Abstract
We evaluated whether delivering educational presentations on human papillomavirus (HPV) to American Indian mothers affected HPV vaccination rates in their adolescent daughters. In March-April 2012, we recruited Hopi mothers or female guardians with daughters aged 9–12 years for a cluster-randomized intervention study on the Hopi Reservation. Participants attended mother-daughter dinners featuring educational presentations for mothers on either HPV (intervention) or juvenile diabetes (control) and completed baseline surveys. Eleven months later, we surveyed mothers on their daughters’ HPV vaccine uptake. We also reviewed aggregated immunization reports from the Indian Health Service to assess community-level HPV vaccination coverage from 2007–2013. Ninety-seven mother-daughter dyads participated; nine mothers reported that their daughters completed the three-dose HPV vaccination series before recruitment. Among the remaining mothers, 63% completed the follow-up survey. Adjusting for household income, the proportion of daughters completing vaccination within 11 months post-intervention was similar in the intervention and control groups (32% vs. 28%, adjusted RR=1.2,95%CI:0.6–2.3). Among unvaccinated daughters, those whose mothers received HPV education were more likely to initiate vaccination (50% vs. 27%, adjusted RR=2.6,95%CI:1.4–4.9) and complete three doses (adjusted RR=4.0,95%CI:1.2-13.1) than girls whose mothers received diabetes education. Community-level data showed that 80% of girls aged 13–17 years and 20% of girls aged 11–12 completed the vaccination series by 2013. HPV vaccine uptake in Hopi girls aged 13–17 years is significantly higher than the U.S. national average. Brief educational presentations on HPV delivered to American Indian mothers might increase HPV vaccination rates in daughters aged 9–12 years.
Keywords: Human papillomavirus (HPV), American Indian/Alaska Native (AI/AN), vaccination, intervention, education
INTRODUCTION
High-risk types of human papillomavirus (HPV) are causally linked to cervical and other anogenital cancers [1], while certain low-risk types cause genital warts [2]. Safe and effective prophylactic HPV vaccines have been widely available in the U.S. since 2006 [3]. In that year, the U.S. Food and Drug Administration [3] approved a quadrivalent vaccine (Gardasil) that protects against HPV-16 and HPV-18, the HPV types linked to 70% of cervical cancers [4], and HPV-6 and HPV-11, the types linked to 90% of genital warts [5]. In 2009, a bivalent vaccine (Cervarix) against HPV-16 and HPV-18 was also approved [3]. Both vaccines are licensed for females and males aged 9–26 years and are administered as a three-dose series over six months. The U.S. Advisory Committee on Immunization Practices (ACIP) recommends vaccination for girls aged 11–12 years, with catch-up vaccination until age 26 years [3]. Despite these recommendations, as well as concerted public health efforts to promote HPV vaccination, uptake in adolescent females is suboptimal [6]. In 2013, according to the U.S. population-based National Immunization Survey-Teen (NIS-Teen), 57% of girls aged 13–17 years had initiated the vaccine series, but only 38% had completed all three doses [6].
HPV vaccine coverage varies across racial and ethnic groups. For example, NIS-Teen reported that receipt of ≥1 HPV vaccine dose was more prevalent among American Indian (AI)/Alaska Native (AN) girls aged 13–17 years than among their non-Hispanic White counterparts (73% vs. 53%). Nevertheless, among those who received ≥1 dose, the proportion who completed the full series was smaller in AI/AN versus non-Hispanic White girls (60% vs. 72%) [7]. Efforts to increase HPV vaccination coverage are particularly important for AI/ANs, because Native women have higher rates of cervical cancer incidence and mortality than non-Hispanic White women [8].
Mother-daughter interactions around health issues in adolescence, including HPV vaccination, have received notable attention in recent research [9, 10, 11]. Findings indicate that study designs engaging parents along with their children result in significantly better health outcomes [12]. For example, a “dyadic” design recognizes the importance of parental knowledge in the acceptability of HPV vaccination [13, 14] and capitalizes on the potential for mothers and daughters to act as reciprocal health educators and co-contributors to health decisions. This approach is especially appropriate in AI/AN contexts, where explicit family involvement in health decisions is a common cultural expectation [15, 16]. Therefore, we conducted a cluster-randomized trial with the Hopi Tribe in Arizona to engage AI mother-daughter dyads in educational social events. We then evaluated whether delivering educational presentations on HPV to mothers affected HPV vaccination rates in their daughters aged 9–12 years.
