Abstract
While various approaches have been proposed in clinical trials aimed at improving motor function after spinal cord injury in humans, there is still limited information regarding the scope, methodological quality, and evidence associated with single-intervention and multi-intervention approaches. A systematic review performed using the PubMed search engine and the key words “spinal cord injury motor recovery” identified 1973 records, of which 39 were selected (18 from the search records and 21 from reference list inspection). Study phase (clinicaltrials.org criteria) and methodological quality (Cochrane criteria) were assessed. Studies included proposed a broad range of single-intervention (encompassing cell therapies, pharmacology, electrical stimulation, rehabilitation) (encompassing cell therapies, pharmacology, electrical stimulation, rehabilitation) and multi-intervention approaches (that combined more than one strategy). The highest evidence level was for Phase III studies supporting the role of multi-intervention approaches that contained a rehabilitation component. Quality appraisal revealed that the percentage of selected studies classified with high risk of bias by Cochrane criteria was as follows: random sequence generation = 64%; allocation concealment = 77%; blinding of participants and personnel = 69%; blinding of outcome assessment = 64%; attrition = 44%; selective reporting = 44%. The current literature contains a high proportion of studies with a limited ability to measure efficacy in a valid manner because of low methodological strength in all items of the Cochrane risk of bias assessment. Recommendations to decrease bias are discussed and include increased methodological rigor in the study design and recruitment of study participants, and the use of electrophysiological and imaging measures that can assess functional integrity of the spinal cord (and may be sufficiently sensitive to detect changes that occur in response to therapeutic interventions).
Key words: : cell transplantation, electrophysiology, human studies, rehabilitation, spinal cord injury
Introduction
An estimated 30 persons sustain an injury to the spinal cord every day in the United States.1 There are approximately 2 million persons living with the consequences of spinal cord injury (SCI) worldwide, a relatively low number thought to reflect the higher mortality associated with acute SCI in developing countries.2 Injury to the spinal cord greatly disrupts information between the supraspinal centers and muscles, leading to varying degrees of paralysis that greatly impact one's functional ability and quality of life.
Despite the great advances in clinical management (including surgical decompression of the spinal cord, pharmacology, and rehabilitation) and investigational efforts, recovery of motor function is still considered limited. The International Standards for Neurological Classification System for Spinal Cord Injury (ISNCSCI) developed by the American Spinal Injury Association (ASIA)3,4 is the most widely used system for the classification of residual neurologic function after SCI. According to the ISNCSCI, approximately 55% of cases are incomplete (i.e., presence of varying degrees of sensory or motor activity below the neurological level of injury), and in the remaining 45% of cases, there is no sensory and/or motor function in the S4-5 sacral segments (classified as motor-complete) as determined by the motor and sensory testing.
Traditionally in SCI research, potential therapeutic approaches targeting motor-incomplete lesions focus on harnessing the neural plasticity of the spared axonal fibers for the activation of muscles below the injury level. While neurological improvements do occur, recovery of function is still variable and difficult to predict. The greatest challenge for the recovery of motor function, however, is axonal regeneration across the injury site and the formation of new functional synapses, the overarching goal of approaches targeting motor-complete SCI. While there is evidence of partial tissue sparing even after complete SCI,5 the adult human injured spinal cord constitutes an inhospitable environment for regeneration because of many factors, including the limited axonal growth response that is because of the presence of many molecules in the myelin debris and glial scar tissue that cause growth cone collapse.6
In addition to the challenges imposed by the lesion itself, the success of potential therapeutic approaches has been linked to the adoption of better practices in scientific design, methodology of research studies, and the development of sensitive outcome measures of spinal cord integrity and residual connectivity.2 These have to complement improved clinical assessments to better characterize the effects of the injury on the nervous system and changes that occur in response to therapeutic interventions.2,7 In addition, the use of combinatorial approaches8,9 may be more effective than single-intervention approaches by simultaneously targeting different injury mechanisms. There is recent promising evidence demonstrating recovery of stepping in rats with a transected spinal cord after a combinatorial approach incorporating locomotor training with electrical neuromodulation and a pharmacology agent.10 There have not been many practical efforts in this direction in humans, however.
The aims of this review were to: (1) perform an appraisal of the methodology of studies targeted at improving motor function in persons with acute and chronic SCI; (2) identify the scope and evidence level associated with single-intervention (encompassing cell therapies, pharmacology, electrical stimulation, rehabilitation) and multi-intervention approaches (that combined more than one strategy) published in the literature to date; (3) identify the number of studies that incorporated an evaluation of spinal cord integrity and residual connectivity; (4) describe potential sources of bias in the selected studies; and (5) make recommendations for future clinical trials in SCI.
Methods
On October 1, 2014, a PubMed search using the following key words “spinal cord injury motor recovery” was performed to identify studies aimed at improving motor function after SCI. In addition, on January 1, 2015, a second search was performed using the following key words “spinal cord injury motor recovery” AND each of the following: “cell therapies,” “electric stimulation,” “pharmacology,” and “rehabilitation.” A filter was applied to limit the search to studies performed in humans. Studies were considered for eligibility if they were written in English and were published from 1990 until the search date (01/10/14). The remaining criteria for inclusion and exclusion can be seen on Table 1, and the protocol for the present review is publicly available.11 Abstracts were screened for eligibility, and relevant studies were reviewed in full by two independent trained examiners (JGO and MC), and discrepancies were resolved in monthly meetings with a third author (APL). Reference lists of the included articles were screened to identify potential articles not captured by the initial search.
Table 1.
Inclusion and Exclusion Criteria Used in the Present Study
| Inclusion criteria | Exclusion criteria | |
|---|---|---|
| Population and condition | Adult human participants (19–70 years, sample size >5 participants) with a history of traumatic SCI: acute/subacute (defined as within min to 12 months post-injury) and/or chronic (after 12 months post-injury). | Studies exclusively in adolescents and children (age <19 years old); Case studies/case series (defined as having a sample size <5 participants); studies performed in animal models or experimental models of SCI; studies including individuals with nontraumatic SCI; studies with participants who have other neurologic, cognitive or orthopedic conditions associated with the SCI (metastatic cancer, etc). |
| Interventions | Cell therapies, pharmacology approaches, electrical stimulation/neuroelectric devices, rehabilitation or a combination of therapies including any of the above strategies. | Studies that: were not designed to assess the effects of an intervention (observational studies, studies carried out to assess an outcome measure); did not include a performance-based measure of motor function; assessed gangliosides and methylprednilosone, surgical decompression of the spinal cord, or were not available in full text. |
| Comparisons of interest | Intervention vs. active or inactive control; pre-intervention/post-intervention (for studies that did not include a control group). | None |
| Study design | Studies with an active control group (comparison group) were preferred, but studies with inactive controls (placebo or wait-list control), and safety/feasibility studies were also included. | Observational studies; studies carried out using retrospective analysis of clinical findings and studies with no original data (reviews) |
| Timing/setting | Longitudinal studies that occur in general and clinical settings. | None |
SCI, spinal cord injury.
This review was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis statement.12 Data extraction of the included studies was performed in adherence to the population intervention comparison outcome framework,12 wherein recommended study characteristics were collected (methods, interventions, participants, and outcomes) using the Revman 5 software (version 5.1, Cochrane Collaboration, Canada) and tables using Excel (Microsoft, Redmond, WA). A semi-quantitative analysis was performed by classifying studies according to the approach used (cell therapy, pharmacology, rehabilitation, electrical stimulation/neuroelectric device, combinatorial), stage of SCI (acute/subacute = study enrollment within minutes to 12 months post-injury; and chronic = study enrollment after 12 months post-injury) and the absence/presence of an assessment of spinal integrity/residual connectivity. Studies were classified according to the phase as per clinicaltrial.org criteria (Table 2).
Table 2.
Criteria Used to Classify Study Phasea
| Study phase | Description |
|---|---|
| I | Study carried out to assess a new approach in a small sample for the first time to evaluate its safety, determine a safe dosage range, and identify side effects |
| II | Approach is given to a larger sample to see if it is effective, further evaluate safety, and determine optimal dose |
| III | Approach is to a large sample to confirm effectiveness, monitor side effects, make comparisons to commonly used treatments, and collect information that will allow the approach to be used safely |
| IV | Studies are done after the drug or treatment has been marketed to gather information on the drug's effect in various populations and any side effects associated with long-term use |
I, II, II, IV, from clinicaltrial.org.
Study quality appraisal and risk of bias assessment were performed as per the Cochrane Handbook for Systematic Reviews and Interventions.13 A “risk of bias” table was constructed with all included studies, containing a description and judgment (low risk, high risk, or unclear risk) for the following potential sources of bias: (1) random sequence generation; (2) allocation concealment; (3) blinding of participants and personnel; (4) blinding of outcome assessment; (5) incomplete outcome data; (6) selective reporting; (7) other sources of bias.
