Abstract
Background
Ethyl acetate extracts of Launaea procumbens is used for the treatment of liver dysfunction as an herbal medicine in Pakistan. In this study, the protective effects of ethyl acetate extracts were evaluated against CCl4-induced liver injuries in rat.
Methods
To examine the protective effects against oxidative stress of carbon tetrachloride in rats, 30 male rats were equally divided into 5 groups (6 rats). Among five groups, one was treated with CCl4 (3 ml/kg i.p. in olive oil b.w.) twice a week for 4 weeks. Others were orally fed with extracts (100, 200 mg/kg b.w.), with CCl4 twice a week for 4 weeks.
Results
Administration of CCl4 altered the serum marker enzymes, lipid profile, CYP 2E1, p53 expression, antioxidant enzymes, nuclear organizer regions (AgNORs), and DNA. Supplement of L. procumbens ameliorated the effects of CCl4, improved CYP 2E1, p53, and increased the activities of antioxidant enzymes while activity of liver marker enzymes (ALP, ALT, AST, g-GT) and contents of lipid per oxidation contents (TBARS), AgNORs, and DNA fragmentation were decreased. Similarly body weight was increased while liver and relative liver weight was decreased with co-administration of various extracts, suggesting that L. procumbens effectively protect liver against the CCl4-induced oxidative damage in rats.
Conclusion
The hepatoprotective and free radical scavenging effects might be due to the presence of bioactive constituents in the extract.
Keywords: carbon tetrachloride, Launaea procumbens, liver, hepatic antioxidants, CYP 2E1, lipid peroxidation, p53, AgNORs
Hepatitis viral infection, food additives, alcohol, toxins, toxic industrial chemicals, and air and water pollutants are the major risk factors of liver toxicity (1). There is increasing evidence that free radicals and reactive oxygen species (ROS) play a crucial role in the various steps that initiate and regulate the progression of liver diseases independently of the agent in its origin (1). CCl4 is a potent environmental hepatotoxin (2) that, in addition to hepatic problems, also causes dysfunction of kidneys, lungs, testes, and brain, as well as generates free radicals in blood (3–5).
CCl4 requires bioactivation by phase I cytochrome P450 system to form reactive metabolic trichloromethyl radical (CCl3*) and peroxy trichloromethyl radical (*OOCCl3). These free radicals can bind with polyunsaturated fatty acid (PUFA) to produce alkoxy (R*) and peroxy radicals (ROO*) that, in turn, generate lipid peroxides that are highly reactive, change enzyme activity, and finally induce injury or necrosis (6). The injuries induced by CCl4 result from free radicals through lipid per oxidation of cell membranes. CCl4 is known to decrease GSH of phase II enzyme, and reduces antioxidant enzyme and antioxidant substrates to induce oxidative stress that is an important factor in acute and chronic injuries in various tissues (6–8). Free radical of carbon tetrachloride reduces glutathione content and antioxidant activity leads to hepatic injuries (9, 10). CCl4 controls the peroxy radicals, thereby depleting the antioxidant enzymes. ROS causes oxidative DNA damages, with the formation of DNA adducts, genetic mutation, strand breakage, and chromosomal alterations (9, 11). Some recent investigation revealed that free radicals induce an increase in the number of nuclear organizer regions (AgNORs); enhance activity of telomerase enzymes activity (9, 12); cause depletion of CYP 2E1 (13); and increase oxo8dG concentration (14). DNA fragmentation causes p53 gene expression; blocks cell cycle, and gives additional time to repair DNA; however, severe DNA damage triggers apoptosis (15).
Launaea procumbens was traditionally used in the treatment of rheumatism (16), kidney and liver disorders (17, 18), eyes diseases (19), and as food (20). Nutritional analysis of L. procumbens reveals the presence of salicylic acid, vanllic acid, synergic acid, 2-methyl-resercinol, and gallic acid (21). Therefore, the present study was arranged to evaluate the traditional use of ethyl acetate extract of L. procumbens versus carbon tetrachloride–induced liver disorders and lipids peroxidation in rats.
Materials and methods
Plant collection
L. procumbens at maturity was collected from Wah Cantt District Rawalpindi (Pakistan). Plants were identified and a specimen was submitted at Herbarium of Quaid-i-Azam University Islamabad, Pakistan. Aerial parts of plant (leaves, stem, flowers, and seeds) were shade dried at room temperature, chopped, and grinded mechanically to mesh size 1 mm.
