Abstract
Opioids produce antinociception by activation of G protein signaling linked to the mu-opioid receptor (MOPr). However, opioid binding to the MOPr also activates β-arrestin signaling. Opioids such as DAMGO and fentanyl differ in their relative efficacy for activation of these signaling cascades, but the behavioral consequences of this differential signaling are not known. The purpose of this study was to evaluate the behavioral significance of G protein and internalization dependent signaling within ventrolateral periaqueductal gray (vlPAG). Antinociception induced by microinjecting DAMGO into the vlPAG was attenuated by blocking Gαi/o protein signaling with administration of pertussis toxin (PTX), preventing internalization with administration of dynamin dominant-negative inhibitory peptide (dyn-DN) or direct inhibition of ERK1/2 with administration of the MEK inhibitor, U0126. In contrast, the antinociceptive effect of microinjecting fentanyl into the vlPAG was not altered by administration of PTX or U0126, and was enhanced by administration of dyn-DN. Microinjection of DAMGO, but not fentanyl, into the vlPAG induced phosphorylation of ERK1/2, which was blocked by inhibiting receptor internalization with administration of dyn-DN, but not by inhibition of Gαi/o proteins. ERK1/2 inhibition also prevented the development and expression of tolerance to repeated DAMGO microinjections, but had no effect on fentanyl tolerance. These data reveal that ERK1/2 activation following MOPr internalization contributes to the antinociceptive effect of some (e.g., DAMGO), but not all opioids (e.g., fentanyl) despite the known similarities for these agonists to induce β-arrestin recruitment and internalization.
Keywords: analgesia, periaqueductal gray, functional selectivity, ERK1/2
1. Introduction
Mu opioid receptor (MOPr) agonists activate and inhibit a number of different intracellular signaling pathways. G protein signaling and the subsequent inhibition of downstream effectors, such as adenylyl cyclase, has been the most thoroughly characterized. In contrast much less is known about β-arrestin signaling following opioid binding. MOPr phosphorylation terminates G protein signaling and recruits β-arrestin to the receptor. β-arrestin binding leads to receptor internalization and activation of a distinct group of signaling proteins such as extracellular signal-regulated kinase (ERK1/2), which is well characterized in adrenergic receptors compared to opioid receptors (Lefkowitz and Shenoy, 2005, Shenoy and Lefkowitz, 2005, Drake et al., 2008). Recent studies have shown that some MOPr agonists such as fentanyl and [D-Ala2, N-MePhe4, Gly-ol]-enkephalin (DAMGO) have high efficacy to recruit β-arrestin and activate of G proteins, whereas other opioids such as morphine are biased toward G protein signaling (McPherson et al., 2010, Molinari et al., 2010, Kelly, 2013, Thompson et al., 2014).
Ligands with high efficacy for receptor internalization correlate inversely with susceptibility to tolerance (Madia et al., 2009) suggesting that β-arrestin signaling contributes to antinociception by preventing the development of tolerance (Finn and Whistler, 2001). Morphine produces limited β-arrestin recruitment and MOPr internalization compared to other opioids such as fentanyl or DAMGO (Williams et al., 2001, Christie, 2008, Williams et al., 2013), but maximal tolerance (He et al., 2002). Although tolerance is observed following administration of morphine, fentanyl, or DAMGO, the signaling proteins underlying tolerance appear to vary. Blockade of G protein associated signaling proteins (c-Jun N-terminal kinase or protein kinase C) prevents tolerance to morphine, but not DAMGO or fentanyl. Conversely, blockade of internalization-dependent signaling pathway prevents tolerance to fentanyl and DAMGO, but not morphine (Hull et al., 2010, Melief et al., 2010, Morgan et al., 2014).
Microinjection of morphine, fentanyl, or DAMGO into the ventrolateral periaqueductal gray (vlPAG) produces antinociception, and repeated administration leads to the development of tolerance (Morgan et al., 2006, Meyer et al., 2007, Bobeck et al., 2012). Although G protein signaling is known to contribute to the antinociceptive effect of opioids, the objective of the present study was to determine whether G protein independent (i.e. β-arrestin and ERK1/2) signaling following administration of these different opioids also contributes to antinociception. Despite minimal activation of ERK1/2 in vitro or in vivo following acute morphine administration, inhibition of ERK1/2 has been shown to prevent or enhance the development of morphine tolerance depending on the site of administration (Macey et al., 2009, Wang et al., 2010, Macey et al., 2014). Given that DAMGO and fentanyl activate ERK1/2 in vitro, we hypothesized that ERK1/2 is activated following DAMGO and fentanyl administration into the vlPAG, and inhibition of this signaling pathway prevents the development of tolerance.
2. Materials and Methods
2.1. Subjects
Male Sprague-Dawley rats (n = 220) weighing 220 – 360 g from Harlan Laboratories (Livermore, CA) were used. Rats were anesthetized with pentobarbital (60 mg/kg, i.p.) and implanted with a guide cannula (23 gauge; 9 mm long) aimed at the vlPAG using stereotaxic techniques (AP: +1.7 mm, ML: −0.6 mm, DV: −4.6 mm from lambda). Two screws were used to anchor the cannula to the skull with dental cement. A 9 mm stylet was inserted into the guide cannula following surgery. Rats were handled daily and allowed to recover for 1 week before testing. Rats were housed in groups of 2 – 5 until surgery and were housed individually on a reverse light cycle (lights off at 7:00 AM) after surgery. Food and water were available at all times except during experimental testing. All procedures were approved by the Washington State University Animal Care and Use Committee and conducted in accordance with the guidelines for animal use described by the International Association for the Study of Pain.
2.2. Behavioral testing
Drugs were administered through a 31-gauge injection cannula extending 2 mm beyond the guide cannula. One day prior to testing, the injector was inserted into the guide cannula without drug administration to habituate the rat to the microinjection procedure. To assess the role of Gαi/o protein signaling, receptor internalization-related signaling, or ERK1/2 activation, different groups of rats were microinjected into the vlPAG with G protein inhibitor pertussis toxin (PTX; 5 or 50 ng/0.4 µL), a myristoylated dominant negative dynamin inhibitory peptide (dyn-DN; 2 µg/0.4 µL) to block formation of the endosome, or a MEK1/2 inhibitor (U0126; 100 ng/0.5 µL) prior to administration of DAMGO or fentanyl. PTX or saline was administered one day prior to opioid administration, whereas U0126, 20% DMSO vehicle, dyn-DN, or the scrambled control peptide (dyn-scr, 2 µg/0.4 µL) were injected 20 minutes prior to opioid administration based on previous studies showing peak effects with microinjections into the vlPAG (Bodnar et al., 1990, Macey et al., 2009, Macey et al., 2010). In preliminary studies 24 hour pretreatment of 5 ng/0.5 µL was sufficient to attenuate morphine induced antinociception within the vlPAG (F(1,92) = 3.95, p < 0.05). Dyn-DN was injected into the vlPAG to disrupt MOPr internalization as we have reported previously using the fluorescent opioid peptide, dermorphin-A594 (Macey et al., 2010). In addition, a higher dose was needed to assure all internalization was blocked and preliminary data showed that dyn-DN (2 µg/0.4 µL) did not alter morphine antinociception (F(1, 98) = 1.88, p = 0.17).
A cumulative dosing procedure was used to assess the antinociceptive effects of DAMGO and fentanyl. Increasing doses of DAMGO were administered every 12 min resulting in third log doses of 0.046, 0.1, 0.22, 0.46, & 1 µg/0.4 µL. Nociception was assessed with the hot plate test 10 min after each injection. Fentanyl has a fast time course of action so was microinjected every 4 min resulting in third log doses of 0.46, 1, 2.2, 4.6, 10 µg/0.4 µL. The hot plate test was conducted 2 min after each injection. Our previous data show clear dose-dependent antinociception using this procedure (Bobeck et al., 2009).
