Abstract
Shaping of the skeleton (modeling) and its maintenance throughout life (remodeling) require coordinated activity among bone forming (osteoblasts) and resorbing cells (osteoclasts) and osteocytes (bone embedded cells). The gap junction protein connexin43 (Cx43) has emerged as a key modulator of skeletal growth and homeostasis. The skeletal developmental abnormalities present in oculodentodigital and craniometaphyseal dysplasias, both linked to Cx43 gene (GJA1) mutations, demonstrate that the skeleton is a major site of Cx43 action. Via direct action on osteolineage cells, including altering production of pro-osteoclastogenic factors, Cx43 contributes to peak bone mass acquisition, cortical modeling of long bones, and maintenance of bone quality. Cx43 also contributes in diverse ways to bone responsiveness to hormonal and mechanical signals. Skeletal biology research has revealed the complexity of Cx43 function; in addition to forming gap junctions and “hemichannels”, Cx43 provides a scaffold for signaling molecules. Hence, Cx43 actively participates in generation and modulation of cellular signals driving skeletal development and homeostasis. Pharmacological interference with Cx43 may in the future help remedy deterioration of bone quality occurring with aging, disuse and hormonal imbalances.
Keywords: Cx43, Cx37, gap junction, bone, signal transduction
1. Introduction
Bone is constantly formed and degraded in order to adapt to mechanical and metabolic demands. The ability to coordinate the function of bone forming and resorbing cells is essential for translating tissue level mechanical and metabolic inputs to single cell effectors for the maintenance of bone quality. Gap junctional intercellular communication is an important mechanism of cell-to-cell communication in bone as in many other tissues. Gap junctions form by the pairing (docking) of two hexameric channels (connexons, or “hemichannels”) on cell membranes of opposing cells to form an aqueous pore that permits the direct exchange of small molecules between the cytoplasm of the coupled cells. Connexons are composed by six subunits, connexins, which dictate the size and charge selective permeability of the gap junction channel [1, 2] (Fig. 1). There are 20 connexin genes in the mouse and 21 (plus one pseudogene) in humans [3] Although most commonly monomeric, connexons can form heteromeric connexons composed of different connexins. Heterotypic channels (docking of two connexons with different connexin composition) can also occur. This allows for large plasticity in the biophysical properties of gap junctions, dictating not only which molecules can be communicated, but also how the open/closed state of the channel is regulated, and what downstream signaling molecules can be recruited to the gap junction [4–6].
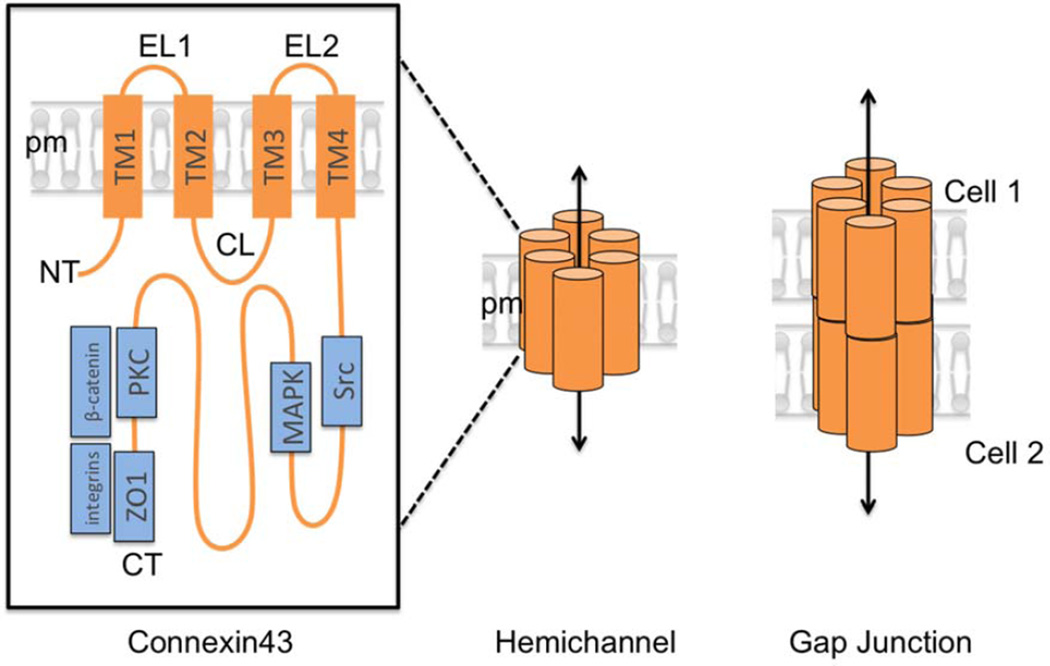
Fig. 1.
Structure and components of gap junctions. Gap junctions are composed of 6 connexin subunits. (Left) Connexins have 4 transmembrane domains (TM1–4), two extracellular loops (EL1–2), a cytoplasmic loop (CL) and cytoplasmic N (NT) and C-termini (CT). Protein complexes known to associate with the Cx43 CT are shown in blue. (Middle) Six connexin monomers assemble to form a connexon, which when inserted at the plasma membrane is called a “hemichannel”, which allows diffusion of small molecules between the cytoplasm and the extracellular space. (Right) When two connexons on the plasma membrane of adjacent cells dock, a continuous channel is formed between the two cells, permitting direct cell-to-cell communication.
A metaphor of a computer network can describe the multicellular ensemble of gap junction-coupled cells. In this analogy, the cells represent the computers and the gap junctions loosely represent the modem/router. Connexin abundance determines the bandwidth, which can be fine-tuned through modulation of the open/closed state or abundance of the gap junction channels to affect actual bandwidth. Much like a computer network, the information exchanged across the intercellular network “administered” by gap junctions is diverse and dictates many functions. Hence, gap junctions can specifically restrict or permit certain types of information being exchanged among cells in the network. As discussed in this review, regulation of bone homeostasis by connexins exemplifies this concept (Fig. 2). Via different molecular mechanisms, connexin43 (Cx43) – the most abundant connexin present in bone cells (Figs. 3, 4), modulates bone modeling and remodeling, the response to hormonal and mechanical stimuli, and the expression of osteo-anabolic and osteo-catabolic genes. While much less is known about other bone connexins, Cx40, Cx45 and Cx46, very recent data reveals distinct roles for Cx37 in bone development and homeostasis.
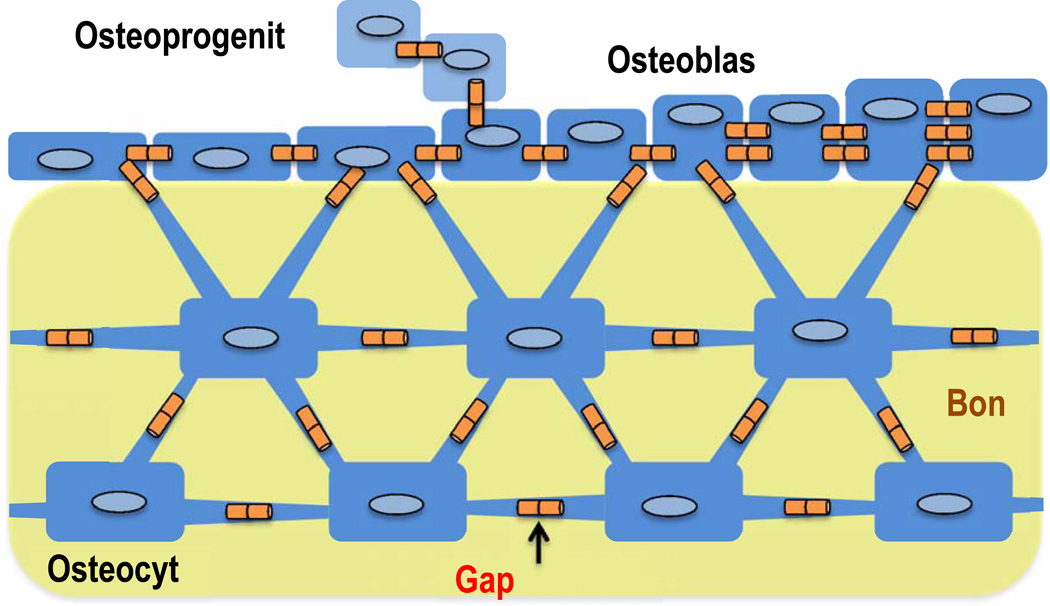
Fig. 2.