Preparation of plant extract
One-kilogram powder of L. procumbens was extracted in 2 L methanol to get crude methanolic extract which was further fractionated to ethyl acetate. The ethyl acetate fraction (LEA) was evaporated under reduced pressure in a rotary evaporator, dried, and stored at 4°C for in vivo studies.
Animals and treatment
A total of 30, six-week-old, male albino rats (180–190 g) were provided by the National Institute of Health Islamabad and were kept in ordinary cages at room temperature of 25±3°C with a 12 h dark/light cycle. They were allowed standard laboratory feed and water. The study protocol was approved by Ethical committee of Quaid-i-Azam University, Islamabad for laboratory animal feed and care.
Experimental design
To study the antioxidant effects of LEA, male albino rats were equally divided into five groups (six rats). Group 1 was given raw water and free access to food materials. Group II received olive oil intraperitoneally (Monday and Thursday) and DMSO orally (Wednesday and Saturday) at a dose of 3 ml/kg body weight. Group III received CCl4 3 ml/kg intraperitoneally in olive oil (Monday and Thursday). Group IV and V were given orally 100; 200 mg/kg b.w. (in DMSO), (LEA) after 48 h of CCl4 treatment (Wednesday and Saturday). After 24 h of the last treatment, all the animals were weighted and sacrificed; their livers were removed, weighted, perfused in ice-cold saline solution, and treated with liquid nitrogen for further analysis.
Assessment of serum profile
Serum marker enzymes (ALT, AST, ALP, γ-GT) and lipid profile (cholesterol, LDL, HDL, triglyceride) were estimated using standard AMP diagnostic kits (Stattogger Strasse 31b 8045 Graz, Austria). CYP 2E1 and p53 concentration was determined with ELISA kit.
Assessment of antioxidant status
Liver tissue (70 mg) was homogenized in 10 volumes of 100 mmol KH2PO4 buffer containing 1 mmol EDTA (pH 7.4) and centrifuged at 12,000× g for 30 min at 4oC. The supernatant was collected and used for determining antioxidant status as described below using concentration of protein estimated with the method of Lowry et al. (22). Antioxidant status including activity of catalase (23), superoxide dismutase (24), glutathione-S-transferase assay (25), glutathione reductase (26), glutathione peroxidase (27), reduced glutathione assay (28), and lipid peroxidation assay (29).
DNA fragmentation % assay
DNA fragmentation % assay was conducted using the procedure of Wu et al. (30) with some modifications. The liver tissue (50 mg) was homogenized in 10 volumes of a TE solution pH 8.0 (5 mmol Tris-HCl, 20 mmol EDTA) and 0.2% triton X-100. One milliliter aliquot of each sample was centrifuged at 27,000×g for 20 min to separate the intact chromatin (pellet B) from the fragmented DNA (supernatant, T). The pellet and supernatant fractions were assayed for DNA content using a freshly prepared DPA (diphenylamine) solution for reaction. Optical density was read at 620 nm with (SmartSpecTM plus Spectrophotometer catalog # 170-2525) a spectrophotometer. The results were expressed as amount of % fragmented DNA by the following formula: % Fragmented DNA=T×100/T+B
AgNORs count
After weighing small pieces, each liver was fixed for 3–4 h in fixative sera followed by dehydration with ascending grades of alcohol (80, 90, and 100%) and transferred to cedar wood oil. When tissue became clear, all tissues were embedded in paraplast and prepared as blocks for further microtomy. Thin slides (3–4 µm) were prepared with microtome and the wax was removed. After complete removal of wax, the slides were hydrated in decreased ethanol concentration (90, 70, and 50%) and washed in distilled water for 10 min and dried in an oven. After drying, slides were treated with one drop of colloidal solution (2% gelatin and 1% formic acid) and two drops of 50% AgNO3 solution onto the slide and incubated at 35°C for about 8–12 min. The progressive staining was followed under a microscope to get golden colored nuclei and brown/black NORs. Then, the slide was washed in distilled water, treated for 1 min with 1% sodium thiosulfate at room temperature to stop the reaction, and washed in tap water. The cells were examined under a light microscope at 100× magnification and the number of AgNORs was counted per cell (31).
Statistical analysis
To determine the treatment effects one-way analysis of variance was carried out by computer software SPSS 13.0. Level of significance among the various treatments was determined by LSD at 0.05% level of probability.
