Abstract
Tuberculosis (TB) granulomas are compact, organized agglomerations of infected and uninfected macrophages, T cells, neutrophils and other immune cells. Within the granuloma, several unique metabolic adaptations occur to modify the behavior of immune cells, potentially favoring bacterial persistence balanced with protection against immunopathology. These include the induction of arginase-1 in macrophages to temper nitric oxide (NO) production and block T cell proliferation, inhibition of oxygen-requiring NO production in hypoxic regions, and induction of tryptophan degrading enzymes that modify T cell proliferation and function. The spatial and time dependent organization of granulomas further influences immunometabolism, for example through lactate production by activated macrophages, which can induce arginase-1. Although complex, the metabolic changes in and around TB granulomas can be potentially modified by host-directed therapies. While elimination of the TB bacilli is often the goal of any anti-TB therapy, host-directed approaches must also account for the possibility of immunopathologic damage to the lung.
Keywords: tuberculosis, granuloma, L-arginine, L-tryptophan, hypoxia, metabolism
Introduction
Tuberculosis (TB) is the leading cause of lethality initiated by a single bacterial pathogen and remains an ongoing public health problem. The causative bacillus, Mycobacterium tuberculosis, has adapted to its primary host – us – by limiting severe pathology for the majority of its host’s life. Most TB researchers conclude active disease is a means to transmit to the next individual. Even though ~one third of the global population is infected with M. tuberculosis, few individuals develop active TB, while the other infected 90% carry a latent infection for the majority of their lives. In rare instances where the immune system is systemically weakened, a faster transition from initial infection to active disease is observed. This type of TB infection includes newborns and infants, elderly, those who are co-infected with immune-debilitating infections (such as HIV), and people with primary immunodeficiencies. Primary immunodeficiencies in people have been instrumental in the search for key regulators of human immunity and many of these genes were discovered on the basis of aberrant responses to immunization against M. tuberculosis via administration of the widely used Mycobacterium bovis bacillus Calmette-Guérin (BCG) live vaccine strain [1]. However, a fundamental question in TB research concerns what happens in otherwise healthy individuals to limit the pathology of TB infection at the expense of complete bacterial elimination.
Following inhalation of M. tuberculosis, cells of the immune system immediately try to contain and kill the pathogen. Instead of sterilizing immunity, infected cells – typically recruited macrophages from the blood monocyte pool – are unable to completely eliminate the bacillus, and an accumulation of host cells forms an organized granuloma that is thought to contain the infection [2]. Therefore in immune competent individuals, living bacilli remain within granulomas. Clinical sterility is considered attained following lengthy courses of antibiotic treatment that can last from 6 months to 2 years depending on the sensitivity of the particular strain of TB, although data suggests M. tuberculosis continues to persist in many treated patients even when declared sputum negative and clinically ‘cured’[3,4].
What is it about the granuloma not only limits escape of the pathogen, but also inhibits a fully effective immune response against M. tuberculosis? Is the immune system dampened to reduce tissue pathology of the host, but at the same time allowing bacterial survival? Is the granuloma beneficial to the host, the pathogen, or both? A more controversial question stemming from the latter concerns possible benefits of latent infections to the host. In this scenario, persisting bacilli within granulomas could help prevent a worse consequence, such as super-infection with more dangerous mycobacteria or other organisms. Such speculation is difficult to dissect because M. tuberculosis is a homotropic pathogen and animal models have limited usefulness for studying an infection that persists for years to decades (discussed briefly below and extensively in other reviews in this series).
Understanding the interface between M. tuberculosis and our immune system is expected to lead to host-directed therapeutic strategies (i.e. host-directed therapy, HDT) that can be used in combination with proven anti-mycobacterials [5]. One central host pathway required for immunity in general is the acquisition of nutrients needed to sustain macrophage and T cell activation [6]. Thus ‘immunometabolism’ is a new area to investigate the interplay between TB, the granuloma and the ongoing immune recognition of bacilli. Here, we review key data concerning the host-dependent nutritional regulation occurring within and surrounding the TB granuloma.
Nutrients and the granuloma
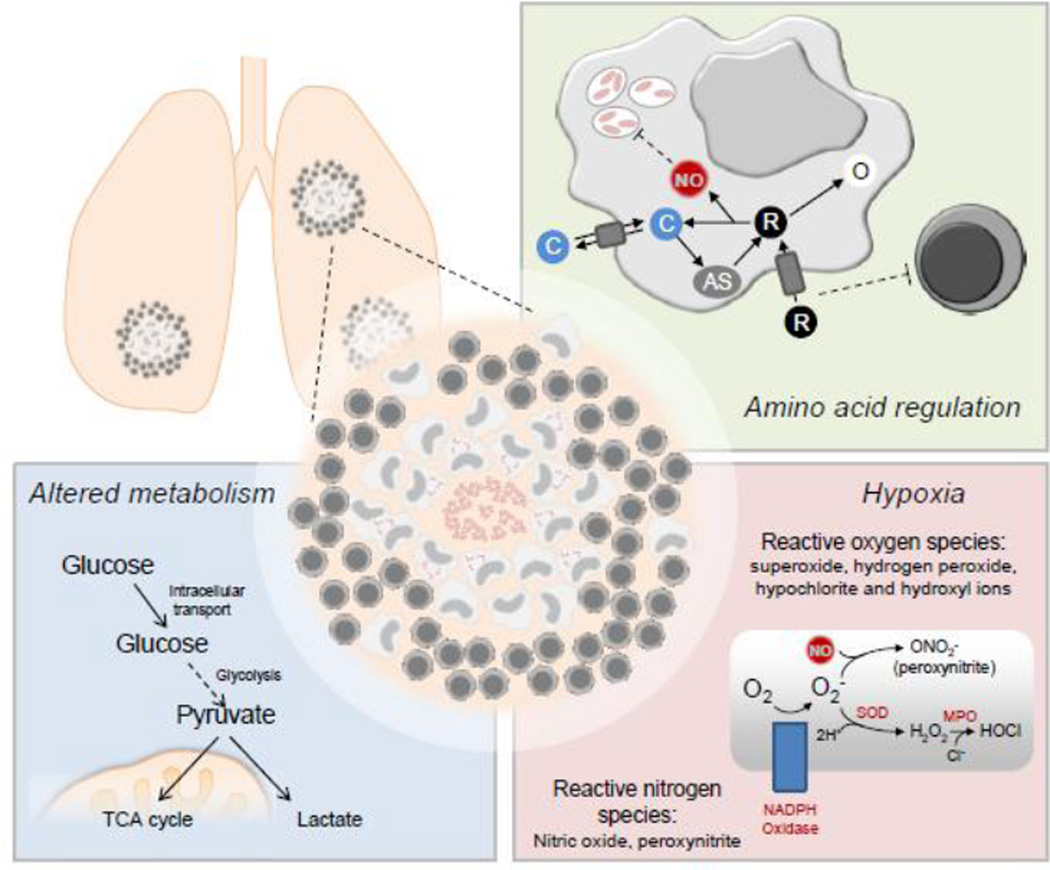
M. tuberculosis is thought to initially infect lung macrophages within the alveoli, leading to the recruitment of other inflammatory myeloid cells from the blood, dominated by monocyte-derived inflammatory macrophages but also neutrophils and dendritic cells [7]. During the first weeks of infection, the bacilli remain within macrophage phagosomes, and at the population level are resistant to anti-microbial mechanisms. A delayed adaptive response ensues, which promotes the mature granuloma structure [8]. Granulomas contain a central core of infected macrophages, surrounded by uninfected macrophages and other recruited myeloid cells, with a cusp of newly recruited lymphocytes present to assist in mycobacterial elimination. As most TB-infected people maintain a bacterial burden for decades, the granulomatous response is inefficient at attaining sterility. Over time the granuloma matures, resulting in a caseous core of dead and dying macrophages and numerous extracellular M. tuberculosis bacilli, which alter their lifestyle to reside within this low oxygen (hypoxic) and diminished nutrient microenvironment (Figure 1). Important work using PET imaging with glucose tracers has further established granulomas are dynamic within a given lung, and can change size and divide [9,10].
Fig. 1. Nutrition and metabolism in tuberculosis granulomas.
The tuberculosis granuloma response balances immune-mediated protection and tissue pathology in many ways, including amino acid regulation, hypoxia, and other metabolism alterations.
Three areas of nutrient acquisition within the TB granuloma have recently been focused on: 1) amino acid restriction and utilization, 2) the responses regulating hypoxia and oxygen supply, and 3) altered cellular respiration within immune cells (Figure 1). Host metabolic pathways involved in granuloma immunometabolism are not mutually exclusive and form a complex and elastic network that contributes to multiple aspects of the immune response to TB across time and space within the lung. Diverse experimental models have been developed to study these and other issues concerning the immune response upon mycobacterial infection, ranging from retrospective and necropsy analysis in the infected human population to infection studies in lower mammals, and invertebrates. Each of these models has benefits and drawbacks, which are summarized in Table 1 and described in detail in other reviews in this issue. The combination of TB models has provided an evolving picture of the immune response to M. tuberculosis, both within and outside of structured granulomas.
Table 1.
Models of M. tuberculosis infection
| Animal model | Pros | Cons | References |
|---|---|---|---|
| zebrafish (M. marinum infection) | inexpensive, fast generation time, trans-luminance, numerous genetic tools | lungs are absent | [106,107] |
| mouse | inexpensive, fast generation time (weeks), similar immune cells involved as in human tuberculosis, numerous genetic tools | does not form necrotic, hypoxic, or calcified granulomas* | [88,108,107,109] |
| guinea pig, rabbit | necrotic granuloma formation | limited genetic tools | [110,88,108,107,109] |
| cattle | necrotic granuloma formation, natural infection model | expensive, limited animal facilities, no genetic tools | [107] |
| non-human primates | necrotic granuloma formation, natural infection model, can develop both active and latent disease | expensive, limited animal facilities, long generation time, no genetic tools | [110,88,107,109] |
| human | natural infection | controlled infection experiments cannot be performed, natural genetic mutations/polymorphism defined retrospectively |
Local amino acid availability and the host response: Procuring L-arginine and L-tryptophan
Amino acids serve as the building blocks of proteins, so their importance in immune activity requiring cell division, cytokine and chemokine production and other de novo protein synthesis requirements is self-evident. In addition to this process, some amino acids, or their downstream metabolites, have been implicated as direct anti-microbial agents [11]. Furthermore, their regulation can serve as growth regulators via amino acid sequestration. Metabolism of two amino acids, L-arginine and L-tryptophan, has dominated discussion of amino acid metabolism in TB patients and in animal models of this disease.
