Abstract
Gap junctions are highly ordered plasma membrane domains that are constantly assembled, remodeled and turned over due to the short half-life of connexins, the integral membrane proteins that form gap junctions. Connexin 43 (Cx43), by far the most widely expressed connexin, is phosphorylated at multiple serine residues in the cytoplasmic, C-terminal region allowing for exquisite cellular control over gap junctional communication. This is evident during epidermal wounding where spatiotemporal changes in connexin expression occur as cells are instructed whether to die, proliferate or migrate to promote repair. Early gap junctional communication is required for initiation of keratinocyte migration, but accelerated Cx43 turnover is also critical for proper wound healing at later stages. These events are controlled via a "kinase program" where sequential phosphorylation of Cx43 leads to reductions in Cx43’s half-life and significant depletion of gap junctions from the plasma membrane within several hours. The complex regulation of gap junction assembly and turnover affords several steps where intervention might speed wound healing.
Keywords: Gap junctions, Connexin43, wound repair, phosphorylation, src, Protein kinase C, Mitogen-activated protein kinase
1. Introduction
1.1. Gap junctions play diverse and essential roles in cells of different tissues
Vertebrate gap junctions are composed of proteins from the 21 gene connexin gene family [1–6]. These collections of intercellular channels, termed gap junction plaques, permit passage of metabolites of less than approximately 1000 Da between cells while macromolecules are excluded (although small RNAs may pass) [7, 8]. Many cell processes effectively require gap junctionalcommunication including control of cell proliferation, embryonic development, cell differentiation and the coordinated contraction of heart and smooth muscle [2, 3, 9–12]. Connexins have been implicated by genetic linkage analysis in at least 14 human diseases - many of which can be recapitulated in mutant connexin mouse models with common forms of hereditary deafness being the most prevalent in humans [12]. Connexins are expressed in a tissue specific manner allowing them to fulfill a variety of physiological roles. Cx43, the focus of this review, is by far the most abundantly and widely expressed gap junction protein (> 34 tissues and 46 cell types [5]). Cx43 knockout mice die within hours of birth [13]. Oculodentodigital dysplasia is caused by many different mutations in the Cx43 gene and exhibits a variety of different symptoms including small eyes, underdeveloped teeth, syndactyly and palmoplantar keratoderma – a skin disease that can be caused by frame shift mutations [14] that result in loss of the C-terminal region where Cx43 is phosphorylated. Wounding of the epidermis leads to significant changes in Cx43 expression that faciliate healing. These changes are, at least in part, a result of altered Cx43 phosphorylation.
1.2 Connexin Phosphorylation
Many connexins (e.g., Cx31, 32, 37, 40, 43, 45, 46, and 50) have multiple phosphorylation sites – with Cx43 being the most prevalent in both tissues and cultured cells. Connexins have 4 transmembrane domains with the N- and C-termini on the cytoplasmic side. Many reports, both in vivo and in cell culture, indicate that Cx43 has a half-life in the range of 1–3 h [5, 15–25] - much faster than typical integral membrane proteins (17–100h) [26, 27]. At least nineteen of the twenty-six serines and 2 of the 6 tyrosines in the C-terminal region of Cx43 have been identified as phosphorylation sites present in cells or tissue, and there has been some progress in the characterization of the network of kinases that phosphorylate Cx43 (Table 1). Cx43 that lacks the last ~140 residues of the C-terminal portion can form gap junctions but the resulting gap junctions have different permeability/electrophysiological properties [28–30]. A “knock-in” mouse expressing Cx43 lacking this C-terminal region developed rigid skin with a defective epidermal layer that readily peels off. Almost all homozygote mutant mice died shortly after birth due to dehydration and Cx43 was found mis-localized throughout the stratified layers of the epidermis rather than restricted to the basal cells [31].
TABLE 1.
Cx43 residues phosphorylated, the kinases involved and their consequences.
| Assembly residues | Kinases | References |
|---|---|---|
| S325/328/330 | CK1, ?? | [41] |
| S364 | ?? | [112, 113] |
| S365 | ?? | [39, 114] |
| S373 | Akt | [60, 114–116] |
| Gating residues | ||
| S255 | MAPK | [117] |
| S262 | p34cdc2, MAPK | [118–120] |
| S279/282 | MAPK | [117] |
| S306 | ?? | [121, 122] |
| S368 | PKC | [116, 121, 123] |
| Dissassembly | ||
| Y247 | Src | [124] |
| S368 | PKC | [116, 121, 123] |
| Unknown | ||
| Y265 | Src | [124] |
| S296 | ?? | [121] |
| S297 | ?? | [121] |
| Y313 | ?? | [125, 126] |
| S369 | Akt,?? | [113–115] |
| S372 | ?? | [114–116] |
2. Cx43 assembly into gap junctions and turnover
2.1. Cx43 lifecycle is dynamic and complex
Cx43 is characterized by a short half-life and extensive regulation that allows the cell to exquisitely control gap junctional communication. This is evident through biochemical analyses, where Cx43 phosphorylation has been shown to regulate protein localization and behavior in a rapid and coordinated manner. Recently developed live cell imaging modalities have been particularly useful for visualizing dynamic interactions of Cx43 with the cytoskeleton and events at the gap junction. An understanding of how different subcellular pools of Cx43 are regulated and feedback on each other is critical to understanding gap junction biology. While intercellular transfer of molecules is a major function of the gap junction plaque, the sheer number of molecules that interact with Cx43 and are found at the gap junction plaque certainly argue for an important role in cell signaling. Gap junction plaques have been suggested to provide a type of "nexus" for coordinating subcellular events [32]. In the subsequent sections of this review, we will follow Cx43 through its lifecycle with a particular emphasis on how its coordinated interactions with kinases appears to regulate and potentially provides feedback to tightly control gap junction assembly and disassembly.
2.2.1. Gap junction assembly
As an integral membrane protein, Cx43 is synthesized and traffics through the endoplasmic reticulum. It lacks a canonical membrane signal sequence and delays oligomerization into a hexameric hemi-channel or “connexon” until reaching the trans-Golgi network [33]. This delay may provide a quality control step and allows feedback control as GJ assembly can be downregulated through Endoplasmic Reticulum Associated Degradation (ERAD) during conditions of cellular stress [34, 35]. Live cell and other imaging modalities show that Cx43 can traffic to the plasma membrane via multiple mechanisms including the secretory pathway or microtubule based vesicle targeting and there is evidence that these pathways can be differentially utilized under different conditions [25, 36, 37]. Cx43 typically moves from the plasma membrane into the periphery of the plaque, thus plaques "grow" from the outside in and the oldest proteins are found in the center of the plaque where they get selectively turned over [38].
2.2.2. Gap junction assembly and Cx43 phosphorylation
Cx43 phosphorylation events can occur within 15 min of synthesis [16] and several kinases appear to affect the assembly of gap junctions. Cx43 migrates as multiple bands in SDS-PAGE with many cell types displaying prominent bands often labeled P0, P1 and P2 which represent sequential stages of Cx43 processing from the plasma membrane to the gap junction in untreated cells. These SDS stable conformational changes in Cx43 structure have been shown to be due to sequential phosphorylation first on S365 then some combination of residues S325/328/330. S365 phosphorylation was found to be present in the P1 and P2 phosphoisoforms and occurs during the transition from the cytoplasm to the plasma membrane [39, 40]; see Fig. 1. Furthermore, phosphorylation at S365 plays a “gatekeeper” role by preventing downregulation of gap junctional communication by subsequent Cx43 phosphorylation at S368 [39]. Conversely, prior phosphorylation at S368 due to PKC activation could decrease gap junction assembly. Activation of cAMP-dependent protein kinase (PKA) can increase Cx43 phosphorylation at S364 and S365 and stimulate trafficking to the plasma membrane resulting in enhanced gap junction assembly (see Fig. 1 and Table 1). Casein kinase 1 (CK1) phosphorylates Cx43 on S325/328/330 during the transition of Cx43 from the plasma membrane into the gap junction – staining with an antibody to these phosphorylated residues exclusively recognizes the P2 form of Cx43 in SDS-PAGE and only Cx43 present in the gap junction [41]. Phosphorylation of serine 373 by Akt allows gap junctions to grow in size (see Fig. 1) but is an event that can be linked to gap junction disassembly (discussed further below). Activation of PKC can halt assembly of new junctions through an unknown mechanism [17]. Thus, the process of gap junction assembly is regulated by a succession of kinases that constitute a “kinase program” with multiple built-in checkpoints.
Fig. 1.
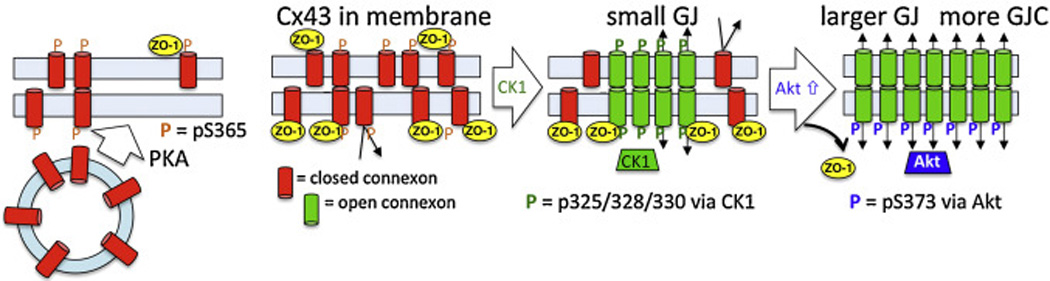
Model of the steps in gap junction assembly regulated by Cx43 phosphorylation. Our model first shows how activation of PKA causes increased trafficking of Cx43 vesicles to the plasma membrane. Cx43 in the plasma membrane then gets phosphorylated first at S365 (shown in orange) and subsequently at S325/328/330 (via CK1, shown in green). ZO-1 can associate with Cx43 and when it is present it keeps junctions small. Phosphorylation of Cx43 at S373 by Akt eliminates ZO-1 interaction and allows Cx43 accumulation into larger gap junctions with increased intercellular communication.
