated with increased morbidity and mortality rates in human immunodeficiency virus (HIV)–infected individuals, primarily in populations with limited access to highly active antiretroviral therapy (HAART) [1]. In the pre-HAART era, anemia was also linked to HIV-associated dementia (HAD) [2]. Because anemia may be caused by inflammation via effects of the iron-regulatory hormone hepcidin on iron transport [1, 3], inflammatory comorbid conditions potentially confound observed associations between anemia and HIV-related outcomes. Older nucleoside reverse-transcriptase inhibitors, notably zidovudine (ZDV), cause anemia and macrocytosis owing to direct effects on iron metabolism [4]. Few studies, however, have ascertained the independent impact of anemia on complications of HIV disease during HAART, such as HIV-associated neurocognitive disorder (HAND) [5, 6]. Although anemia and/or systemic iron levels are associated with neurocognitive disorders in non–HIV-infected persons [7–9], similar studies in HAND are lacking. Two pre-HAART-era studies demonstrated links between anemia and HAD, but HAD is a relatively uncommon form of HAND today [2, 10].
Despite a shift to milder forms of neurocognitive impairment (NCI), HAND still occurs in one-third to one-half of HIV-infected individuals, even in the absence of detectable virus [11]. A “legacy” of neuroinflammation from the acute infection, persistent immune activation, treatment-related mitochondrial toxicity, metabolic derangements, and cerebrovascular disease are implicated to varying degrees, but the pathogenesis of HAND remains perplexing [12, 13]. The nadir CD4+ T-cell count achieved during the course of disease and age at seroconversion remain the most consistently identified risk factors, but significant intraindividual variation in risk suggests the contribution of other factors [6, 12, 14].
Red blood cell (RBC) indices, such as the mean corpuscular volume (MCV) and mean corpuscular hemoglobin (MCH), RBC count, and hemoglobin level are indicators of systemic iron status [15, 16]. Specific measures of RBC size such as MCV, which depend on redox homeostasis and normal biosynthesis of RBC membrane lipids, may also reflect mitochondrial dysfunction [17, 18]. In HIV infection, anemia continues to be observed in individuals with suppressed viremia during HAART, with or without macrocytosis, and it is unexplained by ZDV use [1, 19]. Because iron homeostasis is critical for maintenance of mitochondrial energetics, lipid metabolism, and overall brain function [20] and anemia was a prominent predictor of HAD in the pre-HAART era, we examined the independent contributions of anemia and RBC indices to the risk of HAND in a HAART-era cohort.
PATIENTS AND METHODS
Study Population
The CNS HIV Antiretroviral Therapy Effects Research (CHARTER) Study is a US-based, prospective, observational study of neurobehavioral outcomes of HIV disease, which enrolled ambulatory, HIV-infected adults from 2003 to 2007 at 6 medical centers. Study subjects underwent detailed, structured interviews and laboratory assessments to obtain information on complete blood cell counts, RBC indices, nadir CD4+ T-cell counts, HAART history, exposure to nucleoside reverse-transcriptase inhibitors, history of a major depressive disorder, substance use/dependency, comorbid conditions, and demographics. Data were also collected on current CD4+ T-cell count, HIV RNA levels in plasma, and hepatitis C virus serology. Details regarding CHARTER study eligibility criteria, enrollment, and follow-up procedures are published elsewhere [11, 21]. CHARTER is approved by the institutional review boards of all participating sites and abides by the Declaration of Helsinki; all study subjects provided written informed consent.
Assessment of Neurocognitive Function and Comorbid Conditions
All participants underwent comprehensive neurocognitive assessments in 7 cognitive domains known to be commonly affected by HIV. Effects of age, education, sex, and race/ethnicity were accounted for in the CHARTER test battery, which incorporates the best available normative standards. Composite test scores (global deficit score [GDS]) were derived from demographically corrected standardized scores (T scores) on individual test measures. The GDS is a continuous measure (continuous GDS, cGDS) that reflects the number and severity of impairments on the test battery. A diagnosis of HAND was defined either by a dichotomous GDS-based variable (GDS, ≥0.5 for global NCI vs <0.5 for neurocognitively normal), or by well-established Frascati criteria [11].
To determine the presence and severity of HAND, CHARTER applied a published algorithm that has demonstrated excellent interrater reliability in previous studies [11]. This diagnostic algorithm required (1) absence of confounding neuromedical conditions that preclude a diagnosis of HAND, (2) presence of impairment in ≥2 of 7 ability domains assessed by the neurocognitive battery, and (3) assessment of functional impairment by self-report and performance-based criteria. By these criteria, HAND was classified as asymptomatic NCI, mild neurocognitive disorder (MND), or HAD, in order of increasing severity. Test scores at follow-up visits were corrected for practice effects.
Individuals with neuropsychiatric and severe comorbid conditions that could confound diagnosis of HAND (eg, ongoing substance abuse, prior stroke or cardiovascular complications without return to normal cognition after the event, severe depression with suboptimal effort in cognitive testing, decompensated liver disease) were excluded from analyses. Conditions deemed “incidental/minimal” or “contributing” to NCI were allowed: the latter included chronic stable systemic illness (eg, asthma, hypertension, or diabetes, with mild cognitive and/or functional impairment but with subsequent clear, additional cognitive and functional decline in the context of HIV) [11]. Anemia was defined relatively stringently as a hemoglobin level <11.5 g/dL in women and <13 g/dL in men [22].
Psychiatric Examination
Psychiatric diagnoses were assessed using the computer-assisted Composite International Diagnostic Interview [23], a structured instrument widely used in psychiatric research, which classifies current and lifetime diagnoses of mood disorders and substance use disorders, as well as other mental disorders. Current mood also was assessed with the Beck Depression Inventory-II [11].
Statistical Methods
Normality of variable distributions was evaluated using the Shapiro–Francia test, and summary statistics were presented as means (standard deviations) or medians (interquartile ranges). Standard parametric (t test) or nonparametric statistics (Wilcoxon test for continuous variables and χ2 or Fisher exact test for discrete variables) were used to compare variables at baseline.
Associations of RBC indices with cGDS were analyzed using multiple linear regression, adjusting for age, sex, HAART use, nadir CD4+ T-cell count, ZDV use, plasma HIV RNA concentration, Wide Range Achievement Test III score at entry (an estimate of reading ability, IQ, and educational level), self-reported race/ethnicity, and comorbid conditions (conditions deemed contributing vs incidental to NCI). Logistic regression analyses were conducted to evaluate associations of RBC indices with GDS-defined NCI and HAND, adjusting for the same covariates, to estimate odds ratios (ORs) and their 95% confidence intervals. Similarly adjusted regression analyses were also performed in a subset of participants who were receiving HAART continuously during follow-up.
