Abstract
Variation in the CD38 gene, which regulates secretion of the neuropeptide oxytocin, has been associated with several social phenotypes. Specifically, rs3796863 A allele carriers have demonstrated increased social sensitivity. In 400 older adolescents, we used trait-state-occasion modeling to investigate how rs3796863 genotype, baseline ratings of chronic interpersonal stress, and their gene-environment (GxE) interaction predicted trait social anxiety and depression symptoms over six years. We found significant GxE effects for CD38 A-carrier genotypes and chronic interpersonal stress at baseline predicting greater social anxiety and depression symptoms. A significant GxE effect of smaller magnitude was also found for C/C genotype and chronic interpersonal stress predicting greater depression; however, this effect was small compared to the main effect of chronic interpersonal stress. Thus, in the context of chronic interpersonal stress, heightened social sensitivity associated with the rs3796863 A allele may prospectively predict risk for social anxiety and (to a lesser extent) depression.
Keywords: CD38, social anxiety, depression, trait-state-occasion modeling, interpersonal stress, gene-environment interaction, oxytocin
Recent research has shown that genetic variation in CD38, a multifunctional protein known for its involvement in glucose-mediated insulin secretion (Bruzzone et al., 2008) and immune system functioning (Malavasi et al., 2008), plays a role in regulating social behaviors (Higashida, Yokoyama, Kikuchi, & Munesue, 2012). The social effects of CD38 may result in part from CD38's influence on oxytocin secretion (Higashida et al., 2012; Jin et al., 2007), which has wide-ranging influences on socioemotional processes (Bartz, Zaki, Bolger, & Ochsner, 2011). Building on findings from animal research demonstrating a role for CD38 in social processes, researchers have begun to examine whether genetic variation in CD38 may be associated with clinical disorders in humans characterized by social impairments such as autism spectrum disorders (ASD).
One genetic variant of particular interest has been rs3796863, an adenine to cytosine (A > C) single nucleotide polymorphism (SNP) located in intron 7 of CD38 on chromosome 4p15 (Malavasi et al., 2008). Having one or more rs3796863 C alleles was associated with ASD in a Caucasian sample in the United States (Munesue et al., 2010) and an Israeli sample ( i.e., the rs3796863 C allele was included in several significant haplotype associations; Lerer et al., 2010). Lerer et al. (2010) also found that individuals with ASD had lower levels of CD38 expression in lymophoblastoid cell lines compared to their healthy parents, and among low functioning individuals with ASD, rs3796863 C allele carriers had lower levels of CD38 gene expression. In a non-clinical sample, lower CD38 gene expression was associated with lower plasma oxytocin (Kiss, Levy-Gigi, & Keri, 2011). In addition, parents with the C/C rs3796863 genotype engaged in less parental touch with their infants and had lower levels of plasma oxytocin (Feldman et al., 2012; but see, Szeto et al., 2011 for a discussion of several issues associated with measurement of plasma oxytocin without extraction).
Social sensitivity can be conceptualized as an underlying dimension of normative social functioning in which either extreme sensitivity or insensitivity may represent clinical pathology. The rs3796863 C allele has been generally associated with decreased social sensitivity, as indicated by reduced parental responsiveness and increased risk of ASD (which includes impaired attention to or engagement in social stimuli; Chevallier, Kohls, Troiani, Brodkin, & Schultz, 2012). In addition, the rs3796863 C allele has been associated with lower levels of CD38 gene expression and lower levels of plasma oxytocin. In contrast, the A allele has been associated with increased social sensitivity (i.e., increased attention and engagement in social stimuli), higher levels of CD38 gene expression, and higher levels of plasma oxytocin. Based on these findings and Bartz and McInnes's (2007) suggestion that CD38 may be a promising candidate in understanding the impact of adversity on social behavior, we were interested in examining the interaction between variation in the rs3796863 CD38 polymorphism, chronic interpersonal stress, and two previously unexamined forms of socially-relevant psychopathology: social anxiety and depression.
Because anxiety and depression are highly prevalent (Kessler et al., 2005) and their economic costs are substantial (Simon, Ormel, VonKorff, & Barlow, 1995), efforts to elucidate neurobiological and psychological factors that increase risk for social anxiety disorder and major depression are very important. Twin studies estimate the genetic contribution to social anxiety disorder at .30 (Smoller, Gardner-Schuster, & Misiaszek, 2008) and to major depression at .37 (Sullivan, Neale, & Kendler, 2000). Thus, studies examining the genetic bases of these disorders may improve our ability to identify those at risk and help to inform novel treatments. A core feature of social anxiety disorder is fear of negative evaluation (Clark & McManus, 2002). Similarly, interpersonal sensitivity has been associated with increased risk for major depression (Boyce, Parker, Barnett, Cooney, & Smith, 1991). There is a well-established link between stressful interpersonal life events and depression (Slavich, O'Donovan, Epel, & Kemeny, 2010), including chronic interpersonal stress (e.g., Hammen, Hazel, Brennan, & Najman, 2012). In addition, a gene-environment (GxE) interaction model predicting depression found a key role for interpersonal forms of stress (Vrshek-Schallhorn et al., 2014). Although there is less research on the link between interpersonal stress and anxiety (including social anxiety) than depression, some evidence suggests that interpersonal stressors are associated with social anxiety (e.g., Starr & Davila, 2008). There is also mounting evidence for the role of oxytocin in social anxiety (Heinrichs & Gaab, 2007, Labuschagne et al., 2010). Given the moderate level of heritability in social anxiety disorder and major depression, as well as their relationships with interpersonal stress, genetic predisposition to social sensitivity may increase the impact of interpersonal stress and prospectively predict both disorders.
Based on NIMH Research Domain Criteria (RDoC) initiative to investigate the causes and consequences of mental illness based on dimensions rather than categorical diagnoses (Cuthbert & Kozak, 2013), we chose to investigate prospective longitudinal variation of social anxiety and depression through interviewer-rated and self-report symptom measures. We focused on older adolescents since the transition from adolescence to adulthood represents a critical period that marks the development of many psychiatric disorders (Krueger, 1999). Since cross-sectional assessments of anxiety and depression are subject to normative, transient distress (McGrath, Weill, Robinson, Macrae, & Smoller, 2012), as well as mood-state distortions (Naragon-Gainey, Gallagher, & Brown, 2013), our interest was in predicting the enduring personality-like trait portions of social anxiety and depression over time. Trait-State-Occasion (TSO) structural equation models (Cole, Martin, & Steiger, 2005) were used to separate the stable, trait-like, component (“trait variance”) of symptoms from the more state-like components (“state variance”). Previous work from our research group demonstrated that trait variance accounted for 73.3% of social anxiety symptom variance and 46.3% of depression symptom variance in older adolescents (Prenoveau et al., 2011). TSO modeling is particularly suited to GxE research because improved measurement of outcome variables increases the power to detect GxE associations (Wong, Day, Luan, Chan, & Wareham, 2003). Also, isolation of the stable portion of variance in a dependent variable increases phenotypic precision, and may therefore prove to be a valuable tool for future genetic research. The current report represents the first demonstration of TSO modeling in GxE research.
