Abstract
Osteoarthritis is the leading cause of disability in the US. Consequently, there is a pressing need for restoring the structural and functional properties of diseased articular cartilage. Yet the search for the right combination of proper target cells and growth factors for cartilage regeneration remains challenging. In this study, we first tested the intrinsic chondrogenic differentiation ability of human perivascular stem cells (hPSCs), a novel source of mesenchymal stem cells (MSCs) isolated by fluorescence-activated cell sorting (FACS) from human adipose tissue. A putative prochondrogenic growth factor, NEL-like molecule-1 (NELL-1), was added to the hPSC pellets to upregulate gene expression of chondrogenic markers, including AGGRECAN, COLLAGEN II, and COMP. Furthermore, the addition of NELL-1 to a transforming growth factor beta 3 (TGF-β3) + bone morphogenetic protein-6 (BMP-6) “cocktail” resulted in the best combinatorial stimulation in accelerating the chondrogenic differentiation of hPSCs, as evidenced by increased gene and protein expression of chondrogenic markers in a shortened induction time without elevating expression of hypertrophic, fibrotic, and osteogenic markers. Mechanistically, this acceleration rendered by NELL-1 may be partially attributed to NELL-1's upregulation of BMP receptors and TGF-β receptor type I in hPSCs for increased responsiveness to BMPs + TGF-βs. In conclusion, lipoaspirate-derived hPSCs present a novel and abundant cell source of MSCs for cartilage regeneration, and the combinatorial application of NELL-1, TGF-β3, and BMP-6 with hPSCs may remarkably enhance and accelerate cartilage repair.
Introduction
Articular cartilage is one of the tissues most susceptible to injury and age-related degeneration.1 Naturally, osteoarthritis is the most common cause of disability in American adults.1 Approximately 50 million US adults suffer from arthritis, costing over $128 billion annually.2 Alarmingly, recent studies suggest that younger adults are also suffering from osteoarthritis3 associated with trauma and occupation-related joint stress.4 Thus, there is a pressing need to restore the function of injured and diseased articular cartilage.
To address this unmet need, a multitude of tissue engineering approaches have been attempted. However, they are limited by the availability of cell sources. Cell-based methods using autologous chondrocytes such as Carticel are suboptimal due to donor site morbidity and dedifferentiation during in vitro expansion.5–7 Because the long-term results of the current standard therapy are unsatisfactory, the possibility of using mesenchymal stem cells (MSCs) for treating articular cartilage defects and osteoarthritis is attracting more attention.8–10 However, when used alone or with the chondrogenic inductive growth factors, transforming growth factor beta (TGF-β),11–14 MSCs produce fibrocartilage with osteophyte formation.15–22 In addition, traditional MSC sources, such as those from the bone marrow, not only require prolonged culturing but also lack consistent cell identity, purity, and potency.23–28 Thus, the identification of a new source of stem cells, more specific prochondrogenic factors, and novel therapeutic strategies has been imperative yet challenging.
Perivascular stem cells (PSCs) derived from adipose tissue are highly purified, prospectively isolated type of MSCs that overcome the limitations faced by traditional MSC sources such as long derivation times, highly heterogeneous cell population, and variable potency.23–28 This cell source represents an advantage over other cell sources such as bone marrow stem cells (BMSCs) or primary chondrocytes, both of which require weeks for isolation and expansion. In addition, PSCs are a relatively homogenous MSC population and are free of hematopoietic, endothelial, and fibroblastic cell types.23,24,28 These characteristics of high purity, speedy isolation, and reproducibility make PSCs an ideal cell source in the regeneration of specific types of mesenchymal tissues if provided with the proper microenvironment.29
Nel-like molecule-1 (NELL-1) is a potent, cell- and stage-specific growth factor that promotes osteochondrocyte function. NELL-1 is a secreted molecule that contains putative motifs of several known growth factor families, including an N-terminal thrombospondin-1 module, five von Willebrand factor C domains, and six epidermal growth factor (EGF)-like domains.30 Loss of Nell-1 results in severe bone and cartilage abnormalities in the skull, vertebral column, ribcage, and long bones.31 Particularly, Nell-1 deficiency results in reduced expression of a number of cartilage-related genes, including Collagens, Tenascins, Matrilins, and Chondroadherin.31 Recently published studies demonstrate the successful utility of NELL-1 protein for cartilage tissue engineering: (1) in vitro, NELL-1 can increase cartilage matrix deposition, while preventing chondrocyte dedifferentiation32 and (2) in vivo, NELL-1 promotes healing of rabbit articular cartilage defects with a tissue that closely replicates native articular cartilage with similar patterns of Safranin-O-positive matrix, columnar pattern of chondrocytic cells, and intervening robust type II collagen deposition.33 These features make NELL-1 an ideal candidate growth factor for articular cartilage regeneration.
Because the effects of NELL-1 on the chondrogenic differentiation of MSCs have not been identified, and the side effects of other commonly used molecules such as TGF-βs and bone morphogenetic proteins (BMPs)34–38 have been reported, this study seeks to develop a more efficacious combinatorial strategy than the ones currently available by using prospectively isolated MSCs, namely PSCs, and an experimentally proven prochondrogenic factor, NELL-1. Such strategy will build the foundation of a novel cell-based therapy for cartilage repair that will significantly benefit patients suffering from osteoarthritis and other chondrogenic-deficient conditions.
Materials and Methods
Isolation of human stromal vascular fraction from human lipoaspirate and purification of human perivascular stem cell from human stromal vascular fraction
The isolation of human stromal vascular fraction (SVF) and purification of human perivascular stem cells (hPSCs) followed standard procedures as previously described.27,28,39–42 No patient identifiers were obtained, and therefore no University of California, Los Angeles (UCLA) institutional review board approval was required [45 CFR 46.102(f)]. In brief, human lipoaspirate from 6 cosmetic liposuction patients were collected and digested with collagenase (Sigma-Aldrich, St. Louis, MO) to get human SVF. Adipocytes were separated by centrifugation and excluded. Red blood cells were removed by adding a red cell lysis buffer (eBioscience, San Diego, CA) to SVF. The processed SVF was filtered through a 70-μm cell filter. The resulting hSVF was immediately processed for hPSC purification.
The isolated hSVF was incubated with the following conjugated antibodies: anti-CD34-phycoerythrin (Dako, Glostrup, Denmark), anti-CD45-allophycocyanin (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), and anti-CD146-fluorescein isothiocyanate (AbD Serotec, Raleigh, NC). The resulting cell population was processed on the fluorescence-activated cell sorting (FACS) Aria cell sorter (BD Biosciences). 4′,6-Diamidino-2-phenylindole was used for exclusion of any nonviable cells in cell sorting. Consequently, two populations of cells were sorted according to their cell surface markers to constitute hPSCs: distinct pericytes (CD34−, CD146+, CD45−) and adventitial cells (CD34+, CD146−, CD45−)27,39,40,43,44 (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/tea).
Human articular cartilage sample preparation and human articular chondrocyte isolation
Human articular cartilage samples were obtained from six knee replacement patients. No patient identifiers were obtained, and therefore no University of California, Los Angeles, institutional review board approval was required [45 CFR 46.102(f)]. The samples were fixed in 4% paraformaldehyde (PFA, Sigma-Aldrich) at 4°C overnight and decalcified with 19% ethylenediaminetetraacetic acid (EDTA; Sigma-Aldrich) for 2 weeks before paraffin embedding.
Human articular chondrocytes (hARCs) were isolated from pooled femoral and tibial articular cartilage with healthy appearance from six knee replacement patients by incubating with 1 mg/mL trypsin (Sigma-Aldrich) for 1 h followed by an overnight digestion in 0.5 mg/mL type II collagenase. The following morning, the isolated chondrocytes were washed with a complete medium (MEM-α + 10% FBS) and seeded at the density of 1 × 104 cells/mm2 for culture expansion. The medium was changed every 3 days. After reaching 70% confluence, cells were used for RNA isolation or immunohistochemistry staining of NELL-1.
