Abstract
Adipose-derived mesenchymal stem cells (ASCs) are appealing for cell-based wound therapies because of their accessibility and ease of harvest, but their utility is limited by poor cell survival within the harsh wound microenvironment. In prior work, our laboratory has demonstrated that seeding ASCs within a soft pullulan–collagen hydrogel enhances ASC survival and improves wound healing. To more fully understand the mechanism of this therapy, we examined whether ASC-seeded hydrogels were able to modulate the recruitment and/or functionality of endogenous progenitor cells. Employing a parabiosis model and fluorescence-activated cell sorting analysis, we demonstrate that application of ASC-seeded hydrogels to wounds, when compared with injected ASCs or a noncell control, increased the recruitment of provascular circulating bone marrow-derived mesenchymal progenitor cells (BM-MPCs). BM-MPCs comprised 23.0% of recruited circulating progenitor cells in wounds treated with ASC-seeded hydrogels versus 8.4% and 2.1% in those treated with controls, p < 0.05. Exploring the potential for functional modulation of BM-MPCs, we demonstrate a statistically significant increase in BM-MPC migration, proliferation, and tubulization when exposed to hydrogel-seeded ASC-conditioned medium versus control ASC-conditioned medium (73.8% vs. 51.4% scratch assay closure; 9.1% vs. 1.4% proliferation rate; 10.2 vs. 5.5 tubules/HPF; p < 0.05 for all assays). BM-MPC expression of genes related to cell stemness and angiogenesis was also significantly increased following exposure to hydrogel-seeded ASC-conditioned medium (p < 0.05). These data suggest that ASC-seeded hydrogels improve both progenitor cell recruitment and functionality to effect greater neovascularization.
Introduction
Chronic wounds are an economic and healthcare burden on society.1 In the United States alone, over 6.5 million patients suffer from chronic wounds, resulting in an annual cost of over $25 billion.2 Treatment for these wounds typically includes a combination of dressings, bedside or operative debridement, negative pressure wound therapy, or a skin substitute such as Apligraf®.3–5 In some cases, autologous skin grafting is required to achieve wound closure.6 Unfortunately, these therapeutic approaches are limited by variable effectiveness, cost, anesthetic demands, and donor site morbidity. Stem cell-seeded skin biomaterials can promote neovascularization and wound healing and represent a promising solution to this clinical need.7–10
Adipose-derived mesenchymal stem cells (ASCs) are a multipotent cell population ideally suited for clinical regenerative applications due to their abundance and ease of harvest from liposuction specimens and their proregenerative paracrine function.11 It has been shown that application of ASCs to wounds enhances healing through release of growth factors and cytokines that promote neovascularization.12,13 However, direct injection of ASCs at the wound site is complicated by poor cell engraftment due to mechanical shear and an ischemic wound microenvironment containing reactive oxygen species (ROS) and inflammatory mediators.14 Therefore, optimizing cell delivery is a critical component of a successful cell-based therapy.
Our laboratory has developed a soft pullulan–collagen hydrogel scaffold that functions as a biomimetic stem cell niche15 to effectively deliver these cells in vivo and leverage their regenerative capacity. We have shown that ASCs seeded in this hydrogel demonstrate upregulation of genes related to stemness and neovascularization, display increased survival potential, and accelerate wound closure in vivo.10 Although hydrogels have been previously designed from polymers, such as alginate, hyaluronic acid, and polyethylene glycol, as well as the extracellular matrix components, collagen, elastin, and fibrin, to replicate the three-dimensional structure of the dermis, these constructs have not demonstrated the ideal properties for cutaneous wound healing, including providing the structural context for in situ skin repair.9 Therefore, we explored pullulan, a homopolysaccharide that is biodegradable, nontoxic, retains water for cell delivery, and contains functional groups that permit cytokine delivery, as a promising material for a hydrogel system for wound healing.9 Furthermore, glucans such as pullulan have been shown to quench free radicals, an important property for cell delivery into the harsh wound microenvironment.9,14
Recently it has been shown that bone marrow-derived mesenchymal stem cells (MSCs) delivered to wounds also increase recruitment of resident progenitor cells in the host,16 likely related to the secretion of chemotactic cytokines. Therefore, we investigated whether our biomimetic hydrogel scaffold enhanced the recruitment of endogenous progenitor cells and whether functionality of the recruited progenitor cells changed. We hypothesized that ASC-seeded hydrogels, compared with injected ASCs alone, would increase the recruitment of these progenitor cells and heighten their neovascular functionality.
In this study, we demonstrate here that ASC-seeded hydrogels enhance the recruitment of circulating progenitor cells to the wound in vivo. Focusing on bone marrow-derived mesenchymal progenitor cells (BM-MPCs),17–19 we show that BM-MPCs are selectively recruited as a greater percentage of total recruited circulating progenitor cells in vivo by ASC-seeded hydrogels and that these cells have increased proliferative, stemness, and angiogenic properties in the presence of hydrogel-seeded ASCs. Ultimately, the dual effects of greater BM-MPC recruitment and heightened cell stemness, angiogenesis, and proliferation may explain the improved wound healing observed with our hydrogel-ASC construct.
Materials and Methods
Experimental design
First, in vivo experiments were conducted to determine whether ASC-seeded hydrogels increase the recruitment of endogenous progenitor cells. Specifically, we established cross-circulation between wild-type (WT) and fluorescent reporter mice before assessing progenitor cell recruitment to WT wounds treated with ASC-seeded hydrogels, ASC injections, or saline controls. Fluorescence-activated cell sorting (FACS) and microfluidic single-cell analyses20 were conducted on WT wounds. After finding a recruitment and gene modulatory effect of ASC-seeded hydrogels on progenitor cells within in vivo wounds, we conducted in vitro experiments to determine exactly how ASC-seeded hydrogels modulate the functionality of recruited progenitor cells. Specifically, BM-MPCs were exposed to conditioned medium from either plated or hydrogel-seeded ASCs before running assays relevant to neovascular functionality, including gene and protein expression, proliferation, tubulization, and migration.
Animals
Mice used in this experiment were housed in the Stanford University Veterinary Service Center and NIH and Stanford University animal care guidelines were followed. All procedures were approved by the university's Administrative Panel on Laboratory Animal Care.
Stem cell isolation and culture
ASCs and BM-MPCs were isolated from WT mice. The former were isolated from the stromal vascular fraction of murine inguinal fat pads, prepared by a harvest of these pads, followed by digestion of the tissue for 1 h in collagenase I (Roche Applied Science, Indianapolis, IN) and centrifugation.11 The latter were isolated from the bone marrow of murine femurs, followed by purification using filtration with a 100-μm cell strainer (BD Biosciences, San Jose, CA), as previously described.17 Cells were cultured separately on plastic culture dishes with standard growth medium (high-glucose Dulbecco's modified Eagle's medium) supplemented with 1% antibiotic–antimycotic (ThermoFisher Scientific, Waltham, MA) and 10% fetal bovine serum and grown to approximately full confluence. Media were changed every 2 days, and ASCs were used before or at passage 2, whereas BM-MPCs were used at passage 2.
