Abstract
Rationale
Mitochondria produce ATP, especially critical for survival of highly aerobic cells such as cardiac myocytes. Conversely, opening of mitochondrial high-conductance and long-lasting permeability transition pores (mPTP) causes respiratory uncoupling, mitochondrial injury and cell death. However, low-conductance and transient mPTP openings (tPTP) might limit mitochondrial Ca2+ load and be cardioprotective, but direct evidence for tPTP in cells is limited.
Objective
To directly characterize tPTP occurrence during SR Ca2+ release in adult cardiac myocytes.
Methods and Results
Here, we measured tPTP directly as transient drops in mitochondrial [Ca2+] ([Ca2+]mito) and membrane potential (ΔΨm) in adult cardiac myocytes during cyclical sarcoplasmic reticulum Ca release, by simultaneous live imaging of 500-1,000 individual mitochondria. The frequency of tPTPs rose at higher [Ca2+]mito, [Ca2+]i, with 1 μM peroxide exposure and in myocyte from failing hearts. The tPTPs were suppressed by preventing mitochondrial Ca2+ influx, by mPTP inhibitor cyclosporine A, sanglifehrin and in cyclophilin D knockout mice. These tPTP events were 57 ± 5 s in duration, but were rare (occurring in <0.1% of myocyte mitochondria at any moment) such that the overall energetic cost to the cell is minimal. The tPTP pore size is much smaller than for permanent mPTP, as neither Rhod-2 nor calcien (600 Da) were lost. Thus, proteins and even molecules the size of NADH (663 Da) will be retained during these tPTP.
Conclusions
We conclude that tPTP openings (MitoWinks) may be molecularly related to pathological mPTP, but are likely to be normal physiological manifestation that benefits mitochondrial (and cell) survival by allowing individual mitochondria to reset themselves with little overall energetic cost.
Keywords: Mitochondria, permeability transition pore, cardiac myocytes, calcium, metabolism
INTRODUCTION
Mitochondria sustain cellular life through energy production,1 but also mediate programmed cell death.2 ATP production is mainly via cellular respiration, which is driven by the voltage gradient (ΔΨm) across the inner mitochondrial membrane (IMM), that drives proton flux through the F0F1-ATP synthase. Extremely low resting IMM permeability is critical to maintain high ΔΨm and ATP synthesis rate in living cells. However, under certain stresses, the IMM undergoes a permeability transition pore opening (mPTP), abolishing ΔΨm and allowing molecules of up to 1500 Da in size to freely permeate.3, 4 This causes respiratory uncoupling, metabolite loss (e.g., NADH), cessation of ATP synthesis, increased ATP consumption (via F0F1-ATP synthase), and mitochondrial and cell death.5
The molecular identity of mPTP is unknown, but F0F1-ATP synthase dimers have been proposed as a candidate.6 Much work has shown that mPTP inhibition by cyclosporine (CsA)7-9 or by genetic ablation of a critical mPTP associated protein, cyclophilin D (CypD), protects against mPTP and cell death in response to ischemia-reperfusion injury and amyotrophic lateral sclerosis.8-11 Thus, mPTP is an attractive drug target to protect against cardiac injury. However, mPTP inhibition by CsA during ischemia preconditioning (IPC) abolished the protective effect of IPC.10, 11 Moreover, chronic mPTP inhibition leads to mitochondrial Ca2+ overload and cardiac dysfunction,12 raising the idea that some mPTP openings might be beneficial by allowing Ca2+ release and maintenance of normal physiological mitochondrial [Ca2+] ([Ca2+]mito). But how might that occur without the pathologic consequences of mPTP?
A transient mode of mPTP opening (tPTP), with lower conductance was proposed as protective against pathological Ca2+ overload.2, 13 Unlike prolonged or permanent mPTP (pPTP) openings, these could limit metabolite loss and allow full mitochondrial recovery. Until now, evidence of tPTP openings is restricted to inferences from isolated in vitro mitochondria, mostly in suspensions.13-16 Here we continuously monitor ~800 individual mitochondria in confocal imaging planes of adult cardiac myocytes in physiological conditions, and quantitatively characterize single tPTP openings in mitochondria by measuring [Ca2+]mito and ΔΨm. These MitoWinks are quite rare, last for ~57 s, do not allow solutes >600 Da through (e.g. not NADH) and are modulated by Ca2+, reactive oxygen species (ROS), CypD and CsA like larger pPTP events.17, 18 MitoWinks may serve a physiological role to protect cells against mitochondrial Ca2+ overload or alleviate the cells from accumulated ROS damage.
METHODS
Detailed Methods are in the Online Supplement at http://circres.ahajournals.org.
