Abstract
Serotonin (5-HT) is a potent neuromodulator with multiple receptor types within the cardiorespiratory system, including the nucleus tractus solitarii (nTS) - the central termination site of visceral afferent fibers. The 5-HT7 receptor facilitates cardiorespiratory reflexes through its action in the brainstem and likely in the nTS. However, the mechanism and site of action for these effects is not clear. In this study, we examined the expression and function of 5-HT7 receptors in the nTS of Sprague-Dawley rats. 5-HT7 receptor mRNA and protein were identified across the rostrocaudal extent of the nTS. To determine 5-HT7 receptor function, we examined nTS synaptic properties following 5-HT7 receptor activation in monosynaptic nTS neurons in the in vitro brainstem slice preparation. Application of 5-HT7 receptor agonists altered tractus solitarii evoked and spontaneous excitatory postsynaptic currents which were attenuated with a selective 5-HT7 receptor antagonist. 5-HT7 receptor-mediated changes in excitatory postsynaptic currents were also altered by block of 5-HT1A and GABAA receptors. Interestingly, 5-HT7 receptor activation also reduced the amplitude but not frequency of GABAA-mediated inhibitory currents. Together these results indicate a complex role for 5-HT7 receptors in the nTS that mediate its diverse effects on cardiorespiratory parameters.
Keywords: serotonin receptors, autonomic nervous system, patch clamp, EPSC, IPSC, respiration
1. INTRODUCTION
Serotonin (5-hydroxytryptamine, 5-HT) is a potent neuromodulator in the cardiorespiratory system, with varying and sometimes contradictory effects. Currently there are 14 known 5-HT receptors (5-HTRs) in 7 sub-families. With the exception of 5-HT3R, all of these receptors are G-protein coupled receptors that activate downstream pathways to elicit their physiological effects. The diverse effects of 5-HT are likely due, in part, to the many different 5-HTRs and their activated second messengers within the autonomic nervous and respiratory pathways.
The nucleus tractus solitarii (nTS) contains the first central synapses to receive visceral afferents from baroreceptors and peripheral chemoreceptors, is intrinsically O2 and CO2/pH sensitive, and has reciprocal connections to many central cardiorespiratory nuclei within the brainstem and forebrain (Accorsi-Mendonça and Machado, 2013; Dean, 2010; Kline, 2008; Matott et al., 2014). The nTS is densely innervated by 5-HT fibers originating from the ventral raphe (Thor and Helke, 1988), peripheral ganglia (Nosjean et al., 1990; Thor et al., 1988), and perhaps the nTS itself (Calzà et al., 1985). Several 5-HTRs have been anatomically and functionally identified in the nTS, including the 5-HT1, 2, 3, and 4 receptor subtypes (Raul, 2003). For instance, 5-HT1ARs are located postsynaptically on nTS neurons where they inhibit evoked and spontaneous excitatory and inhibitory postsynaptic currents (EPSCs and IPSCs) and decrease respiration (Ostrowski et al., 2014). Activation of 5-HT2R in the nTS lowers heart rate, blood pressure, and delays the hypoxic ventilatory response (Comet et al., 2007; Kanamaru and Homma, 2009), and activation of 5-HT2AR and 5-HT2CR augment nTS EPSCs (Austgen and Kline, 2013; Austgen et al., 2012). Presynaptic 5-HT3Rs augment nTS neurotransmission by increasing spontaneous glutamate release (Cui et al., 2012) as well as inhibit cardiac reflex responses (Sévoz et al., 1997; Weissheimer and Machado, 2007). 5-HT4Rs in the nTS attenuate cardiopulmonary reflexes (Edwards and Paton, 1999).
5-HT7R, the most recently discovered 5-HT receptor (Bard et al., 1993; Lovenberg et al., 1993; Ruat et al., 1993; Shen et al., 1993), has been demonstrated via in situ hybridization throughout the CNS, including nTS (Gustafson et al., 1996). 5-HT7R has been implicated to have functional or modulatory roles in respiratory (Hoffman and Mitchell, 2013, 2011; Nichols et al., 2012) and cardiovascular reflexes (Damaso et al., 2007; Jordan, 2005; Kellett et al., 2005a; Oskutyte et al., 2009). For example, block of 5-HT7R within the brainstem of anesthetized rats attenuated the vagal-mediated bradycardia evoked by the cardiopulmonary, baro- and chemoreflexes (Kellett et al., 2005a). This was confirmed in conscious rats where brainstem 5-HT7R blockade attenuated the bradycardia and pressor response to cardiopulmonary and peripheral chemoreceptor reflex activation (Damaso et al., 2007). The 5-HT1/7 agonist (5-carboxamidotryptamine, 5-CT) ionophoresed directly onto nTS neurons resulted in a mix of excitatory and inhibitory responses, as well as non-responding neurons (Oskutyte et al., 2009). 5-CT induced neuronal excitation, as well as vagal-afferent induced activity, were reduced or eliminated by 5-HT7R block. Taken together, these studies suggest 5-HT7R within the brainstem enhance several cardiorespiratory reflexes by augmenting neuronal discharge. Given the prominent role of the nTS in integrating visceral reflexes and the identification of 5-HT7R mRNA in this nucleus, the nTS is therefore a likely target for this effect. However, the synaptic mechanisms by which these 5-HT7R mediated effects in the nTS may occur are largely unknown. The goal of the present study was to determine the functional role of 5-HT7Rs on synaptic transmission in monosynaptic second-order nTS neurons.
2. RESULTS
2.1. 5-HT7R is present in the nucleus tractus solitarii
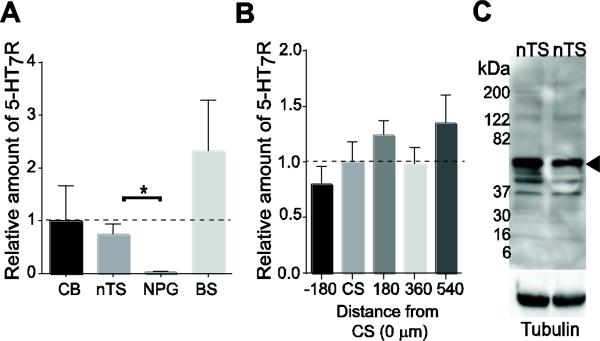
With the exception of one in situ hybridization study (Gustafson et al., 1996), the distribution or presence of 5-HT7R within the nTS has not been described. Therefore, we used RT-PCR and immunoblot to examine the presence and distribution of 5-HT7R mRNA and protein within the caudal nTS. 5-HT7R mRNA was localized in the nTS and was significantly greater compared to the nodose-petrosal ganglia (NPG), the location of visceral afferent somas (Fig 1A, p=0.023 paired t-test). There was not a significant difference in nTS mRNA expression relative to the cerebellum, where abundant 5-HT7R has been shown (Geurts et al., 2002; Kinsey et al., 2001). Likewise, brainstem tissue excluding nTS (medial to ventral brainstem) was not significantly different compared to that of the nTS. In order to determine if there was a pattern in distribution of 5-HT7 receptors within the nTS we examined the expression of 5-HT7R mRNA in paraformaldehyde fixed coronal sections. The rostral-caudal extent of these sections was inclusive of regions recorded electrophysiologically in this study, which include sub-nuclei involved in modulation and integration of cardiorespiratory reflexes. 5-HT7R mRNA was present throughout the rostrocaudal nTS examined with no significant differences among its distribution (Fig 1B). Lastly, immunoblots showed a protein band in the expected range for 5-HT7R (~54 kDa, Fig 1C). These data show 5-HT7R is expressed in the nTS.
Figure 1.
5-HT7R mRNA and protein are present in the nTS. A. Relative 5-HT7R mRNA levels normalized to cerebellum (CB) determined from RT-PCR. The amount of mRNA in the nTS is significantly higher than that found in the nodose-petrosal ganglia (NPG). General brainstem tissue (BS), excluding the nTS, was not different than cerebellum or nTS. ΔΔCT method, n=5 each. B. Relative 5-HT7R mRNA across the rostrocaudal nTS, normalized to calamus scriptorius (CS, 0 μm). ΔΔCT method, n=3 individual sections/level. C. Immunoblot from nTS samples (100 μg) showing band for 5-HT7R protein at expected 54 kDa (arrow). Tubulin loading control is shown below. n=2.
2.2. 5-HT7R activation modulates afferent evoked synaptic transmission
Given the prominent role that 5-HT plays in the modulation of synaptic transmission and cardiorespiratory parameters in the nTS (Comet et al., 2007; Jeggo et al., 2007; Jordan, 2005; Nalivaiko and Sgoifo, 2009; Ostrowski et al., 2014; Takenaka et al., 2011) as well as 5-HT7Rs apparent influence on cardiorespiratory reflexes (Kellett et al., 2005a, 2005b), we sought to determine the role of 5-HT7Rs on nTS synaptic properties. As such, two distinct 5-HT7R agonists, LP 44 (2, 10, and 100 μM) and AS 19 (1 and 10 μM), were used to examine evoked and spontaneous synaptic properties of monosynaptic neurons in the medial and commissural sub-nuclei of the nTS.
The mean baseline cellular parameters, based on values from 97 cells from 60 brainstem slices, consisted of a resting membrane potential (RMP) of −56 ± 1 mV, input resistance (Rin) of 670 ± 43 MΩ, and membrane capacitance of 19.8 ± 0.7 pF. Spontaneous excitatory postsynaptic currents (EPSCs) had amplitudes of −21 ± 1.1 pA and an instantaneous frequency of 23 ± 1.5 Hz. For tractus solitarius (TS) stimulation at 0.5 Hz, EPSC latency was 4.54 ± 0.18 ms, jitter was 182 ± 7 μs, and amplitude was −111.4 ± 6.5 pA. During vehicle time controls (aCSF or 0.01% DMSO in aCSF), all synaptic events (e.g. TS-EPSC amplitude, sEPSC amplitude and frequency, etc.) changed less than ±15% during our 20-25 minute recording period. Thus, cells that respond to 5-HT7R manipulation were defined as those which changed greater than 15% from their baseline for any given variable measured.
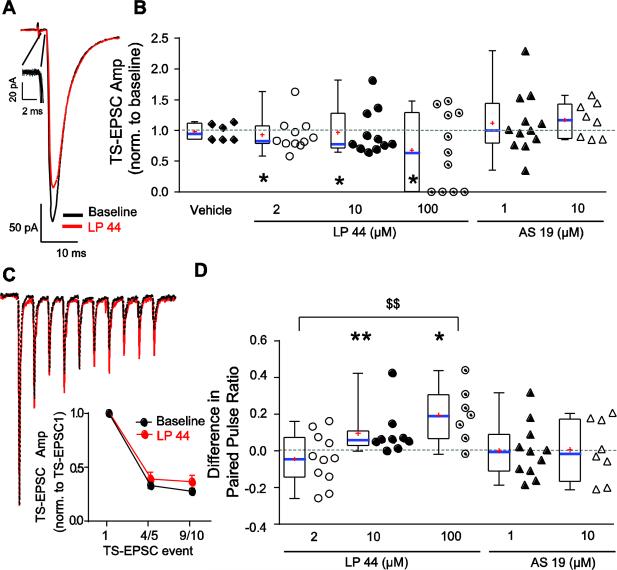
In the presence of LP 44, there were cells that increased and decreased TS-EPSC amplitude from baseline, as well as those that did not respond. Due to these offsetting responses of LP 44, across the three doses of LP 44 there were no significant changes overall from aCSF baseline in the amplitude of single TS-EPSCs. However, the predominant effect of LP 44 was to reduce TS-EPSC amplitude. Fig 2A is a representative example of decreased TS-EPSC amplitude in 10 μM LP 44 from baseline, whereas Fig 2B demonstrates the magnitude and direction of change for all cells tested. No significant increases in TS-EPSC were seen for any concentration of LP 44, yet those cells that reduced their amplitude with LP 44 did so significantly. Decreases in TS-EPSC amplitude were found across all LP 44 concentrations in similar proportions [55% (6/11) for 2, 10 and 100 μM]. Compared to its baseline, 2 μM LP 44 significantly reduced TS-EPSC amplitude for those that decreased (baseline, −109 ± 22 pA vs. LP 44, −81 ± 15 pA, n=6, Wilcoxon test p=0.03); only one neuron increased amplitude. Decreased responders in 10 μM LP 44 also significantly reduced TS-EPSC amplitude compared to baseline (baseline, −103 ± 35 pA vs. LP 44, −76 ± 28 pA, n=6, p = 0.03); three cells increased amplitude. During 100 μM, the TS-EPSC decreased in six neurons with the TS-EPSC completely and irreversibly eliminated in four of these, significantly reducing the mean amplitude (baseline, −121 ± 26 pA vs. LP 44, −13 ± 10 pA, n=6, p=0.03); four cells increased TS-EPSC amplitude.