A time-dependent, Cox proportional-hazards analysis of HAND (defined by dichotomous GDS) as a function of anemia at baseline was conducted to incorporate multiple covariates. Of 1261 study participants, 804 who were unimpaired at baseline were followed up longitudinally and eligible for analysis. The estimated hazard ratio (HR) for anemia was adjusted for age, sex, race/ethnicity, HAART, nadir CD4+ T-cell count, ZDV use, MCV at baseline, premorbid IQ (Wide-Range Achievement Test III score), and comorbid conditions (contributing vs incidental).
RESULTS
General Characteristics of the Study Population
Baseline neurocognitive performance data, RBC indices, and hemoglobin levels were available in 1261 CHARTER subjects without neurologically confounding conditions (median age, 43 years; 23% women; median CD4+ T-cell nadir, 181/μL). In this group, 886 (70.3%) of subjects were receiving HAART. The prevalence of current ZDV use was 18% among those receiving HAART at entry. GDS-defined NCI was present in 457 CHARTER subjects (36.2%) at the baseline visit (Table 1). HAND was diagnosed in 595 (47.2%), and 37 (6.2%) of these persons met criteria for HAD. At baseline, nadir CD4+ T-cell counts, plasma HIV RNA concentrations, and premorbid IQ were slightly lower among individuals classified as impaired. Contributing (vs incidental) comorbid conditions [11], HAART and ZDV use at baseline, and Hispanic ethnicity were more prevalent among GDS-impaired individuals (all P < .05); impairment by both GDS and Frascati criteria was less common among non-Hispanic blacks. The proportion of impaired women was slightly higher (P = .06). Alcohol abuse or dependency at baseline occurred in 22 subjects (1.8%) overall and in 1% of impaired subjects. Approximately 15% of subjects with or without NCI at entry were anemic.
Table 1.
Characteristics of CHARTER Study Participants at Baseline by Neurocognitive Impairment Statusa
| Variable | Not Impaired (n = 804) (GDS <0.5) |
Impaired (n = 457) (GDS ≥0.5) |
P Valueb | No HANDc (n = 665) |
ANI or MND (n = 558) |
HAD (n = 37) |
|---|---|---|---|---|---|---|
| Age, mean (SD), y | 43 (9) | 43 (8) | .53 | 43 (9) | 43 (9) | 44 (6) |
| Sex, No. (%) women | 171 (21) | 119 (26) | .06 | 150 (23) | 131 (24) | 8 (22) |
| Race/ethnicity, No. (%)d | ||||||
| Non-Hispanic black | 416 (52) | 174 (38) | <.01e | 351 (53) | 230 (41) | 8 (22) |
| Non-Hispanic white | 318 (40) | 200 (44) | .17 | 261 (39) | 235 (42) | 22 (59) |
| Hispanic | 53 (7) | 70 (15) | <.05e | 35 (5) | 82 (15) | 6 (16) |
| Nadir CD4+ T-cell count, median (IQR), cells/µL | 190 (56–329) | 162 (41–277) | <.01e | 196 (59–332) | 150 (34–265) | 123 (50–190) |
| Plasma HIV RNA, median (IQR), log10 copies/mL | 2.5 (1.7–4.1) | 2.1 (1.7–3.8) | .01e | 2.5 (1.7–4.1) | 2.2 (1.7–3.9) | 1.9 (1.7–3.7) |
| Receiving HAART, No. (%) | 534 (66) | 352 (77) | <.01e | 442 (66) | 410 (73) | 33 (89) |
| ZDV use, No. (%)f | 122 (15) | 95 (24) | .03e | 109 (16) | 100 (21) | 7 (21) |
| WRAT-III (IQ) score, median (IQR) | 97 (87–105) | 90 (79–102) | <.01e | 98 (87–105) | 92 (81–103) | 87 (77–98) |
| Contributing comorbid condition, No. (%)g | 239 (30) | 215 (47) | <.01e | 191 (29) | 238 (43) | 25 (68) |
| Current alcohol abuse, No. (%) | 18 (2) | 4 (1) | .11 | 18 (3) | 4 (1) | 0 (0) |
| Anemia, No. (%)h | 117 (15) | 74 (16) | .46 | 91 (14) | 86 (15) | 4 (11) |
Abbreviations: ANI, asymptomatic neurocognitive impairment; CHARTER, CNS HIV Antiretroviral Therapy Effects Research; GDS, global deficit score; HAART, highly active antiretroviral therapy; HAD, HIV-associated dementia; HAND, HIV-associated neurocognitive disorder; HIV, human immunodeficiency virus; IQR, interquartile range; MND, mild neurocognitive disorder; SD, standard deviation; WRAT, Wide-Range Achievement Test; y, years; ZDV, zidovudine.
a Data are presented as means (SDs) for normally distributed variables or medians (IQRs) if skewed.
b P values represent comparison between normal and impaired groups.
c One study participant was missing data on HAND assessment.
d A small number of individuals (1% of impaired and 3% of unimpaired subjects) self-identified their race/ethnicity as “other.”
e Statistically significant differences (P < .05; Wilcoxon rank sum test for continuous variables and χ2 or Fisher exact test for discrete variables).
f Data on ZDV use was available in all but 1 subject; 199 subjects were HAART-naive.
g All other comorbid conditions were deemed minimal and unlikely to contribute to neurocognitive impairment.
h Anemia was defined as a hemoglobin level <11.5 g/dL in women and <13 g/dL in men.
Associations of RBC Count and RBC Indices With Neurocognitive Impairment
As presented in Table 2, the RBC count was lower and the MCV and MCH were higher in study participants with GDS-defined NCI than in unimpaired individuals (median [interquartile range] RBC count, 4.4 × 106/μL [4.0–4.8] vs 4.5 × 106/μL [4.1–4.9]; MCV, 94 [88–100] vs 92 [87–97] fL; and MCH, 32 [30–34] vs 31 [29–33] pg/cell; all P < .01). Participants with asymptomatic NCI or MND also had lower RBC counts than those without HAND, and all HAND subgroups had higher MCV and MCH values than individuals without HAND (all P < .05) before multivariable adjustment.
Table 2.