METHODS
Overview
In March–April 2012, we recruited mothers or female legal guardians (hereafter collectively called “mothers”) with daughters aged 9–12 years to attend dinners on the Hopi Reservation. Dinners included an educational presentation for mothers on either HPV (intervention) or juvenile diabetes (control). To assess the effect of the HPV educational presentation on HPV vaccination rates in 9–12 year old daughters, we surveyed all mothers on HPV vaccine uptake (both initiation and completion of the three-dose series) among their daughters approximately 11 months after the intervention. To establish a context for study results, we also reviewed aggregated immunization reports from the Indian Health Service (IHS) from 2007–2013 to assess community-level HPV vaccination coverage among adolescent Hopi girls.
The study protocol was developed in collaboration with tribal partners, local project staff, and community advisors, and was then reviewed and approved by the Hopi Tribal Council and the Institutional Review Boards of Cornell University and the University of Washington. All participants provided written informed consent before undertaking any study procedures.
Study setting
The Hopi Reservation is located in northeastern Arizona, encompassing approximately 1.6 million acres. According to the 2010 U.S. census, more than 7,000 enrolled members of the Hopi Tribe reside on the reservation in 12 village communities situated on or below 3 adjoining mesas. Fifty-one percent of the population are female, 35% are younger than 21 years, and 95% identify as AI.
All enrolled Hopi children <19 years are eligible for free HPV vaccination through the federally-funded Vaccines for Children program. Following the ACIP guidelines, 11–12 years is the target age range for HPV vaccination, although vaccines can be administered as early as age 9 or as late as age 26. Vaccines are administered at schools and IHS facilities. The latter use the Resource Patient Management System (RPMS), an electronic health record system that incorporates a module to record and track immunizations. This module enables public health nurses to track and follow-up patients who need to initiate or complete the HPV vaccination series.
Geographic clustering for intervention assignment
We grouped the 12 Hopi villages into 4 relatively proximate geographic clusters. We randomly assigned two clusters to the intervention group and two to the control group. For each cluster, a single central site was chosen to hold the dinners. Based on village population, two or three dinners were offered for each cluster, for a total of 11 dinners.
Recruitment
We used various recruitment methods, including notices posted in community centers, post offices, local stores and businesses, and other public locations; face-to-face distribution of flyers at community events; public service announcements on the tribal radio station; notices in village newsletters; and in-person presentations at community meetings. All messaging invited interested parties to contact local project staff by telephone for more information.
Using an intake script, staff gave study information to interested women, but did not disclose the study’s full aims. Women who indicated interest in participating were screened for eligibility. Inclusion criteria included age ≥18 years, enrollment in the Hopi Tribe, residence on the reservation, and being a mother or female legal guardian of a girl aged 9–12 years. Participants were given a choice of dinner dates based on village of residence. Before each dinner, participants were mailed letters confirming the date, time, and location of their assigned dinner. A consent form was included along with a stamped, return-addressed envelope. Participants were asked to return the completed consent form by mail or to bring it to the dinner.
Educational intervention
Development of the HPV educational invention was informed by focus group discussions with Hopi parents to assess their knowledge, attitudes, and beliefs about HPV and the HPV vaccine. Focus group methods and results are described in a separate manuscript, currently in preparation. Using the results, study investigators created a PowerPoint presentation with information on HPV prevalence and transmission, HPV vaccine recommendations, dosage schedule, and vaccine efficacy and safety. An educational brochure with similar content was also created to accompany the presentation. After all content was developed, it was reviewed by community advisors, local project staff, staff from the Hopi Office of Prevention and Intervention Cancer Support Services (a tribally run program that offers cancer prevention education and screening), Community Health Representatives (lay health workers who provide outreach health services to tribal members on the reservation), and a small sample of tribal employees, all of whom were tribal members and parents.
The control group presentation was developed by staff from the Hopi Special Diabetes Program, a primary prevention effort supported by the federally-funded Special Diabetes Program for Indians. The presentation was based on material from the IHS Division of Diabetes Treatment and Prevention, with a focus on risk factors for type 2 juvenile diabetes, healthy nutrition, physical activity, and what parents can do to prevent or manage diabetes for their children.
After confirmation of written informed consent, each social event began with activities involving participants and their daughters, followed by dinner and entertainment. After the entertainment, daughters went to a separate, adjoining room where they took part in a health-related activity. Simultaneously, mothers received the educational presentation corresponding to their group assignment (HPV or diabetes) in the dining room. Presentations lasted 30–40 minutes and included time for questions. Research team members delivered the HPV presentation; Hopi Special Diabetes Program staff delivered the diabetes presentation.
Post-education survey
After each presentation, mothers in both groups were asked to complete a brief survey to collect baseline information about their familiarity with HPV before the dinner; their individual and household demographics; their cultural beliefs and practices; and their daughters’ HPV vaccination history.