Results
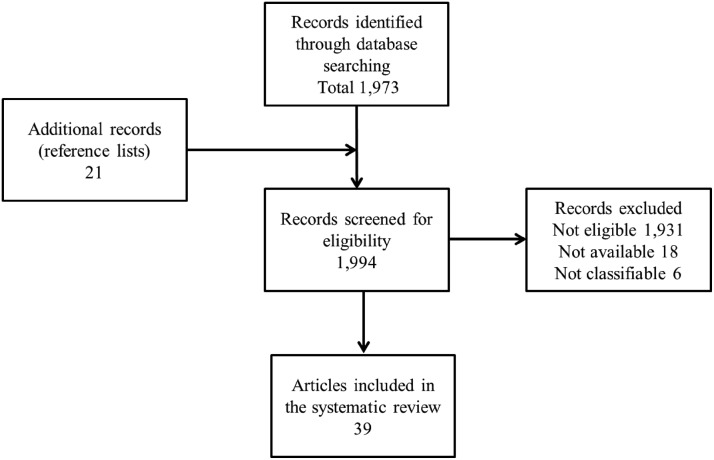
The first and second searches yielded 1973 results (Fig. 1). On examination, 1931 citations that were not relevant to the topic, reviews or opinion articles were excluded. Upon further examination, 24 articles (18 that did not fulfill our inclusion criteria and 6 articles that were not classifiable) were excluded. Eighteen full-text articles met the inclusion criteria. Within the process of the review, 21 additional studies, which pertained to the topic but were not identified in the PubMed search, were included in the study, resulting in the inclusion of 39 full-text articles.
FIG. 1.
Flow chart displaying the search strategy.
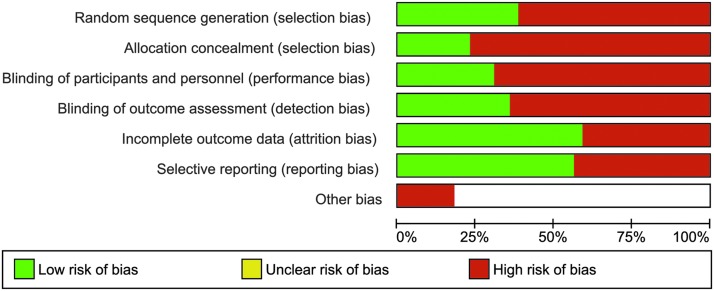
Quality assessment for all studies included can be seen in Figure 2a, and individualized scoring of each study for multiple sources of bias assessed is displayed in Figure 2b. Fourteen (36%) publications had low risk of bias, and the remaining 25 (64%) studies had high risk of bias for random sequence generation. Nine (23%) studies adopted and reported methods of allocation concealment, whereas 30 (77%) studies were at high risk of bias. Twelve (31%) studies adopted and reported blinding methods for the participants and personnel, whereas 27 (69%) studies were associated with high risk of bias. Fourteen (36%) studies adopted and reported methods for blinding of outcome assessment and thus were at low risk for bias from this source. The remaining 25 (64%) studies did not adopt or report methods for minimizing bias arising from outcome assessment.
FIG. 2a.
Risk of bias graph: review authors' judgments about each risk of bias item (random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, other) presented as percentages across all included studies. Color image is available online at www.liebertpub.com/neu
FIG. 2b.
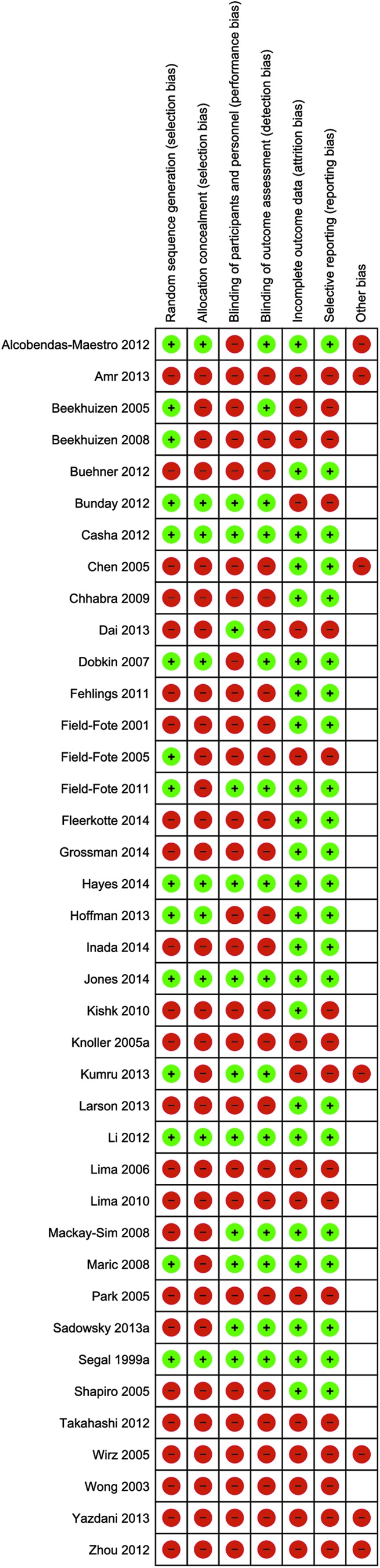
Risk of bias summary: review authors' judgments about each risk of bias item for each included study. Color image is available online at www.liebertpub.com/neu
Twenty-two (56%) studies reported and the remaining 17 (44%) studies did not report whether there was attrition in their samples. For selective reporting, 22 (56%) studies were classified as having low risk of bias, and the remaining 17 (44%) studies were classified as having high risk of bias. Seven (18%) studies were at high risk for other sources of bias, and the remaining 32 (82%) studies were classified as low risk for additional sources of bias.
Figure 3 displays the studies according to the time since injury (acute/subacute and chronic SCI) and study phase (I–IV). It can be seen that 37.5% of studies in the acute stage were Phase I, 18.8% were Phase II, and the remaining 43.8% were Phase III. In the chronic stage, 27.3% of studies were Phase I, 13.6% were Phase II, and 59.1% were Phase III studies. There were no Phase IV studies. Figure 4 displays the approaches used in each study stage, for both acute and chronic SCI.
FIG. 3.
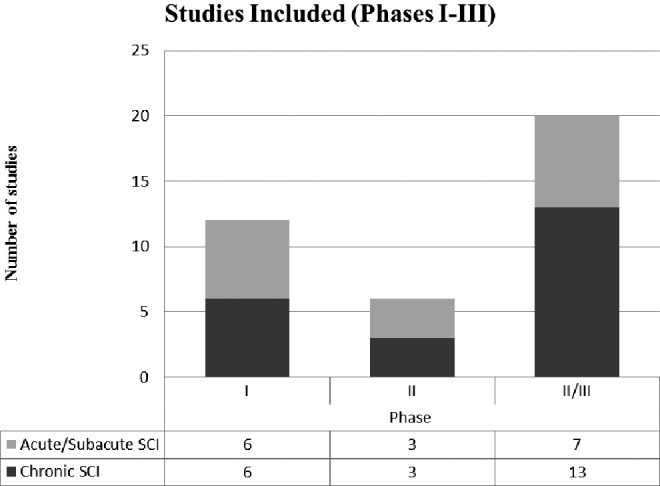
Distribution of studies included in the review separated into acute/subacute spinal cord injury (SCI) (light grey) and chronic SCI (dark grey) and included in the review according to the study phase (I-III, see Methods).
FIG. 4.
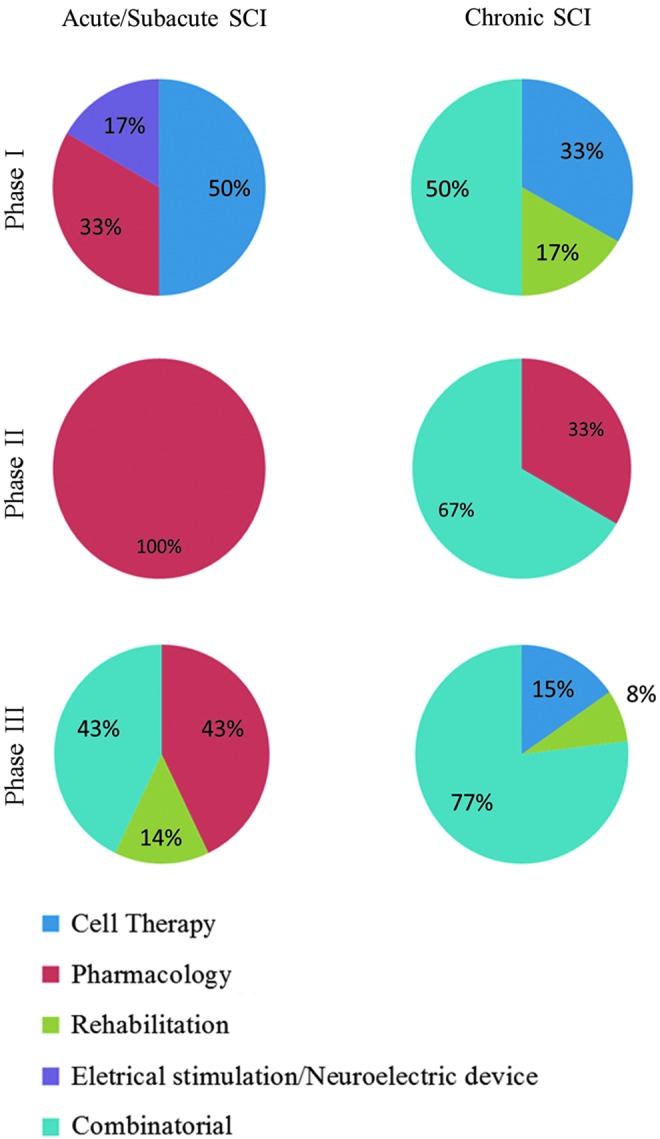
Distribution of studies in acute/subacute and chronic spinal cord injury (SCI) groups divided per phase (see Methods) for all categories (cell therapy, pharmacology, rehabilitation, electrical stimulation/neuroelectric device, combinatorial). Color image is available online at www.liebertpub.com/neu
Twelve studies14–22 assessed spinal integrity using magnetic resonance imaging (MRI) (Tables 3 and 4). Five studies limited the inclusion criteria based on the MRI findings. Two studies proposed limits in the lesion size,18,19 one study excluded participants depending on the presence of tethering in the spinal cord,23 and two studies excluded persons who had complete transections.15,24 Ten studies assessed spinal cord residual connectivity using motor evoked potentials acquired with transcranial magnetic stimulation (TMS).14,17,19,21,25–29
Table 3.