Relationship of RBC Count and RBC Indices to Neurocognitive Impairment and Diagnosis of HAND in CHARTER Subjects
| RBC Measure | Not Impaired (n = 804) (GDS <0.5) |
Impaired (n = 457) (GDS ≥0.5) |
P Valuea | No HAND (n = 665)b |
ANI or MND (n = 558) |
P Valuea | HAD (n = 37) |
P Valuea |
|---|---|---|---|---|---|---|---|---|
| RBC count, median (IQR), ×106/μL | 4.5 (4.1–4.9) | 4.4 (4.0–4.8) | <.01c | 4.5 (4.1–4.9) | 4.4 (4.0–4.8) | <.05c | 4.6 (4.0–4.9) | .55 |
| MCV, median (IQR), fL | 92 (87–97) | 94 (88–100) | <.01c | 92 (87–97) | 93 (89–99) | <.05c | 95 (90–100) | <.05c |
| MCH, median (IQR), pg/cell | 31 (29–33) | 32 (30–34) | <.01c | 31 (29–33) | 32 (30–34) | <.05c | 32 (30–35) | <.05c |
Abbreviations: ANI, asymptomatic neurocognitive impairment; CHARTER, CNS HIV Antiretroviral Therapy Effects Research; GDS, global deficit score; HAD, human immunodeficiency virus (HIV)–associated dementia; HAND, HIV-associated neurocognitive disorder; IQR, interquartile range; MCH, mean corpuscular hemoglobin; MCV, mean corpuscular volume; MND, mild neurocognitive disorder; RBC, red blood cell.
a P values represent comparisons of impaired versus not impaired groups or either ANI/MND or HAD versus no HAND.
b HAND was subclassified as ANI, MND, or HAD.
c Significant difference (univariate P < .05; t test or Wilcoxon rank sum test).
Results of separate multivariable-adjusted linear regression models of cGDS on RBC count, hemoglobin, and RBC indices are shown in Table 3. MCV was independently and positively associated with cGDS (Table 3; adjusted β = .006; P < .01). Lower premorbid IQ, contributing comorbid conditions, and race/ethnicity were also significant factors in this model. (Because alcohol can influence MCV and cognitive function, current alcohol abuse/dependence was tested for its impact on regression models that evaluated MCV associations with HAND, but this variable was dropped owing to low prevalence in the study sample and lack of impact on results.) Similarly adjusted models of cGDS on MCH showed that MCH was also positively associated with cGDS, independent of other factors (Table 3; β = .014; P < .01). No association of cGDS with baseline hemoglobin level was observed, but lower RBC counts tended to be associated with higher cGDS values, indicating more severe NCI (β = −.052; P = .07).
Table 3.
Multivariable-Adjusted Regression Analyses of GDS on Hemoglobin and RBC Indices at Baseline
| Variable | β Coefficient (95% CI)a | P Value | Model P Value (Model R2) |
|---|---|---|---|
| Regression of GDS on MCV | 1.230 × 10−15(0.086) | ||
| MCV | .006 (.002 to .009) | <.01b | … |
| Age | .0003 (−.003 to .004) | .88 | … |
| Sex | −.045 (−.116 to .027) | .22 | … |
| Receiving HAART | .095 (−.006 to .196) | .06 | … |
| Nadir CD4+ T-cell count (per cell/μL ) | −5.7 × 10−5 (−.0002 to .0001) | .56 | … |
| ZDV use | −.054 (−.139 to .032) | .22 | … |
| Plasma HIV RNA (per log10copies/mL) | .008 (−.022 to .037) | .61 | … |
| WRAT-III (premorbid IQ) score | −.004 (−.006 to −.002) | <.01b | … |
| Race/ethnicity | −.235 (−.337 to −.134) | <.01b | … |
| Comorbid condition | −.167 (−.228 to −.105) | <.01b | … |
| Regression of GDS on hemoglobin, RBC count, and MCH | |||
| Hemoglobin level | .008 (−.013 to .029) | .47 | 1.044 × 10−13 (0.078) |
| RBC | −.052 (−.109 to .005) | .07 | 3.102 × 10−14 (0.080) |
| MCH | .014 (.005 to .024) | <.01b | 1.569 × 10−15 (0.086) |
Abbreviations: CI, confidence interval; GDS, global deficit score; HAART, highly active antiretroviral therapy; HIV, human immunodeficiency virus; MCH, mean corpuscular hemoglobin; MCV, mean corpuscular volume; RBC, red blood cell; WRAT, Wide-Range Achievement Test; ZDV, zidovudine.
a Data shown are adjusted β coefficients in separate regression models of GDS on MCV and GDS on hemoglobin, RBC count, or MCH. Each model was adjusted for age, sex (male vs female), HAART (on vs off), nadir CD4+ T-cell count, ZDV use (yes vs no), plasma HIV RNA concentration, WRAT-III score, self-reported race/ethnicity, and comorbid condition (incidental vs contributing).
b Statistically significant differences (P < .05).
In logistic regression models, hemoglobin level at baseline was not associated with GDS-defined NCI or HAND, as shown in Table 4. Though not associated with overall GDS impairment or HAD, higher RBC counts predicted a lower risk of MND (OR, 0.59; P < .05). Adjusted ORs for MCV were 1.03 and 1.06 for association with MND and HAD, respectively (both P < .05), compared with individuals without HAND. Similarly, MCH was associated with GDS-defined impairment and MND (adjusted OR, 1.03 and 1.11, respectively; both P < .01). The association of MCH with HAD was close to statistical significance (OR, 1.14; P = .06). These results indicate a 3% increase in risk of MND and a 6% increase in the risk of HAD per 1-fL rise in MCV; each picogram-per-cell increase in MCH was likewise associated with a 3% increase in risk of GDS-defined HAND and an 11%–14% increase in risk of HAND by Frascati criteria.
Table 4.
Multivariable-adjusted ORs for Associations of Hemoglobin, RBC Count, and RBC Indices With GDS Impairment or a HAND Diagnosis at Baselinea
| Variable | All Neurocognitive Impairment, OR (95% CI) (n = 457) |
P Valueb | MND, OR (95% CI) (n = 103) |
P Valueb | HAD, OR (95% CI) (n = 37) |
P Valueb |
|---|---|---|---|---|---|---|
| Hemoglobin | 1.03 (0.94–1.14) | .48 | 0.96 (.82–1.13) | .65 | 1.26 (.96–1.68) | .10 |
| RBC count | 0.93 (0.72–1.21) | .60 | 0.59 (.38–0.90) | <.05c | 1.02 (.49–2.06) | .96 |
| MCV | 1.01 (0.99–1.03) | .21 | 1.03 (1.00–1.06) | <.05c | 1.06 (1.00–1.11) | <.05c |
| MCH | 1.03 (0.99–1.07) | .18 | 1.11 (1.03–1.19) | <.01c | 1.14 (1.00–1.30) | .06 |
Abbreviations: CI, confidence interval; GDS, global deficit score; HAD, human immunodeficiency virus (HIV)–associated dementia; HAND, HIV-associated neurocognitive disorder; MCH, mean corpuscular hemoglobin; MCV, mean corpuscular volume; MND, mild neurocognitive disorder; OR, odds ratio. RBC, red blood cell.
a Data shown are for separate logistic regression models of RBC, MCV, and MCH, each adjusted for age, sex, highly active antiretroviral therapy (on or off), nadir CD4+ T-cell count, zidovudine use, HIV RNA concentration (plasma), Wide-Range Achievement Test III score, race/ethnicity, and non–HIV-related comorbid conditions that may affect cognition. The “All Neurocognitive Impairment” category was defined as GDS ≥0.5.
b Referent groups for P values are those with no impairment (GDS < 0.5) or no HAND.
c Statistically significant differences (P < .05).
