Abstract
Heat shock protein 90 (Hsp90) is an ATP dependent molecular chaperone protein whose function is critical for maintaining several key proteins involved in survival and proliferation of cancer cells. PU-H71 (1) is a potent purine-scaffold based ATP pocket binding Hsp90 inhibitor which has been shown to have potent activity in a broad range of in vivo cancer models and is currently in Phase I clinical trials in patients with advanced solid malignancies, lymphomas and myeloproliferative neoplasms. In this report we describe the radiosynthesis of [124I]-PU-H71 (5); this was synthesized from the corresponding Boc-protected stannane precursor 3 by iododestannylation with [124I]-NaI using chloramine-T as an oxidant for 2 minutes, followed by Boc deprotection with 6 N HCl at 50 °C for 30 min to yield the final compound. The final product 5 was purified using HPLC and was isolated with an overall yield of 55 ± 6 % (n=6, isolated) from 3, and >98% purity and an average specific activity of 980 mCi/µmol. Our report sets the stage for the introduction of [124I]-PU-H71 as a potential non-invasive probe for understanding biodistribution and pharmacokinetics of PU-H71 in living subjects using PET imaging.
Keywords: iodine-124, PU-H71, purine, heat shock protein 90, PET, radiotracer, cancer, iododestannylation
Introduction
Radiolabeling of a targeted small molecule offers a powerful approach in cancer imaging and in better understanding key pharmacokinetic properties crucial for the successful development of novel anticancer agents. In this regard, they are potent tools to investigate biodistribution that is necessary to assess the clinical viability of an anticancer agent. Hsp90 is an ATP dependent molecular chaperone which facilitates folding of its client proteins to their proper conformation.1 Many of its client proteins are kinases and transcription factors (i.e. HER2, Raf-1, pAkt) involved in key signal transduction processes that are necessary for maintenance and survival of cancer cells.1–2 Because of this, as well as the ability to simultaneously target multiple aberrant signaling pathways, inhibition of Hsp90 has become an attractive target for the treatment of cancer.3 In fact, numerous compounds of different chemotypes are currently being evaluated in clinical trials for diverse cancers.4 Among these is PU-H71 (1), a purine-scaffold Hsp90 inhibitor, that has shown effectiveness in preclinical models of small cell lung carcinoma,5 triple-negative breast cancer (TNBC),6 hepatocellular carcinoma,7 diffuse large B-cell lymphomas,8 myeloproliferative disorders,9 Ewing sarcoma,10 viral cancers11 and acute myeloid leukemias.12 PU-H71 is currently in phase I clinical trials at Memorial Sloan Kettering Cancer Center (NCT01393509) and the National Cancer Institute (NCT01581541).
PU-H71 (1) contains an aryl iodide moiety native to its structure and therefore offers an ideal location for radiolabeling with iodine-124, as aryliodides are relatively more stable metabolically in comparison to alkyl iodides. Given the fact that the radiolabeled molecule 5 is chemically identical to the drug molecule, it may be utilized to obtain information on PU-H71 in vivo biodistribution. Iodine-124 with its relatively long half-life of 4.2 days allows the following of the biodistribution of tracer in vivo for several days. It also permits for non-invasive monitoring of PU-H71 biodistribution by positron emission tomography (PET) imaging. PET is a powerful technique with the capability of delivering quantitative information on the biodistibution of a radiotracer in a living subject with good spatial and temporal resolution. In addition, PET imaging offers a non-invasive and repetitive way for measuring tracer kinetics and distribution.13
In this report, we describe the radiosynthesis of [124I]-PU-H71 (5) as a radiotracer suitable for PET studies of its biodistribution in living subjects. [124I]-PU-H71 is currently being evaluated in a phase 0 clinical trial at Memorial Sloan Kettering Cancer Center (NCT01269593) for dosimetry estimation in patients with solid malignancy or lymphoma.
Experimental
PU-H71 was synthesized as previously reported.14 [124I]-NaI was prepared as previously described15 by the radiochemistry core at MSKCC. 1H spectra were recorded on a Bruker 500 MHz instrument. Chemical shifts are reported in δ values in ppm downfield from TMS as the internal standard. 1H data are reported as follows: chemical shift, multiplicity (s, singlet; d, doublet; t, triplet; q, quartet; br, broad, m, multiplet), coupling constant (Hz), integration. Low-resolution mass spectra were obtained on a Waters Acquity Ultra Performance LC with electrospray ionization and SQ detector. High-performance liquid chromatography (HPLC) analysis of 3 was performed on a Waters Autopurification system with PDA, MicroMass ZQ and ELSD detector and a reversed phase column (Waters X-Bridge C18, 4.6×150 mm, 5 µm). HPLC purification and analysis of [124I]-PU-H71 was performed on a Shimadzu HPLC system equipped with a binary high pressure gradient solvent delivery module LC 20AB and SPD-20A UV dual wavelength detector connected to a Bioscan flow-count radio-HPLC detector system using a specially configured photomultiplier tube with NaI scintillation crystal for gamma detection and a reversed phase column (Phenomenex Gemini NX C18, 4.6×250 mm, 5 µm). Column chromatography was performed using 230–400 mesh silica gel (EMD). Preparatory thin layer chromatography was performed using 1000 µm silica gel plates (Analtech).