Induction of in vitro chondrogenesis
Human BMSCs were purchased from Promo Cell (Catalog Number: C-12975, Lot Number: 1080401.3).
hARCs and hPSCs at passage 0 and hBMSCs at passage 3 were used for pellet culture. Cells were suspended in basic chondrogenic media (DMEM high glucose [Invitrogen, Carlsbad, CA] + 1% ITS + 100 units/mL Penicillin + 100 μg/mL streptomycin + 50 μg/mL ascorbic acid +1 mM sodium pyruvate + 40 μg/mL proline [Sigma-Aldrich]) at a density of 1 × 106 cells/mL. Spheroids of 3 × 105 cells were formed by centrifuging 300 μL of the cell suspension at 500 g in 15-mL centrifuge tubes. The selection and doses of growth factors, including TGF-β3, BMP-6, and NELL-1, were based on previous publications,32,33,45 and the growth factors added to the 500 μL culture medium for each pellet based on the groups are shown in Table 1. The manufacturers and catalog numbers of all the growth factors are listed in Supplementary Table S1. The pellets were incubated at 37°C and 5% CO2 for 0, 7, 14, 21, 28, 35, 42, 49, or 56 days (0–8 weeks). The medium was changed every 3 days.
Table 1.
List of Growth Factors Combination for Each Treatment Group
| Groups | NELL-1 (2000 ng/mL) | IGF-1 (10 ng/mL) | TGF-β1 (25 ng/mL) | TGF-β3 (25 ng/mL) | BMP-2 (500 ng/mL) | BMP-4 (500 ng/mL) | BMP-6 (500 ng/mL) |
|---|---|---|---|---|---|---|---|
| Growth factors | |||||||
| Control | − | − | − | − | − | − | − |
| NELL-1 | + | − | − | − | − | − | − |
| TGF-β1 | − | + | + | − | − | − | − |
| TGF-β3 | − | + | − | + | − | − | − |
| TGF-β1 + BMP-2 | − | − | + | − | + | − | − |
| TGF-β1 + BMP-4 | − | − | + | − | − | + | − |
| TGF-β1 + BMP-6 | − | − | + | − | − | − | + |
| TGF-β3 + BMP-2 | − | − | − | + | + | − | − |
| TGF-β3 + BMP-4 | − | − | − | + | − | + | − |
| TGF-β3 + BMP-6 | − | − | − | + | − | − | + |
| TGF-β3 + BMP-6 + NELL-1 | + | − | − | + | − | − | + |
BMP, bone morphogenetic protein; TGF-β, transforming growth factor beta.
RNA isolation and quantitative real-time polymerase chain reaction
The pellets were placed in TRIzol® Reagent (Invitrogen, Carlsbad, CA; Life Technologies, Carlsbad, CA) and homogenized by using an ultrasonic microtip (Fisher Scientific, Waltham, MA). Total RNA was then isolated following the manufacturer's instruction. DNase (Invitrogen; Life Technologies) treatment was performed, and 1 μg RNA was added for reverse transcription with the SuperScript II Reverse Transcriptase Kit (Invitrogen; Life Technologies). Real-time polymerase chain reaction (PCR) was performed on the 7300 Real-time PCR system with SYBR Green Master mix (Invitrogen; Life Technologies). All the primer sequences used are listed in Table 2. Concomitant Ribosomal protein L13a (RPL13A) was also evaluated in separate tubes for each reverse transcriptase reaction as a housekeeping standard. Relative gene expression was analyzed by ΔΔCT method.46
Table 2.
List of Primers for Quantitative Real-Time Polymerase Chain Reaction
| Gene | Sequence |
|---|---|
| Ribosomal protein L13a (RPL13A) | 5′-AAG TAC CAG GCA GTG ACA G-3′ |
| 5′-CCT GTT TCC GTA GCC TCA TG-3′ | |
| Neural EGFL like 1 (NELL1) | 5′-TAT GAG CGT GTG ATA GAC CCT C-3′ |
| 5′-TCC CAT CTT GGA TGA TCC CTT-3′ | |
| SRY (sex determining region Y)-box 9 (SOX9) | 5′-AGC GAA CGC ACA TCA AGA C-3′ |
| 5′-CTG TAG GCG ATC TGT TGG GG-3′ | |
| Collagen, type II, alpha 1 (COL2) | 5′-TCC TCT GCG ACG ACA TAA TC-3′ |
| 5′-GAG GTC AGT TGG GCA GAT G-3′ | |
| Aggrecan (AGG) | 5′-AAC ATC AGG AGT CCC TGA CC-3′ |
| 5′-CCA CTT CAA CCA AAC TGG TG-3′ | |
| Cartilage oligomeric matrix protein (COMP) | 5′-CAT CAG GGT GCG ATT CTA TG-3′ |
| 5′-TCC TGG GAG AAG CAG AAG AC-3′ | |
| Collagen, type X, alpha 1 (COL10) | 5′-CAG GCA ACA GCA TTA TGA CC-3′ |
| 5′-AGG CCT ACC CAA ACA TGA GT-3′ | |
| Matrix metallopeptidase 13 (MMP13) | 5′-TGA CCC TTC CTT ATC CCT TG-3′ |
| 5′-GCA TCA ACC TGC TGA GGA T-3′ | |
| ADAM metallopeptidase with thrombospondin type 1motif, 4 (ADAMTS4) | 5′-TTT GAC AAG TGC ATG GTG TG-3′ |
| 5′-TCC GTA CCT GAA TTT CCT GA-3′ | |
| Collagen, type I, alpha 1 (COL1) | 5′-CAG CCG CTT CAC CTA CAG C-3′ |
| 5′-TTT TGT ATT CAA TCA CTG TCG CC-3′ | |
| Osteocalcin (OCN) | 5′-GGC GCT ACC TGT ATC AAT GG-3′ |
| 5′-GTG GTC AGC CAA CTC GTC A-3′ | |
| Osteopontin (OPN) | 5′-GAA GTT TCG CAG ACC TGA CAT-3′ |
| 5′-GTA TGC ACC ATT CAA CTC CTC G-3′ | |
| Vascular endothelial growth factor A (VEGF) | 5′-AGG GCA GAA TCA TCA CGA AGT-3′ |
| 5′-AGG GTC TCG ATT GGA TGG CA-3′ | |
| Marker of proliferation Ki-67 (MKI67) | 5′-GCC TGC TCG ACC CTA CAG A-3′ |
| 5′-GCT TGT CAA CTG CGG TTG C-3′ | |
| Proliferating cell nuclear antigen (PCNA) | 5′-GCG TGA ACC TCA CCA GTA TGT-3′ |
| 5′-TCT TCG GCC CTT AGT GTA ATG AT-3′ | |
| Caspase 3, apoptosis-related cysteine peptidase (CASP3) | 5′-AGA GGG GAT CGT TGT AGA AGT C-3′ |
| 5′-ACA GTC CAG TTC TGT ACC ACG-3′ | |
| Bone morphogenetic protein receptor Type IA (BMPRIA) | 5′-AGA TGA CCA GGG AGA AAC CAC-3′ |
| 5′-CAA CAT TCT ATT GTC CGG CGT A-3′ | |
| Bone morphogenetic protein receptor Type IB (BMPRIB) | 5′-ACC AGA CCT CGA TAC AGC ATT-3′ |
| 5′-CCC ATA GCG ACC TTT TCC AAT-3′ | |
| Bone morphogenetic protein receptor Type II (BMPRII) | 5′-GAC AGG AGA CCG TAA ACA AGG-3′ |
| 5′-CCA TAT CGA CCT CGG CCA ATC-3′ | |
| Transforming growth factor β receptor I (TGFβRI) | 5′-GCT GTA TTG CAG ACT TAG GAC TG-3′ |
| 5′-TTT TTG TTC CCA CTC TGT GGT T-3′ |
Glycosaminoglycan quantification
The pellets were digested in 40 mg/mL papain (Invitrogen; Life Technologies), 20 mM ammonium acetate, 1 mM EDTA, and 1 mM dithiothreitol (DTT) (Sigma-Aldrich) for 48 h at 65°C and frozen at −20°C. Samples were then thawed for quantification using dimethylmethylene blue (DMMB) dye binding assay for glycosaminoglycan (GAG) content and DNA quantitation kit for DNA quantification. For the DMMB assay, chondroitin sulfate (Sigma-Aldrich) was used to generate a standard curve. Results were read at a wavelength of 525 nm. DNA content was assessed using a commercial kit: DNA Quantitation Kit–Fluorescence Assay (Cat# DNAQF-1KT; Sigma-Aldrich) in a 96-well plate. The assay was carried out according to the manufacturer's instructions. GAG content was normalized to DNA content.