An elaborate network of gap junction coupled cells allows communication among bone embedded osteocytes, surface osteoblast and osteoprogenitors.
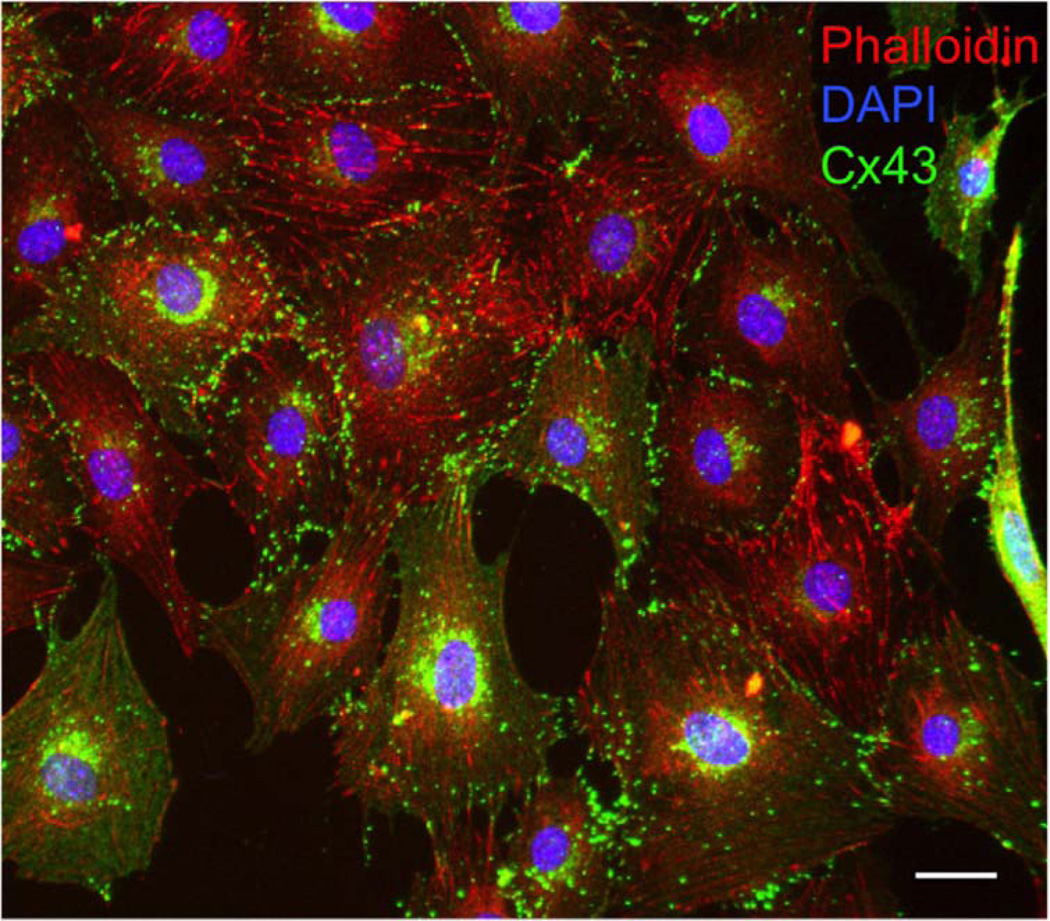
Fig. 3.
Cx43 in cultured MC3T3 osteoblastic cells. Immunofluorescence labeling of Cx43 (green), the actin cytoskeleton (red), and the nuclei (blue). Scale bar = 20 µm.
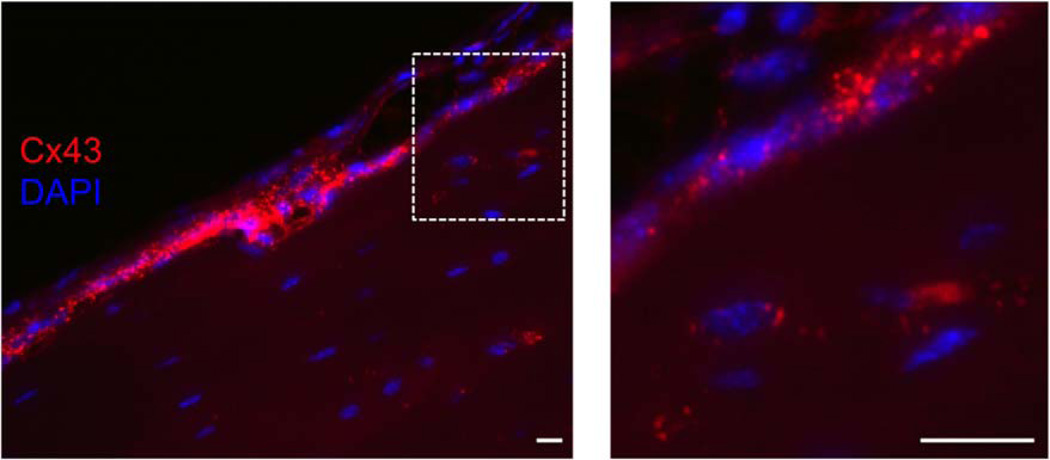
Fig. 4.
Cx43 in cortical bone. Immunofluorescence of Cx43 (red) with DAPI stained nuclei (blue). The boxed area enlarged on the right shows extensive Cx43 signal in surface cells and throughout the osteocytic network. Scale bar = 20 µm.
2. Connexins in Human Skeletal Disease
In the last decade, mutations of Cx43 gene (GJA1) have been identified in patients with skeletal dysplastic syndromes. The increasing number of disease-causing Cx43 mutations and the breadth of the clinical spectrum related to such mutations provide a genetic demonstration that the skeleton is a major site of Cx43 action, a notion that had already emerged from animal studies. Advances in human genetics have also inspired the development of mouse models of human Cx43 disorders.
2.1 Oculodentodigital Dysplasia
A large number of GJA1 mutations have been found in patients with oculodentodigital dysplasia (ODDD), a disease affecting multiple organs but primarily the skeleton, with characteristic craniofacial abnormalities (skull hyperostosis, pointed nose, enamel hypoplasia), aplastic or hypoplastic middle phalanges, syndactyly, and broad tubular long bones [7–9]. Underscoring a predominantly autosomal dominant inheritance, Cx43 mutants found in ODDD typically act as dominant negative; they are assembled in gap junctions but the intercellular channel is functionally defective [10–12]. However in some families, the ODDD phenotype has a recessive inheritance pattern. Further, the ODDD clinical spectrum can be variable, underscoring the complexity of Cx43 function in the skeleton (reviewed in [9]).
Mouse models of ODDD have been developed. A germline mutant generated through N- ethyl-N-nitrosourea mutagenesis, called Gja1Jrt/+, harbors a heterozygous Gja1 mutation (G60S). Although this mutation is not found in humans, the skeletal features of Gja1Jrt/+ mice reproduce many of the features of ODDD patients, including syndactyly, enamel hypoplasia, and craniofacial anomalies [10]. Additionally, bone mineral density is abnormally low in Gja1Jrt/+ mice, with decreased trabecular bone volume and reduced mechanical strength, features not yet described in the human disease. Gja1Jrt/+ mice also exhibit thin cortical bone and enlarged marrow cavity in the femoral diaphysis. In another approach, the Gja1 G138R point mutant – found in several ODDD families – was used to replace one wild type Gja1 allele using the Cre/loxP method. Induction of a global Gja1 to Gja1G138R gene replacement using the ubiquitously expressed PGK-Cre in the mouse (cODDDPGK) produced many of the multi-organ phenotypic features of human ODDD, including craniofacial abnormalities, but also decreased trabecular bone volume [12]. Conditional replacement of one wild type allele with the Gja1G138R allele in cells of the chondro-osteogenic lineage by Dermo1/Twist2-Cre, which is expressed starting at E9.5 (cODDDTW2) recapitulates all the skeletal defects seen in the global cODDDPGK and Gja1Jrt/+ mice, thus demonstrating that the osteogenic lineage is central for Cx43 modulation of skeletal development and homeostasis [13]. Consistent with the other two mouse ODDD models, whole body bone mineral density is reduced in cODDDTW2 mice, and this is associated with cortical thinning and a pronounced widening of diaphyseal cross sectional area; while trabecular bone is essentially unaffected [13]. As discussed more in depth later, expansion of the marrow cavity and cortical thinning are also seen in mice with conditional Gja1 ablation in the osteogenic lineage. Thus, ODDD mutations in the mouse phenocopy most of the human disease, but they also reveal additional features not described in the human disease. The most prominent differences are in the cranial vault. While the skull is thickened in the human disease, mineralization of the skull is delayed and defective at birth in cODDDTW2 mice. Furthermore, low bone density has not been described in humans with ODDD, although the skeletal features of these patients have not been studied in detail. Notably, both the Gja1Jrt and the Gja1G138R mutants are dominant negative for channel function [10] and thus may also interfere with other connexins (e.g., Cx37, Cx40, or Cx45) in forming functional channels in cells that express multiple connexins, such as osteolineage cells. In so doing, they may also interfere with gap junctional communication between osteoblasts (or osteocytes) and other cells present in bone that express a different repertoire of connexins (e.g., vascular endothelial cells).