Tolerance was induced in a separate group of rats by twice daily microinjections of DAMGO (0.5 µg/0.4 µL) or fentanyl (3 µg/0.4 µL) for two consecutive days. On Trial 1, the hot plate test was conducted 20 min after the DAMGO microinjection and 3 min after the fentanyl microinjection. To evaluate the role of ERK1/2 on the development of tolerance, a subset of rats received U0126 or vehicle (20% DMSO in saline, 0.5 µL) 20 min prior to each opioid injection on Trials 1 – 4. Tolerance was assessed on Trial 5 using the cumulative dosing procedure described above. To evaluate the expression of tolerance, a subset of rats received U0126 or vehicle 20 min prior to the cumulative dosing procedure in rats previously treated with twice daily microinjections of DAMGO or fentanyl for two days. We have shown previously that tolerance develops to vlPAG microinjections of DAMGO or fentanyl using this procedure (Meyer et al., 2007, Bobeck et al., 2012).
2.3 Histology and Data Analysis
Following testing, rats received a lethal dose of halothane. Brains were removed and stored in formalin (10%) and sliced coronally (100 µm) at least 2 days later to determine the injection site (Paxinos and Watson, 2005). Only those injection sites in or adjacent to the vlPAG were included in data analysis. Dose-response curves were plotted and the half maximal antinociceptive effect (D50) was calculated for each group using GraphPad (Prism 6). A unique control group was tested alongside the experimental groups for each experiment to control for variability between experiments. All comparisons were made with the control group within each experiment. Significance (α < 0.05) was determined using ANOVA or t-test where appropriate. Bonferroni posthoc analyses were used to compare means when necessary. Data are presented as mean ± SEM unless otherwise stated. To assess the homogeneity of variance the Brown-Forsythe test was used.
2.4. Drugs
All drugs were purchased from Tocris Bioscience except fentanyl citrate and U0126 (Sigma-Aldrich). DAMGO, fentanyl citrate, PTX, dyn-DN, and scr-dyn, were dissolved in sterile saline. U0126 was dissolved in 20% DMSO.
2.5. ERK1/2 Immunohistochemistry
A separate group of rats were deeply anesthetized with pentobarbital (150 mg/kg, i.p.) 20 min after opioid injection and then perfused transcardially through the ascending aorta with 10 mL heparinized saline followed by 400–600 mL of 4% paraformaldehyde in 0.1M phosphate buffer (PB). Brains were postfixed in 4% paraformaldehyde for 30 min and then stored in 0.1M PB for up to 15 hours. Immunohistochemistry was performed on coronal brain slices (40 µm) containing the vlPAG. Sections were incubated in 1% sodium borohydride in 0.1M PB for 30 min followed by another 30 min incubation with 0.3% H2O2 in 0.1M PB. The blocking reagent was then used: 0.5% bovine serum albumin in 0.1M Tris buffered saline for 30 min. The tissue was incubated in primary rabbit antibody against phospho-p44/42 MAPK (ERK 1/2) (1:400; Cell signaling, Beverly, MA) in 0.1M Tris buffered saline containing 0.1% bovine serum albumin and 0.25% TritonX-100, for 42 hours at 4°C. Bound ERK1/2 antibody was visualized with a diaminobenzidine hydrogen peroxidase (DAB-H2O2) reaction. Tissue was incubated in biotinylated goat-anti rabbit IgG secondary antibody (1:400 Vector Laboratories, Burlingame, CA) for 30 min. This was followed by incubation in Avidin-Biotin (Elite Vectastain ABC kit; Vector Laboratories) for 30 min and then DAB-H2O2 for 3 min. Brain slices were mounted, dehydrated and then coverslipped with DPX mounting medium (Sigma-Aldrich, St. Louis, MO). Sections containing the injection site were quantified within a 300 × 300 µm2 region. Depending on the location of the injection, up to 3 separate boxes were used to equal 90,000 µm2 area. To avoid damaged tissue, a region 50 µm away from injection site was chosen. Images were taken with an Olympus DP71 digital camera mounted on an Olympus BX51 microscope. The number of pERK-positive cells was assessed using ImageJ particle analysis to count cell bodies that were larger than 60 pixels2 (National Institutes of Health; Bethesda, MA).
3. Results
3.1. Inhibition of G proteins or internalization differentially alters DAMGO and fentanyl-induced antinociception
To investigate the mechanism of DAMGO and fentanyl induced antinociception, rats were pretreated with either G protein or internalization inhibitors prior to opioid microinjection into the vlPAG. There was no significant difference in baseline hot plate latencies prior to PTX or dyn-DN pretreatment (F(4, 72) = 0.24; p = 0.91) or between opioids (F(1,72) = 0.48; p = 0.49). Mean baseline hot plate latencies for these groups ranged from 14.8 ± 0.9 to 18.4 ± 1.4 s. Treatment with 5 or 50 ng PTX (15.8 ± 2.5 s, 13.37 ± 0.2 s, respectively) did not alter nociception compared to saline controls (15.7 ± 0.9 s; F(2, 49) = 0.54; p = 0.59). Similarly hot plate latencies following dyn-DN (14.9 ± 1.0 s) did not differ from scr-dyn controls (17.3 ± 3.1 s; (t(31) = 1.07; p = 0.29).
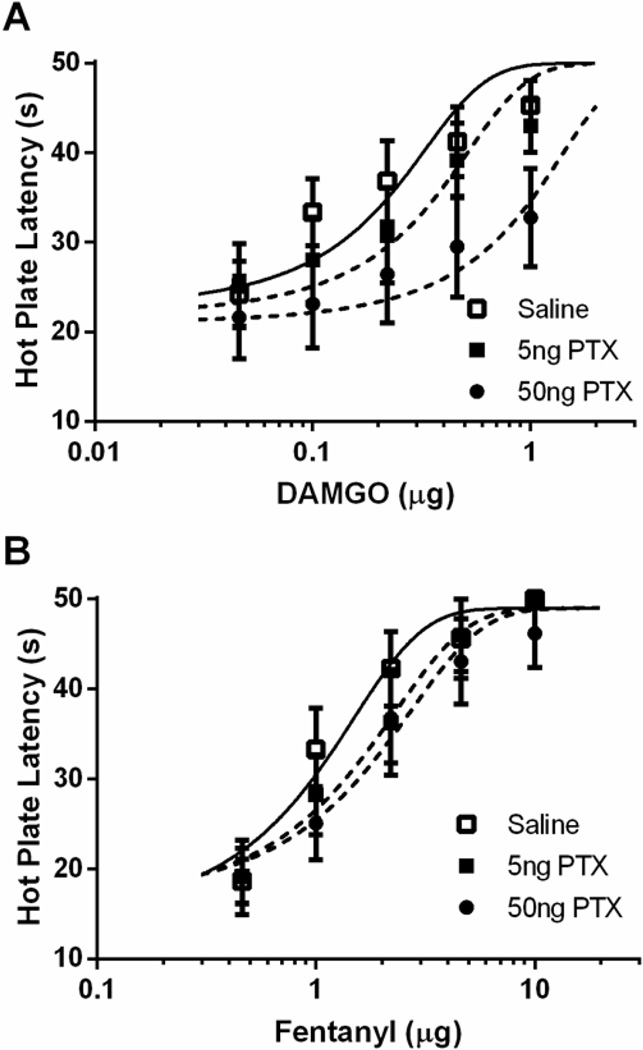
Administration of PTX significantly attenuated the antinociceptive effect of DAMGO, but not fentanyl. PTX caused a rightward shift in the DAMGO dose-response curve (Figure 1A; F(2, 150) = 9.09; p < 0.05), but only the high dose (50 ng) produced a statistically significant difference from saline pretreated rats. Pretreatment with PTX caused a small non-significant rightward shift in the fentanyl dose-response curve (Figure 1B; F(2, 138) = 2.15; p = 0.12).
Figure 1. Differential alteration in antinociception following G protein inhibition.