RESULTS
Transient PTP openings in single mitochondria during cyclical SR Ca2+ release
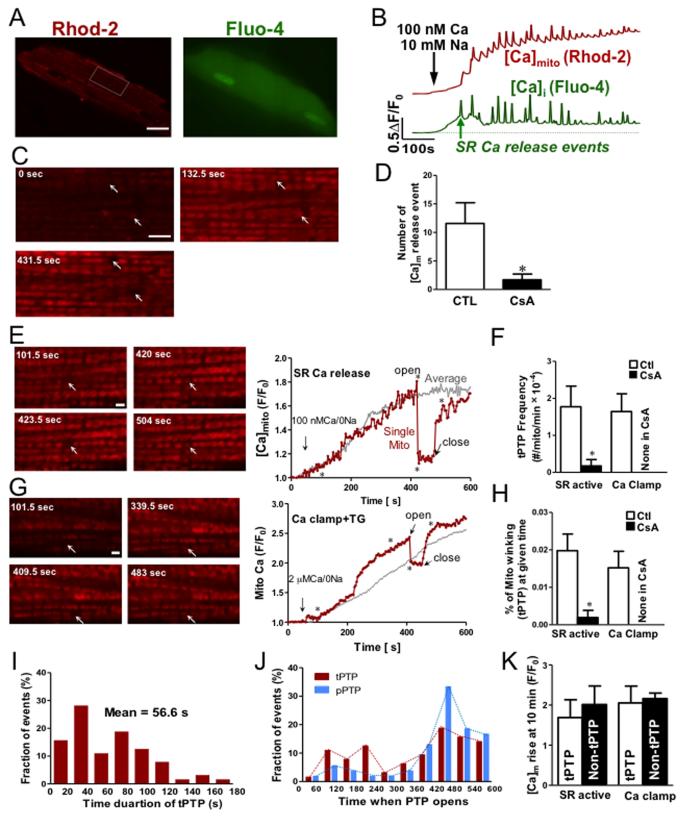
To simultaneously assess mPTP-mediated transient Ca2+ release events in 500-1,000 individual mitochondria in situ, we monitored [Ca2+]mito using Rhod-2 and 2-D confocal microscopy in cardiac myocytes during spontaneous sarcoplasmic reticulum (SR) Ca2+ releases (Fig 1A-B). Our previously validated method19 uses acutely saponin-permeabilized adult ventricular myocytes with physiological intracellular solutions with light Ca2+ buffering (50 μmol/L [EGTA]). Raising [Ca2+]i to 100 nmol/L (Fig 1B) induces spontaneous SR Ca2+ release events (Ca2+ waves) at 5-15 min−1, which creates physiologically relevant Ca2+ releases in a well-controlled system. As [Ca2+]i rises and Ca2+ waves occur [Ca2+]mito rises with each Ca2+ wave, but [Ca2+]mito decline (mainly via Na/Ca exchange, mNCX) is slow, allowing progressive [Ca2+]mito rise. During this protocol individual mitochondria stochastically and suddenly released Ca2+ (Fig 1C), and these events were suppressed by the mPTP inhibitor cyclosporine A (CsA; Fig 1D) and sanglifehrin A (Online Figure II). This demonstrates mPTP-mediated events under relatively physiological conditions.
Figure 1.
Transient PTP openings in single mitochondria. (A) Images of permeabilized cardiac myocyte loaded with Rhod-2 (red) and Fluo-4 (green) (left panel). Scale bar =16 μm. (B) Time course of [Ca2+]mito and [Ca2+]i signals in the same cardiac myocyte, showing mitochondrial Ca2+ uptake (red) induced by cyclical SR Ca2+ release (green) whose time course is not fully captured at this sampling rate. (C) Enlarged images from the indicated myocyte region in A. The 3 frames are from the times indicated along the traces in B (arrows indicate individual mitochondria (or pairs) displaying [Ca2+]mito release (Scale bar =4 μm). (D) Number of mitochondrial Ca2+ release events are decreased by presence of CsA (n=7). (E, G) Transient Ca2+ release events in individual mitochondria, during SR Ca2+ release and Ca2+ clamp protocols. Images are taken from times marked by * during time courses at right. Arrows mark individual (or pairs) of mitochondrial that displayed transient changes in [Ca2+]mito (Scale bar =2 μm). (F) Frequency of tPTP events in the absence and presence of CsA. (H) Percentage of mitochondria in tPTP openings at any time (based on tPTP frequency, mean open duration and total number of mitochondria observed). Histograms of tPTP open duration (I) and time at which tPTP and pPTP openings were observed (J). (K) Amplitude of [Ca2+]mito rise at 10 min for cells that exhibited tPTP vs. those that did not (measured using SR Ca2+ release protocol).
To further examine these events, we used Na+-free internal solution to inhibit mNCX. Fig 1E shows images at times (*) during the [Ca2+]mito trace. While average [Ca2+]mito rises progressively, the highlighted mitochondrion rapidly releases Ca2+ (likely PTP opening) and ~60 s later Ca2+ reuptake resumes. This implies PTP closure and restored ΔΨm (which drives Ca2+uptake). Figure 1G shows similar results using a different protocol, where [Ca2+]i was raised from 0 to 2 μmol/L Ca2+ with [Ca2+]i clamped using 0.5 mmol/L EGTA, and SR function suppressed by 5 μmol/L thapsigargin. This is a typically used protocol, and may simulate tonic cellular Ca2+ loading. The frequency of putative tPTP events was similar for both SR release and Ca-clamp protocols, and in both cases 10 μmol/L CsA inhibited the events, consistent with them being tPTP openings (Fig 1F). These events are rare (~2×10−4 per mitochondria per min) under basal conditions, and our ability to monitor nearly 1,000 individual mitochondria continuously for 10 min was critical for observing these events. The average duration of these tPTP openings is 57 ± 5 s (Fig 1I), and this allows us to quantify that only 0.02% of the myocyte mitochondria experience this tPTP at any moment under physiological conditions (Fig 1H). This means that 99.98% of the mitochondria are busy making ATP, while a tiny percent might be “resting” or resetting (and may be consuming ATP). But this is a quantitatively negligible energetic drain for the myocyte. This agrees with inferences about tPTPs in isolated mitochondrial suspensions where individual events cannot be seen.13
In records where we saw 64 tPTP events we also observed 54 Ca2+ release events that never recovered, and we interpret those as likely permanent PTP events (pPTP). There was some tendency for both tPTP and pPTP to occur later in the 10 min observation period Fig 1J), especially for pPTP (81% were in the last 4 min). In some myocytes we saw no tPTPs, which is a logical consequence of their stochastic rarity, because those cells showed no difference in the [Ca2+]mito reached at the end of the protocol (Fig 1K).