2.2 Craniometaphyseal Dysplasia
Craniometaphyseal dysplasia (CMD) is characterized by cranial hyperostosis and defective modeling of tubular bones, features shared with ODDD, but without syndactyly [14]. The dominant form of CMD is caused by mutations of the ANKH gene, encoding a pyrophosphate transporter [15]. Recently, 4 families with a recessive form of CMD have been reported carrying a GJA1 mutation [16]. Remarkably, patients with recessive CMD have a characteristic flaring and undertrabeculation of the diaphysis of long bones, resembling the Erlenmeyer flask shape present in other metaphyseal dysplastic syndromes, and widening of the phalanges [16]. Intriguingly, while almost all the known ODDD mutations are upstream of the C-terminus of Cx43 and affect channel function [9], the mutant linked to recessive CMD, R239Q is located in the C-terminus, which is important for Cx43 interaction with microtubules and signaling molecules (see section 5.3). Lack of dental, ocular defects and syndactyly suggests that recessive CMD may not be part of the ODDD spectrum, possibly underlying different disease mechanisms linked to interference with specific functions of Cx43. The decreased trabecular bone mass without the cortical phenotype recently reported in mice overexpressing a Cx43 mutant lacking most of the C-terminus tail (see section 3) would be consistent with this hypothesis. Development of mouse models carrying the CMD-causing Gja1 mutations might help clarify these open questions.
2.3 Other Skeletal Disorders Linked to Connexin Mutations
A GJA1 mutation (R76H) has been found in one patient with Hellermann-Streiff syndrome (HSS), whose skeletal features partially overlap those of ODDD, in particular craniofacial dysmorphism, enamel hypoplasia, and clynodactyly [17]. Intriguingly, the R76H and R33X mutations have also been found in patients with ODDD [18]. Since these mutations are in the 3rd transmembrane domain of Cx43, it is likely that that both mutants disrupt Cx43 channel activity; hence, HSS may be part of the ODDD spectrum. Dysmorphic features of the upper extremities with fusion of skeletal elements in the wrist and phalanges are present in mice lacking Cx40 (Gja5−/−) and to a lesser extent in heterozygous Gja5−/+ mice [19]. Notably, similar dysmorphic features are present in mice with haploinsufficiency of T-box transcription factor, Tbx5 [19], a model of Holt-Oram syndrome, a cardiovascular disorder also characterized by dysmorphisms, sternal malformations and fusions of the upper limb bones [20]. Since Cx40 is a downstream target of Tbx5, these observations indicate that Cx40 is involved in upper limb patterning. Whether Cx40 is functionally active in adult bone is unknown. To date, there are no reports of human skeletal disorders that can be linked to Cx37 or Cx45, the other two connexins present in bone cells, although GJA4 (Cx37 gene) polymorphisms are associated with low bone mass in Japanese men [21].
3. Cx43 in Skeletal Development and Homeostasis
Craniofacial abnormalities and developmental defects of the axial skeletal had been observed by Gja1 “knockdown” in chick embryos in the late 1990s using antisense oligonucleotides [22]. However, it was the development of Gja1−/− mice by Drs. Janet Rossant and Gerald Kidder in 1995 [23] that provided the first genetic tool to understand Cx43’s biologic role in the skeleton. Although no major dysmorphisms are evident in Gja1−/− mice at birth, detailed skeletal analysis revealed reduced mineralization of the axial and appendicular skeleton and the cranial vault during embryonic development through E18.5, associated with delayed osteoblast differentiation [24]. These early in vivo studies from our group propelled research on the role of Cx43 in the post-natal skeleton.
3.1 Cx43 Drives Cortical Bone Modeling
To overcome the post-natal lethality in Gja1−/− mice [23], several conditional gene deletion models (cKO) have been generated using promoter-Cre drivers that target the osteogenic lineage. Details on the different conditional Gja1 ablation models have been discussed elsewhere in greater detail [25–27]. While the skeletal phenotype is more severe with broader Gja1 ablation, there is a common long bone phenotype among all the Gja1 cKO models, consisting of widening of the diaphyses, expanded marrow cavity, and decreased bone strength. This cortical modeling defect is secondary to increased endocortical bone resorption and increased periosteal bone formation. The important “take homes” emerging from these mouse genetic studies are as follows: (1) Cx43 in the osteoblast lineage plays an important role for osteoblast function, matrix production, and for modulation of secretory products that regulate osteoclastogenesis [13, 28, 29] and hematopoietic cells [30]. (2) Cortical bone is the primary site of Cx43 action, where it functions to restrain endocortical osteoclast development. It is not yet clear whether the accentuated periosteal bone formation represents an adaptive response to the endocortical expansion or a cell autonomous effect brought about by lack of Cx43. Nonetheless, increased bone resorption is key, as the bone resorption inhibitors, bisphosphonates, can rescue the cortical thinning and improve bone strength in adult mice [31]. (3) The increased endocortical resorption leading to an expanded marrow cavity in Cx43-deficient mice is caused by decreased osteoblast/osteocyte secretion of the osteoclast inhibitor, osteoprotegerin (OPG, Tnfrsf11b), or by increased production of the pro-osteoclastogenic factor, Receptor Activator of Nuclear factor κ-B Ligand (RANKL, Tnfsf11) or both [13, 29, 32]. (4) The mechanism of increased periosteal bone formation in Gja1 cKO mice remains to be determined, even though decreased sclerostin (Sost), a canonical Wnt antagonist and important bone remodeling regulator, may play a role [28, 33]. (5) Differentiated osteoblasts and osteocytes play a predominant role in mediating the effects of Cx43 deficiency on long bone geometry; whereas Gja1 ablation in osteogenic precursors also decreases whole body bone mass, the consequence of increased cortical porosity and production of an under-mineralized matrix with abnormal collagen organization. (5) In a healthy, growing animal, the overall contribution of Cx43 is to supports osteoblast function and bone matrix production, osteocyte survival, and restricting osteoclastic bone resorption.
3.2 Cx43 in Trabecular Bone Remodeling
While Cx43 predominantly acts in cortical bone, in certain conditions an action on the trabecular compartment emerges. Earlier studies using the 2.3kb-Col1A1 promoter to ablate Gja1 (cKOCol1A1) reported reduced mass in skeletal segments high in trabecular bone, such as the spine using dual energy X-ray absorptiometry [34]. However, a trabecular phenotype was not confirmed in other Gja1 cKO models [13, 35]. More to the point, germline mouse mutants mimicking ODDD (Gja1Jrt/+ and Gja1G138R/+) have reduced trabecular bone volume [10, 12, 36]; as have mice harboring a Cx43 truncation mutant that lacks the Cx43 C-terminus (Gja1K258Stop) [37]. Interestingly, since the trabecular phenotype in Gja1K258Stop-carrying mice occurs regardless of the presence or absence of Cx43 in differentiated osteoblasts and osteocytes, it is possible that the mutant acts as dominant negative on Cx43 at earlier stages of osteoblastogenesis. Reduced osteoblast precursor proliferation and/or differentiation would explain the reduced osteoblast number in the trabecular compartment reported in these mice [37], a scenario fully consistent with trabecular abnormalities observed in mice with a germline heterozygous ODDD mutation [10]. These mouse models have opened new questions about the role of Cx43 that requires further investigation.
