SUMMARY
Cholera is an important public health problem in Bangladesh. Interventions to prevent cholera depend on their cost-effectiveness which in turn depends on cholera incidence. Hospital-based diarrhoeal disease surveillance has been ongoing in six Bangladeshi hospitals where a systematic proportion of patients admitted with diarrhoea were enrolled and tested for Vibrio cholerae. However, incidence calculation using only hospital data underestimates the real disease burden because many ill persons seek treatment elsewhere. We conducted a healthcare utilization survey in the catchment areas of surveillance hospitals to estimate the proportion of severe diarrhoeal cases that were admitted to surveillance hospitals and estimated the population-based incidence of severe diarrhoea due to V. cholerae by combining both hospital surveillance and catchment area survey data. The estimated incidence of severe diarrhoea due to cholera ranged from 0·3 to 4·9/1000 population in the catchment area of surveillance hospitals. In children aged <5 years, incidence ranged from 1·0 to 11·-0/1000 children. Diarrhoeal deaths were most common in the Chhatak Hospital’s catchment area (18·5/100 000 population). This study provides a credible estimate of the incidence of severe diarrhoea due to cholera in Bangladesh, which can be used to assess the cost-effectiveness of cholera prevention activities.
Keywords: Cholera, diarrhoea, incidence, vaccines, waterborne infections
INTRODUCTION
Cholera occurs following infection of the intestine by the O1 or O139 serogroups of the bacterium Vibrio cholerae [1–4]. About 20% of infected individuals develop acute, watery diarrhoea and 10–20% of these develop severe watery diarrhoea [5]. Although case-fatality rates have fallen owing to oral and intravenous rehydration therapy, cholera can cause severe disease because of its rapid onset; residents in low-income settings are at particularly high risk of infection in areas where public health systems cannot cope with outbreaks [6].
In 2011, a total of 58 countries reported 589 854 cholera cases, including 7816 deaths, to the World Health Organization (WHO) [7]. However, the WHO considered these figures to be underestimates, as poor surveillance systems and fear of negative impact on trade and tourism in many countries likely led to underreporting [8, 9]. WHO estimates that officially reported cases represent only 5–10% of the actual number occurring worldwide annually [8].
Cholera is a major public health problem in Bangladesh [10], a country located in the heart of the Ganges Delta which is considered the historical home of cholera by many experts [11]. In Bangladesh, cholera occurs year-round with seasonal peaks typically before and after monsoons, and it can be especially devastating during flood years [10, 12]. The true burden of cholera is unknown in Bangladesh due to the lack of a population-based surveillance system.
The estimation of cholera incidence is particularly important to take effective control measures, including the provision of clean water, improved hygiene and sanitation, and introduction of cholera vaccines. Oral cholera vaccines have been found to be safe and effective [13–15]. However, modelling studies have shown that water and sanitation measures may provide an equally viable solution, especially in the long term, since the immunization granted by vaccines wanes over time [16–19]. Two types of inactivated cholera vaccines are currently available: one containing recombinant cholera toxin B subunit and killed cholera whole cells (rBS-WC) and the other containing only killed cholera whole cells (WC) [7, 20]. Field trials demonstrated that both vaccines provided >50% protection for 3 years [21, 22]. However, the WC vaccine is cheaper, at US$1.85 per dose in the public sector, with a protective efficacy of 66% during the third year of follow-up, as reported in a recent study from Kolkata, India [22]. Credible data regarding incidence of cholera is currently unavailable in Bangladesh, which limits the validity of any cost-effectiveness evaluation of a potential intervention programme.
In Bangladesh diarrhoeal disease surveillance with microbiological confirmation has been ongoing in six hospitals. These hospitals enrol a systematic proportion of patients admitted with diarrhoea to test for V. cholerae O1/O139 and other enteric pathogens. However, incidence estimation in the catchment area of a hospital using only hospital data underestimates the real burden of disease and so underestimates the cost-effectiveness of interventions because many sick people may seek care in other facilities. Medical records on patients in other health facilities are poor in Bangladesh where more than one-fourth of patients with serious illness seek care from informal healthcare providers [23]. We conducted a healthcare utilization survey in the catchment areas of surveillance hospitals to estimate the proportion of severe diarrhoeal cases that were admitted to surveillance hospitals. We estimated the population-based incidence of severe diarrhoea due to V. cholera in the hospital catchment areas by adjusting the hospital-based surveillance data by the proportion of severe diarrhoeal cases in the hospital catchment areas that were admitted to surveillance hospitals.
METHODS
Hospital-based diarrhoeal disease surveillance
Of the six diarrhoeal disease surveillance hospitals in Bangladesh, three are specialized diarrhoeal disease hospitals run by icddr,b – Dhaka Hospital, Matlab Hospital and Mirpur Treatment Centre. Kumudini Hospital in Mirzapur subdistrict is a private general hospital, and Chhatak and Mathbaria Upazila Health Complexes are subdistrict-level government general healthcare facilities. However, diarrhoeal disease surveillance in Kumudini, Chhatak and Mathbaria is supported by icddr,b through icddr,b staff, sample collection, and laboratory testing.
Dhaka Hospital and Mirpur Treatment Centre, based in Dhaka, the capital of Bangladesh, mainly serve the urban population of Dhaka and its surrounding area. However, patients with severe diarrhoea from outside Dhaka also seek care at Dhaka Hospital, which is a well-known diarrhoeal disease hospital. Matlab, Kumudini, Chhatak, and Mathbaria hospitals all mainly serve rural populations (Fig. 1). Matlab is in a riverine cholera endemic area and well known for cholera surveillance for more than 45 years; Mirzapur is a plain area that has had no large outbreaks of cholera reported in the last decade. Chhatak is in a low-lying, flood-prone area and Mathbaria is adjacent to the coast of the Bay of Bengal.
Fig. 1.
Map of Bangladesh showing surveillance hospitals.
As part of diarrhoeal disease surveillance, the presence of V. cholerae was tested in specimens of every 50th patient admitted with diarrhoea in Dhaka Hospital, every 10th patient admitted with diarrhoea in Mirpur Treatment Centre, and all patients admitted with diarrhoea from the defined hospital catchment areas (as defined by the Health and Demographic Surveillance System) of Matlab and Kumudini hospitals. Surveillance physicians from the Epidemic Control and Preparedness Unit of icddr,b, Dhaka routinely visited Chhatak and Mathbaria hospitals to collect rectal swabs from all patients with diarrhoea. Based on local cholera seasonality [10, 24], the surveillance physician in Chhatak collected samples 3 days per month during January–August and 3 days per week during September–December; in Mathbaria samples were collected 3 days per month during June–January and 3 days per week during February–May from all patients with acute watery diarrhoea. All admitted patients were considered new cases.