We hypothesized that chronic interpersonal stress at baseline would predict the trait-like components of social anxiety and depression over six years, and that variation in CD38 rs3796863 would moderate this effect. We examined chronic interpersonal stress (Hammen, 2005) at baseline because it captures relatively stable (i.e., chronic) prior stress that might improve the ability to detect gene-environment interactions that predict stable traits. Based on previous findings indicating that individuals who carry the A allele in rs3796863 demonstrate heightened social sensitivity (Feldman et al., 2012; Munesue et al., 2010), we hypothesized that baseline ratings of chronic interpersonal stress would have a greater impact on those with this genotype, contributing to higher levels of social anxiety and depression symptoms over six years.
Methods
Participants
The Youth Emotion Project (YEP) is a longitudinal study of risk factors for anxiety and depression comprised of annual assessments of life stress and symptoms of psychopathology among other measures (for futher details about the YEP study, see Zinbarg et al., 2010). Participants who scored in the approximate top third on neuroticism (measured by the Revised Eysenck Personality Questionnaire; Eysenck, Eysenck, & Barrett, 1985) were oversampled to increase the likelihood of prospective development of anxiety and depression symptomology. Participants were high school students who completed clinical diagnostic and life stress interviews, and symptom questionnaires, annually for six years. In the present study, participants (n = 410) represented a subset of those initially enrolled in the YEP who also provided a saliva sample for DNA analysis. Ten participants were excluded because no genotyping call was possible for rs3796863. This resulted in a total of 400 participants (mean age = 16.12, SD = .43) for all analyses except those involving baseline socioeconomic status, for which seven participants did not provide information (n = 393).
Materials and Procedure
Socioeconomic Status
Due to the association between socioeconomic status and increased risk for psychiatric disorders (McLaughlin et al., 2011), the Hollingshead socioeconomic status index (Hollingshead, 1975) was measured during the baseline interview.
SCID Clinical severity ratings
The Structured Clinical Interview for DSM-IV, non-patient edition (SCID; First, Spitzer, Gibbon, & Williams, 2001) was used to assess lifetime diagnoses at baseline, and Clinical Severity Ratings (CSRs; Di Nardo & Barlow, 1988) captured symptom severity and degree of distress and impairment associated with each diagnosis. Interviewers possessed at least a bachelor's degree, completed extensive training and achieved consensus with gold standard diagnostic and CSR ratings. Diagnoses and CSRs were assigned during supervision with a doctoral level clinical psychologist in a consensus process. CSRs were rated on a nine-point scale with “0” representing no symptoms, distress, or interference, “1-3” representing subclinical severity, and “4-8” representing clinical significance of increasing severity. Follow-up annual SCIDs assessed CSRs for social anxiety disorder and major depression over the time since the participant's previous assessment. Inter-rater reliability was conducted on approximately 10% of all SCIDs (69 cases). Inter-rater Pearson r for CSR ratings was ≥.74 for all disorders and Kappas for major depressive disorder and social anxiety disorder were (.83) and (.65) respectively (Zinbarg et al., 2010).
Social Anxiety
Social anxiety was represented by a latent variable whose indicators included SCID CSR ratings for social anxiety disorder and eight items from the Self-Consciousness subscale of the Social Phobia Scale (SPS-SC; Mattick & Clark, 1998). The SPCSC includes 13 items rated on a five-point Likert scale (e.g., “I feel awkward and tense if I know people are watching me”). The eight items chosen have been shown to uniquely predict social anxiety (Prenoveau et al., 2010). As in Prenoveau et al. (2011), the eight SPS-SC items were separated into two, four-item subscales to improve TSO model convergence rates (see Table S1 in the online Supplemental Material for specific items), which, like all latent variable approaches, require multiple observed indicators (α subscale 1: Mean = .67, SD = .06; α subscale 2: Mean = .75, SD = .04).
Depression
Depression was represented by a latent variable whose indicators included SCID CSR ratings for major depressive disorder, two items from the Inventory to Diagnose Depression (IDD; Zimmerman, Coryell, Corenthal, & Wilson, 1986) and nine items from the Mood and Anxiety Symptom Questionnaire (MASQ; Watson et al., 1995). The specific items from the IDD and MASQ were chosen based on previous research demonstrating that these items uniquely predict depression (Prenoveau et al., 2010). The two IDD items measured discouragement about the future and frequency/intensity of sadness. The MASQ asks individuals to rate the extent to which they have experienced symptoms during the past week (e.g., “felt discouraged”). As in Prenoveau et al. (2011), the 11 items from the IDD and MASQ were separated into two subscales (with 5 and 6-items; see Table S1 in the online Supplemental Material for specific items) to improve TSO model convergence rates (α subscale 1: Mean = .87, SD = .01; α subscale 2: Mean = .84, SD = .02).
Chronic Interpersonal Stress
Past year chronic interpersonal stress was assessed at baseline interview using the UCLA Life Stress Interview (LSI; Hammen, 1991).1 The LSI assesses four interpersonal stress domains: best friend relationship, social circle, romantic relationship, and family relationships. Ratings were assigned by the interviewer for each domain on a scale from 1 to 5 in half-point increments, with 1 representing the least amount of stress, and 5 representing the most. Scores from the interpersonal domains were averaged and centered to create a composite score that represented baseline chronic interpersonal stress, which was analyzed as a prospective predictor of trait symptom factors. Inter-rater reliability (ICCs) for the chronic interpersonal stress composite was .9 within site and .89 cross-site.