L-arginine metabolism
In mammals, L-arginine is a semi-essential amino acid. In healthy adults, L-arginine is synthesized in the kidneys and acquired during protein catabolism. By contrast, in infants, individuals with traumatic stress (e.g. surgery), and in chronic infections (including TB, as discussed below), systemic L-arginine amounts are insufficient and must be acquired externally [12]. In homeostasis, L-arginine has two key biochemical functions: protein biosynthesis and as an intermediate in the liver urea cycle. In the latter, metabolism of L-arginine and the other metabolites in this cycle serve as a nitrogen transfer mechanism for removal of toxic ammonia in the form of non-toxic urea, which is disposed as waste in urine. Deficiency in any one of the urea cycle enzymes results in ammonia toxicity, which can be fatal unless controlled by dietary protein restriction [13].
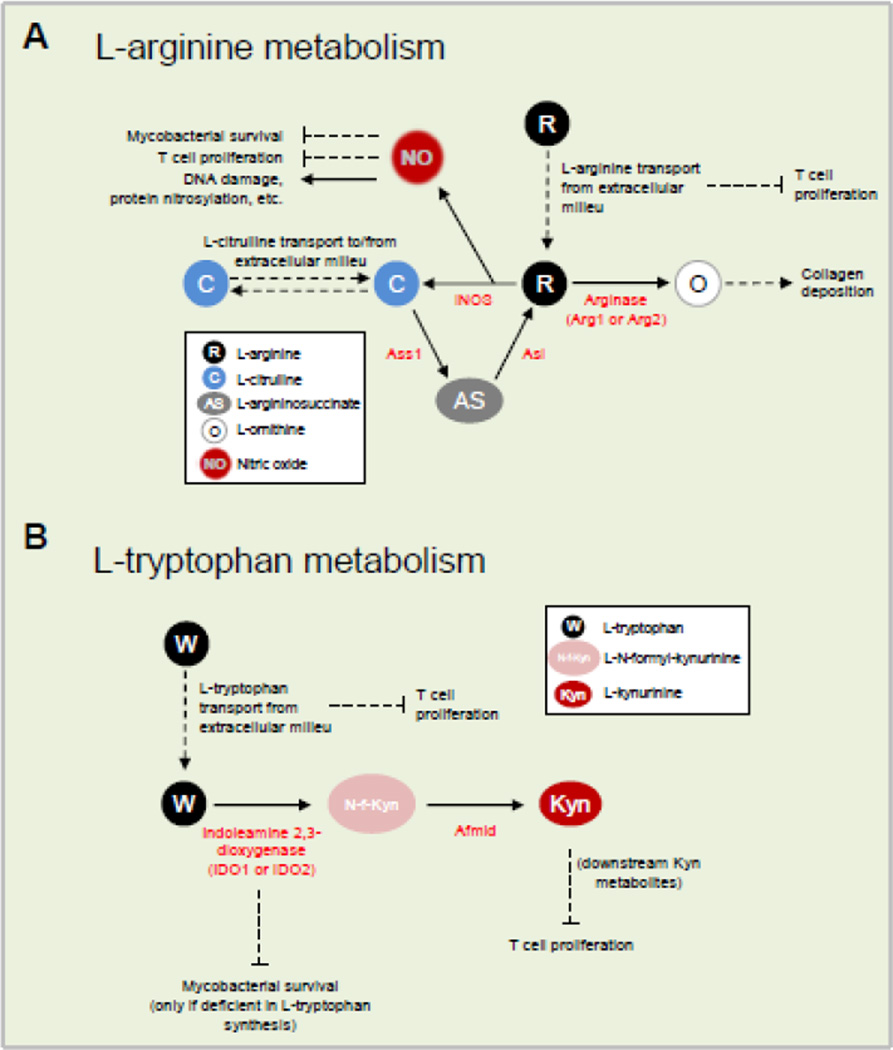
In immune responses, L-arginine has distinct functions. In activated myeloid cells, metabolism of L-arginine occurs by two principal pathways (in addition to protein biosynthesis requirements): iNOS-mediated production of nitric oxide (NO) or arginase-mediated production of L-ornithine (Figure 2A). In TB infections, L-arginine metabolism in activated myeloid cells is decisive in the outcome of the infection.
Fig. 2. Amino acid metabolism in immune cells.
A) L-arginine metabolism. B) L-tryptophan metabolism. Abbreviations: iNOS, inducible nitric oxide synthase; Arg, arginase; Ass1; argininosuccinate synthase; Asl, argininosuccinate lyase; IDO, indoleamine 2,3-dioxygenase; Afmid, kynurenine formamidase.
Production of NO from L-arginine
Inducible nitric oxide synthase, or iNOS, is one of three NO synthase enzymes and the major isoform involved in immune function. iNOS is inducible in immune cells, especially activated macrophages, as part of the anti-microbial pathway [14]. In the mouse, iNOS is upregulated at the mRNA and protein level by IFN-γ and pathogen products signaling through Toll-like receptors. Expression of iNOS results in high output of NO, which has potent mycobactericidal properties [15]. Indeed, mice with a genetic deficiency in Nos2 (encodes iNOS) are susceptible to infection with virulent M. tuberculosis and avirulent M. bovis BCG [16,17] as discussed in depth [18,15].
Reduction of NO production by competition for L-arginine
A central discovery about L-arginine immunometabolism in TB was that mycobacteria-infected macrophages express arginase-1 (Arg1). This cytosolic enzyme that metabolizes L-arginine (Figure 2A) is found in macrophages within TB infected tissue in mice, non-human primates, and humans [19–22]. A second arginase, called Arg2, performs the exact same enzymatic reaction as Arg1, but much less is understood about Arg2, and mice lacking Arg2 have a minimal phenotype [23]. Nevertheless, researchers should always consider the contributions of Arg2, especially in enzyme assays that read out the reaction products of arginase activity. Previous work had closely tied Arg1 expression to M2-like macrophages that do not make NO, and are linked with wound healing and tissue repair. For instance, early proponents of macrophage phenotyping concluded Arg1 and iNOS expression marked diametrically opposite functions of macrophages; an idea we now know to be false [24–26]. In the case of TB infection, Arg1 expression in macrophages has two vital roles. First, Arg1 limits NO output when macrophages coincidently express Arg1 and iNOS. As both enzymes consume L-arginine, less NO is made by activated macrophages. We created conditional Arg1-deficient mice and infected them with M. tuberculosis. Macrophages from these mice produced more NO and displayed enhanced bacilli clearance from the lungs as compared to controls [27]. Second, Arg1 depletes local L-arginine, which deprives lymphocytes of the amino acid. L-arginine deprivation from T cells is a central immunoregulatory pathway in TB (discussed below).
Regulation of L-arginine metabolizing enzymes in TB
Understanding the how, when, and where of gene and protein expression is a key tool in inferring the functions of immunometabolic enzymes. However, a fundamental problem in translating macrophage gene expression findings from mouse to humans lies in the cell types used. Mouse macrophages are easily isolated from tissues and grown in culture from bone marrow progenitors. By contrast, the vast majority of human macrophages and related cells are produced from blood monocytes. As the yield of mouse blood monocytes is low, direct comparisons between human and mouse monocyte-generated macrophages have not been performed, and have led to claims about species differences that cannot be interpreted on the basis of negative data (described below). We predict this complex scenario will be soon resolved, as the ability to analyze mouse monocyte-derived macrophages can be accomplished from single cells. Nevertheless, inferences about the expression of L-arginine metabolizing enzymes in the human TB granuloma can be deduced from indirect evidence.
iNOS transcription and translation is triggered by NF-κB and/or MAPK activity combined with IFN-γ mediated STAT1 signaling [18]. While this pathway has been well dissected in mouse macrophages, the role of iNOS-mediated metabolism of L-arginine in human macrophages is not as straightforward. Human macrophages derived from peripheral blood monocytes make significantly less NO under similar stimulation conditions as mouse macrophages in vitro [28]. And, unlike in mouse macrophages, a direct causation of iNOS function and severity of human TB does not exist. Yet, genome-wide association studies (GWAS) link polymorphisms within NOS2 to TB [29]. In addition, numerous studies have shown the expression of iNOS and NO production in macrophage rich areas within human TB granulomas and from macrophages removed from TB patients [20,30–35]. In recent years focus has been directed at the necessity of the pattern recognition receptor, NOD2, to direct iNOS activity in human macrophages. NOD2 is ligated by muramyl dipeptide, and is necessary for human macrophage control of M. tuberuculosis [36,37] and optimal anti-TB immune responses in mycobacteria-infected mice [38,39]. As with NOS2, NOD2 polymorphisms have also been observed in connection with TB severity [40–43]. Landes et al. observed that iNOS protein and function is upregulated in a NOD2-dependent fashion in human peripheral blood monocyte derived macrophages following in vitro M. tuberculosis infection [44]. Future work will determine if iNOS upregulated in this fashion is necessary for human macrophage-mediated defense to mycobacteria.
Understanding Arg1 expression is equally complex. Arg1 is highly upregulated following STAT6 signaling from either Th2 cytokines, IL-4 or IL-13, in mouse macrophages – a polarization phenotype of macrophages termed M2, or sometimes ‘alternative activation’ [45,46]. These cytokines are likely relevant in human TB as increases in IL-4 and IL-13 are correlated with lung damage [47–49]. In the mouse model of TB, Heitmann et al. used IL-13 overexpressing transgenic mice and observed increased Arg1 expression correlating with mature necrotizing granuloma formation following aerosol infection with virulent M. tuberculosis H37Rv. These mice developed severe disease and died after 20 weeks of infection, whereas the control-infected mice lived at least double that time [50]. However, the lung inflammatory environment in TB is M1 polarized, rather than M2. Therefore, a striking finding was that Arg1 can also be induced in a STAT6-independent manner in mycobacteria-infected macrophages following an autocrine/paracrine production of IL-6, IL-10, and G-CSF, which activate Arg1 via STAT3-mediated cell signaling [22,27,51]. This pathway of Arg1 activation is intriguing because not only the mycobacteria-infected macrophages, but surrounding uninfected macrophages are induced to make Arg1 – setting up a perimeter of L-arginine depletion early in infection. As with iNOS, however, the exact signals necessary for arginase induction in human macrophages surrounding M. tuberculosis granulomas have yet to be identified. In humans, Arg1 is made and secreted from activated neutrophils, and Munder and colleagues have established extracellular Arg1 can deplete L-arginine and block T cell proliferation [52]. Therefore, in human TB, Arg1 may be made from different cellular sources but control the same pathways – inhibiting NO and blocking T cell proliferation.