Laboratory testing
Following rectal swab collection, samples were immediately placed in Cary–Blair transport media. All samples were cultured in the icddr,b laboratory using standard bacteriological methods [24, 25]. Samples collected in Dhaka, Mirpur, Matlab and Kumudini hospitals were cultured on the same day; samples collected in Chhatak and Mathbaria were transported to the icddr,b laboratory in Dhaka within 3 days of collection. In the laboratory, the rectal swabs were incubated in alkaline peptone water (APW) at 37 °C for 4 h. The rectal swabs, as well as the 4-h broth enrichments, were inoculated by streaking on taurocholate-tellurite-gelatin agar (TTGA). Colonies resembling V. cholerae were agglutinated with antisera specific for V. cholerae O1 and V. cholerae O139 [25].
Hospital catchment area identification
Dhaka Hospital and Mirpur Treatment Centre have electronic databases of the home addresses of all patients who have been admitted with diarrhoea. We reviewed hospital databases during September 2009–October 2010 and identified the primary catchment areas of the hospital, defining them as the areas where two-thirds of admitted patients resided. With this criterion, 31 thanas (an administrative unit of a metropolitan area with an average population of 349 000) were identified as the primary catchment area of Dhaka Hospital and two thanas were selected as the primary catchment area of Mirpur Treatment Centre. We reviewed the hospital logbooks of Chhatak and Mathbaria hospitals during November 2009–October 2010 and identified the unions (the smallest administrative unit in rural areas with an average population of 28 000) as the hospital catchment areas where 80% of the admitted patients resided. With this criterion, six unions from Chhatak and eight unions from Mathbaria Hospital were identified as the hospital catchment area. In Matlab, icddr, b’s Health and Demographic Surveillance System (HDSS) was established in 1966 and currently surveillance is ongoing in 142 villages. Although patients with diarrhoea from a wide geographical area come to Matlab Hospital, we considered villages of the HDSS area as the catchment area of Matlab Hospital. Since 2007, icddr,b has also been conducting demographic surveillance in eight unions of Mirzapur which were included in the study as the catchment area for Kumudini Hospital since most patients admitted to that hospital reside in these eight unions.
Healthcare utilization survey in hospital catchment areas
We defined severe diarrhoea as frequent loose or liquid stools for which a person had to be admitted to a healthcare facility, or had to receive intravenous rehydration, or had died as a result of the diarrhoeal illness. We conducted a healthcare utilization survey from December 2010 to April 2011 in the hospital catchment area of surveillance hospitals to estimate the proportion of severe diarrhoeal cases in the defined hospital catchment areas who were admitted to surveillance hospitals in the previous 12 months.
Sample size
We calculated the sample size for healthcare utilization survey in the catchment area of surveillance hospitals by using the sample size calculation formula (with finite population correction) proposed by Daniel et al. [26]. We assumed that in the catchment area of urban-based surveillance hospitals there would be about 100 000 severe diarrhoea patients per year, of which 30% would seek care at surveillance hospital. We also assumed that in the catchment area of rural-based surveillance hospitals there would be about 1000 severe diarrhoea patients per year, of which 50% would seek care at their respective surveillance hospital. Patients in rural area are more likely to seek care at surveillance hospitals because they have fewer healthcare options compared to patients in urban areas. We used a precision estimate of ±5% with 95% confidence level and design effect of 2·0 to calculate a sample size of 644 severe diarrhoeal cases in the catchment area of each of the Dhaka and Mirpur hospitals and 555 cases in the catchment area of each Kumudini, Chhatak and Mathbaria hospitals. We then calculated the number of survey clusters required to reach the sample size; 17 clusters (called mahallas: local geographical units in urban areas) from urban hospital catchment areas and six clusters (unions: local geographical units in rural areas) from rural hospital catchment areas. To randomly select the survey clusters we used a probability proportional to sample size sampling approach. We first listed all the clusters (mahalla or union) in each hospital catchment area by their population size in a spreadsheet and calculated the sampling interval by dividing the total population by the number of required survey clusters, and then selected a random number between 1 and the sampling interval. The cluster having the cumulative population that included the random number was selected as the first cluster. To select the second cluster, the sampling interval was added to the random number and again the list was consulted to see which cluster included that number. This process was repeated until the required number of survey clusters were identified from the defined catchment area of each hospital. We used the 2001 Bangladesh population census as the sampling frame to select the survey clusters from the defined hospital catchment areas.
Survey in urban areas
In the selected survey clusters of Dhaka and Mirpur hospital catchment areas, the field team conducted a house-to-house survey. Starting from the centrepoint of a cluster and proceeding in a randomly chosen direction, the team visited all the households and asked the available household members if anyone in their household had met the case definition of severe diarrhoea in the previous 12 months. The team visited successive households until they collected information on severe diarrhoea for 10 000 people in a selected cluster.
Survey in rural areas
Since the cost of conducting a house-to-house survey in a dispersed rural population is high, the field team did not conduct house-to-house surveys to identify severe diarrhoeal cases in the catchment areas of rural-based Kumudini, Chhatak and Mathbaria hospitals. Instead, they identified severe diarrhoea cases using broader community awareness of serious events in the rural area. People in Bangladeshi rural communities actively discuss community events, such as family illness, and therefore are generally able to report any serious health events experienced by their neighbours. The team first approached local healthcare providers, religious and community leaders, educational institutions and local village markets. Then they walked through the village and met with the residents, especially women, in informal courtyard gatherings. The team explained its definition of severe diarrhoea and asked community members if they knew anybody in their community who met this case definition in the previous 12 months. If the field team received information about anyone with severe diarrhoea, they visited the household and confirmed that the person’s illness met the case definition. Cause of death due to acute diarrhoeal illness was ascertained by inquiries to the household members of the deceased. This approach to identify severely ill patients in rural Bangladeshi communities had been used previously to estimate the incidence of Japanese encephalitis in Bangladesh [27]. The team administered a questionnaire to collect information on symptoms of illness and healthcare utilization along with demographic characteristics. Since icddr, b’s Matlab Hospital is held in high regard by the local people because of its long history of treating patients with diarrhoea and the routine household visits by icddr,b staff in the HDSS villages, we assumed that 100% of severe diarrhoeal patients in the HDSS areas came to Matlab Hospital during their illness. Therefore, the survey was not conducted in the Matlab Hospital catchment area.