Seeding of hydrogel and plated ASCs and conditioned medium harvesting
For in vivo experiments, ASC-seeded hydrogels, ASC injections, and control phosphate-buffered saline (PBS) injections were prepared. Each hydrogel or ASC injection consisted of 2.5 × 105 ASCs. Five percent pullulan–collagen hydrogels were synthesized as described in previous studies.10,15 Briefly, hydrogels were synthesized from the following components: 2 g of pullulan powder, 2 g of sodium trimetaphosphate, 2 g of KCl in 50 mg of NaOH dissolved in deionized H2O up to 10 mL, and with collagen being mixed in at 5% the mass of pullulan.9 Subsequently, the hydrogel was vortexed for half an hour at 4°C to create a homogeneous mixture of polymers. Then, this mixture was poured, compressed to films of 2 mm thickness, and dehydrated in 100% ethanol and dried overnight. Films were washed in PBS at room temperature the next day and stored at 4°C until usage. Overall, a salt-induced phase inversion technique was employed to ensure that hydrogel recapitulated the structure of the skin while remaining soft.9
Then, hydrogels were seeded with 2.5 × 105 ASCs suspended in 15 μL of growth media using a capillary force method, which we have found optimizes time and efficacy of cell seeding.10 For ASC injections, the same number of ASCs suspended in 15 μL of growth media were delivered to the wound, and for the control PBS injection, 15 μL of PBS was delivered to the wound. For in vitro experiments, each ASC-seeded hydrogel was placed in 3 mL of media in a single well of a six-well plate. The other three wells were seeded with 2.5 × 105 ASCs in 15 μL of growth media without hydrogels, as controls. After 48 h of incubation in hypoxia (reflective of the harsh early wound healing environment before development of the vasculature through neovascularization or angiogenesis),21 conditioned media were harvested from the wells with hydrogel-seeded ASCs and from the wells with plated ASCs.
In vivo overview
We employed a series of in vivo experiments to determine how the recruitment of circulating progenitor cells to the wound site as well as colocalization to blood vessels changed when ASCs used to treat the wound were seeded in hydrogel. Briefly, the experiments consisted of a parabiosis model, wounding and treatment, microfluidic single-cell analysis, and FACS and histology.
Parabiosis model, wounding, and treatment
Fluorescent protein (FP)-expressing reporter mice and WT mice were parabiosed, with one reporter and one WT mouse per pair (n = 6), using a standard model (Fig. 1A).22 Mice were joined at their flanks through suturing and staples to skin as a modification of the procedure used by Bunster and Meyer.23–25 In brief, mice were anesthetized and the corresponding lateral aspect of each mouse was shaved and disinfected with Betadine and ethanol solution. Skin incisions from the olecranon to the knee were made on each shaved lateral aspect, and skin and fascia were removed on this corresponding lateral aspect for each mouse. Subsequently, mice were attached using a suture at the olecranon and knee joints and were attached between these joints along their flanks using staples and sutures on both the dorsal and ventral sides.25 Mice were provided with analgesia, an antibiotic diet, and fluids as necessary. After verifying cross-circulation, we created two dorsal excisional wounds on the WT mouse in each pair using a punch biopsy and sutured a silicone ring around each wound.23 At 1 and 4 days postwounding (coinciding with peak neutrophil and macrophage responses), the WT mouse wounds were treated with an ASC-seeded hydrogel (applied directly on top of the wound) or with ASCs through injection without hydrogel or given a control PBS injection. Each form of treatment was prepared directly before application to the wound; for example, ASCs were seeded onto the hydrogel directly before cutaneous application, and for the injection, ASCs were resuspended in 15 μL of growth media directly before delivery. Twenty-four hours after the second treatment, the wounds were harvested and divided into two sections, one used for microfluidics and FACS and the other for histology.
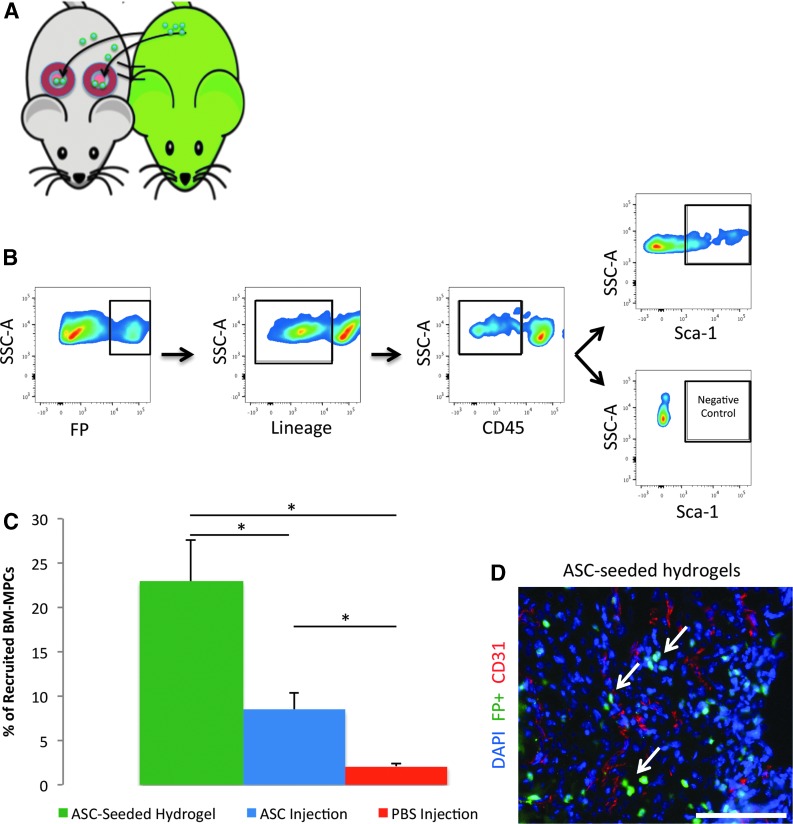
FIG. 1.