Mitochondrial depolarization accompanies tPTP opening
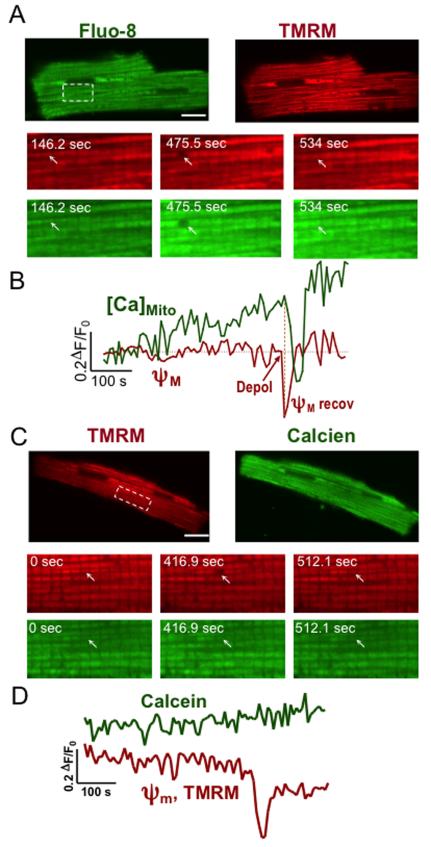
Brief mPTP opening should depolarize ΔΨm and cause mitochondrial Ca2+ release,13, 20 but it was also proposed that Ca2+ enters mitochondria via a sub-conductance mPTP opening associated with partial ΔΨm depolarization.21
We monitored [Ca2+]mito (with Fluo-8 AM) and ΔΨm (with tetramethylrhodamine methylester, TMRM) simultaneously, during protocols as in Fig 1E. Figure 2A-B shows a tPTP event in which rapid ΔΨm depolarization is followed by [Ca2+]mito decline, and then when tPTP closes, proton pumping via cytochromes restores ΔΨm and that allows Ca2+ uptake to resume. Thus tPTP opening rapidly dissipates ΔΨm allowing electrochemically downhill Ca2+ efflux, but the mitochondrion retains functionality, as manifest by ΔΨm recovery. Moreover, simultaneous monitoring of mitochondrial redox state (FAD autofluorescence) and ΔΨm (with TMRM), shows FADH2 oxidation upon pore opening, with gradual reduction on closure (Online Figure I). Hence, during tPTP opening, the mitochondria retain key matrix metabolites that allow respiratory recovery and repolarization once the pore closes, and this differs from pPTP. One possibility is that tPTP openings have lower pore size vs. sustained pPTP openings (in which molecules of 1,500 Da can permeate).13
Figure 2.
Transient PTP openings cause temporary mitochondrial depolarization (ΔΨm) and do not allow calcein permeation. (A) Colocalization of the ΔΨm indicator TMRM (red) and mitochondrial Ca2+ indicator Fluo-8 (green) in permeabilized cardiac myocytes. Enlarged images are from a portion of the above myocyte at times indicated. (B) Traces were obtained from an individual mitochondrion (or pair), as indicated by white arrows. Depolarization preceded Ca2+ loss and gradual repolarization preceded Ca2+ refilling. (C) Similar format for simultaneous mitochondrial ΔΨm indicator (TMRM) and calcein in a permeabilized cardiac myocyte, with enlarged images showing individual mitochondrion (or pair; arrows) in which depolarization and calcein were simultaneously measured (D). Scale bar=16 μm. The SR Ca2+ release protocol (Fig 1) was used.
Since we see full [Ca2+]mito recovery, it is clear that Rhod-2 (MW 869 Da) does not leave the mitochondrial matrix during tPTP opening. To further test the molecular weight cutoff for tPTP, we loaded myocytes with calcein AM and TMRM to monitor ΔΨm and tPTP opening. Fig 2C shows that during tPTP opening, calcien (MW 623 Da) was retained. This indicates that tPTP pore size freely allows ions (protons and Ca2+) to pass through, but prevents efflux of molecules > 600 Da (e.g. NADH, MW 663; FADH2, MW 786). This helps to explain why tPTP openings appear completely reversible and conserve mitochondrial functionality.