Macrophages can make L-arginine to sustain NO production
Generally, when one nutrient is necessary for essential functions, we anticipate multiple pathways exist for acquiring that nutrient. This is certainly the case for L-arginine. Returning to the urea cycle for guidance, L-arginine is synthesized de novo within the cycle from precursor amino acids and then destroyed by Arg1 to create L-ornithine and urea. Interestingly, one of the amino acid intermediates in the urea cycle is L-citrulline – the byproduct of iNOS-mediated L-arginine metabolism. We and others have shown that in L-arginine sufficient conditions, L-citrulline is exported from macrophages following NO synthesis [53–55]. However, once L-arginine becomes limiting in vitro, NO production is sustained by the intracellular generation of L-arginine from reimported L-citrulline (Figure 2A). To determine if this pathway was necessary in vivo, we generated bone marrow chimera mice containing the hematopoietic system of mice deficient in L-citrulline metabolism (Ass1fold/fold mice) and found that these mice were less efficient at clearing either M. tuberculosis or M. bovis BCG compared to control mice [53]. It will be important to determine whether this pathway is active in human macrophages in TB granulomas. In addition, resupplying intracellular L-arginine via L-citrulline metabolism to preserve cellular function extends to T cells [56–58]; however, the significance of this phenomenon has yet to be analyzed in human TB, or in animal models of mycobacterial infection.
Does L-arginine metabolism contribute to collagen production in TB?
Inhibiting NO production is only one outcome for arginase-mediated L-arginine metabolism in macrophages. Arginase activity results in urea plus L-ornithine, and downstream metabolites of L-ornithine include L-proline – a principle component of collagen [12]. Collagen is an extracellular matrix protein that is essential to granuloma formation and stability. While mycobacteria-infected macrophages produce L-ornithine, it is unlikely that they produce significant amounts of collagen because they do not express the enzyme ornithine aminotransferase (Oat), which is necessary to produce the immediate upstream precursor of L-proline called L-glutamate 5-semialdehyde [53]. We and others have demonstrated L-ornithine produced by activated macrophages is exported, similar to L-citrulline [55,59]. Fibroblasts are the major collagen-producing cells, and these cells may utilize L-ornithine expelled from macrophages with arginase activity. Indeed, a recent study using IL-13 overexpressing mice showed an increase in collagen density close to Arg1-expressing macrophages [50]. Other than this work, the specific contribution of macrophages to collagen formation via L-ornithine secretion is relatively unexplored in the context of M. tuberculosis infection.
T cell proliferation in granulomas is controlled by L-arginine
A second mechanism of arginase-mediated immune regulation involves the inhibition of T cell activity by L-arginine restriction. T cell proliferation and function is dependent on L-arginine. Therefore, local macrophages that consume L-arginine by the iNOS and/or Arg1 pathways can deplete L-arginine in poorly perfused areas (i.e. granulomas) and limit the availability to T cells. A key clue about L-arginine consumption in immunity came from genetic-based studies in another granulomatous disease, schitosomiasis. Here, Arg1+ macrophages deprive L-arginine from T cells recruited to worm egg antigens in the liver, reducing their ability to cause immunopathology. When Arg1 was specifically deleted in macrophages, a lethal immunopathologic T cell response was initiated [60]. In TB infections, however, separating the immune regulation of arginase from that of NO production in the context of M. tuberculosis infection is complicated by the absolute requirement for iNOS and NO. Duque-Correa et al. utilized a dermal M. tuberculosis infection model in Nos2−/− mice, which develop mature lung granulomas similar in structure to that found in humans and non-human primates [19,61]. In this and other models of TB in Nos2−/− mice, Arg1 expression is increased compared to iNOS sufficient controls [19,62]. Combining the Nos2−/− dermal infection model with the conditional Arg1-deficient mice mentioned above, mice doubly deficient in Arg1 and iNOS had a T cell-mediated pathology that correlated with increased granuloma number, size, and the number of necrotic granulomas. Furthermore, these mice were not able to clear M. tuberculosis as efficiently as the single Nos2−/− mice. Therefore, Arg1 appears to serve at least two functions during mycobacterial infection: blunt NO production, and limit T cell-mediated pathology. A plausible model for Arg1 function in TB is that both functions contribute to limiting host immunopathology, which is traded off with the host’s need to make abundant NO to kill TB bacilli.
L-tryptophan metabolism
L-tryptophan is essential because mammals do not possess the necessary enzymatic pathways for its synthesis and must acquire it from dietary or microbiota-based sources. In addition to being a building block of proteins, L-tryptophan is also a precursor for serotonin, melatonin, and niacin (vitamin B3) [11,63]. One catabolic pathway for L-tryptophan metabolism is implicated in immune responses to M. tuberculosis infection in human and animal models, and this involves the two enzymes of the indoleamine 2,3-dioxygenase class (IDO1 and IDO2). IDO enzymes perform the rate limiting first step of L-tryptophan metabolism to L-kynurenine and are expressed in different cell types including myeloid cells (Figure 2B). Indeed, downstream metabolites of L-kynurenine suppress T cell proliferation, and T cell apoptosis is induced by metabolites in the L-kynurenine pathway [64–66]. A similar pathway catabolizes L-tryptophan in the liver, and is mediated by L-tryptophan dioxengenase (TDO). IDO1, IDO2 and TDO therefore control L-tryptophan catabolism in different tissues. Both L-kynurenine production and local L-tryptophan depletion by IDO can be immunoregulatory. Discriminating between these two effects remains a contentious area [67]. IDO expression has been shown to inhibit T cell function, and has anti-microbial properties by limiting L-tryptophan to pathogens that are possible L-tryptophan auxotrophs, including select species from the Toxoplasma and Chlamydia genera [67]. However, M. tuberculosis synthesizes L-tryptophan, so IDO-mediated L-tryptophan depletion seems unlikely to be a direct host anti-TB effector.
IDO is induced in multiple cell types, including macrophages, dendritic cells, and endothelial cells. Most importantly IDO1 is induced in response to bacterial molecules and IFN-γ co-stimulation and IDO1 expression seems conserved between mice and humans [68–70]. In response to M. tuberculosis, macrophages express Ido1 both in vitro and in vivo [71,72]. Recently, Suzuki et al. performed correlates of IDO activity in human TB populations and determined IDO activity was a potential prognostic indicator of disease severity. They found L-tryptophan was decreased in the serum and pleural fluid of severe TB patients, with correlating increases in L-kynurenine concentrations [73,74]. In a study involving M. tuberculosis in macaques, Mehra et al. found the expression of IDO was focused in regions of the granuloma that were rich in macrophages [75]. Furthermore, worked performed by Ernst and colleagues described IFN-γ-triggered IDO expression in both hematopoietic and non-hematopoietic cells contribute to host immunity following M. tuberculosis infection in the mouse [76]. Collectively, these data argue IDO expression is a host reaction tied to the anti-TB immune response.
How might IDO activity be affecting the protection and/or pathology associated with M. tuberculosis infection? Similar to L-arginine metabolism, T cell function is impaired when in proximity to IDO-positive cells [77,78]. Furthermore, IDO activity correlates with the induction of regulatory T cells, which serve to reduce immune activity [79]. When IDO is inhibited using 1-methyltryptophan (1-MT), Li et al. found that T cells within the peripheral blood mononuclear cell population were rescued in their ability to produce Th1 cytokines – which were inhibited when these cells were cultured pleural fluid from TB patients [80]. In support of these studies, IDO1 has been shown to protect from T cell mediated disruption of granulomas in a Listeria monocytogenes infection model [81]. Therefore, the role of IDO in M. tuberculosis may be solely to protect the granuloma structure once it has been formed, thereby promoting containment of the infection. To determine the role for Ido1 in M. tuberculosis infection in mice, Blumenthal et al. infected control and Ido1−/− mice, but found genetic disruption of this enzyme did not lead to any changes in mycobacterial burden or mouse survival [71]. Interpretation of the above studies has three caveats. First, 1-MT from different commercial sources, the widely used IDO inhibitor, has recently been shown to be contaminated with L-tryptophan [82]. Thus, ‘rescue’ experiments where IDO is inhibited may reflect resupply of L-tryptophan into the experimental system. Second, the expression and function of both IDO1 and IDO2, which is also inducible in immune cells by a variety of stimuli [83–86], in a given experimental system has yet to be evaluated genetically since Ido1 and Ido2 are tightly linked preventing the efficient generation of a doubly-deficient mouse. Third, our own unpublished studies have shown only a single commercially-available anti-mouse IDO1 antibody (from five tested) is specific for IDO1. Taken together, the role of IDO proteins in TB or any other immunological system, requires systematic re-evaluation with validated reagents.
As mentioned above, M. tuberculosis can synthesize L-tryptophan, so limiting this amino acid by host IDO activity would be unlikely to inhibit mycobacterial growth. However, blocking M. tuberculosis L-tryptophan metabolism in the setting of IDO activity could prove an innovative host-directed strategy. Recent work by Zhang et al. highlighted this possibility [87]. In their model, they infected control mice, or mice lacking CD4+ T cells (H2-Ab−/−, which cannot produce MHC class II molecules) with a collection of M. tuberculosis transposon mutants to determine what mutants survived when the protective (i.e. IFN-γ-producing) CD4+ T cell population was absent as compared to those surviving in immune competent mice. M. tuberculosis mutants that were auxotrophic for L-tryptophan survived in mice lacking CD4+ T cells, but did not survive in control mice. Therefore, the presence of CD4+ T cells creates an environment forcing M. tuberculosis to synthesize its own L-tryptophan. Then, Zhang et al. showed inhibiting bacterial L-tryptophan synthesis with 2-amino-6-fluorobenzoic acid (6-FABA) in synergy with IFN-γ treatment blocked mycobacterial growth in vitro. Mice treated with 6-FABA (and the ester derivative of 6-FABA, which was less toxic in mice) showed improved M. tuberculosis clearance in the lung and spleen following aerosol infection in vivo [87]. Since mammals do not synthesize L-tryptophan, manipulating this pathway therapeutically could be considered in the ongoing fight against TB.