Incidence calculation
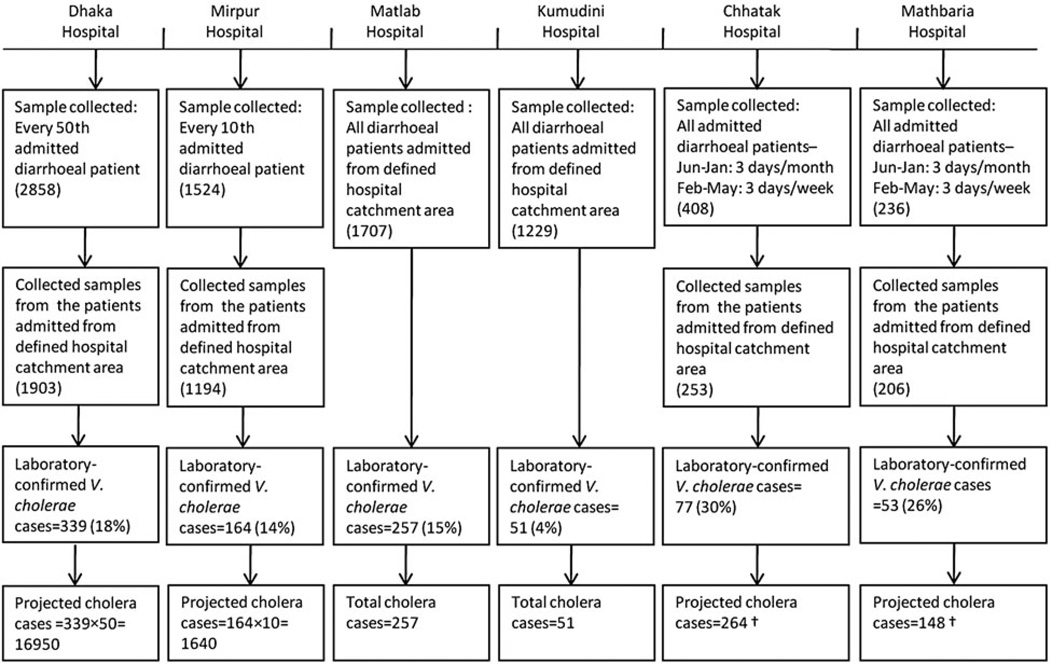
To estimate the incidence of severe diarrhoea due to V. cholerae in the catchment area of surveillance hospitals, we used hospital surveillance data from March 2010 to February 2011. Since only a proportion of admitted patients were enrolled in surveillance and tested for V. cholerae in Dhaka, Mirpur, Chhatak and Mathbaria hospitals, we extrapolated the total number of V. cholerae cases in these hospitals in patients admitted from the hospital catchment area by applying the rate of V. cholerae positivity in surveillance-enrolled patients to all patients with diarrhoea admitted from the hospital catchment area (Fig. 2). We estimated population-based incidence of severe diarrhoea due to V. cholerae in a hospital catchment area by using both hospital-based surveillance and catchment area survey data (see Table 3) applying the following formula. Previous studies have used similar methods to estimate population-based incidence of a disease by extrapolating data from hospital-based surveillance and healthcare utilization surveys in hospital catchment areas [27–29].
| (1) |
where Vc = total or estimated V. cholerae O1/O139 cases over 12 months in a surveillance hospital; Cpop = population in the hospital catchment area; and P = proportion of severe diarrhoea cases in the hospital catchment area that were admitted to a surveillance hospital (obtained from healthcare utilization survey in hospital catchment areas).
Fig. 2.
Stool sample collection methods and cholera cases in six diarrhoeal disease surveillance hospitals in Bangladesh 2010–2011. Reported surveillance period for Dhaka, Mirpur, Matlab and Kumudini hospitals, March 2010–February 2011; for Chhatak Hospital, October 2010–September 2011, and for Mathbaria Hospital, December 2010–November 2011. † Month-wise cholera cases were first extrapolated by dividing the laboratory-confirmed cholera cases by the proportion of number of surveillance days in a month and then the month-wise extrapolated cases were summed to extrapolate the cholera cases during the 12-month surveillance period.
Table 3.
Estimated incidence of severe diarrhoea due to cholera in catchment areas of diarrhoeal disease surveillance hospitals in Bangladesh, 2010–2011
| Dhaka |
Mirpur |
Matlab* |
Kumudini |
Chhatak |
Mathbaria |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristics | <5yr | All ages | <5yr | All ages | <5 yr | All ages | <5yr | All ages | <5yr | All ages | <5yr | All ages |
| Population in hospital catchment areas (in thousands) (Cpop) |
870 | 10 368 | 80 | 993 | 20 | 225 | 22 | 247 | 24 | 176 | 18 | 186 |
| Total/projected cholera cases in surveillance hospital from catchment areas (Vc)† |
2850 | 16 950 | 340 | 1640 | 41 | 257 | 21 | 51 | 47 | 264 | 52 | 148 |
| Proportion of severe diarrhoea cases admitted to surveillance hospital (P) (95% CI)‡ |
0·66 (0·59–0·73) |
0·62 (0·55–0·69) |
0·39 (0·29–0·48) |
0·33 (0·24–0·43) |
- | - | 0·92 (0·88–0·95) |
0·70 (0·55–0·86) |
0·51 (0·37–0·65) |
0·40 (0·27–0·54) |
0·90 (0·85–0·96) |
0·44 (0·27–0·60) |
| Incidence of V. cholerae/1000 population (95% CI)§ |
5·0 (4·5–5·6) |
2·6 (2·4–3·0) |
11·0 (8·8–14·7) |
4·9 (3·8–6·9) |
2·6 | 1·1 | 1·0 (1·0–1·1) |
0·3 (0·2–0·4) |
3·8 (3·0–5·1) |
3·7 (2·8–5·6) |
3·2 (3·0–-3·4) |
1·8 (1·3–2·9) |
Cpop, Population in the hospital catchment area; Vc, total or estimated V. cholerae 01/0139 cases over 12 months in a surveillance hospital; P, proportion of severe diarrhea cases in hospital catchment area that were admitted to a surveillance hospital; CI, confidence interval.