Multivariable-Adjusted, Time-Dependent Associations of Anemia With Neurocognitive Impairment
The likelihood of incident NCI (defined as GDS ≥0.5) was estimated by means of Cox proportional-hazards regression analysis, using repeated assessments from follow-up visits at approximately 6-month intervals and sex-specific cutoffs for anemia (Figure 1). After exclusion of 457 participants with GDS-defined NCI at entry and 5 without available hemoglobin, ZDV, and/or MCV data, 799 persons with a median follow-up of 72 months were evaluable for incident NCI. Baseline and longitudinal follow-up data, including blood cell counts and RBC indices, were available for 2545 visits. Anemic subjects were at significantly higher risk of incident, GDS-defined NCI (adjusted HR for anemia, 1.55; 95% confidence interval, 1.13–2.12; P = .003).
Figure 1.
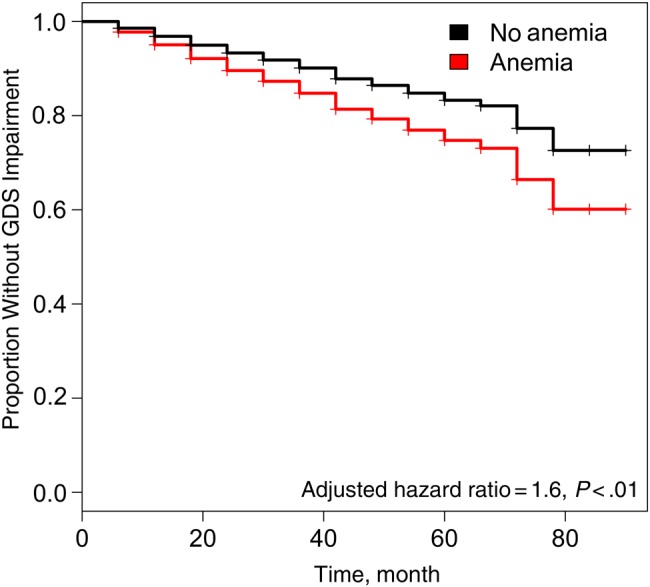
Multivariable-adjusted Cox proportional-hazards analysis of neurocognitive impairment as a function of anemia. This analysis included 799 CHARTER (CNS HIV Antiretroviral Therapy Effects Research) study participants who were neurocognitively normal at baseline and had longitudinal follow-up and data from 2545 visits. Global deficit scores (GDS) obtained after the baseline visit were adjusted for practice effects. Time-dependent, Cox proportional-hazards regression models were adjusted for age, sex, race/ethnicity, highly active antiretroviral therapy (on or off), nadir CD4+ T-cell count, zidovudine use, mean corpuscular volume at baseline, premorbid IQ (Wide-Range Achievement Test III score), and non–human immunodeficiency virus–related comorbid conditions at entry.
Stratified Analyses
Multivariable-adjusted regression modeling of cGDS and HAND on MCH and MCV and time-to-impairment analyses of anemia were also conducted in a subset of individuals receiving continuous HAART; results for RBC indices are shown in Table 5 and Table 6. MCV and MCH were associated with cGDS and with all HAND (GDS ≥0.5 vs <0.5), MND, and HAD (n = 862; cross-sectional analysis). MCH was strongly associated with cGDS (Table 5; β = .020; P = 8 × 10−5), as was the MCV (β = .08; P = 4 × 10−5). Risk estimates for HAND associated with MCV and MCH were similar to estimates in unstratified analyses (Table 6). Each 1-fL rise in MCV conferred a 2% increase in risk of HAND, a 4% increase in risk of MND (OR, 1.04; P < .01), and a 6% increase in risk of HAD (OR, 1.06; P = .02). Each picogram-per-cell increase in MCH was associated with a 5% increase in risk of HAND (OR, 1.05; P = .03), a 14% increase in risk of MND (OR 1.14; P = .002), and a 16% increase in risk of HAD (OR, 1.16; P < .05) among subjects receiving HAART.
Table 5.
Multivariable Analyses Associating RBC Indices With GDS as a Continuous Variable Among 862 Individuals Continuously Receiving HAART, With Use of Repeated Measures During Follow-upa
| Variable | β Coefficient (95% CI) | P Value |
|---|---|---|
| GDS | ||
| MCV | 0.008 (.004–.011) | <.01b |
| MCH | 0.020 (.010–.030) | <.01b |
Abbreviations: CI, confidence interval; GDS, global deficit score; HAART, highly active antiretroviral therapy; MCH, mean corpuscular hemoglobin; MCV, mean corpuscular volume; RBC, red blood cell.
a Regression models were adjusted for the baseline value of MCV (or MCH), age, sex, CD4+ T-cell nadir, zidovudine use, plasma human immunodeficiency virus (HIV) RNA concentration, Wide-Range Achievement Test III score (baseline), self-reported race/ethnicity, and non–HIV-related comorbid conditions (incidental or contributing to neurocognitive impairment).
b Significant difference (P < .05, from multiple linear regression analyses).
Table 6.