ASC-seeded hydrogels increase recruitment of the bone marrow-derived mesenchymal progenitor cell (BM-MPC) population, which is crucial for neovascularization. Using a parabiosis and wounding model and subsequent fluorescence-activated cell sorting, we measured the percentage of cells that were BM-MPCs recruited to wounds by ASC-seeded hydrogels, injected ASCs, and phosphate-buffered saline (PBS) injection. (A) Schematic of parabiosis and wounding model. Through suturing and stables, we joined one reporter and one wild-type (WT) mouse together per parabiosed pair. After verifying cross circulation, we used a punch biopsy to create two dorsal excisional wounds on the WT mouse in each pair and sutured a silicone ring around each wound. (B) Gating scheme of FP+/Lin−/CD45−/Sca1+ used to define BM-MPCs, established in previous work by our laboratory. (C) Percentage of recruited circulating progenitor cells that were FP+/Lin−/CD45−/SCA1+, putative BM-MPCs, was almost 3× greater in wounds treated with ASC-seeded hydrogels compared with those treated with ASC injection (p = 0.017). (D) Recruited fluorescent cells show a predilection for perivascular colocalization. Arrows point to representative recruited perivascular cells. Scale bar = 100 μm. *p ≤ 0.05. ASC, adipose-derived mesenchymal stem cell; FP, fluorescent protein. Color images available online at www.liebertpub.com/tea
FACS and microfluidic single-cell analyses
FACS and microfluidic single-cell analyses were used to determine how treating wounds with WT ASC-seeded hydrogels affected circulating progenitor cell recruitment (as previously described).26,27 Briefly, wound tissue samples were digested in a collagenase solution and stained. Recruited circulating progenitor cells were defined as FP positive (ensuring donor mouse derivation in the parabiosis model) and lineage negative (Lin−; Ter119/CD4/CD8a/Gr-1/CD45R/CD11b, PE-Cy5, eBioscience, San Diego, CA; which excluded differentiated bone marrow-derived cells such as T lymphocytes, B lymphocytes, monocytes/macrophages, natural killer cells, and granulocytes). We then isolated single cells using FACS and assessed recruitment of the neovascular subpopulation of BM-MPCs, defined in prior work as FP+/Lin−/CD45−/Sca1+ progenitor cells (n = 6 pairs/group).17 CD45− gating excluded hematopoietic stem cells. Recruited BM-MPCs were quantified through FACS using the following antibodies: Lin (Pe-Cy5), CD45 (PE-Cy7; eBioscience), and Sca1 (APC; BD Biosciences), according the manufacturer's instructions.
For microfluidic-based single-cell analysis, CellsDirect (Invitrogen, Grand Island, NY) and TaqMan assay primer sets (Applied Biosystems) were used to perform reverse transcription and low cycle preamplification, respectively, on the FP+/Lin− population. Then, quantitative real-time polymerase chain reaction (qPCR) amplification was performed by loading cDNA onto 96.96 Dynamic arrays (Fluidgm, South San Francisco, CA) and using Universal PCR Master Mix (Applied Biosystems) with a uniquely determined TaqMan assay primer set. We then performed unsupervised clustering of the FP+/Lin− cells as previously described (n = 6 pairs per group) and detailed in the Statistical Analysis section.20
Histology
Histology was employed to determine perivascular localization of recruited fluorescent cells, with CD31 staining used to mark endothelial cells in blood vessels (n = 3 pairs/group). After harvesting, wound samples were fixed in 4% paraformaldehyde (PFA) in PBS overnight and dehydrated in a 30% sucrose solution. Then, these samples were fixed in optimal cutting temperature compound (Sakura Finetek U.S., Torrance, CA) and cryosectioned for immunohistochemistry.26 Antigen retrieval was done using a 1% sodium dodecyl sulfate in PBS solution, followed by blocking for 1 h with a 10-fold dilution of PowerBlock in PBST. Then, the primary antibody for CD31 (ab28364; Abcam, Cambridge, MA) was applied overnight, followed by a secondary antibody. Finally, slides were DAPI stained for cell nuclei and mounted. Two sets of three representative images were taken for each wound section, and each set consisted of an image of DAPI-stained cells, an image of fluorescent cells, and an image of CD31-stained blood vessels. An overlay in Photoshop was used to determine whether fluorescent cells colocalized to CD31-stained blood vessels.
In vitro overview
Following the in vivo experiments, we used in vitro studies to focus on the BM-MPC subpopulation of circulating progenitor cells.17 We wanted to determine whether the function of BM-MPCs would change when recruited by ASC-seeded hydrogels. Specifically, we assessed the difference in cell response to conditioned medium from hydrogel-seeded ASCs and conditioned medium from plated ASCs with no hydrogel, with the only difference between the two groups of BM-MPCs being whether they were exposed to conditioned medium from ASCs seeded in hydrogels or those that were plated in a culture dish. Assays included a cell migration assay, a cell proliferation assay, quantitative reverse transcription-PCR (qRT-PCR) for intracellular gene expression, enzyme-linked immunosorbent assays (ELISAs) for intracellular protein expression, and a Matrigel assay to assess tubule formation.
In vitro scratch assay
A scratch assay was used to assess differences in migration between BM-MPCs in conditioned media from hydrogel-seeded ASCs and plated ASCs.28 BM-MPCs were seeded in a six-well plate and grown to full confluence. Twenty-four hours before the assay, cells were serum starved. At time 0, a plus-shaped scratch was made on the bottom of each well using a pipette tip, and cell media were changed to conditioned media from either hydrogel-seeded ASCs or plated ASCs. Mitomycin C (M4287-2 MG) was used as a proliferation inhibitor to ensure that filling of the scratch area was due solely to cell migration. Four representative images were taken for each scratch. Twenty-four hours postscratch, four representative images were taken for each scratch at approximately the same locations as the first images. Change in scratch area was quantified using ImageJ software (NIH, Bethesda, MD).
In vitro cell proliferation
We employed immunocytochemistry, specifically costaining for the cell proliferation marker Ki-67 with DAPI, to determine if there were differences between proliferation rates of BM-MPCs when seeded in conditioned media from hydrogel-seeded ASCs and plated ASCs. BM-MPCs from each group were cultured on three wells of a chamber slide at a concentration of 2.5 × 103 cells/500 μL and were serum starved for 24 h. At time 0, conditioned media from either hydrogel-seeded ASCs or plated ASCs were added to the cells, and after 8 h, cells were fixed in 4% PFA. An anti-Ki-67 antibody (1:50, ab15580; Abcam) was applied overnight, after which secondary antibody was applied for an hour. To quantify, two pairs of representative images were taken per well in the chamber slide, with each set containing a DAPI image and a Ki-67 image of the same region, to determine the fraction of cells that were proliferating. This fraction was defined as the number of Ki-67-positive cells divided by the number of DAPI-positive cells. ImageJ software (NIH) was used for analysis.