Incidence was estimated from hospital-based surveillance data directly; no correction factor was applied.
Reported surveillance period for Dhaka, Mirpur, Matlab and Kumudini hospitals, March 2010-February 2011; for Chhatak Hospital, October 2010-September 2011, and for Mathbaria Hospital, December 2010-November 2011.
Adjusted for cluster effect using linear mixed effect model.
We used the same method to estimate the incidence of V. cholerae in children aged <5 years. However, to calculate Vc for this group, we used the V. cholerae positivity rate in surveillance-enrolled children aged <5 years.
Data analysis
The 2011 Bangladesh Census provided population data for the catchment area of the surveillance hospitals [30]. Since a cluster sampling approach was applied instead of a simple random sampling to identify severe diarrhoea cases in the hospital catchment areas, we used a linear mixed-effect model to adjust for the cluster effects in calculating the proportion of severe diarrhoeal cases that were admitted to study hospitals and in estimating the incidence of cholera with 95% confidence intervals. We compared the demographic characteristics and clinical signs between cases admitted and not admitted to surveillance hospitals by using a two-sample proportion test where reported P values were adjusted for cluster effect using a clustered sandwich estimator [31]. In the first survey cluster in Chhatak and Mathbaria, we found more severe diarrhoeal cases than our assumption in calculating the required sample size. We therefore collected information on demographic characteristics and reported symptoms from every third identified severe diarrhoeal case. However, all diarrhoeal cases were included to calculate the incidence, and proxies of all death cases were interviewed.
Ethical approval
The field team obtained written consent from the identified severe diarrhoeal cases or their guardians. Assent was taken from participants aged between 11 and 17 years. In the surveillance hospitals, consent was also obtained from patients with diarrhoea before collecting the stool specimen. The study protocol was reviewed and approved by the institutional review board of icddr,b.
RESULTS
In Dhaka Hospital, 2858 patients with diarrhoea were tested for V. cholera O1/O139 from March 2010 to February 2011, of which 1903 (67%) were admitted from the defined hospital catchment areas. Of these 1903 patients, 339 (18%) had V. cholera O1 isolated from their stool specimens. Since only 2% of admitted patients with diarrhoea were tested for cholera, we projected 16 950 V. cholerae O1 cases in patients who were admitted from the catchment area of Dhaka Hospital. Similarly, we projected 1640 V. cholerae O1 cases in the catchment area of Mirpur Hospital, 264 cases for Chhatak Hospital and 148 cases for Mathbaria Hospital. On the other hand, 257 (15%) patients at Matlab Hospital and 51 (4%) patients in Kumudini Hospital tested positive for V. cholerae O1 in all admitted and surveillance-enrolled patients with diarrhoea during the 1-year period (Fig. 2). None of the patients tested positive for V. cholerae O139.
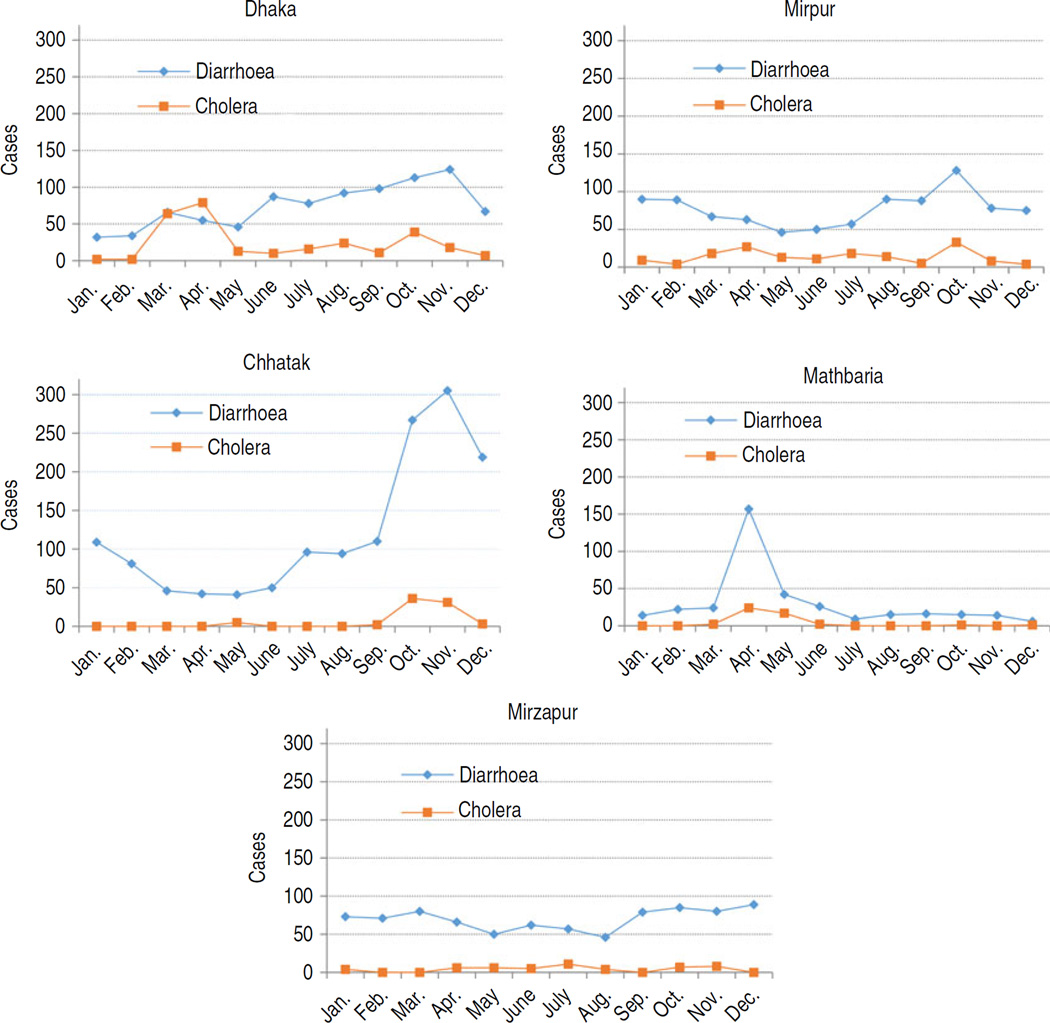
According to the survey in the hospital catchment areas, patients who met the case definition of severe diarrhoea within 12 months of interview ranged from 838 cases (4/1000 population) in Kumudini Hospital to 2708 cases (17/1000 population) in Chhatak Hospital catchment areas (Table 1). Of the severe diarrhoea cases who were admitted to any hospital during their illness, the highest proportion (93%) was identified in Mirpur and the lowest (47%) in Mathbaria. Patients with severe diarrhoea who were admitted to the surveillance hospital ranged from 35% at Mirpur to 67% at Kumudini. The field team also identified 30 diarrhoeal deaths in Chhatak, two in Dhaka and three in each of the Mirpur, Kumudini and Mathbaria catchment areas within 12 months of the interview date (Table 1). The highest incidence of diarrhoeal deaths was found in the catchment area of Chhatak Hospital, which was 18·5/100 000 population. The peak month of diarrhoea and cholera varied by site (Fig. 3).