Figure 2.
5-HT7R activation produces variable effects on TS-EPSCs but reduces synaptic depression. A. Example of representative TS-EPSCs. Traces are averages of 30 sweeps at 0.5 Hz TS stimulation frequency. Stimulus artifact truncated. Inset shows magnification of all 30 control sweeps with high-fidelity low jitter indicative of a monosynaptic recording. Note the decrease in TS-EPSC amplitude with 10 μM LP 44 (red) compared to baseline (aCSF, black). B. Box-and-whisker plot showing normalized TS-EPSC amplitude with vehicle time controls or 5-HT7R activation (LP 44 or AS 19), with respect to each cell's respective baseline (defined as “1”, dashed line). Median indicated by solid blue line, mean by red “+”, quartiles (25 and 75%) by boxes, and the range by the whiskers. Individual data points presented to right of respective box plot. All subsequent plots follow this formatting. The predominant LP 44 response, compared to vehicle and baseline, is a decrease in TS-EPSC amplitude (example shown in panel A). C. Example of representative TS-EPSCs (normalized to initial event) evoked at 20 Hz showing use dependent depression (UDD) under baseline (black) and 10 μM LP 44 (red). Traces are an average of 5 sweeps. Note less UDD following LP 44. Inset: 20 Hz group data normalized to initial TS-EPSC for 10 μM LP 44 (n=9). Events 4/5 and 9/10 are means of their respective TS-EPSCs. Note less UDD with LP 44. D. Box-and-whisker and scatter plot showing change in paired pulse ratio (PPR) with 5-HT7R activation, compared to baseline (denoted as “0”). Note that PPR increases with elevated doses of LP 44, with a significant difference between 100 and 2 μM LP 44. For panel B * p < 0.05 Wilcoxon test, LP 44 vs. its baseline. For panel D $$, p < 0.01, one way ANOVA; * p < 0.05 ** p < 0.01, paired t-test, LP 44 vs. its baseline.
The direction of amplitude change induced by LP 44 did not correlate with the cells’ baseline values for capacitance, holding current, Rin, jitter, latency, or TS-EPSC amplitude (p > 0.05, Pearson correlation). There was a significant decrease in Rin with 2 μM LP 44 (baseline, 694 ± 139 vs. LP 44, 581 ± 111 MΩ), but no difference for 10 μM (baseline, 456 ± 59 vs. LP 44, 445 ± 87 MΩ) or 100 μM LP 44 (baseline, 790 ± 130 vs. LP 44, 700 ± 107 MΩ).
Given the variability of responses to LP 44, we also examined synaptic currents in response to another 5-HT7R agonist, AS 19. While AS 19 is a high affinity 5-HT7R agonist, its affinity for the receptor is lower compared to LP 44 (Brenchat et al., 2009; Leopoldo et al., 2011). As with LP 44, 5-HT7R activation of nTS neurons by 1 and 10 μM AS 19 resulted in neurons which increased and decreased EPSC amplitude, as well as some that did not change. Overall, there was no significant effect of 1 μM AS 19 on 0.5 Hz TS-EPSC amplitude (baseline, −102 ± 18 pA vs. AS 19, −113 ± 28 pA), likely due to these variable responses. Of these recordings 42% (5/12) did not respond, 33% (4/12) increased TS-EPSC amplitude, and 25% (3/12) reduced amplitude (Fig 2B). There was no effect on Rin (baseline, 833 ± 100 vs. AS 19, 787 ± 114 MΩ). Likewise, for 10 μM AS 19 there was no overall significant effect on TS-EPSC amplitude (baseline, −95 ± 20 vs. AS 19, −107 ± 26 pA). Of these neurons, 38% (3/8) did not respond, 50% (4/8) increased, and 13% (1/8) decreased amplitude. There was no effect on Rin (baseline, 579 ± 49 vs. AS 19, 463 ± 152 MΩ). The increased and decreased responders for both concentrations of AS 19 were not significantly different from baseline. Therefore LP 44 was utilized for the majority of 5-HT7R experiments due both to its greater degree of response and greater affinity for 5-HT7R.
Repetitive stimulation that mimics a sustained increase in in vivo sensory activity progressively reduces TS-EPSC amplitudes following the first stimulation event. The magnitude of this use-dependent depression (UDD) during a prolonged stimulus train (Miles, 1986), and the paired pulse ratio between the second and first EPSC [PPR, TS-EPSC2/TS-EPSC1, (Kline et al., 2007)] are indicators of changes in presynaptic release. UDD of TS-EPSCs (20 Hz stimulation) was observed in all cells independent of LP 44 dose subsequently tested, consistent with other studies (Austgen et al., 2012; Miles, 1986). While 5-HT7R activation with LP 44 (10 μM) reduced the initial TS-EPSC amplitude in the majority of cells, it also showed a trend for decreased UDD compared to baseline (Fig 2D). The PPR increased with increasing concentrations of LP 44 (Fig 2D, one way ANOVA p=0.005). For 10 μM LP-44 the PPR increased from 0.40 ± 0.06 (baseline) to 0.51 ± 0.05 (paired t-test, p= 0.05) and for 100 μM increased from 0.40 ± 0.08 (baseline) to 0.60 ± 0.05 (p= 0.014). 2 μM LP 44 and 1 and 10 μM AS 19 did not alter the PPR. These data show that 5-HT7R activation (10 & 100 μM LP 44) reduces the initial TS-EPSC amplitude but has less effect on or even increases subsequent TS-EPSCs.
2.3. 5-HT7R activation does not alter spontaneous EPSCs
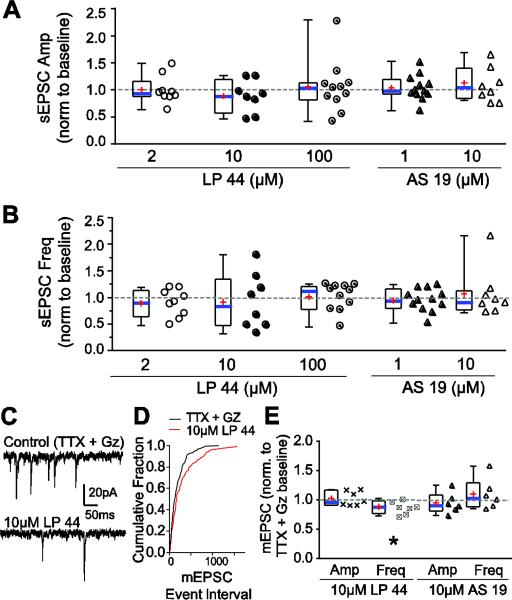
Spontaneous (s)EPSCs represent synaptic events that originate from all neurons which form a synapse on the recorded cell, and may represent network activity (Fortin and Champagnat, 1993). As with TS-EPSCs, sEPSC amplitude and instantaneous frequency overall did not change with 5-HT7R activation but resulted in some neurons that either increased or decreased in amplitude or frequency (Fig 3A, B). For the majority of cells, activation by 3 concentrations of LP 44 or 2 concentrations of AS 19 did not alter sEPSC amplitude or frequency greater than vehicle.
Figure 3.
5-HT7R activation does not alter spontaneous EPSCs but reduces miniature EPSC frequency. A, B. Box-and-whisker and scatter plots showing change in sEPSC amplitude (A) and frequency (B) with 5-HT7R activation by LP 44 (2, 10 or 100 μM) or AS 19 (1 or 10 μM). Data are normalized to each cells respective baseline (denoted as “1”, dashed line). C. Representative current traces showing mEPSCs in TTX and gabazine (Gz) control (top) and after 10 μM LP 44 in TTX and Gz (bottom). Duration of recording is 500ms. Note the decreased mEPSC frequency in response to LP 44. D. Cumulative fraction for inter-event interval from example in C showing an increased interval between events with LP 44. E. Box-and-whisker plots and scatterplots showing changes in mEPSC amplitude and frequency normalized to their TTX + Gz control, during 5-HT7R activation with LP 44 or AS 19. There is a significant decrease in mEPSC frequency (*, p=0.02, paired t-test, vs. TTX + Gz baseline) in response to LP 44.
As shown in Figure 3A, sEPSC amplitude during 5-HT7R activation decreased from their own baseline in 11% (1/9), 38% (3/8) and 27% (3/11) of cells whereas it increased in 22% (2/9), 25% (2/8), and 18% (2/11) of cells with 2, 10 and 100 μM LP 44, respectively. sEPSC amplitude during AS 19, compared to their respective baseline, decreased in 17% (2/12, 1 μM) and 25% (2/8, 10 μM), and increased in 25% (3/12, 1 μM) and 38% (3/8, 10 μM) of neurons. Likewise in Figure 3B, LP 44 decreased sEPSC frequency in 33% (3/9), 50% (4/8) and 27% (3/11) of cells, and increased frequency in 22% (2/9), 38% (3/8) and 45% (5/11, p=0.06 from baseline) of cells during 2, 10 and 100 μM LP 44, respectively. The frequency of sEPSCs during AS 19, compared to their baseline, decreased in in 25% [3/12 (1 μM) and 2/8 (10 μM)], and increased in 25% [3/12 (1 μM) and (2/8 (10 μM)] of neurons. sEPSCs in several cells did not respond to LP 44 or AS 19 by > 15%, and thus were considered non-responders (Fig 3A, B).
Change in sEPSC amplitude or frequency was not concentration dependent (Fig 3A, B). As such the cells were combined within an agonist and the potential correlations between 0.5 Hz TS-EPSC and sEPSC effects by 5-HT7R activation were examined. There was no correlation between 5-HT7R mediated change in amplitude between TS-EPSC and sEPSC. That is, a neuron that decreased 0.5 Hz TS-EPSC amplitude did not necessarily reduce sEPSC amplitude in response to LP 44 or AS 19. There was also no significant correlation between 5-HT7R activation changes in TS-EPSC amplitude and sEPSC instantaneous frequency. There was also no significant correlation between changes in sEPSC amplitude or instantaneous frequency with 5-HT7R activation. These results suggest that if 5-HT7Rs modulate nTS network activity it is independent of any effect on afferent-mediated modulation.