Follow-up survey
Beginning 10 months after the first dinners concluded, study staff telephoned participants to ask if their daughters had been vaccinated against HPV. Participants received up to three telephone calls within a two-week period. For those unreachable by telephone, a follow-up survey was mailed with a stamped, return-addressed envelope. Non-responders were sent a second follow-up mailing two weeks later. Three different versions of the follow-up survey were used, with each tailored to the number of HPV doses reported for the participant’s daughter on the post-education survey (i.e., 0, 1, or 2). Participants who reported that their daughter had already received all three doses of the HPV vaccine at baseline were not contacted for follow-up.
Debriefing
After the follow-up survey, all participants were sent debriefing letters explaining the full purpose of the study. Participants in the control group were also sent the brochure developed for the HPV educational invention. In addition, project team members gave two interviews on the tribal radio station about HPV and HPV vaccination.
Measurement of community-level vaccination coverage
To describe trends in community-level HPV vaccination coverage before and after the mother-daughter dinners (February 1, 2007, through February 1, 2013), we extracted aggregate village-level data from the RPMS immunization module at the IHS Hopi Health Care Center (HHCC). RPMS enables the generation of aggregate data reports on immunization among girls aged 11–12 and 13–17 years. For each calendar year, denominator data were restricted to “active clinic users,” defined as the number of girls in the priority age range who completed ≥2 visits to HHCC in the three years prior to February 1. An estimated 90–95% of Hopi girls living on the Hopi Reservation meet the criteria for active clinic users. The numerator in each group was defined as the number who had received either a minimum of one dose or all three doses of HPV vaccine, either at HHCC or at school, as of February 1 of the calendar year.
Analysis
Poisson regression with robust standard errors was used to compare demographic characteristics, mothers’ familiarity with HPV, and randomization assignment between dyads in which mothers did and did not complete the follow-up survey. Robust standard errors were used because mothers who attended the same dinner might be more similar than mothers who attended different dinners. The same methods were used to compare characteristics between dyads that received HPV versus diabetes education. To protect confidentiality, counts and cell sizes <5 are not reported.
Dyads in which the mother did not complete the follow-up survey were excluded from analyses related to follow-up vaccination status. Among daughters reported to have received 0, 1, 2, or an unknown number of doses of HPV vaccine at baseline, we calculated the proportion who completed the 3-dose series. Among daughters reported to be unvaccinated or to have unknown vaccination status at baseline, we calculated the proportions who initiated the series (received ≥1 dose) and completed it (received all three doses). Poisson regression with robust standard errors was used to estimate the relative risk for HPV vaccination initiation and completion in daughters whose mothers received either HPV or diabetes education. Although group assignment was cluster-randomized, we also performed an analysis adjusting for covariates that showed significant imbalance at baseline between the intervention and control groups.
For community-level prevalence of HPV vaccination, we computed the proportion and exact 95% confidence interval of girls who had initiated and completed the HPV vaccine series by February 1 of each calendar year (2007–2013), stratified by age group (11–12 and 13–17 years).
RESULTS
Ninety-seven mother-daughter dyads participated. Among 88 mothers who reported at baseline that their daughters had received <3 doses of HPV vaccine, 55 (63%) completed the follow-up survey. The mean follow-up time was 11 months (range:10.2–11.8). Demographic characteristics and HPV familiarity were not significantly different in women who did and did not return the follow-up survey (data not shown). Dyads who attended the diabetes dinners were lost to follow-up more often than those who attended the HPV dinners (46% vs. 40%), but the difference was not statistically significant (p=0.28).
The mean age of mothers was 41 (SD:10) years. About half (48%) of daughters were aged 9–10 years, and about half (52%) were aged 11–12 years. Mothers’ baseline demographic characteristics were similar among dyads in the intervention and control groups, except for income (Table 1). Mothers in the intervention group had lower household incomes than mothers in the control group (48% vs. 36% with income <$16,000, p=0.04).
Table 1.