3.3 Cx43 Favors Processes that Improve Bone Quality
There are numerous mechanisms by which Cx43 influences bone quality. In addition to the regulation of the RANKL/OPG ratio, as detailed above, several groups have shown that collagen processing, and specifically collagen cross-linking by lysyl oxidase (Lox), is abnormal in osteoblast Gja1 cKO mice [13, 28], leading to disorganized fibrillar collagen, hypomineralized bone matrix and decreased bone strength; these abnormalities are particularly severe in cKOTw2 mice, where Cx43 is absent in the entire chondro-osteogenic lineage [13]. As already noted, diminished sclerostin expression, a key regulator of bone homeostasis, may explain the enhanced osteo-progenitor proliferation and exuberant periosteal apposition observed Cx43 cKO knockouts [13, 32, 38, 39]. Therefore, Cx43 is a positive regulator of sclerostin, although this action is most likely indirect [32], as a consequence of either increased osteocyte viability [32], or stimulation of osteoblast differentiation [13]. Lastly, in the Gja1Jrt/+ ODDD mice, deposition of a bone matrix markedly enriched in bone sialoprotein caused by osteoprogenitor expansion contributes to increase bone resorption by a direct effect of bone sialoprotein on osteoclastogenesis, an effect abrogated by crossing these mice into a Bsp−/− background [36].
4. Cx43 in Adaptive Responses to Skeletal Stimuli
While Cx43 plays a largely osteoanabolic role in healthy mice, remarkable complexities of Cx43 action in bone have emerged from adaptive responses to various stimuli, including hormonal challenges, mechanical loading and unloading, aging and fracture healing. This research has underscored the need to better identify and decode the unique molecular signals that are propagated by gap junction channels.
4.1 Hormonal Stimulation
The first evidence that Cx43 is involved in the elaboration of hormonallystimulated osteoanabolic signals was provided by the attenuated effect of parathyroid hormone (PTH) in cKOCol1A1 mice [34]. Daily administration of PTH-(1–34) at doses that activate bone formation and increase bone mass in normal mice induces severely attenuated responses in Cx43 deficient mice [34]. Earlier in vitro data demonstrated that interference with Gja1 expression hinders PTH stimulation of cAMP production [40] and matrix mineralization by osteoblastic cell lines [41]. Although the mechanisms by which Cx43 modulates this hormonal responsiveness are not clear, these results underscore the importance of Cx43 in adaptive responses to a hormonal challenge at the tissue level. Intriguingly, Cx43 contributes to the diffusion of survival signals among osteoblasts following treatment with PTH-(1–34) via sequestration of βarrestin by Cx43 [35].
4.2 Mechanical Stimuli
The widened and thinner cortices present in Cx43 deficient bones share striking similarities with changes in bone structure occurring after mechanical unloading (e.g. space flight, prolonged bed rest), as well as in aged bone [42]. There is no evidence that mice with a conditional Gja1 deletion in bone forming cells age prematurely; however, it is quite clear that Cx43 is key for transduction of mechanical signal to skeletal cells. First, the enlarged marrow cavity and cortical thinning evident in Gja1 cKO mice are not present at birth, but develop post-natally and become evident after the animals have been able to ambulate in normal gravity conditions [27], suggesting that the cortical modeling abnormalities reflect a mechano-sensing defect. Observations from different groups have corroborated this premise, leading to the conclusion that in the absence of Cx43 cortical bone perceives normal mechanical loading as a disuse scenario, resulting in abnormal activation of endocortical osteoclasts and eventual expansion of the marrow cavity [13, 29, 32, 33]. These studies have been recently reviewed in detail elsewhere [26, 27, 43]; and overall they provide compelling evidence that osteogenic cells at endocortical and periosteal surfaces respond in different and even opposite ways to application of mechanical load; absence of Cx43 amplifies such a difference. While in an early study from our group a 3-point bending protocol resulted in reduced bone endocortical bone formation in cKOCol1A1 mice [44], an opposite effect has been consistently reporter later at the periosteal surface. Using either cantilever bending of the tibia in cKOOG2 mice – in which Gja1 is ablated by OG2-Cre in mature osteoblasts and osteocytes – [29], or axial tibial compression in cKOTw2 mice [38], or ulna compression in cKODmp1 mice [45], to apply mechanical load, a consistent enhancement of periosteal bone formation response has been reported in Cx43-deficient mice relative to wild type mice, even though with some slight differences among the different experimental settings. Thus, lack of Cx43 increases the sensitivity of periosteal bone formation to mechanical load.
Cx43 also modulates response to skeletal unloading. Our group reported that cortical bone of Gja1 cKOCol1A1 was insensitive to the effect of Botulinum Toxin Type A (BtxA)-induced muscle paralysis, whereas wild type mice experienced a significant expansion of the medullary cavity and cortical thinning associated with increased number of endocortical osteoclasts [33]. Such changes are essentially identical to those caused by loss of Cx43. Indeed, osteoclast number and cortical thickness after induction of muscle paralysis in wild type mice were very similar to control, non-BtxA injected cKOCol1A1 mice. In this study, there was no difference in the profound cancellous bone loss after BtxA injection between Cx43 deficient and wild type mice. These findings have been corroborated, in large part, by another study using the tail suspension method for mechanical unloading, which caused loss of both trabecular and cortical bone in wild type animals, while changes in cortical (and trabecular) bone were attenuated in Gja1 cKOOG2 mice [46]. These findings reinforce the notion that the cortical geometry of Cx43-deficent bones resembles that of disuse bones.
4.3 Fracture Healing
Cx43 expression is upregulated in the fracture callus [47, 48]. Accordingly, fracture healing is impaired in Gjai1 cKOCol1A1 mice [48], which is consistent with delayed osteoblast differentiation and bone formation [34]. This results in decreased bony fraction of the developing callus. There is a transient negative effect on osteoclastogenesis with an unexpected decrease in the RANKL/OPG ratio, the opposite of what is seen in the same and other models of osteolineage specific Gja1 ablation in the context of normal bone physiology [13, 29, 32]. Intriguingly, β-catenin activity is decreased in the fracture callus of Gjai1 cKOCol1A1 mice; and increasing β-catenin stability with LiCl restores normal bone formation in the callus [48]. These data exemplify the concept that Cx43 regulation of bone cell function is context dependent; specifically, Cx43 facilitates new bone formation in fracture healing, while it restrains periosteal bone formation in normal physiology. Likewise, Cx43 restrains bone resorption in normal conditions, while it increases osteoclastogenic signals in fracture healing. This context-dependent function of Cx43 may be related to specific extracellular cues that bone cells are exposed to during facture healing relative to normal physiology. Thus, the signals propagated by Cx43 gap junctions or hemichannels may be different, leading to distinct biologic outcomes.
4.4 Aging
An interesting observation made in Gja1Jrt/+ mice has provided some insights on Cx43 role in the aging skeleton. Bone quality deteriorates with age at a faster rate in Gja1Jrt/+ mice than in wild type mice [36]; trabecular bone mass and strength reach a minimum by 4 months of age, while they continue to decline up to 12 months of age in wild type mice. Somewhat similar results were seen in the cortical compartment. These data can be interpreted to suggest that: (a) loss of gap junctional communication abrogates the exchange of aging, or “catabolic” signals, thus preserving the bone from further deteriorating; or, (b) the Cx43 mutant causes bone quality to decline faster until reaches a minimum that is only reached in wild type mice during aging. In either case, these results strengthen the notion that Cx43 coordinates diffusion of signals that drive either bone anabolism or bone catabolism, depending on the physiologic context. Defining the molecular signals that mediate such changes can potentially uncover fundamental mechanisms by which bone quality can be optimized and maintained.
5. Mechanisms of Signal Modulation by Cx43 in Skeletal Cells
That adaptive skeletal responses to hormonal and mechanical stimuli are Cx43-dependent underscores the importance of the molecular signals that are propagated by Cx43 gap junctions. However, Cx43 connexons can also function as “hemichannels”, through which metabolites and signaling molecules can be released into the extracellular space. A third, emerging mechanism of Cx43 action is to provide a scaffold for signaling pathway component at the cell membrane, thus optimizing downstream signaling.