Approaches Used in Acute/Subacute Spinal Cord Injury
| Study | Study phase | Approach | Classification | Mechanism | Outcome measure/effect (clinical neurological and/or motor performance) | Comparison/control | Direction of results | Follow-up | CS integrity | CS connectivity |
|---|---|---|---|---|---|---|---|---|---|---|
| Fehlings et al, 2011 | Phase I | Cethrin | Pharmacology | ↓ excitoxicity | AIS scores | N/A | N/A | 12 months | MRI | Absent |
| Chen et al, 2005 | Phase I | Raffinee | Pharmacology | ↓ excitoxicity | AIS scores | N/A | N/A | 2–3 weeks | Absent | Absent |
| Shapiro et al, 2005 | Phase I | Implanted oscillating electric field | Electrical stimulation/neuroelectric device | Neural guidance | AIS scores | N/A | N/A | 6 months, 12 months | MRI-excluded complete injuries | Absent |
| Park et al, 2005 | Phase I | Bone-marrow derived mesenchymal stromal cells | Cell therapy | Neural regeneration | AIS scores | N/A | N/A | 6–18 months | MRI-excluded complete injuries | Absent |
| Zhou et al, 2012 | Phase I | Autologous activated Schwann cells | Cell therapy | Neural regeneration | AIS scores, FIM | N/A | N/A | 15–18 weeks, 5–8 years | MRI | MEPs (post-test) |
| Knoller et al, 2005 | Phase I | Autologous macrophages | Cell therapy | Neural regeneration | AIS scores | N/A | N/A | 4–12 months | MRI | MEPs |
| Grossman et al, 2014 | Phase II | Riluzole | Pharmacology | ↓ excitoxicity | AIS motor scores,* SCIM | Database control | * = ↑ Riluzole | 42 days, 3 months, 6 months* | Absent | Absent |
| Takahashi et al, 2012 | Phase II | G-CSF | Pharmacology | ↓ excitoxicity | AIS motor scores† | Database control | † = ↑ (G-CSF) | 3 months | Absent | Absent |
| Casha et al, 2012 | Phase III | Minocycline | Pharmacology | ↓ excitoxicity | AIS scores, FIM, and SCIM | Placebo | No differences | 3, 6, and 12 months | Absent | Absent |
| Inada et al, 2014 | Phase II | G-CSF | Pharmacology | ↓ excitoxicity | AIS motor scores* | Control (no intervention) | * = ↑ (G-CSF) | 1 year | Absent | Absent |
| Kumru et al, 2013 | Phase III | High-frequency rTMS | Combination of electrical stimulation/neuroelectric device + rehabilitation | Plasticity | Gait speed† and LEMS (AIS)† | Sham-rTMS + rehabilitation | † = ↑ rTMS + rehabilitation | 5 weeks | Absent | MEPs |
| Alcobendas-Maestro et al, 2012 | Phase III | Locomotor training (overground vs. robotic orthosis) | Rehabilitation | Plasticity | FIM,† WISCI,† walking distance† and LEMS (AIS)†, walking speed | Overground locomotor training | † = ↑ robotic orthosis | 8 weeks | Absent | Absent |
| Dobkin et al, 2007 | Phase III | Locomotor training (overground vs. treadmill) + Physical therapy, nursing | Combination of rehabilitation+ nursing | Plasticity | Walking speed | Overground locomotor training | No differences | 9 weeks | Absent | Absent |
| Wong et al, 2013 | Phase III | Electrical and auricular acupuncture + rehabilitation | Combination of rehabilitation techniques | Unknown | AIS scores, Ffunctional Independence Measure† | Rehabilitation in isolation | † = ↑rehab + acupuncture | 12 months | Absent | Absent |
| Li et al, 2012 | Phase III | DHYZ | Pharmacology | Unknown | AIS scores* | Placebo | * = ↑ DHYZ group | 12 weeks | Absent | Absent |
| Maric et al, 2009 | Phase III | L-Dopa | Pharmacology | Plasticity | AIS scores, FIM, WISCI | Placebo | No differences | 12 weeks | Absent | Absent |
Refers to between-group differences and †refers to pre-post differences.
Phase I studies do not include a control/comparison group and do not assess effectiveness (direction of results); therefore, these items have been noted as applicable (N/A).
AIS, American Spinal Cord Injury Impairment Scale; MRI, magnetic resonance imaging; MEP, motor evoked potentials; SCIM, Spinal Cord Independence Measure; G-CSF, granulocyte colony-stimulating factor; FIM, Functional Independence Measure; rTMS, repetitive transcranial magnetic stimulation; LEMS, lower extremity motor scores obtained from AIS assessment; WISCI, walking index for spinal cord injury; DHYZ, Di Huang Yin Zi, a traditional Chinese medicine.
Table 4.
Approaches Used in Chronic Spinal Cord Injury
| Study | Study phase | Approach | Classification | Mechanism | Outcome measure (clinical Neurological nnd/or motor performance) | Comparison/control | Direction of results | Follow-up | SC integrity | SC connectivity |
|---|---|---|---|---|---|---|---|---|---|---|
| Lima et al, 2006 | Phase I | Olfactory mucosal autografts | Cell therapy | Neural regeneration | AIS scores | N/A | N/A | 6, 12, and 18 months | MRI | Absent |
| Chhabra et al, 2009 | Phase I | Olfactory ensheathing cells | Cell therapy | Neural regeneration | AIS scores, WISCI, SCIM | N/A | N/A | 6, 12 and 18 months | MRI (only included lesions 2–3 cm) | MEPs |
| Wirz et al, 2005 | Phase I | Locomotor training (gait-driven orthosis) | Rehabilitation | Plasticity | LEMS, 6-min walk test, 10 meter walk, timed up and go, WISCI-II | N/A | N/A | Post-intervention (8 weeks) | Absent | Absent |
| Yazdani et al, 2013 | Phase I | Bone-marrow mesenchymal stromal cells, Schwann cells and rehabilitation | Combination of cell therapy + rehabilitation | Neural regeneration+plasticity | AIS scores | N/A | N/A | 24 months | MRI-excluded stenosis and tethering of the spinal cord | Absent |
| Field-Fote et al, 2001 | Phase I | Body-weight supported treadmill training + electrical stimulation | Combination of rehabilitation+ electrical stimulation/neuroelectric device | Plasticity | 2 min walk (speed), LEMS | N/A | N/A | Post-intervention (12 weeks) | Absent | Absent |
| Fleerkote et al, 2014 | Phase I | Body-weight supported treadmill training (AAN-RO) | Combination of rehabilitation+ electrical stimulation/neuroelectric device | Plasticity | 10 meter walk† (distance), WISCI, TUG†, 6 min walk†, LEMS† | N/A | N/A | 8 weeks | Absent | Absent |
| Segal et al, 1999 | Phase II | 4-amynopiridine (low, high dose) | Pharmacology | Plasticity | AIS motor scores† | Comparison (unblinded high dose) | † = groups collapsed | 3 months | Absent | Absent |
| Buehner et al, 2012 | Phase II | Manual facilitated body weight-supported step training on a treadmill + community reintegration | Combination of rehabilitation + other | Plasticity | LEMS,† UEMS,† 6-min walk (speed and distance),† and 10-meter walk (speed)† | N/A | † = pre-post difference | Mean of 60.3 ± 53.24 sessions (approximately 5 months) | Absent | Absent |
| Bunday et al, 2012 | Phase II | Transcranial magnetic stimulation paired with electrical stimulation | Combination of electrical stimulation/neuroelectric device | Plasticity | NHPT* | Comparison group | * = ↑ TMS + stim | post-test (single session) | Absent | MEPs |
| Beekhuizen et al, 2005 | Phase III | Upper extremity task-specific training and submotor threshold electrical stimulation (TST + stim vs. TST) | Combination of Electrical stimulation/neuroelectric device + rehabilitation | Plasticity | JTT,* WMFT,* pinch strength* | Comparison group (TST) | Between-group difference = * ↑ TST+stim | 3 weeks | Absent | MEPs |
| Beekhuizen et al, 2008 | Phase III | Upper extremity task-specific training and submotor threshold electrical stimulation (TST+stim, TST, stim and wait-list control) | Combination of electrical stimulation/neuroelectric device + rehabilitation | Plasticity | JTT,* WMFT,* pinch strength* | Control group (wait-list) | Between-group difference = * ↑ TST + stim | 3 weeks | Absent | MEPs |
| Hoffman et al, 2010 | Phase III | Upper extremity task-specific training (unimanual, bimanual) and electrical stimulation (submotor threshold and FES), and wait-list control | Combination of electrical stimulation/neuroelectric device + rehabilitation | Plasticity | JTT,† | Rehabilitation in isolation | † = pre-post difference (groups collapsed) | 3 weeks | Absent | MEPs |
| Sadowsky et al, 2013 | Phase III | FES cycling | Combination of rehabilitation + electrical stimulation/neuroelectric device | Plasticity | AIS scores†, AIS motor† scores, FIM† | Control (no intervention) | † = FES | Mean of 29.1 months | Absent | Absent |
| Field-Fote et al, 2005 | Phase III | Body-weight supported treadmill training + electrical stimulation (TM, OG, TS, and LR) | Combination of rehabilitation + electrical stimulation/neuroelectric device | Plasticity | 6 min walk,† 2 min walk † | Comparison group | † = pre-post difference (groups collapsed) | 12 weeks | Absent | Absent |