Demographic characteristics and familiarity with human papillomavirus (HPV) among Hopi mothers a and daughters who participated in mother-daughter educational dinners on the Hopi Reservation between March and April 2012 b
| Baseline characteristic | Intervention | Control | p-value | ||
|---|---|---|---|---|---|
| Mothers/caregivers | (n=43) | (n=54) | |||
|
| |||||
| Age, mean years (standard deviation) | 42 | (12) | 40 | (9) | 0.27 |
| Married or living with partner, n (%) | 0.46 | ||||
| Yes | 24 | (57) | 34 | (63) | |
| No | 18 | (43) | 20 | (37) | |
| Education completed, n (%) | 0.90 | ||||
| High school or less | 18 | (42) | 24 | (45) | |
| 2-year college degree, technical or vocational school | 20 | (47) | 23 | (43) | |
| 4-year college degree or higher | 5 | (12) | 6 | (11) | |
| Working full-time or part-time, n (%) | 0.66 | ||||
| Yes | 32 | (74) | 39 | (72) | |
| No | 11 | (26) | 15 | (28) | |
| Household income for the past 12 months, n (%) | 0.04 | ||||
| Less than $16,000 | 20 | (48) | 19 | (36) | |
| $16,000 – $34,999 | 16 | (38) | 18 | (34) | |
| At least $35,000 | 6 | (14) | 16 | (30) | |
| Ability to speak Hopi or Tewa language, n (%) | 0.62 | ||||
| Not at all | 8 | (19) | 16 | (30) | |
| A little, but not very well | 5 | (12) | 8 | (15) | |
| Moderately well | 15 | (36) | 16 | (30) | |
| Very well | 14 | (33) | 14 | (26) | |
| HPV familiarity c, n (%) | 0.20 | ||||
| Not familiar with HPV | 19 | (44) | 14 | (26) | |
| Somewhat familiar | 15 | (35) | 27 | (50) | |
| Very familiar | 9 | (21) | 13 | (24) | |
|
| |||||
| Daughters | (n=43) | (n=54) | |||
|
| |||||
| Age in years at the time of the dinner, n (%) | 0.25 | ||||
| 9–10 | 24 | (56) | 22 | (42) | |
| 11–12 | 19 | (44) | 31 | (58) | |
Mothers and female legal guardians were enrolled, collectively termed “mothers”;
Numbers might not add to total because of missing values;
The question for the intervention group was phrased, “Before tonight, had you ever heard about HPV?” whereas the question for the control group was phrased, “Have you ever heard of HPV?”
At baseline, all mothers of daughters aged 9–10 years reported that their daughters were unvaccinated or that they did not know their vaccination status. Among mothers of daughters aged 11–12 years, 32% reported that their daughters were unvaccinated, 12% reported that they did not know their vaccination status, and 56% reported that they had received ≥1 dose of HPV vaccine (including 18% reported to have completed the three-dose vaccine series). The proportion of daughters aged 11–12 years reported to have received ≥1 dose of HPV vaccine prior to baseline was higher in the intervention group than in the control group (58% vs. 55%), but the difference was not statistically significant (p=0.74). Most mothers of daughters who were unvaccinated or whose vaccination status was unknown reported that they would consider having their daughters vaccinated (86%). Mothers in the intervention group were more likely to report they would consider having their daughters vaccinated than those in the control group, but the difference was not statistically significant (90% vs. 83%, p=0.42). Among nine mothers who reported that they would not consider the HPV vaccine for their daughters, the most common reasons were insufficient knowledge of the vaccine, belief that their daughters were too young for the vaccine or not sexually active, and worry about vaccine safety.
Among daughters with <3 or an unknown number of vaccine doses reported at baseline, the proportion who completed vaccination during follow-up was similar in the intervention and control groups (32% vs. 28%, RR=1.2, 95%CI:0.6–2.3) (Table 2). Among daughters who were unvaccinated or had unknown vaccination status at baseline, the proportion who initiated vaccination (50% vs. 27%, RR=1.8, 95%CI:0.8–4.4) and who completed all three doses (RR=3.0, 95%CI:0.8-10.8) during follow-up was substantially higher in intervention versus control dyads.
Table 2.
Effect of group assignment on initiation and completion of human papillomavirus (HPV) vaccination a for daughters 11 months after baseline.
| Vaccination initiation or completion
|
Unadjusted RR (95% CI) |
Adjusted b RR (95% CI) |
||||
|---|---|---|---|---|---|---|
| Yes | No | |||||
| Among all daughters: | ||||||
|
| ||||||
| Vaccine completion (3 doses), n (%) | ||||||
| Control group (diabetes education) | 8 | (28) | 21 | (72) | 1.0 | 1.0 |
| Intervention group (HPV education) | 8 | (32) | 17 | (68) | 1.2 (0.6 – 2.3) | 1.1 (0.5 – 2.3) |
|
| ||||||
| Among daughters who had received no vaccinations c at baseline: | ||||||
|
| ||||||
| Vaccine initiation (≥1 dose), n (%) | ||||||
| Control group (diabetes education) | 6 | (27) | 16 | (73) | 1.0 | 1.0 |
| Intervention group (HPV education) | 11 | (50) | 11 | (50) | 1.8 (0.8 – 4.4) | 2.6 (1.4 – 4.9) |
| Vaccine completion (3 doses)d | ||||||
| Control group (diabetes education) | - | - | - | - | 1.0 | 1.0 |
| Intervention group (HPV education) | - | - | - | - | 3.0 (0.8 – 10.8) | 4.0 (1.2 – 13.1) |
Vaccination status was reported by mothers; daughters fully vaccinated at baseline were excluded from analyses;
Adjusted for income;
No vaccinations or mother did not know number of vaccinations at baseline;
Numbers and percentages are not provided for this comparison due to a cell size <5;
CI = confidence interval.