Multivariable Analyses Associating RBC Indices With HAND Among 862 Subjects Continuously Receiving HAART, With Use of Repeated Measures During Follow-up
| Variable | All HAND, OR (95% CI) |
P Value | MND, OR (95% CI) | P value | HAD, OR (95% CI) | P value |
|---|---|---|---|---|---|---|
| HANDb | ||||||
| MCV | 1.02 (1.00–1.03) | <.05c | 1.04 (1.01–1.07) | <.01c | 1.06 (1.01–1.12) | .02c |
| MCH | 1.05 (1.01–1.10) | .03c | 1.14 (1.05–1.23) | <.01c | 1.16 (1.01–1.34) | .04c |
Abbreviations: CI, confidence interval; HAART, highly active antiretroviral therapy; HAD, human immunodeficiency virus (HIV)–associated dementia; HAND, HIV-associated neurocognitive disorder; MCH, mean corpuscular hemoglobin; MCV, mean corpuscular volume; MND, mild neurocognitive disorder; OR, odds ratio; RBC, red blood cell.
a Regression models were adjusted for the baseline value of MCV (or MCH), age, sex, CD4+ T-cell nadir, zidovudine use, plasma HIV RNA concentration, Wide-Range Achievement Test III score (baseline), self-reported race/ethnicity, and non–HIV-related comorbid conditions (incidental or contributing to neurocognitive impairment).
b HAND was defined according to Frascati criteria.
c Significant difference (P < .05, from logistic regression analyses).
In individuals with detectable viremia (n = 479), anemia was associated with an even higher incidence of GDS-defined NCI (adjusted HR, 1.82; 95% confidence interval, 1.24–2.66; P = .002). In the smaller subset with undetectable viral load (n = 314), only 46 were anemic at baseline, and the anemia effect was no longer detectable, but power was low for covariate adjustment in this group; among 492 individuals receiving continuous HAART, most of whom had suppressed viremia, the point estimate for anemia remained in the same direction (adjusted HR, 1.39; P = .14; data not shown).
DISCUSSION
This is the first HAART-era, prospective study to show that anemia predicts HAND, independent of known risk factors for NCI, in HIV-infected persons. Furthermore, routinely available RBC indices (MCV and MCH) were positively associated with GDS-defined HAND. Anemia occurred in approximately 15% of subjects at baseline, lower than previously reported prevalences, although it has been noted that cutoffs used to define anemia (ours being relatively stringent) and proportions of women in older studies have varied considerably [1]. McArthur et al [2] reported that anemia was independently associated with rapid neurocognitive decline in individuals with AIDS, treated largely before the advent of HAART, and that pre-AIDS hemoglobin was the most significant predictor of dementia. The contribution of anemia to HAND in the HAART era has not been well studied, however, possibly because anemia is perceived to be uncommon. Nevertheless, anemia remains one of the most common hematological abnormalities in patients living with HIV infection and is consistently associated with poorer clinical outcomes and more rapid disease progression in both retrospective and prospective studies [1]
Previous studies evaluating the impact of anemia on HIV outcomes focused on overall mortality or outcomes of non-HIV infections [24–26], and most were conducted in populations with limited access to HAART and/or a high prevalence of AIDS and other comorbid conditions. Anemia due to non–HIV-related inflammation, general illness, thalassemias, or malnutrition may be challenging to distinguish from anemia directly attributable to HIV in such studies [27]. Causes of anemia in HAART-naive and HAART-exposed subjects may also differ, with drug regimen and duration of HAART predominating in the latter, as opposed to inflammatory or infectious causes [28]. In this study, the prevalence of AIDS was low, and we adjusted our analyses for the presence of comorbid conditions classified by expert clinicians as either minimal or contributory to NCI. Although residual confounding is possible, hematocrit emerged as an independent predictor of neurocognitive decline in a longitudinal substudy of CHARTER (n = 436), despite inclusion of persons with severe comorbid conditions [21].
In a published study of approximately 600 HIV-infected adults in the United States, the Veterans Aging Cohort Study (VACS) index was associated with concurrent risk of global NCI; in addition to older age and lower CD4+ T-cell count, hemoglobin <12 g/dL was one of the components of the VACS index associated with impairment status [6]. The VACS study was not designed to evaluate the independent impact of anemia on HAND, however, and it incorporated a cross-sectional analysis in which non–HIV-related comorbid conditions and demographic factors previously linked to HAND, such as ethnicity and cardiovascular disease [12, 21], were not included. In this study, anemia was a significant independent predictor of neurocognitive decline. We provide more precise estimates of its impact by excluding severe and likely confounding comorbid conditions [11].
Potential mechanisms linking anemia or RBC indices to NCI in this population include systemic and/or neuroinflammation due to HIV infection [29] and altered cellular iron metabolism that affects brain oxygenation and/or mitochondrial function. Iron transport within the macrophage-monocyte compartment is profoundly influenced by HIV infection as a result of numerous factors including inflammation, hepcidin release, direct actions of viral proteins such as Nef (which benefit intracellular viral replication), and the effects of antiretroviral drugs [4, 30, 31]. Hepcidin-mediated iron sequestration within macrophage-monocytes may contribute to global mitochondrial dysfunction and white matter damage, which are associated with HAND, because iron is essential for mitochondrial energy metabolism [32–34].
Although dysregulation of iron transport may also occur with the use of antiretrovirals such as ZDV, a drug recognized early in the HAART era to cause macrocytic anemia, our analyses adjusted for ZDV use. Suppressing viral replication by initiating HAART may correct the anemia associated with HIV infection [1], and most studies suggest that the benefits of eliminating detectable virus from the plasma and cerebrospinal fluid outweigh potential adverse effects of HAART [35, 36]. The lack of statistical significance of anemia as a predictor of NCI in subgroups continuously receiving HAART or with only undetectable viremia in this study is probably due to the significant loss of sample size for multivariable-adjusted analyses.
Micronutrient deficiencies (eg, iron, folic acid, or vitamin B12), which may underlie anemia and/or changes in erythrocyte indices in HIV-infected persons, deserve mention. We did not measure micronutrient levels in this study, but prior studies showed poor correlation between low serum vitamin B12 levels in HIV infection and true deficiency and a low likelihood that deficiency of this vitamin commonly contributes to HIV-associated neurological disease [37, 38].
Low vitamin B12 levels are also much less prevalent in the HAART era, probably reflecting improved immune status [37]. Others have argued against a major role for nutrient deficiencies in causing macrocytosis or anemia in HIV-infected individuals. For example, pediatric studies have reported that HIV-associated anemia is less likely to be related to iron or vitamin B12 deficiency than to inflammation [1, 39, 40]. Increased, not decreased, MCV and MCH were related to NCI in our study; therefore, these associations are not due to iron deficiency. Furthermore, anemia remained significantly associated with incident GDS impairment after adjustment for baseline MCV, again suggesting that micronutrient deficiencies alone are unlikely to explain our findings. Anemia independently predicted incident dementia in a large cohort of older, non–HIV-infected adults in the United States, in whom adjustment for MCV and RBC distribution width, sensitive indicators of iron and B-vitamin deficiencies, did not alter the associations [7]. At least 1 prior study also excluded a major role for B-vitamin deficiency in HIV-associated macrocytosis [19].