| Field-Fote et al, 2011 | Phase III | Body-weight supported treadmill training + electrical stimulation (TM, OG, TS, and LR) | Combination of rehabilitation+ electrical stimulation/neuroelectric device | Plasticity | 10 meter walk (distance)* | Comparison group | Between-group difference = * ↑ OG | 12 weeks | Absent | Absent |
| Jones et al, 2014 | Phase III | ABT | Rehabilitation | Plasticity | AIS motor score, * LEMS, * 10 meter walk, * 6 min walk, * TUG, SCIM-III, SCI-FAI, * RNL index, | Wait-list control | * = ↑ ABT | 24 weeks | Absent | Absent |
| Dai et al, 2013 | Phase III | Mesenchymal stem cells (OS, CT, control) | Cell therapy | Neural regeneration | AIS scores†1, MEPs, AIS motor scores†2 | Control (no intervention) |
†1 = OS, CT †2 = CT |
6 months | MRI (guided injection site) | MEP |
| Mackay-Sim et al, 2008 | Phase III | Olfactory ensheathing cell transplant | Cell therapy | Neural renegeration | AIS scores, FIM, IADL, COVS | Control group | No differences | 3 years | MRI | MEPs |
| Lima et al, 2010 | Phase III | Olfactory mucosal autografts + locomotor training | Combination of cell therapy + rehabilitation | Plasticity + neural regeneration | LEMS,† FIM† and WISCI† | Comparison group | † = pre-post difference (groups collapsed) | Mean of 27.7 months | MRI (only included lesions 3–4 cm) | Absent |
| Larson et al, 2013 | Phase III | Locomotor training (olfactory mucosal transplant and control) | Combination of cell therapy + rehabilitation | Plasticity + neural regeneration | AIS scores† | Control group | † = pre-post difference (groups collapsed) | 60 days | Absent | Absent |
| Kishk et al, 2010 | Phase III | Bone-marrow + rehabilitation | Combination of cell therapy + rehabilitation | Plasticity + Neural regeneration | AIS scores | Control group | No differences | 1 year | MRI | Absent |
| Hayes et al, 2014 | Phase III | Daih + LT (Daih, Sham-Daih, Daih+LT, Sham-Daih+LT) | Combination of pharmacology + rehabilitation | Plasticity | 10 meter walk (time) †, * 6 min walk†,†1,†2,*1 |
Sham-Daih, Sham-Daih+LT (crossover design) |
† = Daih * = ↑ Daih †1 = Sham-Daih †2 = Daih + LT *1 = ↑ Daih + LT |
Day 1, Day 5, 1 week and 2 weeks | Absent | Absent |
Refers to between-group differences and †refers to pre-post differences.
SC, spinal cord; AIS, American Spinal Cord Injury Impairment Scale; MRI, magnetic resonance imaging; WISCI, walking index for spinal cord injury; SCIM, Spinal Cord Independence Measure; MEP, motor evoked potentials; LEMS, lower extremity motor scores obtained from AIS assessment; AAN-RO, assist-as-needed robotic orthosis; TUG, timed up and go; UEMS, upper extremity motor scores obtained from AIS assessment; TMS, transcranial magnetic stimulation; NHPT, nine-hole peg test; stim, electrical stimulation; TST, task-specific training; WMFT, wolf motor function test; FES, functional electrical stimulation; FIM, Functional Independence Measure; TM, treadmill training with manual assistance; OG, overground training; TS, treadmill training with electrical stimulation; LR, locomat robotic orthosis training; SCI-FAI, Spinal Cord Injury Functional Ambulation Index; RNL Index, Reintegration to Normal Living; ABT, activity-based therapy; OS, open surgery; CT, computed tomography-guided; IADL, Lawton's instrumental activities of daily living; COVS, clinical outcomes variable scale; daily acute intermittent hypoxia; LT, locomotor training.
Approaches used in acute/subacute SCI
Several approaches have been proposed in Phase I studies in acute SCI (Table 3, Fig. 4). Cethrin, a compound that decreases the activity in the rho pathway (involved in the growth cone collapse after central nervous system injury) was tested in one study.30 Raffinee, a drug with free-radical scavenging properties has also been evaluated.31 An electrical stimulation/neuroelectric device that consisted of an implanted oscillating electric field intended to guide neurite growth was also assessed in a study.24 The remainder of Phase I studies have focused on cell therapies, resulting from reports of successful outcomes in experimental models of SCI. These included: cell transplantation of bone marrow derived mesenchymal stromal cells,15 autologous activated Schwann cells,16 and autologous activated macrophages.17
Grossman and associates32 performed a Phase II study to assess the feasibility, safety, and preliminary efficacy of riluzole, a sodium-channel blocking medication shown to reduce excitotoxicity and improve outcomes of motor function in animal models of SCI (and with an established safety profile in humans). Twenty-four persons with SCI (ASIA Injury Impairment Scale [AIS] A–C, within 12 h post-injury) received 50 mg of riluzole (twice daily, for 14 days, enteric administration). Comparisons were made with a database control group, matched for sex, age, and neurological injury level. Persons who received riluzole made significantly greater improvements in AIS motor scores at a 6 months follow-up, when compared with the database control. Greatest improvements were made by those classified AIS B at study entry.32
Takahashi and colleagues33 performed a Phase II study to assess the effects of granulocyte colony-stimulating factor (G-CSF), a compound shown to suppress neuronal apoptosis and expression of inflammatory cytokines. Sixteen participants injured less than 48 h before study enrollment received an intravenous dose of 10 μg/kg/day for 5 consecutive days and demonstrated significant improvements in the ASIA Impairment Scale (AIS) motor scores on the follow-up assessment performed 3 months later when compared with the pre-intervention assessment. No significant differences were found, however, when comparisons were made to a database control group.33
In a subsequent study in 37 participants with incomplete cervical SCI, Inada and coworkers34 found that the same dose of G-CSF as used by Takahashi and colleagues33 was associated with greater improvements in AIS motor scores than a control group at 1 year post-transplantation. Unfortunately, participants were allocated to the experimental group based on the institution they were treated at (in a nonrandomized manner), and it was not possible to assess whether clinical management differed between the sites.
Two studies have assessed the use of pharmacological agents. Casha and associates35 performed a Phase III study with minocycline, following experimental evidence of decreased microglial activation and proliferation (and thus, reduced post-injury excitotoxicity after SCI). Fifty-two adults who had sustained an injury to the cervical or thoracic segments of the spinal cord within 12 h were included in the study and randomized to receive either minocycline (200 mg twice daily) or placebo. The investigators found no between-group differences in AIS scores, Functional Independence Measure (FIM) scores, and Spinal Cord Independence Measure (SCIM) between those treated with minocycline and placebo for up to 1 year post-intervention.35
Following evidence suggesting that dopamine can improve motor task acquisition, Maric and colleagues36 performed a crossover study to compare the effects of L-dopa and placebo. Twelve participants received L-dopa (200 mg L-dopa, 5 days per week for 6 weeks) or placebo before physical therapy (45 min, twice daily for 6 weeks), and there were no differences between the outcomes between the two groups after 12 weeks (AIS scores, FIM, walking index for SCI [WISCI-II]).36
Kumru and coworkers25 performed a Phase III study to assess the influence of noninvasive brain stimulation delivered in the form of high-frequency repetitive transcranial magnetic stimulation (rTMS), aimed at facilitating corticospinal excitability. Seventeen participants who had had a SCI between 3 and 12 months before study enrollment received rTMS or sham-rTMS (15 sessions, 5 days per week for 3 weeks) and engaged in a rehabilitation protocol for 5 h per day, 5 days per week for 5 weeks. There were no between-group differences, but pre-post changes reached significance for gait speed and lower extremity motor scores (LEMS) for the rTMS group.