Ca2+ and reactive oxygen species (ROS) favor tPTP activity
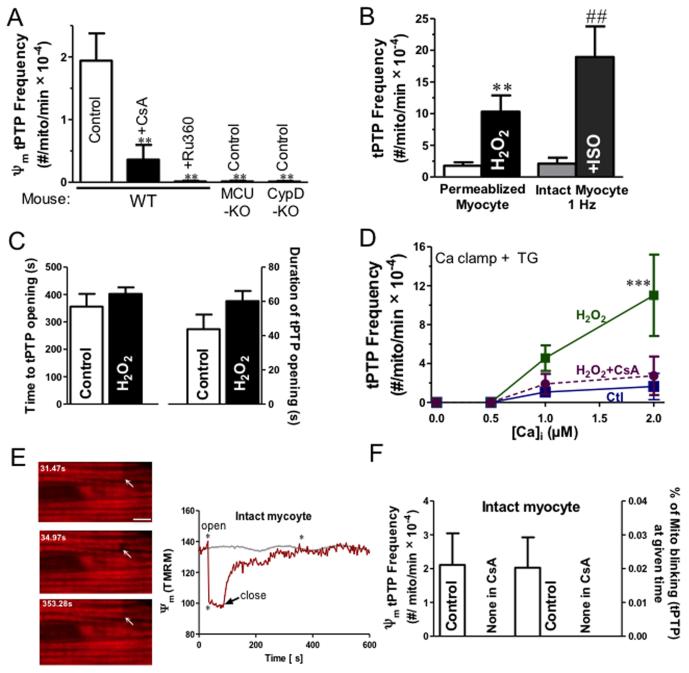
Permanent PTP openings in isolated mitochondria are known to be triggered by increasing matrix Ca2+ and by ROS.13, 14 We tested the involvement of [Ca2+]mito and H2O2 in tPTP activation (Fig 3). The predominant Ca2+ influx pathway in mitochondrial is the mitochondrial Ca2+ uniporter (MCU). When we either inhibit MCU pharmacologically (Ru360) or by genetic deletion (MCU-KO) tPTP events are strongly suppressed during SR Ca2+ releases (Fig 3A). This is consistent with our observation that there are no detectable tPTP openings in Ca2+-free solution in the absence of SR function (Fig 3D). Cyclophilin D (CypD) is known to be an important facilitator of mPTP opening.22 Either pharmacological inhibition of CypD by CsA or genetic ablation of the CypD gene abolished tPTP openings during spontaneous SR Ca release (Fig 3A). So [Ca2+]mito is required, and CypD is essential for tPTP.
Figure 3.
Ca2+, ROS and CypD are involved in tPTP opening. (A) Frequency of tPTP openings (using TMRM to assess ΔΨm) in wild-type (WT) mice in the presence and absence of Ru360 or CsA, and in MCU- and CypD-knockout (KO) mice (**P<0.01, vs CTL, n=6-9). Data were acquired from both permeabilized (with the SR Ca2+ release protocol, as in Fig 1. Influence of 1 μmol/L H2O2 on tPTP frequency) and intact cells (at 1Hz pacing frequency in the absent and the present of ISO) (B), and the time point at which tPTP opening occurred and its duration of opening (C; n= 6-7). (D) Frequency of tPTP openings as a function of [Ca2+]i with and without 1 μmol/L H2O2 (± CsA; n=5-8 for each [Ca2+]i and Ca2+clamp treatment). (E) Transient ΔΨm depolarization in individual mitochondria, during 1Hz pacing in intact myocytes. Images are taken from times marked by * during time course at right. Arrows mark mitochondrion (or pair) that displayed transient ΔΨm changes (Scale bar =4 μm). (F) Frequency of intact myocyte tPTP events and percentage of mitochondria in tPTP openings at any time (±CsA).
ROS, including H2O2 can greatly sensitize the mPTP to Ca.23 Fig 3B shows that even low [H2O2] (1 μmol/L) increased tPTP opening frequency 6-fold (10.0 ± 2.6 vs. 1.6 ± 0.6, H2O2 vs. CTL, **p<0.05), but there were no changes in the average time point at which the pore opened (395 ± 45s vs. 463 ± 24s, CTL vs. H2O2) or the duration of pore opening (52 ± 8 s vs. 45 ± 6 s, CTL vs. H2O2) (Fig 3B-D). Moreover, the frequency of tPTP opening as a function of [Ca2+]i shows that H2O2 increased mPTP sensitivity for Ca (***P<0.001, Figure 3D). Taken together, our data indicate that Ca2+ and moderate oxidative stress via H2O2 synergize in tPTP opening.
To test whether similar tPTP openings could be observed in intact myocytes, we measured CsA-sensitive ΔΨm in electrically stimulated myocytes (1 Hz). The frequency and duration of tPTP openings was comparable in intact vs. permeabilized myocytes (Fig 3B,F vs. Fig 1F,H,I). These results are consistent with our baseline permeabilized myocyte data reflecting basal physiological levels of tPTP openings. Increasing stimulation frequency from 2 to 4 Hz tended to increase tPTPs (1.0 ± 0.6 vs. 5.5 ± 1.1, not significant in ANOVA; Online Figure IIIA), but β-adrenergic stimulation with isoproterenol significantly increased tPTP opening at 1 Hz (nearly 10-fold), and also reduced tPTP duration by ~50% (Fig 3B and Online Figure III). Thus, increased work or stress can favor tPTP opening. Ca2+- and ROS-induced tPTP are increased in heart failure.