tert-Butyl 3-(6-amino-8-(6-iodobenzo[d][1,3]dioxol-5-ylthio)-9H-purin-9-yl)propyl(isopropyl) carbamate [2]
100 mg (0.195 mmol) of 1, 29.5 mg (41 µL, 0.293 mmol) of triethylamine, 46.8 mg (0.215 mmol) of di-tert-butyl dicarbonate in 5 ml of CH2Cl2 was stirred overnight at rt. The solvent was removed under reduced pressure and the residue was purified by column chromatography on silica gel (CHCl3:EtOAc:hexane:MeOH-NH3 (7N), 20:10:20:2) to afford 2 (119 mg, 100%). 1H NMR (500 MHz, CDCl3) δ 8.33 (s, 1H), 7.30 (s, 1H), 6.90 (s, 1H), 5.99 (s, 2H), 5.49 (br s, 2H), 4.23 (m, 2H), 4.12 (m, 1H), 3.10 (m, 2H), 2.04 (m, 2H), 1.41 (s, 9H), 1.07 (d, J = 6.2 Hz, 6H); MS m/z 613.3 (M + H)+.
tert-Butyl 3-(6-amino-8-(6-(trimethylstannyl)benzo[d][1,3]dioxol-5-ylthio)-9H-purin-9-yl)propyl(isopropyl)carbamate [3]
25.3 mg (0.0413 mmol) of 2, 2.4 mg (0.00207 mmol) Pd(PPh3)4, 54.1 mg (34.3 µL,0.1652 mmol) hexamethylditin in 3 ml of dry dioxane was heated at 90 °C for 10.5 hr. The solvent was removed under reduced pressure and the residue was purified by preparatory TLC (CHCl3:EtOAc:hexane:MeOH-NH3 (7N), 2:1:2:0.5) to afford 3 (12 mg, 45%). 1H NMR (500 MHz, CDCl3) δ 8.27 (s, 1H), 7.01 (s, 1H), 6.99 (s, 1H), 5.98 (s, 2H), 5.59 (br s, 2H), 4.20 (m, 2H), 4.12 (m, 1H), 3.14 (m, 2H), 2.06 (m, 2H), 1.43 (s, 9H), 1.09 (d, J = 6.2 Hz, 6H), 0.28 (s, 9H); MS m/z 651.3 (M + H)+; LC-MS: H2O + 0.1% TFA: CH3CN + 0.1% TFA (5-95% in 10 min.) Rt = 9.43 min., m/z 651.1 (M+H)+.
Radiosynthesis of [124I]-PU-H71 [5]
25 µg of tin precursor 3 was dissolved in 20 µL of methanol and added to a vial containing a solution of radioactive sodium iodide [124I]-NaI in 0.1 N NaOH (40 µL). To the resulting solution, 15 µL of chloramine-T solution (1.5 mg/mL in acetic acid) was added and the vial was gently shaken and heated at 50 °C for 2 minutes to ensure complete radioiodination. The vial was allowed to cool to room temperature and 10 µL of methionine methyl ester (0.5 g/mL) in water and 10 µL of concentrated HCl were added and the solution was heated at 50 °C for 30 min for removal of Boc protecting group. The reaction mixture was cooled to room temperature and diluted with 45 µL of deionized water and purified using HPLC. Purification was carried out on a Gemini NX (Phenomenex, Torrance CA) C18 column (4.6×250 mm, 5µ) using 0.1% trifluoroacetic acid (A) and acetonitrile (B) under gradient conditions given below with a flow rate of 1 mL/min. The gradient conditions used are 20% B for 0–3 min; 20–80% B from 3–10 min; 80% B for 10–15 min; 80-20% B from 15–20 min. Under these conditions the product eluted with retention time of 9.7 min (Figure 1). The product was collected and the solvent removed under reduced pressure using a rotary evaporator. The final product was formulated in 5% ethanol in saline (0.9%) and passed through a 0.22 µm filter into a pyrogen free vial equipped with a sterile vent. A portion of the final formulation was withdrawn and used for quality control analysis. The purity was analyzed using HPLC under similar conditions and the identity of the radioactive compound was confirmed by co-elution with the non-radioactive standard using analytical HPLC. The average yields (isolated) of [124I]-PU-H71 were 55 (± 6) % with purity >98% (Starting from 1-4 mCi of [124I]-NaI, we obtained 0.6-2.1 mCi of pure product after HPLC purification). The specific activity of the final formulation was ~980 mCi/µmol.