Two Phase-III studies have proposed locomotor training in acute SCI. Alcobendas-Maestro and colleagues37 compared the outcomes of 40 sessions of locomotor training delivered with a robotic orthosis (30 min per session) to overground walking training (1 h per session) in 75 participants with an SCI of less than 6 months. They found greater improvements in outcomes of walking function in the robotic orthosis group (FIM, WISCII, walking distance, and LEMS), but no between-group difference in walking speed was found.
Dobkin and associates38 enrolled 145 participants with a SCI of at least 8 weeks in a study to compare the effects of a combined approach of locomotor training (either consisting of body weight supported treadmill training or conventional overground mobility training) and physical therapy, occupational therapy, and nursing care delivered in 45 1-h sessions. To minimize bias from differences in spontaneous recovery rates, the analysis was performed separately according to AIS classification at entry.38 There was no between-group difference in walking speed, the primary outcome.38
Two Phase III studies assessed Chinese medicine approaches. Wong and coworkers39 enrolled persons who were injured at approximately 2 months and compared the outcomes of a group whose members received standard rehabilitation program in isolation with a group whose members additionally received electro-acupuncture targeting the Governic meridian. At the end of 1 year, the authors found significant pre-post changes in AIS scores in both groups, but only the acupuncture group exhibited significant pre-post changes in the FIM.39
Li and colleagues40 performed a Phase III study to assess the influence of Di Huang Yin Zi (DHYZ), a pharmacological approach with unclear mechanisms, in 60 persons with acute SCI (approximately 4 weeks post-injury). Participants received either DHYZ or placebo (18 g, twice daily) for 12 weeks, and the authors reported greater increases in AIS motor scores in the DHYZ group when compared with placebo.
Approaches in chronic SCI
Table 4 lists all included studies in chronic SCI. There were a total of six Phase I studies. Lima and coworkers18 proposed the transplantation of olfactory mucosal autografts into the chronically injured spinal cord. A second independent study proposed the use of olfactory ensheathing cells.19 Yazdani and colleagues23 proposed a combinatorial approach that consisted of cell transplantation of bone-marrow mesenchymal stromal cells and Schwann cells, combined with rehabilitation. The remaining Phase I chronic studies included a rehabilitation study that assessed automated locomotor training using a position-controlled gait-driven orthosis,41 an impedance-controlled robotic orthosis,42 and a study that assessed the effects of body weight supported treadmill training combined with electrical stimulation.43
Segal and associates44 performed a Phase II study to assess the effects of 4-aminopyridine, a potassium-channel blocker that has been associated with improved axonal conduction, particularly in demyelinated nerve fibers. Participants (various AIS grades) were randomized to a 3-month regimen of 4-aminopyridine, either delivered in the form of a low dose (6 mg/day) or a high dose (30 mg/day). Comparisons were made with an unblinded group whose members received a high dose (30 mg/day). The authors reported significant pre-post increased AIS motor scores only when all groups were collapsed together.
One Phase II study has assessed the effect of locomotor training in 225 persons who had sustained a chronic incomplete SCI and also examined the effects of a combinatorial approach consisting of manual facilitated body weight supported step training on a treadmill and community reintegration.45 The dose was variable, based on the participant's ambulatory capacity (five times/week for nonambulatory participants, four times/week for participants who needed pronounced assistance, and five times/week for ambulatory participants). After having completed a mean of 60.3 ± 53.24 sessions, the authors found significant pre-post improvements in the following outcome measures: LEMS, upper extremity motor scores (UEMS), 6-min walk (speed and distance), and 10-meter walk (speed). In addition, using pre-established functional walking stratifications,46 33% of nonambulatory participants became walkers, 47% of slow walkers improved to faster walkers, and overall AIS conversion rates from C to D was 28% (for AIS classification, see Table 1).45
Four studies assessed the effects of combinatorial approaches for upper extremity function improvement. One Phase II study assessed the effects of a single-session of transcranial magnetic stimulation paired with electrical stimulation on fine motor hand performance in 10 persons with chronic SCI and found significant improvements on the nine-hole peg test measured at 20 min and 30 min post-stimulation.26 A Phase III study assessed the effects of task specific training (2 h/day, 5 days a week for 3 weeks) delivered in isolation or combined with electrical stimulation of the median nerve in 24 participants, and found greater improvements in hand motor performance in the combined approach.27 In a latter study with the same sample size, the authors found that the combination of task-specific training and electrical stimulation was associated with significantly larger improvements in hand motor performance than either intervention used in isolation.28
The combination of task-specific training and electrical stimulation was further investigated by Hoffman and Field-Fote.29 In this Phase III study, investigators compared the effects of functional electrical stimulation with submotor threshold electrical stimulation, each combined with either unimanual or bimanual task-specific training, using the same dose and sample size (n = 24) as in the previous studies.29 While underpowered to detect changes between the approaches, the authors found that the unimanual group made greater changes in unimanual function, and the bimanual group made greater changes in bimanual function when compared with the control group, irrespective of stimulation type when compared with a wait-list control group.29
Sadowski and associates47 assessed the effects of functional electrical stimulation during cycling in persons with chronic SCI (n = 25) and made comparisons with a control group (n = 20) of persons who were matched for age, sex, and duration, location, and severity of injury. The authors found that those who participated in an FES cycling protocol made significantly greater improvements in AIS total scores, motor scores, and FIM.47
Three Phase III studies proposed rehabilitation combinatorial strategies that included locomotor training. Field-Fote and colleagues48 compared the effects of manually assisted treadmill training, treadmill training assisted with electrical stimulation, overground training assisted with electrical stimulation, and passive locomotor training using a robotic orthosis (all delivered for 1 h a day, 5 days a week for 12 weeks) in 27 persons. The authors found significant pre-post improvements in walking speed and distance (measured by the 2-min walk and 6-min walk) when all groups were collapsed.48 In a follow-up study49 of the same approaches with a larger sample size that was adequately powered to detect statistical differences between the interventions (n = 75), the authors found significant between-group differences for walking distance, with greatest effects observed with the overground training combined with electrical stimulation.
Jones and coworkers50 conducted a study to assess the effects of activity-based therapy (ABT), which consists in an individualized rehabilitation program focused on muscle strengthening (including resistance and endurance) and locomotor training. Using a randomized delayed intervention design, 48 persons (AIS C and D) participated in ABT (up to three 3-h sessions/week over 24 weeks). The average documented treatment time was 89 ± 22.1 h.50 At post-test, there were significantly greater improvements in the ABT condition on AIS motor scores, LEMS, walking speed (10 meter walk) walking distance (6-min walk), and on the Spinal Cord Injury Functional Ambulation Index.50
Five studies assessed the effects of cell therapies, in isolation or combined with rehabilitation interventions, and found mixed results. Dai and associates14 compared the delivery of mesenchymal stem cells using open surgery or delivered (dose: 4 × 107) using CT to an inactive control in 27 persons with chronic SCI with varying characteristics of injury severity. At a follow-up performed 6 months after, the authors reported significant pre-post improvements in AIS scores in the open surgery and CT-guided transplant group, and significant improvements in AIS motor scores only in the CT-guided transplant group.14
McKay-Sim and colleagues21 assessed the effects of transplantation of culture-expanded autologous olfactory ensheathing cells (12–28 million) in 12 participants with thoracic complete injuries (6 persons received the transplants and 6 matched control patients with thoracic level injury 1–3 years before enrollment), and found no functional improvements in any of the outcome measures, which were assessed up to 3 years post-intervention. The authors attributed these results to the lack of a rehabilitation protocol.21
Lima and coworkers20 assessed the influence of olfactory mucosal autografts and locomotor training in 20 participants with complete SCI. All participants engaged in rehabilitation (mean =31.8 ± 6.8 h/week for 34.7 ± 30 weeks) before and after (32.7 ±5.2 h/week for 92 ± 37.6 weeks) the olfactory mucosal autograft transplant. The authors found pre-post improvements in LEMS and walking function (FIM and WISCI). In addition, 15/20 participants (all whom had sustained motor-complete injuries) demonstrated electromyography activation below the level of injury, leading the authors to conclude that there was late neurological recovery.
Larson and associates51 performed a Phase III study to compare outcomes of participation in an intense rehabilitation program between persons with and without a history of a previous olfactory mucosal autograft transplant. Using an open-label design, they recruited persons who had a previous olfactory mucosal cell transplant (privately performed), a matched control group (controlled for age, injury severity, sex, and AIS classification), and a second nonmatched control group. All 23 participants engaged in an intense exercise protocol for an average of 7.1 h/week for approximately 4.6 months (137.3 total hours).51 With all groups collapsed, there were significant improvements in AIS scores at 60 days post-intervention, and no differences were found between those who received olfactory mucosal cell transplant and those who did not.51 The authors concluded that the intense rehabilitation approach was likely a key factor responsible for the functional improvements.51
Kishk and coworkers22 assessed the effects of transplantation of flask-adherent bone marrow stromal cells and rehabilitation in persons who had a SCI at least 6 months before study enrollment. Forty-four participants with variable injury levels and severity received mononuclear cells (dose: 5 × 106 to 10 × 106/kg, administered intrathecally every month for 6 months), and 20 participants who did not agree to study procedures comprised the control group. All persons who participated engaged in rehabilitation 2–3 times/week. The authors found that the intervention group made significantly greater gains in AIS scores, but the amount of improvement was correlated with having an incomplete injury. Because the groups were not balanced for injury severity, this finding was of limited clinical value.