2.3 Cx43 and gap junction turnover
The regulation of connexin turnover and gap junction turnover are still not well understood. This is, in part, due to the fact that while the turnover of connexin molecules is fairly consistent across cell types and conditions, the stability of individual gap junction plaques can be highly variable [42]. This is presumably due to altered kinetics of connexin assembly into and removal from a given plaque, as described in section 2.2.1. What is known is that inhibition of lysosomal degradation can prolong the half-life of Cx43 protein but not necessarily gap junctions while proteasomal inhibition increases and stabilizes Cx43 present in gap junctions in most cell types. In some situations, plaques can remain stable for many hours, presumably through equilibrium of protein moving in and out of the gap junction, while at other times a single plaque can be rapidly and wholly disassembled. As an example, Fig. 2A and the associated movie show a time lapse series of Cx43-green fluorescent protein (GFP) fusion where one gap junction breaks up and is internalized (white arrow) while another gap junction remains relatively stable (white arrowhead) throughout the 30 min movie (frames taken at 20 second intervals). Lysosomes are visualized in red (via LysoTracker) and appear to be partially co-labeling with the gap junction throughout the disassembly process in the time lapse movie. To illustrate the dynamic nature of this interaction, Fig. 2B shows a time lapse series taken at 2 second intervals showing co-labeling of lysosomes with membranes containing Cx43-GFP. The dynamic interactions observed in these types of images suggest that lysosomes may be involved in more than simply engulfing and degrading already internalized membranes containing Cx43.
Fig. 2.
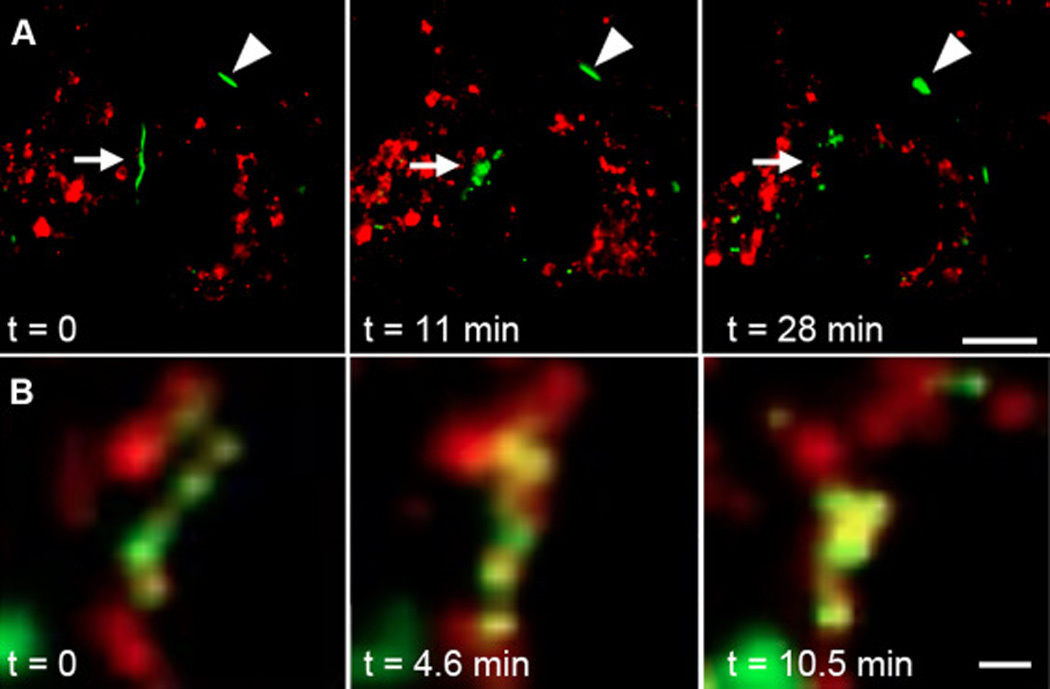
Images from time lapse series visualizing Cx43-GFP and LysoTracker Red. A) Arrow indicates a large gap junction that breaks apart and disappears, while the arrowhead points to a gap junction, in the same cell, that remains stable throughout the time course. In the associated movie, images were collected at 20 second intervals for 30 minutes. Lysosomes are seen in close proximity to the gap junctions throughout the disassembly process. Bar=10µm. B) Magnified view of interactions between Cx43-GFP and lysosomes. In the associated movie, images were collected at 2 second intervals. There appears to be a complex interplay in the membranes between Cx43-GFP and lysosomal compartments. Bar=1µm.
We also do not understand the range of potential cellular machinery involved in connexin and gap junction turnover. There is good evidence that a gap junction can be internalized in its entirety via formation of a double membrane structure termed an annular junction or connexisome [5, 43–50] (see mode 1, Fig. 3). Annular junctions have a distinct structure that is recognizable by electron microscopy, and are increased during the process of autophagy where they co-localize with the clathrin adapter proteins Dab2 [49], Atg14 and 9 [51] and the autophagosome membrane protein LC3 [44, 52, 53]. These structures are generally accepted to be precursors to lysosomal degradation. Whether annular junction formation is the exclusive way gap junctions are turned over and the process is just upregulated during autophagy is less clear. Given that annular junctions appear to vary widely in their prevalence within different cell types and cellular treatments while the half-life of Cx43 appears to be consistently short leads us to hypothesize that annular junction formation may be a mechanism utilized specifically during enhanced turnover, such as reduced nutrient triggered autophagy and growth factor induced gap junction turnover.
Fig. 3.
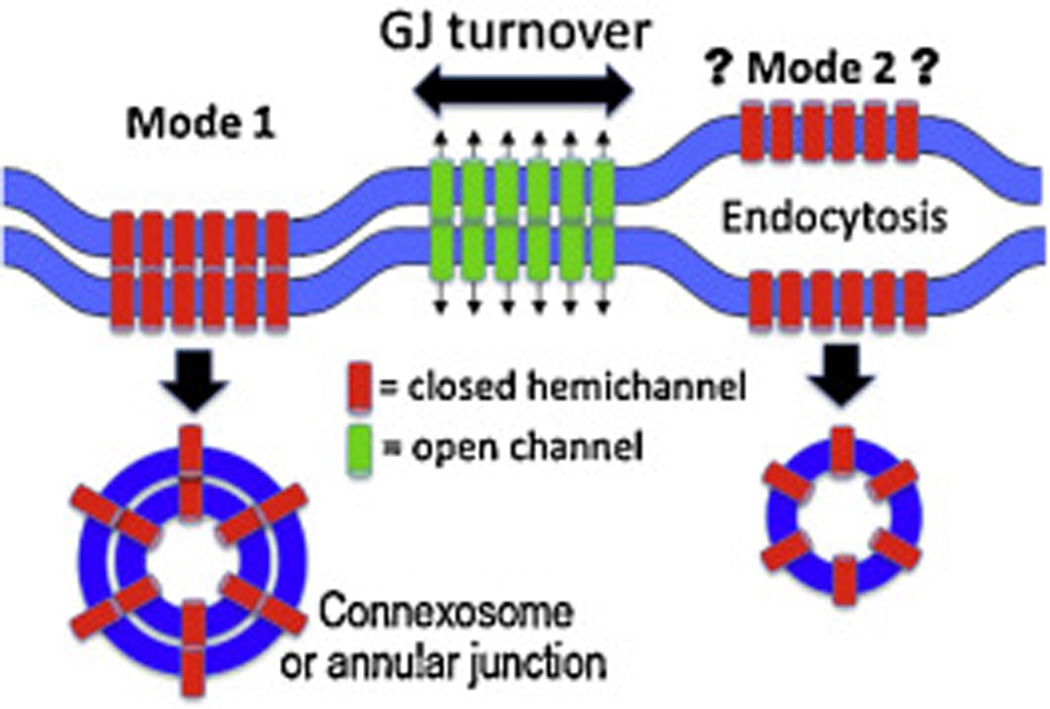
Cartoon of two potential modes of gap junction (GJ) turnover. Mode 1 proceeds through formation of a double membrane structure that will become an annular junction. Mode 2 involves opening of the gap between the two membranes by changes in phosphorylation or ubiquitinylation that destabilize the junction followed by conventional endocytosis.
Another potential mechanism would posit that gap junctions can be turned over via loss of extracellular Cx interactions (i.e., “unzippering” of the gap junction) followed by endocytosis from a single membrane (see mode 2, Fig. 3). At first glance such a mechanism seems unlikely given the stability of isolated gap junctions to relatively harsh conditions and a lack of direct evidence, but time-lapse movies of Cx43-GFP present in gap junctions make it clear that there are dynamic interactions occurring within and around gap junctional plaques as shown in the Fig. 2. It is also clear that kinases and phosphatases regulate the assembly and stability of gap junctions which is, at least, consistent with the idea of an intracellular pathway controlling extracellular disassembly. Finally, a third mechanism for loss of gap junctions would simply result from blockage of new gap junction formation while existing gap junctions are turned over. We would predict that further studies will show that these and perhaps other mechanisms of internalization will be utilized by cells in a context specific manner.