Hypoxia-induced stress: Coping with oxygen tension
One of the more common features of mature granulomas in TB patients is the necrotic core [88]. The core is hypoxic, and the host has numerous mechanisms to provide oxygen to tissues when low oxygen tension is detected. From the host perspective, one might expect forcing hypoxia onto a pathogen would serve some protection; however, mycobacteria survive in this harsh environment. One possible reason for bacterial proliferation in and around the hypoxic core is that key anti-microbial host functions require oxygen for their construction, including reactive oxygen species (ROS), and NO production: if there is no oxygen then iNOS cannot make NO from L-arginine. Furthermore, lack of mature vasculature within hypoxic microenvironment limits delivery of other nutrients to the granuloma, including those mentioned in this review so far, but also delivery of potential anti-mycobacterial drugs. Below we discuss some of the consequences of hypoxia within TB granulomas, how this alters metabolic programming, and how these events may regulate protection and/or pathology associated with disease.
Reactive oxygen and nitrogen species
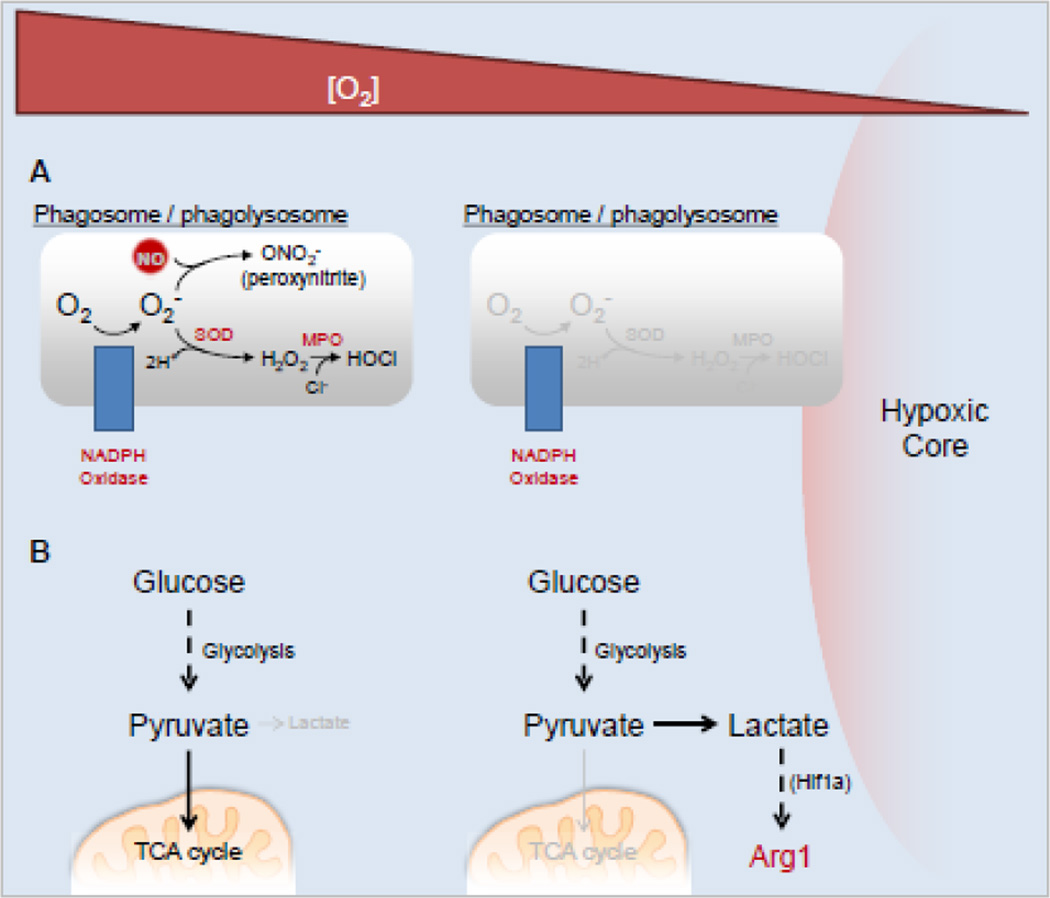
Many myeloid cells, including neutrophils and macrophages, produce ROS following appropriate upstream signaling, including pattern recognition receptor ligation and phagocytosis [89]. These signals trigger the formation of NADPH oxidase (aka, phagocyte oxidase), which converts atmospheric oxygen (O2) within a maturing phagosome to the free radical superoxide, which is then transformed to hydrogen peroxide via super oxide dismutase (Figure 3A). Hydrogen peroxide is then further converted to microbicidal molecules, including hypochlorite and hypobromite ions. In addition, NO (see Figure 2A) can react with superoxide to form cytotoxic peroxynitrite (Figure 3A), demonstrating the combination of immune mechanisms for anti-microbial purposes. Recent work has attempted to address the source of ROS, and the relative contribution of these species to host defense and tissue pathology. In a zebrafish model of TB, using Mycobacterium marinum as a model pathogen, Yang et al. analyzed the contribution of ROS from neutrophils recruited to granulomas [90]. They found that neutrophils were recruited via signals from dying M. marinum-infected macrophages. Some of the recruited neutrophils phagocytosed the dying macrophages, and killed the contained M. marinum. This occurred in a ROS-dependent fashion as NADPH oxidase deficient zebrafish displayed a reduced capacity to kill internalized mycobacteria. It may be that macrophages cannot produce ROS and kill M. marinum due to hypoxia within the granuloma, and rely on newly recruited neutrophils with a fresh supply of O2 to generate ROS and provide the killing mechanisms macrophages were unable to do. This may also be a mechanism to prevent tissue pathology, as ROS have been shown to damage M. tuberculosis-infected tissue [91].
Fig. 3. Altered microbicidal agent production and metabolism in hypoxic microenvironments.
A) ROS and RNS production in cells within tuberculosis granulomas become less efficient approaching areas of hypoxia. B) Anaerobic glycolysis dominates glucose metabolism in hypoxic microenvironments, leading to lactate production and downstream effectors triggered by lactate (e.g. Arg1). Abbreviations: SOD, superoxide dismutase; MPO, myeloperoxidase.
Although the previous model downplays the importance for ROS generation in macrophages, others have shown the role of NADPH oxidase in macrophages is vital. Using mouse models of chronic granulomatous disease (CGD, loss of NADPH function) and a high dose intravenous M. bovis BCG infection, Deffert et al. described a critical role for macrophage ROS production that could not be compensated by production of these species by neutrophils [92]. The authors selectively rescued ROS production in macrophages in Ncf1−/− mice (human CD68 driven wild type Ncf1, encoding the p47phox subunit of NADPH oxidase), but not in neutrophils, and saw that the rescued mice were protected from BCG-induced weight loss and death observed in the CGD control mice, even though bacterial clearance was not drastically altered. The authors also analyzed iNOS and NO production, but found little difference between all infected groups and hypothesized that the production of NO alone may not deliver sufficient mycobactericidal activity without the development of peroxynitrite from ROS production. Regardless, the presence of macrophage ROS production lead to an overall increase in activated phagocyte clustering and development of compact granulomas. The data on ROS in TB correlate with unusual human mutants defective in macrophage ROS production. Bustamante et al. discovered a small number of people with germline mutations in CYBB (encoding the p91phox subunit of the NADPH oxidase). Even though these individuals had a defective neutrophil respiratory burst, the disease phenotype was manifested in monocyte-derived macrophages rather than neutrophils [93].
Glycolysis and cellular respiration
Inflammation and involvement of many metabolically active cells during different stages of TB infection have provided a unique opportunity to track granuloma dynamics by whole body imaging. Activated M1 macrophages (and other cells in and around the granuloma such as actively dividing T cells) have high glycolytic activity, and thus import large amounts of glucose for glycolytic, or Warburg-type, metabolism (Figure 3B). Exploiting computed tomography (CT) in combination with positron emission tomography (PET) scanning with the use of traceable glucose (18F-fluorodeoxyglucose, FDG), researchers have been able to image granulomas in live patients and animals (referred to as FDG PET/CT) [94,95]. Furthermore, depending on the amount of FDG uptake within a granuloma, a correlation between whether the lesion is active or latent can be inferred [95]. Using this technology on macaques infected with a low dose of virulent M. tuberculosis, Coleman et al. found a correlation between the number of FDG+ granulomas that formed early following infection and the likelihood of developing active disease [96]. That is, the more FDG+ granulomas one has early during infection, they are more likely to develop active, versus latent, disease later in life. PET scanning will also be useful in demonstrating responses to antibiotics as Via et al. saw granuloma size and FDG activity decrease following anti-TB therapy with either Rifampin or Isoniazid in rabbits [97].
PET imaging, however, does not differentiate glucose uptake from the type of glycolysis or cellular respiration initiated. Different glycolytic endpoints exist whether cells are in hypoxic, or normoxic, conditions. To determine how cells are metabolizing glucose during M. tuberculosis infection in the guinea pig, Somashekar et al. performed high resolution magnetic angle spinning nuclear magnetic resonance (HRMAS NMR) and detected increases in lactate (lactic acid) elevating as disease progressed in the lungs of infected animals. They suggested lactate detection could represent anaerobic glycolysis, which would be likely as mature granulomas would demand low oxygen metabolism (Figure 3B). Alternatively, the authors suggested the increase in lactate could be from metabolism due to the Warburg effect, generally observed in cancer cells [98]. In this case, briefly, lactate is released from cells following glycolysis, when cells are in aerobic conditions. Higher relative amounts of lactate have also been detected in the serum of M. tuberculosis and M. bovis infected cattle, and in the lung, spleen, and liver of M. tuberculosis infected mice using metabolic profiling, suggesting at least that lactate release (irrespective of anaerobic glycolysis versus Warburg effect) is common in mycobacteria infection across species [99,100]. In addition to lactate indicating an increase in glycolysis, it also has an important link back to amino acid metabolism. Lactate induces a macrophage phenotype characterized by increased Arg1 activity [101], which we hypothesize would further dampen NO production. Hypoxia also increases arginase expression in some systems [102–104] and thus a key anti-microbial effector – NO – takes a ‘double hit’ in the substructures of granulomas where it is needed the most; the high hypoxia, high lactate environment is oxygen limited and L-arginine limited by Arg1. Further work on the spatial segregation of the different cells expressing iNOS, Arg1, IDO correlated with hypoxia and T cells will be an important step in determining the evolution of granuloma structure and how these dynamic processes harm or help the host versus bacteria.