Table 1.
Healthcare utilization of the patients with severe diarrhoea in the catchment area of surveillance hospitals in Bangladesh, 2010–2011
| Hospital catchment area |
|||||
|---|---|---|---|---|---|
| Characteristics | Dhaka n (%) |
Mirpur n (%) |
Kumudini n (%) |
Chhatak n (%) |
Mathbaria n (%) |
| Total population surveyed | 157 493 | 166 020 | 207 566 | 161954 | 150 628 |
| Cases met the case definition of severe diarrhoea* (per 1000 population) |
892 (5·7) | 921 (5·5) | 838 (4·0) | 2708 (16·7) | 1170 (7·8) |
| Cases sought care from qualified healthcare provider† | |||||
| 1st visit | 171 (19) | 175 (19) | 94 (11) | 302 (11) | 117 (10) |
| 2nd visit | 634 (71) | 686 (74) | 365 (44) | 1298 (48) | 447 (38) |
| Any visit | 800 (90) | 881 (96) | 710 (85) | 1794 (66) | 650 (56) |
| Cases admitted to any hospital | 755 (85) | 856 (93) | 697 (83) | 1559 (58) | 552 (47) |
| Cases received intravenous rehydration but not admitted to any hospital |
137 (15) | 64 (7) | 143 (17) | 1149 (42) | 618 (53) |
| Cases admitted to respective surveillance hospital | 565 (63) | 323 (35) | 564 (67) | 1104 (41) | 530 (45) |
| Diarrhoeal death cases (case fatality) | 2 (0·2) | 3 (0·3) | 3 (0·4) | 30 (1·1) | 3 (0·3) |
Defined as persons with frequent loose or watery stools during 12 months preceding the survey for which they had to be admitted to a healthcare facility, or had to receive intravenous rehydration, or died as a result of the new onset diarrhoeal illness.
Buying oral rehydration solution or medicine from pharmacy/drug sellers was considered as a healthcare-seeking event.
Fig. 3.
Number of severe diarrhoea cases in hospital catchment areas during one year preceding the survey and cholera cases in study hospitals during one year of surveillance period, 2010–2011.
About half of the severe diarrhoeal cases identified in hospital catchment areas were male (Table 2). Thirty-two percent of cases were aged <5 years and 58% of cases were aged >15 years. Male patients and children aged <5 years were more likely to be admitted to surveillance hospitals. All of the reported symptoms were similar in both admitted and non-admitted cases (Table 2). Out of 41 death cases, 42% were not admitted to any hospital and 27% did not visit a qualified healthcare provider during their illness. Only 37% of the death cases were admitted to surveillance hospitals. Of the death cases, 56% were aged <5 years, 12% were in the 5–15 years age group and 17% were aged ➮60 years.
Table 2.
Demographic characteristics and reported symptoms of severe diarrhoea cases in the catchment area of six surveillance hospitals in Bangladesh, 2010–2011
| Characteristics | Admitted to surveillance hospitals (N=2172) % |
Not admitted to surveillance hospitals (N= 2299) % |
Total (N=4471) % |
P value* |
|---|---|---|---|---|
| Male | 51 | 47 | 49 | <0·01 |
| Age group, years | ||||
| <5 | 40 | 24 | 32 | <0·001 |
| 5–15 | 10 | 9 | 10 | 0·12 |
| 16–60 | 44 | 57 | 50 | <0·001 |
| >60 | 6 | 10 | 8 | <0·001 |
| Reported symptoms during illness |
||||
| Fever | 58 | 58 | 58 | 0·95 |
| Vomiting | 83 | 80 | 82 | 0·13 |
| Unable to stay awake/ lethargy/drowsiness |
54 | 56 | 55 | 0·57 |
| Loss of consciousness | 18 | 17 | 17 | 0·5 |
| Small amount of urine | 54 | 57 | 56 | 0·23 |
| Sunken eyes | 90 | 89 | 89 | 0·8 |
| Death cases | 0·7 | 1·1 | 41 | 0·27 |
Cluster adjusted two-sample proportion test was applied to compare the characteristics between admitted and not admitted cases at surveillance hospitals.
In 2010, the total population in the defined hospital catchment areas ranged from 176 000 in Chhatak and 10 368 000 in Dhaka hospitals (Table 3). By adjusting the total cholera cases in surveillance hospitals by the proportion of severe diarrhoeal cases identified from the catchment area survey that were admitted to surveillance hospitals, we estimated the incidence of severe diarrhoea due to cholera in the defined catchment areas of surveillance hospitals per 1000 population; the lowest incidence was 0·3/1000 in Kumudini Hospital and highest incidence was 4·9/1000 in Mirpur Hospital catchment areas. For children age <5 years, incidence ranged from 1·0/1000 in Kumudini to 11·0/1000 in Mirpur (Table 3).
DISCUSSION
This study provides population-based data on incidence of severe diarrhoea due to cholera in six areas in Bangladesh which will be useful to inform decisions for effective control measures including the introduction of a cholera vaccine. The study results show variability in incidence at different sites across Bangladesh. Part of the variability in incidence estimates could have resulted from site selection, which was not random but based on existing surveillance systems. Indeed, four of the sites selected for microbiological surveillance (Matlab, Mirpur, Chhatak, Mathbaria) were originally selected because researchers interested in studying cholera believed these were sites with high incidence of cholera. However, we can categorize the estimated cholera incidence based on the geographical locations of the surveillance sites: incidence observed in Dhaka and Mirpur Hospital catchment areas as the cholera incidence in metropolitan cities in Bangladesh where the main source of drinking water is the municipal supply water (2·6–4·9/1000 population); incidence in Chhatak and Matlab as the incidence in flood-prone areas (1·1–3·7/1000 population); incidence in Mathbaria as the incidence in coastal areas (1·8/1000 population); and the incidence observed in Kumudini Hospital catchment area as the incidence in plain rural areas in Bangladesh (0·3/1000 population).