2.4. 5-HT7R activation by LP 44 reduces mEPSC frequency but not amplitude
To examine 5-HT7R activation on neurotransmitter release and receptor efficacy without the potential influence of network activity, miniature (m) EPSCs were recorded in the presence of 1 μM TTX and 25 μM gabazine (Gz) to block sodium channels and GABAA receptors, respectively. Subsequently, 10 μM LP 44 or AS 19 was used to activate 5-HT7Rs. As shown in the representative traces, 5-HT7R activation by LP 44 decreased the frequency of mEPSCs (Fig 3C). There was a significant rightward shift in the cumulative fraction of mEPSC intervals (Fig. 3D; p < 0.05, Kolmogorov-Smirnov, two-sample test) whereas mEPSC amplitudes did not change (not shown). The mean amplitude of the mEPSCs (−20 ± 4 pA, n=7) was unchanged between TTX + Gz baseline and LP 44. However, the mean mEPSC instantaneous frequency was slightly but significantly reduced from 22 ± 3 Hz in TTX + Gz to 20 ± 3 Hz with LP 44 (Fig 3E, paired t-test p=0.02, n=7). A decrease in mEPSC frequency by LP 44 was seen in 86% (6/7, two sample Kolmogorov-Smirnov test) of individual cells with the remaining cell showing no change in frequency. Activation of 5-HT7R with 10 μM AS 19 had no significant effect on either mEPSC amplitude or frequency (Fig 3E). These data provide evidence that 5-HT7R activation by LP 44 reduces mEPSC frequency but does not alter amplitude.
2.5. Effect of 5-HT7R blockade alone and with LP 44 on EPSCs
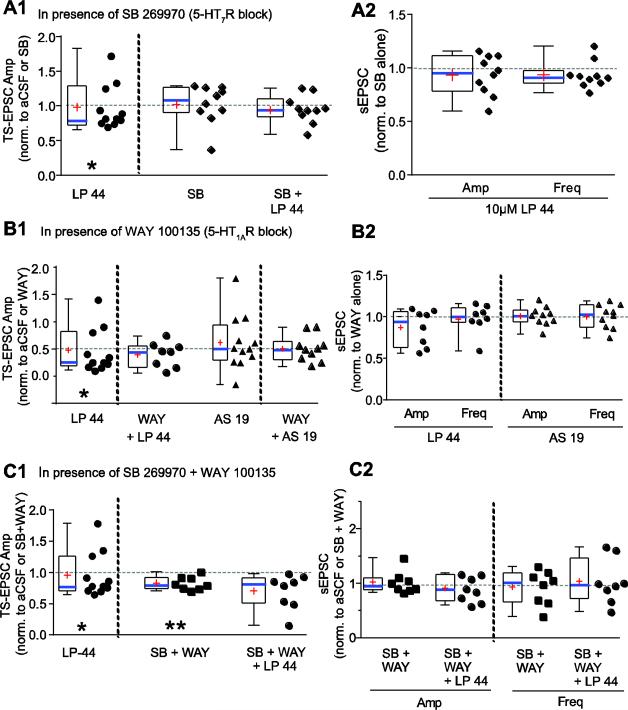
To determine if 5-HT7R tonically modulates synaptic transmission we used the selective antagonist SB 269970 (SB, 1 μM). Compared to aCSF baseline, antagonism of 5-HT7R did not alter mean TS-EPSC amplitude evoked at 0.5 Hz (baseline, −106 ± 10 pA vs. SB, −111 ± 14 pA, Fig 4A1), or sEPSC amplitude (baseline, −19 ± 3.5 pA vs. SB, −16 ± 2.3 pA) or sEPSC frequency (baseline, 29 ± 3 Hz vs. SB, 26 ± 2 Hz) when examined overall or segregated by those which decreased or increased their response. There were also no significant changes in UDD (not shown) or PPR (baseline, 0.57 ± 0.1 vs. SB, 0.57 ± 0.09) in response to SB 269970 alone. Rin also did not change (baseline, 577 ± 79 MΩ vs. SB, 579 ± 85 MΩ). These data suggest 5-HT7Rs do not tonically modulate synaptic transmission in nTS.
Figure 4.
Effect of 5-HT7R agonists in the presence of 5-HT7R and/or 5-HT1AR blockade. Responses to LP 44 or AS 19 in the presence of receptor blockers for (A) 5-HT7R, (B) 5-HT1AR or (C) 5-HT7R and 5-HT1AR for TS-EPSCs (1) or sEPSCs (2). For all panels, box-and-whisker and scatter plots show response of a treatment to its respective baseline (denoted as “1”, dashed line). A1. Changes in 0.5 Hz TS-EPSC amplitudes during LP 44 alone (from Fig 2B for comparison) and SB 269970 (SB) alone normalized to their respective aCSF baseline, and 10 μM LP 44 in the presence of SB 269970 normalized to SB 269970 alone. A2. Magnitude of sEPSC amplitude and frequency response to 10 μM LP 44 in the presence of SB 269970. B1. TS-EPSC amplitude changes during 10 μM LP 44 and 1 μM AS-19 normalized to aCSF (from Fig 2B for comparison), and TS-EPSCs with the same agonists in the presence of the 5-HT1AR antagonist WAY 100135, normalized to WAY 100135 alone. B2. sEPSC amplitude and frequency changes during 10 μM LP 44 and 1 μM AS 19 in the presence of WAY 100135, normalized to WAY 100135 alone. C1. TS-EPSC amplitude changes in response to 10 μM LP 44 (from Fig 2B for comparison), co-application of SB 269970 and WAY 100135 (SB+ WAY, normalized to aCSF) and 10 μM LP 44 in the presence of SB +WAY (normalized to SB + WAY). Note that the combined blockade of 5-HT7R and 5-HT1AR resulted in reduced TS-EPSCs and no increased responders. ** p < 0.01, paired t-test, vs aCSF baseline. C2. sEPSC amplitude and frequency changes for 5-HT7R and 5-HT1AR blockade with SB + WAY (normalized to aCSF) and during 10 μM LP 44 in the presence of SB + WAY (normalized to SB + WAY alone).
In the presence of 5-HT7R blockade with SB 269970, subsequent application of the 5-HT7R agonist LP 44 (10 μM) did not alter TS-EPSCs (Fig 4A1 SB alone, −111 ± 14 pA vs. SB + LP 44, −106 ± 15 pA, n=10). Sixty percent (6/10) of recordings did not respond by more than 15% of SB alone (control) with two recordings each increasing and decreasing (Fig 4A1). This compares to 10 μM LP 44 alone in which there was only one non-responder (Fig 4A1). Additionally, LP 44 in the presence of SB 269970 did not change UDD (not shown) or PPR (SB alone, 0.57 ± 0.09 vs. SB + LP 44, 0.62 ± 0.1). Examining sEPSC amplitude (Fig 4A2), 60% (6/10) of cells did not respond to LP 44 in SB, while 30% decreased amplitude and 10% increased amplitude. For sEPSC frequency (Fig 4A2), 70% (7/10) of cells were non-responders while 20% decreased frequency and 10% increased frequency with LP 44 in SB. There was no significant change in Rin with SB + LP 44 (537 ± 80 MΩ) compared to SB alone (579 ± 85 MΩ).
2.6. 5-HT1AR blockade does not alter LP 44 or AS 19 mediated responses
A study in the hippocampus suggested LP 44 acted as a mixed 5-HT1AR and 5-HT7R agonist (Costa et al., 2012). We previously demonstrated a role for 5-HT1AR in the nTS to decrease EPSCs (Ostrowski et al., 2014). In order to determine if altered synaptic properties elicited by LP 44 in the present study were due, in part, to 5-HT1AR activation, we recorded in the presence of LP 44 (10 μM) and WAY 100135 (10 μM) a selective 5-HT1AR antagonist (Ostrowski et al., 2014). We also recorded AS 19 (1 μM) in the presence of WAY 100135.
As with 5-HT7R activation by LP 44 or AS 19 alone, increased and decreased TS-EPSC amplitudes were seen in response to LP 44 or AS 19 in the presence of WAY 100135, although there was a general reduction in responders compared to LP 44 or AS 19 alone (Fig 4B1). Specifically, TS-EPSC amplitude in the presence of LP 44 and WAY 100135 did not alter 50% (4/8) of cells, decreased 38% (3/8) and increased 12% (1/8). A similar proportion occurred for AS 19 in WAY 100135: 50% (5/10) of cells were non-responders, 30% (3/10) decreased amplitude and 20% (2/10) increased amplitude. In the presence of WAY there was no change in Rin for either LP 44 (WAY alone, 466 ± 171 MΩ vs. WAY+LP 44, 496 ± 151 MΩ) or AS 19 (WAY alone, 700 ± 140 MΩ vs. WAY+AS 19, 711 ± 107 MΩ). Addition of LP 44 or AS 19 in the presence of WAY did not change UDD compared to WAY by itself (not shown). There was no significant difference in PPR for WAY alone (0.66 ± 0.10) or 10 μM LP 44 in the presence of WAY (0.65 ± 0.07) compared to aCSF control (0.61 ± 0.12). Likewise, the PPR did not significantly change between aCSF (0.48 ± 0.04), WAY (0.52 ± 0.06) and AS 19 in WAY (0.53 ± 0.07).
The sEPSCs were not altered by LP 44 or AS 19 in WAY. For sEPSC amplitude during LP 44 in WAY, 62% (5/8) of cells did not change compared to WAY alone while 38% (3/8) decreased amplitude by more than 15% of baseline (Fig 4B2). No increases in sEPSC amplitude were seen with LP 44 in the presence of WAY 100135. Likewise, AS 19 in WAY did not alter sEPSC amplitude in 80% (8/10) of cells, while one decreased and one increased. For sEPSC frequency, 75% (6/8) of LP 44 cells in WAY were non-responders with one increase and one decrease. For AS 19 in WAY, 80% (8/10) of cells did not change sEPSC frequency with one decrease and one increase. Taken together, block of 5-HT7R and 5-HT1AR did not appreciably alter EPSCs alone or following addition of 5-HT7R agonists.
2.7. Co-5-HT7R and 5-HT1AR blockade significantly reduces TS-EPSC amplitude
Since 5-HT7R blockade with SB 269970 and 5-HT1A blockade with WAY 100135 did not separately eliminate all responses to LP 44, experiments were conducted in the presence of both antagonists. Previously, blockade of either 5-HT7R (Fig 4A1) or 5-HT1AR [not shown, and previously (Ostrowski et al., 2014)] alone did not alter TS-EPSCs evoked at 0.5 Hz. However, the combined blockade of 5-HT7 and 5-HT1ARs (SB + WAY) significantly decreased 0.5 Hz TS-EPSCs from −91 ± 12 pA to −77 ± 12 pA (p=0.003, a significant reduction of 17%, Fig 4C1, n=8). There was also a significant reduction in PPR for SB + WAY combined compared to aCSF control (0.54 ± 0.04 to 0.39 ± 0.05, p=0.005), whereas there was no effect on UDD. Rin significantly decreased from 598 ± 101 MΩ to 490 ± 81 MΩ (p=0.05).