Compared to the unadjusted analysis, analyses adjusted for household income showed a similar association between group assignment and vaccine completion among daughters with fewer than three or an unknown number of vaccine doses reported at baseline (RR=1.1, 95%CI:0.5–2.3). Among daughters who were unvaccinated or had unknown vaccination status at baseline, however, analyses adjusted for household income showed a higher likelihood of vaccine initiation (RR=2.6, 95%CI:1.4–4.9) and completion (RR=4.0, 95%CI:1.2–13.1) in intervention versus control participants.
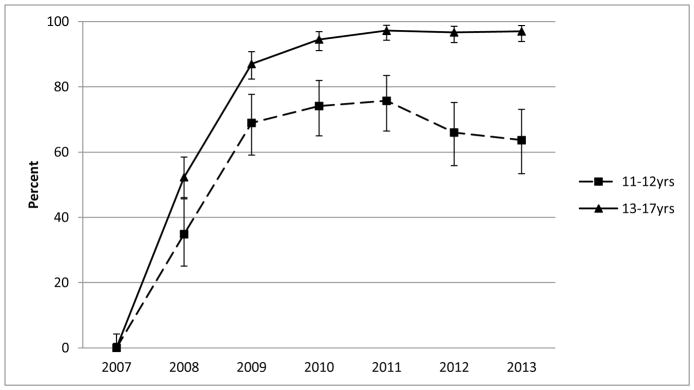
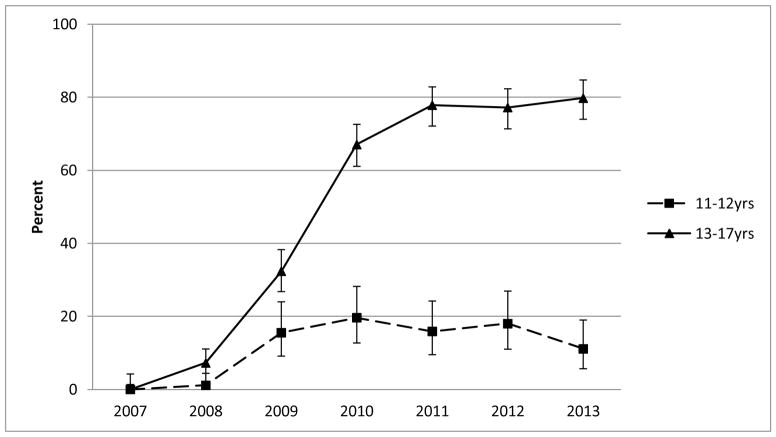
A sharp uptake in the community-level prevalence of vaccine initiation occurred between 2007–2009 for Hopi girls in both age cohorts evaluated (11–12 and 13–17 years; Figure 1). The prevalence of vaccine initiation was relatively stable from 2010–2013, but was higher for the older cohort (range: 95–97%) than the younger cohort (range: 64–76%). From 2010–2013, the community-level prevalence of vaccine completion was higher for girls aged 13–17 years (range: 67–80%) than for girls aged 11–12 years (range: 11–20%) (Figure 2).
Figure 1.
Community-level prevalence of human papillomavirus (HPV) vaccine initiation (minimum of one dose) among Hopi girls according to age (2007–2013). For each data point, the denominator was defined as the number of girls in each age range who had made at least two visits to the Hopi Health Care Center within three years before February 1 of each calendar year, and the numerator was defined as the number who had initiated the HPV vaccine series as of February 1. Error bars represent 95% confidence intervals.
Figure 2.
Community-level prevalence of human papillomavirus (HPV) vaccine completion (three doses) among Hopi girls according to age (2007–2013). For each data point, the denominator was defined as the number of girls in each age range who had made at least two visits to the Hopi Health Care Center within three years before February 1 of each calendar year, and the numerator was defined as the number who had completed the HPV vaccine series as of February 1. Error bars represent 95% confidence intervals.