Results
Body weight, liver weights
CCl4-induced lipid peroxidation plays a key role in the body weight and the organ weight of rats. Administration of CCl4 caused significant diminution (P<0.01) in body weight while amplifying AgNORs, tissue, and relative tissue weight as compared with the non-treated normal control rat. Supplementation of 100 mg/kg and 200 mg/kg b.w. LEA significantly restored (P<0.01) the weight of body and liver as well as relative weight dose dependently (Table 1).
Table 1.
Effect of various fractions of Launaea procumbens on liver weight, relative liver weight, AgNORs count, and DNA fragmentation % in liver of rat
| Treatment | Liver weight (g) | Relative liver weight (g) | % increase in body weight (g) | DNA damages % | AgNORs (NORS/cell) |
|---|---|---|---|---|---|
| Control | 5.78±0.209† | 0.0578±0.00209† | 26.0±0.80† | 5.17±0.94† | 2.167±0.307† |
| DMSO+olive oil | 5.88±0.206† | 0.0588±0.00206† | 25.9±0.63† | 5.00±0.44† | 2.667±0.333† |
| 3 ml/kg CCl4 | 6.96±0.194** | 0.0696±0.00194** | 18.6±0.72** | 35.83±0.14** | 9.000±0.931** |
| 100 mg/kg LPEE+CCl4 | 6.05±0.0861**† | 0.0605±0.00086**† | 22.5±0.42† | 9.83±0.97*† | 7.333±0.667**† |
| 200 mg/kg LPEE+CCl4 | 5.92±0.205† | 0.059±0.00205† | 24.49±0.54† | 7.33±0.67† | 5.333±0.882**† |
| 200 mg/kg LPEE alone | 5.01±0.32† | 0.0523±0.00101† | 27.39±0.21† | 6.01±0.27† | 3.023±0.92† |
Mean±SE (n=6 number).
Significance from the control group at P<0.05 and P<0.01 probability levels.
Significance from the CCl4 group at P<0.01 probability level.
Effects of LEA on lipid profile in rats
The protective effect of LEA on lipid profile is shown in Table 2. Treatment of CCl4 significantly increased (P<0.01) lipid profile (triglycerides, total cholesterol, LDL cholesterol) while extensively decreasing (P<0.01) HDL cholesterol. Reduction of HDL cholesterol was notably (P<0.01) enhanced by 100 mg/kg and 200 mg/kg b.w. LEA, while triglyceride, total cholesterols, and HDL-cholesterol concentration were appreciably (P<0.01) improved to reimburse the CCl4 insult.
Table 2.
Effect of various fractions of Launaea procumbens on serum level of triglycerides, total cholesterol, LDL cholesterol, and HDL cholesterol in rat
| Treatment | Triglycerides (mg/dl) | Total cholesterol (mg/dl) | High-density lipoprotein (mg/dl) | Low-density lipoprotein (mg/dl) |
|---|---|---|---|---|
| Control | 12.3±1.35† | 6.1±0.25† | 8.6±1.71† | 4.8±0.82† |
| DMSO+olive oil | 13.26±2.50† | 6.4±0.22† | 9.5±1.20† | 5.18±0.72† |
| 3 ml/kg CCl4 | 21.53±1.58** | 11.2±0.23** | 5.8±2.18** | 9.8±0.67** |
| 100 mg/kg LPEE+CCl4 | 16.4±1.8*† | 7.0±0.39**† | 7.41±1.9† | 7.3±0.95**† |
| 200 mg/kg LPEE+CCl4 | 14.7±2.09† | 6.5±0.62† | 7.94±0.92† | 6.0±0.59† |
| 200 mg/kg LPEE alone | 12.4±3.21† | 5.8±0.42† | 8.04±0.76† | 5.1±0.15† |
Mean±SE (n=6 number).
Significance from the control group at P<0.05 and P<0.01 probability levels.
Significance from the CCl4 group at P<0.01 probability level.
Indices of hepatotoxicity: serum markers
The expression levels of serum makers, namely ALT, AST, ALP, γ-GT, CYP 2E1, and p53, are susceptible to hepatotoxin and are markers of liver injury and oxidative stress, which promote the release of aminotransferase from hepatocytes into the blood stream. The marked protective effects of LEA on serum marker are shown in Table 3. Induction of CCl4 significantly increases (P<0.01) the activity of liver serum marker enzymes (ALT, AST, ALP, γ-GT) while decreasing (P<0.01) the expression level of CYP 2E1 and p53. The secretion of these enzymes and expression of CYP 2E1 and p53 was significantly improved (P<0.01) by 100 mg/kg and 200 mg/kg b.w. LEA comparatively to control rat viewing that LEA is possessed bioactive hepatoprotectant compounds however marked protection was noted with 200 mg/kg b.w. LEA.