When 10 μM LP 44 was subsequently added in the presence of SB + WAY, there was no significant change in 0.5 Hz TS-EPSC amplitude (Fig 4C1). The decreased PPR observed with SB + WAY combined increased with addition of 10 μM LP 44 (SB + WAY, 0.39 ± 0.05 to SB + WAY+ LP 44, 0.57 ± 0.08, p=0.015) resulting in a PPR not significantly different from aCSF baseline. There was also a significant effect on UDD with the amplitude of LP 44 in SB + WAY lower for each respective stimulus as compared to SB + WAY alone (two-way RM ANOVA, between SB + WAY and LP 44 in SB + WAY p=0.015, interaction effect treatment × event, p=0.014, n=8). Rin significantly increased from 490 ± 81 MΩ (SB + WAY) to 615 ± 81 MΩ in SB + WAY + LP 44 (p=0.005).
Overall, sEPSC amplitude and frequency were not altered by co-block of 5-HT7/1ARs (amplitude aCSF baseline, −17 ± 2 vs. SB + WAY, −17 ± 3 pA, frequency aCSF baseline, 33 ± 6 vs. 29 ± 5 Hz). Addition of LP 44 in SB + WAY also had no effect on overall amplitude and frequency (LP 44 in SB + WAY amplitude, −14 ± 2 pA, frequency, 27 ± 4 Hz). For sEPSC amplitude during LP 44 in SB and WAY, 25% (2/8) of cells did not respond while 50% (4/8) decreased amplitude and 25% increased amplitude (Fig 4C2). For sEPSC frequency, 50% (4/8) of cells in SB and WAY were non-responders with 25% (2/8) increasing and 25% decreasing.
2.8. 5-HT7R activation modulate GABAAR influence on EPSC and GABAergic IPSCs
Given the variable response of 5-HT7R activation, we postulated that part of its effects may be due to the influence of 5-HT7R on inhibitory network activity, as in other nuclei (Harsing et al., 2004; Kawahara et al., 1994). Noting the prominent role of GABAARs on nTS activity (Bailey et al., 2008; Dufour et al., 2010; Zubcevic and Potts, 2010), we examined the influence of IPSCs on 5-HT7R-modulation of EPSCs, as well as 5-HT7R modulation of IPSCs.
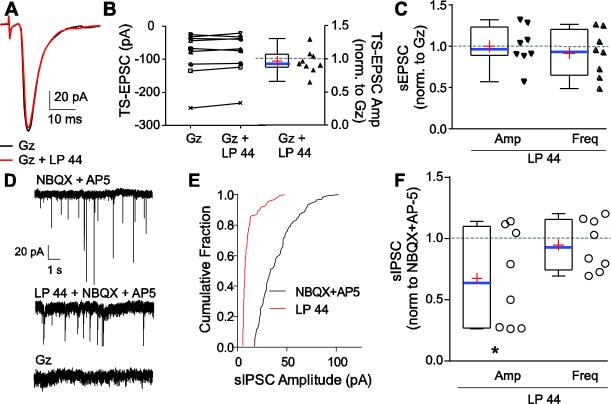
EPSCs following 5-HT7R activation with LP 44 were examined in the presence of GABAAR blockade with gabazine (Gz, 25 μM). Gabazine alone did not alter TS-EPSCs recorded at −60 mV (the chloride reversal potential under our recording conditions, Fig 5A). Addition of 10 μM LP 44 in the presence of Gz did not alter mean TS-EPSC amplitude (Gz, −87 ± 24 pA vs. Gz+LP 44, −82 ± 22pA, Fig 5B, left). Specifically, 67% (6/9) of cells did not show a change in TS-EPSC amplitude (Fig 5B, right). Of the remaining neurons, 11% (1/9) increased and 22% (2/9) decreased TS-EPSC amplitude by more than 15%. Addition of 10 μM LP 44 in the presence of Gz did not alter the magnitude of UDD (not shown) or PPR (Gz, 0.72 ± 0.14 vs. Gz + LP 44, 0.77 ± 0.18). Similarly, the addition of LP 44 in the presence of Gz did not affect overall sEPSC amplitude (15 ± 2pA for both baseline Gz and LP 44 in Gz) or frequency (Gz baseline 21 ± 3 Hz vs. 20 ± 4 Hz, Fig 5C). Specifically, in LP 44 in Gz, 63% (5/8) of cells did not change sEPSC amplitude, while 25% (2/8) increased and 12% (1/8) decreased (Fig 5C). Likewise, 38% (3/8) of cells did not change sEPSC frequency, 25% (2/8) increased and 38% decreased frequency.
Figure 5.
GABAARs modulate 5-HT7R influence on IPSCs. A. Example of representative 0.5 Hz TS-EPSCs during gabazine (Gz) alone and during Gz and LP 44 (black, Gz alone; red, Gz + LP 44). Example is the average of 30 traces. Note the reduced LP 44 response during GABAA receptor blockade. B. Quantitative TS-EPSC amplitude evoked at 0.5 Hz during GABAAR blockade and to addition of 10 μM LP 44. Raw values are presented on the left axis whereas the change in TS-EPSCs in LP 44 in Gz, normalized to Gz alone, is presented on the right axis. Note that addition of LP 44 in Gz did not change TS-EPSC amplitude. C. Change in sEPSC amplitude and frequency of individual responses during 10 μM LP 44 in Gz, normalized to Gz alone (denoted as “1”, dashed line). D. Example of control sIPSCs (top) and their reduction in amplitude with 5-HT7R activation (LP 44, 10 μM, middle) in the presence of NBQX and AP-5. Addition of Gz (bottom) eliminated sIPSCs confirming currents were mediated by GABAAR. E. Cumulative fraction of amplitude events for recording from D. Note that amplitude is reduced with LP 44 in the presence of NBQX and AP-5. F. Normalized group data showing changes in sIPSC amplitude (left) and frequency (right) to LP 44 respective to baseline (NBQX + AP5). Note the significant decrease in sIPSC amplitude. (n=8, * p < 0.05, paired t-test).
We examined the effect of 5-HT7R activation with LP 44 on spontaneous and miniature inhibitory postsynaptic currents (sIPSC and mIPSC) using a high chloride recording solution in the recording pipette and bath blockade of AMPA and NMDA receptors with NBQX (20 μM) and AP5 (50 μM), respectively. Fig 5D is a representative example of sIPSCs under baseline (NBQX+ AP5) and the addition of LP 44, and their elimination by Gz (25 μM). There was a significant leftward shift in the cumulative fraction of sIPSC amplitude (Fig. 5E; p < 0.05, Kolmogorov-Smirnov, two-sample test) whereas sIPSC intervals did not change (not shown). Quantitatively, LP 44 (10 μM) significantly reduced sIPSC amplitude by 33 ± 14% (Fig 5F, left; baseline, −72 ± 25 pA vs. LP 44, −33 ± 9 pA, t-test p=0.05, n=8) but did not change sIPSC frequency (Fig 5F, right; control, 29 ± 5 Hz vs. LP 44, 26 ± 4 Hz). For mIPSCs, recorded in the presence of TTX, NBQX, and AP5, there was no significant effect of LP 44 on amplitude (TTX+NBQX+AP5, −40 ± 7.5 pA vs. LP 44, −54 ± 19 pA) or frequency (TTX+NBQX+AP5, 27 ± 5.8 Hz vs. LP 44, 23 ± 5.7 Hz). Therefore, 5-HT7R activation alters GABAergic signaling reducing the amplitude of inhibitory currents.
3. DISCUSSION
The results from the present study demonstrate 5-HT7Rs are present in the nTS, but their functional role is complex. Specifically, while activation of 5-HT7R modulated the majority of nTS neurons, TS-EPSC amplitude increased as well as decreased. However, during sustained (20 Hz) stimulation 5-HT7R activation reduced the degree of use dependent depression. Miniature EPSCs frequency significantly decreased following 5-HT7R activation with LP 44. Blockade of 5-HT7R did not alter TS-EPSCs tonically, but modestly reduced the number of LP 44 responding cells. By contrast, 5-HT7 receptor activation consistently decreased GABAergic inhibitory network activity. Taken together, these results demonstrate 5-HT7Rs variably modulate excitatory neurotransmission but steadily attenuate inhibitory transmission, suggesting the overall excitatory role of 5-HT7R in nTS in vivo may be due to the shifted balance between these glutamatergic and GABAergic neurotransmitter systems.
In the cortex, 5-HT7R activation increases glutamatergic transmission via a presynaptic mechanism (Béïque et al., 2004; Ciranna and Catania, 2014), while in the hippocampus 5-HT7R modulation of excitatory transmission is either inhibited (Otmakhova et al., 2005) or enhanced (Costa et al., 2012) by a postsynaptic mechanism. We demonstrate in the present study 5-HT7R activation by 2 distinct agonists, LP 44 and AS 19, increase and decrease TS- and sEPSC amplitude, but as a whole did not alter mean amplitude across the cell samples studied. Interestingly, while 5-HT7R activation with LP 44 predominately reduced the initial TS-EPSC in a stimulus train, overall EPSC amplitude was greater in subsequent EPSCs in PPR and UDD protocols which suggests less depression during prolonged stimulation. These changes in PPR and UDD suggest 5-HT7Rs alter presynaptic release properties. This is further supported by the reduction of mEPSC frequency with 5-HT7R LP 44 stimulation. While both LP 44 and AS 19 are potent 5-HT7R agonists, LP 44 has the higher binding affinity than AS 19 for the receptor (Andrade et al., 2015; Leopoldo et al., 2011). Such binding differences may account for the greater effects of LP 44 compared to AS 19 in the present study. Nevertheless, this study demonstrates that the 5-HT7R modulates nTS synaptic activity.
While 5-HT7R mRNA and protein were localized in the nTS, the expression of mRNA was significantly less in the NPG, where the somas of visceral afferents are located. Thus, if 5-HT7Rs are located on presynaptic glutamatergic terminals to alter presynaptic glutamate release, then we might expect higher amounts of 5-HT7R mRNA in the NPG. Several possibilities may explain this apparent contradiction. For instance, 5-HT7R message may be low due to stable protein expression in NPG central afferents. Alternatively, 5-HT7R message may be primarily localized to sensory afferent terminals, as shown with other receptors or channels (Ji et al., 1994; Tohda et al., 2001), and thus the greater message we observe in nTS compared to NPG is in these central boutons. Last, 5-HT7R may be located in nTS astrocytes or interneurons (and thus their fibers and terminals which may impinge on the recorded neuron), and its activation may inhibit neurotransmitter release via a secondary mediator(s). 5-HT7Rs have been identified in CNS astrocytes (Doly et al., 2005; Hirst et al., 1997; Shimizu et al., 1997), but whether they are expressed in nTS astrocytes requires further study. Given that 5-HT7R activation with LP 44 alters mEPSC frequency, it is likely that 5-HT7Rs are located at least on nearby terminals. Nevertheless, our results are consistent with in vivo nTS studies in which ionophoretic application of the non-selective 5-HT7R agonist 5-CT increased and decreased action potential discharge regardless of whether or not the neurons were mono- or polysynaptic to sensory afferents. In those studies, the excitatory response was blocked by a selective 5-HT7R antagonist (Oskutyte et al., 2009). An excitatory role for 5-HT7R in nTS was further suggested by the attenuation of cardiorespiratory reflexes in response to 5-HT block (Damaso et al., 2007; Oskutyte et al., 2009).