Pretreatment with PTX for 24 hours alters antinociception following vlPAG microinjections of DAMGO (A), but not fentanyl (B). Following pretreatment with 50 ng PTX, the DAMGO D50 (0.75 ± 0.45 µg; n = 8) was significantly different from saline treated rats (0.15 ± 0.07 µg; n = 10), however 5 ng PTX (0.26 ± 0.12 µg; n = 8) was not statistically different than saline controls. Pretreatment with PTX caused a slight rightward shift in the fentanyl D50 values following 5 ng (1.62 ± 0.53 µg; n = 8) and 50 ng (1.81 ± 0.67 µg; n = 8) of PTX compared to saline treated rats (1.10 ± 0.32 µg; n = 8), but it did not reach statistical significance.
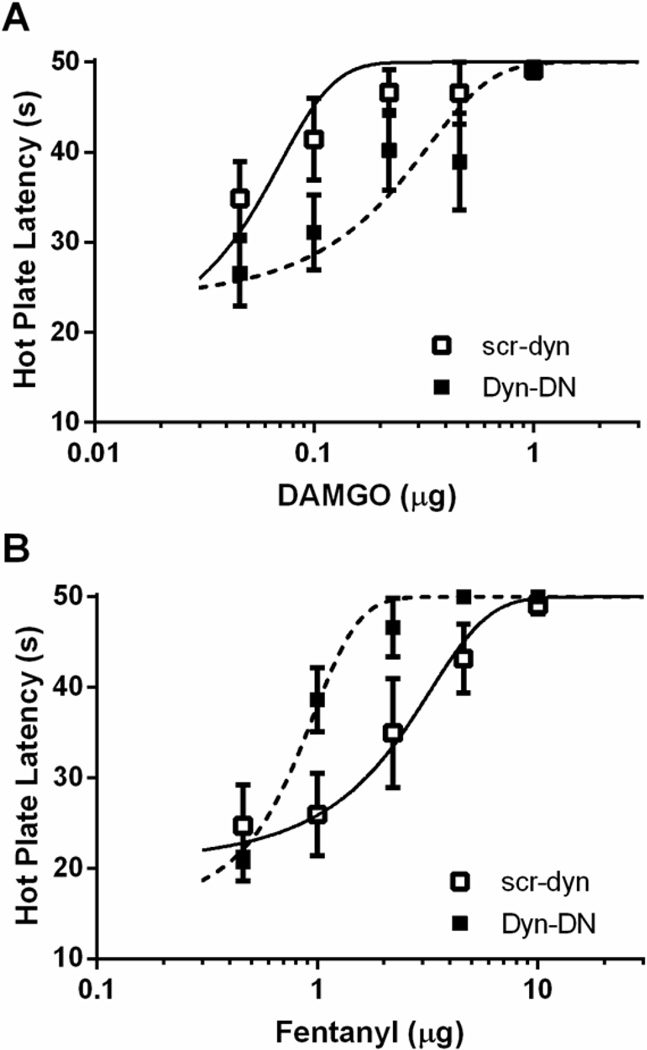
Pretreatment with dyn-DN also had opposite effects on DAMGO and fentanyl-induced antinociception. Administration of dyn-DN reduced DAMGO potency as evident by a rightward shift in the dose-response curve (Figure 2A; F(1,85) = 6.52; p < 0.05). In contrast, administration of dyn-DN enhanced fentanyl antinociception as evident by a leftward shift in the dose-response curve (Figure 2B; F(1, 98) = 13.48; p < 0.05).
Figure 2. Ligand-biased effects on antinociception following inhibition of internalization.
Pretreatment with dyn-DN (2 µg/0.5 µL) 20 min prior to opioid dose-response decreased DAMGO-induced antinociception (A), and enhanced fentanyl-induced antinociception (B) compared to scr-dyn (2 µg/0.5 µL). Pretreatment with dyn-DN shifted the DAMGO D50 to 0.14 ± 0.07 µg (n = 7) compared to pretreatment with scr-dyn (0.046 ± 0.02 µg; n = 8). Conversely, pretreatment with dyn-DN caused a leftward shift in the fentanyl D50 (0.77 ± 0.12 µg; n = 8) compared to saline controls (1.81 ± 0.73 µg; n = 10).
3.2. DAMGO, but not fentanyl, activates ERK1/2 in a dynamin dependent manner
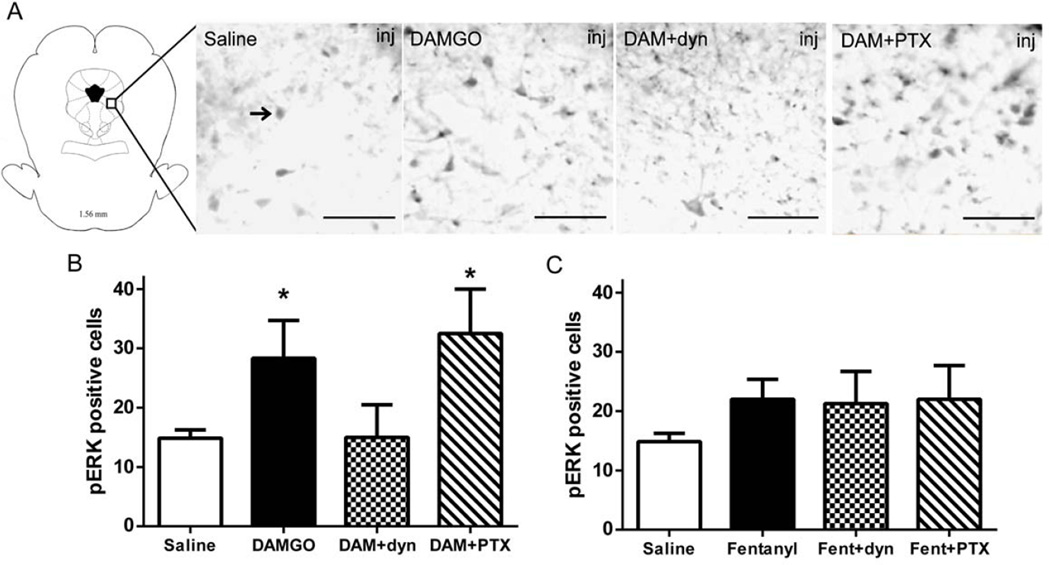
In vitro studies have shown that ERK1/2 can be activated following opioid exposure via G protein or β-arrestin signaling depending on the opioid (Belcheva et al., 2005, Macey et al., 2006, Zheng et al., 2008). To assess whether opioids induce ERK1/2 phosphorylation in vivo, DAMGO or fentanyl were microinjected into the vlPAG followed by immunohistochemical analysis of pERK1/2. DAMGO (n = 3) caused an increase in pERK1/2 immunoreactivity compared to saline (Figure 3A–D: F(3, 11) = 4.62; p < 0.05; n = 7). Microinjection of PTX (n=2) prior to DAMGO into the vlPAG also caused an increase in pERK positive cells compared to saline treated rats (Bonferroni, p < 0.05). In contrast, rats injected with dyn-DN (n = 3) 20 min prior to DAMGO showed a similar number of pERK1/2 positive cells as saline treated rats (Bonferroni, n.s.). The variance in each drug treated group was similar (F(3, 11) = 0.81, p = 0.51).
Figure 3. ERK1/2 activation in vlPAG following opioid microinjections.
Representative photomicrographs (A) of pERK1/2 immunoreactivity in vlPAG following pretreatment of saline, DAMGO, DAMGO+dyn-DN and DAMGO+PTX. Arrow designates a typical pERK1/2 positive cell. Scale bar = 100 µm. Quantification of pERK1/2 immunoreactivity 20 min following microinjection 0.5 µg/0.4 µL DAMGO (B), and 3 µg/0.4 µL fentanyl (C) into the vlPAG. A subset of rats were pretreated with dyn-DN (2 µg/0.5 µL) 20 min prior or PTX (50 ng/0.5 µL) 24 hours prior to opioid pretreatment. DAMGO, but not fentanyl, caused a significant increase in pERK1/2, which was prevented by pretreatment with dyn-DN. *-statistically different from saline.