Table 3.
Effect of ethyl acetate fraction of Launaea procumbens on liver function of rat
| Treatment | ALT (U/L) | AST (U/L) | ALP (U/L) | γ-GT (nM/min/mg protein) | P53 | CYP 2E1 |
|---|---|---|---|---|---|---|
| Control | 32.17±2.12† | 83.83±2.74† | 248.00±3.93† | 70.50±2.23† | 48.3±2.38† | 28.0±2.8† |
| DMSO+olive oil | 32.50±2.05† | 84.67±2.75† | 249.67±3.68† | 71.33±2.04† | 49±2.03† | 29±5.0† |
| 3 ml/kg CCl4 | 91.33±3.42** | 228.00±4.27** | 505.33±6.49** | 119.33±3.12** | 27.6±2.46** | 17.1±2.0** |
| 100 mg/kg LPEE+CCl4 | 77.67±3.36**† | 140.83±3.24**† | 388.33±3.83**† | 106.50±3.77**† | 53±3.78**† | 28±1.2**† |
| 200 mg/kg LPEE+CCl4 | 38.17±2.77† | 102.17±3.96**† | 262.00±2.37† | 78.33±3.30† | 57±2.63*† | 30±2.3*† |
| 200 mg/kg LPEE alone | 35.67±3.01† | 98.15±2.12† | 248.12±2.89† | 71.43±5.10† | 49±1.61† | 29±3.0† |
Mean±SE (n=6 number).
Significance from the control group at P<0.05 and P<0.01 probability levels.
Significance from the CCl4 group at P<0.01 probability level.
CCl4 induction and antioxidant status in rat liver
Antioxidant enzymes system detoxifies ROS and maintains cellular balance. Treatment of CCl4 significantly decreased (P<0.01) the activity of CAT, SOD, GST, GSH-Px, GSR, and GSH while increasing the TBARS content in rat liver. Co-treatment of 100 mg/kg and 200 mg/kg b.w. LEA in rat liver markedly improved (P<0.01) the reduction in the activities of antioxidant, and phase II metabolizing enzymes dose dependently justified that LEA could be used as antioxidant in daily diet (Table 4).
Table 4.
Effect of ethyl acetate fraction of Launaea procumbens on liver antioxidant profile
| Treatment | CAT (U/min) | SOD (U/mg protein) | GST (nM/min/mg protein) | GSH-Px (nM/min/mg protein) | GSH (M/g tissue) | TBARS (nM/min/mg protein) | GSR (nM/min/mg protein) |
|---|---|---|---|---|---|---|---|
| Control | 4.397±0.275† | 24.0±2.27† | 128.50±4.62† | 77.50±3.38† | 0.738±0.0201† | 78.67±6.56† | 147.33±6.01† |
| DMSO+olive oil | 4.242±0.407† | 23.0±2.34† | 126.67±4.21† | 77.0±3.10† | 0.708±0.0105† | 79.00±7.45† | 145.33±6.23† |
| 3 ml/kg CCl4 | 2.590±0.240** | 13.50±1.34** | 68.83±4.57** | 51.83±2.89** | 0.236±0.0066** | 158.83±8.57** | 88.00±3.61** |
| 50 mg/kg Rutin+CCl4 | 4.000±0.163† | 21.0±1.83† | 121.83±3.57† | 71.8±2.39† | 0.728±0.0432† | 87.27±5.23† | 142.17±6.22† |
| 100 mg/kg LPEE+CCl4 | 3.7±0.25**† | 18.667±0.9**† | 97.17±4.09**† | 67.5±2.4*† | 0.550±0.0123**† | 109.50±3.81**† | 107.5±3.3**† |
| 200 mg/kg LPEE+CCl4 | 3.9183±0.06† | 22.17±1.14† | 122.17±3.24† | 70.7±1.62† | 0.655±0.0118† | 94.00±4.50† | 132.33±2.89† |
| 200 mg/kg LPEE alone | 4.63±0.115† | 26.0±2.38† | 131.00±4.12† | 78.8±3.30† | 0.740±0.0101† | 78.0±3.51† | 150.50±6.38† |
Mean±SE (n=6 number).
Significance from the control group at P<0.05 and P<0.01 probability levels.
Significance from the CCl4 group at P<0.01 probability level.