RBC indices are often abnormal in HIV-infected individuals receiving HAART, and MCV has been proposed as a marker of treatment adherence [41]. Indeed, RBC indices are sensitive indicators of changes in iron availability, inflammation, and dysmetabolism [15, 16, 42]. Although RBCs are devoid of mitochondria, increased cell size (MCV) may be a marker for subclinical mitochondrial dysfunction, which is linked to HAND [34, 43]. Increased MCV correlates with hepatic mitochondrial function measured by the methionine breath test in HIV-infected patients receiving nucleoside reverse-transcriptase inhibitors [17].
RBCs also represent an important component of the antioxidant capacity of blood, owing to both enzymatic and nonenzymatic intracellular antioxidants, and studies demonstrating that transfusion of younger RBCs into aged mice results in slower aging suggest a possible role for erythrocytes or their membrane constituents in supporting neurocognitive function that is unrelated to their oxygen-carrying capacity [44]. Anemia may also be a manifestation of the accelerated aging in HIV-infected persons due to chronic immune activation and for which epigenetic evidence has recently been reported [45]. Because increases in proinflammatory cytokines are associated with both HAND and aging, effects attributable to anemia may be inextricably linked to those of inflammation and aging, so-called “inflammaging” [1, 29, 46].
Protease inhibitors have been linked to dyslipidemia and cerebral small-vessel disease, elevated RBC indices [47], and, more recently, HAND [13]. Elevated MCV and MCH indices may indicate HAART neurotoxicity mediated via lipid dysmetabolism. Associations found between MCV or MCH and HAND in our study could reflect subtle mitochondrial toxicity or lipid derangements during HAART, which in turn promote HAND. Dideoxynucleoside analogues besides ZDV are also associated with macrocytosis [19, 48] with or without anemia, suggesting that even in the absence of serious mitochondrial toxic effects of these drugs, such as lactic acidosis and hepatic dysfunction, mitochondrial dysfunction may yet occur, predisposing to HAND.
Notably, substituting other antiretroviral drugs for ZDV does not always resolve macrocytosis. In a 2012 study of 20 HIV-infected subjects, Wobeser et al [49] reported that the MCV correlated strongly with fasting serum lactate levels and was significantly higher in subjects receiving HAART than in those not receiving antiretroviral therapy, or HIV-negative controls. These and other studies support the concept that erythrocyte size and membrane biology may be a sensitive indicator of low-level mitochondrial dysfunction, lipid dyshomeostasis, and/or antiretroviral toxicity. Although subjects in the study by Wobeser et al [49] had a history of ZDV exposure, none was receiving ZDV at the time of the study; antiretroviral regimens were not specified. In the study by McArthur et al [2], the impact of pre-AIDS ZDV use, on HAND was also found to be minimal.
In conclusion, anemia and RBC indices are significant independent predictors of HAND in the HAART era. Although the contributions of MCH and MCV to HAND risk seem small in absolute terms, changes in these indices may reflect mitochondrial toxicity and/or lipid dysmetabolism during treatment, which could have important implications for neurocognitive function over time. Further studies are needed to determine the precise mechanisms underlying these associations. Inexpensive and noninvasive biomarkers are urgently needed for diagnosis, risk stratification, and monitoring of HAND, particularly in low-resource settings; to this end, RBC indices may constitute adjuncts to the clinical armamentarium. Studies are also needed to determine whether treatment of anemia and inflammation in HIV-infected individuals, for example with newer erythropoietic agents that address both [50], may reduce the risk of HAND or ameliorate NCI.
Notes
Acknowledgments. We gratefully acknowledge the contributions of all participants in the CHARTER study.
CHARTER study group. The CHARTER group is affiliated with the Johns Hopkins University, Icahn School of Medicine of Mount Sinai, University of California, San Diego, University of Texas, Galveston, University of Washington, Seattle, and Washington University, St Louis, and it is headquartered at the University of California, San Diego. Director: Igor Grant, MD. Co-directors: J. Allen McCutchan, MD, Ronald J. Ellis, MD, PhD, and Thomas D. Marcotte, PhD. Center manager: Donald Franklin, Jr. Neuromedical component: Ronald J. Ellis, MD, PhD (principal investigator [PI]), J. Allen McCutchan, MD, and Terry Alexander, RN. Laboratory, pharmacology and immunology component: Scott L. Letendre, MD (PI), and Edmund Capparelli, PharmD. Neurobehavioral component: Robert K. Heaton, PhD (PI), J. Hampton Atkinson, MD, Steven Paul Woods, PsyD, and Matthew Dawson. Virology component: David M. Smith, MD (PI). Imaging component: Christine Fennema-Notestine, PhD (co-PI), Michael J. Taylor, PhD (co-PI), and Rebecca Theilmann, PhD. Data management unit: Anthony C. Gamst, PhD (PI), and Clint Cushman. Statistics unit: Ian Abramson, PhD (PI), and Florin Vaida, PhD. Protocol coordinating component: Thomas D. Marcotte, PhD (PI), and Jennifer Marquie-Beck, MPH. Johns Hopkins University site: Justin McArthur (PI), and Vincent Rogalski, RN. Mount Sinai School of Medicine site: Susan Morgello, MD (co-PI), David Simpson, MD (co-PI), and Letty Mintz, NP. University of California, San Diego site: J. Allen McCutchan, MD (PI), and Will Toperoff, NP. University of Washington, Seattle site: Ann Collier, MD (co-PI), Christina Marra, MD (co-PI), Trudy Jones, MN, ARNP. University of Texas, Galveston site: Benjamin Gelman, MD, PhD (PI), and Eleanor Head, RN, BSN. Washington University, St Louis site: David Clifford, MD (PI), Muhammad Al-Lozi, MD, and Mengesha Teshome, MD.
Author contributions. A. R. K. conceived and designed the study and interpreted results; Q. W. and P. J. performed all multivariable statistical analyses, which were also checked by Z. Z., A. R. K., T. H., S. L. L., R. J. E., and J. B.-S. all participated in discussions of results and assisted in manuscript development. D. R. F. provided CHARTER clinical and covariate data at various stages of analysis. Z. Z. and J. B.-S. guided the statistical analyses. All other coauthors were instrumental in recruiting patients onto the CHARTER study and provided helpful comments on the manuscript.
Financial support. The CHARTER study is supported by the National Institutes of Health (contracts N01 MH22005, R01 MH095621 to A. R. K. and T. H., and HHSN271201000036C to I. G.).