Higher incidence of cholera in an area might be related to local ecology as well as faecal contamination of drinking water sources which may differ in rural and urban settings [32, 33]. In the two urban sites, the highest incidence was observed in the Mirpur Hospital catchment area. Mirpur is a densely populated area and has one of the largest concentrations of slums in Dhaka city [34]. Slum settlements often have unhygienic latrines, poor garbage management systems, and sewers that overflow into houses during the rainy season. In most cases, latrines are linked with sewerage lines and municipal water pipes are commonly exposed to sewerage lines which may lead to faecal contamination of the supply water source [34]. In the two surveillance sites in low-lying, flood-prone areas, the highest incidence was observed in Chhatak. During October–December 2010, which is post-monsoon, there was a spike in diarrhoeal cases in Chhatak Hospital catchment area and a spike of cholera cases at Chhatak Hospital indicating a large cholera outbreak (Fig. 3). The lowest incidence was observed in Kumudini Hospital catchment area which is a flatland rural area that has no previous history of repeated cholera outbreaks.
Although diarrhoea is a simple and inexpensive disease to treat with qualified healthcare providers and adequate medical supplies [10], case fatality was markedly higher in flood-prone rural communities, specifically in Chhatak (1·1/100 cases). The higher number of diarrhoeal deaths in Chhatak might be related to delay in seeking treatment from qualified healthcare providers during the severe dehydration stage of diarrhoeal illness. In the rainy season, roads in many areas in Chhatak are inundated by flood water which can make it difficult to access qualified healthcare services. More than half (53%) of the death cases in Chhatak were not admitted to a hospital during their illness which indicates a lack of qualified treatment; however, at what stage of illness the remaining death cases were admitted to a hospital is unknown. Although the cost of vaccination per person would likely be higher in rural areas, the number of deaths averted per vaccination would likely also be higher if the vaccines were targeted to rural communities that have less access to effective curative care compared to urban communities.
In all the study sites, incidence of severe diarrhoea due to cholera was higher in children aged <5 years. Similar to some previous studies, we observed the highest proportion of diarrhoeal cases in children aged <5 years; however, this group included more non-cholera diarrhoeal cases than the patients in the older age groups [6, 10, 35]. Healthcare utilization patterns in different age groups may have influenced detection of cholera cases. During diarrhoeal illness, children are more likely to be taken to healthcare facilities, which is particularly important because children are at increased risk of diarrhoeal death compared to adults. More than half of the death cases in the hospital catchment areas were children aged <5 years. A study in a similar setting in Kolkata observed that children received treatment at a healthcare facility more frequently compared to adults during diarrhoeal illness [36].
There is limited data on population-based incidence of cholera in recent years in Bangladesh. A population-based cholera vaccine trial conducted during 1985–1986 in Matlab, Bangladesh, found cholera incidence in the placebo control group to be 4·6/1000 population which is considerably higher than our study findings in Matlab conducted 25 years later [14]. Another population-based cholera incidence study conducted during 2003–2004 in a small urban area in Kolkata similar to our urban sites found incidence of cholera to be 2·2/1000 population, a little lower than our findings in Dhaka [35]. A recent article on global cholera disease burden estimated cholera incidence in Bangladesh at 2·1/1000 population [37]. However, that estimation was not based on empirical results using methods that could be replicated, but rather was obtained from interviews with in-country experts in Bangladesh. Our study deployed a method to estimate the population-based incidence of cholera in different geographical areas in Bangladesh by combining the hospital-based surveillance and data obtained from a healthcare utilization survey that provides a more credible estimate as well as an approach that would permit this assessment to be repeated in other places and at other times. Although seasonality of cholera in Bangladesh may vary from year to year, we found that the seasonal peaks of cholera in the 2010–2011 study period (Fig. 3) were similar to other reported data from these regions in previous years [10, 24, 38].
We calculated the incidence of severe diarrhoea due to cholera in the catchment area of Matlab Hospital using only the hospital data assuming that all patients with severe diarrhoea in the catchment area were admitted to Matlab Hospital. This is clearly an overestimate as only 10 (8%) of the 119 people who died from diarrhoea in the Matlab Hospital catchment area were admitted to the Matlab Hospital according to a recent verbal autopsy study (2007–2011) (icddr,b, unpublished data). Nevertheless, we did not adjust the 100% estimate because we did not have data to make an evidence-based correction. The calculated estimate should be viewed as a minimum estimate.
There were some additional potential study limitations. First, we used the case definition of severe diarrhoea as a proxy to identify dehydrated patients with diarrhoea in hospital catchment areas during the previous 12 months who required immediate hospitalization and/or intravenous rehydration. It is possible that in order to make money some village healthcare providers occasionally gave intravenous rehydration even when not clinically indicated which would lead to an overestimation of the incidence of severe diarrhoea due to cholera. However, there were little differences in the reported symptoms in admitted and not admitted cases which indicates a similar level of dehydration in both admitted and not admitted diarrhoeal patients. On the other hand, there might have been some other patients infected with cholera who experienced severe diarrhoea, yet who only received oral rehydration or other treatment at home during their illness. This study did not count these cases which would lead to an underestimation of severe diarrhoea due to cholera. Moreover, we did not consider mild gastroenteritis illness and asymptomatic cases. The reported ratio of symptomatic to asymptomatic cholera infections has ranged from 3 to 100 [39]. The total incidence of cholera infections would be higher if we considered asymptomatic and mild cholera infections.
Second, since the number of cholera cases in an area varies over the years [40, 41], our estimated incidence may only be useful for 2010. However, we believe the health-seeking behaviour of community people does not change rapidly. Therefore, our estimated proportion of severe diarrhoea cases admitted to surveillance hospitals would be useful to estimate incidence of severe diarrhoea due to cholera in future years in the catchment areas of surveillance hospitals. Third, since in the catchment area of rural-based Kumudini, Chhatak and Mathbaria hospitals we identified severe diarrhoea cases using broader community awareness instead of house-to-house surveys, it is possible that some severe diarrhoea cases were missed. However, in the incidence calculation formula we used the proportion of severe diarrhoea cases who were admitted to surveillance hospitals instead of total number of severe diarrhoeal cases and therefore, missing cases would be absent in both numerator and denominator. If we consider the missing cases as randomly missing, this would not affect the incidence estimation.