One study assessed the combination of daily intermittent hypoxia (Daih) and rehabilitation on locomotor outcomes in SCI.52 Using a double-blind, randomized crossover study, Daih (15–90 sec intervals, fraction of inspired oxygen = 0.9 for 5 days) or Daih-Sham were delivered in isolation or combined with subsequent walking training, in 19 persons with incomplete SCI.52 The authors found that Daih increased walking speed (measured by the 6-min walk) 1 day and 2 weeks post-administration. Further, the combination of Daih and walking training was associated with greater improvements in walking endurance (measured by the 10-meter walk) than walking training alone and Daih alone at 5 days and 1 week post-administration.52
Discussion
A systematic assessment of the literature reveals that various strategies have been proposed to improve motor function after SCI (including cell therapy, pharmacology, rehabilitation, electrical stimulation/neuroelectric device (delivered individually, or in combinatorial approaches containing each of these strategies). The highest evidence level available (level III) supports combinatorial approaches that contained a rehabilitation component. Quality appraisal of the included literature highlights that there are still few well-designed studies producing high-level evidence that can appropriately answer questions regarding the effectiveness of many approaches proposed. Among the sources of bias encountered, the most concerning were: the inclusion of highly heterogeneous samples; the lack of randomization and concealed allocation procedures; the absence of blinding procedures; and the use of outcomes with limited sensitivity. In addition, only few studies included measures of integrity and residual connectivity of spinal pathways.
The finding that combinatorial approaches comprised 43% of Phase III studies in acute SCI and 77% of studies in chronic SCI is encouraging. Wenger and colleagues10 demonstrated meaningful functional recovery of stepping in rats after a transection to the spinal cord after 4 weeks of body weight supported treadmill training delivered in combination with electrical neurostimulation and serotonin agonists administered systemically. Our study of the human literature supports this experimental evidence10 by demonstrating that the strongest evidence for improved outcomes of motor function comes from trials that used combinatorial approaches containing a rehabilitation component.25,27–29,38,39,48,49 Taken together, we believe that future clinical trials have greater potential for motor recovery if novel therapeutic strategies (such as neurostimulation and pharmacotherapy) are tested as adjuvant to rehabilitation.
The paucity of high-level evidence of studies from other modalities (including cell therapies, pharmacology, electrical stimulation/neuroelectric devices) should not be interpreted as a lack of effectiveness. Instead, this is partially an encouraging finding that reflects the high productivity in SCI research in the last 17 years, characterized by increases in the number of Phase I and Phase II studies suggesting promising novel treatment strategies. While the main objective of Phase I and II trials is the assessment of safety and feasibility of a given intervention, many methodological concerns with the early stage trials were identified, and future trials need to be more carefully designed to allow for inferences regarding preliminary efficacy that are useful in planning Phase III studies.
The inclusion of participants with broad age range and diverse clinical characteristics (time since injury, injury severity) introduces insurmountable heterogeneity that is concerning when inferences regarding recovery are drawn based on group data. Spontaneous recovery rates have been shown to differ considerably based on the level and severity of the injury. There is evidence to support that as many as 10% of persons with complete (AIS A injuries) will convert to AIS C and D (incomplete injuries) within the first year in the absence of any therapeutic intervention.2 For those initially classified as AIS B, 15–40% will convert to AIS C, and approximately 40% of the remaining will convert to AIS D.2 Eighty percent of persons initially classified as AIS C will convert to AIS D within the first year.2 In our opinion it is critical that future studies restrict the inclusion criteria to create homogeneous groups, especially in studies enrolling persons with acute and subacute injuries.53 In studies with larger sample sizes, another option is to perform statistical analyses based on AIS level at entry.38
Further, nearly all studies used the ISNCSCI criteria as the primary outcome measure for neurologic recovery. While the AIS examination is the most widely used neurologic classification for SCI, it has a considerable degree of subjectivity and requires formal training for the investigators/clinical staff to achieve optimal rates in terms of intra-rater and test-retest reliability54 (although rarely reported in the studies assessed). We found that 69% of the studies included did not adopt blinding of participants, and 64% of studies did not adopt blinding procedures for the investigators. This is an important concern, because when recovery occurs, we often do not know why it happens because of the lack of mechanistic discriminative power of our clinical assessments.
It is thus important to develop measures that allow change in particular pathways to be detected. Spinal cord integrity and residual connectivity was only assessed in a few studies (28% and 26%, respectively). Given its importance in motor function, the development of sensitive outcome measures of viability of corticospinal pathways is an important direction given the limited sensitivity of currently available outcome measures to quantify neurologic impairments and changes that may occur in response to novel therapies.
We believe that such neurophysiological and neuroimaging measures should be used to characterize spinal cord structure and physiology of the injury in future clinical trials. Potential approaches for assessing corticospinal conduction may involve the use of noninvasive or minimally invasive brain stimulation, electrophysiology, and imaging techniques.7 For example, the quantification of motor evoked potentials elicited with TMS enables the assessment of functional integrity of the corticospinal tract, spinal nerve roots, and motor pathway's projections to the muscles.55 Moreover, more advanced TMS protocols, particularly the triple-pulse protocol that combines central and peripheral stimuli, enable accurate quantification of the number of corticospinal fibers with preserved conduction across a putative spinal injury level.56 Finally, such neurophysiologic studies can be combined with diffusor tension imaging and other advanced imaging methods57 to provide anatomical characterization of the structural integrity of the corticospinal tract, including alterations in fractional anisotropy (that assesses the axonal count and myelin content), axial diffusivity, and radial diffusivity (that assesses the integrity of axons and myelin).7
Our search strategy captured only 18 or the 39 included articles. This can be explained by the difficulty in designing a search that identifies all of the different interventions of interest without requiring the need to review many thousands of abstracts. Although we used multiple queries and inspected reference lists, it is possible that the search strategy used herein may have been insufficient to detect all published studies for each approach (cell therapies, pharmacology, electrical stimulation, rehabilitation). Another limitation is that we did not include studies that assessed surgical decompression, which is a strategy with established efficacy.58 In addition, the use of the Cochrane criteria of risk of bias may have resulted in a stringent evaluation of Phase I studies. While we acknowledge that the Cochrane criteria were primarily developed for application to randomized clinical trials, we thought it offered valuable insights.
Conclusions
Future research will benefit from addressing the methodological and conceptual concerns highlighted in the present study. The highest available evidence supports the use of combinatorial approaches containing rehabilitation techniques and, thus, novel therapeutic interventions should be tested in combinatorial approaches containing a well-defined rehabilitation component. Future research efforts that assess motor recovery should contain measures of viability of corticospinal fibers. We believe that this will lead to an improved understanding of the functional prognosis and the role of the corticospinal and other pathways critical for motor recovery after SCI, and their response to therapeutic interventions.
Acknowledgments
The authors would like to thank Jordi Valles for his invaluable comments. Work on this study was supported by Fenexy Fundacion para la Curacion de Las Lesiones Medulares, Proyecto Volver a Caminar, Lazarus-Fenexy.
The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic health care centers, the National Institutes of Health or the Sidney R. Baer Jr. Foundation.
Author Disclosure Statement
Dr. Pascual-Leone serves on the scientific advisory boards for Nexstim, Neuronix, Starlab Neuroscience, Neuroelectrics, Axilum Robotics, Magstim Inc., and Neosync and is listed as an inventor on several issued and pending patents on the real-time integration of transcranial magnetic stimulation with electroencephalography and magnetic resonance imaging. Dr. Guest serves on the scientific boards of Bioaxone and In Vivo Therapeutics. Mar Cortes' work is supported by grant NIH R21 HD069776. APL is supported in part by grants from the Sidney R. Baer Jr. Foundation, the National Institutes of Health (R01HD069776, R01NS073601, R21 MH099196, R21 NS082870, R21 NS085491, R21 HD07616), Harvard Catalyst|The Harvard Clinical and Translational Science Center (NCRR and the NCATS NIH, UL1 RR025758). For the remaining author, no competing financial interests exist.