In vitro gene expression using qRT-PCR
BM-MPCs were seeded in a six-well plate, with half of the wells containing hydrogel-seeded ASC-conditioned medium and the other half containing plated ASC-conditioned medium. After 48 h of incubation in hypoxia, RNA was isolated using the RNeasy Mini Kit (Qiagen, Germantown, MD), followed by reverse transcription to cDNA (Superscript First-Strand Synthesis Kit; Invitrogen). qPCR was conducted for murine Mmp3 (matrix metalloproteinase 3, Mm00440295_m1), Cxcl12 (stromal cell-derived factor-1/Sdf-1, Mm00445552_m1), Ccl2 (monocyte chemoattractant protein-1/Mcp-1, Mm00441242_m1), Hgf (hepatocyte growth factor, Mm01135193_m1), Fgf2 (fibroblast growth factor-2, Mm00433287_m1), Angpt1 (angiopoietin 1, Mm00456503_m1), Eng (endoglin, Mm00468256_m1), Vegfa (vascular endothelial growth factor-A, Mm01281447_m1), and Hif1a (hypoxia-inducible factor 1 alpha, Mm01283760_m1). These genes were chosen due to their role in neovascularization, BM-MPC homing and recruitment, and wound healing.10,17,27 Gene expression levels were normalized to Actb (beta actin, Mm01205647_g1). Genes were identified using predesigned primers from TaqMan (Thermo Fisher Scientific, Waltham, MA).
In vitro protein expression using ELISA
From the same BM-MPCs used for qRT-PCR, total protein was harvested using radioimmunoprecipitation assay buffer to lyse cells and a protease inhibitor to collect intracellular protein. Individual ELISAs (Quantikine ELISA) were run for both of the intracellular proteins, HGF and MMP3 (R&D Systems, Minneapolis, MN). Then, a microplate reader was used to determine absorbance at the wavelengths, 450 and 540 nm. Concentrations were determined in comparison with a set of standards by taking the difference between each sample's absorbance at these two wavelengths.
In vitro Matrigel assay
A Matrigel assay, based on a protocol used in previous work done by our laboratory,27 was performed to assess differences in direct tubule formation between BM-MPCs in conditioned media from hydrogel-seeded ASCs and in conditioned media from plated ASCs. First, 500 μL of Matrigel basement membrane (BD Biosciences) was pipetted in several wells in a 24-well plate. The membrane was then stored at 37°C for 1 h for solidification. Following this time period, BM-MPCs were seeded in either hydrogel-seeded ASC-conditioned medium or plated ASC-conditioned medium at a density of 4.0 × 104 cells/500 μL on top of the Matrigel. After 24 h, images of three high-powered fields were taken for each well to assess tubule formation at 10× magnification. The number of tubules in each well was manually counted.
Statistical analysis
A two-tailed unpaired Student's t-test was used to assess statistically significant differences between the control group and treatment group. Results were considered significant if p ≤ 0.05. Microfluidic single-cell transcriptional data were analyzed as previously described.20 Briefly, gene expression data were normalized relative to the median expression for each gene in the pooled sample and converted to base 2 logarithms. Absolute bounds (±5 cycle thresholds from the median, corresponding to 32-fold increases/decreases in expression) were set, and clustergrams were generated using hierarchical clustering (with a complete linkage function and Euclidean distance metric) to facilitate data visualization using MATLAB (R2011b; MathWorks, Natick, MA).
To detect single-cell transcriptional data overlapping patterns, k-means clustering using a standard Euclidean distance metric was employed. Each cell was assigned membership to a specific cluster as dictated by similarities in expression profiles in MATLAB. Following Bonferroni correction for multiple samples using a strict cutoff of p ≤ 0.05, nonparametric two-sample Kolmogorov–Smirnov tests were then used to identify genes with expression patterns that differed significantly between population clusters and/or groups.
Results
ASC-seeded hydrogels increase BM-MPC recruitment to wounds
We employed an in vivo parabiosis model (Fig. 1A) to study the effect of ASC hydrogel seeding on endogenous progenitor cell recruitment and localization of recruited progenitor cells to critical wound structures. FACS quantification of broadly defined circulating progenitor cells (FP+/Lin−) and bone marrow-derived mesenchymal progenitor cells (BM-MPCs, defined as FP+/Lin−/CD45−/SCA1+, with Lin− excluding differentiated bone-marrow derived cells and CD45− excluding hematopoietic stem cells) for each condition within the wounds (Fig. 1B) demonstrated a statistically significant increase in the percentage of BM-MPCs recruited to wounds treated with ASC-seeded hydrogels compared with those treated with injected ASCs alone and control PBS injection (Fig. 1C). Specifically, BM-MPCs comprised 23% of the recruited circulating progenitor cells in wounds treated with ASC-seeded hydrogels, as opposed to 8.4% of such cells in wounds treated with ASC injection alone (p = 0.017) and 2.1% of such cells in wounds treated with PBS alone (p = 0.0012). These data indicated that using the hydrogel in conjunction with ASCs for wound treatment increases BM-MPC recruitment to the wound site.
Separately, using CD31 staining, we assessed colocalization of recruited circulating cells to blood vessels within the wound site and found that recruited fluorescent cells homed to sites of neovascularization when wounds were treated with ASC-seeded hydrogels, consistent with a regenerative role for these recruited cells (Fig. 1D).
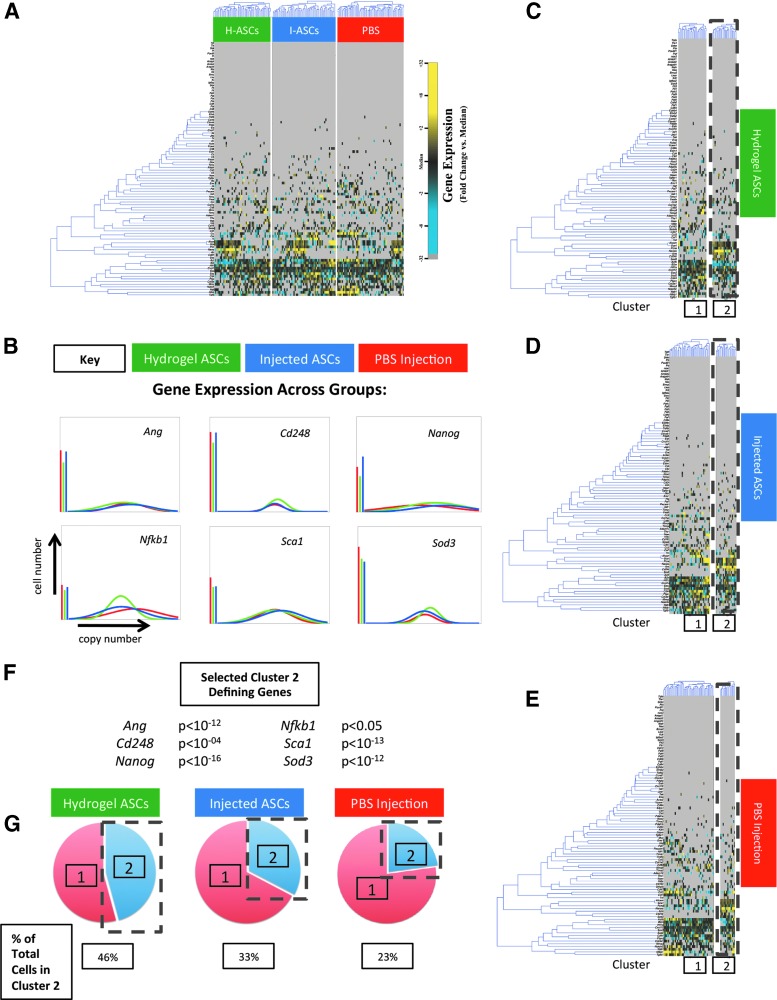
Microfluidic-based single-cell analysis was then used to interrogate the recruitment of circulating progenitor cells in wounds treated with ASC-seeded hydrogels, injected ASCs without hydrogels, or control PBS injection. We characterized the expression profiles in the recruited, broadly defined circulating progenitor cells (FP+/Lin−) for each condition of more than 90 gene targets related to stemness and neovascularization at the single-cell level. This gene list was compiled following an extensive literature search for genes related to neovascularization and stemness, including manuscripts previously published by our laboratory10,17,27 (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/tea). Interestingly, while significant heterogeneity in gene expression was observed within the recruited circulating progenitor cells across all groups (Fig. 2A), population-level analyses between the treatment groups largely did not reach statistical significance (Fig. 2B). Using an unsupervised clustering analysis to uncover potential differences in subsets of recruited cells between the treatment groups, we identified a subpopulation of these recruited circulating progenitor cells defined by the expression of provasculogenic and tissue remodeling genes, including Ang, Cd248, Nanog, Nfkb1, Sca1, and Sod3 (all p < 0.05) (Fig. 2C–F). This population also lacked expression of the pan-hematopoietic cell marker, Cd45, consistent with it being a putative BM-MPC (FP+/Lin−/CD45−/Sca1+). While this putative BM-MPC population was present in all conditions, its relative percentage was markedly increased in wounds following application of ASC-seeded hydrogels (45.8% of recruited circulating progenitor cells) compared with injected ASCs and PBS control (32.7% and 22.8% of recruited circulating progenitor cells, respectively) (Fig. 2G). Collectively, the FACS and microfluidics data are consistent with an increased in vivo capacity of ASC-seeded hydrogels to selectively recruit endogenous BM-MPCs to wounds.