Histology and immunohistochemistry
Monolayer-cultured human PSCs and human ARCs at 50–70% confluence were fixed in 4% PFA (Sigma-Aldrich) at room temperature for 15 min. The pellets for each time point were fixed in 4% PFA (Sigma-Aldrich) at 4°C overnight before paraffin embedding. Hematoxylin and eosin staining was performed on 5-μm sections for histological analyses, while Alcian Blue staining was performed following the standard protocols.32,33 For the immunohistochemistry staining, the slides were blocked with 3% bovine serum albumin before being incubated with primary antibodies. The manufacturer, catalog number, antigen retrieval condition, and the concentration of each of the primary antibodies used for immunohistochemistry staining (IHC) are listed in Supplementary Table S2.
Imaging and image processing
Images were acquired at room temperature with the CellSens software (Olympus, America, Inc., Center Valley, PA) on a microscope (Olympus, America, Inc.) using 10× (dry HC Plan Apochromat, NA 0.30) and 20× (dry HC Plan Apochromat, NA 0.17) objective lenses. Images were processed in Photoshop CS2 and Illustrator CS4 (Adobe Systems Computer Software Company, San Jose, CA) for image merging.
Statistical analysis
Statistical analysis was performed with OriginPro 8 (Origin Lab Corp., Northampton, MA) using the one-way ANOVA test and the two-sample t-test. p < 0.05 was considered statistically significant.
The correlation analysis was performed with OriginPro 8 (Origin Lab Corp.) using the Pearson correlation test. |r| > 0.7 was considered statistically highly related, while r > 0 represented a positive correlation.
Results
Positive correlation between NELL-1 expression and chondrogenesis
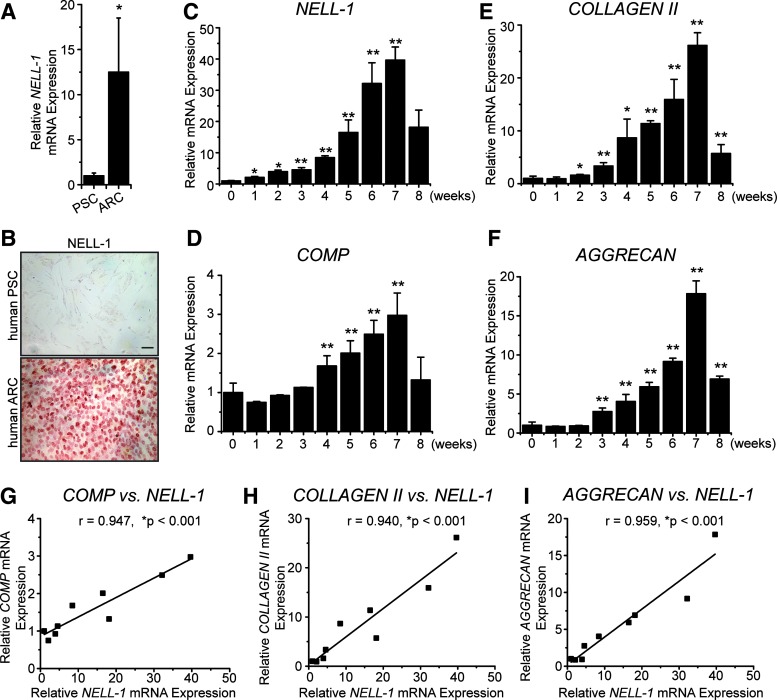
To explore the correlation between NELL-1 and chondrocyte/MSC chondrogenesis, we first tested the expression of NELL-1 in human articular cartilage (Fig. 1). The positive staining of NELL-1 was mainly found in the chondrocytes of the superficial zone, middle zone, and uncalcified deep zone of the articular cartilage. In vitro, the endogenous level of NELL-1 in mature hARCs was 12.5-fold higher than in hPSCs as measured by real-time PCR (Fig. 2A). With respect to the protein level by immunocytochemistry, mature hARCs exhibited a stronger staining intensity of NELL-1 when compared to hPSCs (Fig. 2B and Supplementary Table S3-1). As hPSCs underwent chondrogenesis in basic chondrogenic media for a total of 8 weeks (56 days), the expression level of NELL-1 increased gradually in the first 7 weeks and then dropped at week 8 (Fig. 2C), which paralleled the expression of chondrogenic markers, including cartilage oligomeric matrix protein (COMP, Fig. 2D), COLLAGEN II (Fig. 2E), and AGGRECAN (Fig. 2F) in the hPSC pellets. Thus, not only NELL-1 is highly expressed in articular cartilage and mature chondrocytes but also its expression increases gradually in hPSCs during chondrogenic differentiation. Furthermore, the expression levels of COMP, COLLAGEN II, and AGGRECAN were significantly positively correlated with the expression levels of NELL-1 during the hPSC chondrogenic differentiation (Fig. 2G–I). These findings reveal a gene expression and correlation between NELL-1 and chondrogenesis in hPSCs.
FIG. 1.
NEL-like molecule-1 (NELL-1) immunostaining in human articular cartilage. NELL-1 is mainly present in chondrocytes of the superficial (S) and middle (M) zones (red box) and in the uncalcified (UC) rather than calcified (C) deep (D) zone chondrocytes (blue box). Color images available online at www.liebertpub.com/tea
FIG. 2.
The expression level of NELL-1 is positively related to chondrogenesis. (A) Gene expression levels of NELL-1 in human perivascular stem cells (PSCs) and human articular chondrocytes (hARCs). Mean ± SD of cells from six donors are shown. *p < 0.05 versus ARC NELL-1 expression. (B) Expression of NELL-1 in human PSCs and human ARCs examined by immunocytochemical staining. Scale bar = 50 μm. mRNA levels of NELL-1 (C), COMP (D), COLLAGEN II (E), and AGGRECAN (F) in chondrogenic differentiated hPSCs at different time points. Mean ± SD of cells from six donors are shown. *p < 0.05 versus expression level at week 0, **p < 0.01 versus expression level at week 0. The correlation between the mRNA level of NELL-1 and the mRNA levels of COMP (G), COLLAGEN II (H), and AGGRECAN (I) in chondrogenic differentiated hPSCs analyzed by the Pearson correlation test. *Positive correlation is significant at the 0.001 level (two-tailed). Color images available online at www.liebertpub.com/tea
Recombinant human NELL-1 has prochondrogenic effects on hPSCs
Since NELL-1 expression increased in hPSCs during chondrogenic differentiation, we questioned the chondrogenic differentiation potency of hPSCs and if recombinant human NELL-1 alone could promote chondrogenic differentiation of hPSCs. By comparing the hPSC, hBMSC, and hARC pellets after 7 days of treatment, we found that the expression levels of the chondrogenic markers, including SOX9, COLLAGEN II, AGGRECAN, and COMP, in hPSC pellets cultured in the basic chondrogenic medium were significantly lower than those in hBMSC and hARC pellets (Fig. 3A and Supplementary Table S3-2). Morphologically, the hBMSC and hARC pellets at day 21 showed more intense Alcian Blue staining than hPSC pellets (Fig. 3B). Thus, compared with hBMSCs and hARCs, hPSCs had lower chondrogenic potency.
FIG. 3.