5.1 Gap Junction-Dependent Communication of Second Messengers
Gap junctions formed by Cx43 transmits numerous second messengers and signaling molecules from cell to cell. The first example is represented by mechanically stimulated intracellular Ca2+ waves, which propagate among osteoblasts and osteocytes in culture via gap junctions [49–51]. These observations have been corroborated by ex vivo imaging of Ca2+ signaling in intact bone, where gap junction-dependent propagation of Ca2+ oscillations were recorded in resting conditions and in response to fluid shear stress [52, 53]. These studies elegantly demonstrate that an elaborate interconnected communication network exists among osteocytes and osteoblasts. Indeed, establishing gap junctional communication between osteoblasts and osteocytes in co-culture is sufficient to increase expression of osteoblast genes [49, 54–56]. Furthermore, osteocytes can sense mechanical strain and communicate it to co-cultured osteoblasts to affect their function in a Cx43-dependent manner [49, 56, 57].
One key target of Cx43 action in osteoblasts and osteoprogenitors is Runx2, a master regulator of osteoblastogenesis. Going back to the computer network metaphor, increasing or decreasing the bandwidth of gap junctional intercellular communication by changes in Cx43 expression parallels changes of Runx2 transcriptional activity [58], and requires direct cell-to-cell contact [59]. Cx43 abundance also modulates the ERK and PKCδ pathways [58, 59]. One of the second messengers contributing to the activation of PKCδ, and in turn Runx2, is InsP7, a product of inositol hexakisphosphate kinase [60], and of appropriate size (~740 Da) to pass through Cx43 gap junctions. However, while synthesis of InsP7 is required for Cx43-dependent effects on PKCδ and Runx2, direct evidence of cell-to-cell transfer of InsP7 or related metabolites has not been demonstrated. Nonetheless, involvement of Cx43 in Runx2 regulation is supported by downregulation of Runx2 downstream gene expression in cells isolated from Cx43-deficient mice [34, 61], and by a recent and still preliminary report demonstrating that compound heterozygous Gja1+/−;Runx2+/− mice have a skeletal phenotype that largely phenocopies Gja1 cKO mice [62].
Another mechanisms by which Cx43 regulates osteoblast gene expression is modulation of DNA binding affinity of the transcriptional activator Sp1 via the ERK signaling cascade [63, 64], which also affects transcriptional activity of another master regulator of osteogenesis, Osterix/Sp7 [65]. Among the many pathways that converge on ERK signaling is the cAMP/protein kinase A (PKA) system. Underscoring the importance of cell-cell diffusion of cAMP, interference with Cx43 using antisense oligonucleotides in osteoblastic cells reduces cAMP levels without affecting adenylate cyclase activity in response to PTH [66]. Notably, recent preliminary work suggests that Cx43 can amplify cAMP-dependent signaling and PKA-dependent gene transcription in osteoblasts in a cell-to-cell contact dependent manner [67]. As also noted, Cx43 can bind and sequester β-arrestin, thus preventing β-arrestin inhibition of cAMP signaling [60]. The resistance of Gja1 cKOCol1A1 to the anabolic actions of intermittent PTH administration [34] provides further in vivo evidence of the importance of sharing cAMP among the network of connected osteoblasts and osteocytes.
5.2 Hemichannel-Mediated Paracrine Signaling
Exposure of osteocyte-like cells to fluid flow in culture increases cellular uptake of membrane impermeable fluorescent probes, a phenomenon interpreted as opening of Cx43 hemichannels [68]. This is associated with release of prostaglandins [68, 69] and ATP [70] into the extracellular fluid. Fluid flow-induced hemichannel opening requires physical interaction between Cx43 and α5β1 integrin, triggered by mechanical forces and activation of the Akt pathway [71, 72]. In an attempt to extend these observation in vivo, two transgenic mouse strains were generated using the 10kb-Dmp1 promoter to drive expression of either a Gja1 R76W point mutant, which can form hemichannels but not functional gap junctions, or a mutant lacking the coding sequence for amino acids 130–136 (Δ130–136), which is devoid of both gap junction and hemichannel function [73]. As noted earlier, mutations of R76 have been reported in ODDD patients [9]. The characteristic cortical phenotype of Gja1 cKO mice – cross-sectional expansion and marrow cavity expansion, with increased endocortical bone resorption – was observed in the Δ130–136 expressing mice, but, quite surprisingly, not in those expressing the R76W mutant [73]. The latter result is in direct contrast with the cortical phenotype observed in the two existing ODDD mutants, Gja1Jrt/+ and Gja1G138R/+, both of which phenocopy the cortical abnormalities of Gja1 cKO mice [10, 13]. Indeed, just like R76W, the G138R mutant forms non-functional gap junctions, while “hemichannel” activity (ATP release) is actually enhanced [12]. The use of different approaches (transgenic expression vs. targeted gene replacement) and different promoters to drive the genetic mutation might explain the discrepancy. Notably, the existence of Cx43 hemichannels has been challenged by a study showing that fluid flow-induced PGE2 release and ATP-dependent dye uptake, typically attributed to Cx43 hemichannels, can occur in Cx43-deficient primary osteoblasts and are mediated by a P2X7 receptor and pannexin-1 complex [74]. Thus, the in vivo evidence for a physiologic role of Cx43 hemichannels remains controversial.
5.3 Cx43 as a Docking Platform for Signaling Factors
There is increasing evidence that connexins actively participates in signaling by providing a scaffold for signaling complexes to the gap junction plaque. It is possible that each connexin recruits a unique profile of signaling effectors, thus resulting in specific downstream effects. This model would assign an additional biologic diversity to connexins, in addition to forming channels with specific biophysical properties. As mentioned, at least three signaling complexes interact with the Cx43 C-terminus, PKCδ, which upon FGF2 stimulation translocates from the Cx43 C-terminus to the nucleus [58–60], β-arrestin and α5β1 integrin [35, 71]. Although there is no direct evidence, it is likely that β-catenin and MAPK also interact with Cx43 C-terminus [75]. In osteoblastic cells, the Cx43 C-terminus is necessary but not sufficient to enhance cell signaling in vitro [71, 76]. In this context, the observation that female mice globally expressing the truncated allele Gja1K258Stop have low trabecular bone mass but normal cortical geometry, even in the absence of a wild type allele [37], suggests that the Cx43 C-terminus is dispensable for maintenance of normal cortical geometry, but it may be important for other actions of Cx43 in bone.
5.4 Cx43 and Apoptosis
Increased osteocyte apoptosis and empty osteocyte lacunae have been reported in both cKOhOC and cKODMP1 mice [32]. Since loss of osteocytes leads to increased osteoclast formation and bone resorption [77, 78], these observations would provide a mechanism for the increased endocortical resorption and the reduced abundance of sclerostin, an inhibitor of the Wnt/β-catenin cascade, observed in Cx43-deficient mice [32]. Consistently, Gja1 knockdown by shRNA decreases viability of MLO-Y4 osteocytelike cells, while RANKL production increases and OPG expression is reduced [29, 32]. However, despite relatively conserved phenotypes, osteocyte apoptosis has not been consistently observed in all models of Cx43 loss of function [13, 36]. Accordingly, osteocyte apoptosis may contribute to, but it is not required for the development of the skeletal modeling abnormalities in Cx43 deficiency.
5 Other Connexins in the Skeleton
While significantly less studied than Cx43, recent data demonstrate that Cx37 (Gja4) does have a physiologic role in the skeleton. In contrast to Cx43, Gja4−/− mice have increased bone mass [79]. While both osteoblasts and osteoclasts express Cx37, the phenotype of these mice is secondary to reduced osteoclast differentiation and a concomitant reduction in bone resorption. No changes in osteoblast function were reported, suggesting that if Cx37 plays a role in osteoblasts it may be compensated for by other connexins. Notably, bone quality defects are more pronounced in males than females [79].