FIG. 2.
ASC-seeded hydrogels increase recruitment of a subpopulation of circulating progenitor cells that express stemness and neovascularization-related genes to excisional wounds in vivo. Using a parabiosis model between WT and fluorescent mice and microfluidics, we measured circulating progenitor cell recruitment to dorsal excisional wounds created on the WT mouse that were treated with ASC-seeded hydrogels, injected ASCs (no hydrogel), or a PBS injection. (A) Clustering of cells from wounds treated with ASC-seeded hydrogels, injected ASCs, and PBS injection. Gene expression for over 90 targets in individual cells is shown as a fold change from the median using a color scale we have previously employed: blue (low expression, 32-fold below the median) to yellow (high expression, 32-fold above the median, gray represents nonexpression). Each row displays the expression of a particular gene across all single cells sampled from the wounds treated with ASC-seeded hydrogels (green in the top bar), injected ASCs (blue), and control PBS injection (red). Each column displays a single cell from each group. Supplementary Table S1 displays the complete dataset. (B) Median-centered Gaussian fit curves of genes related to neovascularization and stemness displaying similarities in expression across groups on a population level. (C–E) k-Means clustering revealed two circulating progenitor cells subgroups, which were present in all treatment groups (k = 2). (F) Cluster 2-defining genes and p-values demonstrate a significant increase in the expression of genes related to neovascularization and tissue remodeling in this cluster. This cluster also expressed Sca1 and lacked expression of Cd45, consistent with it being a putative BM-MPC. (G) Percentage of circulating cluster 2 progenitor cells among all cells recruited by each type of treated wound (ASC-seeded hydrogel, ASC injection alone, PBS injection). The percent of cells that were in cluster 2 (the putative BM-MPC subpopulation) was greatest in wounds treated with ASC-seeded hydrogels. Color images available online at www.liebertpub.com/tea
ASC-seeded hydrogels upregulate BM-MPC migration and proliferation in vitro
To better understand these in vivo findings, we assessed the mechanism(s) by which ASC-seeded hydrogels modulate BM-MPC behavior in vitro. Specifically, we studied BM-MPC migration and proliferation.
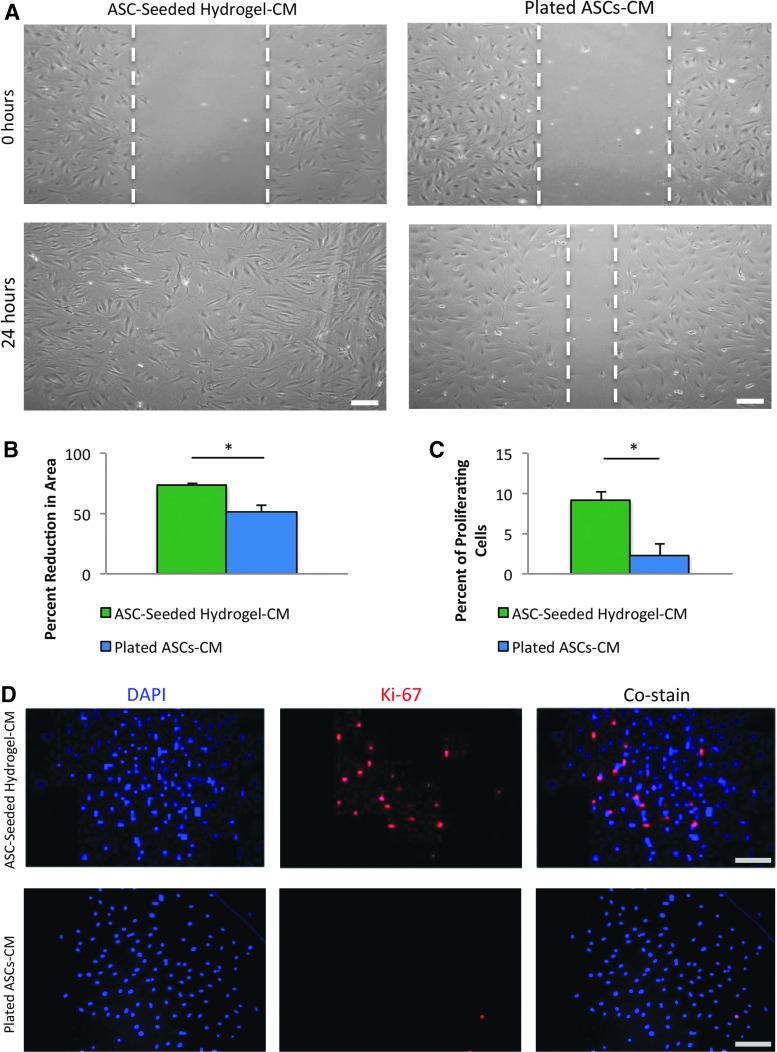
We used a scratch assay to assess BM-MPC migration. Specifically, we compared scratch area reduction between BM-MPCs that were exposed to conditioned medium from hydrogel-seeded ASCs and BM-MPCs that were exposed to conditioned medium from ASCs subjected to standard culture conditions. We noted a statistically significant increase of 44% in BM-MPC scratch area reduction when exposed to conditioned medium from hydrogel-seeded ASCs compared with conditioned medium from plated ASCs (73.8 ± 0.98 vs. 51.4 ± 5.79, p = 0.019) (Fig. 3A, B). This increase in scratch closure (a surrogate for cell mobility critical for both recruitment and movement within the wound bed) suggests that ASC-seeded hydrogels promote BM-MPC migration and is one mechanistic explanation for the increase in BM-MPCs observed in vivo.