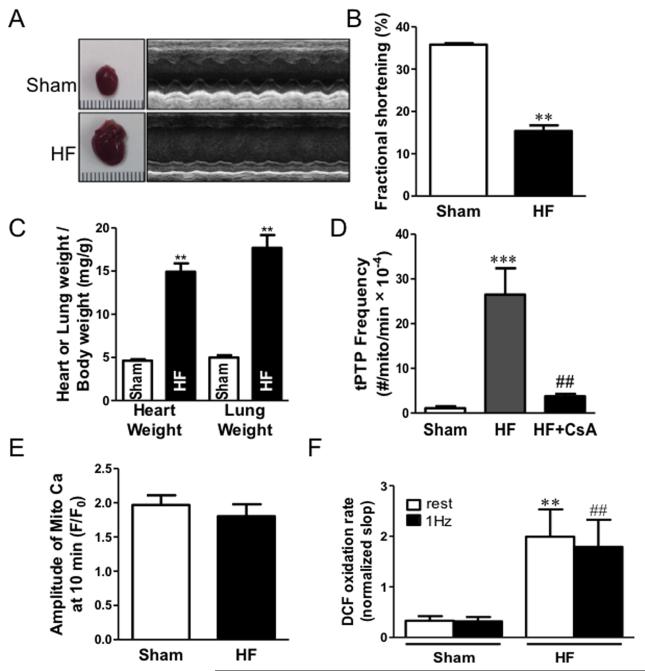
The failing heart exhibits dysregulation of myocyte Ca2+ handling and increased oxidative stress, which could favor tPTP opening. We measured tPTP in HF myocytes. HF was induced by transverse aortic constriction. Systolic function was substantially depressed after 6-8 weeks assessed by echocardiography (Fig 4A-B) and hearts were enlarged (heart: body weight) with pulmonary congestion (lung: body weight; Fig 4C). This is the stage at which we tested tPTP in myocytes.
Figure 4.
Heart failure increases tPTP openings. (A) Characterization of transverse aortic constriction induced HF model in mouse. Heart morphology and function were determined by measuring heart size, echocardiography indices (B) Fractional shortening), heart weight/body weight, and lung weight/body weight ratios (**P<0.01, n=5 hearts; C). (D) Frequency of tPTP openings increased in HF, and could be inhibited by applying CsA. (1.09 ± 0.42 vs. 26.5 ± 5.9 vs. 3.74 ± 0.56, Sham, HF, HF+CsA, ***P<0.001 vs Sham, ##P<0.01 vs HF treatment, n=5-7 cells) (E) Amplitude of [Ca2+]mito rise in sham and HF during 10 min (n=7). (F) Peroxide generation in sham and HF during rest and 1Hz pacing (**P<0.01, n=10-11).
Using the SR Ca release protocol as in Fig 1E, HF vs. sham myocyte exhibited many more tPTP openings, and these could be suppressed by CsA (Fig 4D). To test which factor might be responsible for higher tPTP during HF, we measured mitochondrial Ca2+ uptake and ROS in sham and HF myocytes. The amplitude of [Ca2+]m rise was the same between sham and HF (1.83 ± 0.17 vs. 1.92 ± 0.14, HF vs. Sham; Fig 4E). However, the ROS sensor DCF (2′,7′-dichlorodihydrofluorescein diacetate) indicated that the rate of ROS formation in HF cells was significantly higher than sham, both at rest and during 1 Hz pacing (Fig 4F). Given the potent effects of ROS on tPTP (Fig 3B and D), we suspect that the high tPTP rate in HF could be mediated by increased ROS production. It is possible that the increased number of tPTPs in HF is part of a physiological self-repair mechanism of mitochondria in HF that limits injury to individual mitochondria.
DISCUSSION
The notion of tPTP as a lower conductance and more reversible manifestation of the well-studied and pathological permanent PTP has been suggested by prior work on isolated mitochondria populations,8, 13, 14 and sub-conductance mPTP openings during in vitro voltage clamp.24-26 Mitochondrial superoxide flashes may also involve transient mPTP opening,27 but those events differ in allowing rhod-2 loss (900 Da molecules) and occur at very low [Ca2+]. Here we directly demonstrate physiological tPTP openings in individual mitochondria in situ in cardiac myocytes, measure their duration (~60 s) and pore size cutoff (not allowing 600 Da molecules), both of which are quite distinct from pPTP. However, we also found tPTP to have sensitivity to CsA, CypD, MCU blockade, [Ca2+]i, [Ca2+]mito, H2O2 that are similar to pPTP. Thus, our working hypothesis is that tPTP openings (or MitoWinks) share much of the molecular mechanism and machinery that is involved in the more extensively studied pPTP. Bernardi and colleagues suggested that dimeric F0F1-ATP synthase (complex V) serves also as the pPTP itself,6, 28 but whether this applies to tPTP will require better resolution of detailed molecular basis of pPTP. Disruption of a reported matrix electrical coupling between mitochondria29, 30 might contribute to tPTP, but individual mitochondrial function is the simplest interpretation of these MitoWinks.
An attractive possibility is that tPTP is simply an intermediate state on the transition to pPTP, exhibiting smaller channel pore and greater reversibility. This could be functionally analogous to the kiss-and-run hypothesis of partial, low-conductance vesicle fusion to plasma membrane during exocytosis and neurosecretion.31, 32 We did not observe transitions from tPTP to pPTP, but those might only reasonably be seen as a delayed calcein or Rhod-2 release after ΔΨm depolarization. Since tPTP events are so rare, we cannot unequivocally assess this.
We infer that tPTP openings occur in single mitochondria, but confocal resolution limitations mean that fluorescence from other nearby mitochondria can influence signals in our mitochondrion-sized region of interest. Figure 1G is illustrative. At ~220 s the local [Ca2+]mito rises steeply, which could reflect a burst of Ca2+ influx into one mitochondrion, or a second mitochondrion in close proximity. When the tPTP opening occurs (at 400 s) the [Ca2+]mito decline is incomplete. One likely interpretation is that the total fluorescence is from two (or three) individual mitochondria, only one of which exhibits a MitoWink. This spatial constraint does not influence our conclusions.