DISCUSSION
We observed a positive effect of the HPV educational intervention on uptake of ≥1 dose of the HPV vaccination series, as well as uptake of all three doses, among girls who were unvaccinated or had unknown vaccination status when the intervention began. However, the intervention had no observed effect on the completion of all three doses among girls who had already initiated the HPV vaccination before the study began.
Based on study findings, we estimate that by 2013, 97% of Hopi girls aged 13–17 years and living on the Hopi Reservation had initiated the three-dose HPV vaccination series, and 80% had completed the series. Actual HPV vaccination coverage might be slightly lower, because our sample did not capture an estimated 5–10% of adolescent Hopi girls living on the reservation. Nonetheless, these proportions are significantly higher than those reported for HPV vaccine initiation (57%) and completion (38%) among all U.S. girls aged 13–17 years [6]. Our estimates are also higher than those reported in 2013 among AI/AN girls aged 13–17 years for vaccine initiation (73%, 95%CI:59–88%) and completion (43%, 95%CI:29–57%) [7]. High rates of HPV vaccine coverage in adolescent Hopi girls likely result from a combination of access to free vaccination through the Vaccines for Children program and efforts by public health nurses to track and follow-up adolescents due for vaccination.
However, vaccine coverage was significantly lower in Hopi girls aged 11–12 years, with estimates of 76% for vaccine initiation and 20% for completion in 2013. The differences between the two age groups suggest that fewer Hopi girls are initiating and completing the series at the recommended age of 11–12 years. As the ACIP recommendations were designed to prioritize girls for vaccination before their sexual debut, full protection against the HPV types included in current vaccines might be compromised by delayed vaccination initiation.
In our post-education survey, most mothers of unvaccinated daughters indicated that they would be willing to vaccinate. Among the minority who reported that they would not consider vaccination, the reasons cited were similar to those in previous studies of HPV vaccine acceptability in parents of adolescent girls: insufficient knowledge of the vaccine, beliefs that daughters were too young or not yet sexually active, and concerns about safety [17]. In a survey of health care providers in 12 IHS areas, similar concerns over vaccine safety and daughters’ youth or sexual inactivity were reported as common parental barriers to HPV vaccination, although beliefs regarding youth and sexual inactivity were more common in parents of girls aged 9–12 years versus 13–18 years [18].
To our knowledge, this was the first study to test a parental educational intervention for adolescent HPV vaccine uptake by using both a randomized, controlled design and HPV vaccine uptake as an outcome. Other studies testing parental educational interventions have reported varying results with respect to HPV vaccine uptake and intention to vaccinate. In the single prior investigation with vaccine uptake as an outcome [19], 24 parents of 11–12 year old girls were enrolled in a study with a quasi-experimental design to assess the effect of an educational brochure and reminder system for parents on HPV vaccine uptake. Over 13 months of follow-up, rates of vaccine initiation and series completion were higher in the intervention group than in a historical control group. In addition, two randomized, controlled trials [20, 21] and three quasi-experimental studies [22, 23, 24] have tested parental interventions consisting of printed fact sheets on HPV. The three quasi-experimental designs reported significant increases in HPV vaccination intention among parents who read the fact sheets, whereas the two randomized, controlled trials found no significant effect of the intervention on parental intentions to vaccinate. Another randomized, controlled trial reported that administering a brief radio novela on HPV and HPV vaccination to Latino parents had no significant effect on HPV vaccination intention [25]. Finally, delivery of a one-hour educational presentation on HPV to parents of Appalachian girls was effective in increasing vaccination intention [26].
We note four limitations of the present study. First, statistical power was limited by the small sample size and 37% loss to follow-up for post-dinner ascertainment of HPV vaccine status. The high loss-to-follow-up rate is attributable to several factors. Few Hopi residences have telephone service, and many of the mobile numbers provided during screening were no longer active at follow-up. Furthermore, reservation residences do not have private mailboxes, and the inconvenience of traveling to the post office may have hindered response to mailed follow-up surveys. In addition, the Hopi population is highly mobile.
Second, ascertainment of vaccine uptake was limited to a follow-up survey conducted <12 months after the intervention. We might have seen a stronger intervention effect with a longer follow-up period, especially given the inclusion of dyads with daughters aged 9–10 years. Third, daughters’ vaccination status was ascertained by parental report rather than by medical record review. Nevertheless, the proportion of Hopi girls aged 11–12 years reported to have received ≥1 dose of HPV vaccine at baseline was similar to the proportion estimated from 2012 HHCC medical record data on girls in the same age range. Finally, the process of reading and responding to HPV questions in the post-education survey might have prompted mothers in the control group to vaccinate their daughters or to ask their health care providers about the HPV vaccine, potentially attenuating the observed effect of the intervention.