Genotyping
Participants provided saliva samples using Oragene kits (DNA Genotek, Ontario, Canada) in their homes. Extraction and genotyping for CD38 SNP rs3796863 was performed by Kbioscience (United Kingdom). KASP™ genotyping assays are based on competitive allele-specific PCR and enable bi-allelic scoring of SNPs at specific loci. The assay-specific KASP Primer mix and the universal KASP master mix are added to DNA samples, a thermal cycling reaction is then performed, followed by end-point fluorescent detection. Biallelic discrimination is achieved through the competitive binding of two allele-specific forward primers, each with a unique tail sequence that corresponds with two universal FRET (fluorescence resonant energy transfer) cassettes; one labeled with FAM™ dye and the other with HEX™ dye.
Statistical Analysis
Following the methods outlined by Prenoveau et al. (2011), we created separate TSO models for social anxiety and depression symptoms measured across six annual time points (see Figure S1 in the online Supplemental Material for the social anxiety TSO measurement model).2 State social anxiety (or depression) represented a person's relative standing on a latent social anxiety (or depression) variable at a particular time. The variance for each state social anxiety latent variable (or depression) was then divided into trait social anxiety (or depression) and occasion specific variance (variance that is not explained by either the trait factor or standing on the prior state factor). All of the trait variance that was partitioned out of each state latent variable was then represented by our outcome of interest: latent trait social anxiety or depression (see, Cole et al., 2005). Based on model fit, CSRs were treated as categorical.
TSO structural equation modeling was conducted using Mplus version 7.0 (Muthén & Muthén, 1998-2012) and Full Information Maximum Likelihood was used to estimate missing data and model parameters. We used the false discovery rate correction (Benjamini & Hochberg, 1995) for multiple testing for the two primary GxE tests. As in previous studies (Feldman et al., 2012; Sauer, Montag, Worner, Kirsch, & Reuter, 2012), rs3796863 genotype was dichotomized such that all A allele carriers (A/A and A/C genotype, n = 237, coded 1) were compared to individuals who carried two C alleles (C/C genotype, n = 163, coded 0). In the TSO models, CD38 genotype, chronic interpersonal stress, and the GxE interaction effect were included as predictors of trait social anxiety and trait depression. In addition, self-reported race/ethnicity (Black, Hispanic, Other; Caucasians were designated as the comparison group), genotype x race/ethnicity interactions, gender, and SES were entered as covariates. Race/ethnicity as well as genotype-race/ethnicity interactions were included to statistically reduce the effects of population stratification (Keller, 2014; Tang et al., 2005). P-values < .05 (two-tailed) were considered statistically significant.
Results
Participant demographics and genotype frequencies are shown in Table 1 (for means, standard deviations, and correlations between observed study variables, see Table S2 and Table S3 in the online Supplemental Material). All participants completed the baseline Life Stress Interview. Table 1 also shows the number of annual self-report and interview assessments completed by participants. Genotypes did not depart from Hardy-Weinberg Equilibrium (χ2 = .01, p = .92).
Table 1.
Descriptive Statistics
| Variable | All Participants | CD38 A Allele Carriers | CD38 C/C Homozygotes |
|---|---|---|---|
|
Gender
| |||
| Female | 279 (69.8%) | 112 (68.7%) | 167 (70.5%) |
| Male | 121 (30.3%) | 51 (31.3%) | 70 (29.5%) |
| Race and ethnicity | |||
| Caucasian | 193 (48.0 %) | 94 (57.7%) | 99 (41.8%) |
| Black | 54 (13.5%) | 6 (3.7%) | 48 (20.3%) |
| Hispanic / Latino | 58 (14.7%) | 24 (14.7%) | 34 (14.3%) |
| Other | 95 (23.8%) | 39 (23.9%) | 56 (23.6%) |
| Screener risk level | |||
| Highest tertile | 232 (58.0%) | 96 (58.9%) | 136 (57.4%) |
| Middle tertile | 97 (24.3%) | 39 (23.9%) | 58 (24.5%) |
| Lowest tertile | 71 (17.8%) | 28 (17.2%) | 43 (18.1%) |
| CD38 genotype | |||
| AA | 53 (13.3%) | - | 53 (22.4%) |
| AC | 184 (44.9%) | - | 184 (77.6%) |
| CC | 163 (39.8%) | 163 (100%) | - |
| Age in years at baseline | 16.12 (0.43) | 16.12 (.41) | 16.11 (.45) |
| Baseline EPQ-R Neuroticism score | 11.88 (4.48) | 11.98 (4.49) | 11.81 (4.48) |
| Hollingshead SES Score | 48.47 (12.58) | 49.70 (13.03) | 47.63 (12.22) |
| Annual SCID Interviews completed | 5.31 (.97) | 5.33 (.97) | 5.29 (.97) |
| Self-reported social anxiety symptom items completed | 4.59 (1.27) | 4.65 (1.30) | 4.54 (1.26) |
| Self-reported depression symptom items completed | 4.71 (1.23) | 4.79 (1.23) | 4.65 (1.22) |
We first examined the gene-environment correlation between CD38 genotype and chronic interpersonal stress at baseline. As in other analyses, all covariates (gender, SES, race, genotype-race interactions) were included. No significant relationships were found between rs3796863 genotype (A/C or A/A genotypes coded 1; C/C genotype coded 0) and chronic interpersonal stress (r = -.06, p = .24). Therefore, any gene-environment interactions detected are not due to self-selection into stressful interpersonal conditions as a function of genotype.
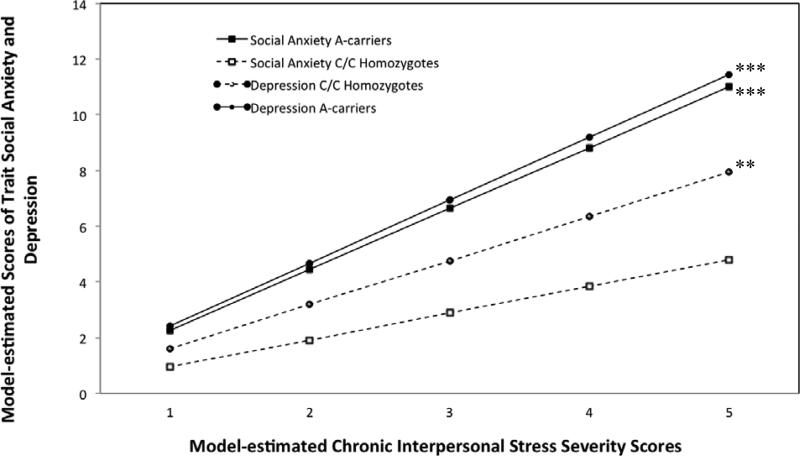
The social anxiety TSO model fit the data well as indicated by RMSEA: .036 (90% CI, .029-.043) and CFI: .927 (Hu & Bentler, 1999). Results for the social anxiety TSO model are shown in Table 2. No main effect was found between CD38 genotype and social anxiety. A main effect between chronic interpersonal stress and social anxiety was qualified by a significant GxE interaction of chronic interpersonal stress and CD38 genotype on trait social anxiety. Both results (main effect and interaction) remained significant when including all covariates in the model. As shown in Table 2, tests of the simple main effects demonstrated that increasing levels of chronic interpersonal stress were significantly associated with increasing levels of trait social anxiety among A allele carriers, but no significant association was found among individuals with the C/C genotype (see Figure 1). Table 2 also shows that results remained unchanged when including all covariates in the simple main effects analysis for A allele carriers and individuals with the C/C genotype. In addition, the GxE interaction between CD38 genotype and chronic interpersonal stress (p = .004) remained significant after multiple test correction.