References
- 1.National Spinal Cord Injury Statistical Center. Spinal cord injury: facts and figures at a glance. Birmingham, AL: Available at: nscisc.uab.edu Accessed: September10, 2015 [Google Scholar]
- 2.Fawcett J.W., Curt A., Steeves J.D., Coleman W.P., Tuszynski M.H., Lammertse D., Bartlett P.F., Blight A.R., Dietz V., Ditunno J., Dobkin B.H., Havton L.A., Ellaway P.H., Fehlings M.G., Privat A., Grossman R., Guest J.D., Kleitman N., Nakamura M., Gaviria M., and Short D. (2007). Guidelines for the conduct of clinical trials for spinal cord injury as developed by the ICCP panel: spontaneous recovery after spinal cord injury and statistical power needed for therapeutic clinical trials. Spinal Cord 45, 190–205 [DOI] [PubMed] [Google Scholar]
- 3.Marino R.J., Barros T., Biering-Sorensen F., Burns S.P., Donovan W.H., Graves D.E., Haak M., Hudson L.M., and Priebe M.M.; ASIA Neurological Standards Committee 2002. (2003). International standards for neurological classification of spinal cord injury. J. Spinal Cord Med. 26, Suppl 1, S50–S56 [DOI] [PubMed] [Google Scholar]
- 4.Waring W.P., 3rd, Biering-Sorensen F., Burns S., Donovan W., Graves D., Jha A., Jones L., Kirshblum S., Marino R., Mulcahey M.J., Reeves R., Scelza W.M., Schmidt-Read M., and Stein A. (2010). 2009 review and revisions of the international standards for the neurological classification of spinal cord injury. J. Spinal Cord Med. 33, 346–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bunge R.P., Puckett W.R., and Hiester E.D. (1997). Observations on the pathology of several types of human spinal cord injury, with emphasis on the astrocyte response to penetrating injuries. Adv. Neurol. 72, 305–315 [PubMed] [Google Scholar]
- 6.Fitch M.T., and Silver J. (2008). CNS injury, glial scars, and inflammation: inhibitory extracellular matrices and regeneration failure. Exp. Neurol. 209, 294–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Steeves J.D., Lammertse D., Curt A., Fawcett J.W., Tuszynski M.H., Ditunno J.F., Ellaway P.H., Fehlings M.G., Guest J.D., Kleitman N., Bartlett P.F., Blight A.R., Dietz V., Dobkin B.H., Grossman R., Short D., Nakamura M., Coleman W.P., Gaviria M., and Privat A.; International Campaign for Cures of Spinal Cord Injury Paralysis. (2007). Guidelines for the conduct of clinical trials for spinal cord injury (SCI) as developed by the ICCP panel: clinical trial outcome measures. Spinal Cord 45, 206–221 [DOI] [PubMed] [Google Scholar]
- 8.Bunge M.B. (2008). Novel combination strategies to repair the injured mammalian spinal cord. J. Spinal Cord Med. 3, 262–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McCreedy D.A., and Sakiyama-Elbert S.E. (2012). Combination therapies in the CNS: engineering the environment. Neurosci. Lett. 519, 115–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wenger N., Moraud E.M., Raspopovic S., Bonizzato M., DiGiovanna J., Musienko P., Morari M., Micera S., and Courtine G. (2014). Closed-loop neuromodulation of spinal sensorimotor circuits controls refined locomotion after complete spinal cord injury. Sci. Transl. Med. 6, 255ra133. [DOI] [PubMed] [Google Scholar]
- 11.Gomes-Osman J, Cortes M, and Pascual-Leone A. (2014). Assessment of different approaches for improving motor function after spinal cord injury in humans. University of York Centre for Reviews and Dissemination, PROSPERO International prospective register of systematic reviews; Available at: http://www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42014009039#.U9uu6x7D9oy Accessed: August1, 2014 [Google Scholar]
- 12.Liberati A., Altman D.G., Tetzlaff J., Mulrow C., Gøtzsche P.C., Ioannidis J.P., Clarke M., Devereaux P.J., Kleijnen J., and Moher D. (2009). The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann. Intern. Med. 151, W65–W94 [DOI] [PubMed] [Google Scholar]
- 13.Higgins J., and Green S. Cochrane Handbook for Systematic Reviews Of Interventions. version 5.1.0. (2011). Available at: http://www.cochrane.org/handbook Accessed: August1, 2014 [Google Scholar]
- 14.Dai G., Liu X., Zhang Z., Wang X., Li M., Cheng H., Hua R., Shi J., Wang R., Qin C., Gao J., and An Y. (2013). Comparative analysis of curative effect of CT-guided stem cell transplantation and open surgical transplantation for sequelae of spinal cord injury. J. Transl. Med. 11, 315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park H.C., Shim Y.S., Ha Y., Yoon S.H., Park S.R., Choi B.H., and Park H.S. (2005). Treatment of complete spinal cord injury patients by autologous bone marrow cell transplantation and administration of granulocyte-macrophage colony stimulating factor. Tissue Eng. 11, 913–922 [DOI] [PubMed] [Google Scholar]
- 16.Zhou X.H., Ning G.Z., Feng S.Q., Kong X.H., Chen J.T., Zheng Y.F., Ban D.X., Liu T., Li H., and Wang P. (2012). Transplantation of autologous activated Schwann cells in the treatment of spinal cord injury: six cases, more than five years of follow-up. Cell Transplant. 21, Suppl 1, S39–S47 [DOI] [PubMed] [Google Scholar]
- 17.Knoller N., Auerbach G., Fulga V., Zelig G., Attias J., Bakimer R., Marder J.B., Yoles E., Belkin M., Schwartz M., and Hadani M. (2005). Clinical experience using incubated autologous macrophages as a treatment for complete spinal cord injury: phase I study results. J. Neurosurg. Spine 3, 173–181 [DOI] [PubMed] [Google Scholar]
- 18.Lima C., Pratas-Vital J., Escada P., Hasse-Ferreira A., Capucho C., and Peduzzi J.D. (2006). Olfactory mucosa autografts in human spinal cord injury: a pilot clinical study. J. Spinal Cord Med. 29, 191–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chhabra H.S., Lima C., Sachdeva S., Mittal A., Nigam V., Chaturvedi D., Arora M., Aggarwal A., Kapur R., and Khan T.A. (2009). Autologous olfactory [corrected] mucosal transplant in chronic spinal cord injury: an indian pilot study. Spinal Cord 47, 887–895 [DOI] [PubMed] [Google Scholar]
- 20.Lima C., Escada P., Pratas-Vital J., Branco C., Arcangeli C.A., Lazzeri G., Maia C.A., Capucho C., Hasse-Ferreira A., and Peduzzi J.D. (2010). Olfactory mucosal autografts and rehabilitation for chronic traumatic spinal cord injury. Neurorehabil. Neural Repair 24, 10–22 [DOI] [PubMed] [Google Scholar]
- 21.Mackay-Sim A., Feron F., Cochrane J., Bassingthwaighte L., Bayliss C., Davies W., Fronek P., Gray C., Kerr G., Licina P., Nowitzke A., Perry C., Silburn P.A., Urquhart S., and Geraghty T. (2008). Autologous olfactory ensheathing cell transplantation in human paraplegia: a 3-year clinical trial. Brain 131, 2376–2386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kishk N.A., Gabr H., Hamdy S., Afifi L., Abokresha N., Mahmoud H., Wafaie A., and Bilal D. (2010). Case control series of intrathecal autologous bone marrow mesenchymal stem cell therapy for chronic spinal cord injury. Neurorehabil. Neural Repair 24, 702–708 [DOI] [PubMed] [Google Scholar]
- 23.Yazdani S.O., Hafizi M., Zali A.R., Atashi A., Ashrafi F., Seddighi A.S., and Soleimania M. (2013). Safety and possible outcome assessment of autologous Schwann cell and bone marrow mesenchymal stromal cell co-transplantation for treatment of patients with chronic spinal cord injury. Cytotherapy 15, 782–791 [DOI] [PubMed] [Google Scholar]
- 24.Shapiro S., Borgens R., Pascuzzi R., Roos K., Groff M., Purvines S., Rodgers R.B., Hagy S., and Nelson P. (2005). Oscillating field stimulation for complete spinal cord injury in humans: A phase 1 trial. J. Neurosurg. Spine 2, 3–10 [DOI] [PubMed] [Google Scholar]
- 25.Kumru H., Benito J., Murillo N., Valls-Sole J., Valles M., Lopez-Blazquez R., Costa U., Tormos J.M., Pascual-Leone A., and Vidal J. (2013) Effects of high-frequency repetitive transcranial magnetic stimulation on motor and gait improvement in incomplete spinal cord injury patients. Neurorehabil. Neural Repair 27, 421–429 [DOI] [PubMed] [Google Scholar]
- 26.Bunday K.L., and Perez M.A. (2012). Motor recovery after spinal cord injury enhanced by strengthening corticospinal synaptic transmission. Curr. Biol. 22, 2355–2361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beekhuizen K.S., and Field-Fote E.C. (2005). Massed practice versus massed practice with stimulation: effects on upper extremity function and cortical plasticity in individuals with incomplete cervical spinal cord injury. Neurorehabil. Neural Repair 19, 33–45 [DOI] [PubMed] [Google Scholar]
- 28.Beekhuizen K.S., and Field-Fote E.C. (2008). Sensory stimulation augments the effects of massed practice training in persons with tetraplegia. Arch. Phys. Med. Rehabil. 89, 602–608 [DOI] [PubMed] [Google Scholar]
- 29.Hoffman L.R., and Field-Fote E.C. (2010). Functional and corticomotor changes in individuals with tetraplegia following unimanual or bimanual massed practice training with somatosensory stimulation: a pilot study. J. Neurol. Phys. Ther. 34, 193–201 [DOI] [PubMed] [Google Scholar]