Potential conflicts of interest. T. H. has served as PI on research grants from Merck to Vanderbilt University. S. L. L. has received support for research projects from Abbott, Merck, Tibotec, Schering-Plough, and GlaxoSmithKline and has received honoraria for speaking from Abbott, GlaxoSmithKline, and Tibotec. R. J. E. gave sponsored talks and received honoraria for serving on the scientific advisory board of GlaxoSmithKline. A. C. C. has received past research support from Schering-Pough, Merck, and Roche Molecular Systems and has served as a data, safety, and monitoring board member for Merck-sponsored studies; she previously owned stock in Abbott Laboratories, Bristol-Myers Squibb, Johnson & Johnson, and Pfizer. C. M. M. receives royalties from Lippincott Williams and Wilkins and from UptoDate. D. B. C. has received support for consulting/advisory boards from Sanofi, Genentech, Quintiles, Inhibikase, Bristol-Myers Squibb, GlaxoSmithKline, Millennium, Biogen Idec, Amgen, Pfizer, AstraZeneca, Cytheris and Merck and receives research support from Lilly, Roche, Bavarian Nordic, Gilead, the Alzheimer Association and the National Institutes of Health (National Institute of Allergy and Infectious Diseases, National Institute of Mental Health, National Institute of Neurological Disorders and Stroke, National Institute on Aging, and National Institute of Nursing Research). J. A. M. authors chapters on HIV for the Merck Manual. D. M. S. has provided consultancy to Astellas, Acorda, Allergan, Merz, and Ipsen; has received speaking honoraria from Allergan and Acorda; and received research grants from Merz, Allergan, Ipsen, Acorda, and Astellas. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
Collaborators: for the CHARTER Study Group, Igor Grant, J. Allen McCutchan, Ronald J. Ellis, Thomas D. Marcotte, Donald Franklin, Terry Alexander, Scott Letendre, Edmund Capparelli, Robert K. Heaton, J. Hampton Atkinson, Steven Paul Woods, Matthew Dawson, David M. Smith, Christine Fennema-Notestine, Michael J. Taylor, Rebecca Theilmann, Anthony C. Gamst, Clint Cushman, Ian Abramson, Florin Vaida, Thomas D. Marcotte, Jennifer Marquie-Beck, Justin McArthur, Vincent Rogalski, Susan Morgello, David Simpson, Letty Mintz, J. Allen McCutchan, Will Toperoff, Ann Collier, Christina Marra, Trudy Jones, Benjamin Gelman, Eleanor Head, David Clifford, Muhammad Al-Lozi, and Mengesha Teshome
References
- 1.Redig AJ, Berliner N. Pathogenesis and clinical implications of HIV-related anemia in 2013. Hematology Am Soc Hematol Educ Program 2013; 2013:377–81. [DOI] [PubMed] [Google Scholar]
- 2.McArthur JC, Hoover DR, Bacellar H et al. Dementia in AIDS patients: incidence and risk factors. Multicenter AIDS Cohort Study. Neurology 1993; 43:2245–52. [DOI] [PubMed] [Google Scholar]
- 3.Gangat N, Wolanskyj AP. Anemia of chronic disease. Semin Hematol 2013; 50:232–8. [DOI] [PubMed] [Google Scholar]
- 4.Bozzi A, Brisdelli F, D'Alessandro AM et al. Effects of AZT on cellular iron homeostasis. Biometals 2004; 17:443–50. [DOI] [PubMed] [Google Scholar]
- 5.Mocroft A, Lifson AR, Touloumi G et al. Haemoglobin and anaemia in the SMART study. Antivir Ther 2011; 16:329–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marquine MJ, Umlauf A, Rooney AS et al. The Veterans Aging Cohort Study index is associated with concurrent risk for neurocognitive impairment. J Acquir Immune Defic Syndr 2014; 65:190–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hong CH, Falvey C, Harris TB et al. Anemia and risk of dementia in older adults: findings from the Health ABC study. Neurology 2013; 81:528–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pichler I, Del Greco MF, Gogele M et al. Serum iron levels and the risk of Parkinson disease: a Mendelian randomization study. PLoS Med 2013; 10:e1001462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crespo AC, Silva B, Marques L et al. Genetic and biochemical markers in patients with Alzheimer's disease support a concerted systemic iron homeostasis dysregulation. Neurobiol Aging 2014; 35:777–85. [DOI] [PubMed] [Google Scholar]
- 10.Qureshi AI, Hanson DL, Jones JL, Janssen RS. Estimation of the temporal probability of human immunodeficiency virus (HIV) dementia after risk stratification for HIV-infected persons. Neurology 1998; 50:392–7. [DOI] [PubMed] [Google Scholar]
- 11.Heaton RK, Franklin DR, Ellis RJ et al. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol 2011; 17:3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clifford DB, Ances BM. HIV-associated neurocognitive disorder. Lancet Infect Dis 2013; 13:976–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soontornniyomkij V, Umlauf A, Chung SA et al. HIV protease inhibitor exposure predicts cerebral small vessel disease. AIDS 2014; 28:1297–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ellis RJ, Badiee J, Vaida F et al. CD4 nadir is a predictor of HIV neurocognitive impairment in the era of combination antiretroviral therapy. AIDS 2011; 25:1747–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alexander HD, Sherlock JP, Bharucha C. Red cell indices as predictors of iron depletion in blood donors. Clin Lab Haematol 2000; 22:253–8. [DOI] [PubMed] [Google Scholar]
- 16.Koorts AM, Levay PF, Becker PJ, Viljoen M. Pro- and anti-inflammatory cytokines during immune stimulation: modulation of iron status and red blood cell profile. Mediators Inflamm 2011; doi:10.1155/2011/716301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sternfeld T, Lorenz A, Schmid M et al. Increased red cell corpuscular volume and hepatic mitochondrial function in NRTI-treated HIV infected patients. Curr HIV Res 2009; 7:336–9. [DOI] [PubMed] [Google Scholar]
- 18.Tsantes AE, Bonovas S, Travlou A, Sitaras NM. Redox imbalance, macrocytosis, and RBC homeostasis. Antioxid Redox Signal 2006; 8:1205–16. [DOI] [PubMed] [Google Scholar]
- 19.Geene D, Sudre P, Anwar D, Goehring C, Saaidia A, Hirschel B. Causes of macrocytosis in HIV-infected patients not treated with zidovudine: Swiss HIV Cohort Study. J Infect 2000; 40:160–3. [DOI] [PubMed] [Google Scholar]
- 20.Atamna H, Frey WH II. Mechanisms of mitochondrial dysfunction and energy deficiency in Alzheimer's disease. Mitochondrion 2007; 7:297–310. [DOI] [PubMed] [Google Scholar]