Table 2.
Social Anxiety TSO Results
| Independent Variable | b | β | SE |
|---|---|---|---|
| CD38 genotype | .07 | .04 | .17 |
| Chronic Interpersonal Stress | .97*** | .42 | .18 |
| With covariates | .96*** | .41 | .19 |
| Genotype × Stress | 1.21** | .38 | .40 |
| With covariates | 1.23** | .37 | .43 |
| Simple Main Effects for A allele carriers | |||
| Chronic Interpersonal Stress | 1.54*** | .63 | .24 |
| With Covariates | 1.49*** | .60 | .27 |
| Simple Main Effects for C/C genotype | |||
| Chronic Interpersonal Stress | .25 | .10 | .32 |
| With Covariates | .25 | .10 | .33 |
Note. Covariates included: self-reported race/ethnicity (Black, Hispanic, Other; Caucasians were designated as the comparison group), genotype × race/ethnicity interactions, gender, and SES.
*p < .05
p < .01
p < .001
Figure 1.
Associations between model-estimated chronic interpersonal stress and model-estimated trait social anxiety and depression symptoms as a function of CD38 genotype. **p < .01; *** p < .001
The depression TSO model fit the data well as indicated by RMSEA: .032 (90% CI, .025-.039) and CFI: .921. Results for the depression TSO model are shown in Table 3. No main effect was found between CD38 genotype and depression. A main effect between chronic interpersonal stress and depression was qualified by a significant GxE interaction of chronic interpersonal stress and CD38 genotype on depression. Both results (main effect and interaction) remained significant when including all covariates. As shown in Table 3, tests of the simple main effects demonstrated that increasing levels of chronic interpersonal stress were significantly associated with increasing levels of trait depression in all individuals, but significantly more strongly among A allele carriers than their C/C counterparts (see Figure 1). Table 3 also shows that results for these simple main effects remained unchanged when including all covariates. In addition, the GxE interaction between CD38 genotype and chronic interpersonal stress (p = .037) remained significant after multiple test correction.
Table 3.
Depression TSO Results
| Independent Variable | b | β | SE |
|---|---|---|---|
| CD38 genotype | .16 | .08 | .16 |
| Chronic Interpersonal Stress | 2.42*** | .75 | .62 |
| With covariates | 1.59*** | .62 | .29 |
| Genotype × Stress | .86* | .24 | .41 |
| With covariates | .67* | .20 | .33 |
| Simple Main Effects for A allele carriers | |||
| Chronic Interpersonal Stress | 2.29*** | .80 | .51 |
| With Covariates | 1.59*** | .62 | .29 |
| Simple Main Effects for C/C genotype | |||
| Chronic Interpersonal Stress | 1.06** | .40 | .35 |
| With Covariates | .91*** | .35 | .25 |
Note. Covariates included: self-reported race/ethnicity (Black, Hispanic, Other; Caucasians were designated as the comparison group), genotype × race/ethnicity interactions, gender, and SES.
p < .05
p < .01
p < .001
Discussion
The present findings are the first to demonstrate that polymorphic variation in the CD38 gene and social sensitivity to interpersonal events at baseline prospectively predict higher trait levels of social anxiety and depression over time. Further, we also show that these effects depend on an individual's social context—a gene environment interaction effect. Although we identified a statistically significant GxE effect that predicted trait depression over time, there are several reasons to be cautious in interpreting this effect. First, the magnitude of the GxE effect was very small compared to the main effect of baseline ratings of chronic interpersonal stress. Second, the main effect of chronic interpersonal stress at baseline compared to the effect of the GxE interaction was much larger in the depression model relative to the social anxiety model. Third, baseline ratings of chronic interpersonal stress were significantly positively associated with increasing levels of trait depression in both genotype groups. These findings led us to question the utility and replicability of the GxE predicting depression symptoms.
To our knowledge, this is also the first study to investigate GxE interactions in the context of a TSO structural equation model. Consistent with evidence of a complex polygenic basis for psychiatric disorders (Demirkan et al., 2011), we did not obtain significant main effects of the CD38 SNP rs3796863 with either form of psychopathology. Our GxE interaction findings are in agreement with previous studies suggesting a link between polymorphic variation in rs3796863 and individual differences in social sensitivity (Feldman et al., 2012; Munesue et al., 2010). In addition, findings accord with previous research demonstrating associations between social sensitivity, depression, and anxiety (Boyce et al., 1991; Sutton et al., 2011).
Specifically, chronic interpersonal stress at baseline was associated with higher levels of trait social anxiety and depression over six years for A allele carriers on rs3796863. In addition, baseline ratings of chronic interpersonal stress were associated with higher levels of depression for individuals with the C/C genotype (though this relationship was of smaller magnitude than the relationship for A allele carriers), and no significant association was found between chronic interpersonal stress and social anxiety symptoms in individuals with the C/C genotype. However, the large main effect of baseline ratings of chronic interpersonal stress on depression appears to be most relevant in the present study, even above and beyond the significant gene-environment interaction findings. Our findings of significant main effects of baseline chronic interpersonal stress on both disorders examined contribute to the large body of work demonstrating the strong association between interpersonal stress and depression (Liu & Alloy, 2010, Slavich et al., 2010), as well as the potential link between interpersonal stress and social anxiety (e.g., Starr & Davila, 2008).