Microinjection of fentanyl (n = 4) into the vlPAG produced a slight increase in the number of pERK1/2 positive cells compared to saline controls (n = 7), but this increase failed to reach significance, and this lack of an effect was not altered by pretreatment with PTX or dyn-DN (F(3, 14) = 1.24; p = 0.33, n = 3–4). The variance in each drug treated was similar (F(3, 14)= 1.22, p = 0.34).
3.3. ERK1/2 inhibition attenuates DAMGO antinociception and tolerance
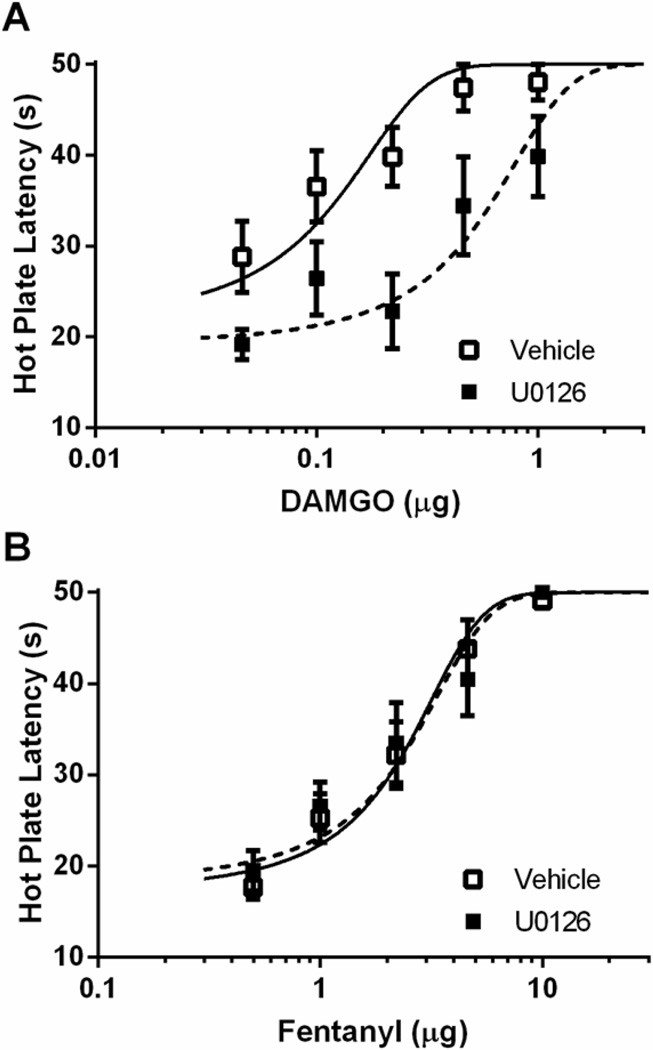
The finding above that DAMGO activation of ERK1/2 is prevented by administration of dyn-DN indicates that DAMGO activates ERK1/2 as a result of MOPr internalization. Moreover, these data raise the possibility that ERK1/2 activation also contributes to DAMGO antinociception and tolerance. This hypothesis was tested by microinjecting the ERK1/2 inhibitor U0126 (100 ng/0.5 µL) into the vlPAG. Administration of U0126 in the absence of an opioid had no effect on nociception compared to vehicle controls (t(45) = 0.97; p = 0.34), but attenuated the antinociceptive effect of DAMGO as indicated by a rightward shift in the DAMGO dose-response curve (Figure 4A; F(1, 98) = 34.10; p < 0.05). In contrast, administration of U0126 had no effect on the fentanyl dose-response curve (Figure 4B; F(1, 128) = .044; p = 0.834).
Figure 4. ERK1/2 inhibition decreases DAMGO-induced antinociception.
Rats were pretreated with 0.4 µL saline twice daily for two days. On Day 3, rats received an injection of vehicle (20% DMSO) or U0126 (100 ng/0.5 µL) 20 min prior to DAMGO (A) or fentanyl (B) dose-response. ERK inhibition caused a decrease in DAMGO-induced antinociception, but had no effect on fentanyl-induced antinociception.
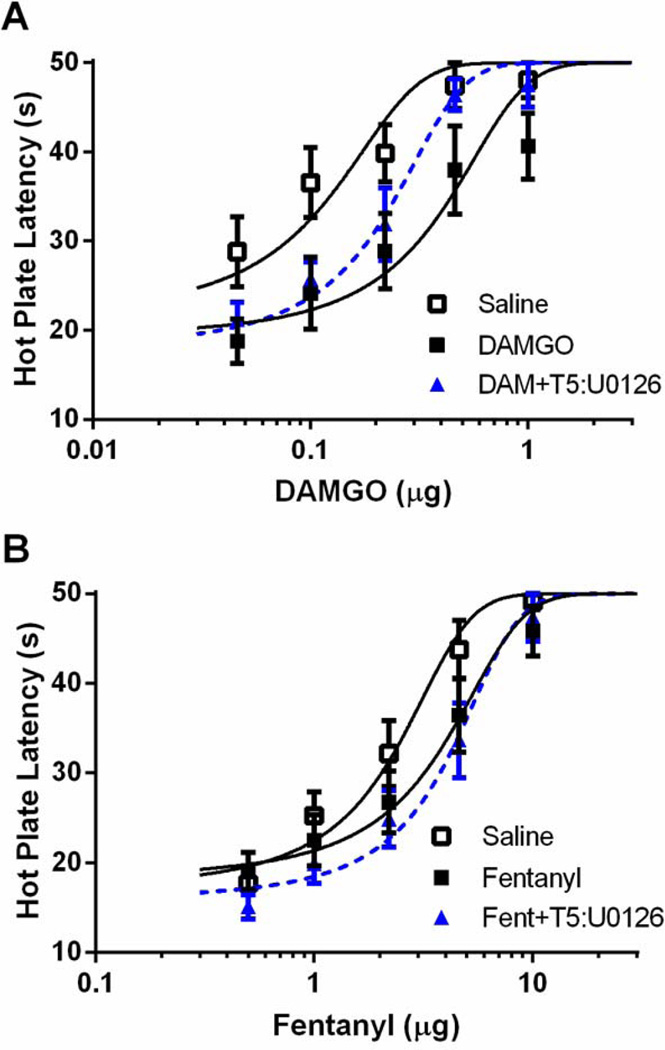
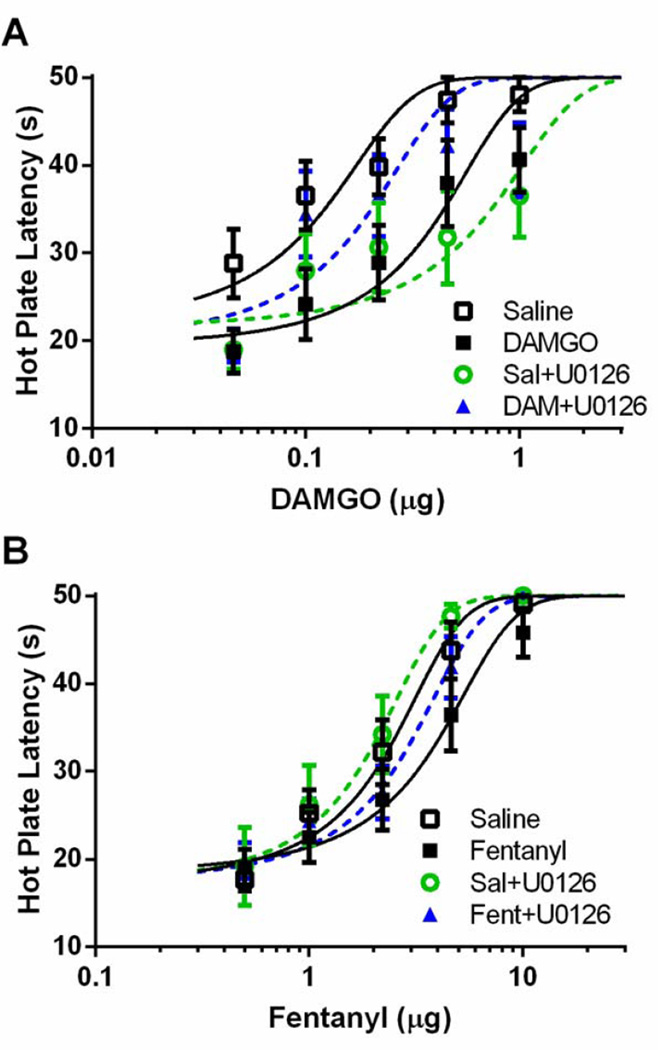
To assess the role of ERK1/2 on the expression of tolerance, U0126 was administered 20 min prior to administration of cumulative doses of DAMGO or fentanyl in rats tolerant to vlPAG microinjections of either opioid. Repeated DAMGO microinjections caused a rightward shift in the DAMGO dose-response curve compared to saline-treated controls. Tolerance to DAMGO was reversed by administration of U0126 prior to testing on Trial 5 (Figure 5A; F(2, 138) = 13.19; p < 0.05). Repeated microinjections of fentanyl also caused a rightward shift in the fentanyl dose response curve (e.g., tolerance) compared to saline treated controls, but microinjection of U0126 prior to fentanyl administration on Trial 5 had no effect on this shift (Figure 5B; F(2, 174) 8.03; p < 0.05).