Effect of LEA on DNA damages (ladder assay, DPA assay)
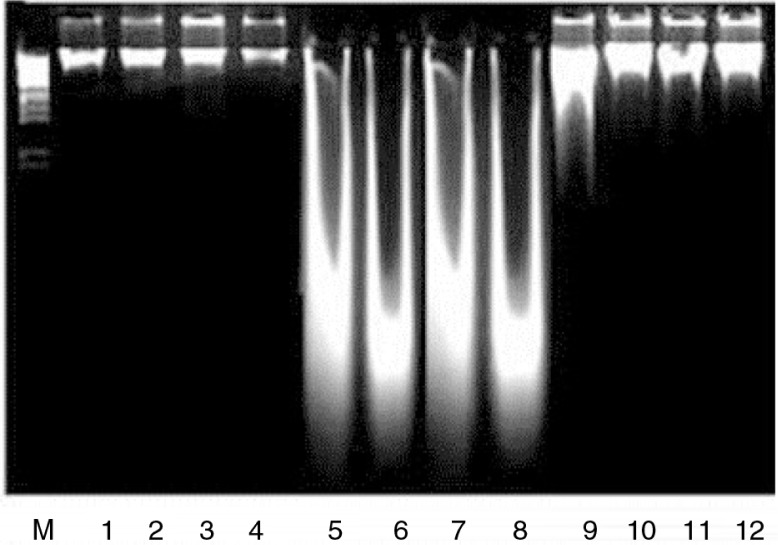
CCl4 free radicals fuse with DNA forming adduct and induce damages/mutation in the liver DNA of rats. The effects of LEA against CCl4 toxicities on DNA damages are shown in Fig. 1. CCl4 administration significantly increased the DNA damage which was significantly improved (P<0.01) by LEA depending on the dose amount as shown by band pattern and quantification (DPA assay) of different groups, when compared with the CCl4 group (Table 1).
Fig. 1.
The protective effects of extract on DNA. Lanes 1–4, non-treated control; 5–8, CCl4 treated rats; 9–10, CCl4+100 mg/kg b.w. LEA; 11–12, CCl4+200 mg/kg b.w. LEA.
Discussion
ROS are extremely reactive molecules, resulting from the metabolism of oxygen. These ROS can cause widespread damage to cells and tissues; causes degenerative disorders, such as cardiovascular disease, oxidative stress, aging; and causes neurodegenerative diseases, such as Alzheimer's disease, mutations, and cancer (32, 33). Free radicals induced from CCl4 during metabolism cause liver, lung, and kidney injuries in experimental animals such as rats (10). CCl4 is metabolized by cytochrome P450 into trichloromethyl (CCl3*) and peroxy trichloromethyl (*OOCCl3) radicals leading to the accumulation of lipid peroxidation products that cause renal and hepatic injuries (6, 34).
Medicinal plants composed of different amounts of antioxidants play the main role in controlling various pathological conditions, including oxidative stress, cancer, cardiovascular diseases, liver diseases, and lipid peroxidation (35, 36). ROS generated due to CCl4 administration are more reactive and toxic than the parent compound. Metabolism of CCl4 occurs in the endoplasmic reticulum and the isoenzyme concerned in this process is CYP 2E1 (37) and elevation of ALT, AST, ALP, and GGT in serum (38, 39). Our results showed that an active free radical of CCl4 caused reduction of CYP 2E1, which was markedly improved by oral supplementation of LEA. Our result was similar to that of a previous investigation which found that polyphenolic natural bioactive compounds are responsible for its fortification (40). Our result coincides with other studies (41). Cholesterol profile is an important marker of free radical–induced toxicity. Maintenance of increased concentration of serum LDH, TG, total cholesterol, and LDL, and decreased HDL at near-normal values with co-treatment of various concentrations of ethyl acetate fraction demonstrated the hepatoprotective effect of L. procumbens. Similar investigations were reported by Lin et al. (41), while working on hepatoprotective effects of bioactive compounds of plants against carbon tetrachloride–induced hepatic injury in rats.
CCl4 free radicals cause the peroxidation of the polyenoic lipids of the endoplasmic reticulum and decrease the activities of antioxidant enzymes (3, 42, 43). Co-administration of LEA markedly erased the toxicity of CCl4 and the enzymatic activities of antioxidant enzymes toward the normal range in this experiment. A similar result has been documented in various studies (44, 45). CCl4 induces lipid peroxidation and increases the TBARS contents in liver cells. TBARS is a major reactive aldehyde occurring during the peroxidation of polyunsaturated fatty acids (PUFA), a useful indicator showing tissue damages including a series of chain reactions (46). Administration of LEA significantly recovered the TBARS content near to control rats as was revealed by other plant extracts (3, 9). Free radicals–induced lipid peroxide react with DNA to form the adduct M1G, the mutagenic pirimedopurinone adduct of deoxyguanosine (47), as was revealed by DNA ladder assay (Fig. 1). Administration of LEA improved the DNA fragmentation, which is in close agreement with other studies (9) (Khan et al., 2009). DNA damage causes expression of p53, blocks cell cycle, and repairs their DNA damage (15).