Finally, for the confirmation of cholera cases, this study used a conventional culture method which remains the gold standard, but this procedure may yield false-negative results in case of inactivation of V. cholerae by in vivo vibriolytic action of the phage and/or non-culturability induced as a result of host response [42–44]. Rapid antigen-based diagnostic tests for cholera dipstick assays have identified 0–32% more cases than the conventional culture method in detecting V. cholerae antigens in stool samples [42, 45–47]. By not accounting for culture-negative V. cholerae cases we are underestimating total cholera incidence, but we did not adjust the incidence calculations for culture negatives because we did not have molecular evidence from this population to estimate the magnitude of the correction.
We identified cholera cases wherever we established diarrhoeal surveillance and throughout the multiple ecological zones in Bangladesh. Cholera is an ongoing public health problem in these communities. Data from this study can help inform assessments of the appropriateness and cost-effectiveness of interventions, including improvements of water quality, sanitation and hygiene, improved clinical services, and introduction of oral cholera vaccine. The study results can also be used by infections disease modellers to more accurately estimate the burden of cholera and so the impact of interventions. Special attention should be directed to high-risk groups, specifically children in urban areas and communities in hard-to-reach areas where case fatality is high. Current oral cholera vaccines are safe and effective [13–15]; Bangladesh should assess the cost-effectiveness of a potential vaccination programme for high-risk populations. The water and sanitation programmes provide a long-term and sustainable solution for the prevention of cholera [2, 48]; Bangladesh should take effective measures to improve the water and sanitation facilities in addition to cholera vaccination.
ACKNOWLEDGEMENTS
This work was funded by core donors which provide unrestricted support to icddr,b for its operation and research. Current donors providing unrestricted support include: Australian Agency for International Development (AusAID); Government of the People’s Republic of Bangladesh; Canadian International Development Agency (CIDA); Swedish International Development Cooperation Agency (Sida); and the Department for International Development, UK (DFID). The authors gratefully acknowledge these donors for their support and commitment to icddr,b’s research efforts. We would like to thank all the study participants, the surveillance hospitals and staff for their contribution in the study. We thank the field staff for their hard work in this study. We are thankful to Meghan Scott for her critical review of the manuscript.
Footnotes
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Snow J. On the mode of communication of cholera, 1855. Salud publica de Mexico. 1991;33:194–201. [PubMed] [Google Scholar]
- 2.Sack DA, et al. Cholera. Lancet. 2004;363:223–233. doi: 10.1016/s0140-6736(03)15328-7. [DOI] [PubMed] [Google Scholar]
- 3.Anon Large epidemic of cholera-like disease in Bangladesh caused by Vibrio cholerae O139 synonym Bengal. Cholera Working Group, International Centre for Diarrhoeal Diseases Research, Bangladesh. Lancet. 1993;342:387–390. [PubMed] [Google Scholar]
- 4.Nair GB, et al. Spread of Vibrio cholerae O139 Bengal in India. Journal of Infectious Diseases. 1994;169:1029–1034. doi: 10.1093/infdis/169.5.1029. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization. Cholera outbreak: assessing the outbreak response and improving preparedness: Global Task Force on Cholera Control. 2004 [Google Scholar]
- 6.Deen JL, et al. The high burden of cholera in children: comparison of incidence from endemic areas in Asia and Africa. PLoS Neglected Tropical Diseases. 2008;2:e173. doi: 10.1371/journal.pntd.0000173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.WHO. Cholera vaccines. Weekly Epidemiological Record. 2011;76:117–124. [Google Scholar]
- 8.WHO. Cholera surveillance and number of cases. Geneva: WHO; [Accessed 22 August 2012]. ( http://www.who.int/topics/cholera/surveillance/en/index.html). [Google Scholar]
- 9.Kimball AM, Wong KY, Taneda K. An evidence base for international health regulations: quantitative measurement of the impacts of epidemic disease on international trade. Revue Scientifique et Technique (International Office of Epizootics) 2005;24:825–832. [PubMed] [Google Scholar]
- 10.Sack RB, et al. A 4-year study of the epidemiology of Vibrio cholerae in four rural areas of Bangladesh. Journal of Infectious Diseases. 2003;187:96–101. doi: 10.1086/345865. [DOI] [PubMed] [Google Scholar]
- 11.Barua D. History of Cholera. New York: Plenum Medical Book; 1992. [Google Scholar]
- 12.Schwartz BS, et al. Diarrheal epidemics in Dhaka, Bangladesh, during three consecutive floods: 1988, 1998, and 2004. American Journal of Tropical Medicine and Hygiene. 2006;74:1067–1073. [PMC free article] [PubMed] [Google Scholar]
- 13.Lopez AL, et al. Cholera vaccines for the developing world. Human Vaccines. 2008;4:165–169. doi: 10.4161/hv.4.2.5122. [DOI] [PubMed] [Google Scholar]
- 14.Clemens JD, et al. Field trial of oral cholera vaccines in Bangladesh: results of one year of follow-up. Journal of Infectious Diseases. 1988;158:60–69. doi: 10.1093/infdis/158.1.60. [DOI] [PubMed] [Google Scholar]
- 15.Sur D, et al. Efficacy and safety of a modified killed-whole-cell oral cholera vaccine in India: an interim analysis of a cluster-randomised, double-blind, placebo-controlled trial. Lancet. 2009;374:1694–1702. doi: 10.1016/S0140-6736(09)61297-6. [DOI] [PubMed] [Google Scholar]
- 16.Andrews JR, Basu S. Transmission dynamics and control of cholera in Haiti: an epidemic model. Lancet. 2011;377:1248–1255. doi: 10.1016/S0140-6736(11)60273-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bertuzzo E, et al. Prediction of the spatial evolution and effects of control measures for the unfolding Haiti cholera outbreak. Geophysical Research Letters. 2011;38 L06403. [Google Scholar]
- 18.Mari L, et al. Modelling cholera epidemics: the role of waterways, human mobility and sanitation. Journal of the Royal Society Interface. 2012;9:376–388. doi: 10.1098/rsif.2011.0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tuite AR, et al. Cholera epidemic in Haiti, 2010: using a transmission model to explain spatial spread of disease and identify optimal control interventions. Annals of Internal Medicine. 2011;154:593–601. doi: 10.7326/0003-4819-154-9-201105030-00334. [DOI] [PubMed] [Google Scholar]