- 30.Fehlings M.G., Theodore N., Harrop J., Maurais G., and Kuntz C. (2011). A phase I/IIa clinical trial of a recombinant Rho protein antagonist in acute spinal cord injury. J. Neurotrauma 28, 787–796 [DOI] [PubMed] [Google Scholar]
- 31.Chen H.Y., Lin J.M., Chuang H.Y., and Chiu W.T. (2005). Raffinee in the treatment of spinal cord injury: an open-labeled clinical trial. Ann. N. Y. Acad. Sci. 1042, 396–402 [DOI] [PubMed] [Google Scholar]
- 32.Grossman R.G., Fehlings M.G, Frankowski R.F., Burau K.D., Chow D.S., Tator C, Teng A., Toups E.G., Harrop J.S., Aarabi B., Shaffrey C.I., Johnson M.M., Harkema S.J., Boakye M., Guest J.D., and Wilson J.R. (2014). A prospective, multicenter, phase I matched-comparison group trial of safety, pharmacokinetics, and preliminary efficacy of riluzole in patients with traumatic spinal cord injury. J. Neurotrauma 31, 239–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takahashi H., Yamazaki M., Okawa A., Sakuma T., Kato K., Hashimoto M., Hayashi K., Furuya T, Fujiyoshi T., Kawabe J., Yamauchi T., Mannoji C., Miyashita T., Kadota R., Hashimoto M., Ito Y., Takahashi K., and Koda M. (2012). Neuroprotective therapy using granulocyte colony-stimulating factor for acute spinal cord injury: A phase I/IIa clinical trial. Eur. Spine J. 21, 2580–2587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Inada T., Takahashi H., Yamazaki M., Okawa A., Sakuma T., Kato K., Hashimoto M., Hayashi K., Furuya T., Fujiyoshi T., Kawabe J., Mannoji C., Miyashita T., Kadota R., Someya Y., Ikeda O., Hashimoto M., Suda K., Kajino T., Ueda H., Ito Y., Ueta T., Hanaoka H., Takahashi K., and Koda M. (2014). Multicenter prospective nonrandomized controlled clinical trial to prove neurotherapeutic effects of granulocyte colony-stimulating factor for acute spinal cord injury: analyses of follow-up cases after at least 1 year. Spine (Phila Pa 1976) 39, 213–219 [DOI] [PubMed] [Google Scholar]
- 35.Casha S., Zygun D., McGowan M.D., Bains I., Yong V.W., and Hurlbert R.J. (2012) Results of a phase II placebo-controlled randomized trial of minocycline in acute spinal cord injury. Brain 135, 1224–1236 [DOI] [PubMed] [Google Scholar]
- 36.Maric O., Zörner B., and Dietz V. (2008). Levodopa therapy in incomplete spinal cord injury. J. Neurotrauma 25, 1303–1307 [DOI] [PubMed] [Google Scholar]
- 37.Alcobendas-Maestro M., Esclarin-Ruz A., Casado-Lopez R.M., Muñoz-González A., Pérez-Mateos G., González-Valdizán E., and Martín J.L. (2012). Lokomat robotic-assisted versus overground training within 3 to 6 months of incomplete spinal cord lesion: randomized controlled trial. Neurorehabil. Neural Repair 26, 1058–1063 [DOI] [PubMed] [Google Scholar]
- 38.Dobkin B., Barbeau H., Deforge D., Ditunno J., Elashoff R., Apple D., Basso M., Behrman A., Harkema S., Saulino M., andScott M.; Spinal Cord Injury Locomotor Trial Group. (2007). The evolution of walking-related outcomes over the first 12 weeks of rehabilitation for incomplete traumatic spinal cord injury: the multicenter randomized Spinal Cord Injury Locomotor Trial. Neurorehabil. Neural Repair 21, 25–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wong A.M., Leong C.P., Su T.Y., Yu S.W., Tsai W.C., and Chen C.P. (2003). Clinical trial of acupuncture for patients with spinal cord injuries. Am. J. Phys. Med. Rehabil. 82, 21–27 [DOI] [PubMed] [Google Scholar]
- 40.Li Y.L., Li L.T., Yu M., Wang Y.Z., Ge H.Y., and Song C.Q. (2012). Beneficial effects of the herbal medicine Di Huang Yin Zi in patients with spinal cord injury: a randomized, placebo-controlled clinical study. J. Int. Med. Res. 40, 1715–1724 [DOI] [PubMed] [Google Scholar]
- 41.Wirz M., Zemon D.H., Rupp R., Scheel A., Colombo G., Dietz V., and Hornby T.G. (2005). Effectiveness of automated locomotor training in patients with chronic incomplete spinal cord injury: a multicenter trial. Arch. Phys. Med. Rehabil. 86, 672–680 [DOI] [PubMed] [Google Scholar]
- 42.Fleerkotte B.M., Koopman B., Buurke J.H., van Asseldonk E.H., van der Kooij H., and Rietman J.S. (2014). The effect of impedance-controlled robotic gait training on walking ability and quality in individuals with chronic incomplete spinal cord injury: an explorative study. J. Neuroeng. Rehabil. 11, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Field-Fote E.C. (2001). Combined use of body weight support, functional electric stimulation, and treadmill training to improve walking ability in individuals with chronic incomplete spinal cord injury. Arch. Phys. Med. Rehabil. 82, 818–824 [DOI] [PubMed] [Google Scholar]
- 44.Segal J.L., Pathak M.S., Hernandez J.P., Himber P.L., Brunnemann S.R., and Charter R.S. (1999). Safety and efficacy of 4-aminopyridine in humans with spinal cord injury: a long-term, controlled trial. Pharmacotherapy 19, 713–723 [DOI] [PubMed] [Google Scholar]
- 45.Buehner J.J., Forrest G.F., Schmidt-Read M., White S., Tansey K., and Basso D.M. (2012). Relationship between ASIA examination and functional outcomes in the NeuroRecovery Network Locomotor Training Program. Arch Phys Med Rehabil. 93, 1530–1540 [DOI] [PubMed] [Google Scholar]
- 46.van Hedel H.J.; EMSCI Study Group. (2009). Gait speed in relation to categories of functional ambulation after spinal cord injury. Neurorehabil. Neural Repair 23, 343–350 [DOI] [PubMed] [Google Scholar]
- 47.Sadowsky C.L., Hammond E.R., Strohl A.B., Commean P.K., Eby S.A., Damiano D.L., Wingert J.R., Bae K.T., McDonald J.W., 3rd. (2013). Lower extremity functional electrical stimulation cycling promotes physical and functional recovery in chronic spinal cord injury. J. Spinal Cord Med. 36, 623–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Field-Fote E.C., Lindley S.D., and Sherman A.L. (2005). Locomotor training approaches for individuals with spinal cord injury: a preliminary report of walking-related outcomes. J. Neurol. Phys. Ther. 29, 127–137 [DOI] [PubMed] [Google Scholar]
- 49.Field-Fote E.C., Roach K.E. (2011). Influence of a locomotor training approach on walking speed and distance in people with chronic spinal cord injury: a randomized clinical trial. Phys. Ther. 91, 48–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jones M.L., Evans N., Tefertiller C., Backus D., Sweatman M., Tansey K., and Morrison S. (2014). Activity-based therapy for recovery of walking in individuals with chronic spinal cord injury: Results from a randomized clinical trial. Arch. Phys. Med. Rehabil. 95, 2239–2246.e2. [DOI] [PubMed] [Google Scholar]
- 51.Larson C.A., and Dension P.M. (2013). Effectiveness of intense, activity-based physical therapy for individuals with spinal cord injury in promoting motor and sensory recovery: is olfactory mucosa autograft a factor? J. Spinal Cord Med. 36, 44–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hayes H.B., Jayaraman A., Herrmann M., Mitchell G.S., Rymer W.Z., and Trumbower R.D. (2014). Daily intermittent hypoxia enhances walking after chronic spinal cord injury: a randomized trial. Neurology 82, 104–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tanadini L.G., Steeves J.D., Hothorn T., Abel R., Maier D., Schubert M., Weidner N., Rupp R., and Curt A. (2014). Identifying homogeneous subgroups in neurological disorders: unbiased recursive partitioning in cervical complete spinal cord injury. Neurorehabil. Neural Repair 28, 507–515 [DOI] [PubMed] [Google Scholar]
- 54.Mulcahey M.J., Gaughan J., Betz R.R., and Vogel L.C. (2007). Rater agreement on the ISCSCI motor and sensory scores obtained before and after formal training in testing technique. J. Spinal Cord Med. 30, Suppl 1, S146–S149 [PMC free article] [PubMed] [Google Scholar]
- 55.Kobayashi M., Pascual-Leone A. (2003). Transcranial magnetic stimulation in neurology. Lancet Neurol. 2, 145–156 [DOI] [PubMed] [Google Scholar]
- 56.Magistris M.R., Rosler K.M., Truffert A., and Myers J.P. (1998). Transcranial stimulation excites virtually all motor neurons supplying the target muscle. A demonstration and a method improving the study of motor evoked potentials. Brain 121, 437–450 [DOI] [PubMed] [Google Scholar]
- 57.Freund P., Curt A., Friston K., and Thompson A. (2013). Tracking changes following spinal cord injury: insights from neuroimaging. Neuroscientist 19, 116–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fehlings M.G., Vaccaro A., Wilson J.R., Singh A., W. Cadotte D., Harrop J.S., Aarabi B., Shaffrey C., Dvorak M., Fisher C., Arnold P., Massicotte E.M., Lewis S., and Rampersaud R. (2012). Early versus delayed decompression for traumatic cervical spinal cord injury: results of the Surgical Timing in Acute Spinal Cord Injury Study (STASCIS). PLoS One 7, e32037. [DOI] [PMC free article] [PubMed] [Google Scholar]