FIG. 3.
Hydrogel-seeded ASC-conditioned medium increases BM-MPC functionality, specifically cell migration and cell proliferation. A scratch assay and Ki-67 immunocytochemistry were performed on BM-MPCs exposed to either hydrogel-seeded ASC-conditioned medium or plated ASC-conditioned medium. (A) Representative images of the scratch assay, with the 0 h time point denoting the full size scratch and the 24 h time point denoting the scratch after migration. Scale bar = 100 μm. (B) Scratch assay demonstrates a statistically significant increase in cell migration of 44%, measured by scratch area closure, in BM-MPCs exposed to hydrogel-seeded ASC-conditioned medium. (C) Ki-67 staining reveals a statistically significant 4× increase in cell proliferation in BM-MPCs exposed to hydrogel-seeded ASC-conditioned medium. (D) Representative images of stained cell nuclei (DAPI), proliferating cells (Ki-67), and the costain for BM-MPCs exposed to hydrogel-seeded ASC-conditioned medium (top row) and for BM-MPCs exposed to plated ASC-conditioned medium (bottom row). Scale bar = 100 μm. *p ≤ 0.05. Color images available online at www.liebertpub.com/tea
We next used immunocytochemistry, staining for DAPI and Ki-67, to determine if cell proliferation was increased in BM-MPCs exposed to conditioned medium from hydrogel-seeded ASCs. We found over fourfold increase in the percent of proliferating cells in BM-MPCs exposed to hydrogel-seeded ASC-conditioned medium compared with their counterparts exposed to plated ASC-conditioned medium (9.17 ± 1.04, 2.26 ± 1.44, p = 0.0027) (Fig. 3C, D). Increased cell proliferation due to enhanced paracrine function of ASCs seeded in hydrogel is another possible mechanism by which ASC-seeded hydrogels increase the recruitment of BM-MPCs to excisional wounds in vivo.
ASC-seeded hydrogels increase angiogenic potential and tubulization of BM-MPCs in vitro
Aside from increasing their recruitment and proliferation, we hypothesized that ASC-seeded hydrogels could also modulate BM-MPC neovascular function. Total RNA and protein were isolated from cultured BM-MPCs exposed to conditioned media from either ASCs seeded in hydrogels or plated ASCs.
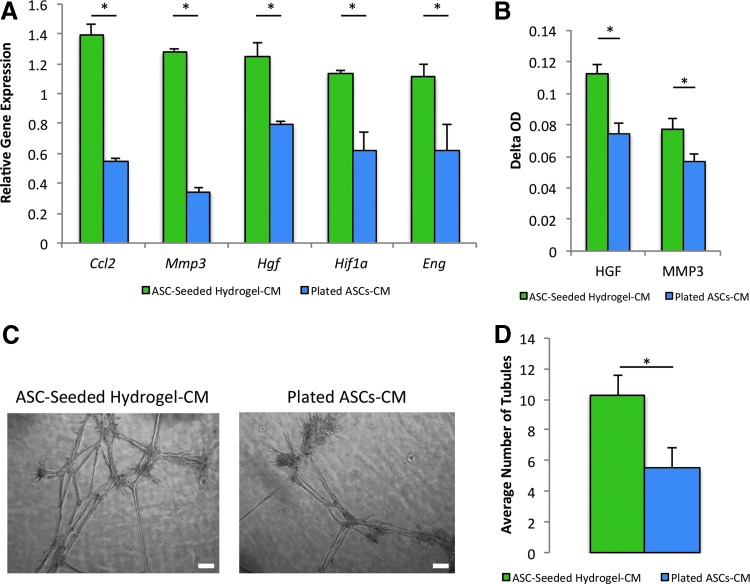
qRT-PCR revealed a statistically significant increase in the expression of several angiogenic genes in BM-MPCs exposed to conditioned media from ASCs seeded in hydrogel. Specifically, the levels of critical cytokines and transcription factors related to wound healing, BM-MPC homing and recruitment, and neovascularization were elevated relative to BM-MPCs exposed to control plated ASC-conditioned medium, including Ccl2 (1.39 ± 0.07 vs. 0.55 ± 0.03, p = 0.0032), Mmp3 (1.28 ± 0.02 vs. 0.34 ± 0.03, p = 0.00014), Hgf (1.25 ± 0.09 vs. 0.79 ± 0.02, p = 0.034), Hif1a (1.14 ± 0.02 vs. 0.61 ± 0.13, p = 0.0076), and Eng (1.11 ± 0.09 vs. 0.62 ± 0.17, p = 0.041) (Fig. 4A).
FIG. 4.
Hydrogel-seeded ASC-conditioned medium upregulates BM-MPC expression of stemness and angiogenic markers and increases BM-MPC tubulization. BM-MPCs were exposed to conditioned medium from either hydrogel-seeded ASCs or plated ASCs, and a qRT-PCR, ELISAs, and Matrigel assay were performed to assess transcriptional and protein expression of markers of stemness and angiogenesis and tubulization. (A) qRT-PCR reveals a statistically significant increase in several markers of stemness and angiogenesis in BM-MPCs exposed to hydrogel-seeded ASC-conditioned medium. (B) ELISAs demonstrate a statistically significant increase in these markers at the protein level. (C) Representative images of tubulization for the Matrigel assay conducted with BM-MPCs exposed to hydrogel-seeded ASC-conditioned medium and BM-MPCs exposed to plated ASC-conditioned medium. Scale bar = 100 μm. (D) Matrigel assay quantification reveals a statistically significant increase of 86% in tubule formation in BM-MPCs exposed to hydrogel-seeded ASC-conditioned medium. *p ≤ 0.05. ELISAs, enzyme-linked immunosorbent assays; qRT-PCR, quantitative reverse transcription-PCR. Color images available online at www.liebertpub.com/tea
We then corroborated the transcriptional data on a protein level. ELISAs demonstrated a statistically significant increase in protein expression for select angiogenesis-related molecules, specifically the matrix remodeling peptidase MMP3 (0.11 ± 0.0065 vs. 0.074 ± 0.0075, p = 0.015) and the growth factor HGF (0.077 ± 0.0065 vs. 0.057 ± 0.0048, p = 0.015) in the BM-MPCs exposed to hydrogel-seeded ASC-conditioned medium (Fig. 4B).