2.4 Cx43 turnover and the role of ubiquitinylation
Cx43 has been reported to undergo ERAD [34, 35] but the process was found to be independent of Cx43 ubiquitinylation as another protein, CIP75, regulated its degradation by the proteasome [34]. Cx43 contains 2 putative tyrosine-based sorting signals (Yxxϕ; where ϕ=hydrophobic) [54] including a key one involving residues 286–289 as Cx43 with a V289D mutation had a 3-fold increase in protein half-life [55]. However, this sorting signal was reported not to regulate ubiquitin-mediated Cx43 internalization [56]. Furthermore, gap junctional stability can be dramatically reduced in response to growth factors and phorbol esters [17, 57–64]. Many different reagents that can efficiently inhibit lysosomal or proteasomal protein degradation partially affect gap junction and Cx43 turnover but do not have complete effects. These and earlier results [65, 66] have led several groups to propose multiple and alternative pathways involving the proteasome and lysosome in Cx43 and gap junction degradation. There are many studies that have shown that if cells are treated with a proteasomal inhibitor and then Cx43 is immunoprecipitated and immunobloted with an antibody to ubiquitin, one sees multiple bands at higher molecular sizes implying poly-ubiquitination [65–67] or multiple instances of mono-ubiquitination [64, 68]. In addition, a recent study indicated that a deubiqutinase plays a key role in regulating gap junction turnover [67] and non-directed screens for ubiquitinated proteins have identified Cx43 [69]. However, we are not aware of reports where inhibition of the proteasome or other factors potentially involved in Cx43 degradation show significant accumulation of Cx43 at higher molecular sizes consistent with addition of ubiquitin when compared to total Cx43 levels with an antibody to Cx43, so it seems that the level of any Cx43 ubiquitination even in the presence of inhibitors is very low. One study that tried to estimate the level of ubiquitinated Cx43 found that it was less than 1% [70] and in another, the putative ubiquitinated species appeared to be even lower [64, 68]. Furthermore, conversion of all 27 of the lysines in Cx43 to arginines did not eliminate the increase in Cx43 in gap junctions in response to proteasomal inhibitors [71] nor did it affect Cx43 turnover due to ERAD [34]. One of the main issues with these inhibitors is that their effects can impact many different pathways. In fact, an indirect role for proteasomal inhibition on Cx43 has been shown where blocking the proteasomal degradation of activated Akt allows it to phosphorylate Cx43 on S373 and eliminates Cx43:ZO-1 interactions thus, resulting in larger junctions [60]. Thus, it seems that direct ubiquitination of Cx43 does not play a very significant role in proteasomal degradation of Cx43, though there may be specific conditions (e.g., autophagy) and cell types where it is important.
2.5. Regulation of Cx43 and gap junction turnover by Cx43 phosphorylation
A variety of stimuli including epidermal growth factor (EGF), TPA (12-O-Tetradecanoylphorbol acetate), src activation, wounding and extracellular ATP [57–62] cause spatiotemporal changes in Cx43 phosphorylation and loss of gap junctions. Many of these treatments result in Cx43 phosphorylation on S368 via PKC and on S255, S279, S282 via MAPK (see Table 1). Phosphorylation at these residues has been shown to affect gap junction channel gating properties and/or is associated with decreased gap junction assembly and increased gap junction turnover. Furthermore, TPA prevents assembly of new gap junctions and reduced the half-life of Cx43 [17]. Transformation of cells by v-Src has also been shown to downregulate gap junctional communication coincident with an increase in tyrosine phosphorylation on Cx43 [16, 72]. Using LA25 cells that express temperature-sensitive v-Src showed that Y247, Y265, S255, S262, S279/282 and S368 are all phosphorylated in response to v-Src activity indicating co-activation of MAPK and PKC [73]. Interestingly, immunofluorescence studies indicated that phosphorylated Y247 appeared to be preferentially present in “larger” gap junction plaques [74]. This distinct pY247 staining could potentially "mark" a portion of the gap junction for internalization, possibly through stimulating interaction with components of the endocytic system.
Recently, we found that if cells are treated with TPA, one also sees a rapid increase in phosphorylation at S373 via Akt [60]. This was somewhat surprising as we also found that phosphorylation at S373 dramatically increased apparent gap junction size and gap junctional communication in a similar manner to proteasomal inhibitor treatment [71]. Results from Gourdie and colleagues have shown that ZO-1 interaction with the C-terminal region of Cx43 near S373 causes a reduction in gap junction size and conversely, elimination of the ZO-1 interaction leads to larger gap junctions [75, 76]. Consistent with this, we found that Akt phosphorylation of Cx43 could regulate ZO-1 interaction, providing a mechanistic explanation for changes in gap junction size during proteasomal inhibition. By mutating Akt phosphorylation sites we found that cells expressing wild type Cx43, S365/369/373A or S373D mutants showed intermediate, extensive and no co-immunoprecipitation of ZO-1 and intermediate, very limited and large gap junctions, respectively. Also extensive Cx43-ZO-1 co-localization was lost in cells expressing the S373D mutant [60].
Thus, at least 4 kinases potentially play a role in the regulation of gap junction turnover – Akt [60], PKC [17, 77, 78], MAPK [79] and Src [73]. Treatment of cells with reagents, such as TPA or EGF, provide a model system to examine spatiotemporal changes in Cx43 phosphorylation during this “activated” or “acute” turnover process. Figure 4 shows that in TPA treated cells, Cx43 phosphorylation at the Akt site is maximal at 5 minutes, shown both by immunoblot, and immunofluorescence, where colocalization of pS373 (red) and total Cx43 (green) antibodies appear as yellow. Phosphorylation at S279/282 follows, peaking at 15–30 min after treatment (Fig. 4). Phosphorylation at S368 (Fig. 4) and Y247 (not shown) show a more gradual and steady increase, perhaps due to effects on newly synthesized Cx43 or protein en route to the plasma membrane.
Fig. 4.
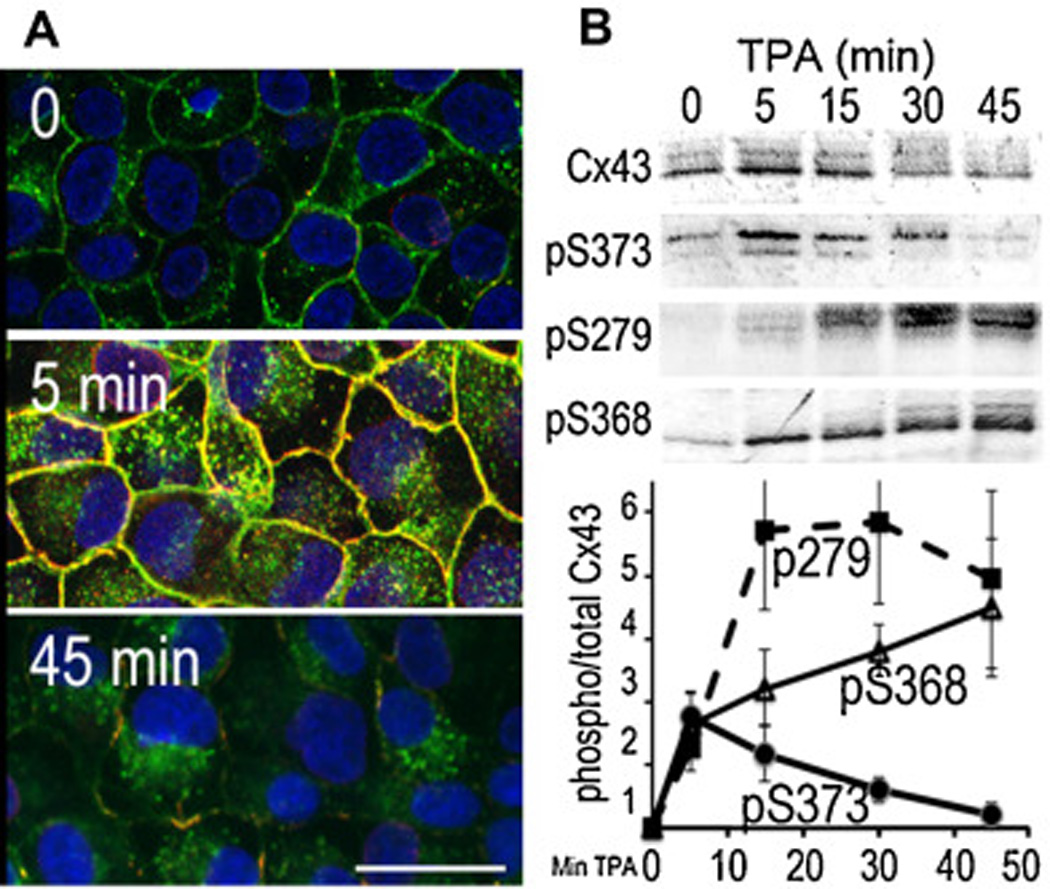
(A) Immunofluorescence staining with antibodies to total Cx43 (green) and phosphoS373 Cx43 (red) 0, 5 and 45 min after 50nM TPA treatment. Nuclei were labeled with DAPI (blue). Bar=25µm. Note the very rapid accumulation of Cx43 into large gap junctions upon Akt activation and phosphorylation at S373. (B) Immunoblot analysis of total Cx43, phosphoS373 (pS373), phosphoS279/282 (pS279) and phospho368 (pS368) levels at 0, 5, 15, 30 and 45 min after TPA treatment
Thus, Cx43 is sequentially phosphorylated by Akt, MAPK, Src and PKC in response to growth factors, wounding and other stimuli which induce acute gap junction turnover. In Fig. 5, we present a model for gap junction turnover that incorporates these phosphorylation events. It seems paradoxical that an initiating signal for gap junction turnover is an Akt-mediated transient increase in gap junction size. However, formation of larger gap junctions could facilitate rapid clearance of Cx43 from the plasma membrane in 2 ways: 1) depletion of the incoming pool of Cx43 by rapid incorporation into a gap junction, and/or 2) to reduce the energetics of annular junction formation/internalization by requiring less membrane curvature during internalization. Once Cx43 is concentrated into the gap junction, the channels are closed via MAPK and PKC phosphorylation (Fig. 5). In keratinocytes, Cx43 is subsequently phosphorylated by Src. We hypothesize that Src phosphorylation of Cx43 at Y247 initiates recruitment of the gap junction internalization machinery (Fig. 5). Inhibition of Src activity via the src kinase inhibitor PP2 blocks growth factor-induced gap junction turnover [80, 81]. Glycyrrhetinic acid-related gap junctional communication inhibitors remodel gap junctions into a looser packing arrangement [82] in a process that involves Src binding [83] and leads to disruption of Cx43-ZO-1 interaction [80]. Src can directly interact with ZO-1 and compete for binding to the C-terminal region of Cx43 [80, 84, 85]. Clearly, Src plays a role in gap junction turnover, but it is not yet clear whether Src phosphorylation of Cx43 plays a direct role. Src phosphorylation of the NMDA receptor, GluN3A [86] has been shown to trigger its endocytosis, so one possibility is that Src similarly stimulates Cx43 internalization. Src phosphorylation of Cx43 may, in fact, direct the endocytic route of internalization through annular gap junction formation (mode 1, Fig. 3) or by “unzippering” gap junctions via loss of extracellular interactions followed by endocytosis and degradation of each connexon within the same cell where it was synthesized (mode 2, Fig. 3). The formation of double membrane endocytic vesicles (i.e., annular junctions) appears to be fairly specific to gap junctions though there are a few reports of “transendocytosis” occurring in dendritic cells [87] and in response to receptor ligand complex formation during neural (Eph:Ephrin [88]) and Drosophila development (Notch:Delta [89], Hedgehog:Patched [90] and Boss:Sevenless [91]).