A major point is that unlike pathological pPTP, these rare tPTP openings are somehow beneficial to a mitochondrion, by allowing a physiological reset by release of excess Ca2+ and perhaps other accumulated harmful factors, but without losing key larger molecules and without harming cell-wide ATP production. That conclusion is clearly appropriate under our quasi-physiological resting conditions where only 0.02% of mitochondria are simultaneously depolarized in this tPTP mode. This percentage increases with mild ROS exposure (6-fold), 1 Hz pacing with isoproterenol (10-fold) and in basal HF myocytes (24-fold), such that nearly 0.5% of cellular mitochondria would be non-functional at any time. Note also that during a tPTP mitochondria would consume rather than make ATP (via F0F1-ATPase),33 which would increase the functional cost of tPTPs on ATP production. So, under in vivo high work-loads combined with pathological stresses, tPTP frequency might become high enough to limit ATP production. Like in control myocytes, there were comparable numbers of tPTP and pPTP openings in HF during the 10 min observation. Thus with the parallel rise in pPTP events in HF or other pathologies, the functional consequences would be further exacerbated, because the pPTP (vs. tPTP) openings are permanent and cumulative.
So, in an individual cardiac mitochondrion how many tPTP events might occur during its normal turnover lifetime (estimated at 17 days34)? Our measurements imply one tPTP every 3 to 83 hr (basal HF vs. control), but again this could be more frequent under in vivo stress. Moreover, if these tPTPs represent the turning point between beneficial refreshment vs. a pPTP and mitochondrial death, it will be important to understand these events in more detail.
We were surprised that tPTP openings lasted ~60 s, thinking that less time would be required to release Ca2+. While it often required >10 s for [Ca2+]mito to reach a minimum, the longer duration might also allow other, beneficial effects to occur to help reset that mitochondrion. Another, possibly related surprise, was that [Ca2+]mito typically rose faster after tPTP closure than it had before. We propose that during tPTP openings mitochondria free [Ca2+]mito drops rapidly, but Ca2+ buffers diffuses out more slowly. Then upon tPTP closure, the same low Ca2+ influx rate would raise [Ca2+]mito faster (i.e. with less intra-mitochondrial Ca2+ buffering). This agrees with slow rises in mitochondrial Ca2+ buffering power during Ca2+ uptake in isolated mitochondrial, which was attributed to slow uptake of phosphates.35 We found that longer tPTP durations had faster subsequent [Ca2+]mito recovery (Online Fig IV), consistent with this idea. The lack of PTP re-opening as [Ca2+]mito recovers might also be due to loss of mitochondrial Ca2+ bound phosphate and polyphosphate, that may both promote PTP opening.21, 35 Hence, tPTPs may also reset mitochondrial Ca2+ buffering in a way that restores acute responsiveness of Ca-dependent dehydrogenase activation during cytosolic Ca2+ changes, while limiting further PTP events.
This characterization of individual MitoWinks in cardiac myocytes demonstrates a potentially beneficial physiological restorative mPTP event, which contrasts functionally with pPTP openings (that lead to mitochondrial and cell death). These initial studies pave the way for future studies that may define the explicit molecular mechanism (e.g. are tPTP and pPTP different functions of the same proteins?) and how tPTP openings integrate into normal mitochondrial function.
Supplementary Material
Novelty and Significance.
What Is Known?
The high-conductance and permanent opening of mitochondrial permeability transition pore (pPTP) causes mitochondrial injury and cell death.
Unlike permanent opening of PTP, transient and low--conductance opening of PTP (tPTP) was proposed as protective against pathological Ca overload.
Direct characterization of tPTP opening in cardiac mycoytes still remained unclear.
What New Information Does this Article Contribute?
tPTP opening in cardiac myocytes is rare (only 0.02% of mitochondria is in tPTP at any given time), last for ~57 s, and respond to PTP regulatory factors like Ca, reactive oxygen species, cyclophilin D and cyclosporine A.
When tPTP opens, molecules > 600 Da can’t pass through (smaller pore than full PTP), and mitochondria can retain small metabolic molecules (e.g. NADH) during tPTP.
tPTP opening frequency increased under higher work or pathological conditions, which could be a physiological mitochondrial self--repair mechanism.
Characterization of tPTP in cardiac mycoytes suggests a physiologically beneficial role of tPTP opening (vs. full pPTP opening).
Metabolism in individual mitochondria is regulated during EC coupling. Opening of long -lasting mitochondrial permeability transition pore (pPTP) causes mitochondria injury, however, transient mPTP openings (tPTP) may protect against cardiac stress. In this study, we visualized the tPTP event in individual mitochondrion directly as transient drops in mitochondrial [Ca2+] ([Ca2+]mito) and voltage ((ΔΨmito), by simultaneous live imaging of 500--1000 mitochondria in situ in adult cardiac myocytes. We quantitatively characterize for the first time key properties of these tPTP (open duration, pore size and regulation) that may be physiologically beneficial in resetting mitochondria. The full recovery of [Ca2+]mito and (ΔΨmito), and the small pore size are in striking contrast to the well--studied permanent PTP openings that lead to mitochondrial dysfunction and cell death. However, the Ca2+, ROS, cyclophilin D and cyclosporine A sensitivity of tPTP resemble those of pPTP. We conclude that these new tPTP openings are mediated by the same molecular components as pPTP, but instead of being the harbinger of death, are beneficial for mitochondrial (and cell) survival by allowing individual mitochondria to reset themselves with negligible overall energetic cost.