Conclusions
Over the past decade a wealth of new knowledge has emerged about TB granuloma structure and dynamics. In addition, new ideas about positive and negative roles of granulomas have been proposed and investigated using human and ever-increasing sophistication of animal modeling. Nevertheless, many questions remain about the host response to TB and how it can be manipulated to reduce the global health burden of TB. From our perspective on the immunology and immunometabolic events in granulomas, we can identify three key questions for the field. These are (i) what is the nature and function of the differential spatial organization of immune cells within the granuloma and how does the expression of the different anti-TB enzyme systems change? Answering this question will require better reagents to track key enzymes, application of reporters coupled to the expression of key genes (e.g. GFP, mCherry) crossed into the newer generation mouse models where granulomas adopt more features of human TB lesions, and an appreciation of the dynamic elasticity of granuloma architecture within the lung of the same host. A second question (ii) concerns how host directed therapy can be used to manipulate the outcome on TB when combined with anti-TB drugs. Although there have been exciting advances in this area, the potential for immunopathology needs to be accounted for. For example, increasing the output of NO (for example, with an arginase inhibitor) may greatly enhance the efficacy of TB drugs but at the same time cause NO-mediated host damage. A third (iii) question concerns granuloma-specific metabolic effects on T cells. As T cells are essential to constrain TB, understanding the influence of local metabolic cues on TB-specific T cells trafficking between granuloma and draining lymph nodes will be imperative. For example, we have already detailed the key role of arginine depletion on blocking the genesis of an immunopathologic T cell response. However, the effects of L-tryptophan depletion and L-kynurenines, glycolysis and glucose, and hypoxia on an efficient T cell response remain largely unexplored. For example, activated CD4+ T cells require glycolysis for IFN-γ production [105]. How can this finding be translated into evaluation of the T cell response within a granuloma?
Acknowledgments
This work was supported by Cincinnati Children’s Hospital Medical Center Trustee Award, the Division of Infectious Diseases, and the American Heart Association Scientist Development Grant 15SDG21550007 (JEQ) and St. Jude Children’s Research Hospital Cancer Center Core Grant and the American Lebanese Syrian Associated Charities (PJM).
References
- 1.Bustamante J, Boisson-Dupuis S, Abel L, Casanova JL. Mendelian susceptibility to mycobacterial disease: genetic, immunological, and clinical features of inborn errors of IFN-gamma immunity. Semin Immunol. 2014;26(6):454–470. doi: 10.1016/j.smim.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Orme IM, Robinson RT, Cooper AM. The balance between protective and pathogenic immune responses in the TB-infected lung. Nat Immunol. 2015;16(1):57–63. doi: 10.1038/ni.3048. [DOI] [PubMed] [Google Scholar]
- 3.Das B, Kashino SS, Pulu I, Kalita D, Swami V, Yeger H, Felsher DW, Campos-Neto A. CD271(+) bone marrow mesenchymal stem cells may provide a niche for dormant Mycobacterium tuberculosis. Sci Transl Med. 2013;5(170):170ra113. doi: 10.1126/scitranslmed.3004912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Panjabi R, Comstock GW, Golub JE. Recurrent tuberculosis and its risk factors: adequately treated patients are still at high risk. Int J Tuberc Lung Dis. 2007;11(8):828–837. [PubMed] [Google Scholar]
- 5.Wallis RS, Hafner R. Advancing host-directed therapy for tuberculosis. Nat Rev Immunol. 2015;15(4):255–263. doi: 10.1038/nri3813. [DOI] [PubMed] [Google Scholar]
- 6.Norata GD, Caligiuri G, Chavakis T, Matarese G, Netea MG, Nicoletti A, O'Neill LA, Marelli-Berg FM. The Cellular and Molecular Basis of Translational Immunometabolism. Immunity. 2015;43(3):421–434. doi: 10.1016/j.immuni.2015.08.023. [DOI] [PubMed] [Google Scholar]
- 7.Dorhoi A, Kaufmann SH. Perspectives on host adaptation in response to Mycobacterium tuberculosis: modulation of inflammation. Semin Immunol. 2014;26(6):533–542. doi: 10.1016/j.smim.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 8.Cooper AM, Torrado E. Protection versus pathology in tuberculosis: recent insights. Curr Opin Immunol. 2012;24(4):431–437. doi: 10.1016/j.coi.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gideon HP, Phuah J, Myers AJ, Bryson BD, Rodgers MA, Coleman MT, Maiello P, Rutledge T, Marino S, Fortune SM, Kirschner DE, Lin PL, Flynn JL. Variability in tuberculosis granuloma T cell responses exists, but a balance of pro- and anti-inflammatory cytokines is associated with sterilization. PLoS Pathog. 2015;11(1):e1004603. doi: 10.1371/journal.ppat.1004603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin PL, Coleman T, Carney JP, Lopresti BJ, Tomko J, Fillmore D, Dartois V, Scanga C, Frye LJ, Janssen C, Klein E, Barry CE, 3rd, Flynn JL. Radiologic responses in cynomolgous macaques for assessing tuberculosis chemotherapy regimens. Antimicrob Agents Chemother. 2013 doi: 10.1128/AAC.00277-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li P, Yin YL, Li D, Kim SW, Wu G. Amino acids and immune function. Br J Nutr. 2007;98(2):237–252. doi: 10.1017/S000711450769936X. [DOI] [PubMed] [Google Scholar]
- 12.Morris SM., Jr Arginine metabolism: boundaries of our knowledge. J Nutr. 2007;137(6) Suppl 2:1602S–1609S. doi: 10.1093/jn/137.6.1602S. [DOI] [PubMed] [Google Scholar]
- 13.Batshaw ML, Tuchman M, Summar M, Seminara J Members of the Urea Cycle Disorders C. A longitudinal study of urea cycle disorders. Mol Genet Metab. 2014;113(1–2):127–130. doi: 10.1016/j.ymgme.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rauch I, Muller M, Decker T. The regulation of inflammation by interferons and their STATs. JAKSTAT. 2013;2(1):e23820. doi: 10.4161/jkst.23820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.MacMicking J, Xie QW, Nathan C. Nitric oxide and macrophage function. Annu Rev Immunol. 1997;15:323–350. doi: 10.1146/annurev.immunol.15.1.323. [DOI] [PubMed] [Google Scholar]
- 16.MacMicking JD, North RJ, LaCourse R, Mudgett JS, Shah SK, Nathan CF. Identification of nitric oxide synthase as a protective locus against tuberculosis. Proc Natl Acad Sci U S A. 1997;94(10):5243–5248. doi: 10.1073/pnas.94.10.5243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garcia I, Guler R, Vesin D, Olleros ML, Vassalli P, Chvatchko Y, Jacobs M, Ryffel B. Lethal Mycobacterium bovis Bacillus Calmette Guerin infection in nitric oxide synthase 2-deficient mice: cell-mediated immunity requires nitric oxide synthase 2. Lab Invest. 2000;80(9):1385–1397. doi: 10.1038/labinvest.3780146. [DOI] [PubMed] [Google Scholar]
- 18.Bogdan C. Nitric oxide synthase in innate and adaptive immunity: an update. Trends Immunol. 2015;36(3):161–178. doi: 10.1016/j.it.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 19.Duque-Correa MA, Kuhl AA, Rodriguez PC, Zedler U, Schommer-Leitner S, Rao M, Weiner J, 3rd, Hurwitz R, Qualls JE, Kosmiadi GA, Murray PJ, Kaufmann SH, Reece ST. Macrophage arginase-1 controls bacterial growth and pathology in hypoxic tuberculosis granulomas. Proc Natl Acad Sci U S A. 2014;111(38):E4024–E4032. doi: 10.1073/pnas.1408839111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mattila JT, Ojo OO, Kepka-Lenhart D, Marino S, Kim JH, Eum SY, Via LE, Barry CE, 3rd, Klein E, Kirschner DE, Morris SM, Jr, Lin PL, Flynn JL. Microenvironments in tuberculous granulomas are delineated by distinct populations of macrophage subsets and expression of nitric oxide synthase and arginase isoforms. J Immunol. 2013;191(2):773–784. doi: 10.4049/jimmunol.1300113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pessanha AP, Martins RA, Mattos-Guaraldi AL, Vianna A, Moreira LO. Arginase-1 expression in granulomas of tuberculosis patients. FEMS Immunol Med Microbiol. 2012;66(2):265–268. doi: 10.1111/j.1574-695X.2012.01012.x. [DOI] [PubMed] [Google Scholar]
- 22.Qualls JE, Neale G, Smith AM, Koo MS, DeFreitas AA, Zhang H, Kaplan G, Watowich SS, Murray PJ. Arginine usage in mycobacteria-infected macrophages depends on autocrine-paracrine cytokine signaling. Sci Signal. 2010;3(135):ra62. doi: 10.1126/scisignal.2000955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shi O, Morris SM, Jr, Zoghbi H, Porter CW, O'Brien WE. Generation of a mouse model for arginase II deficiency by targeted disruption of the arginase II gene. Mol Cell Biol. 2001;21(3):811–813. doi: 10.1128/MCB.21.3.811-813.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mills CD. M1 and M2 Macrophages: Oracles of Health and Disease. Crit Rev Immunol. 2012;32(6):463–488. doi: 10.1615/critrevimmunol.v32.i6.10. [DOI] [PubMed] [Google Scholar]