Conclusion
Ethyl acetate fraction of L. procumbens regulated the activities of serum markers, antioxidant enzymes, CYP 2E1, and p53 protein because of the presence of bioactive constituents. It is therefore suggested that we isolate and purify these compounds to be used in future as a drug against oxidative stress and liver carcinoma.
Conflict of interest and funding
The authors have not received any funding or benefits from industry or elsewhere to conduct this study.
References
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Sahreen S, Khan MR, Khan RA. Hepatoprotective effects of methanol extract of Carissa opaca leaves on CCl4-induced damage in rat. BMC Complement Altern Med. 2011;11:48. doi: 10.1186/1472-6882-11-48. doi: http://dx.doi.org/10.1186/1472-6882-11-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khan MR, Ahmed D. Protective effects of Digera muricata (L.) Mart. On testis against oxidative stress of carbon tetrachloride in rat. Food Chem Toxicol. 2009;47:1393–99. doi: 10.1016/j.fct.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 4.Shah A, Khan RA, Ahmed M, Muhammad N. Hepatoprotective role of Nicotiana plumbaginifolia Linn against carbon tetrachloride-induced injuries. Toxicol Ind Health. 2013;32:292–8. doi: 10.1177/0748233713498448. doi: http://dx.doi.org/10.1177/0748233713498448. [DOI] [PubMed] [Google Scholar]
- 5.Alkreathy HM, Khan RA, Khan MR, Sahreen S. CCl4 induced genotoxicity and DNA oxidative damages in rats: hepatoprotective effect of Sonchus arvensis. BMC Complement Altern Med. 2014;14:452. doi: 10.1186/1472-6882-14-452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weber LW, Boll M, Stampfl A. Hepatotoxicity and mechanism of action of haloalkanes: Carbon tetrachloride as a toxicological model. Crit Rev Toxicol. 2003;33:105–36. doi: 10.1080/713611034. [DOI] [PubMed] [Google Scholar]
- 7.Szymonik-Lesiuk S, Czechowska G, Stryjecka-Zimmer M, Slomka M, Madro A, Celinski K, et al. Catalase, superoxide dismutase, and glutathione peroxidase activities in various rat tissues after carbon tetrachloride intoxication. J Hepatobiliary Pancreat Surg. 2003;10:309–15. doi: 10.1007/s00534-002-0824-5. [DOI] [PubMed] [Google Scholar]
- 8.Preethi KC, Kuttan R. Hepato and reno protective action of Calendula officinalis L. flower extract. Indian J Exp Biol. 2009;47:163–8. [PubMed] [Google Scholar]
- 9.Khan MR, Rizvi W, Khan GN, Khan RA, Shaheen S. Carbon tetrachloride induced nephrotoxicity in rat: Protective role of Digera muricata. J Ethnopharmacol. 2009;122:91–9. doi: 10.1016/j.jep.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 10.Ichi I, Kamikawa C, Nakagawa T, Kobayashi K, Kataoka R, Nagata E, et al. Neutral sphingomyelinase-induced ceramide accumulation by oxidative stress during carbon tetrachloride intoxication. Toxicology. 2009;261:33–40. doi: 10.1016/j.tox.2009.04.040. [DOI] [PubMed] [Google Scholar]
- 11.Jia X, Han C, Chen J. Effect of tea on preneoplastic lesions and cell cycle regulators in rat liver. Cancer Epidemiol Biomarkers Prev. 2002;11:1663–7. [PubMed] [Google Scholar]
- 12.Khanna KK, Jackson SP. DNA double-strand breaks: signaling, repair and cancer connection. Nature Genetics. 2001;27:247–254. doi: 10.1038/85798. [DOI] [PubMed] [Google Scholar]
- 13.Fahmy SR, Hamdi SAH, Abdel-Salam HA. Curative effect of dietary freshwater and marine crustacean extracts on carbon tetrachloride-induced nephrotoxicity. Aust J Basic Appl Sci. 2009;3:2118–29. [Google Scholar]
- 14.Sai K, Tyson CA, Thomas DW, Dabbs JE, Hasegawa R, Kurokawa Y. Oxidative DNA damage induced by potassium bromate in isolated rat renal proximal tubules and renal nuclei. Cancer Lett. 1994;87:1–7. doi: 10.1016/0304-3835(94)90402-2. [DOI] [PubMed] [Google Scholar]