NELL-1 promotes chondrogenic differentiation of hPSCs. (A) Gene expression of chondrogenic markers in different groups of hPSC pellets, human bone marrow stromal cell (hBMSC) pellets, and hARC pellets at day 7. AGG: AGGRECAN, COL II: COLLAGEN II. Mean ± SD of cells from six donors are shown. *p < 0.05 versus gene expression level in hPSC control group; **p < 0.01 versus gene expression level in hPSC control group; #p < 0.05 versus gene expression level in hPSC TGF-β3 group. (B) Hematoxylin and eosin (HE) staining and Alcian Blue staining of the sections of the pellets after 21 days of treatment. *Marks the focal areas within the pellets with intensive stain by Alcian Blue. Color images available online at www.liebertpub.com/tea
For the hPSCs treated with growth factors, the addition of TGF-β3 could only significantly increase the gene expression level of SOX9 in comparison to the control (Fig. 3A). Morphologically, the TGF-β3 treatment group exhibited a dense fibrous tissue shell on the surface of the pellets at day 21 (Fig. 3B). On the other hand, NELL-1 treatment was found not only to dramatically increase the expression of SOX9 to a level comparable to that of the TGF-β3 positive control but also increase the expression levels of COLLAGEN II and AGGRECAN, with significantly higher expression levels compared with the controls at day 7 (Fig. 3A). Notably, focal areas within the pellets with intense Alcian Blue staining were observed in both the NELL-1 and TGF-β3 treatment samples, although typical cartilaginous histology, including chondrocyte-like cells clustered in groups and interterritorial cartilage matrix with darker staining, was not observed at day 21 (Fig. 3B). Indeed, our findings suggest that NELL-1 alone has prochondrogenic effects on hPSCs comparable to, if not significantly greater than, TGF-β3.
NELL-1 significantly enhances and accelerates the chondrogenic differentiation of hPSCs induced by TGF-β3 + BMP-6
Compared with BMSCs, the nonbone marrow-derived MSCs in general are less responsive to chondrogenic induction and unaffected by relevant growth factors such as TGF-β3.12–15,45,47 While chondrogenic induction of hPSCs by TGF-β1 or TGF-β3 alone was also suboptimal, improved chondrogenic differentiation of hPSCs was observed in the presence of TGF-β3 + BMP-6 stimulation for 42 days, as demonstrated by positive staining of COLLAGEN II in obvious hyaline cartilage-like areas (Fig. 4). In addition to chondrogenic differentiation, after 21 days of treatment, TGF-β3 + BMP-6 also increased the size (Fig. 5A) and GAG accumulation (Fig. 5B) of the hPSC pellets in comparison to the control. The addition of NELL-1 further enhanced the SOX9 and COLLAGEN II expression in hPSC pellets (Fig. 4), enlarged the pellets (Fig. 5A), and increased the GAG content (Fig. 5B).
FIG. 4.
The effects of different growth factor combinations on hPSC chondrogenic differentiation. Alcian Blue staining and immunohistochemical staining of COLLAGEN II and SOX9 of the sections of pellets from each treatment after 42 days of culture. Note the hyaline cartilage-like structure with positive staining of COLLAGEN II shown in the section of TGF-β3 + BMP-6-treated pellets and TGF-β3 + BMP-6 + NELL-1-treated pellets. Color images available online at www.liebertpub.com/tea
FIG. 5.
NELL-1 enlarges the size and increases the glycosaminoglycan (GAG) accumulation of hPSC pellets. (A) Photographs of the hPSC pellets at day 21. (B) The longest diameter for the pellets of each group. Box plot of each group shown with maximum, minimum, average, and median values. Six pellets were measured in triplicate for each group. *p < 0.05 versus the control group; **p < 0.01 versus the control group; ##p < 0.01 versus the TGF-β3 group. (C) The quantification of matrix production by GAG analysis. GAG synthesis (μg) normalized against DNA content (μg). Mean ± SD of cells from six donors are shown. *p < 0.05 versus the control group; **p < 0.01 versus the control group; ##p < 0.01 versus the TGF-β3 group. Color images available online at www.liebertpub.com/tea
At the molecular level, the hPSC pellets treated with TGF-β3 + BMP-6 demonstrated a time-dependent increase (from day 7 to 21) in the gene expression of chondrogenic markers, including SOX9, COLLAGEN II, COMP, and AGGRECAN, at levels much higher compared with the control group (increased from 2- to 35-fold) (Fig. 6A and Supplementary Table S3-3). The same trends were also observed by immunohistochemical staining. Specifically, the SOX9, COLLAGEN II, COMP, and AGGRECAN expression was limited in the control pellets (Fig. 6B), but clearly shown in the TGF-β3 + BMP-6-treated pellets (Fig. 6B).
FIG. 6.
NELL-1 enhances the chondrogenic effects of TGF-β3 + BMP-6 on hPSCs. (A) Gene expression of chondrogenic markers in different groups of hPSC pellets at day 7, 14, and 21. Mean ± SD of cells from six donors are shown. *p < 0.05 versus gene expression level in the control group; **p < 0.01 versus gene expression level in the control group; #p < 0.05 versus gene expression level in the TGF-β3 + BMP-6 group; ##p < 0.01 versus gene expression level in the TGF-β3 + BMP-6 group. (B) Fluorescent immunohistochemical staining of SOX9, COLLAGEN II, COMP, and AGGRECAN of the sections of hPSC pellets after 21 days of culture. The staining of hBMSC and hARC pellets were also shown as the positive control. Color images available online at www.liebertpub.com/tea
With the addition of NELL-1, the highest expression levels of SOX9, COLLAGEN II, and AGGRECAN at each time point were observed among the three treatment groups (Fig. 6A). Specifically, at day 21, the gene expression level of SOX9 in the TGF-β3 + BMP-6 + NELL-1 group was 55-fold higher than the control and twofold higher than the TGF-β3 + BMP-6 group; COLLAGEN II in the TGF-β3 + BMP-6 + NELL-1 group was sevenfold higher than the control and twofold higher than the TGF-β3 + BMP-6 group; and AGGRECAN in the TGF-β3 + BMP-6 + NELL-1 group was 50-fold higher than the control and 1.5-fold higher than the TGF-β3 + BMP-6 group. The TGF-β3 + BMP-6 + NELL-1 group also exhibited significantly higher expression levels of COMP compared with the other two groups at day 21 (fivefold higher than the control group, 1.2-fold higher than the TGF-β3 + BMP-6 group) (Fig. 6A). Meanwhile, the addition of NELL-1 increased the staining intensity of SOX9, COLLAGEN II, COMP, and AGGRECAN in hPSC pellets, which was much stronger than the staining of hBMSC pellets, and was at a comparable level with the staining of hARC pellets (Fig. 6B).
The addition of NELL-1 limits the side effects of chondrogenic differentiation caused by TGF-β3 + BMP-6 in hPSCs
As NELL-1 has also shown to enhance bone formation in hPSCs39,42,48 and fibrosis, hypertrophy, and mineralization are unwanted effects that immensely limit the clinical usage of current prochondrogenic growth factors,34–38 we further tested the expression of hypertrophic, fibrotic, osteogenic, and apoptotic markers in pellets from the TGF-β3 + BMP-6 + NELL-1 group.
After 21 days in culture, the addition of TGF-β3 and BMP-6 to the basic chondrogenic medium increased the gene expression of COLLAGEN X and ADAMTS4 as analyzed by real-time PCR (Fig. 7A and Supplementary Table S3-4) and the protein expression of COLLAGEN X and MMP13 as analyzed by IHC (Fig. 7B). However, the addition of NELL-1 did not further increase the expression of these hypertrophic markers. Inversely, NELL-1 could reduce the markers' expression both at gene and protein levels (Fig. 7).
FIG. 7.
NELL-1 does not increase the expression of hypertrophic markers in hPSC pellets. (A) Gene expression of hypertrophic markers in different groups of hPSC pellets at day 21. Mean ± SD of cells from six donors are shown. **p < 0.01 versus gene expression level in the control group; #p < 0.05 versus gene expression level in the TGF-β3 + BMP-6 group; ##p < 0.01 versus gene expression level in the TGF-β3 + BMP-6 group. (B) Fluorescent immunohistochemical staining of COLLAGEN X and MMP13 of the sections of hPSC pellets after 21 days of culture. Color images available online at www.liebertpub.com/tea
The same trends were also observed in the expression of the fibrotic marker COLLAGEN I at day 21. As shown in Figure 8, the hPSC pellets in the TGF-β3 + BMP-6 group showed high expression level of COLLAGEN I, while pellets in the TGF-β3 + BMP-6 + NELL-1 group showed almost the same expression level of COLLAGEN I as the control group (Supplementary Table S3-5).