- 25.Mills CD. Anatomy of a discovery: m1 and m2 macrophages. Front Immunol. 2015;6:212. doi: 10.3389/fimmu.2015.00212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mills CD, Ley K. M1 and M2 macrophages: the chicken and the egg of immunity. J Innate Immun. 2014;6(6):716–726. doi: 10.1159/000364945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.El Kasmi KC, Qualls JE, Pesce JT, Smith AM, Thompson RW, Henao-Tamayo M, Basaraba RJ, Konig T, Schleicher U, Koo MS, Kaplan G, Fitzgerald KA, Tuomanen EI, Orme IM, Kanneganti TD, Bogdan C, Wynn TA, Murray PJ. Toll-like receptor-induced arginase 1 in macrophages thwarts effective immunity against intracellular pathogens. Nat Immunol. 2008;9(12):1399–1406. doi: 10.1038/ni.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thomas AC, Mattila JT. "Of mice and men": arginine metabolism in macrophages. Front Immunol. 2014;5:479. doi: 10.3389/fimmu.2014.00479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Azad AK, Sadee W, Schlesinger LS. Innate immune gene polymorphisms in tuberculosis. Infect Immun. 2012;80(10):3343–3359. doi: 10.1128/IAI.00443-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Choi HS, Rai PR, Chu HW, Cool C, Chan ED. Analysis of nitric oxide synthase and nitrotyrosine expression in human pulmonary tuberculosis. Am J Respir Crit Care Med. 2002;166(2):178–186. doi: 10.1164/rccm.2201023. [DOI] [PubMed] [Google Scholar]
- 31.Jagannath C, Actor JK, Hunter RL., Jr Induction of nitric oxide in human monocytes and monocyte cell lines by Mycobacterium tuberculosis. Nitric Oxide. 1998;2(3):174–186. doi: 10.1006/niox.1998.9999. [DOI] [PubMed] [Google Scholar]
- 32.Nicholson S, Bonecini-Almeida Mda G, Lapa e Silva JR, Nathan C, Xie QW, Mumford R, Weidner JR, Calaycay J, Geng J, Boechat N, Linhares C, Rom W, Ho JL. Inducible nitric oxide synthase in pulmonary alveolar macrophages from patients with tuberculosis. J Exp Med. 1996;183(5):2293–2302. [Google Scholar]
- 33.Ralph AP, Yeo TW, Salome CM, Waramori G, Pontororing GJ, Kenangalem E, Sandjaja, Tjitra E, Lumb R, Maguire GP, Price RN, Chatfield MD, Kelly PM, Anstey NM. Impaired pulmonary nitric oxide bioavailability in pulmonary tuberculosis: association with disease severity and delayed mycobacterial clearance with treatment. J Infect Dis. 2013;208(4):616–626. doi: 10.1093/infdis/jit248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rich EA, Torres M, Sada E, Finegan CK, Hamilton BD, Toossi Z. Mycobacterium tuberculosis (MTB)-stimulated production of nitric oxide by human alveolar macrophages and relationship of nitric oxide production to growth inhibition of MTB. Tuber Lung Dis. 1997;78(5–6):247–255. doi: 10.1016/s0962-8479(97)90005-8. [DOI] [PubMed] [Google Scholar]
- 35.Wang CH, Liu CY, Lin HC, Yu CT, Chung KF, Kuo HP. Increased exhaled nitric oxide in active pulmonary tuberculosis due to inducible NO synthase upregulation in alveolar macrophages. Eur Respir J. 1998;11(4):809–815. doi: 10.1183/09031936.98.11040809. [DOI] [PubMed] [Google Scholar]
- 36.Brooks MN, Rajaram MV, Azad AK, Amer AO, Valdivia-Arenas MA, Park JH, Nunez G, Schlesinger LS. NOD2 controls the nature of the inflammatory response and subsequent fate of Mycobacterium tuberculosis and M. bovis BCG in human macrophages. Cell Microbiol. 2011;13(3):402–418. doi: 10.1111/j.1462-5822.2010.01544.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Juarez E, Carranza C, Hernandez-Sanchez F, Leon-Contreras JC, Hernandez-Pando R, Escobedo D, Torres M, Sada E. NOD2 enhances the innate response of alveolar macrophages to Mycobacterium tuberculosis in humans. Eur J Immunol. 2012;42(4):880–889. doi: 10.1002/eji.201142105. [DOI] [PubMed] [Google Scholar]
- 38.Divangahi M, Mostowy S, Coulombe F, Kozak R, Guillot L, Veyrier F, Kobayashi KS, Flavell RA, Gros P, Behr MA. NOD2-deficient mice have impaired resistance to Mycobacterium tuberculosis infection through defective innate and adaptive immunity. J Immunol. 2008;181(10):7157–7165. doi: 10.4049/jimmunol.181.10.7157. doi:181/10/7157 [pii] [DOI] [PubMed] [Google Scholar]
- 39.Gandotra S, Jang S, Murray PJ, Salgame P, Ehrt S. Nucleotide-binding oligomerization domain protein 2-deficient mice control infection with Mycobacterium tuberculosis. Infect Immun. 2007;75(11):5127–5134. doi: 10.1128/IAI.00458-07. doi:IAI.00458-07 [pii] 10.1128/IAI.00458-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Austin CM, Ma X, Graviss EA. Common nonsynonymous polymorphisms in the NOD2 gene are associated with resistance or susceptibility to tuberculosis disease in African Americans. J Infect Dis. 2008;197(12):1713–1716. doi: 10.1086/588384. [DOI] [PubMed] [Google Scholar]
- 41.Pan H, Ping XC, Zhu HJ, Gong FY, Dong CX, Li NS, Wang LJ, Yang HB. Association of myostatin gene polymorphisms with obesity in Chinese north Han human subjects. Gene. 2011;494(2):237–241. doi: 10.1016/j.gene.2011.10.045. doi:S0378-1119(11)00651-2 [pii] 10.1016/j.gene.2011.10.045. [DOI] [PubMed] [Google Scholar]
- 42.Wang C, Chen ZL, Pan ZF, Wei LL, Xu DD, Jiang TT, Zhang X, Ping ZP, Li ZJ, Li JC. NOD2 polymorphisms and pulmonary tuberculosis susceptibility: a systematic review and meta-analysis. Int J Biol Sci. 2013;10(1):103–108. doi: 10.7150/ijbs.7585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhao M, Jiang F, Zhang W, Li F, Wei L, Liu J, Xue Y, Deng X, Wu F, Zhang L, Zhang X, Zhang Y, Fan D, Sun X, Jiang T, Li JC. A novel single nucleotide polymorphism within the NOD2 gene is associated with pulmonary tuberculosis in the Chinese Han, Uygur and Kazak populations. BMC Infect Dis. 2012;12:91. doi: 10.1186/1471-2334-12-91. doi:1471-2334-12-91 [pii] 10.1186/1471-2334-12-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Landes MB, Rajaram MV, Nguyen H, Schlesinger LS. Role for NOD2 in Mycobacterium tuberculosis-induced iNOS expression and NO production in human macrophages. J Leukoc Biol. 2015;97(6):1111–1119. doi: 10.1189/jlb.3A1114-557R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3(1):23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 46.Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32(5):593–604. doi: 10.1016/j.immuni.2010.05.007. doi:S1074-7613(10)00173-1 [pii] 10.1016/j.immuni.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 47.Rook GA, Hernandez-Pando R, Dheda K, Teng Seah G. IL-4 in tuberculosis: implications for vaccine design. Trends Immunol. 2004;25(9):483–488. doi: 10.1016/j.it.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 48.van Crevel R, Karyadi E, Preyers F, Leenders M, Kullberg BJ, Nelwan RH, van der Meer JW. Increased production of interleukin 4 by CD4+ and CD8+ T cells from patients with tuberculosis is related to the presence of pulmonary cavities. J Infect Dis. 2000;181(3):1194–1197. doi: 10.1086/315325. [DOI] [PubMed] [Google Scholar]
- 49.Ashenafi S, Aderaye G, Bekele A, Zewdie M, Aseffa G, Hoang AT, Carow B, Habtamu M, Wijkander M, Rottenberg M, Aseffa A, Andersson J, Svensson M, Brighenti S. Progression of clinical tuberculosis is associated with a Th2 immune response signature in combination with elevated levels of SOCS3. Clin Immunol. 2014;151(2):84–99. doi: 10.1016/j.clim.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 50.Heitmann L, Abad Dar M, Schreiber T, Erdmann H, Behrends J, McKenzie AN, Brombacher F, Ehlers S, Holscher C. The IL-13/IL-4Ralpha axis is involved in tuberculosis-associated pathology. J Pathol. 2014;234(3):338–350. doi: 10.1002/path.4399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guler R, Parihar SP, Savvi S, Logan E, Schwegmann A, Roy S, Nieuwenhuizen NE, Ozturk M, Schmeier S, Suzuki H, Brombacher F. IL-4Ralpha-dependent alternative activation of macrophages is not decisive for Mycobacterium tuberculosis pathology and bacterial burden in mice. PLoS One. 2015;10(3):e0121070. doi: 10.1371/journal.pone.0121070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Munder M, Schneider H, Luckner C, Giese T, Langhans CD, Fuentes JM, Kropf P, Mueller I, Kolb A, Modolell M, Ho AD. Suppression of T-cell functions by human granulocyte arginase. Blood. 2006;108(5):1627–1634. doi: 10.1182/blood-2006-11-010389. [DOI] [PubMed] [Google Scholar]
- 53.Qualls JE, Subramanian C, Rafi W, Smith AM, Balouzian L, DeFreitas AA, Shirey KA, Reutterer B, Kernbauer E, Stockinger S, Decker T, Miyairi I, Vogel SN, Salgame P, Rock CO, Murray PJ. Sustained generation of nitric oxide and control of mycobacterial infection requires argininosuccinate synthase 1. Cell Host Microbe. 2012;12(3):313–323. doi: 10.1016/j.chom.2012.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hibbs JB, Jr, Taintor RR, Vavrin Z, Rachlin EM. Nitric oxide: a cytotoxic activated macrophage effector molecule. Biochem Biophys Res Commun. 1988;157(1):87–94. doi: 10.1016/s0006-291x(88)80015-9. doi:S0006-291X(88)80015-9 [pii] [DOI] [PubMed] [Google Scholar]
- 55.Benninghoff B, Lehmann V, Eck HP, Droge W. Production of citrulline and ornithine by interferon-gamma treated macrophages. Int Immunol. 1991;3(5):413–417. doi: 10.1093/intimm/3.5.413. [DOI] [PubMed] [Google Scholar]
- 56.Bansal V, Rodriguez P, Wu G, Eichler DC, Zabaleta J, Taheri F, Ochoa JB. Citrulline can preserve proliferation and prevent the loss of CD3 zeta chain under conditions of low arginine. JPEN J Parenter Enteral Nutr. 2004;28(6):423–430. doi: 10.1177/0148607104028006423. [DOI] [PubMed] [Google Scholar]