6 Translational Perspectives
Osteoblasts and osteocytes utilize connexins to communicate and coordinate signals in order to adapt to hormonal, mechanical and local signals. As we gain a better understanding of the information shared among this interconnected cellular network, the anatomic control of tissue modeling and remodeling will come to light. Gap junctions propagate both bone anabolic and catabolic signals depending upon the tissue context and physiological or adaptive conditions. Thus, there is value in designing modulators that could specifically disrupt or enhance the communication of these catabolic or anabolic signals, respectively. New compounds that modify gap junctions have been developed, although their mechanism of action is not completely understood [80]. However, the diversity of the downstream effects of Cx43 in bone and the advancing knowledge on specific signaling pathways regulated by Cx43 indicate that a more focused targeting would be more effective in achieving therapeutic potential, rather than inhibiting or enhancing Cx43 function as a whole. For example, it may be useful to modulate specific interactions between signal complexes and the C-terminus of Cx43 in order to inhibit signals that are catabolic to bone, while preserving the intercellular communication of signals that are anabolic to bone. To this end, defining the communicated information and how the intercellular network formed by gap junctions modulates the function of bone cells adds a new dimension to our understanding of bone modeling and remodeling at the tissue level. Such knowledge opens new avenues in the discovery of molecular targets using Cx43 as a platform for improving bone strength and optimizing response to therapy.
Acknowledgments
Roberto Civitelli receives grant support from Amgen, and owns stock of Eli-Lilly, Merck, and Amgen. Part of the work reported here was supported by NIH grants AR041255 (to RC), AR052719, AR063631 (to JPS), by the Washington University Core Center for Musculoskeletal Biology and Medicine (P30 AR057235), and by grants from the Barnes-Jewish Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES: All other authors state they have no conflict of interest.
REFERENCES
- 1.Koval M, Geist ST, Westphale EM, Kemendy AE, Civitelli R, Beyer EC, et al. Transfected connexin45 alters gap junction permeability in cells expressing endogenous connexin43. J Cell Biol. 1995;130:987–995. doi: 10.1083/jcb.130.4.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Veenstra RD, Wang HZ, Beyer EC, Brink PR. Selective dye and ionic permeability of gap junction channels formed by connexin45. Circ Res. 1994;75:483–490. doi: 10.1161/01.res.75.3.483. [DOI] [PubMed] [Google Scholar]
- 3.Sohl G, Willecke K. An update on connexin genes and their nomenclature in mouse and man. Cell Commun Adhes. 2003;10:173–180. doi: 10.1080/cac.10.4-6.173.180. [DOI] [PubMed] [Google Scholar]
- 4.Veenstra RD, Wang HZ, Westphale EM, Beyer EC. Multiple connexins confer distinct regulatory and conductance properties of gap junctions in developing heart. Circ Res. 1992;71:1277–1283. doi: 10.1161/01.res.71.5.1277. [DOI] [PubMed] [Google Scholar]
- 5.Steinberg TH, Civitelli R, Geist ST, Robertson AJ, Hick E, Veenstra RD, et al. Connexin43 and connexin45 form gap junctions with different molecular permeabilities in osteoblastic cells. EMBO J. 1994;13:744–750. doi: 10.1002/j.1460-2075.1994.tb06316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldberg GS, Valiunas V, Brink PR. Selective permeability of gap junction channels. Biochim Biophys Acta. 2004;1662:96–101. doi: 10.1016/j.bbamem.2003.11.022. [DOI] [PubMed] [Google Scholar]
- 7.Paznekas WA, Boyadjiev SA, Shapiro RE, Daniels O, Wollnik B, Keegan CE, et al. Connexin 43 (GJA1) mutations cause the pleiotropic phenotype of oculodentodigital dysplasia. Am J Hum Genet. 2003;72:408–418. doi: 10.1086/346090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paznekas WA, Karczeski B, Vermeer S, Lowry RB, Delatycki M, Laurence F, et al. GJA1 mutations, variants, and connexin 43 dysfunction as it relates to the oculodentodigital dysplasia phenotype. Hum Mutat. 2009;30:724–733. doi: 10.1002/humu.20958. [DOI] [PubMed] [Google Scholar]
- 9.Laird DW. Syndromic and non-syndromic disease-linked Cx43 mutations. FEBS Lett. 2014;588:1339–1348. doi: 10.1016/j.febslet.2013.12.022. [DOI] [PubMed] [Google Scholar]
- 10.Flenniken AM, Osborne LR, Anderson N, Ciliberti N, Fleming C, Gittens JE, et al. A Gja1 missense mutation in a mouse model of oculodentodigital dysplasia. Development. 2005;132:4375–4386. doi: 10.1242/dev.02011. [DOI] [PubMed] [Google Scholar]
- 11.McLachlan E, Plante I, Shao Q, Tong D, Kidder GM, Bernier SM, et al. ODDD-linked Cx43 mutants reduce endogenous Cx43 expression and function in osteoblasts and inhibit late stage differentiation. J Bone Miner Res. 2008;23:928–938. doi: 10.1359/jbmr.080217. [DOI] [PubMed] [Google Scholar]
- 12.Dobrowolski R, Sasse P, Schrickel JW, Watkins M, Kim JS, Rackauskas M, et al. The conditional connexin43G138R mouse mutant represents a new model of hereditary oculodentodigital dysplasia in humans. Hum Mol Genet. 2008;17:539–554. doi: 10.1093/hmg/ddm329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Watkins M, Grimston SK, Norris JY, Guillotin B, Shaw A, Beniash E, et al. Osteoblast connexin43 modulates skeletal architecture by regulating both arms of bone remodeling. Mol Biol Cell. 2011;22:1240–1251. doi: 10.1091/mbc.E10-07-0571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cai C, Zhang Q, Shen C, Sun G, Wang C. Chiari malformation caused by craniometaphyseal dysplasia: case report and review of literature. Eur J Pediatr Surg. 2008;18:198–201. doi: 10.1055/s-2008-1038536. [DOI] [PubMed] [Google Scholar]
- 15.Chen IP, Wang L, Jiang X, Aguila HL, Reichenberger EJ. A Phe377del mutation in ANK leads to impaired osteoblastogenesis and osteoclastogenesis in a mouse model for craniometaphyseal dysplasia (CMD) Hum Mol Genet. 2011;20:948–961. doi: 10.1093/hmg/ddq541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu Y, Chen IP, de Almeida S, Tiziani V, Do Amaral CM, Gowrishankar K, et al. A novel autosomal recessive GJA1 missense mutation linked to Craniometaphyseal dysplasia. PloS one. 2013;8:e73576. doi: 10.1371/journal.pone.0073576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pizzuti A, Flex E, Mingarelli R, Salpietro C, Zelante L, Dallapiccola B. A homozygous GJA1 gene mutation causes a Hallermann-Streiff/ODDD spectrum phenotype. Hum Mutat. 2004;23:286. doi: 10.1002/humu.9220. [DOI] [PubMed] [Google Scholar]
- 18.Richardson RJ, Joss S, Tomkin S, Ahmed M, Sheridan E, Dixon MJ. A nonsense mutation in the first transmembrane domain of connexin 43 underlies autosomal recessive oculodentodigital syndrome. J Med Genet. 2006;43:e37. doi: 10.1136/jmg.2005.037655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pizard A, Burgon PG, Paul DL, Bruneau BG, Seidman CE, Seidman JG. Connexin 40, a target of transcription factor Tbx5, patterns wrist, digits, and sternum. Mol Cell Biol. 2005;25:5073–5083. doi: 10.1128/MCB.25.12.5073-5083.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Basson CT, Bachinsky DR, Lin RC, Levi T, Elkins JA, Soults J, et al. Mutations in human TBX5 cause limb and cardiac malformation in Holt-Oram syndrome. Nat Genet. 1997;15:30–35. doi: 10.1038/ng0197-30. [DOI] [PubMed] [Google Scholar]
- 21.Yamada Y, Ando F, Shimokata H. Association of candidate gene polymorphisms with bone mineral density in community-dwelling Japanese women and men. Int J Mol Med. 2007;19:791–801. [PubMed] [Google Scholar]
- 22.Becker DL, McGonnell I, Makarenkova HP, Patel K, Tickle C, Lorimer J, et al. Roles for alpha 1 connexin in morphogenesis of chick embryos revealed using a novel antisense approach. Dev Genet. 1999;24:33–42. doi: 10.1002/(SICI)1520-6408(1999)24:1/2<33::AID-DVG5>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 23.Reaume AG, de Sousa PA, Kulkarni S, Langille BL, Zhu D, Davies TC, et al. Cardiac malformation in neonatal mice lacking connexin43. Science. 1995;267:1831–1834. doi: 10.1126/science.7892609. [DOI] [PubMed] [Google Scholar]