FIG. 8.
NELL-1 does not increase the expression of fibrotic marker in hPSC pellets. (A) Gene expression of COLLAGEN I in different groups of hPSC pellets at day 21. Mean ± SD of cells from six donors are shown. *p < 0.05 versus gene expression level in the control group. (B) Fluorescent immunohistochemical staining of COLLAGEN I of the sections of hPSC pellets after 21 days of culture. Color images available online at www.liebertpub.com/tea
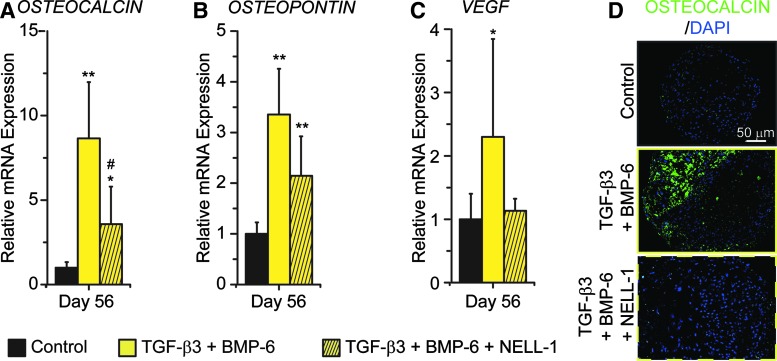
For the long-term treatment (56 days, 8 weeks), the pellets treated with TGF-β3 + BMP-6 exhibited two to sevenfold higher gene expression levels of the osteogenic markers, including OSTEOCALCIN, OSTEOPONTIN, and VEGF, than the control group (Fig. 9A). The TGF-β3 + BMP-6 pellets also had high staining intensity of OSTEOCALCIN (Fig. 9B). On the other hand, the TGF-β3 + BMP-6 + NELL-1 pellets expressed OSTEOCALCIN and OSTEOPONTIN two to threefold higher than the control pellets, but lower than the TGF-β3 + BMP-6 pellets (Fig. 9A). The staining intensity of OSTEOCALCIN in the TGF-β3 + BMP-6 + NELL-1 pellets was almost the same as that in the control pellets (Fig. 9B). Furthermore, the expression level of VEGF in the TGF-β3 + BMP-6 + NELL-1 pellets was also lower compared with the TGF-β3 + BMP-6 pellets (Fig. 9A and Supplementary Table S3-6).
FIG. 9.
NELL-1 does not increase the expression of mineralization markers in hPSC pellets after long-term culture. (A–C) Gene expression of mineralization markers, including OSTEOCALCIN (A), OSTEOPONTIN (B), and VEGF (C), in different groups of hPSC pellets at day 56. Mean ± SD of cells from six donors are shown. *p < 0.05 versus gene expression level in the control group; **p < 0.01 versus gene expression level in the control group; #p < 0.05 versus gene expression level in the TGF-β3 + BMP-6 group. (D) Fluorescent immunohistochemical staining of OSTEOCALCIN of the sections of hPSC pellets after 56 days of culture. Color images available online at www.liebertpub.com/tea
In addition, the usage of NELL-1 did not alter the PSC proliferation (Supplementary Fig. S2 and Supplementary Table S3-9) and apoptosis (Supplementary Fig. S3 and Supplementary Table S3-10) in the pellets.
Collectively, the combination of NELL-1, TGF-β3, and BMP-6 appears to be an improved combinatorial therapeutic strategy that promotes chondrogenic differentiation and limits the hypertrophic, fibrotic, osteogenic, and apoptotic progression of hPSCs over the currently acceptable TGF-β + BMP regimen in articular cartilage repair.15
NELL-1 increases the expression level of BMP receptors and TGF-β receptors in hPSCs
To explore the underlying mechanism of NELL-1 in accelerating and enhancing chondrogenic differentiation of hPSCs, we next sought to investigate the expression of BMP receptors and the TGF-β receptor type I (TGFβRI). Notably, NELL-1 was found to increase the gene and protein expression levels of all the BMP receptors, including BMP receptor type IA (BMPRIA), BMP receptor type IB (BMPRIB), and BMP receptor type II (BMPRII) (Fig. 10A, B and Supplementary Table S3-7). However, NELL-1 alone did not alter the expression of TGFβRI (Fig. 10C, D). In the presence of TGF-β3 + BMP-6, NELL-1 elevated the expression of TGFβRI to a level much higher compared with the control and that of NELL-1 alone (Fig. 10C, D and Supplementary Table S3-8). These results may, at least partially, provide the mode of action of NELL-1 in the enhancement of chondrogenic hPSC differentiation under current testing conditions.
FIG. 10.
NELL-1 increases the expression of BMP receptors and TGF-β receptor type I in hPSCs. (A) Gene expression of BMP receptors in hPSC pellets of control and NELL-1 only groups at day 7. BMPRIA: BMP receptor type IA, BMPRIB: BMP receptor type IB, BMPRII: BMP receptor type II. Mean ± SD of cells from six donors are shown. *p < 0.05 versus gene expression level in the control group. (B) Fluorescent immunohistochemical staining of BMPRs of sections of hPSC pellets after 7 days of culture. (C) Gene expression of TGF-β receptor type I (TGFβRI) in hPSC pellets of different groups at day 7. Mean ± SD of cells from six donors are shown. *p < 0.05 versus gene expression level in the control group. **p < 0.01 versus gene expression level in the control group; ##p < 0.01 versus gene expression level in the TGF-β3 + BMP-6 group. (D) Fluorescent immunohistochemical staining of TGF-β receptor type I of the sections of hPSC pellets after 7 days of culture. Color images available online at www.liebertpub.com/tea
Discussion
As we have previously shown, NELL-1 protein alone results in successful healing of rabbit articular cartilage defects.33 However, for larger and more clinically challenging defects involving cartilage repair, we postulate that MSCs or chondroprogenitor cells are necessary and advantageous.8–10 To overcome the limitations associated with conventionally derived BMSC populations, we have used FACS to purify human perivascular cell populations to a high level of homogeneity from human adipose tissue consisting of microvascular pericytes (CD146+, CD34−, CD45−, CD31−) and adventitial cells (CD146−, CD34+, CD45−, CD31−).28,39,42,49–52 These cells, collectively termed perivascular stem cells or PSCs, not only possess characteristics of conventional MSCs, including multilineage differentiation potential, but also possess unique advantages for tissue engineering.24,27,28,49,53
In this study, not surprisingly, the basic chondrogenic differentiation ability of hPSCs is lower compared with hBMSCs and hARCs. After trying multiple combinations of TGF-βs and BMPs, the hPSC pellets showed hyaline cartilage formation 42 days after being treated with TGF-β3 and BMP-6. This outcome was similar to the results reported by others on the chondrogenic induction of MSCs using the well-known growth factors TGF-βs and BMPs.15 Thus, we confirmed that hPSCs derived from lipoaspirate, a novel and abundant source of MSCs, have the ability to differentiate into chondrocytes and form cartilaginous tissue in pellet cultures. The specific advantages of PSCs over articular chondrocytes or BMSCs are multiple, including: (1) no need for culture, thereby decreasing the risks of immunogenicity, infection, and genetic instability,54 (2) easy accessibility, (3) minimal donor site morbidity, (4) precise characterization of native tissue localization, phenotype, and developmental potential (conversely, MSCs are only retrospectively derived from primary, heterogeneous cell cultures), and (5) improved tropic potency (hPSCs secrete 5–20 times more heparin binding epidermal growth factor [HB-EGF] and FGF-2 than classically derived adipose tissue or cord blood MSCs23). With defined cellular identity, purity (homogeneity), and increased potency, PSCs could become a novel MSC source for cartilage regeneration. Importantly, PSC sources effectively bypass the time-consuming in vitro isolation and expansion techniques required for the traditional MSC sources.