Acknowledgments
SOURCES OF FUNDING
The study was supported by National Institutes of Health grants NIH P01- HL080101, R01-HL30077 and AHA 13PRE16260016.
Nonstandard Abbreviations and Acronyms
- [Ca2+]mito
mitochondrial free Ca2+ concentration
- mPTP
mitochondrial permeability transition pore
- tPTP
transient mode of mitochondrial permeability transition pore opening
- pPTP
permanent opening of mitochondrial permeability transition pore
- ISO
isoproterenol ROS reactive oxygen species
- CypD
cyclophilin D
- CsA
cyclosporine A
Footnotes
DISCLOSURES
None.
REFERENCES
- 1.Harris DA, Das AM. Control of mitochondrial ATP synthesis in the heart. Biochem J. 1991;280(Pt 3):561–73. doi: 10.1042/bj2800561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lesnefsky EJ, Moghaddas S, Tandler B, Kerner J, Hoppel CL. Mitochondrial dysfunction in cardiac disease: ischemia--reperfusion, aging, and heart failure. J Mol Cell Cardiol. 2001;33:1065–89. doi: 10.1006/jmcc.2001.1378. [DOI] [PubMed] [Google Scholar]
- 3.Bernardi P, Broekemeier KM, Pfeiffer DR. Recent progress on regulation of the mitochondrial permeability transition pore; a cyclosporin-sensitive pore in the inner mitochondrial membrane. J Bioenerg Biomembr. 1994;26:509–17. doi: 10.1007/BF00762735. [DOI] [PubMed] [Google Scholar]
- 4.Haworth RA, Hunter DR. The Ca2+-induced membrane transition in mitochondria. II. Nature of the Ca2+ trigger site. Arch Biochem Biophys. 1979;195:460–7. doi: 10.1016/0003-9861(79)90372-2. [DOI] [PubMed] [Google Scholar]
- 5.Zamzami N, Marchetti P, Castedo M, Hirsch T, Susin SA, Masse B, Kroemer G. Inhibitors of permeability transition interfere with the disruption of the mitochondrial transmembrane potential during apoptosis. FEBS Lett. 1996;384:53–7. doi: 10.1016/0014-5793(96)00280-3. [DOI] [PubMed] [Google Scholar]
- 6.Bernardi P, Di Lisa F, Fogolari F, Lippe G. From ATP to PTP and back: A dual function for the mitochondrial ATP synthase. Circ Res. 2015;116:1850–1862. doi: 10.1161/CIRCRESAHA.115.306557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Griffiths EJ, Halestrap AP. Protection by Cyclosporin A of ischemia/reperfusion-induced damage in isolated rat hearts. J Mol Cell Cardiol. 1993;25:1461–9. doi: 10.1006/jmcc.1993.1162. [DOI] [PubMed] [Google Scholar]
- 8.Baines CP, Kaiser RA, Sheiko T, Craigen WJ, Molkentin JD. Voltage-dependent anion channels are dispensable for mitochondrial-dependent cell death. Nat Cell Biol. 2007;9:550–5. doi: 10.1038/ncb1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martin LJ, Gertz B, Pan Y, Price AC, Molkentin JD, Chang Q. The mitochondrial permeability transition pore in motor neurons: involvement in the pathobiology of ALS mice. Exp Neurol. 2009;218:333–46. doi: 10.1016/j.expneurol.2009.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hausenloy D, Wynne A, Duchen M, Yellon D. Transient mitochondrial permeability transition pore opening mediates preconditioning-induced protection. Circulation. 2004;109:1714–7. doi: 10.1161/01.CIR.0000126294.81407.7D. [DOI] [PubMed] [Google Scholar]
- 11.Saotome M, Katoh H, Yaguchi Y, Tanaka T, Urushida T, Satoh H, Hayashi H. Transient opening of mitochondrial permeability transition pore by reactive oxygen species protects myocardium from ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2009;296:H1125–32. doi: 10.1152/ajpheart.00436.2008. [DOI] [PubMed] [Google Scholar]
- 12.Elrod JW, Molkentin JD. Physiologic functions of cyclophilin D and the mitochondrial permeability transition pore. Circ J. 2013;77:1111–22. doi: 10.1253/circj.cj-13-0321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Korge P, Yang L, Yang JH, Wang Y, Qu Z, Weiss JN. Protective role of transient pore openings in calcium handling by cardiac mitochondria. J Biol Chem. 2011;286:34851–7. doi: 10.1074/jbc.M111.239921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ichas F, Jouaville LS, Mazat JP. Mitochondria are excitable organelles capable of generating and conveying electrical and calcium signals. Cell. 1997;89:1145–53. doi: 10.1016/s0092-8674(00)80301-3. [DOI] [PubMed] [Google Scholar]
- 15.Ramesh V, Sharma VK, Sheu SS, Franzini-Armstrong C. Structural proximity of mitochondria to calcium release units in rat ventricular myocardium may suggest a role in Ca2+ sequestration. Ann N Y Acad Sci. 1998;853:341–4. doi: 10.1111/j.1749-6632.1998.tb08295.x. [DOI] [PubMed] [Google Scholar]
- 16.Huser J, Blatter LA. Fluctuations in mitochondrial membrane potential caused by repetitive gating of the permeability transition pore. Biochem J. 1999;343(Pt 2):311–7. [PMC free article] [PubMed] [Google Scholar]