- 24.Lecanda F, Warlow PM, Sheikh S, Furlan F, Steinberg TH, Civitelli R. Connexin43 deficiency causes delayed ossification, craniofacial abnormalities, and osteoblast dysfunction. J Cell Biol. 2000;151:931–944. doi: 10.1083/jcb.151.4.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stains JP, Watkins MP, Grimston SK, Hebert C, Civitelli R. Molecular mechanisms of osteoblast/osteocyte regulation by connexin43. Calcif Tissue Int. 2014;94:55–67. doi: 10.1007/s00223-013-9742-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Plotkin LI, Bellido T. Beyond gap junctions: Connexin43 and bone cell signaling. Bone. 2013;52:157–166. doi: 10.1016/j.bone.2012.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grimston SK, Watkins MP, Stains JP, Civitelli R. Connexin43 modulates post-natal cortical bone modeling and mechano-responsiveness. Bonekey Rep. 2013;2:446. doi: 10.1038/bonekey.2013.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bivi N, Nelson MT, Faillace ME, Li J, Miller LM, Plotkin LI. Deletion of Cx43 from osteocytes results in defective bone material properties but does not decrease extrinsic strength in cortical bone. Calcif Tissue Int. 2012;91:215–224. doi: 10.1007/s00223-012-9628-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang Y, Paul EM, Sathyendra V, Davison A, Sharkey N, Bronson S, et al. Enhanced osteoclastic resorption and responsiveness to mechanical load in gap junction deficient bone. PloS one. 2011;6:e23516. doi: 10.1371/journal.pone.0023516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gonzalez-Nieto D, Li L, Kohler A, Ghiaur G, Ishikawa E, Sengupta A, et al. Connexin-43 in the osteogenic BM niche regulates its cellular composition and the bidirectional traffic of hematopoietic stem cells and progenitors. Blood. 2012;119:5144–5154. doi: 10.1182/blood-2011-07-368506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Watkins MP, Norris JY, Grimston SK, Zhang X, Phipps RJ, Ebetino FH, et al. Bisphosphonates improve trabecular bone mass and normalize cortical thickness in ovariectomized, osteoblast connexin43 deficient mice. Bone. 2012;51:787–794. doi: 10.1016/j.bone.2012.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bivi N, Condon KW, Allen MR, Farlow N, Passeri G, Brun LR, et al. Cell autonomous requirement of connexin 43 for osteocyte survival: consequences for endocortical resorption and periosteal bone formation. J Bone Miner Res. 2012;27:374–389. doi: 10.1002/jbmr.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grimston SK, Goldberg DB, Watkins M, Brodt MD, Silva MJ, Civitelli R. Connexin43 deficiency reduces the sensitivity of cortical bone to the effects of muscle paralysis. J Bone Miner Res. 2011;26:2151–2160. doi: 10.1002/jbmr.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chung DJ, Castro CH, Watkins M, Stains JP, Chung MY, Szejnfeld VL, et al. Low peak bone mass and attenuated anabolic response to parathyroid hormone in mice with an osteoblast-specific deletion of connexin43. J Cell Sci. 2006;119:4187–4198. doi: 10.1242/jcs.03162. [DOI] [PubMed] [Google Scholar]
- 35.Bivi N, Lezcano V, Romanello M, Bellido T, Plotkin LI. Connexin43 interacts with betaarrestin: a pre-requisite for osteoblast survival induced by parathyroid hormone. J Cell Biochem. 2011;112:2920–2930. doi: 10.1002/jcb.23208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zappitelli T, Chen F, Moreno L, Zirngibl RA, Grynpas M, Henderson JE, et al. The G60S connexin 43 mutation activates the osteoblast lineage and results in a resorptionstimulating bone matrix and abrogation of old-age-related bone loss. J Bone Miner Res. 2013;28:2400–2413. doi: 10.1002/jbmr.1965. [DOI] [PubMed] [Google Scholar]
- 37.Pacheco-Costa R, Davis HM, Sorenson C, Hon MC, Hassan I, Reginato RD, et al. Defective cancellous bone structure and abnormal response to PTH in cortical bone of mice lacking Cx43 cytoplasmic C-terminus domain. Bone. 2015;81:632–643. doi: 10.1016/j.bone.2015.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grimston SK, Watkins MP, Brodt MD, Silva MJ, Civitelli R. Enhanced periosteal and endocortical responses to axial tibial compression loading in conditional connexin43 deficient mice. PloS one. 2012;7:e44222. doi: 10.1371/journal.pone.0044222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lloyd SA, Loiselle AE, Zhang Y, Donahue HJ. Connexin 43 deficiency desensitizes bone to the effects of mechanical unloading through modulation of both arms of bone remodeling. Bone. 2013;57:76–83. doi: 10.1016/j.bone.2013.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vander Molen MA, Rubin CT, McLeod KJ, McCauley LK, Donahue HJ. Gap junctional intercellular communication contributes to hormonal responsiveness in osteoblastic networks. J Biol Chem. 1996;271:12165–12171. doi: 10.1074/jbc.271.21.12165. [DOI] [PubMed] [Google Scholar]
- 41.Schiller PC, D'Ippolito G, Balkan W, Roos BA, Howard GA. Gap-junctional communication mediates parathyroid hormone stimulation of mineralization in osteoblastic cultures. Bone. 2001;28:38–44. doi: 10.1016/s8756-3282(00)00412-9. [DOI] [PubMed] [Google Scholar]
- 42.Ruff CB, Hayes WC. Subperiosteal expansion and cortical remodeling of the human femur and tibia with aging. Science. 1982;217:945–948. doi: 10.1126/science.7112107. [DOI] [PubMed] [Google Scholar]
- 43.Loiselle AE, Jiang JX, Donahue HJ. Gap junction and hemichannel functions in osteocytes. Bone. 2013;54:205–212. doi: 10.1016/j.bone.2012.08.132. [DOI] [PubMed] [Google Scholar]
- 44.Grimston SK, Brodt MD, Silva MJ, Civitelli R. Attenuated response to in vivo mechanical loading in mice with conditional osteoblast ablation of the connexin43 gene (Gja1) J Bone Miner Res. 2008;23:879–886. doi: 10.1359/JBMR.080222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bivi N, Pacheco-Costa R, Brun LR, Murphy TR, Farlow NR, Robling AG, et al. Absence of Cx43 Selectively from Osteocytes Enhances Responsiveness to Mechanical Force in Mice. J Orthop Res. 2013 doi: 10.1002/jor.22341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lloyd SA, Lewis GS, Zhang Y, Paul EM, Donahue HJ. Connexin 43 deficiency attenuates loss of trabecular bone and prevents suppression of cortical bone formation during unloading. J Bone Miner Res. 2012;27:2359–2372. doi: 10.1002/jbmr.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gupta RR, Kim H, Chan YK, Hebert C, Gitajn L, Yoo DJ, et al. Axial strain enhances osteotomy repair with a concomitant increase in connexin43 expression. Bone Res. 2015;3:15007. doi: 10.1038/boneres.2015.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Loiselle AE, Lloyd SA, Paul EM, Lewis GS, Donahue HJ. Inhibition of GSK-3beta rescues the impairments in bone formation and mechanical properties associated with fracture healing in osteoblast selective connexin 43 deficient mice. PloS one. 2013;8:e81399. doi: 10.1371/journal.pone.0081399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yellowley CE, Li Z, Zhou Z, Jacobs CR, Donahue HJ. Functional gap junctions between osteocytic and osteoblastic cells. J Bone Miner Res. 2000;15:209–217. doi: 10.1359/jbmr.2000.15.2.209. [DOI] [PubMed] [Google Scholar]