Fig. 5.
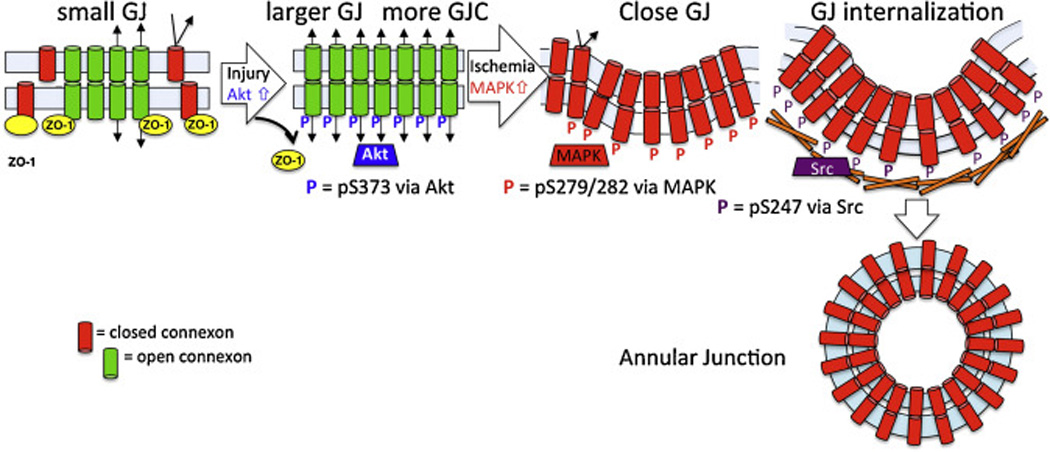
Model of the steps in gap junction turnover regulated by Cx43 phosphorylation. Our model predicts that phosphoS373 (via Akt, shown in blue) first promotes Cx43 accumulation into larger GJs with increased gap junctional communication. Larger junctions could promote annular junction formation through decreased membrane and energy restraints compared to that needed for smaller vesicles. Next, phosphoS279/282 (via MAPK, shown in red) closes the gap junction and then phosphoY247 (via Src, shown in purple) initiates recruitment of the internalization machinery – a timeline consistent with the observed kinetics of phosphorylation at these sites in response to EGF, TPA or wounding.
3. Regulation of wound repair by Cx43 expression and phosphorylation
3.1. Cx43 expression regulates wound repair
Cx43 is abundantly expressed in skin and is known to play a key regulatory role during different stages of the repair process [92–95] via its expression and phosphorylation status changes [78, 96, 97]. Proliferation continually occurs in the basal layer of the epidermis to replace dead keratinocytes and is upregulated dramatically during wounding to provide a source of cells for wound repair. Wounding of the epidermis activates changes in gap junctional communication that synchronize keratinocyte migration across the wound bed [92, 93, 95]. Both rodent [92] and human skin [93] show decreased connexin expression at the edge of a wound within a day and a return to homeostatic levels upon wound closure [92, 98]; see Fig. 6. Many (up to 9) connexins can be detected in epidermis, but Cx43 is the predominant one in vivo and in cultures of human keratinocytes [99]. Cx43 regulation may play the primary role during early stages of wound healing as modulation of Cx43 expression directly affects wound repair [78, 92, 100–106]. Specifically, immediately after wounding there is a requirement for gap junctional communication to initiate efficient migration [78] but a reduction in intercellular communication between cells at the leading edge of the wound rapidly follows. Several results implicate Cx43 as a key regulator of repair. Diabetic mice that display high levels of Cx43 expression [106] or mice with Cx43 overexpression [107] have delayed wound closure. Conversely, mice with reduced epidermal Cx43 can show more rapid healing [104], and Cx43 antisense application accelerated keratinocyte migration and wound repair resulting in less scarring [105].
Fig. 6.
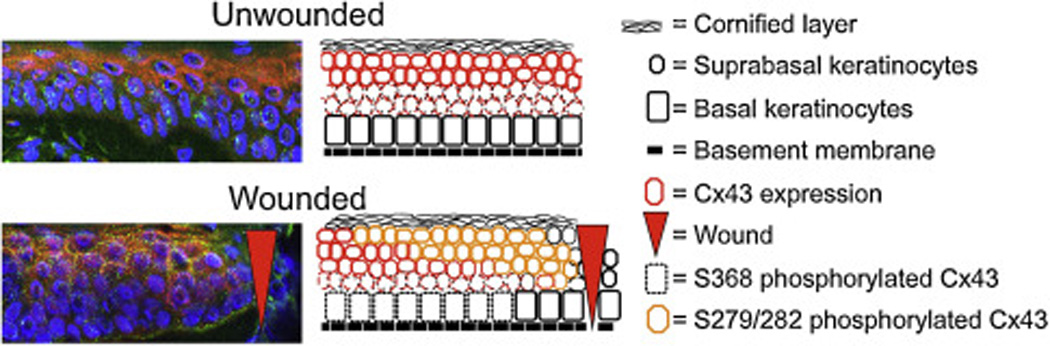
Immunofluorescence of human skin stained with antibodies to total Cx43 (green) and phospho279/282 Cx43 (red). Nuclei were labeled with DAPI (blue). Note the high level of phospho279/282 in suprabasal cells adjacent to the wound edge (noted by red arrowhead). An accompanying cartoon illustrates the changes in phospho279/282 and total Cx43 in basal and suprabasal keratinocytes.
3.2. A model to explain gap junctional upregulation and turnover in response to injury
In response to wounding, we find that Cx43 is sequentially phosphorylated at specific sites by (a) Akt at 5–30 min [60, 71], (b) PKC [78] and MAPK [79] at 15–60 min (Fig. 6), and (c) Src at 30 min–24h. These events are coincident with changes in gap junction localization and function including a transient increase in gap junction size and gap junctional communication followed by gap junction internalization. We hypothesize that this sequence of events represents a wound induced spatiotemporally-regulated “kinase program” that provides a mechanism to promote increased gap junctional communication and signals for keratinocyte activation prior to rapid gap junction internalization. As described above for EGF and TPA and in Fig. 5, the first step is characterized by the increase in gap junction size and gap junctional communication observed early upon activation of Akt [60]. We suggest that this provides the robust gap junctional communication that seems to initiate the changes necessary for cells to adopt a more migratory or proliferative phenotype. Figuring out the role of this first step is potentially important since results with reagents (i.e., potential drugs) that affect Cx43 expression appear to have disparate effects. For example, treatment of wounds with Cx43 antisense results in less inflammation and faster wound healing [108]. However, treatment of diabetic wounds with ACT1 peptide, which mimics the C-terminus (residues 374–382) resulting in increased gap junction size and communication, speeds healing [109]. Part of the issue may be related to timing, as the ACT1 peptide is short-lived and may facilitate early signaling events that promote healing while allowing normal gap junction closure via MAPK and src. Connexin downregulation appears to be important after this early phase so reagents that reduce Cx43 might be helpful at later stages. Another explanation might be related to the target – i.e., whether the reagent is affecting Cx43 connexons (hemichannels) or gap junctions. In either case, it is clear that early events after wounding are dramatically affected by Cx43.
There is strong evidence that a reduction in Cx43 expression and gap junctional communication can be beneficial to wound healing. This evidence includes the observation of what happens to Cx43 during a healthy wound response [93, 94, 101] and how that process is disrupted by Cx43 overexpression or sped by Cx43 downregulation [78, 101, 105–107, 110]. The benefit from reduced Cx43 might be related to the promotion of proliferation and migration or a reduction in inflammatory response. The activation of MAPK and Cx43 phosphorylation at S279 and S282 observed at 15–30 min post wounding would lead to gap junction channel closure [111]. Activation of PKC leads to inhibition of new gap junction assembly [17]. Activation of Src and phosphorylation of Cx43 on Y247 from 30 min to several hours could lead to internalization of gap junctions from the plasma membrane (Fig. 5) [73]. This sequence of Cx43 phosphorylation events during wounding is consistent with what we observe during growth factor treatment. Thus, consistent with the model we propose in Fig. 5, we hypothesize that wound induced gap junction disassembly is driven by a kinase program that regulates the accumulation of Cx43 into gap junctions in preparation for disassembly and “marking” of specific plaque domains for internalization via Cx43 phosphorylation at specific residues by at least 3 kinases. Our model predicts pS373 first promotes Cx43 accumulation into larger gap junctions with increased gap junctional communication. This step also effectively “clears” the plasma membrane of connexons that may exhibit hemichannel activity. Larger junctions could promote annular junction formation through decreased membrane and energy restraints compared to that needed for smaller vesicles. Next, pS279/282 rapidly closes the gap junction and then pY247 initiates accumulation of the internalization machinery – a timeline consistent with the observed kinetics of phosphorylation at these sites during wounding and in response to EGF or TPA, as shown in the previous sections (Fig. 5).