At least one possible model may explain these findings. Results from previous studies have generally demonstrated that individuals who carry the A allele on rs3796863 are characterized by higher levels of social sensitivity and higher levels of peripheral oxytocin when compared to those with the C/C genotype (Feldman et al., 2012; Munesue et al., 2010). One of the leading hypotheses about the effects of oxytocin on social cognition and behavior is that oxytocin increases the salience of social cues (Bartz et al., 2011; Shamay-Tsoory et al., 2009), which can be thought of as increased sensitivity to both positively and negatively valenced social stimuli. Although increased social sensitivity may enhance social cognition and increase prosocial behavior for some individuals in positive environments, the underlying increase in social sensitivity may have detrimental effects for others in negative environments (Bartz et al., 2011; Tabak, 2013). In addition, plasma oxytocin has been positively associated with social anxiety (Hoge, Pollack, Kaufman, Zak, & Simon, 2008), depression (Cyranowski et al., 2008), and relational distress (Tabak, McCullough, Szeto, Mendez, & McCabe, 2011). Therefore, one possible explanation for the present results is that elevations in endogenous oxytocin putatively associated with rs3796863 A-carrier genotypes may heighten social sensitivity, which in the context of higher levels of chronic interpersonal stress, prospectively predicts an increase in social anxiety and (to a lesser extent) depression symptoms over time. Thus, the present findings provide additional evidence for a link between the oxytocin system and social anxiety (Heinrichs & Gaab, 2007; Guastella et al., 2009; Labuschagne et al., 2010).
Although social anxiety and depression are highly comorbid, the present findings also remind us of significant distinctions between these two constructs. To date, social anxiety has appeared to be more empirically proximate to the oxytocin system (in this case as represented by the “G” component of the “GxE”) and to a lesser extent to chronic interpersonal stress (“E”). Although variation in the oxytocin receptor gene has been shown to moderate the association between early life adversity and depression (McQuaid et al., 2013), the present results do not point to a particularly influential role of CD38 variation in the development of depression from late adolescence to early adulthood. Rather, our results showing a strong main effect between ratings of chronic interpersonal stress at baseline and depression confirm previous reports demonstrating that depression is more empirically proximate to environmental influences (represented by the “E” component of the “GxE”), and less so to the oxytocin system (in this case the “G” component). Indeed, the relationship between oxytocinergic signaling and depression is less understood than it is for social anxiety (Neumann & Landgraf, 2012).
Noteworthy strengths of this study include the use of a longitudinal repeated measures design, diagnostic and life stress interviews, dimensional dependent variables, and the application of a latent variable approach through TSO structural equation modeling. This enabled isolation of trait-specific variance in social anxiety and depression symptoms over six years. Limitations included a relatively small sample for a population-based genetic association study. However, our sample is slightly larger than many other GxE interaction studies (Duncan & Keller, 2011), and substantially larger than several recent investigations of CD38 and other social phenotypes (Feldman et al., 2013; Sauer et al., 2012). In addition, our use of a prospective longitudinal design that included both clinician rated social anxiety and depression symptoms as well as interview-based measures of chronic interpersonal stress decreases the likelihood that the present results are false positives (Caspi, Hariri, Holmes, Uher, & Moffitt, 2010). Nonetheless, replication studies with a larger sample size are recommended.
Another limitation is that the present study did not measure endogenous levels of oxytocin, so only speculation is possible regarding the relationship between variation in rs3796963, levels of oxytocin, and social anxiety and depression. Although studies have found an association between lower levels of endogenous oxytocin and the SNP examined here (Feldman et al., 2012, 2013), it is located in an intronic region of the CD38 gene and at present has no known functionality. As a result, this SNP may serve as a marker for a functional variant of CD38 (Lin, Vance, Pericak-Vance, & Martin, 2007). Furthermore, even in the absence of direct connection between rs3796963 genotype and oxytocin levels, the allele-specific effects of genotype by environment interaction with social anxiety/depression remains valid.
It is important to note that our sample was racially/ethnically heterogeneous. Our inclusion of race and genotype-race interactions as covariates reduces the likelihood that our results resulted from false positive findings associations due to population stratification; however, we did not have enough participants from each representative racial group to perform separate analyses. Similarly, our study included an uneven amount of male and female participants. Future studies with larger sample sizes would benefit from examining racial/ethnic and gender specific effects. In addition, while the transition from late adolescence to young adulthood is an optimal period for studying the development of psychopathology (Krueger, 1999), it may also restrict the generalizability of our findings beyond individuals from this developmental period. Future studies would benefit from examining the GxE interaction effect of CD38 and interpersonal stress that occurs before and after late adolescence. Importantly, it should be noted that our suggestion that increased levels of social sensitivity (in the presence of interpersonal stress) may contribute to increased risk for anxiety and depression does not mean that low levels of social sensitivity (e.g., difficulty or disinterest in interpreting the social cues of others that may contribute to decreased social support) do not also contribute to risk for affective psychopathology. The present study also included a subsample of the larger Youth Emotion Project, a study in which participants with high levels of neuroticism were oversampled. Importantly, however, Hauner et al. (2013) demonstrated that oversampling in this fashion does not bias regression effect size estimates.
In summary, using structural equation TSO modeling, we showed a significant association between baseline ratings of chronic interpersonal stress and social anxiety and depression symptoms over six years. The associations with social anxiety and depression were qualified by a significant interaction of CD38 genotype with chronic interpersonal stress at baseline. Our findings suggest that the previously described “protective” A allele on rs3796863 may not represent “protection” in all contexts or for all conditions. Indeed, here it serves as a risk factor for social anxiety, consistent with conceptualizing the A allele as contributing to greater social sensitivity, and the C allele as contributing to less social sensitivity. It further suggests that future research is necessary to continue to unravel the complex role of CD38 and oxytocin in human social functioning (Higashida et al., 2012), particularly attending to environmental context (Tabak, 2013). This study also demonstrates the value of using TSO modeling in genetic association studies.
As coping style moderates the effects of intranasal oxytocin administration on mood response to interpersonal stress (Cordoso et al., 2012), future studies could examine the three-way interaction effect of CD38 genotype, coping style, and interpersonal stress on the development of psychopathology. In addition, future studies would benefit from including a measure of social functioning in addition to the variables included in the present study. This would facilitate an examination of the relationships between CD38, stress, social functioning, anxiety, and depression. Last, future research could build on our findings by examining CD38 and other candidate genes (e.g., 5-HTTLPR; Domschke & Dannlowski, 2010) in gene-gene-environment interactions (Sauer et al., 2012) or multilocus genetic profiles that may further elucidate the complex neurobiology underlying the development and maintenance of social anxiety and depression.
Supplementary Material
Acknowledgements
Dr. Vrshek-Schallhorn is now at the University of North Carolina – Greensboro.