- 50.Jorgensen NR, Geist ST, Civitelli R, Steinberg TH. ATP- and gap junction-dependent intercellular calcium signaling in osteoblastic cells. J Cell Biol. 1997;139:497–506. doi: 10.1083/jcb.139.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huo B, Lu XL, Hung CT, Costa KD, Xu Q, Whitesides GM, et al. Fluid Flow Induced Calcium Response in Bone Cell Network. Cell Mol Bioeng. 2008;1:58–66. doi: 10.1007/s12195-008-0011-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ishihara Y, Sugawara Y, Kamioka H, Kawanabe N, Hayano S, Balam TA, et al. Ex vivo real-time observation of Ca(2+) signaling in living bone in response to shear stress applied on the bone surface. Bone. 2013;53:204–215. doi: 10.1016/j.bone.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 53.Jing D, Lu XL, Luo E, Sajda P, Leong PL, Guo XE. Spatiotemporal properties of intracellular calcium signaling in osteocytic and osteoblastic cell networks under fluid flow. Bone. 2013;53:531–540. doi: 10.1016/j.bone.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nishikawa Y, Akiyama Y, Yamamoto K, Kobayashi M, Watanabe E, Watanabe N, et al. Osteocytes up-regulate the terminal differentiation of pre-osteoblasts via gap junctions. Biochem Biophys Res Commun. 2015;456:1–6. doi: 10.1016/j.bbrc.2014.10.128. [DOI] [PubMed] [Google Scholar]
- 55.Mikami Y, Yamamoto K, Akiyama Y, Kobayashi M, Watanabe E, Watanabe N, et al. Osteogenic gene transcription is regulated via gap junction-mediated cell-cell communication. Stem Cells Dev. 2015;24:214–227. doi: 10.1089/scd.2014.0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vazquez M, Evans BA, Riccardi D, Evans SL, Ralphs JR, Dillingham CM, et al. A new method to investigate how mechanical loading of osteocytes controls osteoblasts. Front Endocrinol (Lausanne) 2014;5:208. doi: 10.3389/fendo.2014.00208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Taylor AF, Saunders MM, Shingle DL, Cimbala JM, Zhou Z, Donahue HJ. Mechanically stimulated osteocytes regulate osteoblastic activity via gap junctions. Am J Physiol Cell Physiol. 2007;292:C545–C552. doi: 10.1152/ajpcell.00611.2005. [DOI] [PubMed] [Google Scholar]
- 58.Lima F, Niger C, Hebert C, Stains JP. Connexin43 potentiates osteoblast responsiveness to fibroblast growth factor 2 via a protein kinase C-delta/Runx2-dependent mechanism. Mol Biol Cell. 2009;20:2697–2708. doi: 10.1091/mbc.E08-10-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Niger C, Buo AM, Hebert C, Duggan BT, Williams MS, Stains JP. ERK acts in parallel to PKCdelta to mediate the connexin43-dependent potentiation of Runx2 activity by FGF2 in MC3T3 osteoblasts. Am J Physiol Cell Physiol. 2012;302:C1035–C1044. doi: 10.1152/ajpcell.00262.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Niger C, Luciotti MA, Buo AM, Hebert C, Ma V, Stains JP. The regulation of Runx2 by FGF2 and connexin43 requires the inositol polyphosphate/protein kinase Cdelta cascade. J Bone Miner Res. 2013 doi: 10.1002/jbmr.1867. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chaible LM, Sanches DS, Cogliati B, Mennecier G, Dagli ML. Delayed osteoblastic differentiation and bone development in Cx43 knockout mice. Toxicol Pathol. 2011;39:1046–1055. doi: 10.1177/0192623311422075. [DOI] [PubMed] [Google Scholar]
- 62.Buo AM, Stains JP. Assessing the skeletal phenotype of compound Gja1+/− Runx2+/− mice. J Bone Miner Res. 2015;30(S1) Available at http://www.asbmr.org/education/AbstractDetail.aspx?aid=44e7cc91-3673-4106-a4e0-8413104f6bc5. [Google Scholar]
- 63.Stains JP, Lecanda F, Screen J, Towler DA, Civitelli R. Gap junctional communication modulates gene transcription by altering the recruitment of Sp1 and Sp3 to connexin-response elements in osteoblast promoters. J Biol Chem. 2003;278:24377–24387. doi: 10.1074/jbc.M212554200. [DOI] [PubMed] [Google Scholar]
- 64.Stains JP, Civitelli R. Gap junctions regulate extracellular signal-regulated kinase signaling to affect gene transcription. Mol Biol Cell. 2005;16:64–72. doi: 10.1091/mbc.E04-04-0339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Niger C, Lima F, Yoo DJ, Gupta RR, Buo AM, Hebert C, et al. The transcriptional activity of osterix requires the recruitment of Sp1 to the osteocalcin proximal promoter. Bone. 2011;49:683–692. doi: 10.1016/j.bone.2011.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vander Molen MA, Donahue HJ, Rubin CT, McLeod KJ. Osteoblastic networks with deficient coupling: differential effects of magnetic and electric field exposure. Bone. 2000;27:227–231. doi: 10.1016/s8756-3282(00)00315-x. [DOI] [PubMed] [Google Scholar]
- 67.Gupta A, Anderson H, Ren M, Stains JP. Communication of cyclic AMP by connexin43 gap junctions influences osteoblast signaling and gene expression. J Bone Miner Res. 2015;30(S1) doi: 10.1016/j.cellsig.2016.04.014. Available at http://www.asbmr.org/education/AbstractDetail.aspx?aid=d1ffcc58-94a1-4139-8df0-e041a0beac09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cherian PP, Siller-Jackson AJ, Gu S, Wang X, Bonewald LF, Sprague E, et al. Mechanical strain opens connexin 43 hemichannels in osteocytes: a novel mechanism for the release of prostaglandin. Mol Biol Cell. 2005;16:3100–3106. doi: 10.1091/mbc.E04-10-0912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Siller-Jackson AJ, Burra S, Gu S, Xia X, Bonewald LF, Sprague E, et al. Adaptation of connexin 43-hemichannel prostaglandin release to mechanical loading. J Biol Chem. 2008;283:26374–26382. doi: 10.1074/jbc.M803136200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Genetos DC, Kephart CJ, Zhang Y, Yellowley CE, Donahue HJ. Oscillating fluid flow activation of gap junction hemichannels induces ATP release from MLO-Y4 osteocytes. J Cell Physiol. 2007;212:207–214. doi: 10.1002/jcp.21021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Batra N, Burra S, Siller-Jackson AJ, Gu S, Xia X, Weber GF, et al. Mechanical stress-activated integrin alpha5beta1 induces opening of connexin 43 hemichannels. Proc Natl Acad Sci U S A. 2012;109:3359–3364. doi: 10.1073/pnas.1115967109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Batra N, Riquelme MA, Burra S, Kar R, Gu S, Jiang JX. Direct regulation of osteocytic connexin 43 hemichannels through AKT kinase activated by mechanical stimulation. J Biol Chem. 2014;289:10582–10591. doi: 10.1074/jbc.M114.550608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Xu H, Gu S, Riquelme MA, Burra S, Callaway D, Cheng H, et al. Connexin 43 channels are essential for normal bone structure and osteocyte viability. J Bone Miner Res. 2015;30:436–448. doi: 10.1002/jbmr.2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Thi MM, Islam S, Suadicani SO, Spray DC. Connexin43 and pannexin1 channels in osteoblasts: who is the "hemichannel"? J Membr Biol. 2012;245:401–409. doi: 10.1007/s00232-012-9462-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Herve JC, Derangeon M, Sarrouilhe D, Giepmans BN, Bourmeyster N. Gap junctional channels are parts of multiprotein complexes. Biochim Biophys Acta. 2012;1818:1844–1865. doi: 10.1016/j.bbamem.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 76.Hebert C, Stains JP. An intact connexin43 is required to enhance signaling and gene expression in osteoblast-like cells. J Cell Biochem. 2013;114:2542–2550. doi: 10.1002/jcb.24603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jilka RL, Noble B, Weinstein RS. Osteocyte apoptosis. Bone. 2013;54:264–271. doi: 10.1016/j.bone.2012.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tatsumi S, Ishii K, Amizuka N, Li M, Kobayashi T, Kohno K, et al. Targeted ablation of osteocytes induces osteoporosis with defective mechanotransduction. Cell Metab. 2007;5:464–475. doi: 10.1016/j.cmet.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 79.Pacheco-Costa R, Hassan I, Reginato RD, Davis HM, Bruzzaniti A, Allen MR, et al. High bone mass in mice lacking Cx37 because of defective osteoclast differentiation. J Biol Chem. 2014;289:8508–8520. doi: 10.1074/jbc.M113.529735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jorgensen NR, Teilmann SC, Henriksen Z, Meier E, Hansen SS, Jensen JE, et al. The antiarrhythmic peptide analog rotigaptide (ZP123) stimulates gap junction intercellular communication in human osteoblasts and prevents decrease in femoral trabecular bone strength in ovariectomized rats. Endocrinol. 2005;146:4745–4754. doi: 10.1210/en.2004-1414. [DOI] [PubMed] [Google Scholar]