Figure 5. ERK1/2 inhibition reverses the expression of DAMGO tolerance but does not alter fentanyl tolerance.
Rats were pretreated with 0.4 µL saline, 0.5 µg/0.4 µL DAMGO, or 3 µg/0.4 µL fentanyl twice daily for two days. On Day 3, rats received an injection of vehicle (20% DMSO) or U0126 (100 ng/0.5 µL) 20 min prior to DAMGO (A) or fentanyl (B) dose-response. The expression of DAMGO, but not fentanyl, tolerance was not reversed by ERK1/2 inhibition.
Furthermore, pretreatment with U0126 20 min prior to each DAMGO injection during tolerance induction prevented the development of tolerance to DAMGO (Figure 6A; F(3, 214) = 10.41; p < 0.05). Similar to what was found with a single injection of U0126, four injections of U0126 caused a rightward shift in the DAMGO dose-response curve in DAMGO-naïve rats injected repeatedly with saline as a control. The rightward shift in the fentanyl dose-response curve that occurred with repeated fentanyl microinjections (Figure 6B; F(3, 226) = 4.86; p < 0.05) was attenuated by administration of U0126 prior to each injection, but this shift in the dose response curve did not reach statistical significance. D50 values are shown in Table 1.
Figure 6. ERK1/2 inhibition prevents the development of tolerance to DAMGO, but not fentanyl.
Rats were pretreated with twice daily microinjections of saline (0.4 µL), DAMGO (0.5 µg/0.4 µL) or fentanyl (3 µg/0.4 µL). A subset of rats received a microinjection of U0126 20 min prior to each DAMGO or fentanyl microinjection. On Day 3, all rats recieved cumulative doses of DAMGO (A) or fentanyl (B). ERK1/2 inhibition in combination with DAMGO prevented the development of tolerance to DAMGO, but had no effect on the development of fentanyl tolerance.
Table 1.
Comparison of D50 values following ERK1/2 inhibition
| Pretreatment | DAMGO D50 ± C.I. (n) | Fentanyl D50 ± C.I. (n) |
|---|---|---|
| Saline | 0.087 ± 0.033 (9) | 2.16 ± 0.44 (11) |
| Opioid | 0.340 ± 0.125 (8)* | 3.42 ± 0.90 (10)* |
| U0126+Saline | 0.530 ± 0.252 (10)* | 1.79 ± 0.48 (8) |
| U0126+Opioid | 0.152 ± 0.064 (10)# | 2.62 ± 0.52 (10) |
| Saline+T5 U0126 | 0.482 ± 0.178 (8)* | 2.24 ± 0.58 (11) |
| Opioid+T5 U0126 | 0.196 ± 0.044 (7)# | 3.96 ± 0.82 (9)* |
Notes:
statistically different from saline (p < 0.05)
statistically different from opioid (p < 0.05)
4. Discussion
The current study found ligand-biased differences in opioid signaling underlying antinociception and tolerance mediated by the vlPAG. The antinociceptive effect of microinjecting DAMGO into the vlPAG was attenuated by blockade of G proteins, MOPr internalization, and ERK1/2 signaling. Blocking dynamin also blocked DAMGO-induced ERK1/2 activation suggesting that ERK1/2 signaling occurs following MOPr internalization. Furthermore, inhibition of ERK1/2 was associated with a reduction in both the expression and development of tolerance to DAMGO. In contrast, these molecular signaling pathways do not appear to contribute to fentanyl antinociception or tolerance. The antinociceptive effect of fentanyl in the vlPAG was enhanced by blocking MOPr internalization and was not dependent on G protein or ERK1/2 signaling. Likewise, ERK1/2 signaling does not appear to contribute to either the development or expression of fentanyl tolerance.
The antinociceptive effect of DAMGO in the vlPAG was attenuated by administration of 50, but not 5 ng of PTX. In contrast, PTX had no significant effect on fentanyl antinociception regardless of dose. A previous study found that 5 ng of PTX was sufficient to attenuate morphine antinociception (Bodnar et al., 1990). Taken together these results within the vlPAG are consistent with previous studies evaluating the role of Gαi/o proteins on antinociception using intracerebroventricular administration. Blockade of Gαi/o proteins with PTX or antisense oligodeoxynucleotides produced a ligand biased attenuation of antinociception in the order of morphine > DAMGO > sufentanil (a fentanyl analog) (Raffa et al., 1994, Goode and Raffa, 1997), which inversely correlated with agonist efficacy to induce antinociception as measured with irreversible antagonists or using a [35S]GTPγS assay (Mjanger and Yaksh, 1991, Ammer and Schulz, 1993, Traynor and Nahorski, 1995, Goode and Raffa, 1997, McPherson et al., 2010, Madia et al., 2012). It is possible that fentanyl-induced antinociception is differentially mediated through a different MOPr splice variant, Gα protein subtype, or even heterodimers (i.e. MOPr/DOPr). Fentanyl analogs have been shown to produce antinociception via Gαs proteins (Goode and Raffa, 1997, Sanchez-Blazquez et al., 2001). In addition fentanyl, but not morphine, antinociception is blocked following deletion of a particular exon on the MOPr, suggesting that certain agonists preferentially activate certain receptor variants (Oldfield et al., 2008, Pan et al., 2009, Xu et al., 2014). It is unknown what variants are present in the vlPAG. It is also possible that certain agonists activate heterodimers (such as MOPr/DOPr) that preferentially signal via ERK1/2 (Rozenfeld and Devi, 2007, Costantino et al., 2012).