Funding
This project was supported by the National Institute of Mental Health to M.G.C. [R01MH065651] and S.M. and R.E.Z. [R01MH065652], and a postdoctoral fellowship for B.A.T. was supported by the T32MH15750 training fellowship in Biobehavioral Issues in Physical and Mental Health at the University of California, Los Angeles.
Footnotes
M.G.C., R.E.Z., and S.M. developed the study concept and design for the larger YEP with E.E.R. and E.K.A. contributing; B.A.T. developed the study concept and design for this article. Data collection was performed by S.V-S. under the supervision of S.M., R.E.Z, and M.G.C. B.A.T. performed the data analysis and interpretation with J.M.P. and R.E.Z. B.A.T. drafted the manuscript, and all authors provided critical revisions. All authors approved the final version of the manuscript for submission.
Declaration of Conflicting Interests
The authors declared that they had no conflicts of interest with respect to their authorship or the publication of this article.
As part of the YEP study, we also measured interpersonal stress at time points following the baseline measurent. However, to account for the temporal dynamics of the study (i.e., using the GxE interaction effect to prospectively predict longitudinal trait social anxiety and depression symptoms), we chose to rely on only ratings of chronic interpersonal stress at baseline.
Given the well-known high degree of comorbidity between social anxiety and depression, we initially attempted to run a unified TSO model that included both. Unfortunately the combined model did not converge, so we present separate TSO models.
References
- Bartz JA, McInnes LA. CD38 regulates oxytocin secretion and complex social behavior. Bioessays. 2007;29:837–841. doi: 10.1002/bies.20623. [DOI] [PubMed] [Google Scholar]
- Bartz JA, Zaki J, Bolger N, Ochsner KN. Social effects of oxytocin in humans: context and person matter. Trends in Cognitive Sciences. 2011;15:301–309. doi: 10.1016/j.tics.2011.05.002. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society. Series B (Methological) 1995;57:289–300. [Google Scholar]
- Boyce P, Parker G, Barnett B, Cooney M, Smith F. Personality as a vulnerability factor to depression. The British Journal of Psychiatry : The Journal of Mental Science. 1991;159:106–114. doi: 10.1192/bjp.159.1.106. [DOI] [PubMed] [Google Scholar]
- Bruzzone S, Bodrato N, Usai C, Guida L, Moreschi I, Nano R, Zocchi E. Abscisic acid is an endogenous stimulator of insulin release from human pancreatic islets with cyclic ADP ribose as second messenger. The Journal of Biological Chemistry. 2008;283:32188–97. doi: 10.1074/jbc.M802603200. [DOI] [PubMed] [Google Scholar]
- Caspi A, Hariri AR, Holmes A, Uher R, Moffitt TE. Genetic sensitivity to the environment: the case of the serotonin transporter gene and its implications for studying complex diseases and traits. American Journal of Psychiatry. 2010;167:509–527. doi: 10.1176/appi.ajp.2010.09101452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevallier C, Kohls G, Troiani V, Brodkin ES, Schultz RT. The social motivation theory of autism. Trends in Cognitive Science. 2012;16:231–239. doi: 10.1016/j.tics.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark DM, McManus F. Information processing in social phobia. Biological Psychiatry. 2002;51:92–100. doi: 10.1016/s0006-3223(01)01296-3. [DOI] [PubMed] [Google Scholar]
- Cole DA, Martin NC, Steiger JH. Empirical and conceptual problems with longitudinal trait-state models: introducing a trait-state-occasion model. Psychological Methods. 2005;10:3–20. doi: 10.1037/1082-989X.10.1.3. [DOI] [PubMed] [Google Scholar]
- Cuthbert BN, Kozak MJ. Constructing constructs for psychopathology: the NIMH research domain criteria. Journal of Abnormal Psychology. 2013;122:928–937. doi: 10.1037/a0034028. [DOI] [PubMed] [Google Scholar]
- Cyranowski JM, Hofkens BA, Frank E, Seltman H, Cai H, Amico J. Evidence of dysregulated peripheral oxytocin release among depressed women. Psychosomatic Medicine. 2008;70:967–975. doi: 10.1097/PSY.0b013e318188ade4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso C, Linnen AM, Joober R, Ellenbogen MA. Coping style moderates the effect of intranasal oxytocin on the mood response to interpersonal stress. Experimental and Clinical Psychopharmacology. 2012;20:84–91. doi: 10.1037/a0025763. [DOI] [PubMed] [Google Scholar]
- Demirkan A, Penninx BWJH, Hek K, Wray NR, Amin N, Aulchenko YS, Middeldorp CM. Genetic risk profiles for depression and anxiety in adult and elderly cohorts. Molecular Psychiatry. 2011;16:773–783. doi: 10.1038/mp.2010.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Nardo PA, Barlow DH. Anxiety Disorders Interview Schedule-Revised (ADIS R) Phobia and Anxiety Disorders Clinic; Albany, NY: 1988. [DOI] [PubMed] [Google Scholar]
- Domschke K, Dannlowski U. Imaging genetics of anxiety disorders. Neuroimage. 2010;53:822–831. doi: 10.1016/j.neuroimage.2009.11.042. [DOI] [PubMed] [Google Scholar]
- Duncan LE, Keller MC. A critical review of the first 10 years of candidate gene-by-environment interaction research in psychiatry. American Journal of Psychiatry. 2011;168:1041–1049. doi: 10.1176/appi.ajp.2011.11020191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eysenck SBG, Eysenck HJ, Barrett P. A revised version of the psychoticism scale. Personality and Individual Differences. 1985;6:21–29. [Google Scholar]
- Feldman R, Gordon I, Influs M, Gutbir T, Ebstein RP. Parental oxytocin and early caregiving jointly shape children's oxytocin response and social reciprocity. Neuropsychopharmacology. 2013;38:1154–1162. doi: 10.1038/npp.2013.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman R, Zagoory-Sharon O, Weisman O, Schneiderman I, Gordon I, Maoz R, Ebstein RP. Sensitive parenting is associated with plasma oxytocin and polymorphisms in the OXTR and CD38 genes. Biological Psychiatry. 2012;72:175–181. doi: 10.1016/j.biopsych.2011.12.025. [DOI] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J. Structured clinical interview for DSM-IV-TR Axis I disorders-non-patient edition. New York State Psychiatric Institute, Bi.; New York: 2001. [Google Scholar]