- 15.Van Gijssel HE, Maassen CB, Mulder GJ, Meerman JH. P53 protein expression by hepatocarcinogens in the rat liver and its potential role in mitoinhibition of normal hepatocytes as a mechanism of hepatic tumor promotion. Carcinogenesis. 1997;18:1027–32. doi: 10.1093/carcin/18.5.1027. [DOI] [PubMed] [Google Scholar]
- 16. Parekh J, Chanda S. Screening of aqueous and alcoholic extracts of some Indian medicinal plants for antibacterial activity. Indian J Pharmaceut sci. 2006;68:835–8. [Google Scholar]
- 17.Ahmad M, Khan MA, Manzoor S, Zafar M, Sultana S. Check list of medicinal flora of Tehsil Isakhel, District Mianwali Pakistan. Ethnobotanical Leaflets. 2006;10:41–8. [Google Scholar]
- 18.Qureshi R, Raza BG. Ethnobotany of plants used by the Thari people of Nara Desert, Pakistan. J Fitoterapia. 2008;79:468–73. doi: 10.1016/j.fitote.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 19.Yousaf Z, Shainwari ZK, Ali SM. Medicinally important flora of Dhibbia Karsal Village (Mianwali District Punjab) Asian J Plant Sci. 2004;3:757–62. [Google Scholar]
- 20.Wazir SM, Saima S, Dasti AA, Subhan S. Ethnobotanical importance of salt range species of district Karak, Pakistan. Pakistan J Plant Sci. 2007;13:29–31. [Google Scholar]
- 21.Shaukat SS, Tajudin Z, Siddiqui IA. Allelopathic potential of Launaea procumbens (Roxb.) Rammaya and Raja: a tropical weed. Pakistan J Biol Sci. 2003;6:225–30. [Google Scholar]
- 22.Lowry OH, Rosenberg NJ, Farr AL, Randall RJ. Protein measurement with Folin Phenol reagent. Journal of Biological Chemistry. 1951;193:265–275. [PubMed] [Google Scholar]
- 23.Chance B, Maehly AC. Assay of catalase and peroxidases. Methods Enzymol. 1955;11:764–75. [Google Scholar]
- 24.Kakkar P, Das B, Viswanathan PN. A modified spectrophotometric assay of superoxide dimutase. Indian J Biochem Biophys. 1984;21:130–2. [PubMed] [Google Scholar]
- 25.Habig WH, Pabst MJ, Jakoby WB. Glutathione-S-transferases: The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974;249:7130–9. [PubMed] [Google Scholar]
- 26.Carlberg I, Mannervik EB. Glutathione level in rat brain. J Biol Chem. 1975;250:4475–80. [PubMed] [Google Scholar]
- 27.Mohandas J, Marshal JJ, Duggin GG, Horvath JS, Tiller DJ. Differential distribution of glutathione and glutathione-related enzymes in rabbit kidney. Possible implications in analgesic nephropathy. Biochem Pharmacol. 1984;33:1801–7. doi: 10.1016/0006-2952(84)90353-8. [DOI] [PubMed] [Google Scholar]
- 28.Jollow DJ, Mitchell JR, Zampaglione N, Gillete JR. Bromobenzene induced liver necrosis. Protective role of glutathione and evidence for 3,4-bromobenzene oxide as a hepatotoxic metabolite. Pharmacology. 1974;11:151–69. doi: 10.1159/000136485. [DOI] [PubMed] [Google Scholar]
- 29.Iqbal M, Sharma SD, Zadeh HR, Hasan N, Abdulla M, Athar M. Glutathione metabolizing enzymes and oxidative stress in ferric nitrilotriacetate (Fe–NTA) mediated hepatic injury. Redox Report. 1996;2:385–91. doi: 10.1080/13510002.1996.11747079. [DOI] [PubMed] [Google Scholar]
- 30.Wu B, Ootani A, Iwakiri R, Sakata Y, Fujise T, Amemori S, Yokoyama F, et al. T cell deficiency leads to liver carcinogenesis in Azoxymethane-treated rats. Exp Biol Med. 2006;231:91–8. doi: 10.1177/153537020623100111. [DOI] [PubMed] [Google Scholar]
- 31.Trere D, Zilbering A, Dittus D, Kim P, Ginsberg PC, Daskal I. AgNOR quantity in needle biopsy specimens of prostatic adenocarcinomas: correlation with proliferation state, Gleason score, clinical stage, and DNA content. Clin Mol Pathol. 1996;49:209–13. doi: 10.1136/mp.49.4.m209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ames B. Micronutrients prevent cancer and delay aging. Toxicol Lett. 1998;102:5–18. doi: 10.1016/s0378-4274(98)00269-0. [DOI] [PubMed] [Google Scholar]