It is well established that certain agonists cause robust MOPr phosphorylation and internalization. In particular, morphine is very weak at inducing MOPr internalization compared to other agonists such as DAMGO and fentanyl (Whistler et al., 1999, Borgland et al., 2003, Celver et al., 2004, McPherson et al., 2010, Melief et al., 2010). Given that both DAMGO and fentanyl produce rapid MOPr internalization, it is surprising that administration of dyn-DN, a GTPase that prevents the formation of endosomes (Herskovits et al., 1993), would have opposite effects. One explanation is that fentanyl is similar to morphine, an opioid that does not induce MOPr internalization, when microinjected into the vlPAG. The antinociceptive efficacy of fentanyl is similar to morphine when microinjected into the vlPAG (Bobeck et al., 2012), whereas its efficacy is much greater than morphine when administered systemically (Madia et al., 2009). We have shown previously that dyn-DN has no effect on morphine antinociception (Macey et al., 2014), whereas in the present study administration of dyn-DN potentiated fentanyl antinociception. Fentanyl produces a rapid (3 min) and short-lived (< 30 min) peak effect in comparison to morphine, DAMGO, and dermorphin, which show peak antinociception at 15–30 min and persists for more than one hour following vlPAG administration (Bobeck et al., 2009, Macey et al., 2010). Blocking MOPr internalization may potentiate fentanyl antinociception by prolonging signaling from the plasma membrane. In contrast, administration of dyn-DN to block MOPr internalization had the opposite effect on DAMGO antinociception. DAMGO-induced antinociception was attenuated by inhibiting dynamin suggesting that MOPr signaling following internalization contributes to antinociception. Although this would indicate a novel signaling mechanism for the MOPr, microinjection of dyn-DN into the vlPAG also attenuated the antinociceptive effect of injecting the high efficacy MOPr agonist dermorphin (Macey et al., 2010). Internalization of the MOPr could contribute to antinociception via β-arrestin signaling from the endosome or by rapid recycling of the receptor to the plasma membrane for additional signaling. Our findings that DAMGO activates ERK1/2 signaling in a dynamin dependent manner and inhibition of ERK1/2 activation attenuates DAMGO antinociception and tolerance indicates that MOPr signaling occurring after internalization contributes to antinociception.
Previous research has shown that ERK1/2 is activated following acute administration of fentanyl or DAMGO, but not morphine (Macey et al., 2006, Zheng et al., 2011, Duraffourd et al., 2014, Macey et al., 2014). Our current data show that fentanyl in the vlPAG caused a small increase in ERK1/2 activation, but this increase was less than that produced by DAMGO and was not attenuated by PTX or dyn-DN administration. It is possible that fentanyl does not activate ERK1/2 in the vlPAG even though fentanyl has this effect in heterologous cell systems and cultured striatal neurons (Macey et al., 2006, Zheng et al., 2008). Morphine has been shown to induce ERK1/2 activation in a brain-region specific manner: an increase in ERK1/2 activation has been reported in the anterior cingulate and locus coeruleus, whereas a decrease occurs in the nucleus accumbens and central amygdala (Eitan et al., 2003). Prolonged, but not acute, morphine treatment showed an increase in pERK1/2 within the vlPAG, but a decrease in a heterologous cell system (Bilecki et al., 2005, Macey et al., 2009, Macey et al., 2014).
Although ERK1/2 is typically considered to be activated in a β-arrestin dependent manner, some agonists such as morphine may activate ERK1/2 via a different signaling mechanism. For example, morphine activates ERK1/2 in a PKC and/or calmodulin dependent mechanism, whereas DAMGO and fentanyl use a dynamin or β-arrestin dependent pathway (Belcheva et al., 2005, Zheng et al., 2011, Duraffourd et al., 2014). The current study using PTX and dyn-DN confirmed that DAMGO activates ERK1/2 via a dynamin dependent mechanism within the vlPAG.
Studies investigating the role of ERK1/2 on morphine tolerance have found mixed results depending on injection site. Co-administration of intrathecal morphine with a MEK inhibitor attenuated the development of tolerance to morphine (Wang et al., 2010), whereas morphine tolerance was not altered by ERK1/2 inhibition following systemic administration (Mouledous, 2007). Inhibition of ERK1/2 activation enhances both the expression and development of tolerance to morphine within the vlPAG (Macey et al., 2009) revealing that activation of ERK1/2 may counteract tolerance. Taken together with the present study, ERK has distinct effects on tolerance within the vlPAG depending on the opioid injected: ERK1/2 activation counteracts (morphine), enhances (DAMGO), or has no effect (fentanyl) on tolerance to MOPr agonists. The previous finding that acute tolerance to DAMGO, but not morphine or fentanyl, was prevented by GRK inhibition (Hull et al., 2010) is consistent with our data given that ERK1/2 is thought to be downstream of GRK/β-arrestin signaling (Macey et al., 2006, Shenoy et al., 2006). The current study is the first to examine the role of ERK1/2 in tolerance to these other MOPr agonists.
It is unclear how phosphorylated ERK1/2 alters opioid function to contribute to DAMGO tolerance. ERK1/2 is known to alter several epigenetic markers and transcription factors including c-fos, brain-derived neurotrophic factor, and cAMP response element binding proteins (CREB) in several brain regions following opioid withdrawal (Wang et al., 2012, Ciccarelli et al., 2013). The adenylyl cyclase-cAMP-CREB pathway is upregulated following prolonged opioid exposure (Cao et al., 2010). ERK1/2 activation may contribute to this upregulation by activation of PKC, although the exact mechanism is unclear (Martin et al., 2011). ERK1/2 also has been found to directly increase synaptic vesicle exocytosis via calcium channels (Subramanian and Morozov, 2011). Given that opioids in the vlPAG produce antinociception by inhibiting GABA release (Depaulis et al., 1987, Morgan et al., 2003, Heinricher et al., 2009), an increase in GABA release via ERK1/2 inhibition would require a higher opioid dose to produce antinociception.
5. Conclusions
The present study shows that both G protein and dynamin/ERK1/2 related signaling contribute to MOPr mediated antinociception in a ligand-biased manner. DAMGO engages both G protein and β-arrestin signaling pathways within the vlPAG to facilitate antinociception and tolerance. DAMGO antinociception and tolerance is dependent on activation of ERK1/2, while fentanyl antinociception and tolerance is not. This adds to the growing body of research on ligand-biased signaling at the MOPr by revealing a distinct role for ERK1/2 using a behavioral approach.
Highlights.
Opioid induced antinociception is regulated by G protein dependent and independent signaling.
Extracellular signal-regulated kinase 1/2 is activated in a ligand-biased manner within the periaqueductal gray.
Antinociceptive tolerance to DAMGO, but not fentanyl, is attenuated by inhibition of ERK1/2.
Acknowledgments
This study was supported in part by the National Institute of Drug Abuse (DA015498; DA027625) and by funds provided for medical and biological research by the State of Washington Initiative Measure No. 171. The authors would like to thank Shauna Schoo, Rachel Reid, and Davina Fitzgibbon for technical assistance.