Block of 5-HT7R (SB 269970) or 5-HT1AR (WAY 100135) did not significantly decrease TS-EPSCs separately, but co-application of these antagonists did significantly attenuate TS-EPSC amplitude and significantly reduced the PPR, revealing a potential tonic role for both receptors that was masked during single receptor blockade. Our previous work examining 5-HT1AR in nTS demonstrated these receptors inhibit TS-EPSC amplitude and sEPSC frequency, which is not influenced by prior block of 5-HT7Rs with SB 269970 (Ostrowski et al., 2014). 5-HT1ARs were identified in nTS somas, and although a portion of them were GABAergic, the remaining cell types were un-phenotyped. The variety of responses observed with LP 44 or AS 19 were similar whether exposed to the 5-HT1A (WAY 100135) or 5-HT7 (SB 269970) receptor antagonists, although the number of responders was attenuated. Given that these receptors activate distinct downstream pathways [e.g., 5-HT1ARs primarily activate the Gi/o signaling pathway to inhibit adenylyl cyclase, whereas 5-HT7R is coupled to Gs to activate adenylyl cyclase, and also G12 to activate the GTPase Rho family (Kvachnina et al., 2005)], one would expect distinct excitatory or inhibitory responses in the presence of either antagonist. This was not the case, and several possibilities may explain these results. First, LP 44, AS 19 and SB 266970 may not be specific for the 5-HT7R, but rather may also bind to the 5-HT1AR, among other receptors (Bosker et al., 2009; Costa et al., 2012; Kim et al., 2012; Leopoldo et al., 2011, 2004). Thus, several offsetting responses on synaptic currents may occur. Second, a physical and functional interaction between 5-HT7R and 5-HT1AR has been reported, including the ability to form heterodimers (Ciranna and Catania, 2014; Renner et al., 2012) to influence each other's activity. It is therefore possible that 5-HT7R may play a regulatory role by interacting with other 5-HTRs. The majority of our data suggest 5-HT7R modulates synaptic events via a presynaptic mechanism(s) while our previous study (Ostrowski et al., 2014) indicates 5-HT1ARs are located postsynaptically in nTS somas (perhaps interneurons). However, although the electrophysiological evidence suggests primarily a pre-synaptic role for 5-HT7R, post-synaptic 5-HT7R is suggested by 5-HT7R induced changes in input resistance (i.e., membrane conductance). Pre- and postsynaptic 5-HT7Rs are found in the rat spinal cord (Doly et al., 2005) while exclusively postsynaptic 5-HT7Rs were suggested in the CA1/CA3 region of the hippocampus (Costa et al., 2012). Also, in other nuclei 5-HT1ARs can alter EPSCs via presynaptic mechanisms (Costa et al., 2012; Dergacheva et al., 2011), which suggests the presence of pre-synaptic 5-HT1AR in the nTS, although unlikely, cannot be ruled out. Last, there is evidence for agonist-independent constitutive activity for 5-HT7R (Krobert and Levy, 2002; Kvachnina et al., 2009) which may help explain the diversity of responses seen with agonist activation both alone and in the presence of selective antagonists for 5-HT7R and 5-HT1AR.
Based on our observations that LP 44 and AS 19 inhibited TS-EPSCs, we explored the degree to which this attenuation occurred via an increase in GABAergic mediated inhibition. While 5-HT7Rs inhibit GABAergic currents in the superchiastmatic (Kawahara et al., 1994) and raphe nuclei (Roberts et al., 2004), 5-HT7R increases sIPSCs in the hippocampus (Tokarski et al., 2011) and globus pallidus (Chen et al., 2008). Blockade of GABAAR with gabazine showed a distinct attenuation of 5-HT7Rs modulation on TS-EPSC amplitude, UDD, and PPR, similar to that seen when blocking 5-HT7R with SB 269970. This may indicate that 5-HT7R activation increases GABA release, possibly at the synapse, to reduce EPSCs. While we held the cell at the chloride reversal potential to minimize GABAergic influence on the recorded cell, 5-HT7R activation may have increased GABAergic influence on the nearby unclamped GABAergic and glutamatergic cells and presynaptic terminals. When directly examining sIPSCs, LP 44 decreased their amplitude to indicate increased 5-HT7R activity reduces spontaneous inhibitory events and allows for more spontaneous excitation. However, there was no change in miniature inhibitory amplitude or frequency which would suggest 5-HT7R activation affects GABAergic neurons within the network (interneurons) and is action potential dependent. 5-HT7Rs may affect GABARs at sites that influence mEPSCs but not TS-EPSCs. Taken together, these data suggest there are both pre- and postsynaptic 5-HT7Rs which can have opposing effects on network activity within the nTS.
In summary, the complexity of these results reflects the complexity of responses elicited by 5-HT within the CNS. Not only can diverse responses occur as a result of the many different receptor sub-types, but it seems based on the results of our study and others that complex responses may occur from a single 5-HT receptor sub-type as well as from interactions between 5-HT receptor sub-types. The exact role of 5-HT7R in the nTS may be better understood once more selective agonists are available or through examination of dissociated nTS neurons to eliminate the potential confounding effect of activating nearby inhibitory and excitatory networks with 5-HT7Rs simultaneously.
4. EXPERIMENTAL PROCEDURE
4.1. Animals
All experiments were conducted following the National Institutes of Health Guide for the Care and Use of Laboratory Animals guidelines and protocols were approved by the University of Missouri Animal Care and Use Committee. Male Sprague-Dawley rats (Harlan, Indianapolis, IN, USA; n=66) aged 4-7 weeks were used. Animals were housed in an in-house animal facility with a 12:12h light-dark cycle with food and water available ad libitum.
4.2. Reverse transcription real-time polymerase chain reaction (RT-PCR)
The presence of 5-HT7R mRNA in the nTS was examined via RT-PCR, similar to our previous studies (Austgen et al., 2012, 2011). Briefly, rats (n=5) were anesthetized with isoflurane, decapitated and the brainstem removed. The brainstem was trimmed and the ventral surface affixed onto the chuck of a vibrating microtome (LeicaVT1000S, Leica) with cyanoacrylate to generate horizontal slices (~400μm) containing nTS. The nTS was trimmed from the slice and snap frozen in liquid nitrogen and stored at −80°C. In addition, brainstem lacking nTS, cerebellum, and the nodose-petrosal ganglia (NPG) were isolated, snap frozen and stored at −80°C. RNA was isolated using the RNAqueous-Micro Kit, following the manufacturer's instructions (Ambion, Life Technologies, Grand Island, NY, USA) and quantified (BioPhotometer plus, Eppendorf, Hauppauge, NY, USA). cDNA was generated from 100ng mRNA (oligo-dT primer set, SuperScript III, Invitrogen). Quantitative real-time PCR amplification of 2μL of cDNA was performed using the SYBR Premix Ex Taq kit (Takara, Mountain View, CA, USA), the SmartCycler System (Cepheid, Sunnyvale, CA, USA) and the following primers: 5-HT7R (forward: TTT ATC TGT GGC ACC TCC TGT, reverse: CCA CAG ACA TGT CCT CTC CA, 10μM; Fisher Scientific, Pittsburgh, PA, USA) and the housekeeping gene β2-microglobulin (B2M) (forward: AGC AGG TTC CTC AAA CAA GG, reverse: TTC TGC CTT GGA GTC CTT TC 10μM; Fisher Scientific). The amount of 5-HT7R mRNA was normalized to B2M using the ΔΔCT method (Livak and Schmittgen, 2001).
The rostral-caudal distribution of 5-HT7R was examined using nTS micro-dissected from 30μm paraformaldehyde fixed coronal tissue slices from 3 rats and extracting mRNA using the RNeasy FFPE Kit (Qiagen #73504, Hildern, Germany) according to manufacturer's instructions. Quantitative RT-PCR was then performed using a High Capacity RNA-to-cDNA kit (Life Technologies #4387406) with primers for 5-HT7R and B2M as above.
4.3. Immunoblot
Presence of 5-HT7R protein in nTS was verified by immunoblot analysis. Rats (n=2) were anesthetized, decapitated, the brainstem removed, and tissue samples prepared and taken as described for RT-PCR. Tissue samples were homogenized in extraction buffer (150 mM NaCl, 100 mM Tris-HCl, 1% Triton X, protease inhibitor cocktail (Complete Mini, EDTA-free, Roche Diagnostics, Indianapolis, IN, USA)), centrifuged (15 min, 13,300 rpm, 4°C), and the supernatant collected. Protein concentrations were determined with the Bio-Rad Protein Assay Dye Reagent (Bio-Rad, Hercules, CA, USA) and 100 μg of protein was separated in a 4-20% precast Mini-PROTEAN TGX gel (Bio-Rad) and transferred to an Immun-Blot PVDF membrane (Bio-Rad). Membranes were subsequently incubated (48 h, 4°C) with primary antibodies against 5-HT7R (rabbit 1:500, Abcam #101913, Cambridge, MA, USA) and tubulin (mouse, 1:100, Abcam #7291), washed, and incubated with HRP-linked secondary antibodies (1:1000, 2h, 23°C, Jackson Immunoresearch Laboratories, West Grove, PA, USA). Blots were developed with ImmunStar WesternC substrate (BioRad, Hercules, CA, USA) and imaged with ChemiDoc XRS+ Imager using Image Lab Software (Version 5.1, Bio-Rad).
4.4. In vitro brain slice nTS preparation, electrophysiology and protocols
Rats were anesthetized with Isoflurane, decapitated, and the brainstem rapidly removed and placed in either ice-cold low-Ca2+, high Mg2+ artificial cerebrospinal fluid (aCSF, in mM: 124 NaCl, 3 KCl, 1.2 NaH2PO4, 1.2 MgSO4, 25 NaHCO3, 11 D-glucose, 0.4 L-ascorbic acid, 2 MgCl2, and 1 CaCl2, aerated with 95% O2 + 5% CO2, pH 7.4, 300-310 mOsm) or an N-methyl-D-glucamine (NMDG)-HEPES cutting solution (in mM: 93 NMDG, 2.5 KCl, 1.2 NaH2PO4, 10 MgSO4, 30 NaHCO3,20 HEPES, 25 D-glucose, 5 L-ascorbic acid, 2 thiourea, 3 sodium pyruvate and 0.5 CaCl2, aerated with 95% O2 + 5% CO2, pH 7.4, 300-310 mOsm). The brainstem was trimmed and the ventral surface affixed with cyanoacrylate onto the chuck of a vibrating microtome to generate horizontal slices (~280 μm) that contained the nTS and a long length of the afferent containing tractus solitarii (TS) in the same plane.
Slices were placed in a heated recording chamber (Warner Instruments, TC-344B, Hamden, CT, USA) and secured with a nylon mesh harp. Slices were superfused with recording aCSF (in mM: 124 NaCl, 3 KCl, 1.2 NaH2PO4, 1.2 MgSO4, 25 NaHCO3, 11 D-glucose, 0.4 L-ascorbic acid, and 2 CaCl2, aerated with 95% O2 + 5% CO2, pH 7.4, 300-310 mOsm) at 32-35°C and allowed to recover a minimum of 30 minutes following slicing before recording.
Recording electrodes (3-5 MΩ) were pulled from borosilicate glass (#8250 King Precision Glass, Inc., Claremont, CA, USA) on a horizontal pipette puller (Sutter Instruments P-97, Novato, CA, USA). Neurons were visualized on an Olympus BX51WI fixed stage upright microscope with DIC optics. Recording electrodes were lowered to the surface of the neuron by a piezoelectric micromanipulator (Burleigh PCS-6000, Mississauga, Ontario, Canada), under positive pressure. A tight giga-ohm seal was formed prior to the whole-cell configuration. Neurons were voltage clamped at −60mV. Cell capacitance, input resistance (Rin), series resistance (Rs), resting membrane potential (RMP), and holding current were measured initially and monitored throughout recordings. An initial Rs > 25 MΩ was compensated for prior to control recordings. Signals were filtered at 2 kHz and acquired at 10 kHz using a Multiclamp 700B amplifier controlled by Clampex 10 software by a Digidata 1440 interface (Molecular Devices, Sunnyvale, CA, USA).