In conclusion, mothers’ receipt of a brief educational presentation on HPV appeared to increase HPV vaccine uptake in their unvaccinated daughters. Given high rates of cervical cancer in AI/AN women [8], parental education delivered through a parent-child dyadic approach should be considered in other AI/AN communities as a strategy to improve HPV vaccination uptake. Additional strategies should be explored for increasing completion of the three-dose HPV vaccination series among AI/AN adolescents who initiate the series.
Acknowledgments
This research was performed under the auspices of the Collaborative to Improve Native Cancer Outcomes, a P50 program project sponsored by the National Cancer Institute (grant no. 1P50CA148110). The National Cancer Institute had no involvement in the study design; collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
We are grateful to the Hopi and Tewa women who participated in this project, and to the Hopi Tribal Council and Lorencita Joshweseoma for their support. We thank Phyllis Winans at the Hopi Health Care Center for her assistance in extracting the community-level HPV vaccination data; we thank Carey Onsae at the Hopi Health Care Center for her assistance leading the health activity for daughters attending the dinners; we thank our local project coordinators, Olivia Dennis and Lorene Vicente, for their coordination efforts; and we thank our community advisors, Carrie Watahomagie, Lisa Lomavaya, and Marilyn Fredericks, for their input and advice. For editing the final manuscript, we also thank Raymond Harris.
Funding: This research was performed under the auspices of the Collaborative to Improve Native Cancer Outcomes, a P50 program project sponsored by the National Cancer Institute (grant no. 1P50CA148110).
Footnotes
Conflict of Interest: The authors declare that they have no conflict of interest.
The Collaborative to Improve Native Cancer Outcomes includes D. Buchwald, D.R. Flum, E.M. Garroutte, A.A. Gonzales, J.A. Henderson, P. Nez Henderson, D.L. Patrick, S.P. Tu, and R.L. Winer.
References
- 1.Forman D, de Martel C, Lacey CJ, Soerjomataram I, Lortet-Tieulent J, Bruni L, et al. Global burden of human papillomavirus and related diseases. Vaccine. 2012;30(Suppl 5):F12–23. doi: 10.1016/j.vaccine.2012.07.055. [DOI] [PubMed] [Google Scholar]
- 2.Gissmann L, Wolnik L, Ikenberg H, Koldovsky U, Schnurch HG, zur Hausen H. Human papillomavirus types 6 and 11 DNA sequences in genital and laryngeal papillomas and in some cervical cancers. Proc Natl Acad Sci U S A. 1983;80(2):560–563. doi: 10.1073/pnas.80.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Markowitz LE, Dunne EF, Saraiya M, Chesson HW, Curtis CR, Gee J, et al. Human papillomavirus vaccination: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2014;63(RR-05):1–30. [PubMed] [Google Scholar]
- 4.Munoz N, Bosch FX, Castellsague X, Diaz M, De Sanjose S, Hammouda D, et al. Against which human papillomavirus types shall we vaccinate and screen? the international perspective. Int J Cancer. 2004;111(2):278–285. doi: 10.1002/ijc.20244. [DOI] [PubMed] [Google Scholar]
- 5.Bryan JT. Developing an HPV vaccine to prevent cervical cancer and genital warts. Vaccine. 2007;25(16):3001–3006. doi: 10.1016/j.vaccine.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 6.Stokley S, Jeyarajah J, Yankey D, Cano M, Gee J, Roark J, et al. Human papillomavirus vaccination coverage among adolescents, 2007–2013, and postlicensure vaccine safety monitoring, 2006–2014--United States. MMWR Morb Mortal Wkly Rep. 2014;63(29):620–624. [PMC free article] [PubMed] [Google Scholar]
- 7.Elam-Evans LD, Yankey D, Jeyarajah J, Singleton JA, Curtis RC, MacNeil J, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years--United States, 2013. MMWR Morb Mortal Wkly Rep. 2014;63(29):625–633. [PMC free article] [PubMed] [Google Scholar]