4. Summary and Perspective
Gap junction biology affects several fundamental cell processes including wound repair. This review mainly discussed the short terms effects of Cx43 phosphorylation on the wound healing process but Cx43 is dynamically regulated for at least 72 h after wounding [78, 92, 93, 101] so those effects warrant further investigation. For example, wound-dependent phosphorylation at the PKC site S368 at 24 hours creates specialized communication boundaries within the basal cells of the epidermis [78]. In addition our discussion has only considered a few Cx43 interacting proteins, but CASK and CADM1 directly interact with hypo-phosphorylated Cx43 one hour post-wounding in human keratinocytes to apparently regulate activation and migration [96]. So at this point, we are left with a number of questions related to the role of Cx43 in wound healing: i) What is the role of the increased gap junctional communication early after wounding?; ii) How long is gap junctional communication necessary after the initial wounding event to maximize wound healing?; iii) Can we effectively target specific cells such as migratory or proliferative cells with connexin reagents?; iv) Can we target specific subcellular pools of Cx43 to induce the desired results?
Obviously, a more complete understanding of Cx43 biology in the epidermis would allow for more targeted and rational drug design to facilitate wound repair. Some of our understanding of the regulation of Cx43 assembly and disassembly has been affected by the use of broad specificity reagents that might be affecting the expression of many different Cx43 interacting and regulating proteins. Through the use of more targeted reagents such as phosphospecific antibodies, mutant versions of the protein and targeted knockdown of the connexin interacting protein, the field is starting to, at least, identify the important steps and players if not to fully grasp their implications. Furthermore, drugs based on that knowledge could target Cx43, the kinase important at a particular step, important interacting proteins or other key actors. Given the extensive pharmaceutical development of kinase inhibitors, we believe it will be important to test whether kinase or gap junction activators/inhibitors could be topically applied in a manner dictated by the wound status (i.e., fresh, ulcerated, diabetic, etc.) to yield better healing and reduce the need for amputations. For example, we envision speeding up the wound healing process via the use of different “band aids” embedded with distinct gap junction or kinase inhibitors/activators. One would utilize a specific bandage on a fresh wound (i.e., applied within 0–30 min of wounding and kept on for 4 hours) and then change to a second type of bandage that might be applied 4–24 hours post wounding and a third that would be good until healing is complete and might be specifically designed to reduce scarring. In this way, development of a clear spatiotemporal map of Cx43 phosphorylation and activation of its cognate kinases during pathogenic processes could yield real clinical value.
Supplementary Material
Acknowledgments
Research performed in the Lampe lab was supported by a grant from the National Institutes of Health (GM55632).
Abbreviations
- Da
daltons
- cAMP
cyclic adenosine monophosphate
- cGMP
cyclic guanosine monophosphate
- Cx43
Connexin 43
- SDS
sodium dodecyl sulfate
- PAGE
polyacrylamide gel electrophoresis
- EGF
epidermal growth factor
- TPA
12-O-Tetradecanoylphorbol acetate
- ERAD
Endoplasmic Reticulum Associated Degradation
- PKA
cAMP-dependent protein kinase
- PKC
protein kinase C
- CK1
casein kinase 1
- MAPK
mitogen-activated protein kinase
- ZO-1
zonula occludens-1
- GFP
green fluorescent protein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bruzzone R, White TW, Paul DL. Connections with connexins: The molecular basis of direct intercellular signaling. Eur J Biochem. 1996;238:1–27. doi: 10.1111/j.1432-1033.1996.0001q.x. [DOI] [PubMed] [Google Scholar]
- 2.Willecke K, Eiberger J, Degen J, Eckardt D, Romualdi A, Guldenagel M, et al. Structural and functional diversity of connexin genes in the mouse and human genome. Biol Chem. 2002;383:725–737. doi: 10.1515/BC.2002.076. [DOI] [PubMed] [Google Scholar]
- 3.Saez JC, Berthoud VM, Branes MC, Martinez AD, Beyer EC. Plasma membrane channels formed by connexins: their regulation and functions. Physiol Rev. 2003;83:1359–1400. doi: 10.1152/physrev.00007.2003. [DOI] [PubMed] [Google Scholar]
- 4.Loewenstein WR. Junctional intercellular communication: The cell-to-cell membrane channel. Physiol Rev. 1981;61:829–913. doi: 10.1152/physrev.1981.61.4.829. [DOI] [PubMed] [Google Scholar]
- 5.Laird DW. Life cycle of connexins in health and disease. Biochem J. 2006;394:527–543. doi: 10.1042/BJ20051922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goodenough DA, Paul DL. Gap junctions. Cold Spring Harb Perspect Biol. 2009;1:a002576. doi: 10.1101/cshperspect.a002576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kelsey L, Katoch P, Johnson KE, Batra SK, Mehta PP. Retinoids regulate the formation and degradation of gap junctions in androgen-responsive human prostate cancer cells. PLOS One. 2012;7:e32846. doi: 10.1371/journal.pone.0032846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kizana E, Cingolani E, Marban E. Non-cell-autonomous effects of vector-expressed regulatory RNAs in mammalian heart cells. Gene therapy. 2009;16:1163–1168. doi: 10.1038/gt.2009.64. [DOI] [PubMed] [Google Scholar]
- 9.Goodenough DA, Goliger JA, Paul DL. Connexins, connexons, and intercellular communication. Ann Rev Biochem. 1996;65:475–502. doi: 10.1146/annurev.bi.65.070196.002355. [DOI] [PubMed] [Google Scholar]
- 10.Kumar NM, Gilula NB. The gap junction communication channel. Cell. 1996;84:381–388. doi: 10.1016/s0092-8674(00)81282-9. [DOI] [PubMed] [Google Scholar]
- 11.White T, Paul D. Genetic diseases and gene knockouts reveal diverse connexin functions. Ann Rev Physiology. 1999;61:283–310. doi: 10.1146/annurev.physiol.61.1.283. [DOI] [PubMed] [Google Scholar]
- 12.Dobrowolski R, Willecke K. Connexin-caused genetic diseases and corresponding mouse models. Antioxid Redox Signal. 2009;11:283–295. doi: 10.1089/ars.2008.2128. [DOI] [PubMed] [Google Scholar]
- 13.Reaume AG, de Sousa PA, Kulkarni S, Langille BL, Zhu D, Davies TC, et al. Cardiac malformation in neonatal mice lacking connexin43. Science. 1995;267:1831–1834. doi: 10.1126/science.7892609. [DOI] [PubMed] [Google Scholar]
- 14.Vreeburg M, de Zwart-Storm EA, Schouten MI, Nellen RG, Marcus-Soekarman D, Devies M, et al. Skin changes in oculo-dento-digital dysplasia are correlated with C-terminal truncations of connexin 43. American journal of medical genetics Part A. 2007;143:360–363. doi: 10.1002/ajmg.a.31558. [DOI] [PubMed] [Google Scholar]
- 15.Laird DW, Puranam KL, Revel JP. Turnover and phosphorylation dynamics of connexin43 gap junction protein in cultured cardiac myocytes. Biochem J. 1991;273:67–72. doi: 10.1042/bj2730067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crow DS, Beyer EC, Paul DL, Kobe SS, Lau AF. Phosphorylation of connexin43 gap junction protein in uninfected and Rous sarcoma virus-transformed mammalian fibroblasts. Mol Cell Biol. 1990;10:1754–1763. doi: 10.1128/mcb.10.4.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lampe PD. Analyzing phorbol ester effects on gap junction communication: A dramatic inhibition of assembly. J Cell Biol. 1994;127:1895–1905. doi: 10.1083/jcb.127.6.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Musil LS, Beyer EC, Goodenough DA. Expression of the gap junction protein connexin43 in embryonic chick lens: Molecular cloning, ultrastructural localization, and post-translational phosphorylation. J Membr Biol. 1990;116:163–175. doi: 10.1007/BF01868674. [DOI] [PubMed] [Google Scholar]
- 19.Beardslee M, Laing J, Beyer E, Saffitz J. Rapid turnover of connexin43 in the adult rat heart. Circ Res. 1998;83:629–635. doi: 10.1161/01.res.83.6.629. [DOI] [PubMed] [Google Scholar]
- 20.Hertlein B, Butterweck A, Haubrich S, Willecke K, Traub O. Phosphorylated carboxy terminal serine residues stabilize the mouse gap junction protein connexin45 against degradation. J Membrane Biol. 1998;162:247–257. doi: 10.1007/s002329900362. [DOI] [PubMed] [Google Scholar]
- 21.Darrow BJ, Laing JG, Lampe PD, Saffitz JE, Beyer EC. Expression of multiple connexins in cultured neonatal rat ventricular myocytes. Circ Res. 1995;76:381–387. doi: 10.1161/01.res.76.3.381. [DOI] [PubMed] [Google Scholar]
- 22.Laird DW. The gap junction proteome and its relationship to disease. Trends Cell Biol. 2010;20:92–101. doi: 10.1016/j.tcb.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 23.Solan JL, Lampe PD. Biochemistry of Connexins. In: Harris A, Locke D, editors. Connexins: A Guide. New York: Humana Press; 2009. pp. 263–286. [Google Scholar]