To examine EPSCs, recording electrodes were filled with (in mM) 130 K+-gluconate, 10 NaCl, 11 EGTA, 10 HEPES, 1 MgCl2, 1 CaCl2, 2 MgATP, 0.2 NaGTP. Tractus solitarii (TS) evoked excitatory postsynaptic currents (TS-EPSCs) were induced by placing a bipolar stimulating electrode (FHC, Bowdoin, ME, USA) on the afferent containing TS and generating stimulus trains at 0.5 or 20 Hz, the latter mimicking increases in afferent activity. For each recording, TS stimulus intensity was progressively increased until an EPSC was evoked. Final intensity was ~25% above this minimum in order to ensure consistent evoked responses while avoiding polysynaptic inputs. Spontaneous EPSCs (sEPSCs) were recorded for 1 minute in gap-free recordings without stimulation. Spontaneous miniature EPSCs (mEPSCs) were examined by adding 1 μM tetrodotoxin (TTX, Na+ channel blocker) and 25 μM gabazine (GABAA antagonist) to the aCSF. Inhibitory postsynaptic currents (IPSCs) were recorded by using a high Cl− filling solution (in mM 140 CsCl, 5 NaCl, 10 EGTA, 10 HEPES, 1.2 MgSO4, 3 K-ATP, 0.2 Na-GTP, 5 lidocaine, Ereversal ~ 0 mV) in the recording electrode and addition of 20 μM NBQX (AMPA antagonist) and 50 μM DL-AP5 (NMDA antagonist) to the perfusion media (Chen et al., 2009; Ostrowski et al., 2014). Miniature IPSCs (mIPSCs) were recorded by adding 1 μM TTX to the perfusion media along with NBQX and DL-AP5.
4.5. Drugs
Recorded cells, typically 1-2 cell layers deep, were exposed to a minimum of 5 minutes of pharmacological treatment. 5-HT7R pharmacological agents were initially prepared as stock solutions in DMSO or double distilled water, and subsequently diluted to given working concentrations in aCSF. The final concentration of DMSO in the working solutions was ≤ 0.01% which has been shown to have no effect on neuronal function (Clark et al., 2011; Kang and Schuman, 1995). Vehicle time controls contained the same concentration of DMSO in aCSF. All drugs were bath applied via superfusion by means of a valve controller (Warner Instruments VC-6). To examine the effect of 5-HT7R activation, the commercially available 5-HT7R agonists LP 44 (Leopoldo et al., 2007) or AS 19 (Brenchat et al., 2009) were applied. LP 44 has greater affinity and specificity for 5-HT7R than AS 19, therefore LP 44 was used for the majority of experiments (Brenchat et al., 2010, 2009; Leopoldo et al., 2011). Concentrations of LP 44 were based on literature and preliminary experiments (Costa et al., 2012; Hawkins et al., 2015). Blockade of 5-HT7R was accomplished by use of the selective antagonist SB 269970 [1 μM (Thomas et al., 2000)], whereas 5-HT1ARs were blocked by the antagonist WAY 100135 (10 μM, Ostrowski et al., 2014). LP 44, AS 19, TTX, NBQX, DL-AP5, WAY 100135, and SB 269970 were purchased from Tocris Bioscience (R&D Systems, Inc., Minneapolis, MN, USA). All other general chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA) and Fisher Scientific (Pittsburgh, PA, USA).
4.6. Data Analysis
Recordings were not used if the neuron initially had a RMP more depolarized than −40mV, Rs greater than 25 MΩ, or a holding current greater than −100pA. Data were analyzed off-line using Clampfit 10.4 (Molecular Devices) and Excel. Variables analyzed included EPSC peak amplitude, instantaneous frequency, paired pulse interval (PPR), and use dependent depression (UDD) as well as Rin. Latency of TS-EPSCs was measured as the time from stimulus artifact to the start of the inward current over 30 events. Only nTS cells connected to TS-afferents with a single synapse (i.e., monosynaptic) were included in this study. Recorded neurons were considered monosynaptic if the standard deviation of the latency time (“jitter”) was less than 300 μs over 30 events (Austgen et al., 2012; Ramirez-Navarro et al., 2011). Neurons in the present study were monosynaptic second-order as indicated by low jitter and latency. For 5-HT7R activation with LP 44 or AS 19, aCSF was used for the baseline control (defined as “1”). Determining the effects of 5-HT7R blockade, aCSF was used as baseline with SB 269970, whereas SB 269970 was then used as baseline for LP 44 in the presence of SB 269970. Similarly, WAY 100135 and gabazine served as respective baseline when examining the impact of 5-HT1AR and GABAAR blockade on 5-HT7R activation. A change from baseline of ±15% was considered to be a response; this value determined from vehicle time trials. Due to cell-to-cell variability, magnitude changes are given as normalized to its respective baseline value with values above 1 indicating an increase (excitatory) and values below 1 indicating a decrease (inhibitory) from baseline. Statistical analyses were performed using Prism 6 (GraphPad Software, La Jolla, CA, USA) software. Data from those neurons which respond to pharmacological manipulation were compared using Wilcoxon's text, Student's t-test, one way ANOVA, or one way repeated-measures ANOVA as appropriate. UDD was analyzed using two-way repeated-measures ANOVA. P values < 0.05 were considered significant. All data within the text are presented as mean ± standard error. Graphical representations of the data were made with box-and-whisker plots with mean, median and quartiles indicated. Whiskers delineate minimum and maximum values for respective data sets.
Highlights.
Activation of nTS 5-HT7 receptors produces both inhibitory and excitatory responses
Paired pulse ratio increased with LP 44, suggesting pre-synaptic action for 5-HT7R
5-HT7R activation reduces mEPSC frequency and modulates GABA effect on nTS neurons
Co-blockade of 5-HT7&1ARs reduces peak amplitude of evoked TS currents
ACKNOWLEDGEMENTS
We thank Heather A. Dantzler and Christine Schramm for their technical expertise in RT-PCR and immunoblots. Funded by RO1 HL085108 and RO1 HL098602.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions: M.P.M. and D.D.K. designed the study, interpreted the data, edited the manuscript and approved final version. M.P.M. performed the experiments, analyzed the data, prepared figures and drafted the manuscript.
REFERENCES
- Accorsi-Mendonça D, Machado BH. Synaptic transmission of baro- and chemoreceptors afferents in the NTS second order neurons. Auton. Neurosci. Basic Clin. 2013;175:3–8. doi: 10.1016/j.autneu.2012.12.002. doi:10.1016/j.autneu.2012.12.002. [DOI] [PubMed] [Google Scholar]
- Andrade R, Barnes NM, Baxter G, Bockaert J, Branchek T, Cohen ML, Dumuis A, Eglen RM, Göthert M, Hamblin M, Hamon M, Hartig PR, Hen R, Herrick-Davis K, Hills R, Hoyer D, Humphrey PPA, Latté KP, Maroteaux L, Martin GR, Middlemiss DN, Mylecharane E, Peroutka SJ, Saxena PR, Sleight A, Villalon CM, Yocca F. 5-Hydroxytryptamine receptors: 5-HT7 receptor [WWW Document]. [1.1.16];IUPHAR/BPS Guid. to Pharmacol. 2015 URL http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=12.
- Austgen JR, Dantzler HA, Barger BK, Kline DD. 5-Hydroxytryptamine 2C receptors tonically augment synaptic currents in the nucleus tractus solitarii. J. Neurophysiol. 2012;108:2292–305. doi: 10.1152/jn.00049.2012. doi:10.1152/jn.00049.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austgen JR, Hermann GE, Dantzler HA, Rogers RC, Kline DD. Hydrogen sulfide augments synaptic neurotransmission in the nucleus of the solitary tract. J. Neurophysiol. 2011;106:1822–32. doi: 10.1152/jn.00463.2011. doi:10.1152/jn.00463.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austgen JR, Kline DD. Endocannabinoids blunt the augmentation of synaptic transmission by serotonin 2A receptors in the nucleus tractus solitarii (nTS). Brain Res. 2013;1537:27–36. doi: 10.1016/j.brainres.2013.09.006. doi:10.1016/j.brainres.2013.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey TW, Appleyard SM, Jin Y-H, Andresen MC. Organization and properties of GABAergic neurons in solitary tract nucleus (NTS). J. Neurophysiol. 2008;99:1712–22. doi: 10.1152/jn.00038.2008. doi:10.1152/jn.00038.2008. [DOI] [PubMed] [Google Scholar]
- Bard JA, Zgombick J, Adham N, Vaysse P, Branchek TA, Weinshank RL. Cloning of a novel human serotonin receptor (5-HT7) positively linked to adenylate cyclase. J. Biol. Chem. 1993;268:23422–23426. [PubMed] [Google Scholar]
- Béïque J-C, Chapin-Penick EM, Mladenovic L, Andrade R. Serotonergic facilitation of synaptic activity in the developing rat prefrontal cortex. J. Physiol. 2004;556:739–54. doi: 10.1113/jphysiol.2003.051284. doi:10.1113/jphysiol.2003.051284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosker FJ, Folgering JHA, Gladkevich AV, Schmidt A, van der Hart MCG, Sprouse J, den Boer JA, Westerink BHC, Cremers TIFH. Antagonism of 5-HT(1A) receptors uncovers an excitatory effect of SSRIs on 5-HT neuronal activity, an action probably mediated by 5-HT(7) receptors. J. Neurochem. 2009;108:1126–35. doi: 10.1111/j.1471-4159.2008.05850.x. doi:10.1111/j.1471-4159.2008.05850.x. [DOI] [PubMed] [Google Scholar]
- Brenchat A, Nadal X, Romero L, Ovalle S, Muro A, Sánchez-Arroyos R, Portillo-Salido E, Pujol M, Montero A, Codony X, Burgueño J, Zamanillo D, Hamon M, Maldonado R, Vela JM. Pharmacological activation of 5-HT7 receptors reduces nerve injury-induced mechanical and thermal hypersensitivity. Pain. 2010;149:483–94. doi: 10.1016/j.pain.2010.03.007. doi:10.1016/j.pain.2010.03.007. [DOI] [PubMed] [Google Scholar]
- Brenchat A, Romero L, García M, Pujol M, Burgueño J, Torrens A, Hamon M, Baeyens JM, Buschmann H, Zamanillo D, Vela JM. 5-HT7 receptor activation inhibits mechanical hypersensitivity secondary to capsaicin sensitization in mice. Pain. 2009;141:239–47. doi: 10.1016/j.pain.2008.11.009. doi:10.1016/j.pain.2008.11.009. [DOI] [PubMed] [Google Scholar]