- 8.Watson M, Benard V, Thomas C, Brayboy A, Paisano R, Becker T. Cervical cancer incidence and mortality among American Indian and Alaska Native women, 1999–2009. Am J Public Health. 2014;104(Suppl 3):S415–422. doi: 10.2105/AJPH.2013.301681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dempsey AF, Abraham LM, Dalton V, Ruffin M. Understanding the reasons why mothers do or do not have their adolescent daughters vaccinated against human papillomavirus. Ann Epidemiol. 2009;19(8):531–538. doi: 10.1016/j.annepidem.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mosavel M, Thomas T. Daughter-initiated health advice to mothers: perceptions of African-American and Latina daughters. Health Educ Res. 2009;24(5):799–810. doi: 10.1093/her/cyp015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mosavel M. The feasibility of mothers accepting health advice from their adolescent daughters. J Health Care Poor Underserved. 2009;20(1):42–49. doi: 10.1353/hpu.0.0102. [DOI] [PubMed] [Google Scholar]
- 12.Evans D, Clark NM, Levison MJ, Levin B, Mellins RB. Can children teach their parents about asthma? Health Educ Behav. 2001;28(4):500–511. doi: 10.1177/109019810102800409. [DOI] [PubMed] [Google Scholar]
- 13.Fazekas KI, Brewer NT, Smith JS. HPV vaccine acceptability in a rural Southern area. J Womens Health (Larchmt) 2008;17(4):539–548. doi: 10.1089/jwh.2007.0489. [DOI] [PubMed] [Google Scholar]
- 14.Marlow LA, Waller J, Wardle J. Trust and experience as predictors of HPV vaccine acceptance. Hum Vaccin. 2007;3(5):171–175. doi: 10.4161/hv.3.5.4310. [DOI] [PubMed] [Google Scholar]
- 15.Cohen K. Native American medicine. Altern Ther Health Med. 1998;4(6):45–57. [PubMed] [Google Scholar]
- 16.Reid R, Rhoades ER. Cultural considerations in providing care to American Indians. In: Rhoades ER, editor. American Indian Health: Innovations in health care, promotion and policy. Baltimore: Johns Hopkins University Press; 2000. pp. 418–25. [Google Scholar]
- 17.Walhart T. Parents, adolescents, children and the human papillomavirus vaccine: a review. Int Nurs Rev. 2012;59(3):305–311. doi: 10.1111/j.1466-7657.2012.00991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jim CC, Lee JW, Groom AV, Espey DK, Saraiya M, Holve S, et al. Human papillomavirus vaccination practices among providers in Indian health service, tribal and urban Indian healthcare facilities. J Womens Health (Larchmt) 2012;21(4):372–378. doi: 10.1089/jwh.2011.3417. [DOI] [PubMed] [Google Scholar]
- 19.Cassidy B, Braxter B, Charron-Prochownik D, Schlenk EA. A quality improvement initiative to increase HPV vaccine rates using an educational and reminder strategy with parents of preteen girls. J Pediatr Health Care. 2014;28(2):155–164. doi: 10.1016/j.pedhc.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 20.Dempsey AF, Zimet GD, Davis RL, Koutsky L. Factors that are associated with parental acceptance of human papillomavirus vaccines: a randomized intervention study of written information about HPV. Pediatrics. 2006;117(5):1486–1493. doi: 10.1542/peds.2005-1381. [DOI] [PubMed] [Google Scholar]
- 21.Kennedy A, Sapsis KF, Stokley S, Curtis CR, Gust D. Parental attitudes toward human papillomavirus vaccination: evaluation of an educational intervention, 2008. J Health Commun. 2011;16(3):300–313. doi: 10.1080/10810730.2010.532296. [DOI] [PubMed] [Google Scholar]
- 22.Basu P, Mittal S. Acceptability of human papillomavirus vaccine among the urban, affluent and educated parents of young girls residing in Kolkata, Eastern India. J Obstet Gynaecol Res. 2011;37(5):393–401. doi: 10.1111/j.1447-0756.2010.01371.x. [DOI] [PubMed] [Google Scholar]
- 23.Chan SS, Cheung TH, Lo WK, Chung TK. Women’s attitudes on human papillomavirus vaccination to their daughters. J Adolesc Health. 2007;41(2):204–207. doi: 10.1016/j.jadohealth.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 24.Davis K, Dickman ED, Ferris D, Dias JK. Human papillomavirus vaccine acceptability among parents of 10- to 15-year-old adolescents. J Low Genit Tract Dis. 2004;8(3):188–194. doi: 10.1097/00128360-200407000-00005. [DOI] [PubMed] [Google Scholar]
- 25.Kepka D, Coronado GD, Rodriguez HP, Thompson B. Evaluation of a radionovela to promote HPV vaccine awareness and knowledge among Hispanic parents. J Community Health. 2011;36(6):957–965. doi: 10.1007/s10900-011-9395-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spleen AM, Kluhsman BC, Clark AD, Dignan MB, Lengerich EJ, Force AHCT. An increase in HPV-related knowledge and vaccination intent among parental and non-parental caregivers of adolescent girls, age 9–17 years, in Appalachian Pennsylvania. J Cancer Educ. 2012;27(2):312–319. doi: 10.1007/s13187-011-0294-z. [DOI] [PubMed] [Google Scholar]