- Guastella AJ, Howard AL, Dadds MR, Mitchell P, Carson DS. A randomized controlled trial of intranasal oxytocin as an adjunct to exposure therapy for social anxiety disorder. Psychoneuroendocrinology. 2009;34:917–923. doi: 10.1016/j.psyneuen.2009.01.005. [DOI] [PubMed] [Google Scholar]
- Hammen C. Generation of stress in the course of unipolar depression. Journal of Abnormal Psychology. 1991;100:555–561. doi: 10.1037//0021-843x.100.4.555. [DOI] [PubMed] [Google Scholar]
- Hammen C. Stress and depression. Annual Review of Clinical Psychology. 2005;1:293–319. doi: 10.1146/annurev.clinpsy.1.102803.143938. [DOI] [PubMed] [Google Scholar]
- Hammen C, Hazel NA, Brennan PA, Najman J. Intergenerational transmission and continuity of stress and depression: depressed women and their offspring in 20 years of follow-up. Psychological Medicine. 2012;42(5):931–942. doi: 10.1017/S0033291711001978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauner KK, Revelle W, Zinbarg RE. A latent variable model approach to estimating systematic bias in the oversampling method. Behavioral Research Methods. 2013;46:786–797. doi: 10.3758/s13428-013-0402-6. [DOI] [PubMed] [Google Scholar]
- Heinrichs M, Gaab J. Neuroendocrine mechanisms of stress and social interaction: implications for mental disorders. Current Opinion in Psychiatry. 2007;20:158–162. doi: 10.1097/YCO.0b013e3280146a13. [DOI] [PubMed] [Google Scholar]
- Higashida H, Yokoyama S, Kikuchi M, Munesue T. CD38 and its role in oxytocin secretion and social behavior. Hormones and Behavior. 2012;61:351–358. doi: 10.1016/j.yhbeh.2011.12.011. [DOI] [PubMed] [Google Scholar]
- Hoge EA, Pollack MH, Kaufman RE, Zak PJ, Simon NM. Oxytocin levels in social anxiety disorder. CNS Neuroscience and Therapeutics. 2008;14:165–170. doi: 10.1111/j.1755-5949.2008.00051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingshead AB. A four factor index of social status. Yale University; New Haven: 1975. Unpublished manuscript. [Google Scholar]
- Hu L, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling. 1999;6:1–55. [Google Scholar]
- Jin D, Liu HX, Hirai H, Torashima T, Nagai T, Lopatina O, Higashida H. CD38 is critical for social behaviour by regulating oxytocin secretion. Nature. 2007;446:41–45. doi: 10.1038/nature05526. [DOI] [PubMed] [Google Scholar]
- Keller MC. Gene × Environment Interaction Studies Have Not Properly Controlled for Potential Confounders: The Problem and the (Simple) Solution. Biological Psychiatry. 2014;75:18–24. doi: 10.1016/j.biopsych.2013.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Prescott CA, Myers J, Neale MC. The structure of genetic and environmental risk factors for common psychiatric and substance use disorders in men and women. Archives of General Psychiatry. 2003;60:929–937. doi: 10.1001/archpsyc.60.9.929. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Kiss I, Levy-Gigi E, Keri S. CD 38 expression, attachment style and habituation of arousal in relation to trust-related oxytocin release. Biological Psychology. 2011;88:223–226. doi: 10.1016/j.biopsycho.2011.08.005. [DOI] [PubMed] [Google Scholar]
- Krueger RF. Personality traits in late adolescence predict mental disorders in early adulthood: a prospective-epidemiological study. Journal of Personality. 1999;67:39–65. doi: 10.1111/1467-6494.00047. [DOI] [PubMed] [Google Scholar]
- Labuschagne I, Phan KL, Wood A, Angstadt M, Chua P, Heinrichs M, et al. Oxytocin attenuates amygdala reactivity to fear in generalized social anxiety disorder. Neuropsychopharmacology. 2010;35:2403–2413. doi: 10.1038/npp.2010.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerer E, Levi S, Israel S, Yaari M, Nemanov L, Mankuta D, et al. Low CD38 expression in lymphoblastoid cells and haplotypes are both associated with autism in a family-based study. Autism Research. 2010;3:293–302. doi: 10.1002/aur.156. [DOI] [PubMed] [Google Scholar]
- Lin PI, Vance JM, Pericak-Vance MA, Martin ER. No gene is an island: the flip-flop phenomenon. American Journal of Human Genetics. 2007;80:531–538. doi: 10.1086/512133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu RT, Alloy LB. Stress generation in depression: A systematic review of the empirical literature and recommendations for future study. Clinical Psychology Review. 2010;30:582–593. doi: 10.1016/j.cpr.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malavasi F, Deaglio S, Funaro A, Ferrero E, Horenstein AL, Ortolan E, et al. Evolution and function of the ADP ribosyl cyclase/CD38 gene family in physiology and pathology. Physiological Reviews. 2008;88:841–886. doi: 10.1152/physrev.00035.2007. [DOI] [PubMed] [Google Scholar]
- Mattick RP, Clark JC. Development and validation of measures of social phobia scrutiny fear and social interaction anxiety. Behaviour Research and Therapy. 1998;36:455–470. doi: 10.1016/s0005-7967(97)10031-6. [DOI] [PubMed] [Google Scholar]
- McGrath LM, Weill S, Robinson EB, Macrae R, Smoller JW. Bringing a developmental perspective to anxiety genetics. Development and Psychopathology. 2012;24:1179–1193. doi: 10.1017/S0954579412000636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Breslau J, Green JG, Lakoma MD, Sampson NA, Zaslavsky AM, Kessler RC. Childhood socio-economic status and the onset, persistence, and severity of DSM-IV mental disorders in a US national sample. Social Science and Medicine. 2011;73:1088–1096. doi: 10.1016/j.socscimed.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuaid RJ, McInnis OA, Stead JD, Matheson K, Anisman H. A paradoxical association of an oxytocin receptor gene polymorphism: early-life adversity and vulnerability to depression. Frontiers in Neuroscience. 2013;7:128. doi: 10.3389/fnins.2013.00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middeldorp CM, Cath DC, Van Dyck R, Boomsma DI. The co-morbidity of anxiety and depression in the perspective of genetic epidemiology. A review of twin and family studies. Psychological Medicine. 2005;35:611–624. doi: 10.1017/s003329170400412x. [DOI] [PubMed] [Google Scholar]