- 20.Chaignat CL, Monti V. Use of oral cholera vaccine in complex emergencies: what next? Summary report of an expert meeting and recommendations of WHO. Journal of Health, Population, and Nutrition. 2007;25:244–261. [PMC free article] [PubMed] [Google Scholar]
- 21.Clemens JD, et al. Field trial of oral cholera vaccines in Bangladesh: results from three-year follow-up. Lancet. 1990;335:270–273. doi: 10.1016/0140-6736(90)90080-o. [DOI] [PubMed] [Google Scholar]
- 22.Sur D, et al. Efficacy of a low-cost, inactivated whole-cell oral cholera vaccine: results from 3 years of follow-up of a randomized, controlled trial. PLoS Neglected Tropical Diseases. 2011;5:e1289. doi: 10.1371/journal.pntd.0001289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anwar I. Perceptions of quality of care for serious illness at different levels of facilities in a rural area of Bangladesh. Journal of Health, Population, and Nutrition. 2009;27:396–405. doi: 10.3329/jhpn.v27i3.3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alam M, et al. Seasonal cholera caused by Vibrio cholerae serogroups O1 and O139 in the coastal aquatic environment of Bangladesh. Applied and Environmental Microbiology. 2006;72:4096–4104. doi: 10.1128/AEM.00066-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.WHO. World Health Organization Guidelines for the laboratory diagnosis of cholera. Geneva: WHO Bacterial Disease Unit; 1974. [Google Scholar]
- 26.Daniel WW. Biostatistics: A Foundation for Analysis in the Health Sciences. 7th edn. New York: John Wiley & Sons; 1999. [Google Scholar]
- 27.Paul RC, et al. A novel low-cost approach to estimate the incidence of Japanese encephalitis in the catchment area of three hospitals in Bangladesh. American Journal of Tropical Medicine and Hygiene. 2011;85:379–385. doi: 10.4269/ajtmh.2011.10-0706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Homaira N, et al. Influenza-associated mortality in 2009 in four sentinel sites in Bangladesh. Bulletin of the World Health Organization. 2012;90:272–278. doi: 10.2471/BLT.11.095653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Biggs HM, et al. Estimating leptospirosis incidence using hospital-based surveillance and a population-based health care utilization survey in Tanzania. PLoS Neglected Tropical Diseases. 2013;7:e2589. doi: 10.1371/journal.pntd.0002589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bangladesh Population and Housing Census. [Accessed 21 August 2012];2011 ( http://www.bbs.gov.bd/Home.aspx).
- 31.Rogers WH. Regression standard errors in clustered samples. Stata Technical Bulletin. 1993;13:19–23. [Google Scholar]
- 32.Akanda AS, Jutla AS, Islam S. Dual peak cholera transmission in Bengal Delta: a hydroclimatological explanation. Geophysical Research Letters. 2009;36(19) [Google Scholar]
- 33.Bertuzzo E, et al. Hydroclimatology of dual-peak annual cholera incidence: insights from a spatially explicit model. Geophysical Research Letters. 2012;39(5) [Google Scholar]
- 34.Islam N, et al. Slums of urban Bangladesh: mapping and census. Dhaka: Centre for Urban Studies; 2005. [Google Scholar]
- 35.Sur D, et al. The burden of cholera in the slums of Kolkata, India: data from a prospective, community based study. Archives of Disease in Childhood. 2005;90:1175–1181. doi: 10.1136/adc.2004.071316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sur D, et al. Correlates for diarrhoea and treatment uptake in an impoverished slum area of Kolkata, India. Journal of Health, Population, and Nutrition. 2004;22:130–138. [PubMed] [Google Scholar]
- 37.Ali M, et al. The global burden of cholera. Bulletin of the World Health Organization. 2012;90:209–218A. doi: 10.2471/BLT.11.093427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Das SK, et al. Geographical diversity in seasonality of major diarrhoeal pathogens in Bangladesh observed between 2010 and 2012. Epidemiology and Infection. 2014;142:2530–2541. doi: 10.1017/S095026881400017X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.King AA, et al. Inapparent infections and cholera dynamics. Nature. 2008;454:877–880. doi: 10.1038/nature07084. [DOI] [PubMed] [Google Scholar]
- 40.Longini IM, Jr, et al. Epidemic and endemic cholera trends over a 33-year period in Bangladesh. Journal of Infectious Diseases. 2002;186:246–251. doi: 10.1086/341206. [DOI] [PubMed] [Google Scholar]
- 41.Carrel M, et al. Spatio-temporal clustering of cholera: the impact of flood control in Matlab, Bangladesh, 1983–2003. Health & Place. 2009;15:741–752. doi: 10.1016/j.healthplace.2008.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alam M, et al. Diagnostic limitations to accurate diagnosis of cholera. Journal of Clinical Microbiology. 2010;48:3918–3922. doi: 10.1128/JCM.00616-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Colwell RR, et al. Viable but non-culturable Vibrio cholerae O1 revert to a cultivable state in the human intestine. World Journal of Microbiology & Biotechnology. 1996;12:28–31. doi: 10.1007/BF00327795. [DOI] [PubMed] [Google Scholar]
- 44.Faruque SM, et al. Transmissibility of cholera: in vivo-formed biofilms and their relationship to infectivity and persistence in the environment. Proceedings of the National Academy of Sciences USA. 2006;103:6350–6355. doi: 10.1073/pnas.0601277103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boncy J, et al. Performance and utility of a rapid diagnostic test for cholera: notes from Haiti. Diagnostic Microbiology and Infectious Disease. 2013;76:521–523. doi: 10.1016/j.diagmicrobio.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 46.Ley B, et al. Evaluation of a rapid dipstick (Crystal VC) for the diagnosis of cholera in Zanzibar and a comparison with previous studies. PLoS ONE. 2012;7:e36930. doi: 10.1371/journal.pone.0036930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nato F, et al. One-step immunochromatographic dipstick tests for rapid detection of Vibrio cholerae O1 and O139 in stool samples. Clinical and Diagnostic Laboratory Immunology. 2003;10:476–478. doi: 10.1128/CDLI.10.3.476-478.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Waldman RJ, Mintz ED, Papowitz HE. The cure for cholera – improving access to safe water and sanitation. New England Journal of Medicine. 2013;368:592–594. doi: 10.1056/NEJMp1214179. [DOI] [PubMed] [Google Scholar]