Figure 1.
HPLC chromatogram of purified [124I]-PU-H71 (5).
Results and Discussion
Radioiodination by iododemetallation of stannane derivatives is a very convenient and high yielding process and it was chosen as our preferred method. For the synthesis of [124I]-PU-H71 (5) this requires access to a stannane precursor such as 3 (Scheme 1). This precursor can be conveniently obtained in two steps from PU-H71 (1, Scheme 1). 1 contains a secondary alkyl amine which can interfere during the synthesis of the corresponding trimethylstannyl derivative using palladium (0) catalyst resulting in unwanted side reactions. Therefore, the free amine was Boc-protected using standard conditions to give 2 in quantitative yield (Scheme 1). Boc-protected PU-H71 (2) was converted in 45% yield to the corresponding trimethylstannane derivative 3 via a Pd-mediated cross coupling with hexamethylditin using tetrakis(triphenylphosphine)Pd(0) as a catalyst (Scheme 1). The Boc-protected stannyl compound 3 was used as precursor for radioiodination.
Scheme 1.
Synthesis of stannane precursor 3. Reagents and conditions: a. Et3N, (Boc)2O, CH2Cl2, rt; b. Pd(PPh3)4, hexamethylditin, dioxane, 90 °C.
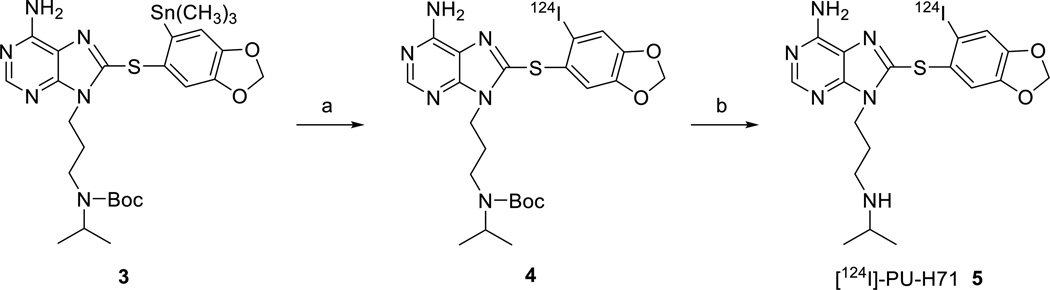
Radioiododestannylation of trimethyltin precursor 3 with [124I]-NaI in the presence of chloramine-T gave Boc-protected [124I]-PU-H71 (4, Scheme 2) in quantitative yield as indicated by HPLC. The radioiodinated Boc-protected derivative 4 was not isolated and was directly used for the subsequent deprotection reaction. Boc-deprotection was carried out using 6M HCl at 50 °C for 30 min to yield [124I]-PU-H71 (5, Scheme 2), which was purified using HPLC (Figure 1). The radioiodination was high yielding, however, unidentified byproducts were observed during the deprotection step which decreased the overall yields. The radiochemical yield of [124I]-PU-H71 was 55 ± 6 % (n=6, isolated), with purity >98% and average specific activity of 980 mCi/µmol.
Scheme 2.
Synthesis of [124I]-PU-H71. Reagents and conditions: a. [124I]-NaI, chloramine-T, 50 °C; b. 6M HCl, 50 °C.
Conclusions
[124I]-PU-H71 (5) was synthesized by iododestannylation of Boc-protected trimethyltin precursor 3 with [124I]-NaI in the presence of chloramine-T, followed by deprotection with 6M HCl and purified by HPLC in >50% overall yield and >98% purity. Since the chemical identity of the radiotracer is identical with the therapeutic agent, it may be used as a PET imaging agent for understanding biodistribution and pharmacokinetics of PU-H71 in living subjects.
Acknowledgments
This work was supported in part by P50-CA86438 (G.C., S.M.L.), R01 CA172546 (GC, NVP K, JSL), R01 CA155226 (GC), P50 CA192937 (GC), David Rubenstein Center for Pancreatic Cancer Research (GC and TT) and R03-BC085588 (G.C.). T Taldone acknowledges grant support from Susan G. Komen for the Cure, and the Department of Defense (PDF-BC093421) and A Bolaender from MSKCC Brain Tumor Center.