- 57.Rodriguez PC, Quiceno DG, Ochoa AC. L-arginine availability regulates T-lymphocyte cell-cycle progression. Blood. 2007;109(4):1568–1573. doi: 10.1182/blood-2006-06-031856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tarasenko TN, Gomez-Rodriguez J, McGuire PJ. Impaired T cell function in argininosuccinate synthetase deficiency. J Leukoc Biol. 2015;97(2):273–278. doi: 10.1189/jlb.1AB0714-365R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rapovy SM, Zhao J, Bricker RL, Schmidt SM, Setchell KD, Qualls JE. Differential Requirements for l-Citrulline and l-Arginine during Antimycobacterial Macrophage Activity. J Immunol. 2015 doi: 10.4049/jimmunol.1500800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pesce JT, Ramalingam TR, Mentink-Kane MM, Wilson MS, El Kasmi KC, Smith AM, Thompson RW, Cheever AW, Murray PJ, Wynn TA. Arginase-1-expressing macrophages suppress Th2 cytokine-driven inflammation and fibrosis. PLoS Pathog. 2009;5(4):e1000371. doi: 10.1371/journal.ppat.1000371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Reece ST, Loddenkemper C, Askew DJ, Zedler U, Schommer-Leitner S, Stein M, Mir FA, Dorhoi A, Mollenkopf HJ, Silverman GA, Kaufmann SH. Serine protease activity contributes to control of Mycobacterium tuberculosis in hypoxic lung granulomas in mice. J Clin Invest. 2010;120(9):3365–3376. doi: 10.1172/JCI42796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Obregon-Henao A, Henao-Tamayo M, Orme IM, Ordway DJ. Gr1(int)CD11b+ myeloid-derived suppressor cells in Mycobacterium tuberculosis infection. PLoS One. 2013;8(11):e80669. doi: 10.1371/journal.pone.0080669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Le Floc'h N, Otten W, Merlot E. Tryptophan metabolism, from nutrition to potential therapeutic applications. Amino Acids. 2011;41(5):1195–1205. doi: 10.1007/s00726-010-0752-7. [DOI] [PubMed] [Google Scholar]
- 64.Munn DH, Shafizadeh E, Attwood JT, Bondarev I, Pashine A, Mellor AL. Inhibition of T cell proliferation by macrophage tryptophan catabolism. J Exp Med. 1999;189(9):1363–1372. doi: 10.1084/jem.189.9.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lee GK, Park HJ, Macleod M, Chandler P, Munn DH, Mellor AL. Tryptophan deprivation sensitizes activated T cells to apoptosis prior to cell division. Immunology. 2002;107(4):452–460. doi: 10.1046/j.1365-2567.2002.01526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fallarino F, Grohmann U, Vacca C, Bianchi R, Orabona C, Spreca A, Fioretti MC, Puccetti P. T cell apoptosis by tryptophan catabolism. Cell Death Differ. 2002;9(10):1069–1077. doi: 10.1038/sj.cdd.4401073. [DOI] [PubMed] [Google Scholar]
- 67.Munn DH, Mellor AL. Indoleamine 2,3 dioxygenase and metabolic control of immune responses. Trends Immunol. 2013;34(3):137–143. doi: 10.1016/j.it.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mellor AL, Munn DH. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat Rev Immunol. 2004;4(10):762–774. doi: 10.1038/nri1457. [DOI] [PubMed] [Google Scholar]
- 69.Wang Y, Liu H, McKenzie G, Witting PK, Stasch JP, Hahn M, Changsirivathanathamrong D, Wu BJ, Ball HJ, Thomas SR, Kapoor V, Celermajer DS, Mellor AL, Keaney JF, Jr, Hunt NH, Stocker R. Kynurenine is an endothelium-derived relaxing factor produced during inflammation. Nat Med. 2010;16(3):279–285. doi: 10.1038/nm.2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zelante T, Fallarino F, Bistoni F, Puccetti P, Romani L. Indoleamine 2,3-dioxygenase in infection: the paradox of an evasive strategy that benefits the host. Microbes Infect. 2009;11(1):133–141. doi: 10.1016/j.micinf.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 71.Blumenthal A, Nagalingam G, Huch JH, Walker L, Guillemin GJ, Smythe GA, Ehrt S, Britton WJ, Saunders BM. M. tuberculosis induces potent activation of IDO-1, but this is not essential for the immunological control of infection. PLoS One. 2012;7(5):e37314. doi: 10.1371/journal.pone.0037314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mehra S, Pahar B, Dutta NK, Conerly CN, Philippi-Falkenstein K, Alvarez X, Kaushal D. Transcriptional reprogramming in nonhuman primate (rhesus macaque) tuberculosis granulomas. PLoS One. 2010;5(8):e12266. doi: 10.1371/journal.pone.0012266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Suzuki Y, Miwa S, Akamatsu T, Suzuki M, Fujie M, Nakamura Y, Inui N, Hayakawa H, Chida K, Suda T. Indoleamine 2,3-dioxygenase in the pathogenesis of tuberculous pleurisy. Int J Tuberc Lung Dis. 2013;17(11):1501–1506. doi: 10.5588/ijtld.13.0082. [DOI] [PubMed] [Google Scholar]
- 74.Suzuki Y, Suda T, Asada K, Miwa S, Suzuki M, Fujie M, Furuhashi K, Nakamura Y, Inui N, Shirai T, Hayakawa H, Nakamura H, Chida K. Serum indoleamine 2,3-dioxygenase activity predicts prognosis of pulmonary tuberculosis. Clin Vaccine Immunol. 2012;19(3):436–442. doi: 10.1128/CVI.05402-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mehra S, Alvarez X, Didier PJ, Doyle LA, Blanchard JL, Lackner AA, Kaushal D. Granuloma correlates of protection against tuberculosis and mechanisms of immune modulation by Mycobacterium tuberculosis. J Infect Dis. 2013;207(7):1115–1127. doi: 10.1093/infdis/jis778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Desvignes L, Ernst JD. Interferon-gamma-responsive nonhematopoietic cells regulate the immune response to Mycobacterium tuberculosis. Immunity. 2009;31(6):974–985. doi: 10.1016/j.immuni.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Munn DH, Sharma MD, Lee JR, Jhaver KG, Johnson TS, Keskin DB, Marshall B, Chandler P, Antonia SJ, Burgess R, Slingluff CL, Jr, Mellor AL. Potential regulatory function of human dendritic cells expressing indoleamine 2,3-dioxygenase. Science. 2002;297(5588):1867–1870. doi: 10.1126/science.1073514. [DOI] [PubMed] [Google Scholar]
- 78.Terness P, Bauer TM, Rose L, Dufter C, Watzlik A, Simon H, Opelz G. Inhibition of allogeneic T cell proliferation by indoleamine 2,3-dioxygenase-expressing dendritic cells: mediation of suppression by tryptophan metabolites. J Exp Med. 2002;196(4):447–457. doi: 10.1084/jem.20020052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fallarino F, Grohmann U, You S, McGrath BC, Cavener DR, Vacca C, Orabona C, Bianchi R, Belladonna ML, Volpi C, Fioretti MC, Puccetti P. Tryptophan catabolism generates autoimmune-preventive regulatory T cells. Transpl Immunol. 2006;17(1):58–60. doi: 10.1016/j.trim.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 80.Li Q, Li L, Liu Y, Fu X, Qiao D, Wang H, Lao S, Huang F, Wu C. Pleural fluid from tuberculous pleurisy inhibits the functions of T cells and the differentiation of Th1 cells via immunosuppressive factors. Cell Mol Immunol. 2011;8(2):172–180. doi: 10.1038/cmi.2010.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Popov A, Abdullah Z, Wickenhauser C, Saric T, Driesen J, Hanisch FG, Domann E, Raven EL, Dehus O, Hermann C, Eggle D, Debey S, Chakraborty T, Kronke M, Utermohlen O, Schultze JL. Indoleamine 2,3-dioxygenase-expressing dendritic cells form suppurative granulomas following Listeria monocytogenes infection. J Clin Invest. 2006;116(12):3160–3170. doi: 10.1172/JCI28996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schmidt SK, Siepmann S, Kuhlmann K, Meyer HE, Metzger S, Pudelko S, Leineweber M, Daubener W. Influence of tryptophan contained in 1-Methyl-Tryptophan on antimicrobial and immunoregulatory functions of indoleamine 2,3-dioxygenase. PLoS One. 2012;7(9):e44797. doi: 10.1371/journal.pone.0044797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ball HJ, Sanchez-Perez A, Weiser S, Austin CJ, Astelbauer F, Miu J, McQuillan JA, Stocker R, Jermiin LS, Hunt NH. Characterization of an indoleamine 2,3-dioxygenase-like protein found in humans and mice. Gene. 2007;396(1):203–213. doi: 10.1016/j.gene.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 84.Divanovic S, Sawtell NM, Trompette A, Warning JI, Dias A, Cooper AM, Yap GS, Arditi M, Shimada K, Duhadaway JB, Prendergast GC, Basaraba RJ, Mellor AL, Munn DH, Aliberti J, Karp CL. Opposing biological functions of tryptophan catabolizing enzymes during intracellular infection. J Infect Dis. 2012;205(1):152–161. doi: 10.1093/infdis/jir621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Metz R, Duhadaway JB, Kamasani U, Laury-Kleintop L, Muller AJ, Prendergast GC. Novel tryptophan catabolic enzyme IDO2 is the preferred biochemical target of the antitumor indoleamine 2,3-dioxygenase inhibitory compound D-1-methyl-tryptophan. Cancer Res. 2007;67(15):7082–7087. doi: 10.1158/0008-5472.CAN-07-1872. [DOI] [PubMed] [Google Scholar]