- 21.Heaton RK, Franklin DR Jr, Deutsch R et al. Neurocognitive change in the era of HIV combination antiretroviral therapy: the longitudinal CHARTER study. Clin Infect Dis 2015; 60:473–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McLean E, Cogswell M, Egli I, Wojdyla D, de Benoist B. Worldwide prevalence of anaemia, WHO Vitamin and Mineral Nutrition Information System, 1993–2005. Public Health Nutr 2009; 12:444–54. [DOI] [PubMed] [Google Scholar]
- 23.Grant I, Franklin DR Jr, Deutsch R et al. Asymptomatic HIV-associated neurocognitive impairment increases risk for symptomatic decline. Neurology 2014; 82:2055–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoffmann CJ, Fielding KL, Johnston V et al. Changing predictors of mortality over time from cART start: implications for care. J Acquir Immune Defic Syndr 2011; 58:269–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kerkhoff AD, Wood R, Vogt M, Lawn SD. Predictive value of anemia for tuberculosis in HIV-infected patients in sub-Saharan Africa: an indication for routine microbiological investigation using new rapid assays. J Acquir Immune Defic Syndr 2014; 66:33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van Geertruyden JP, Mulenga M, Chalwe V et al. Impact of HIV-1 infection on the hematological recovery after clinical malaria. J Acquir Immune Defic Syndr 2009; 50:200–5. [DOI] [PubMed] [Google Scholar]
- 27.Borges AH, Weitz JI, Collins G et al. Markers of inflammation and activation of coagulation are associated with anaemia in antiretroviral-treated HIV disease. AIDS 2014; 28:1791–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gedefaw L, Yemane T, Sahlemariam Z, Yilma D. Anemia and risk factors in HAART naive and HAART experienced HIV positive persons in south west Ethiopia: a comparative study. PLoS One 2013; 8:e72202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cassol E, Misra V, Dutta A, Morgello S, Gabuzda D. Cerebrospinal fluid metabolomics reveals altered waste clearance and accelerated aging in HIV patients with neurocognitive impairment. AIDS 2014; 28:1579–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kallianpur AR, Jia P, Ellis RJ et al. Genetic variation in iron metabolism is associated with neuropathic pain and pain severity in HIV-infected patients on antiretroviral therapy. PLoS One 2014; 9:e103123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Armitage AE, Stacey AR, Giannoulatou E et al. Distinct patterns of hepcidin and iron regulation during HIV-1, HBV, and HCV infections. Proc Natl Acad Sci U S A 2014; 111:12187–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Levine AJ, Miller JA, Shapshak P et al. Systems analysis of human brain gene expression: mechanisms for HIV-associated neurocognitive impairment and common pathways with Alzheimer's disease. BMC Med Genomics 2013; 6:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gongvatana A, Cohen RA, Correia S et al. Clinical contributors to cerebral white matter integrity in HIV-infected individuals. J Neurovirol 2011; 17:477–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kallianpur AR, Levine AJ. Host Genetic Factors Predisposing to HIV-Associated Neurocognitive Disorder. Curr HIV/AIDS Rep 2014; 11:336–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marra CM, Zhao Y, Clifford DB et al. Impact of combination antiretroviral therapy on cerebrospinal fluid HIV RNA and neurocognitive performance. AIDS 2009; 23:1359–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smurzynski M, Wu K, Letendre S et al. Effects of central nervous system antiretroviral penetration on cognitive functioning in the ALLRT cohort. AIDS 2011; 25:357–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Remacha AF, Cadafalch J, Sarda P, Barcelo M, Fuster M. Vitamin B-12 metabolism in HIV-infected patients in the age of highly active antiretroviral therapy: role of homocysteine in assessing vitamin B-12 status. Am J Clin Nutr 2003; 77:420–4. [DOI] [PubMed] [Google Scholar]
- 38.Robertson KR, Stern RA, Hall CD et al. Vitamin B12 deficiency and nervous system disease in HIV infection. Arch Neurol 1993; 50:807–11. [DOI] [PubMed] [Google Scholar]
- 39.Malik ZA, Abadi J, Sansary J, Rosenberg M. Elevated levels of vitamin B12 and folate in vertically infected children with HIV-1. AIDS 2009; 23:403–7. [DOI] [PubMed] [Google Scholar]
- 40.Shet A, Arumugam K, Rajagopalan N et al. The prevalence and etiology of anemia among HIV-infected children in India. Eur J Pediatr 2012; 171:531–40. [DOI] [PubMed] [Google Scholar]
- 41.Kim AH, Jang W, Kim Y, Park YJ, Han K, Oh EJ. Mean corpuscular volume (MCV) values reflect therapeutic effectiveness in zidovudine-receiving HIV patients. J Clin Lab Anal 2013; 27:373–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bovy C, Gothot A, Krzesinski JM, Beguin Y. Mature erythrocyte indices: new markers of iron availability. Haematologica 2005; 90:549–51. [PubMed] [Google Scholar]
- 43.Hulgan T, Samuels DC, Bush W et al. Mitochondrial DNA haplogroups and neurocognitive impairment during HIV infection. Clin Infect Dis 2015; 61:1476–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kaiser J. “Rejuvenation factor” in blood turns back the clock in old mice. Science 2014; 344:570–1. [DOI] [PubMed] [Google Scholar]
- 45.Horvath S, Levine AJ. HIV-1 infection accelerates age according to the epigenetic clock. J Infect Dis 2015; 212:1563–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Steele AK, Lee EJ, Vestal B et al. Contribution of intestinal barrier damage, microbial translocation and HIV-1 infection status to an inflammaging signature. PLoS One 2014; 9:e97171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lai S, Lai H, Celentano DD et al. Factors associated with accelerated atherosclerosis in HIV-1-infected persons treated with protease inhibitors. AIDS Patient Care STDS 2003; 17:211–9. [DOI] [PubMed] [Google Scholar]
- 48.Eyer-Silva WA, Arabe J, Pinto JF, Morais-De-Sa CA. Macrocytosis in patients on stavudine. Scand J Infect Dis 2001; 33:239–40. [DOI] [PubMed] [Google Scholar]
- 49.Wobeser W, Morgan E, Rumman A, Ford PM. Macrocytosis is a predictor of resting lactate concentrations in persons on dideoxynucleoside therapy for HIV infection. Int J Infect Dis 2012; 16:e225–7. [DOI] [PubMed] [Google Scholar]
- 50.Weiss G. Anemia of chronic disorders: new diagnostic tools and new treatment strategies. Semin Hematol 2015; 52:313–20. [DOI] [PubMed] [Google Scholar]