- 24.Jordan K, Solan JL, Dominguez M, Sia M, Hand A, Lampe PD, et al. Trafficking, assembly and function of a connexin43-green fluorescent protein chimera in live mammalian cells. Mol Biol Cell. 1999;10:2033–2050. doi: 10.1091/mbc.10.6.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lauf U, Giepmans BN, Lopez P, Braconnot S, Chen SC, Falk MM. Dynamic trafficking and delivery of connexons to the plasma membrane and accretion to gap junctions in living cells. Proc Natl Acad Sci U S A. 2002;99:10446–10451. doi: 10.1073/pnas.162055899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chu F-F, Doyle D. Turnover of plasma membrane proteins in rat hepatoma cells and primary cultures of rat hepatocytes. J Biol Chem. 1985;260:3097–3107. [PubMed] [Google Scholar]
- 27.Hare JF, Taylor K. Mechanisms of plasma membrane protein degradation: Recycling proteins are degraded more rapidly than those confined to the cell surface. Proc Nat Acad Sci USA. 1991;88:5902–5906. doi: 10.1073/pnas.88.13.5902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fishman GI, Moreno AP, Spray DC, Leinwand LA. Functional analysis of human cardiac gap junction channel mutants. Proc Natl Acad Sci. 1991;88:3525–3529. doi: 10.1073/pnas.88.9.3525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dunham B, Liu S, Taffet S, Trabka-Janik E, Delmar M, Petryshyn R, et al. Immunolocalization and expression of functional and nonfunctional cell-to-cell channels from wild-type and mutant rat heart connexin43 cDNA. Circ Res. 1992;70:1233–1243. doi: 10.1161/01.res.70.6.1233. [DOI] [PubMed] [Google Scholar]
- 30.Moreno AP, Chanson M, Elenes S, Anumonwo J, Scerri I, Gu H, et al. Role of the carboxyl terminal of connexin43 in transjunctional fast voltage gating. Circ Res. 2002;90:450–457. doi: 10.1161/hh0402.105667. [DOI] [PubMed] [Google Scholar]
- 31.Maass K, Ghanem A, Kim JS, Saathoff M, Urschel S, Kirfel G, et al. Defective epidermal barrier in neonatal mice lacking the C-terminal region of connexin43. Mol Biol Cell. 2004;15:4597–4608. doi: 10.1091/mbc.E04-04-0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Duffy HS, Delmar M, Spray DC. Formation of the gap junction nexus: binding partners for connexins. Journal of physiology, Paris. 2002;96:243–249. doi: 10.1016/s0928-4257(02)00012-8. [DOI] [PubMed] [Google Scholar]
- 33.Musil LS, Goodenough DA. Multisubunit assembly of an integral plasma membrane channel protein, gap junction connexin43, occurs after exit from the ER. Cell. 1993;74:1065–1077. doi: 10.1016/0092-8674(93)90728-9. [DOI] [PubMed] [Google Scholar]
- 34.Su V, Nakagawa R, Koval M, Lau AF. Ubiquitin-independent Proteasomal Degradation of Endoplasmic Reticulum-localized Connexin43 Mediated by CIP75. J Biol Chem. 2010;285:40979–40990. doi: 10.1074/jbc.M110.170753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.VanSlyke JK, Musil LS. Dislocation and degradation from the ER are regulated by cytosolic stress. J Cell Biol. 2002;157:381–394. doi: 10.1083/jcb.200111045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johnson RG, Meyer RA, Li XR, Preus DM, Tan L, Grunenwald H, et al. Gap junctions assemble in the presence of cytoskeletal inhibitors, but enhanced assembly requires microtubules. Exp Cell Res. 2002;275:67–80. doi: 10.1006/excr.2002.5480. [DOI] [PubMed] [Google Scholar]
- 37.Shaw RM, Fay AJ, Puthenveedu MA, von Zastrow M, Jan YN, Jan LY. Microtubule plus-end-tracking proteins target gap junctions directly from the cell interior to adherens junctions. Cell. 2007;128:547–560. doi: 10.1016/j.cell.2006.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gaietta G, Deerinck TJ, Adams SR, Bouwer J, Tour O, Laird DW, et al. Multicolor and electron microscopic imaging of connexin trafficking. Science. 2002;296:503–507. doi: 10.1126/science.1068793. [DOI] [PubMed] [Google Scholar]
- 39.Solan JL, Marquez-Rosado L, Sorgen PL, Thornton PJ, Gafken PR, Lampe PD. Phosphorylation of Cx43 at S365 is a gatekeeper event that changes the structure of Cx43 and prevents downregulation by PKC. J Cell Biol. 2007;179:1301–1309. doi: 10.1083/jcb.200707060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sosinsky GE, Solan JL, Gaietta GM, Ngan L, Lee GJ, Mackey MR, et al. The C-terminus of Connexin43 adopts different conformations in the golgi and gap junction as detected with structure specific antibodies. Biochem J. 2007;408:375–385. doi: 10.1042/BJ20070550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cooper CD, Lampe PD. Casein kinase 1 regulates connexin43 gap junction assembly. J Biol Chem. 2002;277:44962–44968. doi: 10.1074/jbc.M209427200. [DOI] [PubMed] [Google Scholar]
- 42.Falk MM. Connexin-specific distribution within gap junctions revealed in living cells. J Cell Sci. 2000;113(Pt 22):4109–4120. doi: 10.1242/jcs.113.22.4109. [DOI] [PubMed] [Google Scholar]
- 43.Archard HO, Denys FR. Development of annular gap junctions in guinea pig epithelia. J Oral Pathol. 1979;8:187–197. doi: 10.1111/j.1600-0714.1979.tb01885.x. [DOI] [PubMed] [Google Scholar]
- 44.Fong JT, Kells RM, Gumpert AM, Marzillier JY, Davidson MW, Falk MM. Internalized gap junctions are degraded by autophagy. Autophagy. 2012;8:794–811. doi: 10.4161/auto.19390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Johnson KE, Mitra S, Katoch P, Kelsey LS, Johnson KR, Mehta PP. Phosphorylation on Ser-279 and Ser-282 of connexin43 regulates endocytosis and gap junction assembly in pancreatic cancer cells. Mol Biol Cell. 2013;24:715–733. doi: 10.1091/mbc.E12-07-0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jordan K, Chodock R, Hand AR, Laird DW. The origin of annular junctions: a mechanism of gap junction internalization. J Cell Sci. 2001;114:763–773. doi: 10.1242/jcs.114.4.763. [DOI] [PubMed] [Google Scholar]
- 47.Leithe E, Brech A, Rivedal E. Endocytic processing of connexin43 gap junctions: a morphological study. Biochem J. 2006;393:59–67. doi: 10.1042/BJ20050674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nickel B, Boller M, Schneider K, Shakespeare T, Gay V, Murray SA. Visualizing the effect of dynamin inhibition on annular gap vesicle formation and fission. J Cell Sci. 2013;126:2607–2616. doi: 10.1242/jcs.116269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Piehl M, Lehmann C, Gumpert A, Denizot JP, Segretain D, Falk MM. Internalization of large double-membrane intercellular vesicles by a clathrin-dependent endocytic process. Mol Biol Cell. 2007;18:337–347. doi: 10.1091/mbc.E06-06-0487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Severs NJ, Shovel KS, Slade AM, Powell T, Twist VW, Green CR. Fate of gap junctions in isolated adult mammalian cardiomyocytes. Circ Res. 1989;65:22–42. doi: 10.1161/01.res.65.1.22. [DOI] [PubMed] [Google Scholar]
- 51.Bejarano E, Yuste A, Patel B, Stout RF, Jr, Spray DC, Cuervo AM. Connexins modulate autophagosome biogenesis. Nat Cell Biol. 2014;16:401–414. doi: 10.1038/ncb2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lichtenstein A, Minogue PJ, Beyer EC, Berthoud VM. Autophagy: a pathway that contributes to connexin degradation. J Cell Sci. 2011;124:910–920. doi: 10.1242/jcs.073072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hesketh GG, Shah MH, Halperin VL, Cooke CA, Akar FG, Yen TE, et al. Ultrastructure and regulation of lateralized connexin43 in the failing heart. Circ Res. 2010;106:1153–1163. doi: 10.1161/CIRCRESAHA.108.182147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fong JT, Kells RM, Falk MM. Two tyrosine-based sorting signals in the Cx43 C-terminus cooperate to mediate gap junction endocytosis. Mol Biol Cell. 2013;24:2834–2848. doi: 10.1091/mbc.E13-02-0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thomas MA, Zosso N, Scerri I, Demaurex N, Chanson M, Staub O. A tyrosine-based sorting signal is involved in connexin43 stability and gap junction turnover. J Cell Sci. 2003;116:2213–2222. doi: 10.1242/jcs.00440. [DOI] [PubMed] [Google Scholar]
- 56.Catarino S, Ramalho JS, Marques C, Pereira P, Girao H. Ubiquitin-mediated internalization of connexin43 is independent of the canonical endocytic tyrosine-sorting signal. Biochem J. 2011;437:255–267. doi: 10.1042/BJ20102059. [DOI] [PubMed] [Google Scholar]
- 57.Kanemitsu MY, Lau AF. Epidermal growth factor stimulates the disruption of gap junctional communication and connexin43 phosphorylation independent of 12-O-tetradecanoyl 13-acetate-sensitive protein kinase C: The possible involvement of mitogen-activated protein kinase. Mol Biol Cell. 1993;4:837–848. doi: 10.1091/mbc.4.8.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ruch RJ, Trosko JE, Madhukar BV. Inhibition of connexin43 gap junctional intercellular communication by TPA requires ERK activation. J Cell Biochem. 2001;83:163–169. doi: 10.1002/jcb.1227. [DOI] [PubMed] [Google Scholar]