- 86.Metz R, Smith C, DuHadaway JB, Chandler P, Baban B, Merlo LM, Pigott E, Keough MP, Rust S, Mellor AL, Mandik-Nayak L, Muller AJ, Prendergast GC. IDO2 is critical for IDO1-mediated T-cell regulation and exerts a non-redundant function in inflammation. Int Immunol. 2014;26(7):357–367. doi: 10.1093/intimm/dxt073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhang YJ, Reddy MC, Ioerger TR, Rothchild AC, Dartois V, Schuster BM, Trauner A, Wallis D, Galaviz S, Huttenhower C, Sacchettini JC, Behar SM, Rubin EJ. Tryptophan biosynthesis protects mycobacteria from CD4 T-cell-mediated killing. Cell. 2013;155(6):1296–1308. doi: 10.1016/j.cell.2013.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Orme IM, Basaraba RJ. The formation of the granuloma in tuberculosis infection. Semin Immunol. 2014;26(6):601–609. doi: 10.1016/j.smim.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 89.Deffert C, Cachat J, Krause KH. Phagocyte NADPH oxidase, chronic granulomatous disease and mycobacterial infections. Cell Microbiol. 2014;16(8):1168–1178. doi: 10.1111/cmi.12322. [DOI] [PubMed] [Google Scholar]
- 90.Yang CT, Cambier CJ, Davis JM, Hall CJ, Crosier PS, Ramakrishnan L. Neutrophils exert protection in the early tuberculous granuloma by oxidative killing of mycobacteria phagocytosed from infected macrophages. Cell Host Microbe. 2012;12(3):301–312. doi: 10.1016/j.chom.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Palanisamy GS, Kirk NM, Ackart DF, Shanley CA, Orme IM, Basaraba RJ. Evidence for oxidative stress and defective antioxidant response in guinea pigs with tuberculosis. PLoS One. 2011;6(10):e26254. doi: 10.1371/journal.pone.0026254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Deffert C, Schappi MG, Pache JC, Cachat J, Vesin D, Bisig R, Ma Mulone X, Kelkka T, Holmdahl R, Garcia I, Olleros ML, Krause KH. Bacillus calmette-guerin infection in NADPH oxidase deficiency: defective mycobacterial sequestration and granuloma formation. PLoS Pathog. 2014;10(9):e1004325. doi: 10.1371/journal.ppat.1004325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bustamante J, Arias AA, Vogt G, Picard C, Galicia LB, Prando C, Grant AV, Marchal CC, Hubeau M, Chapgier A, de Beaucoudrey L, Puel A, Feinberg J, Valinetz E, Janniere L, Besse C, Boland A, Brisseau JM, Blanche S, Lortholary O, Fieschi C, Emile JF, Boisson-Dupuis S, Al-Muhsen S, Woda B, Newburger PE, Condino-Neto A, Dinauer MC, Abel L, Casanova JL. Germline CYBB mutations that selectively affect macrophages in kindreds with X-linked predisposition to tuberculous mycobacterial disease. Nat Immunol. 2011;12(3):213–221. doi: 10.1038/ni.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Davis SL, Nuermberger EL, Um PK, Vidal C, Jedynak B, Pomper MG, Bishai WR, Jain SK. Noninvasive pulmonary [18F]-2-fluoro-deoxy-D-glucose positron emission tomography correlates with bactericidal activity of tuberculosis drug treatment. Antimicrob Agents Chemother. 2009;53(11):4879–4884. doi: 10.1128/AAC.00789-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kim IJ, Lee JS, Kim SJ, Kim YK, Jeong YJ, Jun S, Nam HY, Kim JS. Double-phase 18F-FDG PET-CT for determination of pulmonary tuberculoma activity. Eur J Nucl Med Mol Imaging. 2008;35(4):808–814. doi: 10.1007/s00259-007-0585-0. [DOI] [PubMed] [Google Scholar]
- 96.Coleman MT, Maiello P, Tomko J, Frye LJ, Fillmore D, Janssen C, Klein E, Lin PL. Early Changes by (18)Fluorodeoxyglucose positron emission tomography coregistered with computed tomography predict outcome after Mycobacterium tuberculosis infection in cynomolgus macaques. Infect Immun. 2014;82(6):2400–2404. doi: 10.1128/IAI.01599-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Via LE, Schimel D, Weiner DM, Dartois V, Dayao E, Cai Y, Yoon YS, Dreher MR, Kastenmayer RJ, Laymon CM, Carny JE, Flynn JL, Herscovitch P, Barry CE., 3rd Infection dynamics and response to chemotherapy in a rabbit model of tuberculosis using [(1)(8)F]2-fluoro-deoxy-D-glucose positron emission tomography and computed tomography. Antimicrob Agents Chemother. 2012;56(8):4391–4402. doi: 10.1128/AAC.00531-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Palsson-McDermott EM, O'Neill LA. The Warburg effect then and now: from cancer to inflammatory diseases. Bioessays. 2013;35(11):965–973. doi: 10.1002/bies.201300084. [DOI] [PubMed] [Google Scholar]
- 99.Shin JH, Yang JY, Jeon BY, Yoon YJ, Cho SN, Kang YH, Ryu do H, Hwang GS. (1)H NMR-based metabolomic profiling in mice infected with Mycobacterium tuberculosis. J Proteome Res. 2011;10(5):2238–2247. doi: 10.1021/pr101054m. [DOI] [PubMed] [Google Scholar]
- 100.Chen Y, Wu J, Tu L, Xiong X, Hu X, Huang J, Xu Z, Zhang X, Hu C, Hu X, Guo A, Wang Y, Chen H. (1)H-NMR spectroscopy revealed Mycobacterium tuberculosis caused abnormal serum metabolic profile of cattle. PLoS One. 2013;8(9):e74507. doi: 10.1371/journal.pone.0074507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Colegio OR, Chu NQ, Szabo AL, Chu T, Rhebergen AM, Jairam V, Cyrus N, Brokowski CE, Eisenbarth SC, Phillips GM, Cline GW, Phillips AJ, Medzhitov R. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature. 2014;513(7519):559–563. doi: 10.1038/nature13490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Albina JE, Henry WL, Jr, Mastrofrancesco B, Martin BA, Reichner JS. Macrophage activation by culture in an anoxic environment. J Immunol. 1995;155(9):4391–4396. [PubMed] [Google Scholar]
- 103.Jin Y, Calvert TJ, Chen B, Chicoine LG, Joshi M, Bauer JA, Liu Y, Nelin LD. Mice deficient in Mkp-1 develop more severe pulmonary hypertension and greater lung protein levels of arginase in response to chronic hypoxia. Am J Physiol Heart Circ Physiol. 2010;298(5):H1518–H1528. doi: 10.1152/ajpheart.00813.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Vergadi E, Chang MS, Lee C, Liang OD, Liu X, Fernandez-Gonzalez A, Mitsialis SA, Kourembanas S. Early macrophage recruitment and alternative activation are critical for the later development of hypoxia-induced pulmonary hypertension. Circulation. 2011;123(18):1986–1995. doi: 10.1161/CIRCULATIONAHA.110.978627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chang CH, Curtis JD, Maggi LB, Jr, Faubert B, Villarino AV, O'Sullivan D, Huang SC, van der Windt GJ, Blagih J, Qiu J, Weber JD, Pearce EJ, Jones RG, Pearce EL. Posttranscriptional control of T cell effector function by aerobic glycolysis. Cell. 2013;153(6):1239–1251. doi: 10.1016/j.cell.2013.05.016. doi:S0092-8674(13)00582-5 [pii] 10.1016/j.cell.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ramakrishnan L. Revisiting the role of the granuloma in tuberculosis. Nat Rev Immunol. 2012;12(5):352–366. doi: 10.1038/nri3211. [DOI] [PubMed] [Google Scholar]
- 107.Young D. Animal models of tuberculosis. Eur J Immunol. 2009;39(8):2011–2014. doi: 10.1002/eji.200939542. [DOI] [PubMed] [Google Scholar]
- 108.Dharmadhikari AS, Nardell EA. What animal models teach humans about tuberculosis. Am J Respir Cell Mol Biol. 2008;39(5):503–508. doi: 10.1165/rcmb.2008-0154TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gupta UD, Katoch VM. Animal models of tuberculosis. Tuberculosis (Edinb) 2005;85(5–6):277–293. doi: 10.1016/j.tube.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 110.Dorhoi A, Reece ST, Kaufmann SH. For better or for worse: the immune response against Mycobacterium tuberculosis balances pathology and protection. Immunol Rev. 2011;240(1):235–251. doi: 10.1111/j.1600-065X.2010.00994.x. [DOI] [PubMed] [Google Scholar]
- 111.Cooper AM, Dalton DK, Stewart TA, Griffin JP, Russell DG, Orme IM. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J Exp Med. 1993;178(6):2243–2247. doi: 10.1084/jem.178.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cooper AM, Pearl JE, Brooks JV, Ehlers S, Orme IM. Expression of the nitric oxide synthase 2 gene is not essential for early control of Mycobacterium tuberculosis in the murine lung. Infect Immun. 2000;68(12):6879–6882. doi: 10.1128/iai.68.12.6879-6882.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Dutta NK, Illei PB, Jain SK, Karakousis PC. Characterization of a novel necrotic granuloma model of latent tuberculosis infection and reactivation in mice. Am J Pathol. 2014;184(7):2045–2055. doi: 10.1016/j.ajpath.2014.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Harper J, Skerry C, Davis SL, Tasneen R, Weir M, Kramnik I, Bishai WR, Pomper MG, Nuermberger EL, Jain SK. Mouse model of necrotic tuberculosis granulomas develops hypoxic lesions. J Infect Dis. 2012;205(4):595–602. doi: 10.1093/infdis/jir786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Pan H, Yan BS, Rojas M, Shebzukhov YV, Zhou H, Kobzik L, Higgins DE, Daly MJ, Bloom BR, Kramnik I. Ipr1 gene mediates innate immunity to tuberculosis. Nature. 2005;434(7034):767–772. doi: 10.1038/nature03419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Calderon VE, Valbuena G, Goez Y, Judy BM, Huante MB, Sutjita P, Johnston RK, Estes DM, Hunter RL, Actor JK, Cirillo JD, Endsley JJ. A humanized mouse model of tuberculosis. PLoS One. 2013;8(5):e63331. doi: 10.1371/journal.pone.0063331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cyktor JC, Carruthers B, Kominsky RA, Beamer GL, Stromberg P, Turner J. IL-10 inhibits mature fibrotic granuloma formation during Mycobacterium tuberculosis infection. J Immunol. 2013;190(6):2778–2790. doi: 10.4049/jimmunol.1202722. [DOI] [PMC free article] [PubMed] [Google Scholar]