Finally, we employed a Matrigel assay to assess differences in tubulization (an in vitro surrogate for blood vessel formation) of BM-MPCs when exposed to conditioned medium from plated ASCs or from ASCs seeded in hydrogels (Fig. 4C). The assay revealed a statistically significant increase in tubule formation of 86% for BM-MPCs exposed to conditioned medium from ASCs seeded in hydrogel (10.26 ± 1.38, 5.51 ± 1.28, p = 0.045) (Fig. 4D).
Discussion
Hydrogel delivery of stem cells has been shown to significantly enhance wound healing.14,15,29–32 Hydrogels improve stem cell survival within the wound,14 increase the local production of growth factors and chemokines, sequester secreted growth factors, and slowly release these bioactive molecules through the progressive phases of wound healing.29,30 Furthermore, hydrogels can maintain the morphology of seeded stem cells and control the differentiation of these cells into specific lineages based on cellular interactions with small-molecule functional groups present in the hydrogel.31 Last, hydrogels address a commonly cited limitation of cell-based therapies–the lack of an effective delivery system–and in doing so, improve the viability of cells applied to the wound.15,32
Using a pullulan–collagen hydrogel developed in our laboratory, we previously reported enhancement of the viability of transplanted MSCs and ASCs within the wound.10,15 We believe shielding seeded cells from ROS and inflammatory factors, resulting in enhanced cell survival, is responsible for this effect.14 Furthermore, we have shown that seeding ASCs in the hydrogel environment results in increased expression of the embryonic transcription factor, Oct4, enhancing the stemness of cells delivered to the wound.10 Lastly, pullulan has water retention and cross-linking properties that allow for the delivery of seeded ASCs and small molecules, such as cytokines produced by ASCs, to promote neovascularization and subsequent healing.9 Accordingly, by enhancing seeded cell survival and delivery of cytokines to the wound, the pullulan–collagen hydrogel creates a high concentration of therapeutic small molecules and cytokines produced by ASCs at the wound such as VEGF-A and MCP-1, which play roles in BM-MPC recruitment to the wound site.15 Therefore, we hypothesized in this work that ASC-seeded hydrogels, compared with injected ASCs alone, would increase the recruitment of progenitor cells and heighten their neovascular functionality by enhancing the stemness and angiogenic properties of these recruited cells.
Additionally, we have shown that seeding ASCs in the pullulan–collagen hydrogel upregulates their expression of the master chemokine, Sdf-1.10 The SDF-1/CXCR4 pathway is a likely means of mobilizing BM-MPCs and other progenitors,32 making ASC-seeded hydrogel therapy a potentially promising method of promoting endogenous progenitor cell recruitment to the wound.10 We and others have described how SDF-1 interaction with its receptor, CXCR-4, mobilizes bone marrow cells to the wound site.32–34 In brief, hypoxia-inducible factor-1 (HIF-1) regulates the expression of SDF-1, creating expression of the cytokine in ischemic tissue. This process increases the homing, migration, and adhesion of circulating progenitor cells from the bone marrow that express CXCR-4 to ischemic tissue such as the wound site.33 In this current study, SDF-1 produced by ASCs seeded in hydrogel and delivered by the hydrogel is posited to increase the homing, migration, and adhesion of CXCR-4-positive BM-MPCs to the wound site.
In this study, we investigated the potential of ASC-seeded hydrogels to increase endogenous progenitor cell recruitment to the wound and enhance the function of the recruited progenitor cells. Microfluidics and FACS were employed in single-cell transcriptional analysis of these recruited progenitor cells (FP+/Lin−), comparing the expression levels of over 90 genes related to stemness and neovascularization between treatment groups, and both were crucial to identifying changes at the cell subpopulation level. Specifically, using these methods, we found that although transcriptional differences between the total population of FP+/Lin− cells recruited by the treatment and control groups did not reach statistical significance, the recruitment of a subset of the FP+/Lin− population, identified as putative BM-MPCs, was selectively increased in wounds treated with ASC-seeded hydrogels compared with controls. These results have implications for the treatment of chronic wounds as BM-MPCs not only promote neovascularization but also produce anti-inflammatory cytokines that have the potential to advance chronic wounds past the chronic low-grade inflammation typically associated with poorly healing diabetic ulcers.35–37
Several methods have previously been used to recruit endogenous progenitor cells, including injection of cytokines involved in stem cell homing, such as SDF or HGF, and gene therapy.32,38 Polymeric hydrogels seeded with stem cells, however, display several advantages over these methods. Hydrogels promote sustained expression of cytokines involved in progenitor cell recruitment, rather than the transitory increase achieved through injection, and they avoid the safety concerns involved in gene therapy, such as the use of viral vectors and the regulation of transgene expression after completing therapeutics.32 Additionally, our in vitro experiments using conditioned medium from ASC-seeded hydrogels and plated ASCs to assess BM-MPC neovascular functionality suggest that ASC-seeded hydrogels enhance the function of recruited progenitor cells, increasing the expression of angiogenic and stemness-related genes, as well as their capacity for proliferation, migration, and tubulization. We have previously shown how seeding ASCs in the pullulan–collagen hydrogel results in increased expression of growth factors and cytokines related to wound healing, including SDF1, VEGF-A, and HGF,10 indicating that the increased presence of such factors in the ASC-seeded hydrogel-conditioned medium likely heightened the properties of BM-MPCs relevant to recruitment such as migration and proliferation. Ultimately, hydrogels, such as our pullulan–collagen biomaterial, serve as a medium for exogenous stem cell delivery while also promoting endogenous stem cell recruitment to wounds.
Limitations
This study has demonstrated that ASC-seeded hydrogels effect greater neovascularization and wound healing, in part, by increasing the recruitment and neovascular functionality of endogenous progenitor cells, specifically BM-MPCs. However, the specific role of these BM-MPCs upon homing to the wound is beyond the scope of this study and to be elucidated. In particular, it is unclear whether these BM-MPCs differentiate into endothelial cells, for example, and become part of the vasculature, or if they play a different supportive role at the wound site in promoting neovascularization and wound healing. Currently, our laboratory is conducting further work that addresses this question.
Conclusion
In elucidating the properties by which ASC-seeded hydrogels effect neovascularization, our work has demonstrated that this biomaterial has promise in leveraging the recruitment of endogenous progenitor cells and upregulating the stemness and angiogenic properties of these cells, leading to improved wound healing. In summary, our experiments supported our hypothesis in demonstrating that ASC-seeded hydrogels effect greater neovascularization through both increasing BM-MPC recruitment and upregulating BM-MPC angiogenic functionality.