- 59.Rivedal E, Leithe E. Connexin43 synthesis, phosphorylation, and degradation in regulation of transient inhibition of gap junction intercellular communication by the phorbol ester TPA in rat liver epithelial cells. Exp Cell Res. 2005;302:143–152. doi: 10.1016/j.yexcr.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 60.Dunn CA, Lampe PD. Injury-triggered Akt phosphorylation of Cx43: a ZO-1-driven molecular switch that regulates gap junction size. J Cell Sci. 2014;127:455–464. doi: 10.1242/jcs.142497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schwiebert EM, Zsembery A. Extracellular ATP as a signaling molecule for epithelial cells. Biochim Biophys Acta. 2003;1615:7–32. doi: 10.1016/s0005-2736(03)00210-4. [DOI] [PubMed] [Google Scholar]
- 62.Chang SJ, Tzeng CR, Lee YH, Tai CJ. Extracellular ATP activates the PLC/PKC/ERK signaling pathway through the P2Y2 purinergic receptor leading to the induction of early growth response 1 expression and the inhibition of viability in human endometrial stromal cells. Cell Signal. 2008;20:1248–1255. doi: 10.1016/j.cellsig.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 63.Leithe E, Rivedal E. Epidermal growth factor regulates ubiquitination, internalization and proteasome-dependent degradation of connexin43. J Cell Sci. 2004;117:1211–1220. doi: 10.1242/jcs.00951. [DOI] [PubMed] [Google Scholar]
- 64.Leithe E, Rivedal E. Ubiquitination and down-regulation of gap junction protein connexin-43 in response to 12-O-tetradecanoylphorbol 13-acetate treatment. J Biol Chem. 2004;279:50089–50096. doi: 10.1074/jbc.M402006200. [DOI] [PubMed] [Google Scholar]
- 65.Laing JG, Beyer EC. The gap junction protein connexin43 is degraded via the ubiquitin proteasome pathway. J Biol Chem. 1995;270:26399–26403. doi: 10.1074/jbc.270.44.26399. [DOI] [PubMed] [Google Scholar]
- 66.Laing JG, Tadros PN, Westphale EM, Beyer EC. Degradation of connexin43 gap junctions involves both the proteasome and the lysosome. Exp Cell Res. 1997;236:482–492. doi: 10.1006/excr.1997.3747. [DOI] [PubMed] [Google Scholar]
- 67.Ribeiro-Rodrigues TM, Catarino S, Marques C, Ferreira JV, Martins-Marques T, Pereira P, et al. AMSH-mediated deubiquitination of Cx43 regulates internalization and degradation of gap junctions. FASEB J. 2014;28:4629–4641. doi: 10.1096/fj.13-248963. [DOI] [PubMed] [Google Scholar]
- 68.Girao H, Catarino S, Pereira P. Eps15 interacts with ubiquitinated Cx43 and mediates its internalization. Exp Cell Res. 2009;315:3587–3597. doi: 10.1016/j.yexcr.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 69.Wagner SA, Beli P, Weinert BT, Nielsen ML, Cox J, Mann M, et al. A proteome-wide, quantitative survey of in vivo ubiquitylation sites reveals widespread regulatory roles. Mol Cell Proteomics. 2011;10:M111 013284. doi: 10.1074/mcp.M111.013284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen VC, Kristensen AR, Foster LJ, Naus CC. Association of connexin43 with E3 ubiquitin ligase TRIM21 reveals a mechanism for gap junction phosphodegron control. Journal of proteome research. 2012;11:6134–6146. doi: 10.1021/pr300790h. [DOI] [PubMed] [Google Scholar]
- 71.Dunn CA, Su V, Lau AF, Lampe PD. Activation of Akt, not connexin 43 protein ubiquitination, regulates gap junction stability. J Biol Chem. 2012;287:2600–2607. doi: 10.1074/jbc.M111.276261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Swenson KI, Piwnica-Worms H, McNamee H, Paul DL. Tyrosine phosphorylation of the gap junction protein connexin43 is required for pp60src-induced inhibition of communication. Cell Regul. 1990;1:989–1002. doi: 10.1091/mbc.1.13.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Solan JL, Lampe PD. Connexin 43 in LA-25 cells with active v-src is phosphorylated on Y247, Y265, S262, S279/282, and S368 via multiple signaling pathways. Cell Commun Adhes. 2008;15:75–84. doi: 10.1080/15419060802014016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Solan JL, Lampe PD. Specific Cx43 phosphorylation events regulate gap junction turnover in vivo. FEBS Lett. 2014;588:1423–1429. doi: 10.1016/j.febslet.2014.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hunter AW, Barker RJ, Zhu C, Gourdie RG. Zonula occludens-1 alters connexin43 gap junction size and organization by influencing channel accretion. Mol Biol Cell. 2005;16:5686–5698. doi: 10.1091/mbc.E05-08-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rhett JM, Jourdan J, Gourdie RG. Connexin 43 connexon to gap junction transition is regulated by zonula occludens-1. Mol Biol Cell. 2011;22:1516–1528. doi: 10.1091/mbc.E10-06-0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Solan JL, Fry MD, TenBroek EM, Lampe PD. Connexin43 phosphorylation at S368 is acute during S and G2/M and in response to protein kinase C activation. J Cell Sci. 2003;116:2203–2211. doi: 10.1242/jcs.00428. [DOI] [PubMed] [Google Scholar]
- 78.Richards TS, Dunn CA, Carter WG, Usui ML, Olerud JE, Lampe PD. Protein kinase C spatially and temporally regulates gap junctional communication during human wound repair via phosphorylation of connexin43 on serine368. J Cell Biol. 2004;167:555–562. doi: 10.1083/jcb.200404142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Johnstone SR, Kroncke BM, Straub AC, Best AK, Dunn CA, Mitchell LA, et al. MAPK phosphorylation of connexin 43 promotes binding of cyclin e and smooth muscle cell proliferation. Circ Res. 2012;111:201–211. doi: 10.1161/CIRCRESAHA.112.272302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gilleron J, Fiorini C, Carette D, Avondet C, Falk MM, Segretain D, et al. Molecular reorganization of Cx43, Zo-1 and Src complexes during the endocytosis of gap junction plaques in response to a non-genomic carcinogen. J Cell Sci. 2008;121:4069–4078. doi: 10.1242/jcs.033373. [DOI] [PubMed] [Google Scholar]
- 81.Spinella F, Rosano L, Di Castro V, Nicotra MR, Natali PG, Bagnato A. Endothelin-1 decreases gap junctional intercellular communication by inducing phosphorylation of connexin 43 in human ovarian carcinoma cells. J Biol Chem. 2003;278:41294–41301. doi: 10.1074/jbc.M304785200. [DOI] [PubMed] [Google Scholar]
- 82.Goldberg GS, Moreno AP, Bechberger JF, Hearn SS, Shivers RR, MacPhee DJ, et al. Evidence that disruption of connexon particle arrangements in gap junction plaques is associated with inhibition of gap junctional communication by a glycyrrhetinic acid derivative. Exp Cell Res. 1996;222:48–53. doi: 10.1006/excr.1996.0006. [DOI] [PubMed] [Google Scholar]
- 83.Chung TH, Wang SM, Chang YC, Chen YL, Wu JC. 18beta-glycyrrhetinic acid promotes src interaction with connexin43 in rat cardiomyocytes. J Cell Biochem. 2007;100:653–664. doi: 10.1002/jcb.21018. [DOI] [PubMed] [Google Scholar]
- 84.Kieken F, Mutsaers N, Dolmatova E, Virgil K, Wit AL, Kellezi A, et al. Structural and molecular mechanisms of gap junction remodeling in epicardial border zone myocytes following myocardial infarction. Circ Res. 2009;104:1103–1112. doi: 10.1161/CIRCRESAHA.108.190454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sorgen PL, Duffy HS, Sahoo P, Coombs W, Delmar M, Spray DC. Structural changes in the carboxyl terminus of the gap junction protein connexin43 indicates signaling between binding domains for c-Src and zonula occludens-1. J Biol Chem. 2004;279:54695–54701. doi: 10.1074/jbc.M409552200. [DOI] [PubMed] [Google Scholar]
- 86.Chowdhury D, Marco S, Brooks IM, Zandueta A, Rao Y, Haucke V, et al. Tyrosine phosphorylation regulates the endocytosis and surface expression of GluN3A-containing NMDA receptors. J Neurosci. 2013;33:4151–4164. doi: 10.1523/JNEUROSCI.2721-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Spacek J, Harris KM. Trans-endocytosis via spinules in adult rat hippocampus. J Neurosci. 2004;24:4233–4241. doi: 10.1523/JNEUROSCI.0287-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Marston DJ, Dickinson S, Nobes CD. Rac-dependent trans-endocytosis of ephrinBs regulates Eph-ephrin contact repulsion. Nat Cell Biol. 2003;5:879–888. doi: 10.1038/ncb1044. [DOI] [PubMed] [Google Scholar]
- 89.Klueg KM, Parody TR, Muskavitch MA. Complex proteolytic processing acts on Delta, a transmembrane ligand for Notch, during Drosophila development. Mol Biol Cell. 1998;9:1709–1723. doi: 10.1091/mbc.9.7.1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Incardona JP, Lee JH, Robertson CP, Enga K, Kapur RP, Roelink H. Receptor-mediated endocytosis of soluble and membrane-tethered Sonic hedgehog by Patched-1. Proc Natl Acad Sci U S A. 2000;97:12044–12049. doi: 10.1073/pnas.220251997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cagan RL, Kramer H, Hart AC, Zipursky SL. The bride of sevenless and sevenless interaction: internalization of a transmembrane ligand. Cell. 1992;69:393–399. doi: 10.1016/0092-8674(92)90442-f. [DOI] [PubMed] [Google Scholar]