Despite the encouraging effects of NELL-1 on cartilage regeneration in a challenging rabbit cartilage defect model,33 the importance and effects of NELL-1 on PSC/MSC chondrogenesis are not well defined. In this study, we identified and validated the gene and protein expression of NELL-1 in human articular cartilage and mature hARCs. Specifically, we demonstrated the time-dependent, increasing expression levels of NELL-1 during the chondrogenic differentiation of hPSCs along with the expression of chondrogenic markers: COLLAGEN II, COMP, and AGGRECAN. These results clearly demonstrated the high correlation between NELL-1 and chondrogenesis.
Functionally, NELL-1 alone can dramatically increase chondrogenic marker expression in PSC pellets when compared with the control and TGF-β3 only groups, which demonstrate that NELL-1 is a prochondrogenic protein for PSCs/MSCs. Countless research projects have focused on finding the best combination of growth factors, dosages, and time courses for MSC chondrogenesis.14,15,17,22,35,47,55–57 However, numerous studies have indicated that the current strategies delivering TGF-βs and BMPs result in an inadequate quantity and quality of regenerated cartilage.58–62 To find a better combination of available growth factors for hPSC chondrogenesis and to shorten the in vitro inducing time, which may also expedite in vivo cartilage formation, we introduced a NELL-1 + TGF-β3 + BMP-6 combination.
Grossly, NELL-1 enlarged the size of hPSC pellets in combination with TGF-β3 and BMP-6 in vitro. The addition of NELL-1 to TGF-β3 + BMP-6 also enhanced the GAG accumulation and increased the gene expression of chondrogenic markers as early as 7 days postinduction. At 21 days postinduction, the NELL-1 + TGF-β3 + BMP-6-treated pellets showed the highest gene and protein expression levels of all the chondrogenic markers in comparison to the pellets in the control and TGF-β3 + BMP-6 groups. Thus, the combination of NELL-1 + TGF-β3 + BMP-6 significantly reduces the time course of cartilage formation with hPSCs in comparison to the 42 days required for TGF-β3 + BMP-6. Furthermore, the addition of NELL-1 also restricted the unwanted effects that commonly accompany chondrogenic differentiation of MSCs when treated with TGFβs and BMPs, including hypertrophy, fibrosis, osteogenesis (mineralization), and apoptosis. This result indicates the superior prochondrogenic potential of NELL-1 that may contribute significantly to the current regimen of driving MSCs toward chondrogenesis.
Hennig et al.63 described that MSCs originating from adipose tissue (ATSC) exhibited a reduced chondrogenic potential under standard culture conditions driven by TGF-β due to the lack of TGF-β receptor type I expression when compared with BMSCs, and BMP-6 treatment can elevate TGF-β receptor type I expression levels to facilitate TGF-β effects on ATSC.63 To explore how NELL-1 could accelerate and enhance cartilage formation in a manner similar to BMSCs, we tested the effects of NELL-1 on BMP and TGF-β receptor expression levels in hPSCs. NELL-1 treatment alone did not significantly increase the expression level of TGF-β receptor type I. However, the addition of NELL-1 dramatically enhanced TGF-β receptor type I expression in the presence of BMP-6. Moreover, NELL-1 stimulation revealed higher expression levels of all BMP receptors, including BMP receptor type IA, BMP receptor type IB, and BMP receptor type II when compared to the control group. Collectively, with the combinatorial application of NELL-1 + TGF-β3 + BMP-6, we propose that NELL-1 increases the expression of BMP receptors in hPSCs to enhance the receptors' availability to BMP-6 stimulation. This, in turn, elevates the expression level of TGF-β receptor type I for increased responsiveness to TGF-β stimulation of hPSCs.
Additional studies are required to decipher the precise mode of action of this novel combinatorial strategy using NELL-1 + TGF-β3 + BMP-6 in hPSCs/MSCs for cartilage regeneration. In addition, the human lipoaspirates containing abundant hPSCs that are usually discarded as waste and readily obtainable may become a superior reliable cell source over conventional bone marrow aspirates. Together, this combinatorial application of NELL-1, TGF-β3, and BMP-6 using hPSCs derived from lipoaspirates may have a profound impact on the future of cartilage regeneration.
Supplementary Material
Acknowledgments
We would like to thank Eric Chen, Maxwell C. Murphy, and Kevin S. Lee at the UCLA School of Dentistry for article editing. This work was supported by the CIRM Early Translational II Research Award TR2-01821, NIH/NIDCR (grants R21 DE0177711 and RO1 DE01607), UC Discovery Grant 07-10677, Eli & Edythe Broad Center of Regenerative Medicine and Stem Cell Research at UCLA Innovation Award, and International S&T Cooperation Program of China (Grant No. 2013DFB30360).
Disclosure Statement
Drs. X.Z., K.T., and C.S. are inventors of Nell-1-related patents and K.T., B.P., and C.S. are inventors of perivascular stem cell-related patents filed from UCLA. Drs. X.Z., K.T., and C.S. are founders and/or board members of Bone Biologics, Inc., which sublicenses Nell-1 patents from the UC Regents, who also hold equity in Bone Biologics. Drs. K.T. and C.S. are founders of Scarless Laboratories, Inc., which sublicenses perivascular stem cell-related patents from the UC Regents, who also hold equity in Scarless Laboratories. Dr. Chia Soo is also an officer of Scarless Laboratories, Inc.
References
- 1.Pleis J.R., Ward B.W., and Lucas J.W. Summary health statistics for U.S. adults: National Health Interview Survey, 2009. Vital Health Stat 10 1, 2010 [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention (CDC). National and state medical expenditures and lost earnings attributable to arthritis and other rheumatic conditions—United States, 2003. MMWR Morb Mortal Wkly Rep 56, 4, 2007 [PubMed] [Google Scholar]
- 3.Kopec J.A., Rahman M.M., Berthelot J.M., Le Petit C., Aghajanian J., Sayre E.C., Cibere J., Anis A.H., and Badley E.M. Descriptive epidemiology of osteoarthritis in British Columbia, Canada. J Rheumatol 34, 386, 2007 [PubMed] [Google Scholar]
- 4.Lohmander L.S., Englund P.M., Dahl L.L., and Roos E.M. The long-term consequence of anterior cruciate ligament and meniscus injuries: osteoarthritis. Am J Sports Med 35, 1756, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Matricali G.A., Dereymaeker G.P., and Luyten F.P. Donor site morbidity after articular cartilage repair procedures: a review. Acta Orthop Bel 76, 669, 2010 [PubMed] [Google Scholar]
- 6.Darling E.M., and Athanasiou K.A. Rapid phenotypic changes in passaged articular chondrocyte subpopulations. J Orthop Res 23, 425, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Goessler U.R., Bieback K., Bugert P., Naim R., Schafer C., Sadick H., Hormann K., and Riedel F. Human chondrocytes differentially express matrix modulators during in vitro expansion for tissue engineering. Int J Mol Med 16, 509, 2005 [PubMed] [Google Scholar]
- 8.Khan W.S., Johnson D.S., and Hardingham T.E. The potential of stem cells in the treatment of knee cartilage defects. Knee 17, 369, 2010 [DOI] [PubMed] [Google Scholar]
- 9.Wang Y., Yuan M., Guo Q.Y., Lu S.B., and Peng J. Mesenchymal stem cells for treating articular cartilage defects and osteoarthritis. Cell Transplant 24, 1661, 2015 [DOI] [PubMed] [Google Scholar]
- 10.Vinardell T., Thorpe S.D., Buckley C.T., and Kelly D.J. Chondrogenesis and integration of mesenchymal stem cells within an in vitro cartilage defect repair model. Ann Biomed Eng 37, 2556, 2009 [DOI] [PubMed] [Google Scholar]