Supplementary Material
Acknowledgments
The authors are grateful for the grant awarded to G.C.G from the Armed Forces Institute of Regenerative Medicine, DOD #W81XWH-08–2-0032, the grant awarded to R.K. from Stanford University's Office of Undergraduate Advising and Research, the 2014 Major Grant, the Hagey Laboratory for Pediatric Regenerative Medicine, and the Oak Foundation. The authors would also like to thank Yujin Park for her assistance with histologic processing and staining. All cell sorting was completed at the Stanford Shared FACS Facility.
Disclosure Statement
The authors have nothing to disclose.
References
- 1.Gurtner G.C., Werner S., Barrandon Y., and Longaker M.T. Wound repair and regeneration. Nature 453, 314, 2008 [DOI] [PubMed] [Google Scholar]
- 2.Sen C.K., et al. Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regen 17, 763, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wong V.W., and Gurtner G.C. Tissue engineering for the management of chronic wounds: current concepts and future perspectives. Exp Dermatol 21, 729, 2012 [DOI] [PubMed] [Google Scholar]
- 4.Zaulyanov L., and Kirsner R.S. A review of a bi-layered living cell treatment (Apligraf) in the treatment of venous leg ulcers and diabetic foot ulcers. Clin Interv Aging 2, 93, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schlatterer D., and Hirshorn K. Negative pressure wound therapy with reticulated open cell foam-adjunctive treatment in the management of traumatic wounds of the leg: a review of the literature. J Orthop Trauma 22, S152, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Capla J.M., et al. Skin graft vascularization involves precisely regulated regression and replacement of endothelial cells through both angiogenesis and vasculogenesis. Plast Reconstr Surg 117, 836, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Griffith L.G., and Naughton G. Tissue engineering—current challenges and expanding opportunities. Science 295, 1009, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Celiz A.D., et al. Materials for stem cell factories of the future. Nat Mater 13, 570, 2014 [DOI] [PubMed] [Google Scholar]
- 9.Wong V.W., et al. Engineered pullulan-collagen composite dermal hydrogels improve early cutaneous wound healing. Tissue Eng Part A 17, 631, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garg R.K., et al. Capillary force seeding of hydrogels for adipose-derived stem cell delivery in wounds. Stem Cells Transl Med 3, 1079, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Glotzbach J.P., Wong V.W., Gurtner G.C., and Longaker M.T. Regenerative medicine. Curr Probl Surg 48, 148, 2011 [DOI] [PubMed] [Google Scholar]
- 12.Levi B., et al. Human adipose derived stromal cells heal critical size mouse calvarial defects. PLoS One 5, e11177, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rehman J., et al. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation 109, 1292, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Wong V.W., et al. Pullulan hydrogels improve mesenchymal stem cell delivery into high-oxidative-stress wounds. Macromol Biosci 11, 1458, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rustad K.C., et al. Enhancement of mesenchymal stem cell angiogenic capacity and stemness by a biomimetic hydrogel scaffold. Biomaterials 33, 80, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shin L., and Peterson D.A. Human mesenchymal stem cell grafts enhance normal and impaired wound healing by recruiting existing endogenous tissue stem/progenitor cells. Stem Cells Transl Med 2, 33, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Januszyk M., et al. Diabetes irreversibly depletes bone marrow-derived mesenchymal progenitor cell subpopulations. Diabetes 63, 3047, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen J.S., Wong V.W., and Gurtner G.C. Therapeutic potential of bone marrow-derived mesenchymal stem cells for cutaneous wound healing. Front Immunol 3, 192, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hamou C., et al. Mesenchymal stem cells can participate in ischemic neovascularization. Plast Reconstr Surg 123, 45S, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Glotzbach J.P., et al. An information theoretic, microfluidic-based single cell analysis permits identification of subpopulations among putatively homogeneous stem cells. PLoS One 6, e21211, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hong W.X., et al. The role of hypoxia-inducible factor in wound healing. Adv Wound Care 3, 390, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kamran P., et al. Parabiosis in mice: a detailed protocol. J Vis Exp 2013. [Epub ahead of print]; DOI: 10.3791/50556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wong V.W., Sorkin M., Glotzbach J.P., Longaker M.T., and Gurtner G.C. Surgical approaches to create murine models of human wound healing. J Biomed Biotechnol 2011, 969618, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bunster E., and Meyer R.K. An improved method of parabiosis. Anat Rec 57, 339, 1933 [Google Scholar]
- 25.Maan Z.N., et al. Cell recruitment by amnion chorion grafts promotes neovascularization. J Surg Res 193, 953, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suga H., et al. Tracking the elusive fibrocyte: identification and characterization of collagen-producing hematopoietic lineage cells during murine wound healing. Stem Cells 32, 1347, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rennert R.C., et al. Diabetes impairs the angiogenic potential of adipose-derived stem cells by selectively depleting cellular subpopulations. Stem Cell Res Ther 5, 79, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liang C.C., Park A.Y., and Guan J.L. In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc 2, 329, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Kim J., et al. Bone regeneration using hyaluronic acid-based hydrogel with bone morphogenic protein-2 and human mesenchymal stem cells. Biomaterials 28, 1830, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Park H., Temenoff J.S., Tabata Y., Caplan A.I., and Mikos A.G. Injectable biodegradable hydrogel composites for rabbit marrow mesenchymal stem cell and growth factor delivery for cartilage tissue engineering. Biomaterials 28, 3217, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Benoit D.S., Schwartz M.P., Durney A.R., and Anseth K.S. Small functional groups for controlled differentiation of hydrogel-encapsulated human mesenchymal stem cells. Nat Mater 7, 816, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rennert R.C., Sorkin M., Garg R.K., and Gurtner G.C. Stem cell recruitment after injury: lessons for regenerative medicine. Regen Med 7, 833, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ceradini D.J., et al. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med 10, 858, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Abbott J.D., et al. Stromal cell-derived factor-1alpha plays a critical role in stem cell recruitment to the heart after myocardial infarction but is not sufficient to induce homing in the absence of injury. Circulation 110, 3300, 2004 [DOI] [PubMed] [Google Scholar]
- 35.Aggarwal S., and Pittenger M.F. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 105, 1815, 2005 [DOI] [PubMed] [Google Scholar]
- 36.Rahavi H., Hashemi S.M., Soleimani M., Mohammadi J., and Tajik N. Adipose tissue-derived mesenchymal stem cells exert in vitro immunomodulatory and beta cell protective functions in streptozotocin-induced diabetic mice model. J Diabetes Res 2015, 878535, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yolanda M.-M., Maria A.-V., Amaia F.-G., Marcos P.-B., Silvia P.L., Dolores E., and Jesús O.-H. Adult stem cell therapy in chronic wound healing. J Stem Cell Res Ther 4, 162, 2014 [Google Scholar]
- 38.Zhao J., Zhang N., Prestwich G.D., and Wen X. Recruitment of endogenous stem cells for tissue repair. Macromol Biosci 8, 836, 2008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.