- Calzà L, Giardino L, Grimaldi R, Rigoli M, Steinbusch HW, Tiengo M. Presence of 5-HT-positive neurons in the medial nuclei of the solitary tract. Brain Res. 1985;347:135–139. doi: 10.1016/0006-8993(85)90900-x. doi:10.1016/0006-8993(85)90900-X. [DOI] [PubMed] [Google Scholar]
- Chen C-Y, Bechtold AG, Tabor J, Bonham AC. Exercise reduces GABA synaptic input onto nucleus tractus solitarii baroreceptor second-order neurons via NK1 receptor internalization in spontaneously hypertensive rats. J. Neurosci. 2009;29:2754–61. doi: 10.1523/JNEUROSCI.4413-08.2009. doi:10.1523/JNEUROSCI.4413-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Yung KKL, Chan YS, Yung WH. 5-HT excites globus pallidus neurons by multiple receptor mechanisms. Neuroscience. 2008;151:439–51. doi: 10.1016/j.neuroscience.2007.11.003. doi:10.1016/j.neuroscience.2007.11.003. [DOI] [PubMed] [Google Scholar]
- Ciranna L, Catania MV. 5-HT7 receptors as modulators of neuronal excitability, synaptic transmission and plasticity: physiological role and possible implications in autism spectrum disorders. Front. Cell. Neurosci. 2014;8:250. doi: 10.3389/fncel.2014.00250. doi:10.3389/fncel.2014.00250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark CG, Hasser EM, Kunze DL, Katz DM, Kline DD. Endogenous brain-derived neurotrophic factor in the nucleus tractus solitarius tonically regulates synaptic and autonomic function. J. Neurosci. 2011;31:12318–29. doi: 10.1523/JNEUROSCI.0746-11.2011. doi:10.1523/JNEUROSCI.0746-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comet M-A, Bernard JF, Hamon M, Laguzzi R, Sévoz-Couche C. Activation of nucleus tractus solitarius 5-HT2A but not other 5-HT2 receptor subtypes inhibits the sympathetic activity in rats. Eur. J. Neurosci. 2007;26:345–54. doi: 10.1111/j.1460-9568.2007.05673.x. doi:10.1111/j.1460-9568.2007.05673.x. [DOI] [PubMed] [Google Scholar]
- Costa L, Trovato C, Musumeci SA, Catania MV, Ciranna L. 5-HT(1A) and 5-HT(7) receptors differently modulate AMPA receptor-mediated hippocampal synaptic transmission. Hippocampus. 2012;22:790–801. doi: 10.1002/hipo.20940. doi:10.1002/hipo.20940. [DOI] [PubMed] [Google Scholar]
- Cui RJ, Roberts BL, Zhao H, Zhu M, Appleyard SM. Serotonin activates catecholamine neurons in the solitary tract nucleus by increasing spontaneous glutamate inputs. J. Neurosci. 2012;32:16530–8. doi: 10.1523/JNEUROSCI.1372-12.2012. doi:10.1523/JNEUROSCI.1372-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damaso EL, Bonagamba LGH, Kellett DO, Jordan D, Ramage AG, Machado BH. Involvement of central 5-HT7 receptors in modulation of cardiovascular reflexes in awake rats. Brain Res. 2007;1144:82–90. doi: 10.1016/j.brainres.2007.01.088. doi:10.1016/j.brainres.2007.01.088. [DOI] [PubMed] [Google Scholar]
- Dean JB. Hypercapnia causes cellular oxidation and nitrosation in addition to acidosis: implications for CO2 chemoreceptor function and dysfunction. J. Appl. Physiol. 2010;108:1786–95. doi: 10.1152/japplphysiol.01337.2009. doi:10.1152/japplphysiol.01337.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dergacheva O, Kamendi HW, Wang X, Mendelowitz D. 5HT1A receptors inhibit glutamate inputs to cardiac vagal neurons post-hypoxia/hypercapnia. Respir. Physiol. Neurobiol. 2011;179:254–8. doi: 10.1016/j.resp.2011.09.005. doi:10.1016/j.resp.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doly S, Fischer J, Brisorgueil M-J, Vergé D, Conrath M. Pre- and postsynaptic localization of the 5-HT7 receptor in rat dorsal spinal cord: immunocytochemical evidence. J. Comp. Neurol. 2005;490:256–69. doi: 10.1002/cne.20667. doi:10.1002/cne.20667. [DOI] [PubMed] [Google Scholar]
- Dufour A, Tell F, Kessler J-P, Baude A. Mixed GABA-glycine synapses delineate a specific topography in the nucleus tractus solitarii of adult rat. J. Physiol. 2010;588:1097–115. doi: 10.1113/jphysiol.2009.184838. doi:10.1113/jphysiol.2009.184838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards E, Paton JF. 5-HT(4) receptors in nucleus tractus solitarii attenuate cardiopulmonary reflex in anesthetized rats. Am. J. Physiol. 1999;277:H1914–H1923. doi: 10.1152/ajpheart.1999.277.5.H1914. [DOI] [PubMed] [Google Scholar]
- Fortin G, Champagnat J. Spontaneous synaptic activities in rat nucleus tractus solitarius neurons in vitro: evidence for re-excitatory processing. Brain Res. 1993;630:125–135. doi: 10.1016/0006-8993(93)90650-c. doi:10.1016/0006-8993(93)90650-C. [DOI] [PubMed] [Google Scholar]
- Geurts FJ, De Schutter E, Timmermans JP. Localization of 5-HT2A, 5-HT3, 5-HT5A and 5-HT7 receptor-like immunoreactivity in the rat cerebellum. J. Chem. Neuroanat. 2002;24:65–74. doi: 10.1016/s0891-0618(02)00020-0. doi:10.1016/S0891-0618(02)00020-0. [DOI] [PubMed] [Google Scholar]
- Gustafson EL, Durkin MM, Bard JA, Zgombick J, Branchek TA. A receptor autoradiographic and in situ hybridization analysis of the distribution of the 5-ht7 receptor in rat brain. Br. J. Pharmacol. 1996;117:657–666. doi: 10.1111/j.1476-5381.1996.tb15241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harsing LG, Prauda I, Barkoczy J, Matyus P, Juranyi Z. A 5-HT 7 heteroreceptor-mediated inhibition of [3H]serotonin release in raphe nuclei slices of the rat: Evidence for a serotonergic-glutamatergic interaction. Neurochem. Res. 2004;29:1487–1497. doi: 10.1023/b:nere.0000029560.14262.39. doi:10.1023/B:NERE.0000029560.14262.39. [DOI] [PubMed] [Google Scholar]
- Hawkins VE, Hawryluk JM, Takakura AC, Tzingounis AV, Moreira TS, Mulkey DK. HCN channels contribute to serotonergic modulation of ventral surface chemosensitive neurons and respiratory activity. J. Neurophysiol. 2015;113:1195–1205. doi: 10.1152/jn.00487.2014. doi:10.1152/jn.00487.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst WD, Price GW, Rattray M, Wilkin GP. Identification of 5-hydroxytryptamine receptors positively coupled to adenylyl cyclase in rat cultured astrocytes. Br. J. Pharmacol. 1997;120:509–515. doi: 10.1038/sj.bjp.0700921. doi:10.1038/sj.bjp.0700921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman MS, Mitchell GS. Spinal 5-HT7 receptors and protein kinase A constrain intermittent hypoxia-induced phrenic long-term facilitation. Neuroscience. 2013;250:632–643. doi: 10.1016/j.neuroscience.2013.06.068. doi:10.1016/j.neuroscience.2013.06.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman MS, Mitchell GS. Spinal 5-HT7 receptor activation induces long-lasting phrenic motor facilitation. J. Physiol. 2011;589:1397–407. doi: 10.1113/jphysiol.2010.201657. doi:10.1113/jphysiol.2010.201657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeggo RD, Wang Y, Jordan D, Ramage AG. Activation of 5-HT1B and 5-HT1D receptors in the rat nucleus tractus solitarius: opposing action on neurones that receive an excitatory vagal C-fibre afferent input. Br. J. Pharmacol. 2007;150:987–95. doi: 10.1038/sj.bjp.0707169. doi:10.1038/sj.bjp.0707169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji RR, Zhang X, Wiesenfeld-Hallin Z, Hökfelt T. Expression of neuropeptide Y and neuropeptide Y (Y1) receptor mRNA in rat spinal cord and dorsal root ganglia following peripheral tissue inflammation. J. Neurosci. 1994;14:6423–6434. doi: 10.1523/JNEUROSCI.14-11-06423.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan D. Vagal control of the heart: central serotonergic (5-HT) mechanisms. Exp. Physiol. 2005;90:175–81. doi: 10.1113/expphysiol.2004.029058. doi:10.1113/expphysiol.2004.029058. [DOI] [PubMed] [Google Scholar]
- Kanamaru M, Homma I. Dorsomedial medullary 5-HT2 receptors mediate immediate onset of initial hyperventilation, airway dilation, and ventilatory decline during hypoxia in mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009;297:R34–41. doi: 10.1152/ajpregu.90802.2008. doi:10.1152/ajpregu.90802.2008. [DOI] [PubMed] [Google Scholar]
- Kang H, Schuman EM. Long-lasting neurotrophin-induced enhancement of synaptic transmission in the adult hippocampus. Science. 1995;267:1658–1662. doi: 10.1126/science.7886457. doi:10.1126/science.7886457. [DOI] [PubMed] [Google Scholar]
- Kawahara F, Saito H, Katsuki H. Inhibition by 5-HT7 receptor stimulation of GABAA receptor-activated current in cultured rat suprachiasmatic neurones. J. Physiol. 1994;478(Pt 1):67–73. doi: 10.1113/jphysiol.1994.sp020230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellett DO, Ramage AG, Jordan D. Central 5-HT7 receptors are critical for reflex activation of cardiac vagal drive in anaesthetized rats. J. Physiol. 2005a;563:319–31. doi: 10.1113/jphysiol.2004.076521. doi:10.1113/jphysiol.2004.076521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellett DO, Stanford SC, Machado BH, Jordan D, Ramage AG. Effect of 5-HT depletion on cardiovascular vagal reflex sensitivity in awake and anesthetized rats. Brain Res. 2005b;1054:61–72. doi: 10.1016/j.brainres.2005.06.063. doi:10.1016/j.brainres.2005.06.063. [DOI] [PubMed] [Google Scholar]
- Kim MK, Lee HS, Kim S, Cho SY, Roth BL, Chong Y, Choo H. 4-Aminoethylpiperazinyl aryl ketones with 5-HT1A/5-HT7 selectivity. Bioorg. Med. Chem. 2012;20:1139–48. doi: 10.1016/j.bmc.2011.11.005. doi:10.1016/j.bmc.2011.11.005. [DOI] [PubMed] [Google Scholar]
- Kinsey AM, Wainwright A, Heavens R, Sirinathsinghji DJS, Oliver KR. Distribution of 5-ht5A, 5-ht5B, 5-ht6 and 5-HT7 receptor mRNAs in the rat brain. Mol. Brain Res. 2001;88:194–198. doi: 10.1016/s0169-328x(01)00034-1. doi:10.1016/S0169-328X(01)00034-1. [DOI] [PubMed] [Google Scholar]