- 92.Goliger JA, Paul DL. Wounding alters epidermal connexin expression and gap junctionmediated intercellular communication. Mol Biol Cell. 1995;6:1491–1501. doi: 10.1091/mbc.6.11.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lampe PD, Nguyen BP, Gil S, Usui M, Olerud J, Takada Y, et al. Cellular interaction of integrin a3b1 with laminin 5 promotes gap junctional communication. J Cell Biol. 1998;143:1735–1747. doi: 10.1083/jcb.143.6.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Clark RAF. Cutaneous tissue repair: basic biological considerations. J Am Acad Derm. 1985;13:701–725. doi: 10.1016/s0190-9622(85)70213-7. [DOI] [PubMed] [Google Scholar]
- 95.Grinnell F. Wound repair, keratinocyte activation and integrin modulation. J Cell Sci. 1992;101:1–5. doi: 10.1242/jcs.101.1.1. [DOI] [PubMed] [Google Scholar]
- 96.Marquez-Rosado L, Singh D, Rincon-Arano H, Solan JL, Lampe PD. CASK (LIN2) interacts with Cx43 in wounded skin and their coexpression affects cell migration. J Cell Sci. 2012;125:695–702. doi: 10.1242/jcs.084400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Marquez-Rosado L, Solan JL, Dunn CA, Norris RP, Lampe PD. Connexin43 phosphorylation in brain, cardiac, endothelial and epithelial tissues. Biochim Biophys Acta. 2012;1818:1985–1992. doi: 10.1016/j.bbamem.2011.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Saitoh M, Oyamada M, Oyamada Y, Kaku T, Mori M. Changes in the expression of gap junction proteins (connexins) in hamster tongue epithelium during would healing and carcinogenesis. Carcinogenesis. 1997;18:1319–1328. doi: 10.1093/carcin/18.7.1319. [DOI] [PubMed] [Google Scholar]
- 99.Fitzgerald DJ, Fusenig NE, Boukamp P, Piccoli C, Mesnil M, Yamasaki H. Expression and function of connexin in normal and transformed human keratinocytes in culture. Carcinogenesis. 1994;15:1859–1865. doi: 10.1093/carcin/15.9.1859. [DOI] [PubMed] [Google Scholar]
- 100.Brandner JM, Houdek P, Husing B, Kaiser C, Moll I. Connexins 26, 30, and 43: differences among spontaneous, chronic, and accelerated human wound healing. J Invest Dermatol. 2004;122:1310–1320. doi: 10.1111/j.0022-202X.2004.22529.x. [DOI] [PubMed] [Google Scholar]
- 101.Coutinho P, Qiu C, Frank S, Tamber K, Becker D. Dynamic changes in connexin expression correlate with key events in the wound healing process. Cell biology international. 2003;27:525–541. doi: 10.1016/s1065-6995(03)00077-5. [DOI] [PubMed] [Google Scholar]
- 102.Ghatnekar GS, O'Quinn MP, Jourdan LJ, Gurjarpadhye AA, Draughn RL, Gourdie RG. Connexin43 carboxyl-terminal peptides reduce scar progenitor and promote regenerative healing following skin wounding. Regenerative medicine. 2009;4:205–223. doi: 10.2217/17460751.4.2.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.King TJ, Lampe PD. Temporal regulation of connexin phosphorylation in embryonic and adult tissues. Biochim Biophys Acta. 2005;1719:24–35. doi: 10.1016/j.bbamem.2005.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kretz M, Euwens C, Hombach S, Eckardt D, Teubner B, Traub O, et al. Altered connexin expression and wound healing in the epidermis of connexin-deficient mice. J Cell Sci. 2003;116:3443–3452. doi: 10.1242/jcs.00638. [DOI] [PubMed] [Google Scholar]
- 105.Qiu C, Coutinho P, Frank S, Franke S, Law LY, Martin P, et al. Targeting connexin43 expression accelerates the rate of wound repair. Curr Biol. 2003;13:1697–1703. doi: 10.1016/j.cub.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 106.Wang CM, Lincoln J, Cook JE, Becker DL. Abnormal connexin expression underlies delayed wound healing in diabetic skin. Diabetes. 2007;56:2809–2817. doi: 10.2337/db07-0613. [DOI] [PubMed] [Google Scholar]
- 107.Nakano Y, Oyamada M, Dai P, Nakagami T, Kinoshita S, Takamatsu T. Connexin43 knockdown accelerates wound healing but inhibits mesenchymal transition after corneal endothelial injury in vivo. Investigative ophthalmology & visual science. 2008;49:93–104. doi: 10.1167/iovs.07-0255. [DOI] [PubMed] [Google Scholar]
- 108.Grupcheva CN, Laux WT, Rupenthal ID, McGhee J, McGhee CN, Green CR. Improved corneal wound healing through modulation of gap junction communication using connexin43-specific antisense oligodeoxynucleotides. Investigative ophthalmology & visual science. 2012;53:1130–1138. doi: 10.1167/iovs.11-8711. [DOI] [PubMed] [Google Scholar]
- 109.Grek CL, Prasad GM, Viswanathan V, Armstrong DG, Gourdie RG, Ghatnekar GS. Topical administration of a connexin43-based peptide augments healing of chronic neuropathic diabetic foot ulcers: A multicenter, randomized trial. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2015;23:203–212. doi: 10.1111/wrr.12275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Becker DL, Thrasivoulou C, Phillips AR. Connexins in wound healing; perspectives in diabetic patients. Biochim Biophys Acta. 2012;1818:2068–2075. doi: 10.1016/j.bbamem.2011.11.017. [DOI] [PubMed] [Google Scholar]
- 111.Warn-Cramer BJ, Cottrell GT, Burt JM, Lau AF. Regulation of connexin-43 gap junctional intercellular communication by mitogen-activated protein kinase. J Biol Chem. 1998;273:9188–9196. doi: 10.1074/jbc.273.15.9188. [DOI] [PubMed] [Google Scholar]
- 112.TenBroek EM, Lampe PD, Solan JL, Reynhout JK, Johnson RG. Ser364 of connexin43 and the upregulation of gap junction assembly by cAMP. J Cell Biol. 2001;155:1307–1318. doi: 10.1083/jcb.200102017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Shah MM, Martinez A-M, Fletcher WH. The connexin43 gap junction protein is phosphorylated by protein kinase A and protein kinase C: In vivo and in vtitro studies. Mol Cell Biochem. 2002;238:57–68. doi: 10.1023/a:1019902920693. [DOI] [PubMed] [Google Scholar]
- 114.Yogo K, Ogawa T, Akiyama M, Ishida N, Takeya T. Identification and functional analysis of novel phosphorylation sites in Cx43 in rat primary granulosa cells. FEBS Lett. 2002;531:132–136. doi: 10.1016/s0014-5793(02)03441-5. [DOI] [PubMed] [Google Scholar]
- 115.Park DJ, Wallick CJ, Martyn KD, Lau AF, Jin C, Warn-Cramer BJ. Akt phosphorylates Connexin43 on Ser373, a "mode-1" binding site for 14-3-3. Cell Commun Adhes. 2007;14:211–226. doi: 10.1080/15419060701755958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Saez JC, Nairn AC, Czernik AJ, Fishman GI, Spray DC, Hertzberg EL. Phosphorylation of connexin43 and the regulation of neonatal rat cardiac myocyte gap junctions. J Mol Cell Cardiol. 1997;29:2131–2145. doi: 10.1006/jmcc.1997.0447. [DOI] [PubMed] [Google Scholar]
- 117.Warn-Cramer BJ, Lampe PD, Kurata WE, Kanemitsu MY, Loo LWM, Eckhart W, et al. Characterization of the MAP kinase phosphorylation sites on the connexin43 gap junction protein. J Biol Chem. 1996;271:3779–3786. doi: 10.1074/jbc.271.7.3779. [DOI] [PubMed] [Google Scholar]
- 118.Doble BW, Dang X, Ping P, Fandrich RR, Nickel BE, Jin Y, et al. Phosphorylation of serine 262 in the gap junction protein connexin-43 regulates DNA synthesis in cell-cell contact forming cardiomyocytes. J Cell Sci. 2004;117:507–514. doi: 10.1242/jcs.00889. [DOI] [PubMed] [Google Scholar]
- 119.Lampe PD, Kurata WE, Warn-Cramer B, Lau AF. Formation of a distinct connexin43 phosphoisoform in mitotic cells is dependent upon p34cdc2 kinase. J Cell Sci. 1998;111:833–841. doi: 10.1242/jcs.111.6.833. [DOI] [PubMed] [Google Scholar]
- 120.Kanemitsu MY, Jiang W, Eckhart W. Cdc2-mediated phosphorylation of the gap junction protein, connexin43, during mitosis. Cell Growth Differ. 1998;9:13–21. [PubMed] [Google Scholar]
- 121.Axelsen LN, Stahlhut M, Mohammed S, Larsen BD, Nielsen MS, Holstein-Rathlou NH, et al. Identification of ischemia-regulated phosphorylation sites in connexin43: A possible target for the antiarrhythmic peptide analogue rotigaptide (ZP123) J Mol Cell Cardiol. 2006;40:790–798. doi: 10.1016/j.yjmcc.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 122.Procida K, Jorgensen L, Schmitt N, Delmar M, Taffet SM, Holstein-Rathlou NH, et al. Phosphorylation of connexin43 on serine 306 regulates electrical coupling. Heart Rhythm. 2009;6:1632–1638. doi: 10.1016/j.hrthm.2009.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lampe PD, TenBroek EM, Burt JM, Kurata WE, Johnson RG, Lau AF. Phosphorylation of connexin43 on serine368 by protein kinase C regulates gap junctional communication. J Cell Biol. 2000;126:1503–1512. doi: 10.1083/jcb.149.7.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lin R, Warn-Cramer BJ, Kurata WE, Lau AF. v-Src phosphorylation of connexin 43 on Tyr247 and Tyr265 disrupts gap junctional communication. J Cell Biol. 2001;154:815–827. doi: 10.1083/jcb.200102027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bonnette PC, Robinson BS, Silva JC, Stokes MP, Brosius AD, Baumann A, et al. Phosphoproteomic characterization of PYK2 signaling pathways involved in osteogenesis. Journal of proteomics. 2010;73:1306–1320. doi: 10.1016/j.jprot.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 126.Ballif BA, Carey GR, Sunyaev SR, Gygi SP. Large-scale identification and evolution indexing of tyrosine phosphorylation sites from murine brain. Journal of proteome research. 2008;7:311–318. doi: 10.1021/pr0701254. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