- 33.Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–47. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 34.Aleynik SI, Leo MA, Ma X, Aleynick MK, Leiber CS. Polyenoyl phasphatidylcholine prevents CCl4 induced lipid peroxidation while it attenuates liver fibrosis. J Hepatol. 1997;27:554–61. doi: 10.1016/s0168-8278(97)80361-3. [DOI] [PubMed] [Google Scholar]
- 35.Martin GR, Danner DB, Holbrook NJ. Hepatoprotective activity of phenylthanoids from Cistanche deserticola . Planta Med. 1993;64:120–5. doi: 10.1055/s-2006-957387. [DOI] [PubMed] [Google Scholar]
- 36.Agbor AG, Oben JE, Ngogang JY. Effect of water extract of Hibiscus cannabinus leaves on phenyl hydrazine induced haemolytic anaemia. Paper presented at the 8th symposium of the Cameroon Society for Biochemistry and Molecular Biology; University of Dschang; 2001. pp. 25–6. [Google Scholar]
- 37.Al-Shabanah OA, Alam K, Nagi MN, Al-Rikabi AC, Al-Bekairi AM. Protective effect of aminoguanidine, a nitric oxide synthetase inhibiter against CCl4 induced hepatotoxicity in mice. Life Sci. 2000;66:265–70. doi: 10.1016/s0024-3205(99)00589-5. [DOI] [PubMed] [Google Scholar]
- 38.Singh B, Saxena AK, Chandan BK, Agarwal SG, Bhatia MS, Anand KK. Hepatoprotective effect of ethanolic extract of Eclipta alba on experimental liver damage in rats and mice. Phototherapy Research. 1993;7:154–158. [Google Scholar]
- 39.Shenoy KA, Somayaji SN, Bairy KL. Hepatoprotective effects of Ginko biloba against carbon tetra chloride induced hepatic injury in rats. Indian J Pharmacol. 2001;33:260–6. [Google Scholar]
- 40.Sreelatha S, Padma PR, Umadevi M. Protective effects of Coriandrum sativum extracts on CCl4-induced hepatotoxicity in rats. Food Chem Toxicol. 2009;48:702–708. doi: 10.1016/j.fct.2008.12.022. doi: http://dx.doi.org/10.1016/j.fct.2008.12.022. [DOI] [PubMed] [Google Scholar]
- 41.Lin HM, Tseng HC, Wang CJ, Lin JJ, Lo CW, Chou FP. Hepatoprotective effects of Solanum nigrum Linn. extract against CCl4-induced oxidative damage in rats. Chem Biol Interact. 2008;171:283–93. doi: 10.1016/j.cbi.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 42.Sheweita SA, Abd El-Gabar M, Bastawy M. Carbon tetrachloride changes the activity of cytochrome P450 system in the liver of male rats: role of antioxidants. Toxicology. 2001;169:83–92. doi: 10.1016/s0300-483x(01)00473-5. [DOI] [PubMed] [Google Scholar]
- 43.Adewole SO, Salako AA, Doherty OW, Naicker T. Effect of melatonin on carbon tetrachloride-induced kidney injury in Wistar rats. Afr J Biomed Res. 2007;10:153–64. [Google Scholar]
- 44.Manna P, Sinha M, Sil PC. Aqueous extract of Terminalia arjuna prevents carbon tetrachloride induced hepatic and renal disorders. BMC Complement Altern Med. 2006;6:33. doi: 10.1186/1472-6882-6-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hassan SA, Rizk MZ, El-Sharkawi F, Badary O, Kadry MO. The possible synergestic role of phytic acid and catechin in ameliorating the deteriorative biochemical effects induced by carbon tetrachloride in rats. J Appl Sci Res. 2007;3:1449–59. [Google Scholar]
- 46.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxidation in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–8. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 47.Marnett LJ. Oxyradicals and DNA damage. Carcinogenesis. 2000;21:361–70. doi: 10.1093/carcin/21.3.361. [DOI] [PubMed] [Google Scholar]