- 11.Leonard C.M., Fuld H.M., Frenz D.A., Downie S.A., Massague J., and Newman S.A. Role of transforming growth factor-beta in chondrogenic pattern formation in the embryonic limb: stimulation of mesenchymal condensation and fibronectin gene expression by exogenenous TGF-beta and evidence for endogenous TGF-beta-like activity. Dev Biol 145, 99, 1991 [DOI] [PubMed] [Google Scholar]
- 12.Thorpe S.D., Buckley C.T., Vinardell T., O'Brien F.J., Campbell V.A., and Kelly D.J. The response of bone marrow-derived mesenchymal stem cells to dynamic compression following TGF-beta3 induced chondrogenic differentiation. Ann Biomed Eng 38, 2896, 2010 [DOI] [PubMed] [Google Scholar]
- 13.Bian L., Zhai D.Y., Tous E., Rai R., Mauck R.L., and Burdick J.A. Enhanced MSC chondrogenesis following delivery of TGF-beta3 from alginate microspheres within hyaluronic acid hydrogels in vitro and in vivo. Biomaterials 32, 6425, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dahlin R.L., Ni M., Meretoja V.V., Kasper F.K., and Mikos A.G. TGF-beta3-induced chondrogenesis in co-cultures of chondrocytes and mesenchymal stem cells on biodegradable scaffolds. Biomaterials 35, 123, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Danisovic L., Varga I., and Polak S. Growth factors and chondrogenic differentiation of mesenchymal stem cells. Tissue Cell 44, 69, 2012 [DOI] [PubMed] [Google Scholar]
- 16.Erickson I.E., Huang A.H., Chung C., Li R.T., Burdick J.A., and Mauck R.L. Differential maturation and structure-function relationships in mesenchymal stem cell- and chondrocyte-seeded hydrogels. Tissue Eng Part A 15, 1041, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gadjanski I., Spiller K., and Vunjak-Novakovic G. Time-dependent processes in stem cell-based tissue engineering of articular cartilage. Stem Cell Rev 8, 863, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mauck R.L., Yuan X., and Tuan R.S. Chondrogenic differentiation and functional maturation of bovine mesenchymal stem cells in long-term agarose culture. Osteoarthritis Cartilage 14, 179, 2006 [DOI] [PubMed] [Google Scholar]
- 19.McIlwraith C.W., Frisbie D.D., Rodkey W.G., Kisiday J.D., Werpy N.M., Kawcak C.E., and Steadman J.R. Evaluation of intra-articular mesenchymal stem cells to augment healing of microfractured chondral defects. Arthroscopy 27, 1552, 2011 [DOI] [PubMed] [Google Scholar]
- 20.Studer D., Millan C., Ozturk E., Maniura-Weber K., and Zenobi-Wong M. Molecular and biophysical mechanisms regulating hypertrophic differentiation in chondrocytes and mesenchymal stem cells. Eur Cells Mater 24, 118, 2012 [DOI] [PubMed] [Google Scholar]
- 21.Vadala G., Sowa G., Hubert M., Gilbertson L.G., Denaro V., and Kang J.D. Mesenchymal stem cells injection in degenerated intervertebral disc: cell leakage may induce osteophyte formation. J Tissue Eng Regen Med 6, 348, 2012 [DOI] [PubMed] [Google Scholar]
- 22.Zhang X., Zhang Y., Wang Z., Li Q., and Li B. The effect of non-growth factors on chondrogenic differentiation of mesenchymal stem cells. Cell Tissue Bank 15, 319, 2014 [DOI] [PubMed] [Google Scholar]
- 23.Chen C.W., Montelatici E., Crisan M., Corselli M., Huard J., Lazzari L., and Peault B. Perivascular multi-lineage progenitor cells in human organs: regenerative units, cytokine sources or both? Cytokine Growth Factor Rev 20, 429, 2009 [DOI] [PubMed] [Google Scholar]
- 24.Corselli M., Chen C.W., Crisan M., Lazzari L., and Peault B. Perivascular ancestors of adult multipotent stem cells. Arterioscler Thromb Vasc Biol 30, 1104, 2010 [DOI] [PubMed] [Google Scholar]
- 25.Crisan M., Deasy B., Gavina M., Zheng B., Huard J., Lazzari L., and Peault B. Purification and long-term culture of multipotent progenitor cells affiliated with the walls of human blood vessels: myoendothelial cells and pericytes. Methods Cell Biol 86, 295, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Crisan M., Huard J., Zheng B., Sun B., Yap S., Logar A., Giacobino J.P., Casteilla L., and Peault B. Purification and culture of human blood vessel-associated progenitor cells. Curr Protoc Stem Cell Biol Chapter 2, Unit 2B 2.1, 2008 [DOI] [PubMed] [Google Scholar]
- 27.James A.W., Zara J.N., Corselli M., Askarinam A., Zhou A.M., Hourfar A., Nguyen A., Megerdichian S., Asatrian G., Pang S., Stoker D., Zhang X., Wu B., Ting K., Peault B., and Soo C. An abundant perivascular source of stem cells for bone tissue engineering. Stem Cells Transl Med 1, 673, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.James A.W., Zara J.N., Zhang X., Askarinam A., Goyal R., Chiang M., Yuan W., Chang L., Corselli M., Shen J., Pang S., Stoker D., Wu B., Ting K., Peault B., and Soo C. Perivascular stem cells: a prospectively purified mesenchymal stem cell population for bone tissue engineering. Stem Cells Transl Med 1, 510, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barthes J., Ozcelik H., Hindie M., Ndreu-Halili A., Hasan A., and Vrana N.E. Cell microenvironment engineering and monitoring for tissue engineering and regenerative medicine: the recent advances. BioMed Res Int 2014, 921905, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuroda S., and Tanizawa K. Involvement of epidermal growth factor-like domain of NELL proteins in the novel protein-protein interaction with protein kinase C. Biochem Biophys Res Commun 265, 752, 1999 [DOI] [PubMed] [Google Scholar]
- 31.Desai J., Shannon M.E., Johnson M.D., Ruff D.W., Hughes L.A., Kerley M.K., Carpenter D.A., Johnson D.K., Rinchik E.M., and Culiat C.T. Nell1-deficient mice have reduced expression of extracellular matrix proteins causing cranial and vertebral defects. Hum Mol Genet 15, 1329, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Lee M., Siu R.K., Ting K., and Wu B.M. Effect of Nell-1 delivery on chondrocyte proliferation and cartilaginous extracellular matrix deposition. Tissue Eng Part A 16, 1791, 2010 [DOI] [PubMed] [Google Scholar]
- 33.Siu R.K., Zara J.N., Hou Y., James A.W., Kwak J., Zhang X., Ting K., Wu B.M., Soo C., and Lee M. NELL-1 promotes cartilage regeneration in an in vivo rabbit model. Tissue Eng Part A 18, 252, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang T., Wen F., Wu Y., Goh G.S., Ge Z., Tan L.P., Hui J.H., and Yang Z. Cross-talk between TGF-beta/SMAD and integrin signaling pathways in regulating hypertrophy of mesenchymal stem cell chondrogenesis under deferral dynamic compression. Biomaterials 38, 72, 2015 [DOI] [PubMed] [Google Scholar]
- 35.Steinert A.F., Weissenberger M., Kunz M., Gilbert F., Ghivizzani S.C., Gobel S., Jakob F., Noth U., and Rudert M. Indian hedgehog gene transfer is a chondrogenic inducer of human mesenchymal stem cells. Arthritis Res Ther 14, R168, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Steinert A.F., Proffen B., Kunz M., Hendrich C., Ghivizzani S.C., Noth U., Rethwilm A., Eulert J., and Evans C.H. Hypertrophy is induced during the in vitro chondrogenic differentiation of human mesenchymal stem cells by bone morphogenetic protein-2 and bone morphogenetic protein-4 gene transfer. Arthritis Res Ther 11, R148, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mueller M.B., Fischer M., Zellner J., Berner A., Dienstknecht T., Prantl L., Kujat R., Nerlich M., Tuan R.S., and Angele P. Hypertrophy in mesenchymal stem cell chondrogenesis: effect of TGF-beta isoforms and chondrogenic conditioning. Cells Tissues Organs 192, 158, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dickhut A., Pelttari K., Janicki P., Wagner W., Eckstein V., Egermann M., and Richter W. Calcification or dedifferentiation: requirement to lock mesenchymal stem cells in a desired differentiation stage. J Cell Physiol 219, 219, 2009 [DOI] [PubMed] [Google Scholar]