- Kline DD. Plasticity in glutamatergic NTS neurotransmission. Respir. Physiol. Neurobiol. 2008;164:105–11. doi: 10.1016/j.resp.2008.04.013. doi:10.1016/j.resp.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline DD, Ramirez-Navarro A, Kunze DL. Adaptive depression in synaptic transmission in the nucleus of the solitary tract after in vivo chronic intermittent hypoxia: evidence for homeostatic plasticity. J. Neurosci. 2007;27:4663–73. doi: 10.1523/JNEUROSCI.4946-06.2007. doi:10.1523/JNEUROSCI.4946-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krobert KA, Levy FO. The human 5-HT7 serotonin receptor splice variants: constitutive activity and inverse agonist effects. Br. J. Pharmacol. 2002;135:1563–1571. doi: 10.1038/sj.bjp.0704588. doi:10.1038/sj.bjp.0704588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvachnina E, Dumuis A, Wlodarczyk J, Renner U, Cochet M, Richter DW, Ponimaskin E. Constitutive Gs-mediated, but not G12-mediated, activity of the 5-hydroxytryptamine 5-HT7(a) receptor is modulated by the palmitoylation of its C-terminal domain. Biochim. Biophys. Acta - Mol. Cell Res. 2009;1793:1646–1655. doi: 10.1016/j.bbamcr.2009.08.008. doi:10.1016/j.bbamcr.2009.08.008. [DOI] [PubMed] [Google Scholar]
- Kvachnina E, Liu G, Dityatev A, Renner U, Dumuis A, Richter DW, Dityateva G, Schachner M, Voyno-Yasenetskaya TA, Ponimaskin EG. 5-HT7 receptor is coupled to G alpha subunits of heterotrimeric G12-protein to regulate gene transcription and neuronal morphology. J. Neurosci. 2005;25:7821–7830. doi: 10.1523/JNEUROSCI.1790-05.2005. doi:10.1523/JNEUROSCI.1790-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leopoldo M, Berardi F, Colabufo NA, Contino M, Lacivita E, Niso M, Perrone R, Tortorella V. Structure-affinity relationship study on N-(1,2,3,4-tetrahydronaphthalen-1-yl)-4-aryl-1-piperazinealkylamides, a new class of 5-hydroxytryptamine7 receptor agents. J. Med. Chem. 2004;47:6616–6624. doi: 10.1021/jm049702f. doi:10.1021/jm049702f. [DOI] [PubMed] [Google Scholar]
- Leopoldo M, Lacivita E, Berardi F, Perrone R, Hedlund PB. Serotonin 5-HT7 receptor agents: Structure-activity relationships and potential therapeutic applications in central nervous system disorders. Pharmacol. Ther. 2011;129:120–48. doi: 10.1016/j.pharmthera.2010.08.013. doi:10.1016/j.pharmthera.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leopoldo M, Lacivita E, Contino M, Colabufo NA, Berardi F, Perrone R. Structure-activity relationship study on N-(1,2,3,4-tetrahydronaphthalen-1- yl)-4-aryl-1-piperazinehexanamides, a class of 5-HT7 receptor agents. 2. J. Med. Chem. 2007;50:4214–4221. doi: 10.1021/jm070487n. doi:10.1021/jm070487n. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. doi:10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lovenberg TW, Baron BM, de Lecea L, Miller JD, Prosser RA, Rea MA, Foye PE, Racke M, Slone AL, Siegel BW. A novel adenylyl cyclase-activating serotonin receptor (5-HT7) implicated in the regulation of mammalian circadian rhythms. Neuron. 1993;11:449–458. doi: 10.1016/0896-6273(93)90149-l. doi:10.1016/0896-6273(93)90149-L. [DOI] [PubMed] [Google Scholar]
- Matott MP, Ciarlone GE, Putnam RW, Dean JB. Normobaric hyperoxia (95% O2) stimulates CO2-sensitive and CO2-insensitive neurons in the caudal solitary complex of rat medullary tissue slices maintained in 40% O2. Neuroscience. 2014;270C:98–122. doi: 10.1016/j.neuroscience.2014.03.017. doi:10.1016/j.neuroscience.2014.03.017. [DOI] [PubMed] [Google Scholar]
- Miles R. Frequency dependence of synaptic transmission in nucleus of the solitary tract in vitro. J. Neurophysiol. 1986;55:1076–1090. doi: 10.1152/jn.1986.55.5.1076. [DOI] [PubMed] [Google Scholar]
- Nalivaiko E, Sgoifo A. Central 5-HT receptors in cardiovascular control during stress. Neurosci. Biobehav. Rev. 2009;33:95–106. doi: 10.1016/j.neubiorev.2008.05.026. doi:10.1016/j.neubiorev.2008.05.026. [DOI] [PubMed] [Google Scholar]
- Nichols NL, Dale EA, Mitchell GS. Severe acute intermittent hypoxia elicits phrenic long-term facilitation by a novel adenosine-dependent mechanism. J. Appl. Physiol. 2012;112:1678–88. doi: 10.1152/japplphysiol.00060.2012. doi:10.1152/japplphysiol.00060.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosjean A, Compoint C, Buisseret-Delmas C, Orer HS, Merahi N, Puizillout JJ, Laguzzi R. Serotonergic projections from the nodose ganglia to the nucleus tractus solitarius: An immunohistochemical and double labeling study in the rat. Neurosci. Lett. 1990;114:22–26. doi: 10.1016/0304-3940(90)90422-6. doi:10.1016/0304-3940(90)90422-6. [DOI] [PubMed] [Google Scholar]
- Oskutyte D, Jordan D, Ramage AG. Evidence that 5-hydroxytryptamine(7) receptors play a role in the mediation of afferent transmission within the nucleus tractus solitarius in anaesthetized rats. Br. J. Pharmacol. 2009;158:1387–94. doi: 10.1111/j.1476-5381.2009.00410.x. doi:10.1111/j.1476-5381.2009.00410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrowski TD, Ostrowski D, Hasser EM, Kline DD. Depressed GABA and glutamate synaptic signaling by 5-HT1A receptors in the nucleus tractus solitarii and their role in cardiorespiratory function. J. Neurophysiol. 2014;111:2493–504. doi: 10.1152/jn.00764.2013. doi:10.1152/jn.00764.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otmakhova NA, Lewey J, Asrican B, Lisman JE. Inhibition of perforant path input to the CA1 region by serotonin and noradrenaline. J. Neurophysiol. 2005;94:1413–22. doi: 10.1152/jn.00217.2005. doi:10.1152/jn.00217.2005. [DOI] [PubMed] [Google Scholar]
- Ramirez-Navarro A, Glazebrook PA, Kane-Sutton M, Padro C, Kline DD, Kunze DL. Kv1.3 channels regulate synaptic transmission in the nucleus of solitary tract. J. Neurophysiol. 2011;105:2772–80. doi: 10.1152/jn.00494.2010. doi:10.1152/jn.00494.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raul L. Serotonin2 receptors in the nucleus tractus solitarius: Characterization and role in the baroreceptor reflex arc. Cell. Mol. Neurobiol. 2003 doi: 10.1023/A:1025096718559. doi:10.1023/A:1025096718559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renner U, Zeug A, Woehler A, Niebert M, Dityatev A, Dityateva G, Gorinski N, Guseva D, Abdel-Galil D, Fröhlich M, Döring F, Wischmeyer E, Richter DW, Neher E, Ponimaskin EG. Heterodimerization of serotonin receptors 5-HT1A and 5-HT7 differentially regulates receptor signalling and trafficking. J. Cell Sci. 2012;125:2486–99. doi: 10.1242/jcs.101337. doi:10.1242/jcs.101337. [DOI] [PubMed] [Google Scholar]
- Roberts C, Thomas DR, Bate ST, Kew JNC. GABAergic modulation of 5-HT7 receptor-mediated effects on 5-HT efflux in the guinea-pig dorsal raphe nucleus. Neuropharmacology. 2004;46:935–41. doi: 10.1016/j.neuropharm.2004.01.010. doi:10.1016/j.neuropharm.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Ruat M, Traiffort E, Leurs R, Tardivel-Lacombe J, Diaz J, Arrang JM, Schwartz JC. Molecular cloning, characterization, and localization of a high-affinity serotonin receptor (5-HT7) activating cAMP formation. Proc. Natl. Acad. Sci. U. S. A. 1993;90:8547–8551. doi: 10.1073/pnas.90.18.8547. doi:10.1073/pnas.90.18.8547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sévoz C, Callera JC, Machado BH, Hamon M, Laguzzi R. Role of serotonin3 receptors in the nucleus tractus solitarii on the carotid chemoreflex. Am. J. Physiol. 1997;272:H1250–H1259. doi: 10.1152/ajpheart.1997.272.3.H1250. [DOI] [PubMed] [Google Scholar]
- Shen Y, Monsma FJ, Metcalf MA, Jose PA, Hamblin MW, Sibley DR. Molecular cloning and expression of a 5-hydroxytryptamine7 serotonin receptor subtype. J. Biol. Chem. 1993;268:18200–18204. [PubMed] [Google Scholar]
- Shimizu M, Nishida A, Zensho H, Miyata M, Yamawaki S. Down-regulation of 5-hydroxytryptamine7 receptors by dexamethasone in rat frontocortical astrocytes. J. Neurochem. 1997;68:2604–2609. doi: 10.1046/j.1471-4159.1997.68062604.x. doi:10.1046/j.1471-4159.1997.68062604.x. [DOI] [PubMed] [Google Scholar]
- Takenaka R, Ohi Y, Haji A. Distinct modulatory effects of 5-HT on excitatory synaptic transmissions in the nucleus tractus solitarius of the rat. Eur. J. Pharmacol. 2011;671:45–52. doi: 10.1016/j.ejphar.2011.09.164. doi:10.1016/j.ejphar.2011.09.164. [DOI] [PubMed] [Google Scholar]
- Thomas DR, Atkinson PJ, Ho M, Bromidge SM, Lovell PJ, Villani AJ, Hagan JJ, Middlemiss DN, Price GW. [(3)H]-SB-269970--A selective antagonist radioligand for 5-HT(7) receptors. Br. J. Pharmacol. 2000;130:409–17. doi: 10.1038/sj.bjp.0703318. doi:10.1038/sj.bjp.0703318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thor KB, Helke CJ. Serotonin and substance P colocalization in medullary projections to the nucleus tractus solitarius: dual-colour immunohistochemistry combined with retrograde tracing. J. Chem. Neuroanat. 1988;2:139–148. [PubMed] [Google Scholar]
- Thor KB, Hill KM, Harrod C, Helke CJ. Immunohistochemical and Biochemical Analysis of Serotonin and Substance P Colocalization in the Nucleus Tractus Solitarii and Associated Afferent Ganglia of the Rat. Synapse. 1988;231:225–231. doi: 10.1002/syn.890020309. doi:10.1002/syn.890020309. [DOI] [PubMed] [Google Scholar]
- Tohda C, Sasaki M, Konemur T, Sasamura T, Itoh M, Kuraishi Y. Axonal transport of VR1 capsaicin receptor mRNA in primary afferents and its participation in inflammation-induced increase in capsaicin sensitivity. J. Neurochem. 2001;76:1628–1635. doi: 10.1046/j.1471-4159.2001.00193.x. doi:10.1046/j.1471-4159.2001.00193.x. [DOI] [PubMed] [Google Scholar]
- Tokarski K, Kusek M, Hess G. 5-HT7 receptors modulate GABAergic transmission in rat hippocampal CA1 area. J. Physiol. Pharmacol. 2011;62:535–40. [PubMed] [Google Scholar]
- Weissheimer KV, Machado BH. Inhibitory modulation of chemoreflex bradycardia by stimulation of the nucleus raphe obscurus is mediated by 5-HT3 receptors in the NTS of awake rats. Auton. Neurosci. 2007;132:27–36. doi: 10.1016/j.autneu.2006.09.002. doi:10.1016/j.autneu.2006.09.002. [DOI] [PubMed] [Google Scholar]
- Zubcevic J, Potts JT. Role of GABAergic neurones in the nucleus tractus solitarii in modulation of cardiovascular activity. Exp. Physiol. 2010;95:909–18. doi: 10.1113/expphysiol.2010.054007. doi:10.1113/expphysiol.2010.054007. [DOI] [PubMed] [Google Scholar]