Abbreviations
- DAMGO
[D-Ala2, N-MePhe4, Gly-ol]-enkephalin
- dyn-DN
dominant negative dynamin inhibitory peptide
- ERK1/2
extracellular signal-regulated kinase 1 and 2
- PTX
pertussis toxin
- scr-dyn
scrambled control peptide
- vlPAG
ventrolateral periaqueductal gray
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ammer H, Schulz R. Alterations in the expression of G-proteins and regulation of adenylate cyclase in human neuroblastoma SH-SY5Y cells chronically exposed to low-efficacy mu-opioids. Biochem J. 1993;295(Pt 1):263–271. doi: 10.1042/bj2950263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belcheva MM, Clark AL, Haas PD, Serna JS, Hahn JW, Kiss A, Coscia CJ. Mu and kappa opioid receptors activate ERK/MAPK via different protein kinase C isoforms and secondary messengers in astrocytes. J Biol Chem. 2005;280:27662–27669. doi: 10.1074/jbc.M502593200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilecki W, Zapart G, Ligeza A, Wawrzczak-Bargiela A, Urbanski MJ, Przewlocki R. Regulation of the extracellular signal-regulated kinases following acute and chronic opioid treatment. Cell Mol Life Sci. 2005;62:2369–2375. doi: 10.1007/s00018-005-5277-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobeck EN, Haseman RA, Hong D, Ingram SL, Morgan MM. Differential development of antinociceptive tolerance to morphine and fentanyl is not linked to efficacy in the ventrolateral periaqueductal gray of the rat. J Pain. 2012;13:799–807. doi: 10.1016/j.jpain.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobeck EN, McNeal AL, Morgan MM. Drug dependent sex-differences in periaqueducatal gray mediated antinociception in the rat. Pain. 2009;147:210–216. doi: 10.1016/j.pain.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodnar RJ, Paul D, Rosenblum M, Liu L, Pasternak GW. Blockade of morphine analgesia by both pertussis and cholera toxins in the periaqueductal gray and locus coeruleus. Brain Res. 1990;529:324–328. doi: 10.1016/0006-8993(90)90845-3. [DOI] [PubMed] [Google Scholar]
- Borgland SL, Connor M, Osborne PB, Furness JB, Christie MJ. Opioid agonists have different efficacy profiles for G protein activation, rapid desensitization, and endocytosis of mu-opioid receptors. J Biol Chem. 2003;278:18776–18784. doi: 10.1074/jbc.M300525200. [DOI] [PubMed] [Google Scholar]
- Cao JL, Vialou VF, Lobo MK, Robison AJ, Neve RL, Cooper DC, Nestler EJ, Han MH. Essential role of the cAMP-cAMP response-element binding protein pathway in opiate-induced homeostatic adaptations of locus coeruleus neurons. Proc Natl Acad Sci U S A. 2010;107:17011–17016. doi: 10.1073/pnas.1010077107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celver J, Xu M, Jin W, Lowe J, Chavkin C. Distinct domains of the mu-opioid receptor control uncoupling and internalization. Mol Pharmacol. 2004;65:528–537. doi: 10.1124/mol.65.3.528. [DOI] [PubMed] [Google Scholar]
- Christie MJ. Cellular neuroadaptations to chronic opioids: tolerance, withdrawal and addiction. Br J Pharmacol. 2008;154:384–396. doi: 10.1038/bjp.2008.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccarelli A, Calza A, Santoru F, Grasso F, Concas A, Sassoe-Pognetto M, Giustetto M. Morphine withdrawal produces ERK-dependent and ERK-independent epigenetic marks in neurons of the nucleus accumbens and lateral septum. Neuropharmacology. 2013;70C:168–179. doi: 10.1016/j.neuropharm.2012.12.010. [DOI] [PubMed] [Google Scholar]
- Costantino CM, Gomes I, Stockton SD, Lim MP, Devi LA. Opioid receptor heteromers in analgesia. Expert Rev Mol Med. 2012;14:e9. doi: 10.1017/erm.2012.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depaulis A, Morgan MM, Liebeskind JC. GABAergic modulation of the analgesic effects of morphine microinjected in the ventral periaqueductal gray matter of the rat. Brain Res. 1987;436:223–228. doi: 10.1016/0006-8993(87)91665-9. [DOI] [PubMed] [Google Scholar]
- Drake MT, Violin JD, Whalen EJ, Wisler JW, Shenoy SK, Lefkowitz RJ. beta-arrestin-biased agonism at the beta2-adrenergic receptor. J Biol Chem. 2008;283:5669–5676. doi: 10.1074/jbc.M708118200. [DOI] [PubMed] [Google Scholar]
- Duraffourd C, Kumala E, Anselmi L, Brecha NC, Sternini C. Opioid-induced mitogen-activated protein kinase signaling in rat enteric neurons following chronic morphine treatment. PloS one. 2014;9:e110230. doi: 10.1371/journal.pone.0110230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eitan S, Bryant CD, Saliminejad N, Yang YC, Vojdani E, Keith D, Jr, Polakiewicz R, Evans CJ. Brain region-specific mechanisms for acute morphine-induced mitogen-activated protein kinase modulation and distinct patterns of activation during analgesic tolerance and locomotor sensitization. J Neurosci. 2003;23:8360–8369. doi: 10.1523/JNEUROSCI.23-23-08360.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn AK, Whistler JL. Endocytosis of the mu opioid receptor reduces tolerance and a cellular hallmark of opiate withdrawal. Neuron. 2001;32:829–839. doi: 10.1016/s0896-6273(01)00517-7. [DOI] [PubMed] [Google Scholar]
- Goode TL, Raffa RB. An examination of the relationship between mu-opioid antinociceptive efficacy and G-protein coupling using pertussis and cholera toxins. Life Sci. 1997;60:PL107–PL113. doi: 10.1016/s0024-3205(96)00684-4. [DOI] [PubMed] [Google Scholar]
- He L, Fong J, von Zastrow M, Whistler JL. Regulation of opioid receptor trafficking and morphine tolerance by receptor oligomerization. Cell. 2002;108:271–282. doi: 10.1016/s0092-8674(02)00613-x. [DOI] [PubMed] [Google Scholar]
- Heinricher MM, Tavares I, Leith JL, Lumb BM. Descending control of nociception: Specificity, recruitment and plasticity. Brain Res Rev. 2009;60:214–225. doi: 10.1016/j.brainresrev.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herskovits JS, Burgess CC, Obar RA, Vallee RB. Effects of mutant rat dynamin on endocytosis. J Cell Biol. 1993;122:565–578. doi: 10.1083/jcb.122.3.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull LC, Llorente J, Gabra BH, Smith FL, Kelly E, Bailey C, Henderson G, Dewey WL. The effect of protein kinase C and G protein-coupled receptor kinase inhibition on tolerance induced by mu-opioid agonists of different efficacy. J Pharmacol Exp Ther. 2010;332:1127–1135. doi: 10.1124/jpet.109.161455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly E. Efficacy and ligand bias at the mu-opioid receptor. Br J Pharmacol. 2013;169:1430–1446. doi: 10.1111/bph.12222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefkowitz RJ, Shenoy SK. Transduction of receptor signals by beta-arrestins. Science. 2005;308:512–517. doi: 10.1126/science.1109237. [DOI] [PubMed] [Google Scholar]
- Macey TA, Bobeck EN, Hegarty DM, Aicher SA, Ingram SL, Morgan MM. ERK1/2 activation counteracts morphine tolerance in the periaqueductal gray of the rat. J Pharmacol Exp Ther. 2009 doi: 10.1124/jpet.109.152157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macey TA, Bobeck EN, Suchland KL, Morgan MM, Ingram SL. Change in functional selectivity of morphine with the development of antinociceptive tolerance. Br J Pharmacol. 2014 doi: 10.1111/bph.12703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macey TA, Ingram SL, Bobeck EN, Hegarty DM, Aicher SA, Arttamangkul S, Morgan MM. Opioid receptor internalization contributes to dermorphin-mediated antinociception. Neuroscience. 2010;168:543–550. doi: 10.1016/j.neuroscience.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macey TA, Lowe JD, Chavkin C. Mu opioid receptor activation of ERK1/2 is GRK3 and arrestin dependent in striatal neurons. J Biol Chem. 2006;281:34515–34524. doi: 10.1074/jbc.M604278200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madia PA, Dighe SV, Sirohi S, Walker EA, Yoburn BC. Dosing protocol and analgesic efficacy determine opioid tolerance in the mouse. Psychopharmacology (Berl) 2009 doi: 10.1007/s00213-009-1673-6. [DOI] [PubMed] [Google Scholar]