- 39.Askarinam A., James A.W., Zara J.N., Goyal R., Corselli M., Pan A., Liang P., Chang L., Rackohn T., Stoker D., Zhang X., Ting K., Peault B., and Soo C. Human perivascular stem cells show enhanced osteogenesis and vasculogenesis with Nel-like molecule I protein. Tissue Eng Part A 19, 1386, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chung C.G., James A.W., Asatrian G., Chang L., Nguyen A., Le K., Bayani G., Lee R., Stoker D., Zhang X., Ting K., Peault B., and Soo C. Human perivascular stem cell-based bone graft substitute induces rat spinal fusion. Stem Cells Transl Med 3, 1231, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.James A.W., Zara J.N., Corselli M., Chiang M., Yuan W., Nguyen V., Askarinam A., Goyal R., Siu R.K., Scott V., Lee M., Ting K., Peault B., and Soo C. Use of human perivascular stem cells for bone regeneration. J Vis Exp, e2952, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang X., Peault B., Chen W., Li W., Corselli M., James A.W., Lee M., Siu R.K., Shen P., Zheng Z., Shen J., Kwak J., Zara J.N., Chen F., Zhang H., Yin Z., Wu B., Ting K., and Soo C. The Nell-1 growth factor stimulates bone formation by purified human perivascular cells. Tissue Eng Part A 17, 2497, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee S., Zhang X., Shen J., James A.W., Chung C.G., Hardy R., Li C., Girgius C., Zhang Y., Stoker D., Wang H., Wu B.M., Peault B., Ting K., and Soo C. Brief report: human perivascular stem cells and Nel-like protein-1 synergistically enhance spinal fusion in osteoporotic rats. Stem Cells 33, 3158, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chung C.G., James A.W., Asatrian G., Chang L., Nguyen A., Le K., Bayani G., Lee R., Stoker D., Pang S., Zhang X., Ting K., Peault B., and Soo C. Human perivascular stem cell-based bone graft substitute induces rat spinal fusion. Stem Cells Transl Med 4, 538, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Puetzer J.L., Petitte J.N., and Loboa E.G. Comparative review of growth factors for induction of three-dimensional in vitro chondrogenesis in human mesenchymal stem cells isolated from bone marrow and adipose tissue. Tissue Eng Part B Rev 16, 435, 2010 [DOI] [PubMed] [Google Scholar]
- 46.Livak K.J., and Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25, 402, 2001 [DOI] [PubMed] [Google Scholar]
- 47.Sang Y., Zang W., Yan Y., Liu Y., Fu Q., Wang K., Chen Y., and Qi N. Study of differential effects of TGF-beta3/BMP2 on chondrogenesis in MSC cells by gene microarray data analysis. Mol Cell Biochem 385, 191, 2014 [DOI] [PubMed] [Google Scholar]
- 48.Vos M.B., Colvin R., Belt P., Molleston J.P., Murray K.F., Rosenthal P., Schwimmer J.B., Tonascia J., Unalp A., Lavine J.E., and Group N.C.R. Correlation of vitamin E, uric acid, and diet composition with histologic features of pediatric NAFLD. J Pediatr Gastroenterol Nutr 54, 90, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Crisan M., Yap S., Casteilla L., Chen C.W., Corselli M., Park T.S., Andriolo G., Sun B., Zheng B., Zhang L., Norotte C., Teng P.N., Traas J., Schugar R., Deasy B.M., Badylak S., Buhring H.J., Giacobino J.P., Lazzari L., Huard J., and Peault B. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 3, 301, 2008 [DOI] [PubMed] [Google Scholar]
- 50.Montemurro T., Andriolo G., Montelatici E., Weissmann G., Crisan M., Colnaghi M.R., Rebulla P., Mosca F., Peault B., and Lazzari L. Differentiation and migration properties of human foetal umbilical cord perivascular cells: potential for lung repair. J Cell Mol Med 15, 796, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Park T.S., Gavina M., Chen C.W., Sun B., Teng P.N., Huard J., Deasy B.M., Zimmerlin L., and Peault B. Placental perivascular cells for human muscle regeneration. Stem Cells Dev 20, 451, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tottey S., Corselli M., Jeffries E.M., Londono R., Peault B., and Badylak S.F. Extracellular matrix degradation products and low-oxygen conditions enhance the regenerative potential of perivascular stem cells. Tissue Eng Part A 17, 37, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Corselli M., Chen C.W., Sun B., Yap S., Rubin J.P., and Peault B. The tunica adventitia of human arteries and veins as a source of mesenchymal stem cells. Stem Cells Dev 21, 1299, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dahl J.A., Duggal S., Coulston N., Millar D., Melki J., Shahdadfar A., Brinchmann J.E., and Collas P. Genetic and epigenetic instability of human bone marrow mesenchymal stem cells expanded in autologous serum or fetal bovine serum. Int J Dev Biol 52, 1033, 2008 [DOI] [PubMed] [Google Scholar]
- 55.Cheon H., Yu S.J., Yoo D.H., Chae I.J., Song G.G., and Sohn J. Increased expression of pro-inflammatory cytokines and metalloproteinase-1 by TGF-beta1 in synovial fibroblasts from rheumatoid arthritis and normal individuals. Clin Exp Immunol 127, 547, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yamanishi Y., Boyle D.L., Clark M., Maki R.A., Tortorella M.D., Arner E.C., and Firestein G.S. Expression and regulation of aggrecanase in arthritis: the role of TGF-beta. J Immunol 168, 1405, 2002 [DOI] [PubMed] [Google Scholar]
- 57.Murphy M.K., Huey D.J., Hu J.C., and Athanasiou K.A. TGF-beta1, GDF-5, and BMP-2 stimulation induces chondrogenesis in expanded human articular chondrocytes and marrow-derived stromal cells. Stem Cells 33, 762, 2015 [DOI] [PubMed] [Google Scholar]
- 58.Olivos-Meza A., Fitzsimmons J.S., Casper M.E., Chen Q., An K.N., Ruesink T.J., O'Driscoll S.W., and Reinholz G.G. Pretreatment of periosteum with TGF-beta1 in situ enhances the quality of osteochondral tissue regenerated from transplanted periosteal grafts in adult rabbits. Osteoarthritis Cartilage 18, 1183, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Holland T.A., Bodde E.W., Baggett L.S., Tabata Y., Mikos A.G., and Jansen J.A. Osteochondral repair in the rabbit model utilizing bilayered, degradable oligo(poly(ethylene glycol) fumarate) hydrogel scaffolds. J Biomed Mater Res A 75, 156, 2005 [DOI] [PubMed] [Google Scholar]
- 60.Abe T., Yamada H., Nakajima H., Kikuchi T., Takaishi H., Tadakuma T., Fujikawa K., and Toyama Y. Repair of full-thickness cartilage defects using liposomal transforming growth factor-beta1. J Orthop Sci 8, 92, 2003 [DOI] [PubMed] [Google Scholar]
- 61.Sellers R.S., Zhang R., Glasson S.S., Kim H.D., Peluso D., D'Augusta D.A., Beckwith K., and Morris E.A. Repair of articular cartilage defects one year after treatment with recombinant human bone morphogenetic protein-2 (rhBMP-2). J Bone Joint Surg Am 82, 151, 2000 [DOI] [PubMed] [Google Scholar]
- 62.Menendez M.I., Clark D.J., Carlton M., Flanigan D.C., Jia G., Sammet S., Weisbrode S.E., Knopp M.V., and Bertone A.L. Direct delayed human adenoviral BMP-2 or BMP-6 gene therapy for bone and cartilage regeneration in a pony osteochondral model. Osteoarthritis Cartilage 19, 1066, 2011 [DOI] [PubMed] [Google Scholar]
- 63.Hennig T., Lorenz H., Thiel A., Goetzke K., Dickhut A., Geiger F., and Richter W. Reduced chondrogenic potential of adipose tissue derived stromal cells correlates with an altered TGFbeta receptor and BMP profile and is overcome by BMP-6. J Cell Physiol 211, 682, 2007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.