References
- 1.Trepel J, Mollapour M, Giaccone G, Neckers L. Nat. Rev. Cancer. 2010;10:537–549. doi: 10.1038/nrc2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whitesell L, Lindquist SL. Nat. Rev. Cancer. 2005;5:761–772. doi: 10.1038/nrc1716. [DOI] [PubMed] [Google Scholar]
- 3.Janin Y. Drug Discov. Today. 2010;15:342–353. doi: 10.1016/j.drudis.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 4.(a) Biamonte MA, Van de Water R, Arndt JW, Scannevin RH, Perret D, Lee WC. J. Med. Chem. 2010;53:3–17. doi: 10.1021/jm9004708. [DOI] [PubMed] [Google Scholar]; (b) Jhaveri K, Taldone T, Modi S, Chiosis G. Biochim. Biophys. Acta. 2012;1823:742–755. doi: 10.1016/j.bbamcr.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rodina A, Vilenchik M, Moulick K, Aguirre J, Kim J, Chiang A, Litz J, Clement CC, Kang Y, She Y, Wu N, Felts S, Wipf P, Massague J, Jiang X, Brodsky JL, Krystal GW, Chiosis G. Nat. Chem. Biol. 2007;3:498–507. doi: 10.1038/nchembio.2007.10. [DOI] [PubMed] [Google Scholar]
- 6.Caldas-Lopes E, Cerchietti L, Ahn JH, Clement CC, Robles AI, Rodina A, Moulick K, Taldone T, Gozman A, Guo Y, Wu N, de Stanchina E, White J, Gross SS, Ma Y, Varticovski L, Melnick A, Chiosis G. Proc. Natl. Acad. Sci. USA. 2009;106:8368–8373. doi: 10.1073/pnas.0903392106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Breinig M, Caldas-Lopes E, Goeppert B, Malz M, Rieker R, Bergmann F, Schirmacher P, Mayer M, Chiosis G, Kern MA. Hepatology. 2009;50:102–112. doi: 10.1002/hep.22912. [DOI] [PubMed] [Google Scholar]
- 8.Cerchietti LC, Lopes E-C, Yang SN, Hatzi K, Bunting KL, Tsikitas LA, Mallik A, Robles AI, Walling J, Varticovski L, Shaknovich R, Bhalla KN, Chiosis G, Melnick A. Nat. Med. 2009;15:1369–1376. doi: 10.1038/nm.2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.(a) Marubayashi S, Koppikar P, Taldone T, Abdel-Wahab O, West N, Bhagwat N, Lopes-Vazquez EC, Ross KN, Gönen M, Gozman A, Ahn J, Rodina A, Ouerfelli O, Yang G, Hedvat C, Bradner JE, Chiosis G, Levine RL. J. Clin. Invest. 2010;120:3578–3593. doi: 10.1172/JCI42442. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Bhagwat N, Koppikar P, Keller M, Marubayashi S, Shank K, Rampal R, Qi J, Kleppe M, Patel HJ, Shah SK, Taldone T, Bradner JE, Chiosis G, Levine RL. Blood. 2014;123:2075–2083. doi: 10.1182/blood-2014-01-547760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ambati SR, Lopes EC, Kosugi K, Mony U, Zehir A, Shah SK, Taldone T, Moreira AL, Meyers PA, Chiosis G, Moore MA. Mol. Oncol. 2014;8:323–336. doi: 10.1016/j.molonc.2013.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nayar U, Lu P, Goldstein RL, Vider J, Ballon G, Rodina A, Taldone T, Erdjument-Bromage H, Chomet M, Blasberg R, Melnick A, Cerchietti L, Chiosis G, Wang YL, Cesarman E. Blood. 2013;122:2837–2847. doi: 10.1182/blood-2013-01-479972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zong H, Gozman A, Caldas-Lopes E, Taldone T, Sturgill E, Brennan S, Ochiana SO, Gomes-DaGama EM, Sen S, Rodina A, Koren J, III, Becker MW, Rudin CM, Melnick A, Levine RL, Roboz GJ, Nimer SD, Chiosis G, Guzman ML. Cell Reports. doi: 10.1016/j.celrep.2015.10.073. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zanzonico PB, Nehmeh SA. Introduction to clinical and laboratory (small-animal) image registration and fusion. Conf Proc IEEE Eng Med Biol Soc. 2006;1:1580–1583. doi: 10.1109/IEMBS.2006.259649. [DOI] [PubMed] [Google Scholar]
- 14.He H, Zatorska D, Kim J, Aguirre J, Llauger L, She Y, Wu N, Immormino RM, Gewirth DT, Chiosis G. J. Med. Chem. 2006;49:381–390. doi: 10.1021/jm0508078. [DOI] [PubMed] [Google Scholar]
- 15.Sheh Y, Koziorowski J, Balatoni J, Lom C, Dahl JR, Finn RD. Radiochim. Acta. 2000;88:169–173. [Google Scholar]