- Madia PA, Navani DM, Yoburn BC. [(35)S]GTPgammaS binding and opioid tolerance and efficacy in mouse spinal cord. Pharmacol Biochem Behav. 2012;101:155–165. doi: 10.1016/j.pbb.2011.11.001. [DOI] [PubMed] [Google Scholar]
- Martin F, Mora L, Laorden M, Milanes M. Protein kinase C phosphorylates the cAMP response element binding protein in the hypothalamic paraventricular nucleus during morphine withdrawal. Br J Pharmacol. 2011;163:857–875. doi: 10.1111/j.1476-5381.2011.01287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherson J, Rivero G, Baptist M, Llorente J, Al-Sabah S, Krasel C, Dewey WL, Bailey CP, Rosethorne EM, Charlton SJ, Henderson G, Kelly E. mu-opioid receptors: correlation of agonist efficacy for signalling with ability to activate internalization. Mol Pharmacol. 2010;78:756–766. doi: 10.1124/mol.110.066613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melief EJ, Miyatake M, Bruchas MR, Chavkin C. Ligand-directed c-Jun N-terminal kinase activation disrupts opioid receptor signaling. Proc Natl Acad Sci U S A. 2010;107:11608–11613. doi: 10.1073/pnas.1000751107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer PJ, Fossum EN, Ingram SL, Morgan MM. Analgesic tolerance to microinjection of the mu-opioid agonist DAMGO into the ventrolateral periaqueductal gray. Neuropharmacology. 2007;52:1580–1585. doi: 10.1016/j.neuropharm.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mjanger E, Yaksh TL. Characteristics of dose-dependent antagonism by beta-funaltrexamine of the antinociceptive effects of intrathecal mu agonists. J Pharmacol Exp Ther. 1991;258:544–550. [PubMed] [Google Scholar]
- Molinari P, Vezzi V, Sbraccia M, Gro C, Riitano D, Ambrosio C, Casella I, Costa T. Morphine-like opiates selectively antagonize receptor-arrestin interactions. J Biol Chem. 2010;285:12522–12535. doi: 10.1074/jbc.M109.059410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan MM, Clayton CC, Lane DA. Behavioral evidence linking opioid-sensitive GABAergic neurons in the ventrolateral periaqueductal gray to morphine tolerance. Neuroscience. 2003;118:227–232. doi: 10.1016/s0306-4522(02)00822-9. [DOI] [PubMed] [Google Scholar]
- Morgan MM, Fossum EN, Levine CS, Ingram SL. Antinociceptive tolerance revealed by cumulative intracranial microinjections of morphine into the periaqueductal gray in the rat. Pharmacol Biochem Behav. 2006;85:214–219. doi: 10.1016/j.pbb.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Morgan MM, Reid RA, Saville KA. Functionally selective signaling for morphine and fentanyl antinociception and tolerance mediated by the rat periaqueductal gray. PloS one. 2014;9:e114269. doi: 10.1371/journal.pone.0114269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouledous L, Diaz, Miguel F, Gutstein, Howard B. Extracellular signal-regulated kinase (ERK) inhibition does not prevent the development or expression of tolerance to and dependence on morphine in the mouse. Pharmacology, Biochemistry, and Behavior. 2007;88:39–46. doi: 10.1016/j.pbb.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield S, Braksator E, Rodriguez-Martin I, Bailey CP, Donaldson LF, Henderson G, Kelly E. C-terminal splice variants of the mu-opioid receptor: existence, distribution and functional characteristics. J Neurochem. 2008;104:937–945. doi: 10.1111/j.1471-4159.2007.05057.x. [DOI] [PubMed] [Google Scholar]
- Pan YX, Xu J, Xu M, Rossi GC, Matulonis JE, Pasternak GW. Involvement of exon 11-associated variants of the mu opioid receptor MOR-1 in heroin, but not morphine, actions. Proc Natl Acad Sci U S A. 2009;106:4917–4922. doi: 10.1073/pnas.0811586106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson SJ. The rat brain, in stereotaxic coordinates. Sydney: Academic Press; 2005. [Google Scholar]
- Raffa RB, Martinez RP, Connelly CD. G-protein antisense oligodeoxyribonucleotides and mu-opioid supraspinal antinociception. Eur J Pharmacol. 1994;258:R5–R7. doi: 10.1016/0014-2999(94)90073-6. [DOI] [PubMed] [Google Scholar]
- Rozenfeld R, Devi LA. Receptor heterodimerization leads to a switch in signaling: beta-arrestin2-mediated ERK activation by mu-delta opioid receptor heterodimers. Faseb J. 2007;21:2455–2465. doi: 10.1096/fj.06-7793com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Blazquez P, Gomez-Serranillos P, Garzon J. Agonists determine the pattern of G-protein activation in mu-opioid receptor-mediated supraspinal analgesia. Brain Res Bull. 2001;54:229–235. doi: 10.1016/s0361-9230(00)00448-2. [DOI] [PubMed] [Google Scholar]
- Shenoy SK, Drake MT, Nelson CD, Houtz DA, Xiao K, Madabushi S, Reiter E, Premont RT, Lichtarge O, Lefkowitz RJ. beta-Arrestin-dependent, G Protein-independent ERK1/2 Activation by the beta2 Adrenergic Receptor. J Biol Chem. 2006;281:1261–1273. doi: 10.1074/jbc.M506576200. [DOI] [PubMed] [Google Scholar]
- Shenoy SK, Lefkowitz RJ. Seven-transmembrane receptor signaling through beta-arrestin. Sci STKE. 2005;2005:cm10. doi: 10.1126/stke.2005/308/cm10. [DOI] [PubMed] [Google Scholar]
- Subramanian J, Morozov A. Erk1/2 inhibit synaptic vesicle exocytosis through L-type calcium channels. J Neurosci. 2011;31:4755–4764. doi: 10.1523/JNEUROSCI.6594-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson GL, Kelly E, Christopoulos A, Canals M. Novel GPCR paradigms at the mu-opioid receptor. Br J Pharmacol. 2014 doi: 10.1111/bph.12600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traynor JR, Nahorski SR. Modulation by mu-opioid agonists of guanosine-5'-O-(3-[35S]thio)triphosphate binding to membranes from human neuroblastoma SH-SY5Y cells. Mol Pharmacol. 1995;47:848–854. [PubMed] [Google Scholar]
- Wang WS, Kang S, Liu WT, Li M, Liu Y, Yu C, Chen J, Chi ZQ, He L, Liu JG. Extinction of aversive memories associated with morphine withdrawal requires ERK-mediated epigenetic regulation of brain-derived neurotrophic factor transcription in the rat ventromedial prefrontal cortex. J Neurosci. 2012;32:13763–13775. doi: 10.1523/JNEUROSCI.1991-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Ma W, Chabot JG, Quirion R. Calcitonin gene-related peptide as a regulator of neuronal CaMKII-CREB, microglial p38-NFkappaB and astroglial ERK-Stat1/3 cascades mediating the development of tolerance to morphine-induced analgesia. Pain. 2010;151:194–205. doi: 10.1016/j.pain.2010.07.006. [DOI] [PubMed] [Google Scholar]
- Whistler JL, Chuang HH, Chu P, Jan LY, von Zastrow M. Functional dissociation of mu opioid receptor signaling and endocytosis: implications for the biology of opiate tolerance and addiction. Neuron. 1999;23:737–746. doi: 10.1016/s0896-6273(01)80032-5. [DOI] [PubMed] [Google Scholar]
- Williams JT, Christie MJ, Manzoni O. Cellular and synaptic adaptations mediating opioid dependence. Physiol Rev. 2001;81:299–343. doi: 10.1152/physrev.2001.81.1.299. [DOI] [PubMed] [Google Scholar]
- Williams JT, Ingram SL, Henderson G, Chavkin C, von Zastrow M, Schulz S, Koch T, Evans CJ, Christie MJ. Regulation of mu-opioid receptors: desensitization, phosphorylation, internalization, and tolerance. Pharmacol Rev. 2013;65:223–254. doi: 10.1124/pr.112.005942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Xu M, Bolan E, Gilbert AK, Pasternak GW, Pan YX. Isolating and characterizing three alternatively spliced mu opioid receptor variants: mMOR-1A, mMOR-1O, and mMOR-1P. Synapse. 2014;68:144–152. doi: 10.1002/syn.21727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Chu J, Zhang Y, Loh HH, Law PY. Modulating micro-opioid receptor phosphorylation switches agonist-dependent signaling as reflected in PKCepsilon activation and dendritic spine stability. J Biol Chem. 2011;286:12724–12733. doi: 10.1074/jbc.M110.177089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Loh HH, Law PY. Beta-arrestin-dependent mu-opioid receptor-activated extracellular signal-regulated kinases (ERKs) Translocate to Nucleus in Contrast to G protein-dependent ERK activation. Mol Pharmacol. 2008;73:178–190. doi: 10.1124/mol.107.039842. [DOI] [PMC free article] [PubMed] [Google Scholar]