- Munesue T, Yokoyama S, Nakamura K, Anitha A, Yamada K, Hayashi K, et al. Two genetic variants of CD38 in subjects with autism spectrum disorder and controls. Neuroscience Research. 2010;67:181–191. doi: 10.1016/j.neures.2010.03.004. [DOI] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus User's Guide (Seventh.) Muthen & Muthen; Los Angeles, CA: 1998-2012. [Google Scholar]
- Naragon-Gainey K, Gallagher MW, Brown TA. Stable “trait” variance of temperament as a predictor of the temporal course of depression and social phobia. Journal of Abnormal Psychology. 2013;122:611–623. doi: 10.1037/a0032997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann ID, Landgraf R. Balance of brain oxytocin and vasopressin: implications for anxiety, depression, and social behaviors. Trends in Neuroscience. 35:649–659. doi: 10.1016/j.tins.2012.08.004. (20120. [DOI] [PubMed] [Google Scholar]
- Prenoveau JM, Craske MG, Zinbarg RE, Mineka S, Rose RD, Griffith JW. Are anxiety and depression just as stable as personality during late adolescence? Results from a three-year longitudinal latent variable study. Journal of Abnormal Psychology. 2011;120:832–843. doi: 10.1037/a0023939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prenoveau JM, Zinbarg RE, Craske MG, Mineka S, Griffith JW, Epstein AM. Testing a hierarchical model of anxiety and depression in adolescents: a tri-level model. Journal of Anxiety Disorders. 2010;24:334–344. doi: 10.1016/j.janxdis.2010.01.006. [DOI] [PubMed] [Google Scholar]
- Sauer C, Montag C, Worner C, Kirsch P, Reuter M. Effects of a common variant in the CD38 gene on social processing in an oxytocin challenge study: possible links to autism. Neuropsychopharmacology. 2012;37:1474–1482. doi: 10.1038/npp.2011.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, Fischer M, Dvash J, Hariri AR, Perach-Bloom N, Levkovitz Y. Intranasal administration of oxytocin increases envy and schadenfreude (gloating). Biological Psychiatry. 2009;66:864–870. doi: 10.1016/j.biopsych.2009.06.009. [DOI] [PubMed] [Google Scholar]
- Simon G, Ormel J, VonKorff M, Barlow W. Health care costs associated with depressive and anxiety disorders in primary care. The American Journal of Psychiatry. 1995;152:352–357. doi: 10.1176/ajp.152.3.352. [DOI] [PubMed] [Google Scholar]
- Slavich GM, O'Donovan A, Epel ES, Kemeny ME. Black sheep get the blues: a psychobiological model of social rejection and depression. Neuroscience and Biobehavioral Reviews. 2010;35:39–45. doi: 10.1016/j.neubiorev.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smoller JW, Gardner-Schuster E, Misiaszek M. Genetics of anxiety: would the genome recognize the DSM? Depression and Anxiety. 2008;25(4):368–377. doi: 10.1002/da.20492. [DOI] [PubMed] [Google Scholar]
- Starr LR, Davila J. Differentiating interpersonal correlates of depressive symptoms and social anxiety in adolescence: implications for models of comorbidity. Journal of Clinical Child and Adolescent Psycholology. 2008;37:337–349. doi: 10.1080/15374410801955854. [DOI] [PubMed] [Google Scholar]
- Sullivan PF, Neale MC, Kendler KS. Genetic epidemiology of major depression: review and meta-analysis. American Journal of Psychiatry. 2000;157:1552–1562. doi: 10.1176/appi.ajp.157.10.1552. [DOI] [PubMed] [Google Scholar]
- Sutton JM, Mineka S, Zinbarg RE, Craske MG, Griffith JW, Rose RD, et al. The Relationships of Personality and Cognitive Styles with Self-Reported Symptoms of Depression and Anxiety. Cognitive Therapy and Research. 2011;35:381–393. doi: 10.1007/s10608-010-9336-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szeto A, McCabe PM, Nation DA, Tabak BA, Rossetti MA, McCullough ME, Mendez AJ. Evaluation of enzyme immunoassay and radioimmunoassay methods for the measurement of plasma oxytocin. Psychosomatic Medicine. 2011;73:393–400. doi: 10.1097/PSY.0b013e31821df0c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabak BA. Oxytocin and social salience: a call for gene-environment interaction research. Frontiers in Neuroscience. 2013;7:199. doi: 10.3389/fnins.2013.00199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabak BA, McCullough ME, Szeto A, Mendez AJ, McCabe PM. Oxytocin indexes relational distress following interpersonal harms in women. Psychoneuroendocrinology. 2011;36:115–122. doi: 10.1016/j.psyneuen.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang H, Quertermous T, Rodriguez B, Kardia SL, Zhu X, Brown A, Risch NJ. Genetic structure, self-identified race/ethnicity, and confounding in case-control association studies. American Journal of Human Genetics. 2005;76:268–275. doi: 10.1086/427888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrshek-Schallhorn S, Mineka S, Zinbarg RE, Craske MG, Griffith JW, Sutton J, Adam EK. Refining the Candidate Environment: Interpersonal Stress, the Serotonin Transporter Polymorphism, and Gene-Environment Interactions in Major Depression. Clinical Psychological Science. 2014;2:235–248. doi: 10.1177/2167702613499329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Weber K, Assenheimer JS, Clark LA, Strauss ME, McCormick RA. Testing a tripartite model: I. Evaluating the convergent and discriminant validity of anxiety and depression symptom scales. Journal of Abnormal Psychology. 1995;104:3–14. doi: 10.1037//0021-843x.104.1.3. [DOI] [PubMed] [Google Scholar]
- Wong MY, Day NE, Luan JA, Chan KP, Wareham NJ. The detection of gene-environment interaction for continuous traits: should we deal with measurement error by bigger studies or better measurement? International Journal of Epidemiology. 2003;32:51–57. doi: 10.1093/ije/dyg002. [DOI] [PubMed] [Google Scholar]
- Zimmerman M, Coryell W, Corenthal C, Wilson S. A self-report scale to diagnose major depressive disorder. Archives of General Psychiatry. 1986;43:1076–1081. doi: 10.1001/archpsyc.1986.01800110062008. [DOI] [PubMed] [Google Scholar]
- Zinbarg RE, Mineka S, Craske MG, Griffith JW, Sutton J, Rose RD, et al. The Northwestern-UCLA youth emotion project: Associations of cognitive vulnerabilities, neuroticism and gender with past diagnoses of emotional disorders in adolescents. Behaviour Research and Therapy. 2010;48:347–358. doi: 10.1016/j.brat.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.