- 17.Halestrap AP. Mitochondria and reperfusion injury of the heart--a holey death but not beyond salvation. J Bioenerg Biomembr. 2009;41:113–21. doi: 10.1007/s10863-009-9206-x. [DOI] [PubMed] [Google Scholar]
- 18.Bernardi P, von Stockum S. The permeability transition pore as a Ca2+ release channel: new answers to an old question. Cell Calcium. 2012;52:22–7. doi: 10.1016/j.ceca.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andrienko TN, Picht E, Bers DM. Mitochondrial free calcium regulation during sarcoplasmic reticulum calcium release in rat cardiac myocytes. J Mol Cell Cardiol. 2009;46:1027–36. doi: 10.1016/j.yjmcc.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huser J, Rechenmacher CE, Blatter LA. Imaging the permeability pore transition in single mitochondria. Biophys J. 1998;74:2129–37. doi: 10.1016/S0006-3495(98)77920-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seidlmayer LK, Juettner VV, Kettlewell S, Pavlov EV, Blatter LA, Dedkova EN. Distinct mPTP activation mechanisms in ischaemia-reperfusion: contributions of Ca2+, ROS, pH, and inorganic polyphosphate. Cardiovasc Res. 2015 doi: 10.1093/cvr/cvv097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, Brunskill EW, Sayen MR, Gottlieb RA, Dorn GW, Robbins J, Molkentin JD. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature. 2005;434:658–62. doi: 10.1038/nature03434. [DOI] [PubMed] [Google Scholar]
- 23.Zorov DB, Juhaszova M, Sollott SJ. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol Rev. 2014;94:909–50. doi: 10.1152/physrev.00026.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petronilli V, Szabo I, Zoratti M. The inner mitochondrial membrane contains ion-conducting channels similar to those found in bacteria. FEBS Lett. 1989;259:137–43. doi: 10.1016/0014-5793(89)81513-3. [DOI] [PubMed] [Google Scholar]
- 25.Kinnally KW, Zorov D, Antonenko Y, Perini S. Calcium modulation of mitochondrial inner membrane channel activity. Biochem Biophys Res Commun. 1991;176:1183–8. doi: 10.1016/0006-291x(91)90410-9. [DOI] [PubMed] [Google Scholar]
- 26.Giorgio V, von Stockum S, Antoniel M, Fabbro A, Fogolari F, Forte M, Glick GD, Petronilli V, Zoratti M, Szabo I, Lippe G, Bernardi P. Dimers of mitochondrial ATP synthase form the permeability transition pore. Proc Natl Acad Sci U S A. 2013;110:5887–92. doi: 10.1073/pnas.1217823110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang W, Fang H, Groom L, Cheng A, Zhang W, Liu J, Wang X, Li K, Han P, Zheng M, Yin J, Wang W, Mattson MP, Kao JP, Lakatta EG, Sheu SS, Ouyang K, Chen J, Dirksen RT, Cheng H. Superoxide flashes in single mitochondria. Cell. 2008;134:279–90. doi: 10.1016/j.cell.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bernardi P, Di Lisa F. The mitochondrial permeability transition pore: molecular nature and role as a target in cardioprotection. J Mol Cell Cardiol. 2015;78:100–6. doi: 10.1016/j.yjmcc.2014.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang X, Sun L, Ji S, Zhao T, Zhang W, Xu J, Zhang J, Wang Y, Wang X, Franzini-Armstrong C, Zheng M, Cheng H. Kissing and nanotunneling mediate intermitochondrial communication in the heart. Proc Natl Acad Sci U S A. 2013;110:2846–51. doi: 10.1073/pnas.1300741110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Glancy B, Hartnell LM, Malide D, Yu ZX, Combs CA, Connelly PS, Subramaniam S, Balaban RS. Mitochondrial reticulum for cellular energy distribution in muscle. Nature. 2015;523:617–20. doi: 10.1038/nature14614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alabi AA, Tsien RW. Perspectives on kiss-and-run: role in exocytosis, endocytosis, and neurotransmission. Annu Rev Physiol. 2013;75:393–422. doi: 10.1146/annurev-physiol-020911-153305. [DOI] [PubMed] [Google Scholar]
- 32.Wu LG, Hamid E, Shin W, Chiang HC. Exocytosis and endocytosis: modes, functions, and coupling mechanisms. Annu Rev Physiol. 2014;76:301–31. doi: 10.1146/annurev-physiol-021113-170305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cross RL, Muller V. The evolution of A-, F-, and V-type ATP synthases and ATPases: reversals in function and changes in the H+/ATP coupling ratio. FEBS Lett. 2004;576:1–4. doi: 10.1016/j.febslet.2004.08.065. [DOI] [PubMed] [Google Scholar]
- 34.Kim TY, Wang D, Kim AK, Lau E, Lin AJ, Liem DA, Zhang J, Zong NC, Lam MP, Ping P. Metabolic labeling reveals proteome dynamics of mouse mitochondria. Mol Cell Proteomics. 2012;11:1586–94. doi: 10.1074/mcp.M112.021162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wei AC, Liu T, Winslow RL, O'Rourke B. Dynamics of matrix-free Ca2+ in cardiac mitochondria: two components of Ca2+ uptake and role of phosphate buffering. J Gen Physiol. 2012;139:465–78. doi: 10.1085/jgp.201210784. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.